Abstract
The aim of this study was to assess if the dose and exposure duration of the anabolic androgenic steroids (AAS) boldenone (BOL) and stanazolol (ST) affected memory, anxiety, and social interaction, as well as acetylcholinesterase (AChE) activity and oxidative stress in the cerebral cortex (CC) and hippocampus (HC). Male Wistar rats (90 animals) were randomly assigned to three treatment protocols: (I) 5 mg/kg BOL or ST, once a week for 4 weeks; (II) 2.5 mg/kg BOL or ST, once a week for 8 weeks; and (III) 1.25 mg/kg BOL or ST, once a week for 12 weeks. Each treatment protocol included a control group that received an olive oil injection (vehicle control) and AAS were administered intramuscularly (a total volume of 0.2 ml) once a week in all three treatment protocols. In the BOL and ST groups, a higher anxiety level was observed only for Protocol I. BOL and ST significantly affected social interaction in all protocols. Memory deficits and increased AChE activity in the CC and HC were found in the BOL groups treated according to Protocol III only. In addition, BOL and ST significantly increased oxidative stress in both the CC and HC in the groups treated according to Protocol I and III. In conclusion, our findings show that the impact of BOL and ST on memory, anxiety, and social interaction depends on the dose and exposure duration of these AAS.
Introduction
Anabolic androgenic steroids (AAS) form a large class of synthetic androgens that mimic the effects of male sex hormones such as testosterone and dihydrotestosterone. AAS are widely used by athletes to increase muscle mass and enhance physical performance, and by non-athletes for esthetic reasons. Both young athletes and non-athletes may take 10–100-fold the physiological dose of AAS [1]. Further, several studies have investigated the potentially severe side-effects of AAS abuse [2, 3]. In fact, the number of adolescents using AAS has grown significantly over the past 10 years, with estimates of use ranging between 4–12% among adolescents.
AAS in supraphysiological doses affect several central nervous system- (CNS) related behaviors such as memory, aggression, anxiety, and depression [2, 4]. Studies investigating the mechanisms underlying AAS demonstrated that AAS influence neurotransmission in the CNS by directly affecting the cellular membrane, modulating synthesis and degradation of neurotransmitters, and altering neurotransmitter metabolism [5, 6]. In addition, androgen receptors are expressed in brain structures such as the hippocampus (HC), amygdala, and cerebral cortex (CC). Further, the use of AAS interferes with important signaling and neurotransmission systems, such as glutamatergic [7], cholinergic [8], and opioid systems, that modulate animal behavior [9].
Behavioral responses to AAS depend on several factors, including the chemical structure of the steroid administered, whether a single compound or cocktail is administered, the recipient’s age, and treatment duration [10]. While several controlled studies have described the behavioral changes induced by AAS, these studies primarily used hormone cocktails. Therefore, the aim of this study was to comparatively and separately assess the effects of the AAS boldenone (BOL) and stanazolol (ST) on behavioral tasks to determine if one or both altered learning, memory, anxiolytic-like behavior, and dominant or submissive social behavior. In addition, in an attempt to simulate different user groups, we analyzed the effects of three different treatment protocols that varied in dose and treatment duration to determine their impact on user outcomes.
Materials and methods
Animals
Male Wistar rats (45 days of age) weighing +/-200 g were used in the study. The animals were maintained in the Central Animal House of the Federal University of Santa Maria in colony cages at an ambient temperature of 23 ± 2°C and a relative humidity of 45–55% under a 12-h light/dark cycle. The animals had access to a standard rodent pellet diet and water ad libitum.
Experimental treatment protocols
Stanazolol (EstrombolTM, Fundación Lab, Argentina) and boldenone undecylenate (EquipoiseTM, Fort Dodge Lab, USA) treatments were administered according to Protocol I, II and III. Both the vehicle (olive oil) and AAS were administered intramuscularly (a total volume of 0.2 ml) once a week in all three treatment protocols. A total of 90 animals were used to protocol I, II, or III, with rats being randomly allocated to 3 groups: vehicle (VE, n = 10), Boldenone (BOL, n = 10) and stanazolol (ST, n = 10).
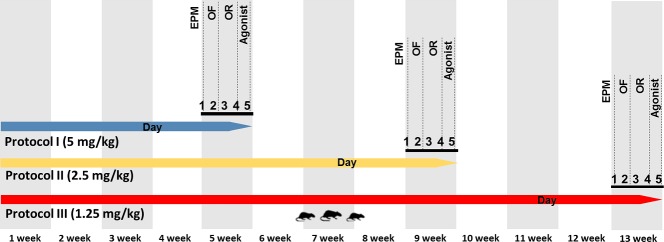
Protocol I: 5 mg/kg of ST or BOL for 4 weeks. Protocol II: 2.5 mg/kg of ST or BOL for 8 weeks. Protocol III: 1.25 mg/kg of ST or BOL for 12 weeks. A representative treatment scheme is shown in Fig 1. The animals were anesthetized using halothane before being euthanized by total exsanguination. The CC and HC of each rat were dissected and homogenized in a Tris–HCl 10 mM solution, pH 7.4, on ice [11].
Fig 1.
Experimental Protocols: Protocol I: Intramuscular injection of vehicle (olive oil, 0.2 ml), stanazolol (ST, 5 mg/kg), or boldenone (BOL, 5 mg/kg) once a week for 4 weeks. Protocol II: Intramuscular injection of vehicle (olive oil, 0.2 ml), stanazolol (ST, 2.5 mg/kg), or boldenone (BOL, 2.5 mg/kg) once a week for 8 weeks. Protocol III: Intramuscular injection of vehicle (olive oil, 0.2 ml), stanazolol (ST, 1.25 mg/kg) or boldenone (BOL, 1.25 mg/kg) once a week for 12 weeks. Day 1: elevated plus maze task (EPM). Day 2: object recognition task, habituation. Day 3: object recognition task, training. Day 4: object recognition task, testing. Day 5: agonist behavior (resident–intruder paradigm), (n = 10).
Behavioral tests
Elevated plus maze task
On the first day of the evaluation of the behavioral parameters, anxiolytic-like behavior was evaluated using the elevated plus maze (DAY 1) as previously described [12]. The apparatus consisted of a wooden structure raised to 50 cm from the floor, and was composed of four equally sized arms (two closed arms [walls 40 cm] and two open arms). The rats were placed on the central platform of the maze facing an open arm, and were allowed 5 min to explore the apparatus. The time spent and the number of entries in the open and closed arms were recorded. The apparatus was thoroughly cleaned with 30% ethanol between each session.
Open field test
Locomotor and exploratory activities were evaluated in the apparatus used for the object recognition task as previously described [13]. The number of crossings and rearings was assessed on the first day of exposure to the apparatus (habituation day, DAY 2). The animals were transferred to an apparatus with an open field of 100 cm × 100 cm (floor; divided into 16 squares of 6.25 cm2 each) × 50 cm (walls). During the 5-min open-field session, the number of crossings and rearings was recorded.
Object recognition test
The object recognition task makes use of the spontaneous tendency of a rat to explore its environment and does not require punishment or reward [14]. Here, it consisted of three sessions: habituation (DAY 2), training (DAY 3), and testing (DAY 3), and was conducted as previously described [15]. Briefly, the rats were left to freely explore a square arena (length: 100 cm × 100 cm, height: 50 × 50 cm) for 10 min in the absence of any objects. Twenty-four hours after habituation, the training session was conducted by placing one rat into the arena in which two identical objects (objects A1 and A2) were positioned at two adjacent sides of the arena. The rat was allowed to explore the arena and objects for 10 min. Generally, during the training session, a rat should explore each object for 40–60% of the time spent in the arena, or otherwise be excluded from the experiments. Here, during the test session performed 24 h after the training session, the rats were allowed 10 min to explore the arena in which one of the familiar objects used during the training session was replaced by a novel object (object B). The discrimination index was then calculated, taking into account the difference of time spent exploring the novel (B) and the familiar (A) object x 100 divided by the sum of time spent exploring the novel (B) and the familiar (A), and used as a cognitive parameter ([(Tnovel–Tfamiliar)/(Tnovel X Tfamiliar)]/100) [20]. The objects were made of odorless plastic and were similar in size. Between each trial, the objects and the arena were cleaned with a 30% ethanol solution. The total time spent sniffing or touching each object with the nose and/or forepaws and the number of rearings were analyzed.
Agonistic behavior test
The rats were tested for agonistic behavior using the resident–intruder paradigm described by Salas-Ramires et al. [10]. After a 5-min acclimation period, an age- and weight-matched male intruder was placed among the rats in the vehicle, BOL, and ST home cage. The task was recorded and the recordings were analyzed. The duration and number of occurrences of dominant behavior over the intruder (contact team and offensive posturing) and dominant behavior over the territory (number of flank marks) were recorded. Aggressive behavior was determined by the number of attacks and bites over the intruder. Submissive behavior was determined by the frequency of defensive posturing, walking with tail upward, and escape dashes. The intruders were used for more than one behavioral test. All rats were tested during the first 4 h of the dark cycle under dimmed red light conditions to control for circadian influences on behavioral responses [16].
Sample preparation for biochemical parameters
The CC and HC were dissected and placed in a 10-mM Tris–HCl solution and 0.1 mM EDTA, pH 7.4, on ice, followed by homogenization in a glass potter in Tris–HCl solution [17]. Aliquots of the homogenate were separated. After centrifugation at 1’500 × g at 4°C for 15 min, aliquots of the supernatant were stored at −80°C until use.
Measurement of intracellular ROS production
2′-7′-Dichlorofluorescein diacetate (DCFH-DA) levels were determined as markers of intracellular ROS production. Aliquots (50 μl) of the supernatants of the CC and HC were added to a medium containing Tris–HCl buffer (10 mM, pH 7.4) and 1 mM DCFH-DA. After the addition of DCFH-DA, the medium was incubated in the dark for 1 h until fluorescence was measured (excitation at 488 nm and emission at 525 nm, with slit widths of 1.5 nm). DCFH-DA levels were determined using a standard curve consisting of DCF; the results were normalized to the total protein content [18].
MDA levels
MDA levels in the CC and HC homogenates were measured using the thiobarbituric acid reactive species (TBARS) method as previously described [19], with minor modifications [20]. Briefly, the reaction mixture contained 200 μl of homogenate or standard (MDA, 0.03 mM), 200 μl of 8.1% sodium dodecylsulfate (SDS), 750 μl of acetic acid solution (2.5 M HCl, pH 3.5), and 750 μl of 0.8% thiobarbituric acid (TBA). The mixtures were heated at 95°C for 90 min. After centrifugation at 1’700 × g for 5 min, the absorbance was measured at 532 nm. The MDA tissue levels were expressed as µmol MDA/mg of protein.
GSH levels
GSH levels were determined in the CC and HC as previously described [21]. Aliquots of the supernatant (100 μl) adjusted to 1 mg/ml of protein content were added to 85 μl of a phosphate buffer (300 mM, pH 7.4), 50 μl of a 10-mM 5,5′-dithiobisnitrobenzoic acid (DTNB) solution were added, and the reaction was read at 412 nm. The results were expressed as μmol of GSH/mg of protein.
NPSH levels
Tissue NPSH levels were determined in the CC and HC as previously described [21]. Briefly, the supernatant was diluted (1:1) with 10% trichloroacetic acid (TCA), homogenized, and centrifuged at 2’000 × g for 10 min. Subsequently, the supernatant was incubated with 10 mM DTNB in a final volume of 2 ml, and the absorbance was read at 412 nm. A cysteine solution was used as the reference standard. The NPSH levels were expressed as µmol SH/mg of tissue.
Determination of cerebral AChE activity
AChE activity was determined as previously described [22], with a modification of the spectrophotometric method [23]. The reaction mixture (2 ml final volume) contained 100 mM K+-phosphate buffer, pH 7.5, and 1 mM DTNB. This method is based on the formation of the yellow anion 5,5′-dithio-bis-acid-nitrobenzoic as measured by its absorbance at 412 nm during a 2-min incubation at 25°C. The enzyme (40–50 μg of protein) was pre-incubated for 2 min. The reaction was initiated by adding 0.8 mM acetylthiocholine iodide (AcSCh). All samples were run in triplicate and enzyme activity was expressed in μmol AcSCh/h/mg of protein. The protein concentration was determined beforehand in a piece of the respective brain tissue: CC, 0.7 mg/ml and HC, 0.8 mg/ml as previously described [24].
Protein concentration determination
Protein concentrations were measured by the Coomassie Blue method [25] using bovine serum albumin as the standard.
Statistical analysis
The Shapiro-Wilk test was included in the statistical analysis for the determination of the normal distribution of data (SPSS Statistics 22.0). One-way ANOVA followed by Tukey's test was used for the analysis of behavioral results, stress parameters, and enzyme activity tests. P<0.05 was considered significant in all experiments (GraphPad Prism 5.0). All data were expressed as the mean ± SEM.
Ethical approval
All procedures were performed according to the NIH Guide for the Care and Use of Laboratory Animals and the Brazilian Society for Neuroscience and Behavior (SBNeC) recommendations for animal care. This work was approved by the ethical committee of the Federal University of Santa Maria, Brazil (protocol number 032/2014).
Results
Anxiogenic-like behavior of rats treated with BOL and ST according to Protocol I, II, and III
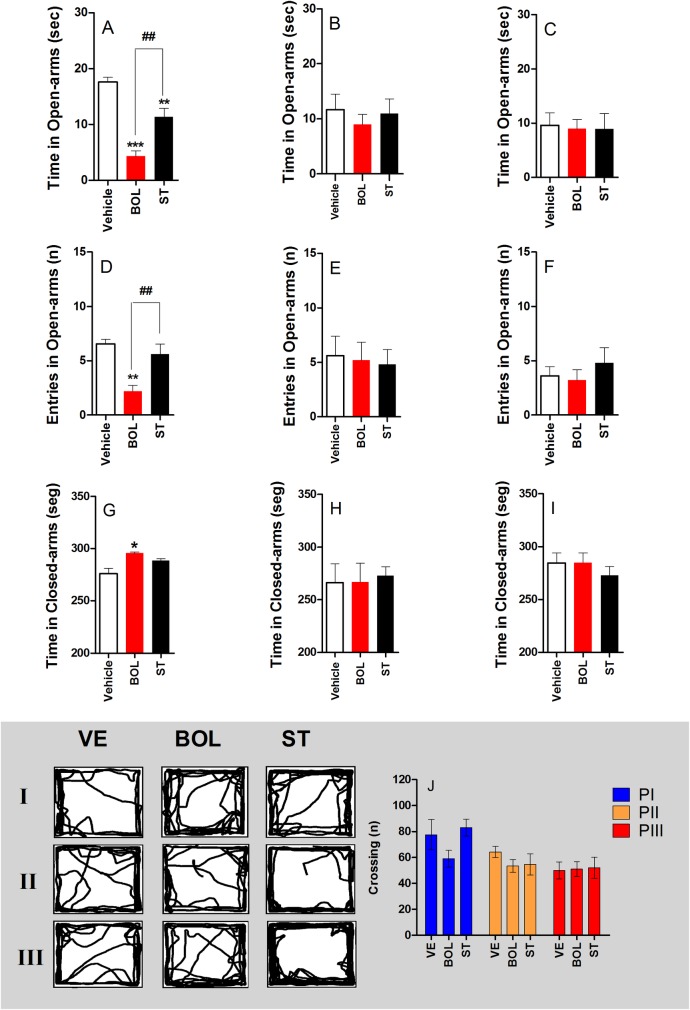
Fig 2 shows the anxiogenic-like behavior of rats in the elevated plus maze. Rats receiving BOL and ST according to Protocol I showed a decrease in the time spent in the open arms [F(2,29) = 30.53, p<0.001, graph A], while those receiving BOL and ST according to Protocols II and III did not show differences in the time spent in the open-arms [F(2,29) = 0.301, graph B and F(2,29) = 0.027, graph C, respectively]. Rats receiving BOL according to Protocol I showed a decrease in the number of open-arm entries [F(2,29) = 11.14, p<0.01, graph D]. However, rats receiving ST according to Protocol I and those receiving BOL and ST according to Protocol II [F(2,29) = 0.0612, graph E] and III [F(2,29) = 0.517, graph F] did not show a change in the number of open-arm entries. Rats receiving BOL according to Protocol I spent more time in the closed-arm [F(2,29) = 10.86, p<0.01, graph G]. BOL and ST administered according to Protocol II did not affect the time spent in the closed arm [F(2,29) = 0.057, graph H] and III [F(2,29) = 0.557, graph I]. Further, treatment with BOL and ST according to Protocol I, II, or III did not affect crossing numbers [F(2,29) = 1.855, P1; F(2,29) = 0.824, P2 and F(2,29) = 0.025, P3, graph J].
Fig 2.
Anxiogenic-like behavior of rats (n = 10) treated intramuscularly with vehicle (olive oil, 0.2 ml), boldenone (BOL), or stanazolol (ST) once a week according to Protocol I (4 weeks, 5 mg/kg), Protocol II (8 weeks, 2.5 mg/kg), or Protocol III (12 weeks, 1.25 mg/kg) as assessed in the elevated plus maze task. Time spent in the open-arms (A, B, and C), entry to open-arms (D, E, and F), time spent in closed-arms (G, H, and I), and crossing numbers (J; P1, P2 and P3). *Denotes significant difference from the vehicle group. # Denotes significant difference between BOL and ST groups.
Dominant behavior over an intruder of rats treated with BOL and ST according to Protocol I, II, and III
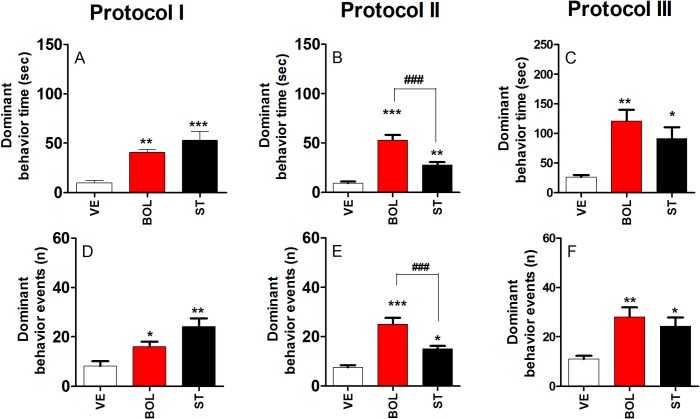
Fig 3 shows the time (A, B, and C) and events (D, E, and F) of dominant behavior over an intruder after treatment with BOL or ST according to Protocol I, II, and III. The duration of dominant behavior was increased for rats treated with BOL and ST according to Protocol I, II, and III [F(2,29) = 14.74, p<0.001, graph A; F(2,29) = 36.22, p<0.001, graph B; and F(2,29) = 9.623, p<0.01, graph C, respectively]. Similarly, rats treated with BOL and ST according to Protocol I, II, and III, showed an increase in dominant behavior events [F(2,29) = 10.04, p<0.01, graph D; F(2,29) = 25.49, p<0.001, graph E; F(2,29) = 8.263, p<0.01, graph F, respectively].
Fig 3.
Dominant behavior over the intruder of rats (n = 10) reated intramuscularly with vehicle (olive oil, 0.2 ml), boldenone (BOL), or stanazolol (ST) once a week according to Protocol I (4 weeks, 5 mg/kg), Protocol II (8 weeks, 2.5 mg/kg), or Protocol III (12 weeks, 1.25 mg/kg). Dominant behavior time for Protocol I (A), II (B), and III (C) and dominant behavior events for Protocol I (D), II (E), and III (F). *Denotes significant difference from the vehicle group. # Denotes significant difference between BOL and ST groups.
Territorial dominant behavior of rats treated with BOL and ST according to Protocol I, II, and III
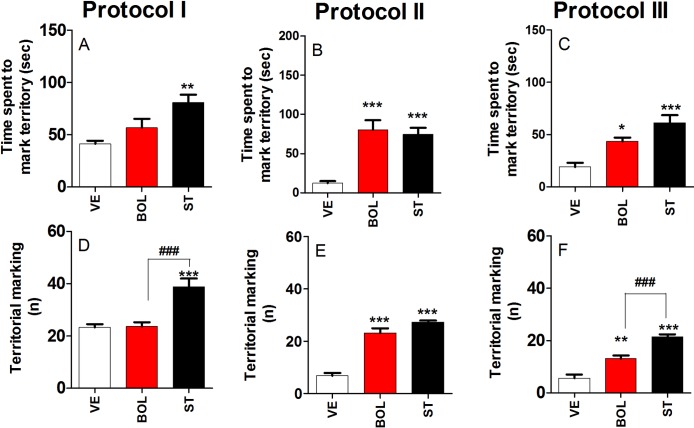
Fig 4 shows the time spent to mark territory (A, B, and C) and the number of these events (D, E, and F) after treatment with BOL or ST according to Protocol I, II, and III. Rats treated with ST according to Protocol I, II, and III spent an increased amount of time marking their territory [F(2,29) = 8.815, p<0.01, graph A; F(2,29) = 20.38, p<0.001, graph B; and F(2,29) = 14.73, p<0.001, graph C, respectively], which was not observed for rats treated with BOL according to Protocol I. In addition, rats treated with BOL according to Protocol II and III spent significantly more time marking their territory [F(2,29) = 20.38, p<0.001, graph B; and F(2,29) = 14.73, p<0.01, graph C, respectively].
Fig 4.
Dominant behavior over territory in rats treated intramuscularly with vehicle (olive oil, 0.2 ml), boldenone (BOL), or stanazolol (ST) once a week according to Protocol I (4 weeks, 5 mg/kg), Protocol II (8 weeks, 2.5 mg/kg), or Protocol III (12 weeks, 1.25 mg/kg). Time spent marking territory for Protocol I (A), II (B), and III (C) and territory-marking events for Protocol I (D), II (E), and III (F). *Denotes significant difference from the vehicle group. # Denotes significant difference between BOL and ST groups.
Rats treated with ST according to Protocol I, II, and III displayed a significant increase in the number of territorial-marking events [F(2,29) = 16.37, p<0.001, graph D; F(2,29) = 74.68, p<0.001, graph E; and F(2,29) = 42.89, p<0.001, graph F, respectively], which was not observed for rats treated with BOL according to Protocol I [graph D]. However, these numbers were significantly increased in rats receiving BOL according to Protocol II and III [F(2,29) = 74.68, p<0.001, graph E; and F(2,29) = 42.89, p<0.001, graph F, respectively].
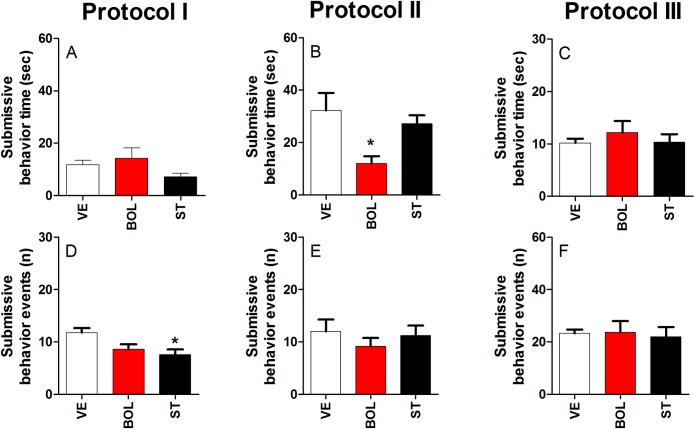
Submissive behavior of rats treated with BOL and ST according to Protocol I, II, and III
Fig 5 shows the time (A, B, and C) and events (D, E, and F) of submissive behavior after treatment with BOL or ST according to Protocol I, II, and III. Rats treated with ST according to Protocol I did not show a change in submissive behavior time [graphs A, B, and C]. Treatment with BOL decreased submissive behavior time only when administered according to Protocol II [F(2,29) = 5.164, p<0.05, graph B], while treatment with ST reduced the number of submissive behavior events only when administered according to Protocol I [F(2,29) = 5.045, p<0.05, graph D].
Fig 5.
Submissive behavior of rats (n = 10) treated intramuscularly with vehicle (olive oil, 0.2 ml), boldenone (BOL), or stanazolol (ST) once a week according to Protocol I (4 weeks, 5 mg/kg), Protocol II (8 weeks, 2.5 mg/kg), or Protocol III (12 weeks, 1.25 mg/kg). Submissive behavior time for Protocol I (A), II (B), and III (C) and submissive behavior events for Protocol I (D), II (E), and III (F). *Denotes significant difference from the vehicle group. # Denotes significant difference between BOL and ST groups.
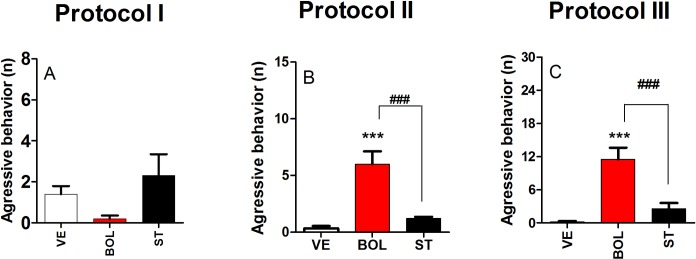
Aggressive behavior of rats treated with BOL and ST according to Protocol I, II, and III
Fig 6 shows aggressive behavior events after treatment with BOL or ST according to Protocol I, II, and III. Rats receiving ST according to Protocol I, II, and III did not show changes in aggressive behavior (graph A, graph B, and graph C, respectively). However, an increase in aggressive behavior was observed for rats treated with BOL according to Protocol II and III [F(2,29) = 23.38, p<0.001, graph B; and F(2,29) = 21.92, p<0.001, graph C, respectively].
Fig 6.
Aggressive behavior of rats (n = 10) treated intramuscularly with vehicle (olive oil, 0.2 ml), boldenone (BOL), or stanazolol (ST) once a week according to Protocol I (4 weeks, 5 mg/kg, graph A), Protocol II (8 weeks, 2.5 mg/kg, graph B), or Protocol III (12 weeks, 1.25 mg/kg, graph C). *Denotes significant difference from the vehicle group. # Denotes significant difference between BOL and ST groups.
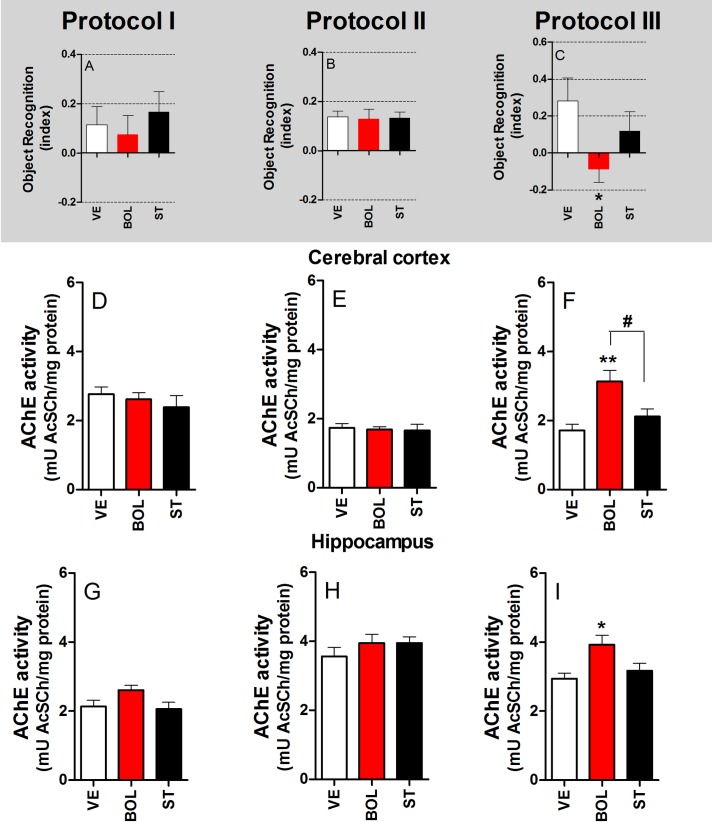
Object recognition task indices and acetylcholinesterase (AChE) activity in the brain of rats treated with BOL and ST according to Protocol I, II, and III
Fig 7 shows the index value of the object recognition task of the rats, as well as the AChE activity in the CC and HC of rats treated with BOL and ST according to Protocol I, II, and III. Rats treated with BOL and ST according to Protocol I and II showed no significant differences in the object recognition task index or AChE activity in the CC and HC [graphs A and B, D and E, and G and H, respectively). However, rats treated with BOL according to Protocol III had reduced index scores in the object recognition task [F(2,29) = 3.601, p<0.05, graph C] and increased AChE activity in the CC [F(2,29) = 8.321, p<0.01, graph F] and hippocampus [F(2,29) = 4.968, p<0.05, graph I].
Fig 7.
Object recognition task index and acetylcholinesterase (AChE) activity in the brain of rats (n = 10) treated intramuscularly with vehicle (olive oil, 0.2 ml), boldenone (BOL), or stanazolol (ST) once a week (intramuscular) according to Protocol I (4 weeks, 5 mg/kg, graph A), Protocol II (8 weeks, 2.5 mg/kg, graph B), or Protocol III (12 weeks, 1.25 mg/kg, graph C). Object recognition task index for Protocol I (A), II (B), and III (C). AChE activity in the cerebral cortex for Protocol I (D), II (E), and III (F) and hippocampus for Protocol I (G), II (H), and III (I). *Denotes significant difference from the vehicle group. # Denotes significant difference between BOL and ST groups.
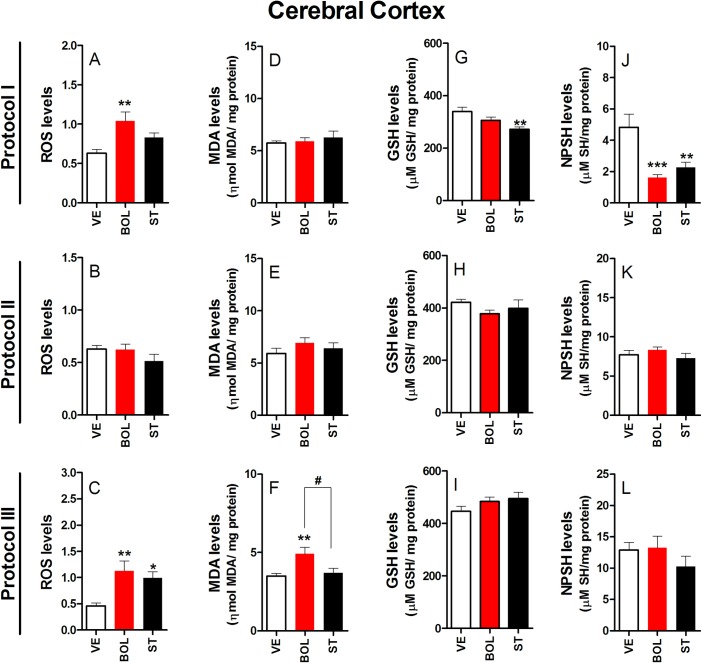
Oxidative stress parameters in the CC of rats treated with BOL or ST according to Protocol I, II, and III
Increased levels of reactive oxygen species (ROS) were observed in rats treated with BOL according to Protocol I [F(2,29) = 7.547, p<0.01, graph A] and III [F(2,29) = 7.910, p<0.01, graph C] (Fig 8). In contrast, treatment with ST resulted in increased ROS levels only when administered according to Protocol III [F(2,29) = 7.910, p<0.05, graph C]. No significant differences in ROS levels were observed in rats receiving BOL and ST according to Protocol II [F(2,29) = 1.550, graph B]. A significant increase in malondialdehyde (MDA) levels was observed in rats treated with BOL according to Protocol III [F(2,29) = 6.561, p<0.01, graph F]; however, no significant differences in MDA levels were observed in rats treated with BOL and ST according to Protocol I [F(2,29) = 0.490, graph D] and II [F(2,29) = 1.106, graph E]. Reduced glutathione (GSH) levels were only observed in rats treated with ST according to Protocol I [F(2,29) = 6.495, p<0.01, graph G]. No significant differences were observed in the GSH levels of rats receiving BOL and ST according to Protocol II [F(2,29) = 0.945, graph H] and III [F(2,29) = 1.537, graph I]. Non-protein thiols (NPSH) levels decreased in rats receiving BOL and ST according to Protocol I [F(2,29) = 10.09, p<0.001, graph J]. No significant differences were observed in rats receiving these two drugs according to Protocol II [F(2,29) = 0.968, graph K] and III [F(2,29) = 1.126, graph L].
Fig 8.
Parameters of oxidative stress in the cerebral cortex of rats (n = 10) treated intramuscularly with vehicle (olive oil, 0.2 ml), boldenone (BOL), or stanazolol (ST) once a week according to Protocol I (4 weeks, 5 mg/kg, graph A), Protocol II (8 weeks, 2.5 mg/kg, graph B), or Protocol III (12 weeks, 1.25 mg/kg, graph C). Reactive oxygen species (ROS) production for Protocol I (A), II (B), and II (C); malondialdehyde (MDA) levels for Protocol I (D), II (E), and III (F); reduced glutathione (GSH) levels for Protocol I (G), II (H), and III (I); non-protein thiol (NPSH) levels for Protocol I (J), II (K), and III (L). *Denotes significant difference from the vehicle group. # Denotes significant difference between BOL and ST groups.
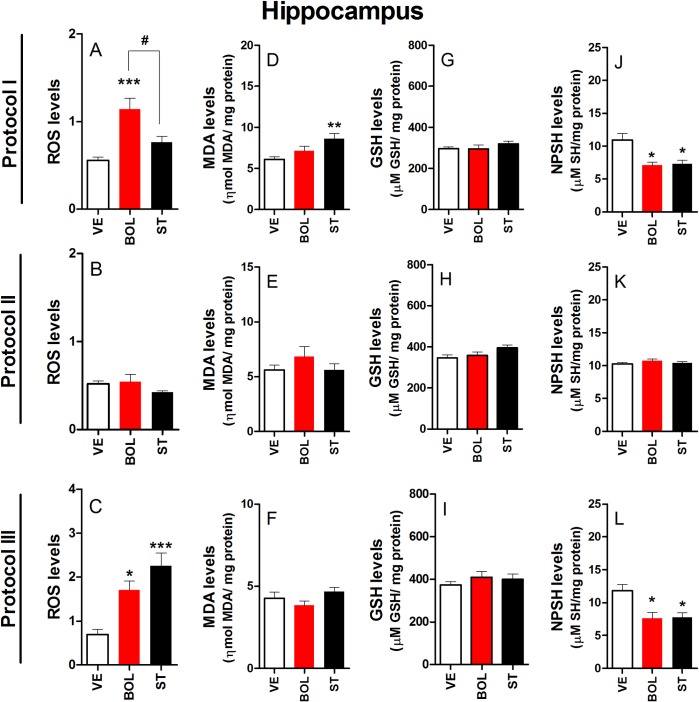
Oxidative stress parameters in the HC of rats treated with BOL or ST according to Protocol I, II, and III
Fig 9 shows that ROS levels increased in rats treated with BOL according to Protocol I [F(2,29) = 12.21, p<0.001, graph A] and III [F(2,29) = 12.67, p<0.05, graph C]. In contrast, ROS levels increased in rats treated with ST according to Protocol III only [F(2,29) = 12.67, p<0.001, graph C]. No significant differences in ROS levels were observed in rats treated with ST according to Protocol II [F(2,29) = 0.176, graph B]. Increased levels of MDA were observed in rats treated with ST according to Protocol I [F(2,29) = 6.610, p<0.01, graph D]. There were no significant differences in MDA levels in rats treated with BOL and ST according to Protocol II [F(2,29) = 1.113, graph E] and III [F(2,29) = 1.1973, graph F]. GSH levels were unchanged in rats treated with BOL and ST according to Protocol I [F(2,29) = 0.863, graph G], II [F(2,29) = 2.347,graph H], and III [F(2,29) = 0.675, graph I]. NPSH levels decreased in rats treated with BOL and ST according to Protocol I [F(2,29) = 7.682, p<0.01, graph J] and III [F(2,29) = 6.902, p<0.01, graph L]. In contrast, no significant differences in these levels were observed in rats treated with BOL and ST according to Protocol II [F(2,29) = 1.202, graph K].
Fig 9.
Parameters of oxidative stress in the hippocampus of rats (n = 10) treated intramuscularly with vehicle (olive oil, 0.2 ml), boldenone (BOL), or stanazolol (ST) once a week according to Protocol I (4 weeks, 5 mg/kg, graph A), Protocol II (8 weeks, 2.5 mg/kg, graph B), or Protocol III (12 weeks, 1.25 mg/kg, graph C). Reactive oxygen species (ROS) production for Protocol I (A), II (B), and II (C); malondialdehyde (MDA) levels for Protocol I (D), II (E), and III (F); reduced glutathione (GSH) levels for Protocol I (G), II (H), and III (I); non-protein thiol (NPSH) levels for Protocol I (J), II (K), and III (L). *Denotes significant difference from the vehicle group. # Denotes significant difference between BOL and ST groups.
Discussion
In the present study, we measured the physiological effects of BOL and ST using three different treatment protocols. Protocol I consisted of a dose higher than the recommended dose administered over a shorter time interval. Protocol II consisted of a moderate dose administered over an intermediate period, and Protocol III consisted of a reduced dose administered over an extended period [26]. These protocols were based on steroid user groups known to use widely ranging steroid doses and treatment durations [1, 27].
Our research focused on comparing the behavioral changes in rats receiving BOL and ST according to the three protocols described. Locomotion parameters were unchanged in the rats receiving BOL and ST, regardless of the treatment protocol used. In contrast, Bronson and Bronson [28] and colleagues [29] have reported that female mice treated with a combination of AAS at low and high doses for either 9 weeks or 6 months (high dose only) exhibited significantly reduced spontaneous activity in a running wheel relative to that observed for the controls. Further, these authors hypothesized that female-specific effects of AAS on locomotion might reflect AAS antagonism of estrogen-induced spontaneous activity [28, 29].
Here, we showed that the anxiety levels were significantly increased in the rats receiving BOL and ST according to Protocol I since these rats spent less time in the open arms of the elevated plus maze. Bitran and colleagues performed the first study of the effects of AAS on anxiety [30]. Another study showed that high doses of testosterone propionate induced an anxiogenic effect after 6 days of treatment; however, after 14 days of treatment the animals did not behave differently from those in the control group [31]. These results are indicative of a transitional character treatment, which might explain the fact that the relatively longer treatments at a relatively low dose used in our study did not increase anxious behavior. Nevertheless, Minkin and colleagues showed that nandrolone administered for 8 weeks increased anxious behavior, contradicting the findings of anxiolytic activity of some steroids [32].
Dominant behavior over the intruder and territorial dominance increased in all protocols tested, while submissive behavior was not affected by the BOL and ST treatments according to Protocol I and III. However, submissive behavior events decreased only when the animals were exposed to the higher dose of ST (PI). Further, the treatment with BOL according to Protocol II and III affected aggressiveness. The findings of Kalinine et al. show that a neural circuit composed of several regions including the prefrontal cortex, amygdala, HC, hypothalamus, anterior cingulated cortex, and other interconnected structures is implicated in the regulation of emotions [33]. Furthermore, it is plausible that functional or structural abnormalities in these regions can increase the susceptibility to impulsive aggression and violence [34]. AAS also affect aggressive behavior in rodents [35]. Single compounds such as testosterone and testosterone propionate increase dominance and aggression in healthy adult male rats and mice [36, 37]. Further, adolescent male Syrian hamsters exposed to a cocktail of AAS for 2 or 4 weeks showed increased aggressive behavior [10]. Consequently, the choice of a specific protocol for drug administration (both time and dose dependent), as well as the type of AAS used can affect the brain differently, resulting in a greater or lesser degree of dominance and aggressiveness.
The array of physiological and behavioral effects of these chemically disparate drugs is vastly compounded not only by complexity in the patterns of self-administration, but also by the heterogeneity of the subjects that take these drugs [38, 39]. Symptoms resulting from the chronic use of supra-therapeutic doses of AAS include mania, increased anxiety, irritability, extreme mood swings, and abnormal levels of aggression, depression, and even suicide. In particular, individuals self-administering the highest doses of AAS have elevated scores on the “Symptom Check List-90”, a self-report system that includes a number of different dimensions of anxiety [40]. These findings support our data that clearly show different behavioral responses of the rats treated with BOL and ST depending on the exposure duration and the dose. However, to investigate the effect of BOL and ST on cognitive processes, additional experimental techniques can be used. Since gonadal hormones are known to play a crucial role in cognitive processes such as spatial learning performance [41] and extinction responses in a passive avoidance task [42], it is conceivable that BOL and ST also influence cognitive functions. In particular, the current literature contains several controversial data regarding the absence of cognitive disorders in animals treated with AAS [43, 44], while other studies have clearly shown that individuals abusing AAS have a high risk of developing cognitive disorders and important morphological changes in the amygdala, HC, and forebrain [45, 46]. Thus, the present study evaluated memory performance of rats treated with BOL and ST in the object recognition task. Only rats receiving BOL according to Protocol III had a significant memory deficit compared to that of rats in the control and ST groups. Therefore, the use of BOL at a reduced dose but for a relatively long duration had a higher impact on learning and memory compared to that observed for ST treatment.
The biochemical mechanisms underlying the effects of AAS are still poorly understood. The mesocorticolimbic dopaminergic pathway is considered to play an important role in the reinforcement circuitry of the brain [47], and a connection between AAS and central dopaminergic and serotoninergic activity has been found in animal studies [48, 49]. However, only little information is available to explain the potential role of the cholinergic system in the biochemical mechanisms underlying the CNS effects of AAS. Such information is, however, especially important given that the cholinergic system plays an essential role in memory formation and learning [50, 51], and perhaps also in the behavioral changes we observed according to the type, treatment duration, and dose of the AAS used. Because acetylcholine coordinates the neuronal network response, modulation of the cholinergic system is an essential mechanism underlying complex behaviors; stimulation of presynaptic nicotinic acetylcholine receptors can increase the release of glutamate, ˠ-aminobutyric acid (GABA), dopamine, acetylcholine, norepinephrine, and serotonin [52, 53]. AChE is an important enzyme that regulates the concentration of acetylcholine in the synaptic cleft. We observed an increase in AChE activity in the CC and HC of animals treated with BOL (protocol III). These results are supported by those of other studies that have shown that the anabolic androgenic steroid methandrostenolone changes the expression of neuronal growth factor (NGF) and its receptors while reducing the activity of choline acetyltransferase (ChAT). Methandrostenolone also induces the synthesis of acetylcholine in the basal forebrain and impairs behavioral performance in the Morris water maze task [46].
Experimental studies in animals suggest that the redox status may be involved in AAS-induced neurotoxicity [54]. To the best of our knowledge, our results are the first to demonstrate that both BOL and ST change ROS, MDA, GSH, and NPSH levels in the CC and HC of rats. However, we found no significant changes in the CC and HC of rats that had received BOL and ST according to Protocol II. We hypothesize that both BOL and ST cause a physiological adaptation via changing of the redox status homeostasis.
The main limitations of this preliminary study were that we did not explore the underlying molecular mechanisms of the effects observed, and that we did not assess the expression of superoxide dismutase and glutathione peroxidase and reductase in the brain. Measuring antioxidant enzymes in brain tissue may aid in finding a potential correlation between the ROS, GSH, and NPSH levels in the treatment protocols we used. However, we found that a high dose or chronic use of BOL or ST changed oxidative stress parameters, resulting in deleterious effects on the HC and CC. Similar results were reported by Tugyan and colleagues who demonstrated that nandrolone increased MDA levels and reduced GPX activity, thereby negatively affecting the consequences of brain injury [55]. Holmes and colleagues suggested that androgens are neuroprotective at minimal oxidative stress levels, but that they exacerbate oxidative stress damage at elevated oxidative stress levels [56]. Additionally, Cunningham et al. demonstrated that in a preexisting oxidative stress environment, androgens could further exacerbate oxidative stress damage [57, 58].
In summary, our findings highlight that the impact of BOL and ST on cognition, anxiety, and social interaction depends on their dose and exposure duration. Furthermore, it is plausible that there is a correlation between the use of anabolic steroids and increased oxidative stress in the brain. More studies are necessary to understand the mechanisms by which BOL and ST affect brain function.
Acknowledgments
We would like to thank Editage (http://www.editage.com.br) for English language editing.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Lumia AR, McGinnis MY. Impact of anabolic androgenic steroids on adolescent males. Physiology & behavior. 2010;100(3):199–204. [DOI] [PubMed] [Google Scholar]
- 2.Kouvelas D, Pourzitaki C, Papazisis G, Dagklis T, Dimou K, Kraus MM. Nandrolone abuse decreases anxiety and impairs memory in rats via central androgenic receptors. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2008;11(7):925–34. [DOI] [PubMed] [Google Scholar]
- 3.Tanehkar F, Rashidy-Pour A, Vafaei AA, Sameni HR, Haghighi S, Miladi-Gorji H, et al. Voluntary exercise does not ameliorate spatial learning and memory deficits induced by chronic administration of nandrolone decanoate in rats. Hormones and behavior. 2013;63(1):158–65. Epub 2012/10/17. doi: 10.1016/j.yhbeh.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 4.Ramos-Pratts K, Rosa-Gonzalez D, Perez-Acevedo NL, Cintron-Lopez D, Barreto-Estrada JL. Sex-specific effect of the anabolic steroid, 17alpha-methyltestosterone, on inhibitory avoidance learning in periadolescent rats. Behavioural processes. 2013;99:73–80. PubMed Central PMCID: PMC3791152. doi: 10.1016/j.beproc.2013.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poling MC, Kauffman AS. Organizational and activational effects of sex steroids on kisspeptin neuron development. Frontiers in neuroendocrinology. 2013;34(1):3–17. PubMed Central PMCID: PMC3725275. doi: 10.1016/j.yfrne.2012.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McEwen BS, Parsons B. Gonadal steroid action on the brain: neurochemistry and neuropharmacology. Annual review of pharmacology and toxicology. 1982;22:555–98. doi: 10.1146/annurev.pa.22.040182.003011 [DOI] [PubMed] [Google Scholar]
- 7.Carrillo M, Ricci LA, Melloni RH Jr. Adolescent anabolic androgenic steroids reorganize the glutamatergic neural circuitry in the hypothalamus. Brain research. 2009;1249:118–27. doi: 10.1016/j.brainres.2008.10.053 [DOI] [PubMed] [Google Scholar]
- 8.Richmond V, Murray AP, Maier MS. Synthesis and acetylcholinesterase inhibitory activity of polyhydroxylated sulfated steroids: structure/activity studies. Steroids. 2013;78(11):1141–7. doi: 10.1016/j.steroids.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 9.Magnusson K, Birgner C, Bergstrom L, Nyberg F, Hallberg M. Nandrolone decanoate administration dose-dependently affects the density of kappa opioid peptide receptors in the rat brain determined by autoradiography. Neuropeptides. 2009;43(2):105–11. doi: 10.1016/j.npep.2008.12.005 [DOI] [PubMed] [Google Scholar]
- 10.Salas-Ramirez KY, Montalto PR, Sisk CL. Anabolic androgenic steroids differentially affect social behaviors in adolescent and adult male Syrian hamsters. Hormones and behavior. 2008;53(2):378–85. PubMed Central PMCID: PMC2883314. doi: 10.1016/j.yhbeh.2007.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carvalho FB, Gutierres JM, Bohnert C, Zago AM, Abdalla FH, Vieira JM, et al. Anthocyanins suppress the secretion of proinflammatory mediators and oxidative stress, and restore ion pump activities in demyelination. The Journal of nutritional biochemistry. 2015;26(4):378–90. doi: 10.1016/j.jnutbio.2014.11.006 [DOI] [PubMed] [Google Scholar]
- 12.Gutierres JM, Carvalho FB, Schetinger MR, Agostinho P, Marisco PC, Vieira JM, et al. Neuroprotective effect of anthocyanins on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia in rats. International journal of developmental neuroscience: the official journal of the International Society for Developmental Neuroscience. 2014;33:88–97. [DOI] [PubMed] [Google Scholar]
- 13.Gutierres JM, Carvalho FB, Schetinger MR, Marisco P, Agostinho P, Rodrigues M, et al. Anthocyanins restore behavioral and biochemical changes caused by streptozotocin-induced sporadic dementia of Alzheimer's type. Life sciences. 2014;96(1–2):7–17. doi: 10.1016/j.lfs.2013.11.014 [DOI] [PubMed] [Google Scholar]
- 14.Velloso NA, Dalmolin GD, Gomes GM, Rubin MA, Canas PM, Cunha RA, et al. Spermine improves recognition memory deficit in a rodent model of Huntington's disease. Neurobiology of learning and memory. 2009;92(4):574–80. Epub 2009/07/28. doi: 10.1016/j.nlm.2009.07.006 [DOI] [PubMed] [Google Scholar]
- 15.Marisco PC, Carvalho FB, Rosa MM, Girardi BA, Gutierres JM, Jaques JA, et al. Piracetam prevents scopolamine-induced memory impairment and decrease of NTPDase, 5'-nucleotidase and adenosine deaminase activities. Neurochemical research. 2013;38(8):1704–14. doi: 10.1007/s11064-013-1072-6 [DOI] [PubMed] [Google Scholar]
- 16.Morrison TR, Ricci LA, Melloni RH Jr. Anabolic/androgenic steroid administration during adolescence and adulthood differentially modulates aggression and anxiety. Hormones and behavior. 2015;69:132–8. Epub 2015/02/07. PubMed Central PMCID: PMC4359666. doi: 10.1016/j.yhbeh.2015.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutierres JM, Carvalho FB, Rosa MM, Schmatz R, Rodrigues MV, Vieira JM, et al. Protective effect of α-Tocopherol on memory deficits and Na+,K+-ATPase and acetylcholinesterase activities in rats with diet-induced hypercholesterolemia. Biomedicine & Aging Pathology. 2012;2(3):73–80. [Google Scholar]
- 18.Myhre O, Andersen JM, Aarnes H, Fonnum F. Evaluation of the probes 2',7'-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochemical pharmacology. 2003;65(10):1575–82. [DOI] [PubMed] [Google Scholar]
- 19.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical biochemistry. 1979;95(2):351–8. [DOI] [PubMed] [Google Scholar]
- 20.Rossato JI, Zeni G, Mello CF, Rubin MA, Rocha JB. Ebselen blocks the quinolinic acid-induced production of thiobarbituric acid reactive species but does not prevent the behavioral alterations produced by intra-striatal quinolinic acid administration in the rat. Neuroscience letters. 2002;318(3):137–40. [DOI] [PubMed] [Google Scholar]
- 21.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–7. Epub 1959/05/01. [DOI] [PubMed] [Google Scholar]
- 22.Ellman GL, Courtney KD, Andres V Jr., Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical pharmacology. 1961;7:88–95. Epub 1961/07/01. [DOI] [PubMed] [Google Scholar]
- 23.Rocha JB, Emanuelli T, Pereira ME. Effects of early undernutrition on kinetic parameters of brain acetylcholinesterase from adult rats. Acta neurobiologiae experimentalis. 1993;53(3):431–7. [PubMed] [Google Scholar]
- 24.Stefanello N, Schmatz R, Pereira LB, Rubin MA, da Rocha JB, Facco G, et al. Effects of chlorogenic acid, caffeine, and coffee on behavioral and biochemical parameters of diabetic rats. Molecular and cellular biochemistry. 2014;388(1–2):277–86. doi: 10.1007/s11010-013-1919-9 [DOI] [PubMed] [Google Scholar]
- 25.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical biochemistry. 1976;72:248–54. [DOI] [PubMed] [Google Scholar]
- 26.Bueno A, Carvalho FB, Gutierres JM, Lhamas CL, Brusco I, Oliveira SM, et al. Impacts of dose and time of boldenone and stanazolol exposure in inflammatory markers, oxidative and nitrosative stress and histopathological changes in the rat testes. Theriogenology. 2017;90:101–8. doi: 10.1016/j.theriogenology.2016.11.024 [DOI] [PubMed] [Google Scholar]
- 27.McGinnis MY. Anabolic androgenic steroids and aggression: studies using animal models. Annals of the New York Academy of Sciences. 2004;1036:399–415. doi: 10.1196/annals.1330.024 [DOI] [PubMed] [Google Scholar]
- 28.Bronson FH. Effects of prolonged exposure to anabolic steroids on the behavior of male and female mice. Pharmacology, biochemistry, and behavior. 1996;53(2):329–34. [DOI] [PubMed] [Google Scholar]
- 29.Bronson FH, Nguyen KQ, De La Rosa J. Effect of anabolic steroids on behavior and physiological characteristics of female mice. Physiology & behavior. 1996;59(1):49–55. [DOI] [PubMed] [Google Scholar]
- 30.Bitran D, Hilvers RJ, Frye CA, Erskine MS. Chronic anabolic-androgenic steroid treatment affects brain GABA(A) receptor-gated chloride ion transport. Life sciences. 1996;58(7):573–83. [DOI] [PubMed] [Google Scholar]
- 31.Bitran D, Kellogg CK, Hilvers RJ. Treatment with an anabolic-androgenic steroid affects anxiety-related behavior and alters the sensitivity of cortical GABAA receptors in the rat. Hormones and behavior. 1993;27(4):568–83. doi: 10.1006/hbeh.1993.1041 [DOI] [PubMed] [Google Scholar]
- 32.Minkin DM, Meyer ME, van Haaren F. Behavioral effects of long-term administration of an anabolic steroid in intact and castrated male Wistar rats. Pharmacology, biochemistry, and behavior. 1993;44(4):959–63. [DOI] [PubMed] [Google Scholar]
- 33.Kalinine E, Zimmer ER, Zenki KC, Kalinine I, Kazlauckas V, Haas CB, et al. Nandrolone-induced aggressive behavior is associated with alterations in extracellular glutamate homeostasis in mice. Hormones and behavior. 2014;66(2):383–92. doi: 10.1016/j.yhbeh.2014.06.005 [DOI] [PubMed] [Google Scholar]
- 34.Chiavegatto S, Dawson VL, Mamounas LA, Koliatsos VE, Dawson TM, Nelson RJ. Brain serotonin dysfunction accounts for aggression in male mice lacking neuronal nitric oxide synthase. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(3):1277–81. PubMed Central PMCID: PMC14745. doi: 10.1073/pnas.98.3.1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chichinadze K, Chichinadze N, Gachechiladze L, Lazarashvili A. The role of androgens in regulating emotional state and aggressive behavior. Reviews in the neurosciences. 2012;23(2):123–33. doi: 10.1515/revneuro-2012-0026 [DOI] [PubMed] [Google Scholar]
- 36.Albert DJ, Dyson EM, Walsh ML. Intermale social aggression: reinstatement in castrated rats by implants of testosterone propionate in the medial hypothalamus. Physiology & behavior. 1987;39(5):555–60. [DOI] [PubMed] [Google Scholar]
- 37.Farrell SF, McGinnis MY. Effects of pubertal anabolic-androgenic steroid (AAS) administration on reproductive and aggressive behaviors in male rats. Behavioral neuroscience. 2003;117(5):904–11. doi: 10.1037/0735-7044.117.5.904 [DOI] [PubMed] [Google Scholar]
- 38.Sagoe D, Andreassen CS, Molde H, Torsheim T, Pallesen S. Prevalence and correlates of anabolic-androgenic steroid use in a nationally representative sample of 17-year-old Norwegian adolescents. Substance use & misuse. 2015;50(2):139–47. [DOI] [PubMed] [Google Scholar]
- 39.Kuehn BM. Teen steroid, supplement use targeted: officials look to prevention and better oversight. Jama. 2009;302(21):2301–3. doi: 10.1001/jama.2009.1711 [DOI] [PubMed] [Google Scholar]
- 40.Pagonis TA, Angelopoulos NV, Koukoulis GN, Hadjichristodoulou CS. Psychiatric side effects induced by supraphysiological doses of combinations of anabolic steroids correlate to the severity of abuse. European psychiatry: the journal of the Association of European Psychiatrists. 2006;21(8):551–62. [DOI] [PubMed] [Google Scholar]
- 41.Naghdi N, Nafisy N, Majlessi N. The effects of intrahippocampal testosterone and flutamide on spatial localization in the Morris water maze. Brain research. 2001;897(1–2):44–51. [DOI] [PubMed] [Google Scholar]
- 42.Vazquez-Pereyra F, Rivas-Arancibia S, Loaeza-Del Castillo A, Schneider-Rivas S. Modulation of short term and long term memory by steroid sexual hormones. Life sciences. 1995;56(14):PL255–60. [DOI] [PubMed] [Google Scholar]
- 43.Clark AS, Mitre MC, Brinck-Johnsen T. Anabolic-androgenic steroid and adrenal steroid effects on hippocampal plasticity. Brain research. 1995;679(1):64–71. [DOI] [PubMed] [Google Scholar]
- 44.Fernandez L, Chirino R, Boada LD, Navarro D, Cabrera N, del Rio I, et al. Stanozolol and danazol, unlike natural androgens, interact with the low affinity glucocorticoid-binding sites from male rat liver microsomes. Endocrinology. 1994;134(3):1401–8. doi: 10.1210/endo.134.3.8119180 [DOI] [PubMed] [Google Scholar]
- 45.Kaufman MJ, Janes AC, Hudson JI, Brennan BP, Kanayama G, Kerrigan AR, et al. Brain and cognition abnormalities in long-term anabolic-androgenic steroid users. Drug and alcohol dependence. 2015;152:47–56. PubMed Central PMCID: PMC4458166. doi: 10.1016/j.drugalcdep.2015.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pieretti S, Mastriota M, Tucci P, Battaglia G, Trabace L, Nicoletti F, et al. Brain nerve growth factor unbalance induced by anabolic androgenic steroids in rats. Medicine and science in sports and exercise. 2013;45(1):29–35. doi: 10.1249/MSS.0b013e31826c60ea [DOI] [PubMed] [Google Scholar]
- 47.Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends in pharmacological sciences. 1992;13(5):177–84. [DOI] [PubMed] [Google Scholar]
- 48.Kindlundh AM, Hagekull B, Isacson DG, Nyberg F. Adolescent use of anabolic-androgenic steroids and relations to self-reports of social, personality and health aspects. European journal of public health. 2001;11(3):322–8. [DOI] [PubMed] [Google Scholar]
- 49.Kindlundh AM, Lindblom J, Bergstrom L, Wikberg JE, Nyberg F. The anabolic-androgenic steroid nandrolone decanoate affects the density of dopamine receptors in the male rat brain. The European journal of neuroscience. 2001;13(2):291–6. [DOI] [PubMed] [Google Scholar]
- 50.Jaques JA, Rezer JF, Carvalho FB, da Rosa MM, Gutierres JM, Goncalves JF, et al. Curcumin protects against cigarette smoke-induced cognitive impairment and increased acetylcholinesterase activity in rats. Physiology & behavior. 2012;106(5):664–9. [DOI] [PubMed] [Google Scholar]
- 51.Anand P, Singh B. A review on cholinesterase inhibitors for Alzheimer's disease. Archives of pharmacal research. 2013;36(4):375–99. doi: 10.1007/s12272-013-0036-3 [DOI] [PubMed] [Google Scholar]
- 52.Wonnacott S, Barik J, Dickinson J, Jones IW. Nicotinic receptors modulate transmitter cross talk in the CNS: nicotinic modulation of transmitters. Journal of molecular neuroscience: MN. 2006;30(1–2):137–40. doi: 10.1385/JMN:30:1:137 [DOI] [PubMed] [Google Scholar]
- 53.McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269(5231):1692–6. [DOI] [PubMed] [Google Scholar]
- 54.Pomara C, Neri M, Bello S, Fiore C, Riezzo I, Turillazzi E. Neurotoxicity by Synthetic Androgen Steroids: Oxidative Stress, Apoptosis, and Neuropathology: A Review. Current neuropharmacology. 2015;13(1):132–45. PubMed Central PMCID: PMC4462038. doi: 10.2174/1570159X13666141210221434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tugyan K, Ozbal S, Cilaker S, Kiray M, Pekcetin C, Ergur BU, et al. Neuroprotective effect of erythropoietin on nandrolone decanoate-induced brain injury in rats. Neuroscience letters. 2013;533:28–33. doi: 10.1016/j.neulet.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 56.Holmes S, Abbassi B, Su C, Singh M, Cunningham RL. Oxidative stress defines the neuroprotective or neurotoxic properties of androgens in immortalized female rat dopaminergic neuronal cells. Endocrinology. 2013;154(11):4281–92. PubMed Central PMCID: PMC3800758. doi: 10.1210/en.2013-1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cunningham RL, Singh M, O'Bryant SE, Hall JR, Barber RC. Oxidative stress, testosterone, and cognition among Caucasian and Mexican-American men with and without Alzheimer's disease. Journal of Alzheimer's disease: JAD. 2014;40(3):563–73. PubMed Central PMCID: PMC4219556. doi: 10.3233/JAD-131994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cunningham RL, Macheda T, Watts LT, Poteet E, Singh M, Roberts JL, et al. Androgens exacerbate motor asymmetry in male rats with unilateral 6-hydroxydopamine lesion. Hormones and behavior. 2011;60(5):617–24. PubMed Central PMCID: PMC3210335. doi: 10.1016/j.yhbeh.2011.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.