Abstract
Objective
To assess the efficacy of probiotic Lactobacillus on serum lipids using a meta-analysis of randomized, controlled trials.
Methods
Fifteen studies containing 15 trials, with 976 subjects were included. The pooled WMD was calculated by random effects model.
Results
Probiotic Lactobacillus consumption significantly reduced TC by 0.26mmol/l (95% CI, -0.40 to -0.12) and LDL-C by 0.23mmol/l (95% CI, -0.36 to -0.10). Subgroup analysis of trials found significantly reduction of TC using L. plantarum and reduction of LDL-C using L. plantarum or L. reuteri. No significant effects were found on TG and HDL-C levels after supplementation with probiotic Lactobacillus. While, subgroup analysis found significantly beneficial effects on TG and HDL-C by consuming synbiotic food, containing L. sporogenes and inulin.
Conclusion
Consuming probiotic Lactobacillus, especially L. reuteri and L. plantarm, could reduce TC and LDL-C significantly. The study also suggested significantly beneficial effects on TG and HDL-C by consuming synbiotic food, containing L. sporogenes and inulin.
Introduction
As we know, coronary artery disease (CAD) is one of the most important causes of deaths all over the world. Epidemiological reports have demonstrated that the high risk of CAD is associated with dyslipidemia, especially the high level of low density lipoprotein-cholesterol (LDL-C)[1]. One of the considerable first line for treating dyslipidemia is dietary regulations and recommendations; however, using these methods, only a modest amelioration can be achieved[2].
Probiotics are called as live microorganisms which are beneficial to human health when consumed in adequate quantities [3]. Specific strains of probiotic bacteria, such as Lactobacillus, have been demonstrated to improve anti-diarrheal and anti-inflammatory, potentiate immune, and delay diabetes[4]. Several Lactobacillus strains have been found to reduce total cholesterol (TC) and triglyceride (TG) concentrations in rats[5, 6], while in human clinical studies there is no consensus on the effects of consumption of Lactobacillus strains on lipid profile. One previous study has shown that three weeks daily administration of 200ml milk containing Lactobacillus acidophilus L1 was associated with the reduction of TC level in hypercholesterolemic individuals[7]. Further, Fuentes et al. reported that daily intake of L. plantarumin a capsule containing 1.2×109 CFU lowered TC and LDL-C concentrations in participants with hypercholesterolemia after 12 weeks[8]. However, Hove et al. reported that 12 weeks intake of milk fermented with L. helveticus had no effect on serum lipids in type 2 diabetes patients[9]. As we know, there has been no meta-analysis on the efficacy of probiotic Lactobacillus for lipid control. In this paper, a meta-analysis was carried out to assess the functional effects of supplementation of probiotic Lactobacillus on lipid profile, which may provide further information on the efficacy of specific strains of Lactobacillus required to lipid control.
Experimental section
Literature search
Studies exploring the effects of probiotic Lactobacillus consumption on serum lipid were searched in PubMed, Web of Knowledge, Cochrane Library, and Embase databases. The search was last updated in July 2016 and involved only full-text articles published in English. The search was performed, for example in PubMed, as following: (((((((((((((((cholesterol) OR (((("Cholesterol"[Mesh]) OR "Cholesterol, VLDL"[Mesh]) OR "Cholesterol, LDL"[Mesh]) OR "Cholesterol, HDL"[Mesh])) OR plasma lipids) OR triglycerides) OR "Triglycerides"[Mesh]) OR serum lipids) OR HDL-C) OR LDL-C) OR low density cholesterol) OR high density lipoprotein cholesterol) OR low density lipoprotein cholesterol) OR very low density lipoprotein cholesterol) OR VLDL-C)) AND (("Lactobacillus"[Mesh]) OR Lactobacillus)) AND (("Randomized Controlled Trial" [Publication Type]) OR "Randomized Controlled Trials as Topic"[Mesh]). All the references of included articles were also established by a hand search.
Study eligibility
According to the PRISMA guidelines, the inclusion criteria was as following: (1) randomized controlled trial (RCTs), (2) adults, (3) the serum lipid changes and standard deviation (SD) were available, (4) interventions of the articles only used specific strains of Lactobacillus, (5) articles were published in English language. Exclusion criteria: (1) unpublished reports, (2) review articles, case reports, editorials, and letters, (3) not include a control group.
Data extraction
Firstly, initial screening of studies was done independently by two researchers. Then, abstracts and full texts were reviewed according to the eligibility criteria. Finally, the included articles were determined by the two reviewers together. The data were extracted from each study as following: the first author, study design, publication year, characteristics of subjects, species of Lactobacillus strains, dose and duration of Lactobacillus strains supplementation, and baseline measures and changes from baseline of serum lipid. When the study has two or more comparisons, we chose the one whose follow-up period is the longest. There is no restriction on minimum number of interventions or controls in our meta-analysis. The study flow is showed as Fig 1. Wu YC and Zhang QQ conducted the search, data extraction, and analysis of study quality.
Fig 1. The study flow diagram.
Risk of bias
S1 Table shows the risk of bias of included articles, which was assessed by the Cochrane Collaboration’s tool. Two studies clearly describe the method of randomization (13.3%, n = 2). The risk of bias regarding concealed allocation and selective reporting was unclear (100%, n = 15). Low risk of bias was revealed on incomplete outcome data (93.3%, n = 14), blinding of participants (100%, n = 15), and other sources of bias (93.3%, n = 14).
Statistical analysis
SD of the changes of serum lipid from baseline, if not reported, was calculated using the formula below (1). Statistical analysis was conducted using STATA 11.0 (Stata, College Station, TX, USA). The effect of Lactobacillus strains on serum lipid was showed as the weight mean difference (WMD) of serum lipid changes between the treatment groups and control groups, which was calculated as the difference in the mean outcome between the groups divided by SD of outcome among participants and analyzed by the random effects model regardless of heterogeneity. Heterogeneity across the included studies was assessed using the I2 statistics, representing the percentage of actual variation in relation to total variation. Subgroup analysis was also performed. P < 0.05 was considered statistically significant. Additionally we assessed the probability of publication bias with Begg’s funnel plots and the Egger’s test, with p value < 0.10 considered representative of statistically significant publication bias.
| (1) |
Results
Characteristics of included studies
Fig 1 shows the search flow for this meta-analysis. The literature search yielded 87 citations, of which 37 articles were retrieved after preliminary screening. Finally, 15 articles were included in this meta-analysis[7–21].
Fifteen studies, with 976 subjects in all, were included in this meta-analysis. All the included studies were randomized, controlled trials with double-blind design. Of the 15 studies, 13 were parallel and the other 2 were cross-over.
Table 1 shows the basic characteristics of included trials. Of the 15 studies, 5 used subjects with hypercholesterolemia, 3 were healthy participants, 2 were diabetes mellitus patients, 1 included smokers, 2 included obese subjects, 1 included pregnant women and 1 included patients with gestational diabetes mellitus. Of the 15 studies, 13 studies used probiotics, including L. plantarum, L. acidophilus, L. fermentum, L. helveticus, L. salivarius, L. reuteri, and L. rhamnosus, and the other 2 studies used synbiotics containing L. sporogenes and inulin. The duration and dose of probiotic Lactobacillus consumption varied between trials. The total daily dose changed from 107 to 1011 colony-forming units. The duration changed from 3 to 24 weeks. To be mentioned, different trials give the different definitions about hypercholesterolemia that we didn’t make a subgroup analysis, so it is not quite necessary to give a new definition.
Table 1. Characteristics of included trials.
| Study (publish year) | Design | Participants | Intervention | duration (wk) |
|---|---|---|---|---|
| Bukowska, H.(1998) | DB, PC, P | 30 HC (42.6±2.8 y) | 95:5 (v/v) fruit and fermented oatmeal soups and contains: 5×10^7 CFU/ml of Lactobacillus plantarum 299 v, 80 mg/dl oat fibers and rose hip drink | 6 |
| Schaafsma, G.(1998) | DB, C | 30 healthy (33–64 y) | two strains of Lactobacillus acidophilus and contained 2.5% fructo-oligosaccharides, 0.5% vegetable oil and 0.5% milk fat. (10^7–10^8 CFU/g) | 3 |
| Anderson, J.W.(1999) | DB, C | 40 HC (52–61 y) | Fermented Milk containing the L. acidophilus L1 strain (L1 FM) | 4 |
| de Roos, N.M.(1999) | DB, PC, P | 78 healthy (18–65 y) | Lactobacillus acidophilus L-1(4.8×10^9 to 2.7×10^10 CFU) | 6 |
| Naruszewicz, M.(2002) | DB, PC, P | 36 smokers (35–45 y) | Lactobacillus plantarum 299v (2×10^9 CFU) | 6 |
| Simons, L.A.(2006) | DB, PC, P | 44 HC (30–75 y) | Lactobacillus fermentum(4×10^9 CFU) | 8–10 |
| Jones, M.L.(a)(2012) | DB, PC, P | 127 HC (20–75 y) | Lactobacillus reuteri NCIMB 30242, 2.0× 10^9 CFU | 9 |
| Jones, M.L.(b)(2012) | DB, PC, P | 131 healthy (20–75 y) | L. reuteri NCIMB 30242, 2.9×10^9 CFU | 9 |
| Fuentes, M.C.(2013) | DB, PC, P | 60 HC (18–65 y) | Lactobacillus plantarum CECT 7527, 7528 and 7529, 1.2 ×10^9 CFU | 12 |
| Sharafedtinov, K.K.(2013) | DB, PC, P | 40 obesity (30–69 y) | L. plantarum TENSIA, 1.5x10^11 CFU/gx50g | 3 |
| Taghizadeh, M.(2014) | DB, PC, P | 52 pregnant women (18–35 y) | Lactobacillus sporogenes, 1×10^7 CFU, 0.04 g inulin (HPX)/g as the prebiotic | 9 |
| Shakeri, H.(2014) | DB, PC, P | 52 DM (35–70 y) | L. sporogenes, 1.2×10^10 CFU | 8 |
| Hove, K.D.(2015) | DB, PC, P | 41 DM (40–70 y) | 300 ml L. helveticus Cardi04 yogurt | 12 |
| Lindsay, K.L.(2015) | DB, PC, P | 100 women with gestational DM, >18 y | Lactobacillus salivarius UCC118 | 4–6 |
| Sanchez, M.(2014) | DB, PC, P | 125 obesity (18–55 y) | Lactobacillus rhamnosus CGMCC1.3724, 1.6 × 10^8 CFU with oligofructose and inulin | 24 |
C, cross-over; CFU, colony-forming unit; DB, double blind; HC, hypercholesterolemia; P, parallel; PC, placebo control; and DM, diabetes mellitus.
Main outcomes
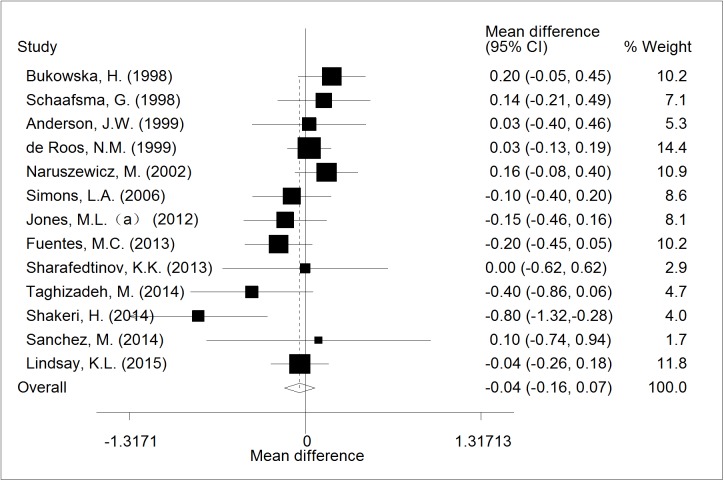
Of 13 RCTs, a random-effects meta-analysis did not show the effect of supplementation with probiotic Lactobacillus on TG levels (WMD = -0.04; CI,-0.16 to 0.07; p = 0.481). The forest plot of the effect was presented in Fig 2. The included studies showed highly significant homogeneity (I2 = 42.0%; p = 0.055).
Fig 2. The effect of consumption of probiotic Lactobacillus on TG.
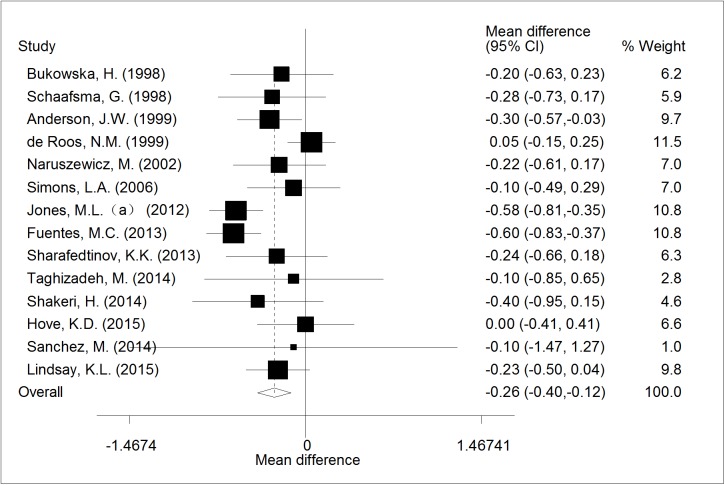
Of 14 RCTs, the meta-analysis result showed a significant reduction of 0.26mmol/l (95% CI, -0.40 to -0.12; p < 0.001) in TC compared with control (Fig 3), the heterogeneity of the included studies was moderate (I2 = 53.6%; p = 0.033).
Fig 3. The effect of consumption of probiotic Lactobacillus on TC.
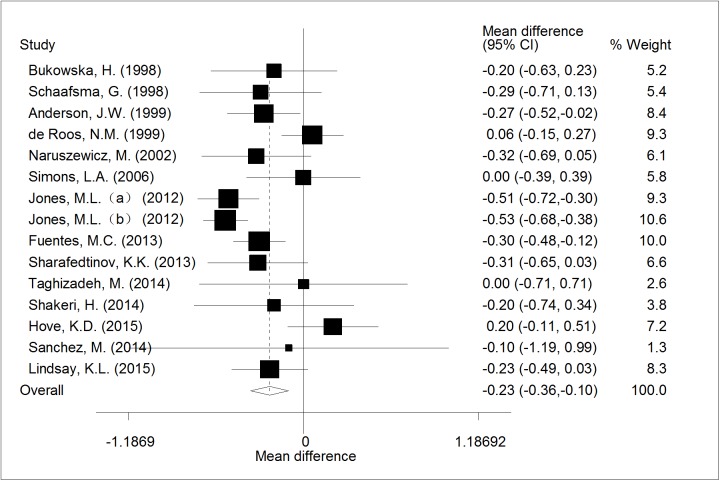
A significant reduction of LDL-C by 0.23mmol/l (95% CI, -0.36 to -0.10; p< 0.001)was showed after meta-analysis of 15 trials, with high heterogeneity (I2 = 62.8%; p = 0.034). The forest plot of the effect was presented in Fig 4.
Fig 4. The effect of consumption of probiotic Lactobacillus on LDL.
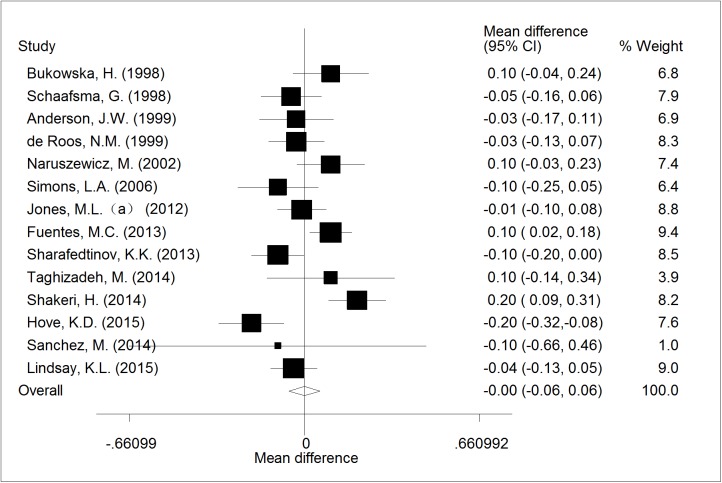
Fourteen RCTs reported the effect of probiotic Lactobacillus onhigh density lipoprotein cholesterol (HDL-C) levels. A pooled effect was found to be nonsignificant (WMD = -0.00; CI,-0.06 to 0.06; p = 0.99; I2 = 68.7%; p = 0.008 for heterogeneity, Fig 5).
Fig 5. The effect of consumption of probiotic Lactobacillus on HDL.
Evaluation of subgroup analysis
A random-effects meta-analysis of 4 RCTs showed a significant decrease TC and LDL-C levels after the consumption of L. plantarum. (WMD = -0.37; CI, -0.60 to -0.13; p = 0.002; WMD = -0.29; CI, -0.43 to -0.16; p < 0.001; respectively, Table 2). The included studies showed highly significant homogeneity (I2 = 41.2%, p = 0.022; I2 = 0.0%, p <0.001, respectively).
Table 2. Evaluation of subgroup analysis.
| Subgroup Analysis | Weight Mean Difference (95% Confidence Interval) |
No. of study | p value |
|---|---|---|---|
| TG | |||
| L. plantarum | 0.05(-0.16, 0.26) | 4 | 0.636 |
| L. acidophilus | 0.05(-0.09, 0.19) | 3 | 0.504 |
| L. fermentum | -0.10(-0.40, 0.20) | 1 | 0.508 |
| L. reuteri | -0.15 (-0.46,0.16) | 1 | 0.346 |
| L. sporogenes | -0.58(-0.97, -0.19) | 2 | 0.003 |
| L. salivarius | -0.04 (-0.26, 0.18) | 1 | 0.717 |
| L. rhamnosus | 0.10(-0.74, 0.94) | 1 | 0.816 |
| All trials | -0.02(-0.10, 0.07) | 13 | 0.481 |
| TC | |||
| L. plantarum | -0.37(-0.60, -0.13) | 4 | 0.002 |
| L. acidophilus | -0.15(-0.41, 0.12) | 3 | 0.275 |
| L. fermentum | -0.10(-0.49, 0.29) | 1 | 0.613 |
| L. reuteri | -0.58(-0.81, -0.35) | 1 | <0.001 |
| L. sporogenes | -0.30(-0.74, 0.15) | 2 | 0.189 |
| L. helveticus | 0.00(-0.41, 0.41) | 1 | 1.000 |
| L. salivarius | -0.23(-0.50, 0.04) | 1 | 0.091 |
| L. rhamnosus | -0.10(-1.47, 1.27) | 1 | 0.886 |
| All trials | -0.26(-0.40, -0.12) | 14 | <0.001 |
| LDL-C | |||
| L. plantarum | -0.29 (-0.43, -0.16) | 4 | <0.001 |
| L. acidophilus | -0.14(-0.39, 0.11) | 3 | 0.275 |
| L. fermentum | 0.00(-0.39, 0.39) | 1 | 1.000 |
| L. reuteri | -0.52(-0.65, -0.40) | 2 | <0.001 |
| L. sporogenes | -0.13(-0.56, 0.31) | 2 | 0.567 |
| L. helveticus | 0.20(-0.11, 0.51) | 1 | 0.204 |
| L. salivarius | -0.23(-0.49, 0.03) | 1 | 0.082 |
| L. rhamnosus | -0.10(-1.19, 0.99) | 1 | 0.857 |
| All trials | -0.23(-0.36, -0.10) | 15 | <0.001 |
| HDL-C | |||
| L. plantarum | 0.05(-0.06, 0.15) | 4 | 0.385 |
| L. acidophilus | -0.04(-0.11, 0.03) | 3 | 0.285 |
| L. fermentum | -0.10(-0.25, 0.05) | 1 | 0.198 |
| L. reuteri | -0.01(-0.10, 0.08) | 1 | 0.833 |
| L. sporogenes | 0.18(0.08, 0.28) | 2 | <0.001 |
| L. helveticus | -0.20(-0.32, -0.08) | 1 | 0.001 |
| L. salivarius | -0.04(-0.13, 0.05) | 1 | 0.375 |
| L. rhamnosus | -0.10(-0.66,0.46 | 1 | 0.727 |
| All trials | -0.00(-0.06, 0.06) | 14 | 0.990 |
A pooled analysis of 2 RCTs showed a significant reduction in LDL levels receiving L. reuteri compared with those receiving placebo (WMD = -0.52; CI, -0.65 to -0.40; p <0.001; Table 2). The included studies showed highly significant homogeneity (I2 = 0.0%, p = 0.880).
A pooled analysis of 2 RCTs demonstrated a significant decrease in TG levels and increase in HDL-C levels after the use of the synbiotic food containing L. sporogenes and inulin compared with control groups (WMD = -0.58; CI, -0.97 to -0.19; p = 0.003; WMD = 0.18; CI, 0.08 to 0.28; p < 0.001; respectively, Table 2). The included studies showed highly significant homogeneity (I2 = 22.0%, p = 0.258; I2 = 0.0%, p = 0.460, respectively).
Besides, subgroup analysis with the givensubjects of non-pregnancy, non-diabetes/obesityordiabetes/obesitywas performed in supplemental material (S2 Table). Most of the results remained similar to the main outcomes, except LDL-C in the diabetes/obesity subjects became nonsignificant.
Publication bias diagnostics
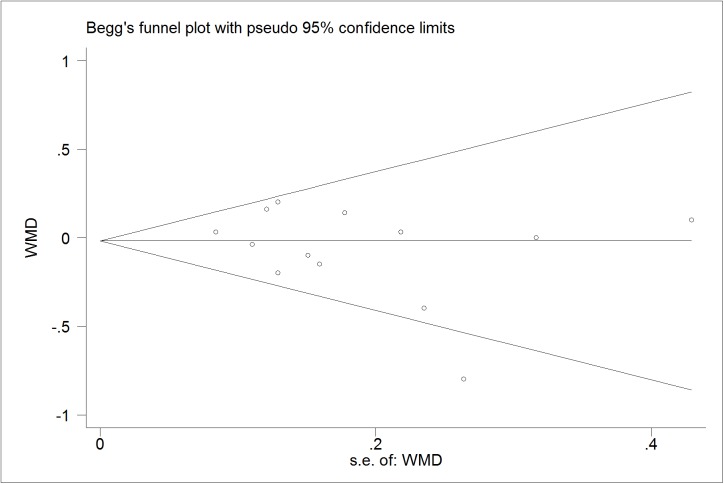
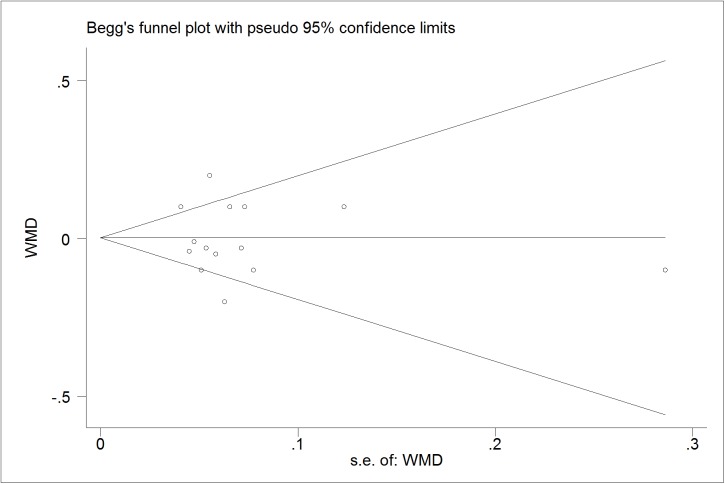
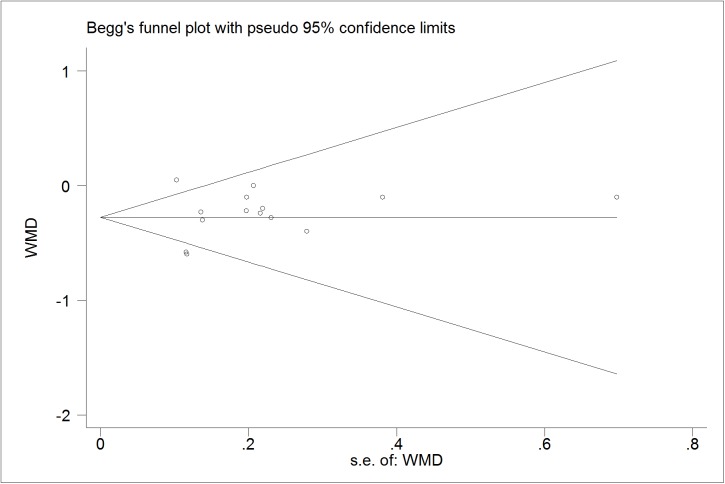
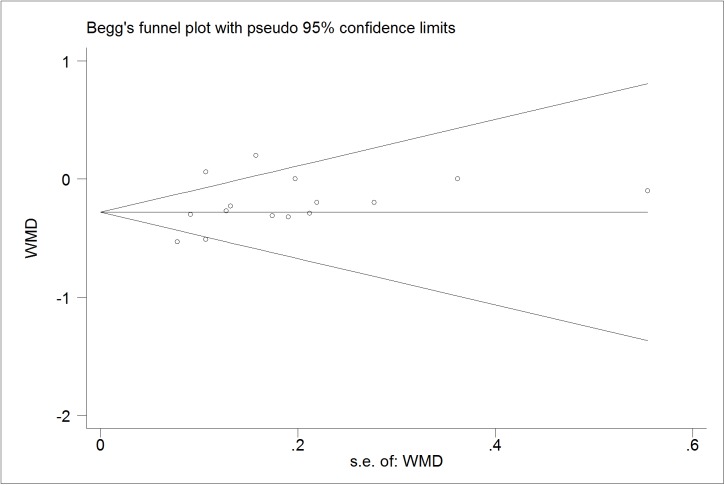
We further identify the potential publication biases of literatures by Egger’s test and Begg’s funnel plot. In all trials, the shapes of funnel plot indicated no obvious asymmetry (Figs 6–9). And Egger’s test provided statistical evidence for the funnel plot symmetry. No significant publication bias was found in the trials (p = 0.218 for TG; p = 0.599 for TC; p = 0.141 for LDL-C; p = 0.785 for HDL-C).
Fig 6. Begg’s funnel plot for publication bias in trials on the effect of probiotics Lactobacillus on TG.
Fig 9. Begg’s funnel plot for publication bias in trials on the effect of probiotics Lactobacillus on HDL.
Fig 7. Begg’s funnel plot for publication bias in trials on the effect of probiotics Lactobacillus on TC.
Fig 8. Begg’s funnel plot for publication bias in trials on the effect of probiotics Lactobacillus on LDL.
Discussion
The effects of probiotics consumption on lipid control have attracted increasing interest recently [22, 23]. A meta-analysis of 13 randomized controlled trials reported that probiotics consumption was able to decrease total-cholesterol and LDL-cholesterol levels effectively [24].Previous studies show that the gut microbiome may play an important role in the variation in serum lipids, supporting the potential of therapies altering the gut microbiome to control triglycerides and high-density lipoproteins[25, 26]. While, there are many different probiotic strains on the market. It is of practical interest to know the effect of specific strains rather than probiotics as ‘a whole group’. So, we conducted this meta-analysis to explore the effects of probiotic Lactobacillus consumption on lipid profile.
This study demonstrated that consumption of Lactobacillus has beneficial effects on serum TC and LDL-C levels, while no obvious changes in serum HDL-C and TG levels. As we know, this is the first meta-analysis assessing the effect of probiotics Lactobacillus on lipid profile. The mechanisms of reducing cholesterol levels may be as following: reduction of the enterohepatic circulation of bile salts; assimilation of cholesterol in the gastrointestinal tract; production of propionic acid which could decrease blood lipids; conversion of cholesterol into coprostanol in the gut[27–29]. Furthermore, it appears that activation of FXRα, nuclear receptor of bile acids, can also improve triglyceride control in animal models[30]. Besides, activation of the bile acid cell membrane receptor TGR5 may also activate thyroid hormone in brown adipose tissue and muscle, which increases energy expenditure and therefore improves serum lipids[31, 32].
The present meta-analysis found that consumption of L. reuteri and L. plantarum could decrease the TC and LDL-C levels effectively. This is in accordance with the findings by Singh TP et al.[33]who reported that the values for TC and LDL-C of pigs were reduced significantly in group fed with L. reuteri LR6, and also in line with findings by Salaj R et al. [34]who showed that Lactobacillus plantarum LS/07 reduced TC and LDL in rats fed with a high fat diet. Few studies have explored the mechanisms of decreasing TC and LDL-C levels by L. reuteri and L. plantarum. Yamaoka-Tojo M et al.[35] provided possible mechanism that bile acid-binding resins decrease the concentration of bile acids returned to the liver via enterohepatic cycling and therefore stimulate the conversion of cholesterol to bile acids.
Moreover, in the present study, consumption of the synbiotic food, containing L. sporogenes and inulin, significantly decreased serum TG and increased serum HDL-C levels, while had no impact on serum TC and LDL-C compared with control. Similar findings have also been shown in hypercholesterolemic pigs fed with a synbiotic food containing L. acidophilus, fructooligosaccharide, inulin and mannitol for 8 weeks[36]. The mechanisms of decreasing TG and increasing HDL-C levels by synbiotics remained unclear. One of the possible mechanisms was inhibition of synthesis of fatty acids in the liver by producing short-chain fatty acid (SCFA)[37]. Next, inulin played a determinant role in decreasing expression of lipogenic enzymes[38]. Moreover, inulin and probiotics may have a synergistic effect when administered as a synbiotic[39]. Besides, we also found that consumption of L. helveticus significantly decreased serum HDL-C levels, reminding us that not all Lactobacillus are beneficial to health.
There are some limitations to this meta-analysis. Primarily, the bias of the included studies exists in some aspects such as the dietary restrictions and exercise. And crossover studies may bring additional biases. Besides, some studies had a small number of participants and a quite short duration of Lactobacillus consumption. Last, the insufficient databases were searched, and it was absence of papers in other languages and pre-published protocol. Further researches with larger sample and longer duration are required to verify the effect of probiotic Lactobacillus on lipid profile.
Conclusion
This meta-analysis showed that consumption of probiotic Lactobacillus, especially L. reuteri and L. plantarm, could reduce TC and LDL-C significantly. The study also suggested significantly beneficial effects on TG and HDL-C by consuming synbiotic food, containing L. sporogenes and inulin. The effect of probiotic Lactobacillus on serum lipids, as well as their mechanisms involved, required further investigation. This study provides useful information on the effects of probiotic Lactobacillus on serum lipids, which could make a contribution to the application of probiotic Lactobacillus, especially L. reuteri, L. plantarm and synbiotics, as novel therapies in lipids control.
Supporting information
+ low risk of bias (plausible bias unlikely to seriously alter the results),—high risk of bias (plausible bias that seriously weakens confidence in the results),? unclear risk of bias (plausible bias that raises some doubt about the results).
(DOCX)
(DOCX)
(DOCX)
(DOC)
Acknowledgments
We thank all the participants in this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Ladeia AM, Guimaraes AC, Lima JC. [The lipid profile and coronary artery disease]. Arquivos brasileiros de cardiologia. 1994;63(2):101–6. Epub 1994/08/01. . [PubMed] [Google Scholar]
- 2.DiNicolantonio JJ, Harcombe Z, O'Keefe JH. Problems with the 2015 Dietary Guidelines for Americans: An Alternative. Missouri medicine. 2016;113(2):93–7. Epub 2016/06/18. . [PMC free article] [PubMed] [Google Scholar]
- 3.Sanchez B, Delgado S, Blanco-Miguez A, Lourenco A, Gueimonde M, Margolles A. Probiotics, gut microbiota, and their influence on host health and disease. Molecular nutrition & food research. 2016. Epub 2016/08/09. 10.1002/mnfr.201600240 . [DOI] [PubMed] [Google Scholar]
- 4.Aguirre M, Bussolo de Souza C, Venema K. The Gut Microbiota from Lean and Obese Subjects Contribute Differently to the Fermentation of Arabinogalactan and Inulin. PloS one. 2016;11(7):e0159236 Epub 2016/07/15. PubMed Central PMCID: PMC4943740. 10.1371/journal.pone.0159236 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie N, Cui Y, Yin YN, Zhao X, Yang JW, Wang ZG, et al. Effects of two Lactobacillus strains on lipid metabolism and intestinal microflora in rats fed a high-cholesterol diet. BMC complementary and alternative medicine. 2011;11:53 Epub 2011/07/05. PubMed Central PMCID: PMC3144010. 10.1186/1472-6882-11-53 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben Salah R, Trabelsi I, Hamden K, Chouayekh H, Bejar S. Lactobacillus plantarum TN8 exhibits protective effects on lipid, hepatic and renal profiles in obese rat. Anaerobe. 2013;23:55–61. Epub 2013/07/31. 10.1016/j.anaerobe.2013.07.003 . [DOI] [PubMed] [Google Scholar]
- 7.Anderson JW, Gilliland SE. Effect of fermented milk (yogurt) containing Lactobacillus acidophilus L1 on serum cholesterol in hypercholesterolemic humans. Journal of the American College of Nutrition. 1999;18(1):43–50. Epub 1999/03/06. . [DOI] [PubMed] [Google Scholar]
- 8.Fuentes MC, Lajo T, Carrion JM, Cune J. Cholesterol-lowering efficacy of Lactobacillus plantarum CECT 7527, 7528 and 7529 in hypercholesterolaemic adults. The British journal of nutrition. 2013;109(10):1866–72. Epub 2012/09/29. 10.1017/S000711451200373X . [DOI] [PubMed] [Google Scholar]
- 9.Hove KD, Brons C, Faerch K, Lund SS, Rossing P, Vaag A. Effects of 12 weeks of treatment with fermented milk on blood pressure, glucose metabolism and markers of cardiovascular risk in patients with type 2 diabetes: a randomised double-blind placebo-controlled study. European journal of endocrinology / European Federation of Endocrine Societies. 2015;172(1):11–20. Epub 2014/10/11. 10.1530/EJE-14-0554 . [DOI] [PubMed] [Google Scholar]
- 10.Sanchez M, Darimont C, Drapeau V, Emady-Azar S, Lepage M, Rezzonico E, et al. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. The British journal of nutrition. 2014;111(8):1507–19. Epub 2013/12/05. 10.1017/S0007114513003875 . [DOI] [PubMed] [Google Scholar]
- 11.Bukowska H, Pieczul-Mroz J, Jastrzebska M, Chelstowski K, Naruszewicz M. Decrease in fibrinogen and LDL-cholesterol levels upon supplementation of diet with Lactobacillus plantarum in subjects with moderately elevated cholesterol. Atherosclerosis. 1998;137(2):437–8. Epub 1998/06/11. . [DOI] [PubMed] [Google Scholar]
- 12.de Roos NM, Schouten G, Katan MB. Yoghurt enriched with Lactobacillus acidophilus does not lower blood lipids in healthy men and women with normal to borderline high serum cholesterol levels. European journal of clinical nutrition. 1999;53(4):277–80. Epub 1999/05/20. . [DOI] [PubMed] [Google Scholar]
- 13.Schaafsma G, Meuling WJ, van Dokkum W, Bouley C. Effects of a milk product, fermented by Lactobacillus acidophilus and with fructo-oligosaccharides added, on blood lipids in male volunteers. European journal of clinical nutrition. 1998;52(6):436–40. Epub 1998/07/31. . [DOI] [PubMed] [Google Scholar]
- 14.Jones ML, Martoni CJ, Di Pietro E, Simon RR, Prakash S. Evaluation of clinical safety and tolerance of a Lactobacillus reuteri NCIMB 30242 supplement capsule: a randomized control trial. Regulatory toxicology and pharmacology: RTP. 2012;63(2):313–20. Epub 2012/05/09. 10.1016/j.yrtph.2012.04.003 . [DOI] [PubMed] [Google Scholar]
- 15.Jones ML, Martoni CJ, Prakash S. Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: a randomized controlled trial. European journal of clinical nutrition. 2012;66(11):1234–41. Epub 2012/09/20. 10.1038/ejcn.2012.126 . [DOI] [PubMed] [Google Scholar]
- 16.Lindsay KL, Brennan L, Kennelly MA, Maguire OC, Smith T, Curran S, et al. Impact of probiotics in women with gestational diabetes mellitus on metabolic health: a randomized controlled trial. American journal of obstetrics and gynecology. 2015;212(4):496 e1–11. Epub 2015/02/18. 10.1016/j.ajog.2015.02.008 . [DOI] [PubMed] [Google Scholar]
- 17.Naruszewicz M, Johansson ML, Zapolska-Downar D, Bukowska H. Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. The American journal of clinical nutrition. 2002;76(6):1249–55. Epub 2002/11/27. . [DOI] [PubMed] [Google Scholar]
- 18.Shakeri H, Hadaegh H, Abedi F, Tajabadi-Ebrahimi M, Mazroii N, Ghandi Y, et al. Consumption of synbiotic bread decreases triacylglycerol and VLDL levels while increasing HDL levels in serum from patients with type-2 diabetes. Lipids. 2014;49(7):695–701. Epub 2014/04/08. 10.1007/s11745-014-3901-z . [DOI] [PubMed] [Google Scholar]
- 19.Sharafedtinov KK, Plotnikova OA, Alexeeva RI, Sentsova TB, Songisepp E, Stsepetova J, et al. Hypocaloric diet supplemented with probiotic cheese improves body mass index and blood pressure indices of obese hypertensive patients—a randomized double-blind placebo-controlled pilot study. Nutrition journal. 2013;12:138 Epub 2013/10/15. PubMed Central PMCID: PMC3852723. 10.1186/1475-2891-12-138 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simons LA, Amansec SG, Conway P. Effect of Lactobacillus fermentum on serum lipids in subjects with elevated serum cholesterol. Nutrition, metabolism, and cardiovascular diseases: NMCD. 2006;16(8):531–5. Epub 2006/11/28. 10.1016/j.numecd.2005.10.009 . [DOI] [PubMed] [Google Scholar]
- 21.Taghizadeh M, Hashemi T, Shakeri H, Abedi F, Sabihi SS, Alizadeh SA, et al. Synbiotic food consumption reduces levels of triacylglycerols and VLDL, but not cholesterol, LDL, or HDL in plasma from pregnant women. Lipids. 2014;49(2):155–61. Epub 2013/11/26. 10.1007/s11745-013-3867-2 . [DOI] [PubMed] [Google Scholar]
- 22.de Roos NM, Katan MB. Effects of probiotic bacteria on diarrhea, lipid metabolism, and carcinogenesis: a review of papers published between 1988 and 1998. The American journal of clinical nutrition. 2000;71(2):405–11. Epub 2000/01/29. . [DOI] [PubMed] [Google Scholar]
- 23.Kiessling G, Schneider J, Jahreis G. Long-term consumption of fermented dairy products over 6 months increases HDL cholesterol. European journal of clinical nutrition. 2002;56(9):843–9. Epub 2002/09/05. 10.1038/sj.ejcn.1601399 . [DOI] [PubMed] [Google Scholar]
- 24.Guo Z, Liu XM, Zhang QX, Shen Z, Tian FW, Zhang H, et al. Influence of consumption of probiotics on the plasma lipid profile: a meta-analysis of randomised controlled trials. Nutrition, metabolism, and cardiovascular diseases: NMCD. 2011;21(11):844–50. Epub 2011/09/21. 10.1016/j.numecd.2011.04.008 . [DOI] [PubMed] [Google Scholar]
- 25.Fu J, Bonder MJ, Cenit MC, Tigchelaar EF, Maatman A, Dekens JA, et al. The Gut Microbiome Contributes to a Substantial Proportion of the Variation in Blood Lipids. Circulation research. 2015;117(9):817–24. Epub 2015/09/12. PubMed Central PMCID: PMC4596485. 10.1161/CIRCRESAHA.115.306807 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allayee H, Hazen SL. Contribution of Gut Bacteria to Lipid Levels: Another Metabolic Role for Microbes? Circulation research. 2015;117(9):750–4. Epub 2015/10/10. PubMed Central PMCID: PMC4705836. 10.1161/CIRCRESAHA.115.307409 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Rodas BZ, Gilliland SE, Maxwell CV. Hypocholesterolemic action of Lactobacillus acidophilus ATCC 43121 and calcium in swine with hypercholesterolemia induced by diet. Journal of dairy science. 1996;79(12):2121–8. Epub 1996/12/01. 10.3168/jds.S0022-0302(96)76586-4 . [DOI] [PubMed] [Google Scholar]
- 28.Kumar R, Grover S, Batish VK. Bile Salt Hydrolase (Bsh) Activity Screening of Lactobacilli: In Vitro Selection of Indigenous Lactobacillus Strains with Potential Bile Salt Hydrolysing and Cholesterol-Lowering Ability. Probiotics and antimicrobial proteins. 2012;4(3):162–72. Epub 2012/09/01. 10.1007/s12602-012-9101-3 . [DOI] [PubMed] [Google Scholar]
- 29.Gilliland SE, Nelson CR, Maxwell C. Assimilation of cholesterol by Lactobacillus acidophilus. Applied and environmental microbiology. 1985;49(2):377–81. Epub 1985/02/01. PubMed Central PMCID: PMC238411. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Del Bas JM, Ricketts ML, Vaque M, Sala E, Quesada H, Ardevol A, et al. Dietary procyanidins enhance transcriptional activity of bile acid-activated FXR in vitro and reduce triglyceridemia in vivo in a FXR-dependent manner. Molecular nutrition & food research. 2009;53(7):805–14. Epub 2009/06/06. 10.1002/mnfr.200800364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pols TW, Noriega LG, Nomura M, Auwerx J, Schoonjans K. The bile acid membrane receptor TGR5: a valuable metabolic target. Dig Dis. 2011;29(1):37–44. Epub 2011/06/22. PubMed Central PMCID: PMC3128138. 10.1159/000324126 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staley C, Weingarden AR, Khoruts A, Sadowsky MJ. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Applied microbiology and biotechnology. 2017;101(1):47–64. Epub 2016/11/27. PubMed Central PMCID: PMC5203956. 10.1007/s00253-016-8006-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh TP, Malik RK, Katkamwar SG, Kaur G. Hypocholesterolemic effects of Lactobacillus reuteri LR6 in rats fed on high-cholesterol diet. International journal of food sciences and nutrition. 2015;66(1):71–5. Epub 2014/09/30. 10.3109/09637486.2014.953450 . [DOI] [PubMed] [Google Scholar]
- 34.Salaj R, Stofilova J, Soltesova A, Hertelyova Z, Hijova E, Bertkova I, et al. The effects of two Lactobacillus plantarum strains on rat lipid metabolism receiving a high fat diet. TheScientificWorldJournal. 2013;2013:135142 Epub 2014/01/29. PubMed Central PMCID: PMC3891428. 10.1155/2013/135142 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamaoka-Tojo M, Tojo T, Izumi T. Beyond cholesterol lowering: pleiotropic effects of bile acid binding resins against cardiovascular disease risk factors in patients with metabolic syndrome. Current vascular pharmacology. 2008;6(4):271–81. Epub 2008/10/16. . [DOI] [PubMed] [Google Scholar]
- 36.Liong MT, Dunshea FR, Shah NP. Effects of a synbiotic containing Lactobacillus acidophilus ATCC 4962 on plasma lipid profiles and morphology of erythrocytes in hypercholesterolaemic pigs on high- and low-fat diets. The British journal of nutrition. 2007;98(4):736–44. Epub 2007/05/11. 10.1017/S0007114507747803 . [DOI] [PubMed] [Google Scholar]
- 37.Nielsen TS, Jensen BB, Purup S, Jackson S, Saarinen M, Lyra A, et al. A search for synbiotics: effects of enzymatically modified arabinoxylan and Butyrivibrio fibrisolvens on short-chain fatty acids in the cecum content and plasma of rats. Food & function. 2016;7(4):1839–48. Epub 2016/03/19. 10.1039/c6fo00114a . [DOI] [PubMed] [Google Scholar]
- 38.Delzenne NM, Daubioul C, Neyrinck A, Lasa M, Taper HS. Inulin and oligofructose modulate lipid metabolism in animals: review of biochemical events and future prospects. The British journal of nutrition. 2002;87 Suppl 2:S255–9. Epub 2002/06/29. 10.1079/BJNBJN/2002545 . [DOI] [PubMed] [Google Scholar]
- 39.de Vrese M, Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Advances in biochemical engineering/biotechnology. 2008;111:1–66. Epub 2008/05/08. 10.1007/10_2008_097 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
+ low risk of bias (plausible bias unlikely to seriously alter the results),—high risk of bias (plausible bias that seriously weakens confidence in the results),? unclear risk of bias (plausible bias that raises some doubt about the results).
(DOCX)
(DOCX)
(DOCX)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.