Abstract
Knowledge of the diet and trophic ecology of apex predators is key for the implementation of effective ecosystem as well as species-based management initiatives. Using a combination of stomach content data and stable isotope analysis (δ15N and δ13C) the current study provides information on size-based and sex-specific variations in diet, trophic position (TP) and foraging habitat of tiger sharks (Galeocerdo cuvier) caught in the KwaZulu-Natal Sharks Board bather protection program. This study presents the longest time-series and most detailed analysis of stomach content data for G. cuvier worldwide. Prey identified from 628 non-empty stomachs revealed a size-based shift in diet. Reptiles, birds, mysticetes, and large shark species increased in dietary importance with G. cuvier size, concomitant with a decrease in smaller prey such as batoids and teleosts. Seasonal and decadal shifts in diet driven primarily by changes in the importance of elasmobranchs and mammal (cetacean) prey were recorded for medium sized (150–220 cm) G. cuvier. Both stomach content and stable isotope analysis indicated that G. cuvier is a generalist feeder at the population level. Size-based δ13C profiles indicated a movement to offshore foraging habitats by larger G. cuvier. Calculated TP varied by method ranging from 4.0 to 5.0 (TPSCA for stomach contents) and from 3.6 to 4.5 (TPscaled and TPadditive for δ15N). Large (> 220 cm) G. cuvier did not feed at discrete trophic levels, but rather throughout the food web. These data provide key information on the ecological role of G. cuvier to improve the accuracy of regional food web modelling. This will enable a better understanding of the ecological impacts related to changes in the abundance of this predator.
Introduction
Large sharks are one of the most ecologically important group of animals in coastal and open ocean systems [1, 2, 3]. Overfishing and habitat loss, however, has increasingly resulted in declines in some populations [4, 5, 6]. Sharks can affect the structure and function of the ecosystem at the community level through both direct predation and risk effects [1, 7]. As a result, understanding the potential of top predator removal is critical. Some studies suggest their potential for driving large scale cascading effects within food webs [2, 3, 8] while more recent analysis suggests this may not be the case [9, 10, 11]. To improve our understanding of the wider ecological consequences of predator removal, detailed information is required on species’ ecological roles. In particular, information is required on diet and dietary switches with body size and sex as these ultimately determine the species trophic position [2, 12, 13].
The diet and trophic ecology of large sharks can be assessed using a range of techniques. Each technique, however, has its own limitations and biases, which need to be considered when designing studies and interpreting results. Direct observation of feeding behavior provides the most accurate information on food ingested, however, this is impractical for almost all shark species as they are difficult to observe, highly mobile and wide ranging. Traditionally, stomach content analysis (SCA) has been used to provide an insight into the type and diversity of prey consumed [14, 15, 16]. Although it provides detailed taxonomic resolution of prey, limitations arise due to the snapshot nature of recently consumed prey, regurgitation of prey upon capture, differential digestion rates of prey and the misidentification of prey [12, 17, 18].
More recently, stable carbon and nitrogen isotope analysis (SIA) has emerged as a complementary tool to SCA and has provided new insights into the trophic relationships among sharks and the ecosystems they inhabit [19, 20]. Stable isotope analysis is based on the fact that ratios of carbon (13C/12C) and nitrogen (15N/14N) isotopes in a predator’s tissues reflect the isotopic composition of its prey and foraging location over both time and space [19, 21, 22]. Carbon isotopes reflect variation in baseline producers and hence foraging habitat of the predator [23, 24, 25] whereas nitrogen isotopes indicate its relative trophic position (TP) within the food web [13, 26, 27].
Most studies to date utilise the isotopic values within muscle tissue, collected from multiple animals, to examine feeding behaviour at the population level [28, 29]. However, there is growing evidence that individuals within a population may exhibit different dietary preferences and foraging behaviors [21, 29, 30]. Analysis of multiple tissue types with varying turnover rates allows for the investigation of isotopic variation within and among individuals [22, 31, 32]. Determining the proportion of specialist and generalist feeders within a population is required to better understand the full range of trophic roles a population utilises [33, 34].
Tiger sharks (Galeocerdo cuvier) are found worldwide in tropical and warm-temperate coastal and pelagic waters [35]. In the West Indian Ocean (WIO), they occur from the Red Sea to the east coast of South Africa, as well as off Madagascar [36]. In South Africa, their principal range extends from the Mozambique border to Cape St Francis [37, 38]. They are one of the largest apex predators growing to at least 550 cm total length (TL) [39, 40, 41] and are known to consume a wide variety of both invertebrate and vertebrate prey [37, 42, 43]. Ontogenetic shifts in their diet have been identified with larger prey becoming more important with increasing shark size [42, 43, 44].
The movement patterns and foraging habitat of G. cuvier has been studied at various locations including Australia [45, 46], Hawaii [47, 48, 49], the southwest Pacific [50], and the northwest Atlantic [51]. These studies indicate that G. cuvier utilize large home ranges incorporating a variety of both coastal and oceanic habitats. However, comparatively little is known about the habitat use and trophic ecology of G. cuvier within the WIO, specifically in South Africa where it is one of the commonly caught species within the bather protection program of the KwaZulu-Natal Sharks Board (KZNSB) [41, 52, 53].
Previous studies utilising SCA to investgate the diet and feeding ecology of G. cuvier have been limited by either low sample sizes [37, 54], or the inability to identify prey to species level [42, 43, 44]. Studies utilising SIA have investigated the TP of G. cuvier within the broader context of the large shark assemblage in the WIO [13] and examined size-based variation in inter-tissue isotopic values and individual dietary specialization in Western Australia [22]. Despite this SCA and SIA research, there is still uncertainty related to aspects of size, sex and individual-based variation in the trophic ecology of G. cuvier in South African waters.
Through access to long term data on tiger shark stomach contents (1983 to 2014) combined with multiple tissues sampled from recent captures (2006 to 2014), this paper provides a detailed investigation of the diet and trophic ecology of G. cuvier off KwaZulu-Natal (KZN), South Africa. The overall aim of the investigation was to examine size-based and sex-specific variations in diet, TP and foraging habitat of G. cuvier at the individual and population level through a combined SCA and SIA (δ15N and δ13C) approach. Given the unique time series of stomach content data, decadal shifts in diet were also investigated. These data will provide knowledge to help future efforts to model the ecosystem consequences of depletions or recoveries of G. cuvier in the region.
Materials and methods
Ethics statement
All research in this investigation was conducted under anually renewed operating (OC/OCS/020) and research permits issued by the Department of Environmental Affairs, South Africa. Samples were collected from dead specimens, caught in the KZN bather protection programme, and hence ethical approval was not required.
Study site and sample collection
All G. cuvier were sampled from animals incidentally caught in the KZN bather protection programme. The program currently uses shark nets, or a combination of nets and drumlines at 37 beaches along the KZN coastline (Fig 1). The majority of nets are 213.5 m long, 6.3 m deep, with a stretched mesh of 51cm. All nets are set parallel and approximately 300–500 m from the shore in a water depth of 10–14 m. More details of the netting operation are given by [55]. Drumlines were introduced as a replacement to nets at some installations in 2007. As of December 2014 there were 79 drumlines installed at 18 of the 37 beaches along the coast. Each drumline is anchored adjacent to the nets and consists of a single Mustad 4480DT 14/0 J hook (Gjøvik, Norway) suspended 4 m beneath a large float [56, 57]. The hooks are baited with southern rover (Emmelichthys nitidus) or jacopever species (Scorpaenidae).
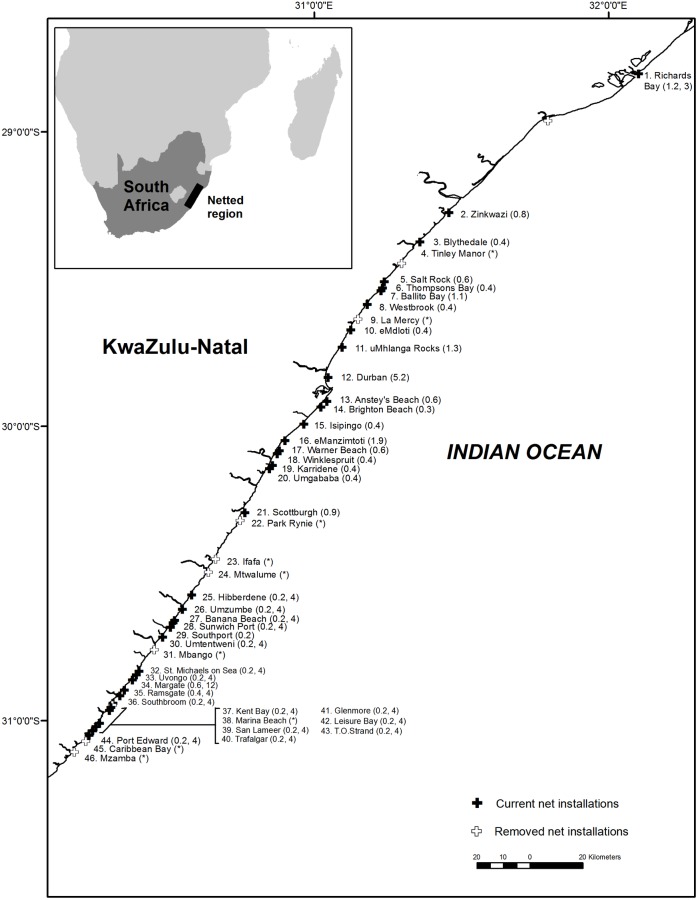
Fig 1. Netted beaches on the KwaZulu-Natal coast and, in parenthesis, the length of nets in kilometres and number of drumlines as of December 2014.
Several net installations (*) were removed permanently during the study period 1983–2014. Insert shows the locality of the netted region in relation to the South African coast.
Recently caught sharks, which were dead but not yet decomposed, were retrieved and transported to the KZNSB laboratory where they were stored frozen (–20°C) until dissection. On arrival at the laboratory, basic data on size, sex, maturity status and morphological measurements, were recorded. Precaudal length (PCL) was measured in centimeters as the straight-line distance between perpendiculars to the snout and the precaudal notch [58]. Maturity status was visually assessed using published criteria [37] and the state of the reproductive organs according to published criteria [59].
Stomach content analysis
Stomach content data was collected from 1983 to 2014. For specific details on catch rates and seasonality of capture, refer to [41]. For each G. cuvier, the complete stomach was removed and prey items identified to the lowest possible taxon, counted, and weighed (wet mass) to the nearest 1.0 g. Prey was identified at various levels of digestion including whole animals, teleost otoliths [60] and cephalopod beaks [61, 62]. Cumulative prey curves were constructed to determine if a sufficient number of stomachs had been collected for accurately describing total diet and diet by size class. The order in which the stomachs were analysed was randomised 500 times and the mean cumulative number of new prey items plotted against the number of stomachs sampled.
Diet composition was calculated as percentage number (%N), percentage mass (%M), percentage frequency of occurrence (%F) and percentage index of relative importance (%IRI) of prey, from non-empty stomachs, according to the definitions of [14]. Stomach contents containing prey items in conjunction with shark net twine, which suggested they had been scavenged from the nets, were excluded from all analyses. Otoliths and beaks may remain undigested for long periods of time. As a result, stomachs containing only these hard prey items were considered empty and excluded from the analyses to avoid any bias [63, 64]. This procedure has been followed in all previously published KZNSB dietary studies. The exclusion of cephalopod beaks, however, would result in the loss of a wealth of information on the species of cephalopods consumed by G. cuvier [64]. As a result, their contribution (%N and %F) to the diet of G. cuvier were analysed separately.
For statistical analysis, prey items were grouped to family level and then further categorised into eight functional prey groups: elasmobranch, teleost, cephalopod, crustacean, reptile, mammal, bird, and miscellaneous items as defined by [18]. To examine whether G. cuvier undergo a size-based diet shift, individuals were grouped into three size classes: small (< 150 cm), medium (150–220 cm) and large (> 220 cm). The length frequency distribution of sharks sampled can be found in [42]. These size classes were chosen to enable a comparison with previously published dietary studies on G. cuvier [42, 43, 44]. Seasonal and decadal shifts in diet, for each size class of shark, were investigated between Summer (December to February), Autumn (March to May), Winter (June to August) and Spring (September to November) and between 1983 to 1992, 1993 to 2003 and 2004 to 2014, respectively.
Dietary index (%F, %M, %N and %IRI) data for each prey group and size class of shark were subjected to nonmetric multidimensional scaling (nMDS) ordination. To overcome the problem of low prey diversity in the stomachs sampled, dietary data for groups of animals (approximately 5 sharks per group) were randomly pooled within each size class, herein referred to as dietary samples [65, 66]. Prior to nMDS ordination, dietary samples were square root-transformed, and a similarity matrix was constructed using the Bray–Curtis similarity coefficient [67]. A one-way analysis of similarities (ANOSIM) test was then employed to determine any statistical differences in diet composition between size classes [66, 68]. There was no signficant effect of sex on initial ANOSIM tests (%F: R = 0.024, p = 0.26, %M: R = 0.009, p = 0.59, and %N: R = 0.049, p = 0.08); consequently sexes were combined for all analyses. The resultant global R statistic ranges from -1 to +1 and provides a measure of similarity among groups, with 0 indicating no difference in groups. Similarity percentage analysis (SIMPER, [69]) was used to determine the functional prey groups most responsible for any significant multivariate differences in diet between size classes, seasons and decades.
Ordination means plots with approximate 95% regions were constructed through bootstrap averages (n = 100) using metric MDS (mMDS) [69]. These plots provide a much clearer and intuitively easier structure to interpret than plots, which contain a point for each dietary sample. Mean plots also have lower stress values (better representation), as well as providing information on the magnitude of differences both between and within group locations. All analyses were performed in PRIMER (PRIMER-E Ltd., Ivybridge, UK).
Stable isotope analysis
For a subset of G. cuvier caught between 2006 and 2014 (n = 56), a tissue plug was excised from the white muscle block anterior to the first dorsal fin adjacent to the vertebral column for SIA. For 17 individuals, additional liver and skin samples were excised from the central region of either the right or left liver lobe and anterior of the first dorsal fin, respectively. All samples were immediately stored at -20°C. Each sample was then freeze dried and muscle and liver tissue were ground to a fine homologous powder using a hand-held mortar and pestle. For skin tissue, surgical scissors were used to cut the tissue into a fine material. Muscle and liver tissue were lipid extracted (LE) using a modified chloroform methanol treatment outlined by [70], following [20]. Skin tissue was not lipid extracted given expected low lipid content [71]. Following LE, muscle and liver were placed in a fume hood to evaporate remaining chloroform methanol and then freeze dried a second time. While water washing is recomended to remove urea from elasmobranch tissue [70] it was not undertaken in the current study to maintain consistency in sampling protocols with previously archived stable isotope data for this region. In addition, LE is known to remove the majority of urea [71, 72] and a previous SIA examination of sharks caught in the KZNSB nets suggested urea may have started to break down prior to analysis and consequently had limited effect on δ15N values (72). Between 400 and 600 μg of each tissue sample was weighed into tin cups and carbon and nitrogen stable isotopes analyzed using a Thermo Finnigan DeltaPlus mass-spectrometer (Thermo Finnigan, San Jose, CA, USA) coupled with an elemental analyzer (Costech, Valencia, CA, USA) at the Great Lakes Institute for Environmental Research, Windsor, Canada. Stable isotope results are expressed in standard delta notation (δ; parts per thousand) according to the following equation;
| (1) |
where R is the ratio of heavy to light isotopes in the sample and standards. The standard reference material was atmospheric nitrogen for N2, and Pee Dee Belemnite carbonate for CO2. The analytical precision based on the standard deviation of two standards (NIST 8414 and internal fish muscle lab standard; n = 76) was 0.10‰ and 0.21‰ for δ15N and 0.06‰ and 0.09‰ for δ13C, respectively. Analytical accuracy based on the analysis of NIST standards, performed with muscle tissue sample, sucrose (NIST 8542), and bovine liver and muscle samples (n = 3 for each), were within 0.07‰ for δ15N and 0.01‰ for δ13C of certified values.
Single tissue diet (δ15N) and habitat (δ13C) ontogenetic profiles
To examine ontogenetic shifts in relative TP (absolute δ15N values) and foraging habitat (δ13C) of G. cuvier, muscle isotope data for all individuals (n = 56) were grouped by sex and plotted by size (PCL) and body mass. Both linear and polynomial regression models were then tested to examine significant relationships and the best model fit presented. Both regression types were tested following [22], given the fact that there can be a lag in isotopic tissue incorporation of diet and G. cuvier have previously been reported to undertake a dietary shift [42, 43, 44]. Mean δ13C values for scalloped hammerheads (Sphyrna lewini) that have known nursery grounds on the KZN continental shelf were used following [20] as a proxy for the range of expected δ13C values for the KZN coastal habitat.
Multi tissue stable isotope analysis to infer individual and population level feeding behavior
To examine the effects of sex, body size (PCL), body mass, maturity state, tissue type and year of capture on δ15N and δ13C values, a linear mixed-effects model was constructed for the subset of 17 G. cuvier with multiple tissue samples. Each isotope was modeled independently with all factors set as fixed effects with the exception of individual shark ID, which was included as a random effect. The optimal model was then identified by conducting sequential likelihood ratio tests. Non-significant fixed effects were removed in a stepwise manner until minimum adequate models containing only significant factors remained. Tissue type included muscle, liver and skin representing different turnover or isotope integration periods. These periods range from more than a year for muscle [29, 73] to approximately 6 months for liver [73]. The current isotope turnover rate of skin is unknown but is thought to lie somewhere between muscle and liver [25, 71]. Given uncertainties over skin turnover rates, two linear models were constructed. The first included only two time points (muscle and liver isotope values per individual) while the second included three time points (muscle, skin and liver per individual). Prior to each model run, all tissue data were standardized to remove the effects of tissue-specific isotope values. LE muscle and liver isotope data were corrected with known diet-tissue discrimination factors (DTDF) of 2.3‰ and 1.5‰, respectively, for δ15N, and 0.9‰ and 0.2‰, respectively for δ13C, according to [72, 74]. DTDF values for BULK skin isotopes are unknown, but are thought to be similar to cartilage (vertebrae) [20]; therefore DTDFs of 1.5‰ for δ15N and 3.8‰ for δ13C were used [74].
To further investigate the feeding behavior of G. cuvier on a generalist to specialist continuum, (i.e. diet consistency of individuals over time), mixed model variance component analysis was used to estimate the total observed variability for the population (total isotopic niche width–TNW) by summing the intercept variability (between individual component–BIC) and residual variability (within individual component—WIC) in the random effect of the linear models above [34, 75]. This was calculated for both sets of models (two and three tissue). BIC indicates the dietary variability among individuals, while WIC indicates dietary consistency of an individual over time (34). Specialist feeding behavior is indicated by a higher BIC than TNW value and generalist behavior vice versa. The absolute measure of individual specialization for all G. cuvier, measured on a scale of 0–1, was then calculated as the ratio of WIC/TNW. A value of 0 (0%) represents specialized feeding behavior, a value of 1 (100%) generalist feeding behavior and values in between, a continuum between the two end points (i.e., 0–49.9%—specialist and 50–100%—generalist) [34]. Statistical analyses were performed in R v. 3.2.3 (R Development Core Team 2015) using the nlme package v. 3.1–124 [76] with an α of 0.05.
Trophic position estimation from stomach contents (TPSCA) and stable isotope analysis (TPSIA)
To estimate TP of G. cuvier from stomach content analysis (TPSCA),—a measure of the position an organism occupies in the food web, the following equation was used [15]:
| (2) |
where TPSCA is diet-calculated TP per dietary sample, pi is the proportion of each prey category in the total diet (expressed as %M), and TPi is the TP for each functional prey category. The TP of functional prey categories were defined [15] as birds (3.87), cephalopods (3.20), crustaceans (2.52), elasmobranchs (3.65), mammals (4.02), reptiles (2.40), and teleosts (3.24). The miscellaneous functional prey group was excluded from all TP calculations.
To estimate TP of G. cuvier using nitrogen stable isotopes, a scaled Δ15N framework approach (TPscaled) based on a dietary δ15N value-dependent model was used [77, 78]. With knowledge of the δ15N value of a known baseline consumer (δ15Nbase), the δ15N value of the consumer (individual G. cuvier; δ15Nconsumer), the dietary δ15N value at which δ15N incorporation and δ15N elimination are equal (δ15Nlim) and the rate at which the ratio between δ15N incorporation and δ15N elimination changes relative to dietary δ15N averaged across the food-web (k), TPscaled is calculated as follows:
| (3) |
For comparative purposes, G. cuvier TP was also estimated using a constant discrimination factor of 3.4‰ in an additive framework (TPadditive) following [79];
| (4) |
Where TPadditive is the estimated TP of the consumer of interest, δ15Nconsumer is the δ15N value of the individual consumer, δ15Nbase is the δ15N value of a baseline species, and 3.4‰ is the fixed discrimination value.
Three baseline species were used to estimate TPscaled and TPadditive and an average TP value presented to increase confidence in TP estimation. Baseline species included zooplankton (copepod; Euphausia frigida and mysid; Undinula vulgaris; n = 16; mean δ15N ± SD = 5.2‰ +- 0.8), whale sharks (Rhincodon typus; n = 3; mean δ15N ± SD = 9.9‰ +- 0.5) and manta rays (manta sp.: n = 8; mean δ15N ± SD = 9.8‰ +- 0.5). Whale sharks and Manta rays, which are known zooplanktivores [80, 81, 82], were included as TP3 baseline consumers (TP = 3), while zooplankton was included as a TP2 consumer (77). For the TPscaled approach, a value of k = 0.14 and δ15Nlim = 21.9 were used following a meta-analysis of experimental isotopic studies [77, 78].
Results
Stomach content analysis
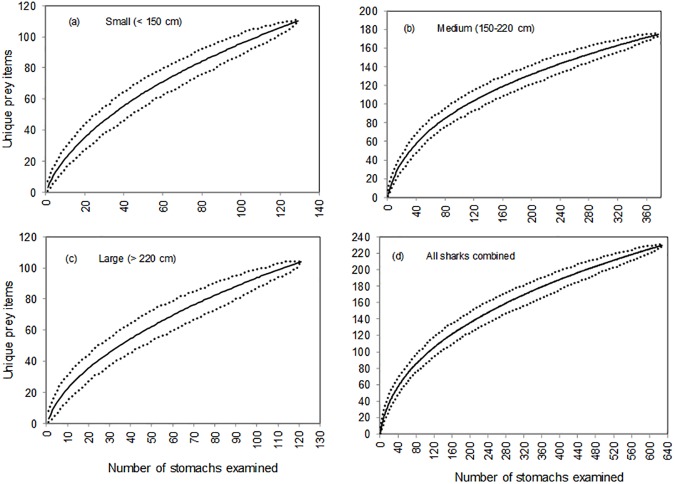
A total of 778 G. cuvier, ranging in size from 94 to 335 cm PCL (mean = 185.8 cm, SD = 39.4), were examined. None of these sharks were either neonate, or pregnant and less than 1.5% were considered mature. Of these, 81 (10.4%) had empty stomachs and 69 (8.9%) had regurgitated during capture. Cumulative prey curves were constructed using data from the remaining 628 stomachs that contained food items. When examining each of the size classes as well as all sharks combined, none of the curves (Fig 2A–2D) reached an asymptote indicating that a greater number of individuals would be required to completely describe G. cuvier diet for this region.
Fig 2. Randomized cumulative prey curves derived from the stomach contents of G. cuvier caught in the KwaZulu-Natal shark nets and drumlines, 1983–2014.
a) Small, b) medium, c) large size classes and d) all sharks combined. The order in which the stomachs were analysed was randomised 500 times and the means (solid lines) and 95% confidence levels (dashed lines) presented.
A diverse range of 192 prey items were identified from the stomach contents of G. cuvier (S1 Table, Fig 3). Prey items ranged in size from small unidentified shrimps and bivalves to various large whale species including Physeter macrocephalus (sperm whale) and Megaptera novaeangliae (humpback whale).
Fig 3. Stomach contents retrieved from G. cuvier caught in the KwaZulu-Natal shark nets and drumlines, 1983–2014.
(a) Philantomba monticola (blue duiker) (240 cm female). (b) Sousa plumbea (humpback dolphin) and unidentified seabird (195 cm female). (c) Spheniscus demersus (African penguin), skate egg case, unidentified shark, Megaptera novaeangliae (humpback whale) (194 cm female). (d) Morus capensis (Cape gannet) and unidentified porcupine fish (232 cm male).
Elasmobranchs were the most important functional prey group (%IRI) for medium and large G. cuvier (Table 1). A total of 20 shark and 18 batoid species were identified (S1 Table). With increasing G. cuvier body size (small to large), the number of identified shark prey species increased from 5 to 12, whereas the number of batoid prey species decreased from 11 to 7 (S1 Table). Although all size classes of G. cuvier commonly preyed on unidentified dasyatid (stingray), Manta birostris (oceanic manta) and Carcharhinus obscurus (dusky shark), the dietary importance of these and other elasmobranch prey varied with size class. There was a general decrease in the importance of typically small inshore elasmobranchs such as dasyatids, Rhinobatos sp. (guitarfish), neonate C. obscurus and an increase in larger and more offshore species such as Carcharias taurus (raggedtooth shark), and Squatina africana (African angelshark) with increasing G. cuvier body size (S1 Table).
Table 1. Stomach content composition of G. cuvier caught in the KwaZulu-Natal shark nets and drumlines, 1983–2014.
Results are summarized for eight functional prey groups and presented by frequency of occurrence (%F), by mass (%M), by number (N%) and index of relative importance (%IRI). Totals represent number of non-empty stomachs (F), mass prey items (M, kg) and number of unique prey items recorded (N).
| Predator category | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prey category | All | Small (<150 cm) | Medium (150–220 cm) | Large (>220 cm) | ||||||||||||
| %F | %M | %N | % IRI | %F | %M | %N | % IRI | %F | %M | %N | % IRI | %F | %M | %N | % IRI | |
| Elasmobranchs | 54.74 | 54.35 | 14.72 | 3780.64 | 44.26 | 52.79 | 18.27 | 3144.97 | 60.70 | 55.77 | 16.07 | 4361.39 | 47.11 | 52.25 | 9.74 | 2920.35 |
| Teleosts | 51.31 | 7.44 | 19.89 | 1402.25 | 72.95 | 18.18 | 35.60 | 3923.22 | 47.15 | 7.97 | 14.12 | 1041.63 | 42.15 | 4.73 | 24.92 | 1249.52 |
| Reptiles | 6.21 | 1.65 | 1.60 | 20.20 | 1.64 | 0.21 | 0.62 | 1.36 | 5.69 | 1.68 | 1.54 | 18.32 | 11.57 | 1.84 | 2.08 | 45.33 |
| Birds | 26.96 | 6.38 | 6.48 | 346.64 | 15.57 | 3.51 | 3.72 | 112.59 | 27.91 | 4.40 | 6.85 | 313.92 | 35.54 | 10.16 | 7.03 | 610.97 |
| Mammals | 40.69 | 27.83 | 9.92 | 1536.23 | 29.51 | 20.99 | 10.22 | 920.98 | 43.36 | 28.02 | 10.76 | 1681.66 | 44.63 | 28.70 | 8.15 | 1644.40 |
| Cephalopods | 15.52 | 0.86 | 29.94 | 478.14 | 14.75 | 1.59 | 12.38 | 206.22 | 17.62 | 1.10 | 35.43 | 643.53 | 10.74 | 0.33 | 26.36 | 286.78 |
| Crustaceans | 12.75 | 0.64 | 7.86 | 108.39 | 9.02 | 0.50 | 4.33 | 43.55 | 14.36 | 0.56 | 7.27 | 112.44 | 11.57 | 0.80 | 11.02 | 136.76 |
| Micellaneous items | 37.91 | 0.84 | 9.59 | 395.32 | 54.10 | 2.23 | 14.86 | 924.51 | 33.88 | 0.49 | 7.97 | 286.41 | 33.88 | 1.19 | 10.70 | 402.95 |
| Totals | 612 | 1341.59 | 192 | 122 | 80.67 | 83 | 369 | 787.06 | 148 | 121 | 473.85 | 91 | ||||
Teleosts were the most important functional prey group (%IRI) for small G. cuvier (Table 1). Representatives from 28 families and 67 species were identified (S1 Table). Although a wide variety of prey were consumed many of them had a relatively low incidence. Prey included inshore soft bottom demersal and benthic fishes (e.g. Galeichthys feliceps, Pomadasys olivaceum), reef associated species (e.g. Sarpa salpa, Epinephelus andersoni), as well as more offshore epipelagic species (e.g. istiophorid sp., Thunnus albacares). The most common species recorded from stomachs (%F and %N) for all size classes of G. cuvier were from the families Diodontidae (porcupinefish), Tetraodontidae (pufferfish) and Ostraciidae (boxfish). However, the importance of these families in the diet of G. cuvier decreased with size. Otoliths without any associated soft tissue (excluded from S1 Table) were only identified for single samples of the following: Otolithes ruber (snapper kob), Cheilodonichthys sp. (gurnards), unidentified synodontid (lizardfish) and unidentified macrourid (grenadiers or rattails). The latter are benthic species typically occurring on the outer-continental shelf and slope at depths of more than 200 m.
Mammals became an increasingly important prey group (%IRI) for both medium and large G. cuvier (Table 1). A total of 20 prey items was identified representing at least 7 marine and 8 terrestrial species (S1 Table). Small odontocetes (e.g. Tursiops aduncus and Delphinus delphis) were the most commonly consumed prey in terms of %IRI for small and medium size class G. cuvier. As body size increased mysticetes (e.g. Megaptera novaeangliae) became the more dominant prey. Interesting terrestrial species recorded in stomach contents included: Cryptomys hottentotus (Common mole-rat), Philantomba monticola (blue duiker) and Hystrix africaeaustralis (South African porcupine). Human (Homo sapiens) remains, comprising parts of tibia, fibula and pelvis bones were recorded from the stomachs of two sharks with lengths of 2.1 and 2.3 m (S1 Table).
Birds increased in dietary importance (%IRI) with G. cuvier body size (Table 1) with the Cape gannet (Morus capensis) being the most commonly consumed species (S1 Table). Reptiles were one of the least important functional prey groups, however, their importance increased with G. cuvier body size (Table 1). The most common turtle species were Chelonia mydas (green turtle) and Caretta caretta (loggerhead turtle). At least two reptile and five bird species recorded had terrestrial origins (S1 Table).
A wide variety of Miscellaneous items were recorded from stomach samples, particularly from small G. cuvier (S1 Table). Items included unidentified gastropods, molluscs and seaweed as well as junk food (e.g. sweet and potato crisp packets), terrestrial/flood garbage (e.g. condoms, chamois leather, cigarettes) and butcher's bones (e.g. bags of chicken gizzards, cut abattoir bones). Crustaceans, comprising mainly brachyuran crabs, were recorded from all size classes of G. cuvier in relatively low numbers (Table 1).
Cephalopods were an important functional prey group (%IRI) in all size classes of G. cuvier, especially medium sized sharks (Table 1). Cuttlefish (Sepiidae) as well as 28 species (14 families) of squid (Teuthoidea) and 5 species (2 families) of octopus (Octopodidae) were identified from beaks (S2 Table). The %F and %N of cuttlefish were similar in all size classes of G. cuvier whereas the proportion of oceanic squid and neritic octopi species increased and decreased with shark size, respectively (S2 Table). The most commonly recorded squid was Ancistrocheirus lesueurii (sharpear enope squid), an oceanic deep water species. Other lower epipelagic to mesopelagic species identified included Onykia robsoni (rugose hooked squid), Sthenoteuthis oulaniensis (purpleback flying squid) and Histioteuthis miranda. The most commonly recorded octopus species was Octopus cyanea (big blue octopus), (S2 Table).
Multivariate analysis of stomach content data
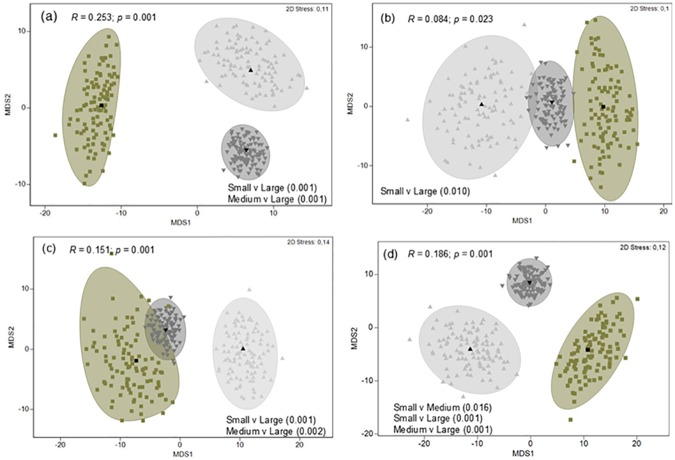
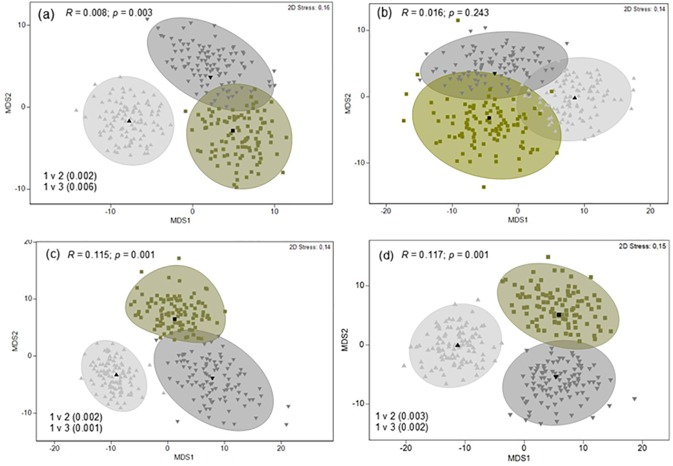
MDS ordination of dietary samples (small n = 24, medium n = 75 and large n = 24) highlighted a level of dietary separation between small and large G. cuvier, but the level of separation was moderate, as indicated by the low global R statistic values (Fig 4). ANOSIM pairwise comparisons indicated significant differences between small and large G. cuvier for all dietary indexes and between medium and large sharks for all indexes except %M. Small and medium sized sharks only exhibited a significant separation for %IRI. Similarity percentage analysis (for all dietary indexes) identified birds, mammals and elasmobranchs as the principal functional prey groups driving this separation.
Fig 4. Metric multidimensional scaling (mMDS) ordinations of dietary samples with approximate 95% region estimates fitted to bootstrap averages for small (< 150 cm), medium (150–220 cm) and large (> 220 cm) G. cuvier.
(a) Percentage frequency of occurrence (%F), (b) Percentage mass (%M), (c) Percentage number (%N) and (d) Percentage index of relative importance (%IRI). R, ANOSIM global R statistic and associated p value. Significant pairwise tests (with p value in brackets) are detailed in each figure.
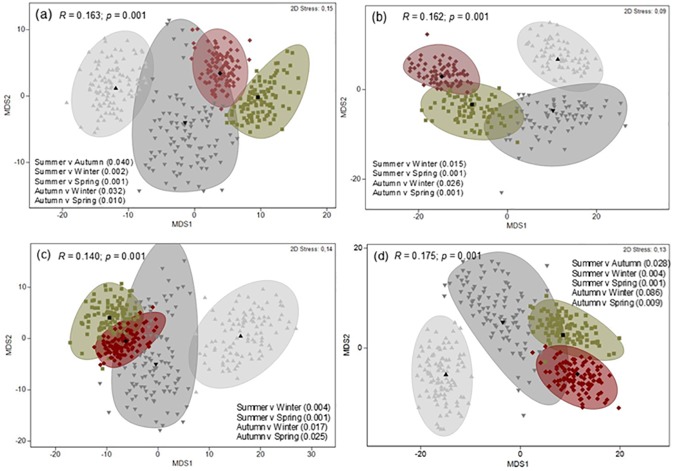
Seasonal levels of dietary separation were only investigated for medium sized sharks, due to sufficiently large sample sizes (summer n = 21, autumn n = 12, winter n = 14, and spring n = 26). MDS ordination of dietary samples indicated a level of separation (for at least 2 of the dietary indexes) between all seasons except winter and spring (Fig 5). ANOSIM pairwise comparisons indicated these differences were significant. However, a high degree of overlap between all seasons was evident as indicated by the low global R statistic values. Similarity percentage analysis (in terms of %IRI) identified elasmobranchs and mammals as the two principal prey groups driving this separation. Elasmobranchs were the dominant dietary component in summer and autumn whereas the importance of mammals increased in winter becoming the dominant group in spring.
Fig 5. Metric multidimensional scaling (mMDS) ordinations of dietary samples with approximate 95% region estimates fitted to bootstrap averages by season for medium (150–220 cm) G. cuvier.
(a) Percentage frequency of occurrence (%F), (b) Percentage mass (%M), (c) Percentage number (%N) and (d) Percentage index of relative importance (%IRI). R, ANOSIM global R statistic and associated p value. Significant pairwise tests (with p value in brackets) are detailed in each figure.
Decadal levels of dietary separation were also only investigated for medium sharks, due to sufficiently large sample sizes (decade 1 n = 30, decade 2 n = 23 and decade 3 n = 21). MDS ordination for all dietary indexes (except %M) indicated a level of separation between decades 1 and 2 and 1 and 3 (Fig 6). ANOSIM pairwise comparisons indicated these differences were significant. However, a high degree of overlap between all decades was evident as indicated by the low global R statistic values (Fig 6). Similarity percentage analysis identified elasmobranchs and mammals as the principal functional prey groups driving this separation. Elasmobranchs were the dominant dietary component in Decade 1 whereas the importance of mammals increased in Decades 2 and 3.
Fig 6. Metric multidimensional scaling (mMDS) ordinations of size class 2 (medium) G. cuvier dietary samples with approximate 95% region estimates fitted to bootstrap averages for decades 1 (1983–1992), 2 (1993–2003) and 3 (2004–2014).
(a) Percentage frequency of occurrence (%F), (b) Percentage mass (%M), (c) Percentage number (%N) and d) Percentage index of relative importance (%IRI). R, ANOSIM global R statistic and associated p value. Significant pairwise tests (with p value in brackets) are detailed in each figure.
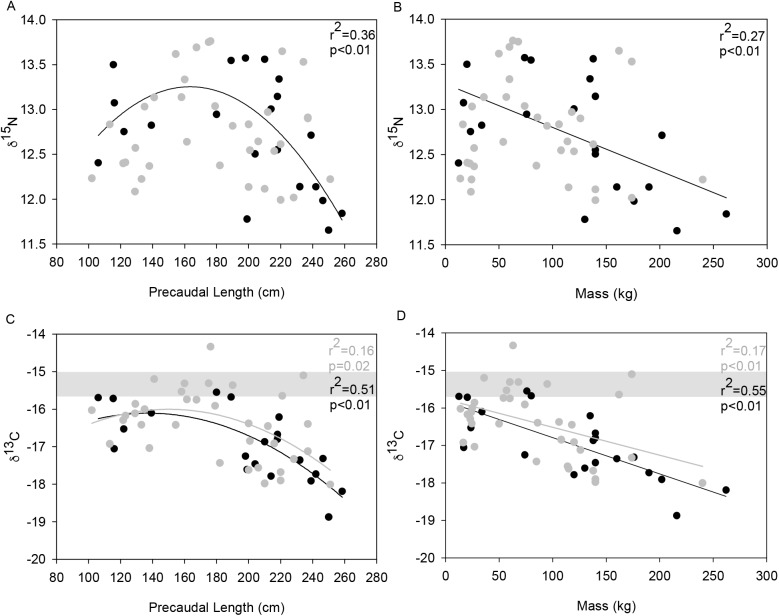
Single tissue diet (δ15N) and habitat (δ13C) ontogenetic profiles for G. cuvier
For males, there was a significant relationship between δ15N values and PCL (r2 = 0.36, p<0.01) that increased until 160–170 cm and then decreased with increasing body size (Fig 7A). There was also a significant negative relationship between δ15N and mass (r2 = 0.27, p<0.01) for males (Fig 7B). For female sharks, there was no significant relationship between δ15N and PCL, or mass. The relationship between δ13C and PCL was significant for both sexes, however it was stronger in males (r2 = 0.51, p < 0.01) than females (r2 = 0.16, p = 0.02), likely related to sample size (Fig 7C). Similarly, the significant negative relationship between δ13C and mass was stronger for males (r2 = 0.55, p< 0.01) than females (r2 = 0.17, p < 0.01) (Fig 7D).
Fig 7.
δ15N (a and b) and δ13C (c and d) ontogenetic profiles for G. cuvier by sex (black circles represent males, grey circles represent females). Linear and polynomial regression models (where appropriate) were fitted to both sexes. Grey bar depicts the predicted δ13C range of the KwaZulu-Natal (KZN) coastal habitat of G. cuvier.
Multi tissue stable isotope analysis to infer individual and population level feeding behavior
Linear mixed-effects models identified that there was a significant effect of tissue type on DTDF corrected G. cuvier δ15N values and a significant effect of PCL on DTDF corrected δ13C values for both the two-tissue and three-tissue models (Table 2). All remaining parameters were not significant.
Table 2. Results of linear mixed-effects models for G. cuvier δ13C and δ15N values for two tissue (muscle and liver) and three tissue (muscle, liver and skin) models with mass, maturity state, precaudal length, sex, tissue and capture year as the fixed effects and shark ID as a random effect.
Only significant variables were retained in the optimal model. SE: Standard error.
| Muscle and Liver | Muscle, Liver, and Skin | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Slope ± SE | df | t-statistic | p-value | Slope ± SE | df | t-statistic | p-value | ||
| δ13C (‰) | |||||||||
| Precaudal length | -0.002 ± 0.0002 | 15 | -7.97 | <0.01 | -0.002 ± 0.0002 | 15 | -7.18 | <0.01 | |
| δ15N (‰) | |||||||||
| Tissue | 0.79 ± 0.11 | 16 | 7.19 | <0.01 | 0.80 ± 0.06 | 33 | 12.42 | <0.01 | |
When δ13C and δ15N values were both included in the analysis, the total residual variance (WIC) accounted for 60% and 64% of the variation in the two-tissue and three-tissue models, respectively. This indicates that G. cuvier captured in KZN are generalists (Table 3). When δ15N was considered alone the WIC accounted for 42% and 48% of the variation in the two-tissue and three-tissue models, respectively, indicating G. cuvier are borderline specialists in terms of their diet (Table 3). For δ13C, the WIC accounted for 71% and 73% of the variation in the two-tissue and three-tissue models, respectively, indicating G. cuvier are generalized in terms of foraging location (Table 3).
Table 3. Variance component analysis from linear mixed-model analysis for G. cuvier δ13C and δ15N for two tissue (muscle and liver) and three tissue (muscle, liver and skin) models.
The between-individual component (BIC) represents the total intercept variance and the within-individual component (WIC) represents the residual variance. Total niche width (TNW) is the sum of the intercept and residual variances for δ13C and δ15N. Total BIC and total WIC are calculated by combining the intercept variances for δ13C and δ15N and then dividing by TNW. Proportion of WIC and BIC that explained TNW is in parentheses.
| δ13C | δ15N | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | BIC | WIC | TNW | WIC/TNW | BIC | WIC | TNW | WIC/TNW | BIC (%) | WIC (%) | TNW | WIC/TNW |
| Two Tissue | 0.12 | 0.29 | 0.41 | 0.71 | 0.14 | 0.10 | 0.24 | 0.42 | 0.26 (40) | 0.39 (60) | 0.65 | 0.60 |
| Three Tissue | 0.12 | 0.33 | 0.45 | 0.73 | 0.15 | 0.14 | 0.29 | 0.48 | 0.27 (36) | 0.47(64) | 0.74 | 0.64 |
Trophic-level estimation: Stomach contents (TPSCA) and δ15N (TPscaled and TPadditive)
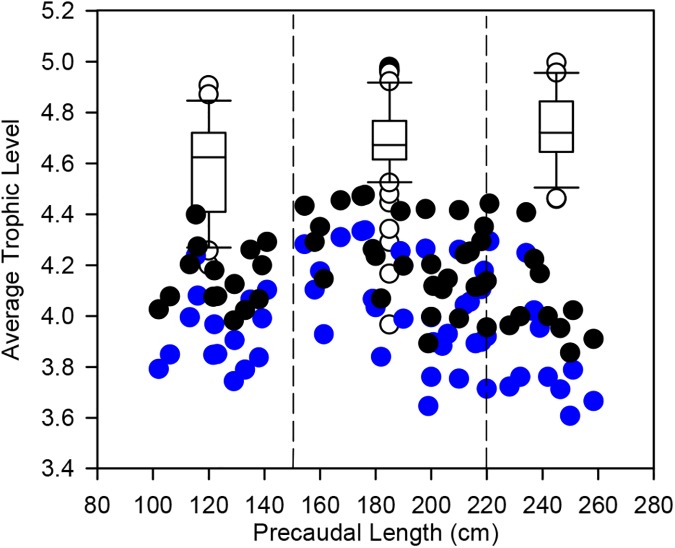
Overall TP calculated using stomach content data (TPSCA) ranged from 4.0 to 5.0 (mean ± SD, 4.7 ± 0.18). There was a minor increase in average TPSCA with increasing body size: small (mean ± SD, 4.6 ± 0.2), medium (mean ± SD, 4.7 ± 0.17), and large (mean ± SD, 4.7 ± 0.14). Overall TPSCA predicted G. cuvier of all sizes feeding across one trophic level (Fig 8). TP calculated using a scaled Δ15N framework (TPscaled) predicted that G. cuvier were feeding across 0.7 of a trophic level with TP ranging from 3.6 to 4.3 (mean ± SD: 4.0 ± 0.2). Estimated TP using the additive framework (TPadditive) predicted a similar, but slightly higher TP range of 3.9 to 4.5 (range = 0.6 of a TL; 4.3 ± 0.2) (Fig 8).
Fig 8. The relationship between TP and increasing body size of G. cuvier.
Stomach content calculated trophic position (TPSCA) for each size class is indicated by the white box. The solid black line in each box represents the median, outliers are indicated by open circles. TP estimated using a scaled δ15N framework (TPscaled) is indicated by blue circles and TP estimated using a standard additive trophic framework (TPadditive) is indicated by black circles. Vertical dashed black lines indicate the predetermined size classes of G. cuvier used in the stomach content analysis (<150 cm), medium (150–220 cm) and large (>220 cm).
Discussion
Stomach content data for G. cuvier indicates they consume a wide variety of different sized prey of both marine and terrestrial origins along the KZN coast. Although none of the cumulative prey curves in this study reached an asymptote the number of prey items recorded (n = 193) is higher than that recorded for any other species of elasmobranch. From an extensive search of the literature the next highest number of identified prey items (n = 121) was from the dusky shark (Carcharhinus obscurus) [58]. In addition, aside from the white shark (Carcharodon carcharias) [59, 72, 83], no other shark species has been reported to feed on prey from all eight functional prey groups as defined by [15].
The habitat of the prey, within the functional prey categories, consumed by G. cuvier was also highly diverse. Teleosts included reef, pelagic and demersal species. Cephalopods and crustaceans included both benthic and pelagic species and reptiles and mammals included terrestrial and marine species. The broad spectrum of prey consumed and the relatively low incidence of most items indicates that G. cuvier is a generalist feeder, foraging in a variety of different habitats, as previously reported by [43] and [44]. Evidence for this was further provided by the higher intra-individual variation compared to inter-individual variation in δ13C values at multiple time scales (i.e. variable tissue turnover rates). This is similar to the generalist strategy previously observed for this species in Australia [30]. Tiger sharks have large home ranges [48, 50, 84] and are known to forage over a wide vertical range exhibiting yo-yo diving behavior [85, 86, 87]. As generalist feeders, these movement patterns likely provide an optimal search strategy to encounter a variety of prey [85], as well as prey with either a low abundance or patchy distribution, which are typical in pelagic waters.
The generalist feeding strategy of G. cuvier, may in part be related to the seasonal abundance of prey [42, 47, 86]. Interestingly, there was a significant shift in the diet of medium sized G. cuvier from one dominated by elasmobranchs (in summer and autumn) to one of mammals (mysticetes) (in winter and spring). This coincides with the northward reproductive migration of humpback whales (Megaptera novaeangliae) in the winter as they move from the Antarctic to breeding grounds in Mozambique and their return southward migration in the spring [88, 89]. In the North Atlantic and Hawaiian Archipelago G. cuvier have been shown to switch movement patterns and foraging strategies to take advantage of loggerhead turtles [90] and predictable seasonal congregations of fledging albatross (Phoebastria sp.) [48, 91], respectively. In the Abrolhos Islands, Western Australia, G. cuvier have learnt to exploit the seasonal abundance of discarded bait from the rock lobster fishing industry [44].
Tiger sharks demonstrated asymmetric feeding behavior, whereby larger prey were consumed with increasing predator size, but small prey items were retained in the diet. For example, a variety of both cephalopod and crustacean species were recorded from the stomachs of both small and large G. cuvier whereas larger prey such as whale sharks and some species of mysticete were found only in large sharks. Although signs of predatory attacks on these larger prey species are rare [92, 93] these events may occur more frequently than existing literature suggests [94, 95, 96]. As such, it is difficult to determine with any certainty whether pieces (up to 14 kg) of these prey items were the result of scavenging or predation. The fact that the diet of small G. cuvier is a subset of larger individuals is a contributing factor to the high degree of overlap of functional prey categories across size classes. This is similar to that observed for white sharks [72] and most predatory fish [97].
Despite the high degree of dietary overlap between the three size classes there was a clear size based expansion and shift in diet. Reptiles, birds, mysticetes, and large shark species increased in dietary importance with shark size, concomitant with a decrease in smaller prey such as batoids and teleosts. Ontogenetic dietary shifts in G. cuvier, with larger prey becoming increasingly important with shark size, have been reported in New Caledonia [98], Australia [42, 44] and Hawaii [43]. It has been postulated that these ontogenetic changes are attributable to: 1) larger sharks capable of capturing and consuming larger and more mobile prey [43], 2) increased size of the mouth, jaw and teeth enable the consumption of larger prey and those with a tough shell (e.g. turtles) [44, 99], 3) acquisition of hunting skills required to predate larger, as well as air-breathing prey e.g. turtles and birds [16], and 4) shifts in foraging habitats with shark size [43]. These attributes, together with the fact that human remains were recorded from the stomach contents of a medium sized shark, suggest that G. cuvier of 150 cm PCL (approximately 203 cm TL) and above are potentially the greatest threat to humans. This is similar to the size of G. cuvier (230 cm TL) postulated to be the greatest threat in Hawaii [43].
In addition to ontogenetic and seasonal shifts in diet, SCA also indicated a decadal change driven primarily by a decrease in elasmobranch and an increase in mammal (cetacean) prey. Data from the KZNSB bather protection program have shown that there have been declines in several species of sharks, with the exception of G. cuvier, over the past 30 years, [41, 53]. Declines of shark populations along the coast are likely the result of over-exploitation in a variety of commercial, artisanal and recreational fisheries [100, 101]. Over a similar time period the humpback whale population in the WIO has increased at a rate of 9–11.5% [102]. Although it is difficult to draw strong conclusions from the data available, it is possible, that G. cuvier (as generalist and opportunistic feeders) are able to take advantage of changes in the relative abundance of different prey through time.
Tiger sharks are regarded as the least discriminate feeders of all shark species [42, 43, 44]. The discovery of a variety of non-digestible anthropogenic as well as digestible terrestrial prey items in this study further confirm its ability to scavenge and forage opportunistically. These attributes may be one of the reasons for the high incidence of ostraciids, tetraodontids and diodontids in their diet. Although of low calorific value these small prey species are likely easy to predate and confer an energetic advantage in achieveing the required daily ration of 0.56% their body weight [103].
It is interesting to note that G. cuvier is the only species of shark to exhibit a mass capture phenomenon (7 to 14 sharks caught simultaneously at the same netted installation) related to the scavenging of mysticete carcasses in the vicinity of the KZN bather protection nets [41]. It is likely that their foraging strategies confer a competitive advantage over other shark species by benefiting from these unpredictable events and would explain the high dietary occurrence of large prey species such as mysticetes in its diet. Although G. cuvier are also able to scavenge net-caught manta species the high incidence of shark-inflicted bite marks (76.3%) recorded from reef manta’s (Manta alfredi) off Southern Mozambique [104] suggests that most are actively predated.
As G. cuvier increase in size they range over a wider variety of habitats, which is probably related to the exploration of potential new foraging grounds [41, 49, 86]. Stomach contents indicated that small sharks had a higher proportion of prey typical of inshore and shallow habitats e.g. batoids, benthic octopi and miscellaneous items (transported down rivers) than large sharks. In contrast the stomach contents of larger sharks contained more elasmobranch species, oceanic and deep water squid as well as teleost species typically only found at depths of more than 200 m. These results suggest that larger sharks are spending more time further offshore in the pelagic environment than smaller sharks. This hypothesis is supported by three independent datasets: Firstly, by the fact that very few adult G. cuvier are caught close inshore in the KZN bather protection programme [41], secondly, by the overall trend of decreasing δ13C with increasing size, as offshore waters are typically depleted in δ13C [105] and thirdly, tag recaptures of G. cuvier, indicate they begin to move offshore at a size of about 170 cm PCL [41]. In addition, PCL explained a significant amount of the variability in δ13C values providing further support for changes in foraging habitat with size.
Tissue type was the only factor that explained a significant amount of the observed variation in δ15N values. Individual tissue types integrate stable isotopes over different time scales, highlighting the requirement to assess diet on long-term scales to fully capture the breadth of a predator’s diet. The slightly higher inter-individual variation observed in δ15N than intra-individual variation for both the two-tissue and three-tissue models suggests borderline generalist/specialist feeding behaviour that could be driven by differences between the potential preference of individual G. cuvier to certain prey types e.g. humpback whales, Cape gannets, shark or batoid species. However, the combined stable isotope results indicate that G. cuvier along the South African coast is a generalist feeder at the population level, in agreement with stomach content data and previous diet studies [22, 106].
Overall, there was an increasing trend in TPSCA with increasing shark size, as larger G. cuvier consumed a greater proportion of larger prey from higher trophic levels. This is consistent with ontogenetic shifts recorded in G. cuvier dietary studies elsewhere in the world [42, 43, 44]. TPSCA calculated from dietary samples, however, varied markedly across the size range of sharks sampled and the size based shift in TP was only slight. This likely reflects the high degree of overlap of functional prey categories consumed across size classes of shark, as well as the wide-ranging movement patterns among various foraging habitats exhibited by G. cuvier. As a result, changes in the abundance of G. cuvier may not result in a simple top-down trophic cascade. This highlights the importance of obtaining information on the diet and resource use of G. cuvier, and indeed any top predator, to better predict the ecological consequences of shark depletions or recoveries [1, 2, 3].
The absolute values of TPSCA estimated for G. cuvier were similar to those recorded for white sharks in this region [72], supporting the role of this species as a top predator along the South African coastline. Similar to TPSCA, both the TPscaled and TPadditive estimates increased initially for the small to medium size class G. cuvier. However, there was then a trend of decreasing TP (for both TPscaled and TPadditive) from the medium to the large size class of G. cuvier. The observed decrease in TPSIA for the largest sharks likely represents the long-term incorporation rate of isotope values into muscle tissue and consequently it may represent an integration of variable feeding strategies including foraging in offshore pelagic food webs. Overall, the TPscaled and TPadditive estimates were lower and less variable than those of TPSCA. SCA represents more recent feeding while SIA integrates dietary data over a longer time frame. Therefore, it is likely that offshore pelagic dietary items (i.e., lower δ15N values as a result of lower baseline δ13C values in pelagic ecosystems) are better represented by long-term (SIA) data. It is also possible that this results in TPscaled and TPadditive underestimating the actual TP value for G. cuvier, given a single baseline model was used to estimate TP. Alternatively, lower δ15N values and associated TP values of larger G. cuvier could relate to their increased foraging on turtle and certain mammalian prey (for example humpback whales) that typically have lower δ15N values. The δ15N values for green and loggerhead turtles are approximately 1.7–8.1‰ and 4.0–12.0‰, respectively [107, 108]. The differences observed among these methods to estimate TP highlight the requirement of multiple methods to capture the full breadth of diet and to provide an overall TP range.
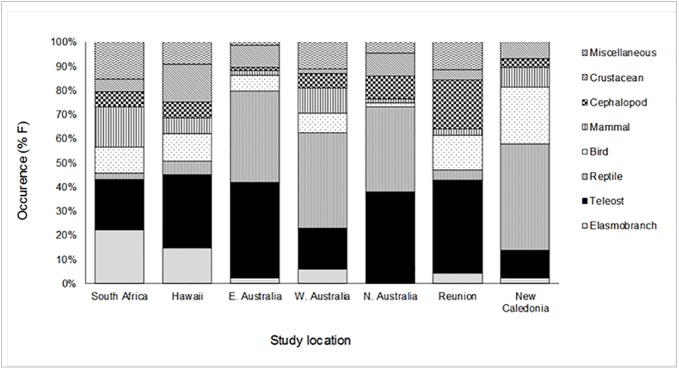
Comparing the findings from diet studies among regions is complicated due to differences in sampling methods e.g. necropsy [42, 43, 44] or regurgitation [54], the habitats they are sampled from e.g. inshore [42, 43] or offshore [44, 109], seagrass meadows [54], or rocky and coral reefs [44, 106] as well as the size and number of sharks sampled. Despite these limitations, distinct differences in the dietary composition of the functional prey groups were evident between regional populations of G. cuvier (South Africa, Hawaii, Eastern, Western and Northern Australia, Reunion and New Caledonia), (Fig 9).
Fig 9. Comparison in the relative percentage frequency of occurrence (%F) of functional prey groups to the diet of G. cuvier caught in the current study (South Africa) to other geographic regions.
Although teleosts were an important prey group in all regions, there was a noticeable difference in the importance of elasmobranchs to the diet of G. cuvier from South Africa and Hawaii, reptiles (turtles and sea snakes) in the Australian and New Caledonia studies and cephalopods from Reunion. In Australia and New Caledonia, G. cuvier were sampled from regions encompassing large rookeries (and high densities) of both loggerhead and green turtles [110]. In South Africa, Hawaii and Reunion sharks were sampled more than 200 kilometers away from any major turtle nesting sites [110, 111]. In Australia and New Caledonia over 32 and 15 species of sea snakes have been recorded respectively, many of which are known to congregate in shallow coastal waters [112]. In comparison, the only species found in South Africa, Hawaii and Reunion is the yellow-bellied sea snake (Pelamis platura), which is pelagic and relatively rare close inshore. As a result, regional differences in diet, at least in part, are a result of differences in the abundance of prey. However, the prevalence of reptiles (especially turtles) in the diet of G. cuvier from Australia, despite the availability of elasmobranch prey, suggests that diet is not only determined by prey abundance, but also prey preference and catchability. It has been suggested that G. cuvier are specialist turtle predators [113, 114]. The selective predation of turtles over another abundant species was noted by [44] and postulated to account for the geographic variation in G. cuvier diet along the west coast of Australia.
This study presents one of the longest time-series and most detailed analysis of stomach content data for G. cuvier worldwide. It indicates that G. cuvier in South African waters is a generalist predator, which exhibits an ontogenetic expansion and shift in diet, as previously documented in other geographic localities. There was greater variation in stable isotope values within individual G. cuvier than among individuals further supporting the concept of a generalist feeding strategy. Stomach content analysis in combination with SIA in this study provides information on the diet and TP for a large apex predator, which exerts influence across multiple components of marine ecosystems. Knowledge of the diet and trophic ecology of G. cuvier is key for the effective implementation of ecosystem as well as species management initiatives in South Africa and the WIO. The key question that remains, however, is whether the trends observed in this study are indeed indicative of the larger WIO population. This highlights the importance of future studies (such as long-term satellite-tracking) to better understand the level of population connectivity within the region.
Supporting information
(DOCX)
Details of the prey are presented by frequency of occurrence (%F) and by number (N%). Totals represent number of non-empty stomachs (F) and number of unique prey items recorded (N).
(DOCX)
Acknowledgments
We thank the Operations staff of the KwaZulu-Natal Sharks Board for providing specimens and information associated with their capture. We particularly thank Phillip Zungu and his staff who dissected many of the sharks. We are grateful for comments made by three anonymous reviewers and Neil Hammerschlag, which greatly improved this paper.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Heithaus MR, Frid A, Wirsing AJ, Worm B. Predicting ecological consequences of marine top predator declines. Trends Ecol Evol. 2008;23(4): 202–210. doi: 10.1016/j.tree.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 2.Baum JK, Worm B. Cascading top-down effects of changing oceanic predator abundances. J Anim Ecol. 2009;78(4): 699–714. doi: 10.1111/j.1365-2656.2009.01531.x [DOI] [PubMed] [Google Scholar]
- 3.Ferretti F, Worm B, Britten GL, Heithaus MR, Lotze HK. Patterns and ecosystem consequences of shark declines in the ocean. Ecol Lett. 2010;13: 1055–1071. doi: 10.1111/j.1461-0248.2010.01489.x [DOI] [PubMed] [Google Scholar]
- 4.Baum JK, Myers RA, Kehler DG, Worm B, Harley SJ, Doherty PA. Collapse and conservation of shark populations in the Northwest Atlantic. Science. 2003;299(5605): 389–92. doi: 10.1126/science.1079777 [DOI] [PubMed] [Google Scholar]
- 5.Dulvy NK, Baum JK, Clarke S, Compagno LJV, Cortes E, Domingo A, et al. You can swim but you can’t hide: the global status and conservation of oceanic pelagic sharks and rays. Aquat Conserv Mar Freshwat Ecosyst. 2008;18(5): 459–482. doi: 10.1002/aqc.975 [Google Scholar]
- 6.Worm B, Davis B, Kettemer L, Ward-Paige CA, Chapman DD, Heithaus MR, et al. Global catches, exploitation rates, and rebuilding options for sharks. Mar Policy. 2013;40: 194–204. [Google Scholar]
- 7.Creel S. and Christianson D. Relationships between direct predation and risk effects. Trends Ecol Evol. 2008;23 doi: 10.1016/j.tree.2007.12.004 [DOI] [PubMed] [Google Scholar]
- 8.Myers RA, Baum JK, Shepherd TD, Powers SP, Peterson CH. Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science. 2007;315(5820): 1846–1850. doi: 10.1126/science.1138657 [DOI] [PubMed] [Google Scholar]
- 9.Mac Nally R, Albano C, Fleishman E. A scrutiny of the evidence for pressure-induced state shifts in estuarine and nearshore ecosystems. Austral Ecol. 2014; 39: 898–906. [Google Scholar]
- 10.Capon SJ, A. Jasmyn J. Lynch b, Bond N, Chessman BC, Davis J, Davidson N, Finlayson M et al. Regime shifts, thresholds and multiple stable states in freshwater ecosystems; a critical appraisal of the evidence. Sci Total Environ. 2015: https://doi.org/10.1016/j.scitotenv.2015.02.045 [DOI] [PubMed] [Google Scholar]
- 11.Grubbs RD, Carlson JK, Romine JG, Curtis TH, McElroy WD, McCandless CT, Cotton CF, Musick JA. Critical assessment and ramifications of a purported marine trophic cascade. 2016. Sci. Rep. 6, 20970; doi: 10.1038/srep20970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevens JD, Bonfil R, Dulvy NK, Walker PA. The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J Mar Sci. 2000;57(3): 476–494. [Google Scholar]
- 13.Hussey NE, MacNeil MA, Siple MC, Popp BN, Dudley SFJ, Fisk AT. Expanded trophic complexity among large sharks. Food Webs. 2015;4: 1–7. [Google Scholar]
- 14.Hyslop EJ. Stomach contents analysis—a review of methods and their application. J Fish Biol. 1980;17: 411–429. [Google Scholar]
- 15.Cortes E. Standardized diet compositions and trophic levels of sharks. ICES J Mar Sci. 1999;56: 707–717. [Google Scholar]
- 16.Wetherbee B, Cortes E. Food Consumption and Feeding Habits In: Carrier JC, Musick JA, Heithaus MR, editors. Biology of sharks and their relatives. CRC Press; 2004. pp. 225–246 [Google Scholar]
- 17.Cortés E. A critical review of methods of studying fish feeding based on analysis of stomach contents: application to elasmobranch fishes. Can J Fish Aquat Sci. 1997; 54:726–738. [Google Scholar]
- 18.Estrada JA, Lutcavage M. Thorrold SR. Diet and trophic position of Atlantic Bluefin tuna (Thunnus thynnus) inferred from stable carbon and nitrogen isotope analysis. Mar Biol. 2005;147: 37–45. [Google Scholar]
- 19.Fisk AT, Tittlemier SA, Pranschke JL, Norstrom RJ. Using anthropogenic contaminants and stable isotopes to assess the feeding ecology of Greenland sharks. Ecology. 2002;83(8): 2162–2172. [Google Scholar]
- 20.Hussey NE, Dudley SFJ, McCarthy ID, Cliff G, Fisk AT. Stable isotope profiles of large marine predators: viable indicators of trophic position, diet and movement in sharks. Can J Fish Aquat Sci. 2011;68: 2029–2045. [Google Scholar]
- 21.Estrada JA, Rice AN, Natanson LJ, Skomal GB. Use of isotopic analysis of vertebrae in reconstructing ontogenetic feeding ecology in white sharks. Ecology. 2006;87(4): 829–834. [DOI] [PubMed] [Google Scholar]
- 22.Matich P, Heithaus MR, Layman CA. Size-based variation in intertissue comparisons of stable carbon and nitrogen isotopic signatures of bull sharks (Carcharhinus leucas) and tiger sharks (Galeocerdo cuvier). Can J Fish Aquat Sci. 2010;67(5): 877–885. [Google Scholar]
- 23.DeNiro MJ, Epstein S. Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta. 1978;42: 495–506. [Google Scholar]
- 24.Peterson BJ, Fry B. Stable isotopes in ecosystem studies. Annu Rev Ecol Syst. 1987;18: 293–320. [Google Scholar]
- 25.Carlisle AB, Kim SL, Semmens BX, Madigan DJ, Jorgensen SJ, Perle CR, et al. Using stable isotope analysis to understand the migration and trophic ecology of northeastern Pacific white sharks (Carcharodon carcharias). PLoS ONE. 2012;7(2): e30492 doi: 10.1371/journal.pone.0030492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Post DM. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology. 2002;83(3): 703–718. [Google Scholar]
- 27.Estrada JA, Rice AN, Lutcavage ME, Skomal GB. Predicting trophic position in sharks of the north-west Atlantic Ocean using stable isotope analysis. J Mar Biol Assoc UK.2003;83: 1347–1350. [Google Scholar]
- 28.Newsome SD, Martínez del Rio C, Bearhop S, Phillips DL. A niche for isotopic ecology. Front Ecol Environ. 2007;5: 429–436. [Google Scholar]
- 29.Kim SL, Tinker MT, Estes JA, Koch PL. Ontogenetic and among-individual variation in foraging strategies of northeast Pacific white sharks based on stable isotope analysis. PLoS ONE. 2012;7(9): e45068 doi: 10.1371/journal.pone.0045068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matich P, Heithaus MR, Layman CA. Contrasting patterns of individual specialization and trophic coupling in two marine apex predators. J Anim Ecol. 2011;80(1): 294–305. doi: 10.1111/j.1365-2656.2010.01753.x [DOI] [PubMed] [Google Scholar]
- 31.MacNeil MA, Drouillard KG, Fisk AT. Variable uptake and elimination of stable nitrogen isotopes between tissues in fish. Can. J. Fish. Aquat. Sci. 2006;63(2): 345–353. [Google Scholar]
- 32.Logan JM, Lutcavage ME. Stable isotope dynamics in elasmobranch fishes. Hydrobiologia. 2010; 644(1): 231–244. [Google Scholar]
- 33.Bolnick DI, Amarasekare P, Araújo MS, Bürger R, Levine JM et al. Why intraspecific trait variation matters in community ecology. Trends Ecol Evol. 2011;26: 183–192. doi: 10.1016/j.tree.2011.01.009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newsome SD, Etnier MA, Monson DH, Fogel ML. Retrospective characterization of ontogenetic shifts in killer whale diets via δ13C and δ15N analysis of teeth. Mar Ecol Prog Ser. 2009;374: 229–242. [Google Scholar]
- 35.Randall JE. Review of the biology of the tiger shark (Galeocerdo cuvier). Aust J Mar Freshwat Res. 1992;43: 21–31. [Google Scholar]
- 36.Compagno LJV. FAO species catalogue. Sharks of the world: An annotated and illustrated catalogue of shark species known to date. II. Carcharhiniformes. FAO Fish Synop. 1984;4(125): 251–655. [Google Scholar]
- 37.Bass AJ, D’Aubrey JD, Kistnasamy N. Sharks of the east coast of southern Africa. III. The families Carcharhinidae (excluding Carcharhinus and Mustelus) and Sphyrnidae. Invest Rep Oceanogr Res Inst. 1975;38: 100. [Google Scholar]
- 38.Compagno LJV, Ebert DA, Smale MJ. Guide to the sharks and rays of southern Africa. Cape Town: Struik; 1989. [Google Scholar]
- 39.Holmes BJ, Sumpton WD, Mayer DG, Tibbetts IR, Neil DT, Bennett MB. Declining trends in annual catch rates of the tiger shark (Galeocerdo cuvier) in Queensland, Australia. Fish Res. 2012;129–130: 38–45. [Google Scholar]
- 40.Meyer CG, O'Malley JM, Papastamatiou YP, Dale JJ, Hutchinson MR, Anderson JM, et al. Growth and maximum size of tiger sharks (Galeocerdo cuvier) in Hawaii. PLoS ONE. 2014;9(1): e84799 doi: 10.1371/journal.pone.0084799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dicken ML, Cliff G, Winker H. Sharks caught in the KwaZulu-Natal bather protection programme, South Africa. 13. The tiger shark Galeocerdo cuvier. Afr J Mar Sci. 2016;38(3): 1–17. [Google Scholar]
- 42.Simpfendorfer C. Biology of tiger sharks (Galeocerdo cuvier) caught by the Queensland shark meshing program off Townsville, Australia. Aust J Mar Freshwater Res. 1992;43(33–43). [Google Scholar]
- 43.Lowe CG, Wetherbee BM, Crow GL, Tester AL. Ontogenetic dietary shifts and feeding behaviour of the tiger shark, Galeocerdo cuvier, in Hawaiian waters. Environ Biol Fish. 1996;(47): 203–211. [Google Scholar]
- 44.Simpfendorfer CA, Goodreid AB, McAuley RB. Size, sex and geographic variation in the diet of the tiger shark, Galeocerdo cuvier, from Western Australian waters. Environ Biol Fish. 2001(61): 37–46. [Google Scholar]
- 45.Heithaus MR, Wirsing AJ, Dill LM, Heithaus LI. Long- term movements of tiger sharks satellite-tagged in Shark Bay, Western Australia. Mar Biol. 2007;151: 1455–1461 [Google Scholar]
- 46.Holmes BJ, Pepperell JG, Griffiths SP, Jaine FRA, Tibbetts IR, Bennett MB. Tiger shark (Galeocerdo cuvier) movement patterns and habitat use determined by satellite tagging in eastern Australian waters. Mar Biol. 2014;161(11): 2645–58. [Google Scholar]
- 47.Lowe CG, Wetherbee BM, Meyer CG. Using acoustic telemetry monitoring techniques to quantify movement patterns and site fidelity of sharks and giant trevally around French Frigate Shoals and Midway Atoll. Atoll Res Bull. 2006;543: 281–303 [Google Scholar]
- 48.Meyer CG, Clark TB, Papastamatiou YP, Whitney NM, Holland KN. Long-term movement patterns of tiger sharks Galeocerdo cuvier in Hawaii. Mar Ecol Prog Ser. 2009;381: 223–235. [Google Scholar]
- 49.Fitzpatrick R, Thums M, Bell I, Meekan MG, Stevens JD, Barnett A. A comparison of the seasonal movements of tiger sharks and green turtles provides insight into their predator-prey relationship. PLoS ONE. 2012;7(12): e51927 doi: 10.1371/journal.pone.0051927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Werry JM, Planes S, Berumen ML, Lee KA, Braun CD, Clua E. Reef-fidelity and migration of tiger sharks, Galeocerdo cuvier, across the Coral Sea. PLoS ONE. 2014;9(1): e83249 doi: 10.1371/journal.pone.0083249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hammerschlag N, Gallagher AJ, Wester J, Luo J, Ault JS. Don’t bite the hand that feeds: assessing ecological impacts of provisioning ecotourism on an apex marine predator. Funct Ecol. 2012;26(3): 567–576. [Google Scholar]
- 52.Wintner SP, Dudley SFJ. Age and growth estimates for the tiger shark, Galeocerdo cuvier, from the east coast of South Africa. Mar Freshwater Res. 2000;51: 43–53. [Google Scholar]
- 53.Dudley SFJ, Simpfendorfer CA. Population status of 14 shark species caught in the protective gillnets off KwaZulu-Natal beaches, South Africa, 1978–2003. Mar Freshwater Res. 2006; 57: 225–240. [Google Scholar]
- 54.Heithaus MR. The biology of tiger sharks, Galeocerdo cuvier, in Shark Bay, Western Australia: sex ratio, size distribution, diet, and seasonal changes in catch rates. Environ Biol Fish. 2001;61: 25–36. [Google Scholar]
- 55.Cliff G, Dudley SFJ. Sharks caught in the protective gill nets of Natal, South Africa. 6. The copper shark (Carcharhinus brachyurus) (Gunther). S Afr J Mar Sci. 1992;12: 663–674. [Google Scholar]
- 56.Dudley SFJ, Haestier RC, Cox KR, Murray M. Shark control: experimental fishing with baited drumlines. Mar Freshwater Res.1998;49: 653–661. [Google Scholar]
- 57.Cliff G, Dudley SFJ. Reducing the environmental impact of shark control programs: a case study from KwaZulu-Natal, South Africa. Mar Freshwater Res. 2011;62: 700. [Google Scholar]
- 58.Dudley S, Cliff G, Zungu MP, Smale M. Sharks caught in the protective gill nets off KwaZulu-Natal, South Africa. 10. The dusky shark Carcharhinus obscurus (Lesueur 1818). Afr J Mar Sci. 2005;27(1): 107–127. [Google Scholar]
- 59.Cliff G, Dudley SFJ, Davis B. Sharks caught in the protective gill nets of Natal, South Africa. 2. The great white shark (Carcharodon carcharias) (Linnaeus). S Afr J Mar Sci. 1989;8: 131–144. [Google Scholar]
- 60.Smale MJ, Watson G, Hecht T. Otolith atlas of southern African marine fishes. Ichthyol. Monogr. J. L. B. Smith Inst. Ichthyol. 1995;1: 1–253 [Google Scholar]
- 61.Clarke MR. Handbook for the identification of cephalopod beaks. Oxford: Oxford University Press; 1986. [Google Scholar]
- 62.Smale MJ, Clarke MR, Klages NTW, Roeleveld MAC. Octopod beak identification-resolution at a regional level (Cephalopoda, Octopoda: southern Africa). S Afr J Mar Sci. 1993;13(1): 269–293. [Google Scholar]
- 63.Smale MJ. Occurrence and feeding of three shark species, Carcharhinus brachyurus, C. obscurus and Sphyrna zygaena, on the Eastern Cape coast of South Africa. S. Afr. J. mar. Sci. 1991; 11: 31–42. [Google Scholar]
- 64.Smale MJ, Cliff G. Cephalopods in the diets of four shark species (Galeocerdo cuvier, Sphyrna lewini, S. zygaena and S. mokarran) from KwaZuluNatal, South Africa. S. Afr. J. mar. Sci. 1998; 20: 241–253. [Google Scholar]
- 65.Platell ME, Potter IC. Partitioning of food resources amongst 18 abundant benthic carnivorous fish species in marine waters on the lower west coast of Australia. J Exp Mar Biol Ecol. 2001;261: 31–54 [DOI] [PubMed] [Google Scholar]
- 66.White WT, Platell ME, Potter IC. Comparisons between the diets of four abundant species of elasmobranchs in a subtropical embayment: implications for resource partitioning. Mar Biol. 2004;144(3): 439–448. [Google Scholar]
- 67.Bray RJ, Curtis JT. An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr. 1957;27: 325–349. [Google Scholar]
- 68.Huveneers C, Otway NM, Gibbs SE, Harcourt RG. Quantitative diet assessment of wobbegong sharks (genus Orectolobus) in New South Wales, Australia. ICES J M Sci. 2007;64: 1272–1281. [Google Scholar]
- 69.Clarke KR, Gorley RN, Somerfield PJ, Warwick RM. Change in marine communities: An approach to statistical analysis and interpretation 3rd edition PRIMER-E: Plymouth, UK: 2014. [Google Scholar]
- 70.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Physiol Pharmacol. 1959;37: 911–917. [DOI] [PubMed] [Google Scholar]
- 71.Li Y, Zhang Y, Hussey NE, Dai X. Urea and lipid extraction treatment effects on δ15N and δ13C values in pelagic sharks. Rapid Commun Mass Spectrom. 2015;30: 1–8 [DOI] [PubMed] [Google Scholar]
- 72.Hussey NE, MacNeil MA, Olin JA, McMeans BC, Kinney MJ, Chapman DD, et al. Stable isotopes in elasmobranchs: tissue types, methods, applications and assumptions. J Fish Biol. 2012;80: 1449–1484. doi: 10.1111/j.1095-8649.2012.03251.x [DOI] [PubMed] [Google Scholar]
- 73.MacNeil MA, Skomal GB, Fisk AT (2005) Stable isotopes from multiple tissues reveal diet switching in sharks. Mar Ecol Prog Ser 302:199–206. doi: 10.3354/meps302199 [Google Scholar]
- 74.Hussey NE, Brush J, McCarthy ID, Fisk AT. δ15N and δ13C diet-tissue discrimination factors for large sharks under semi-controlled conditions. Comp Biochem Physiol A Mol Integr Physiol. 2010;155(4): 445–453. doi: 10.1016/j.cbpa.2009.09.023 [DOI] [PubMed] [Google Scholar]
- 75.Roughgarden J. Evolution of Niche Width. Am Nat. 1972;106: 683–687. [Google Scholar]
- 76.Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RC. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–128. 2016
- 77.Hussey NE, MacNeil MA, McMeans BC, Olin JA, Dudley SFJ, Cliff G, et al. Rescaling the trophic structure of marine food webs. Ecol Lett. 2014a;17: 239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hussey NE, MacNeil MA, McMeans BC, Olin JA, Dudley SFJ, Cliff G, et al. Corrigendum to Hussey et al. (2014). Ecol Lett. 2014b;17: 768–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vander Zanden MJ, Cabana G, Rasmussen JB. Comparing trophic position of freshwater fish calculated using stable nitrogen isotope ratios (δ15N) and literature dietary data. Can J Fish Aquat Sci. 1997;54: 1142–1158. [Google Scholar]
- 80.Wilson SG, Newbound DR. Two whale shark faecal samples from Ningaloo Reef, Western Australia. Bull Mar Sci. 2001;68: 361–362. [Google Scholar]
- 81.Hoffmayer ER, Franks JS, Driggers WB III, Oswald KJ, Quattro JM. Observations of a feeding aggregation of whale sharks, Rhincodon typus, in the north central Gulf of Mexico. Gulf Caribb Res. 2007;19: 69–73. [Google Scholar]
- 82.Couturier LIE, Dudgeon CL, Pollock KH, Jaine FRA, Bennett MB. Population dynamics of the reef manta ray Manta alfredi in eastern Australia. Coral Reefs. 2014;33:329–342. [Google Scholar]
- 83.Malcolm H, Bruce BD, Stevens JD. A review of the biology and status of white sharks in Australian waters. Report to Environment Australia, Marine Species Protection Program, CSIRO Marine Research, Hobart. 2001.
- 84.Holland KN, Wetherbee BM, Lowe CG, Meyer CG. Movements of tiger sharks (Galeocerdo cuvier) in coastal Hawaiian waters. Mar Biol. 1999;134: 665–673 [Google Scholar]
- 85.Nakamura I, Watanabe YY, Papastamatiou YP, Sato K, Meyer CG. Yoyo vertical movements suggest a foraging strategy for tiger sharks Galeocerdo cuvier. Mar Ecol Prog Ser. 2011;424: 237–246. [Google Scholar]
- 86.Meyer CG, Papastamatiou YP, Holland KN. A multiple instrument approach to quantifying the movement patterns and habitat use of tiger (Galeocerdo cuvier) and Galapagos sharks (Carcharhinus galapagensis) at French Frigate Shoals, Hawaii. Mar Biol. 2010;157: 1857–1868. [Google Scholar]
- 87.Vaudo JJ, Wetherbee BM, Harvey G, Nemeth RS, Aming C, Burnie N, et al. Intraspecific variation in vertical habitat use by tiger sharks (Galeocerdo cuvier) in the western North Atlantic. Ecol. Evol. 2014;4: 1768–1786. doi: 10.1002/ece3.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Best PB, Findlay KP, Sekiguchi K, Peddemors VM, Rakotonirina B, Rossouw A, et al. Winter distribution and possible migration routes of humpback whales Megaptera novaeangliae in the southwest Indian Ocean. Mar Ecol Progr Ser. 1998;162: 287–299. [Google Scholar]
- 89.Clapham PJ, Baker CS. Modern whaling In: Perrin WF, Würsig B, Thewissen JGM, editors. Encyclopedia of marine mammals. New York; Academic Press; 2002. pp. 1328–1332. [Google Scholar]
- 90.Hammerschlag N, Broderick AC, Coker JW, Coyne MS, Dodd M, Frick MG, Godfrey MH, Godley BJ, Griffin DB, Hartog K, Murphy SR. Evaluating the landscape of fear between apex predatory sharks and mobile sea turtles across a large dynamic seascape. Ecology. 2015; 96(8): 2117–26. [DOI] [PubMed] [Google Scholar]
- 91.Tricas TC, Taylor LR, Naftel G. Diel behavior of the tiger shark, Galeocerdo cuvier, at French Frigate Shoals, Hawaiian Islands. Copeia. 1981: 904–908. [Google Scholar]
- 92.Mazzuca L, Atkinson S, Nitta E. Deaths and entanglements of humpback whales, Megaptera novaeangliae, in the main Hawaiian Islands, 1972–1996. 1998. Pacific Science;52: 1–13. [Google Scholar]
- 93.Fitzpatrick B, Meekan M, Richards A. Shark attacks on a whale shark (Rhincodon typus) at Ningaloo Reef, Western Australia. Bull. Mar. Sci. 2006;78(2): 397–402. [Google Scholar]
- 94.Naessig PJ, Lanyon JM. Levels and probable origin of predatory scarring on humpback whales (Megaptera novaeangliae) in east Australian waters. Wildl. Res. 2004;31(2): 163–170. [Google Scholar]
- 95.Bornatowski H, Wedekin LL, Heithaus MR, Marcondes MCC, Rossi-Santos MR. Shark scavenging and predation on cetaceans at Abrolhos Bank, eastern Brazil. Journal of the Marine Biological Association of the United Kingdom. 2012;92(8): 1767–1772. [Google Scholar]
- 96.Dicken ML, Kock AA, Hardenberg M. First observations of dusky sharks (Carcharhinus obscurus) attacking a humpback whale (Megaptera novaeangliae) calf. Mar Freshwater Res. 2015;66(12): 1211–5. [Google Scholar]
- 97.Scharf FS, Juanes F, Rountree RA. Predator size-prey size relationships of marine fish predators: interspecific variation and effects of ontogeny and body size on trophic- niche breadth. Mar Ecol Prog Ser. 2000;208: 229–248 [Google Scholar]
- 98.Rancurel P. Intes A. Le requin tigre, Galeocerdo cuvieri Lacepede, des eaux Neocaledoniennes examen des contenus stomacaux. Tethys. 1982;10(3): 195–199 [Google Scholar]
- 99.Fu AL, Hammerschlag N, Lauder GV, Wilga CD, Kuo CY, Irschick DJ. Ontogeny of head and caudal fin shape of an apex marine predator: The tiger shark (Galeocerdo cuvier). J. Morphol. 2016;277: 556–564 doi: 10.1002/jmor.20515 [DOI] [PubMed] [Google Scholar]
- 100.Sousa MI, Marshall NT, Smale MJ. The shark trade in Mozambique In: Marshall NT, Barnett R, editors. The Trade in Sharks and Shark Products in the Western Indian and Southeast Atlantic Oceans. TRAFFIC East/Southern Africa: Nairobi: 1997. pp. 67–69 [Google Scholar]
- 101.da Silva C, Booth AJ, Dudley SFJ, Kerwath SE, Lamberth SJ, Leslie RW, McCord ME, Sauer WHH, Zweig T. The current status and management of South Africa's chondrichthyan fisheries. S Afr J Mar Sci. 2015;37(2): 233–248. [Google Scholar]
- 102.Findlay KP, Best PB, Meÿer MA. Migrations of humpback whales past Cape Vidal, South Africa, and an estimate of the population increase rate (1988–2002). Afr. J Mar Sci. 2011;33: 375–392. [Google Scholar]
- 103.Hammerschlag N, Gallagher AJ, Carlson JK. A revised estimate of daily ration in the tiger shark with implication for assessing ecosystem impacts of apex predators. Funct. Ecol. 2013;27(5): 1273–4. [Google Scholar]
- 104.Marshall AD, Bennett MB. The frequency and effect of shark-inflicted bite injuries to the reef manta ray Manta alfredi. Afr J Mar Sci. 2010;32(3): 573–580. [Google Scholar]
- 105.Sherwood GD, Rose GA. Stable isotope analysis of some representative fish and invertebrates of the Newfoundland and Labrador continental shelf food web. Estuar. Coast. Shelf Sci. 2005; 63(4): 537–549. [Google Scholar]
- 106.Trystram C, Rogers KM, Soria M, Jaquemet S. Feeding patterns of two sympatric shark predators in coastal ecosystems of an oceanic island. Can J Fish Aquat Sci. 2016; Available from: http://www.nrcresearchpress.com/doi/abs/10.1139/cjfas-2016-105#.WCxRrOZ95PY [Google Scholar]
- 107.Vander Zanden HB, Bjorndal KA, Reich KJ, Bolten AB. Individual specialists in a generalist population: results from a long-term stable isotope series. Biol Lett 2010;6: 711–714. 2010. doi: 10.1098/rsbl.2010.0124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vander Zanden HB, Arthur KE, Bolten AB, Popp BN, Lagueux CJ, Harrison E, Campbell CL, Bjorndal KA. Trophic ecology of a green turtle breeding population. Mar Ecol Prog Ser. 2013;476: 237–249. [Google Scholar]
- 109.Carlson JK, Grace MA, Lago PK. An observation of juvenile tiger sharks feeding on clapper rails off the south- eastern coast of the United States. Southeast Nat. 2002;1(3): 307–10. [Google Scholar]
- 110.Márquez MR. FAO Species Catalogue. Sea turtles of the world; An annotated and illustrated catalogue of sea turtles species known to date. FAO Fish Synop. 1990;11(125): 1–81. [Google Scholar]
- 111.Hughes GR. The sea turtles of south-east Africa. 1. Status, morphology and distributions. Invest Rep oceanogr Res Inst S Afr. 1974;35: 144 pp. [Google Scholar]
- 112.Ineich I, Laboute P. Les serpents marins de Nouvelle-Caledonie/Sea snakes of New Caledonia. Paris: I.R.D. (Institut pour la Recherche et le Developpement) and M.N.H.N. (Museum national d'Histoire naturelle) 2002. 302 pp. [Google Scholar]
- 113.Heithaus MR, Dill LM, Marshall GJ, Buhleier B. Habitat use and foraging behaviour of tiger sharks (Galeocerdo cuvier) in a seagrass ecosystem. Mar Biol. 2002;140: 237–248 [Google Scholar]
- 114.Heithaus MR, Frid A. Optimal diving under the risk of predation. J. Theor. Biol. 2003;223: 79–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Details of the prey are presented by frequency of occurrence (%F) and by number (N%). Totals represent number of non-empty stomachs (F) and number of unique prey items recorded (N).
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.