Abstract
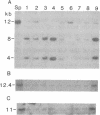
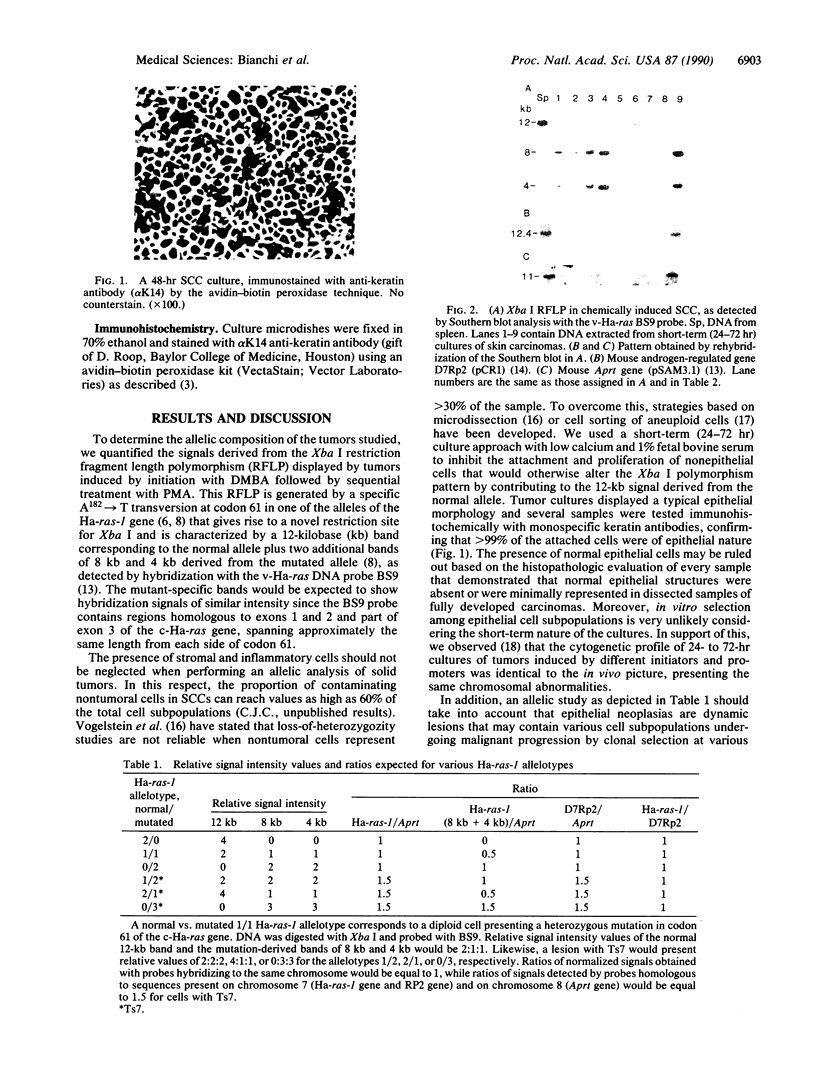
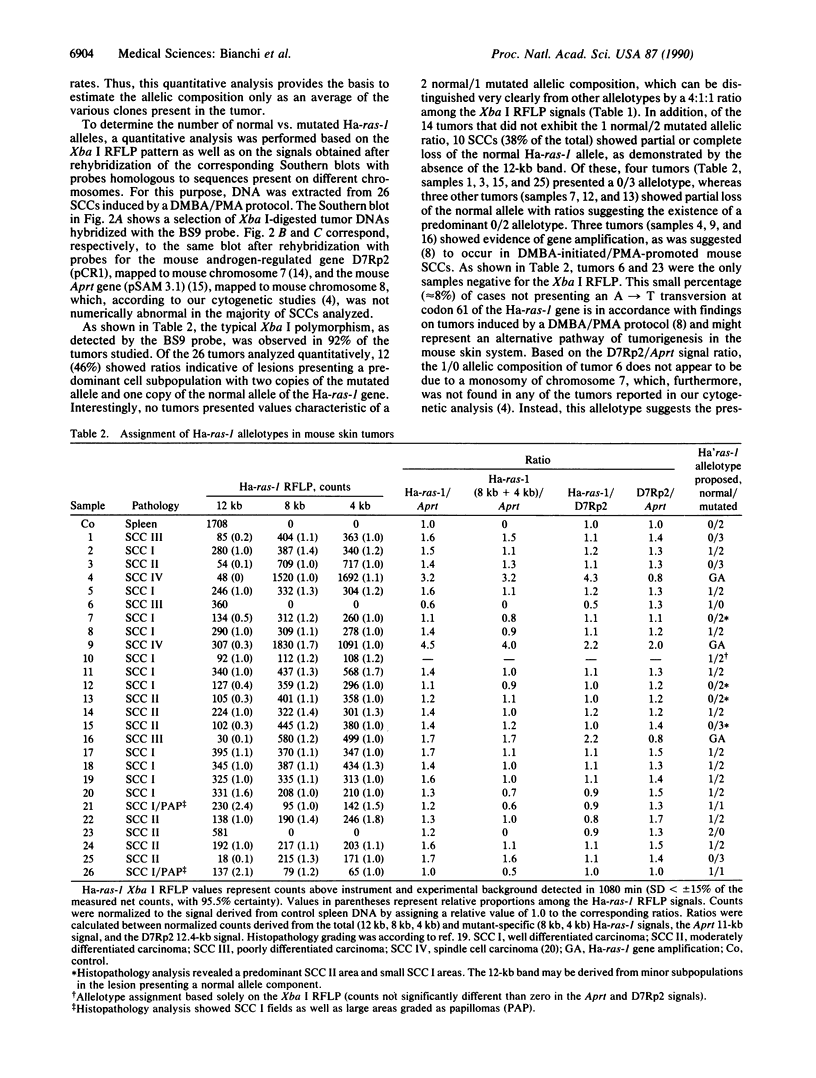
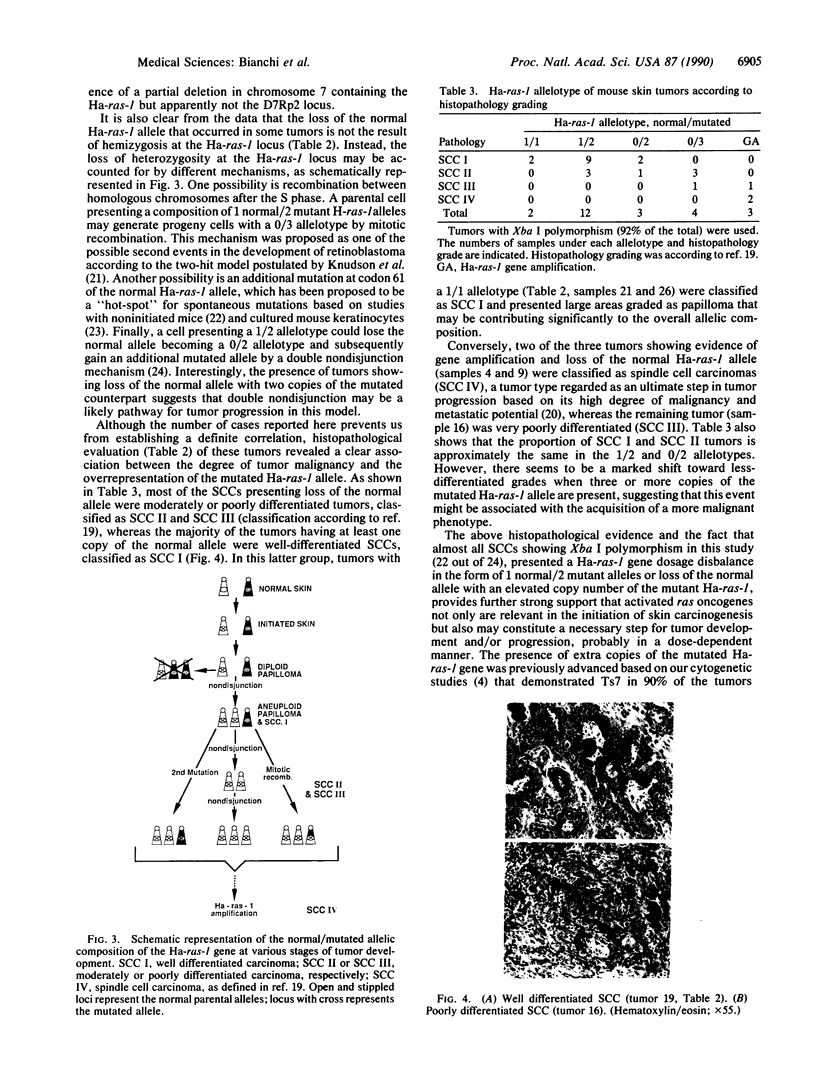
We analyzed the normal/mutated allelic ratio of the Ha-ras-1 gene in mouse skin squamous cell carcinomas induced by initiation with dimethylbenz[a]anthracene and promotion with phorbol 12-myristate 13-acetate. DNA for these studies was obtained from short-term tumor cultures (24-72 hr) to eliminate the contribution of stromal and inflammatory cells to the sample. The allelotypic analysis was performed in 25 squamous cell carcinomas by quantitative radio-analysis of the Xba I restriction fragment length polymorphism as detected by BS9, a v-Ha-ras probe, and rehybridization of the Southern blots with probes for chromosomes 7 and 8. Approximately 85% of the tumors presented overrepresentation of the mutated allele in the form of 1 normal/2 mutated (12 tumors), 0 normal/3 mutated (4 tumors), 0 normal/2 mutated (3 tumors), and gene amplification (3 tumors). No tumor was found with a 2 normal/1 mutated allelic ratio. These results support our previous cytogenetic studies, indicating that trisomy of chromosome 7 is present in the majority of these tumors and show that nonrandom duplication of the chromosome carrying the mutated Ha-ras-1 allele appears to be a major mechanism by which the mutated gene is overrepresented.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldaz C. M., Conti C. J., Klein-Szanto A. J., Slaga T. J. A direct cytogenetic technique for mouse skin carcinomas and papillomas. Cancer Genet Cytogenet. 1986 Feb 15;20(3-4):223–229. doi: 10.1016/0165-4608(86)90077-4. [DOI] [PubMed] [Google Scholar]
- Aldaz C. M., Conti C. J., Klein-Szanto A. J., Slaga T. J. Progressive dysplasia and aneuploidy are hallmarks of mouse skin papillomas: relevance to malignancy. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2029–2032. doi: 10.1073/pnas.84.7.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldaz C. M., Trono D., Larcher F., Slaga T. J., Conti C. J. Sequential trisomization of chromosomes 6 and 7 in mouse skin premalignant lesions. Mol Carcinog. 1989;2(1):22–26. doi: 10.1002/mc.2940020104. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Fearon E. R., Nigro J. M., Hamilton S. R., Preisinger A. C., Jessup J. M., vanTuinen P., Ledbetter D. H., Barker D. F., Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Balmain A., Pragnell I. B. Mouse skin carcinomas induced in vivo by chemical carcinogens have a transforming Harvey-ras oncogene. Nature. 1983 May 5;303(5912):72–74. doi: 10.1038/303072a0. [DOI] [PubMed] [Google Scholar]
- Benedict W. F., Weissman B. E., Mark C., Stanbridge E. J. Tumorigenicity of human HT1080 fibrosarcoma X normal fibroblast hybrids: chromosome dosage dependency. Cancer Res. 1984 Aug;44(8):3471–3479. [PubMed] [Google Scholar]
- Bizub D., Blair D., Alvord G., Skalka A. M. Correlation between H-ras p21TLeu61 protein content and tumorigenicity of NIH3T3 cells. Oncogene. 1988 Oct;3(4):443–448. [PubMed] [Google Scholar]
- Bizub D., Wood A. W., Skalka A. M. Mutagenesis of the Ha-ras oncogene in mouse skin tumors induced by polycyclic aromatic hydrocarbons. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6048–6052. doi: 10.1073/pnas.83.16.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti C. J., Aldaz C. M., O'Connell J., Klein-Szanto A. J., Slaga T. J. Aneuploidy, an early event in mouse skin tumor development. Carcinogenesis. 1986 Nov;7(11):1845–1848. doi: 10.1093/carcin/7.11.1845. [DOI] [PubMed] [Google Scholar]
- Dush M. K., Sikela J. M., Khan S. A., Tischfield J. A., Stambrook P. J. Nucleotide sequence and organization of the mouse adenine phosphoribosyltransferase gene: presence of a coding region common to animal and bacterial phosphoribosyltransferases that has a variable intron/exon arrangement. Proc Natl Acad Sci U S A. 1985 May;82(9):2731–2735. doi: 10.1073/pnas.82.9.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R. W., Berger F. G. DNA sequence polymorphism in an androgen-regulated gene is associated with alteration in the encoded RNAs. Proc Natl Acad Sci U S A. 1983 Jan;80(2):501–504. doi: 10.1073/pnas.80.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. W., DeFeo D., Maryak J. M., Young H. A., Shih T. Y., Chang E. H., Lowy D. R., Scolnick E. M. Dual evolutionary origin for the rat genetic sequences of Harvey murine sarcoma virus. J Virol. 1980 Nov;36(2):408–420. doi: 10.1128/jvi.36.2.408-420.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Greenhalgh D. A., Welty D. J., Strickland J. E., Yuspa S. H. Spontaneous Ha-ras gene activation in cultured primary murine keratinocytes: consequences of Ha-ras gene activation in malignant conversion and malignant progression. Mol Carcinog. 1989;2(4):199–207. doi: 10.1002/mc.2940020406. [DOI] [PubMed] [Google Scholar]
- Klein-Szanto A. J., Larcher F., Bonfil R. D., Conti C. J. Multistage chemical carcinogenesis protocols produce spindle cell carcinomas of the mouse skin. Carcinogenesis. 1989 Nov;10(11):2169–2172. doi: 10.1093/carcin/10.11.2169. [DOI] [PubMed] [Google Scholar]
- Klein G. Specific chromosomal translocations and the genesis of B-cell-derived tumors in mice and men. Cell. 1983 Feb;32(2):311–315. doi: 10.1016/0092-8674(83)90449-x. [DOI] [PubMed] [Google Scholar]
- Klein G. The role of gene dosage and genetic transpositions in carcinogenesis. Nature. 1981 Nov 26;294(5839):313–318. doi: 10.1038/294313a0. [DOI] [PubMed] [Google Scholar]
- Klinger H. P., Kaelbling M. Suppression of tumorigenicity in somatic cell hybrids. IV. Chromosomes of normal human cells associated with suppression of tumorigenicity in hybrids with D98AH2 carcinoma cells. Cytogenet Cell Genet. 1986;42(4):225–235. doi: 10.1159/000132283. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Genetics of human cancer. Annu Rev Genet. 1986;20:231–251. doi: 10.1146/annurev.ge.20.120186.001311. [DOI] [PubMed] [Google Scholar]
- Kozak C. A., Sears J. F., Hoggan M. D. Genetic mapping of the mouse oncogenes c-Ha-ras-1 and c-fes to chromosome 7. J Virol. 1983 Jul;47(1):217–220. doi: 10.1128/jvi.47.1.217-220.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruszewski F. H., Conti C. J., DiGiovanni J. Characterization of skin tumor promotion and progression by chrysarobin in SENCAR mice. Cancer Res. 1987 Jul 15;47(14):3783–3790. [PubMed] [Google Scholar]
- Miller D. R., Viaje A., Aldaz C. M., Conti C. J., Slaga T. J. Terminal differentiation-resistant epidermal cells in mice undergoing two-stage carcinogenesis. Cancer Res. 1987 Apr 1;47(7):1935–1940. [PubMed] [Google Scholar]
- Monpezat J. P., Delattre O., Bernard A., Grunwald D., Remvikos Y., Muleris M., Salmon R. J., Frelat G., Dutrillaux B., Thomas G. Loss of alleles on chromosome 18 and on the short arm of chromosome 17 in polyploid colorectal carcinomas. Int J Cancer. 1988 Mar 15;41(3):404–408. doi: 10.1002/ijc.2910410315. [DOI] [PubMed] [Google Scholar]
- Oshimura M., Barrett J. C. Double nondisjunction during karyotypic progression of chemically induced Syrian hamster cell lines. Cancer Genet Cytogenet. 1985 Oct;18(2):131–139. doi: 10.1016/0165-4608(85)90063-9. [DOI] [PubMed] [Google Scholar]
- Pelling J. C., Neades R., Strawhecker J. Epidermal papillomas and carcinomas induced in uninitiated mouse skin by tumor promoters alone contain a point mutation in the 61st codon of the Ha-ras oncogene. Carcinogenesis. 1988 Apr;9(4):665–667. doi: 10.1093/carcin/9.4.665. [DOI] [PubMed] [Google Scholar]
- Quintanilla M., Brown K., Ramsden M., Balmain A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986 Jul 3;322(6074):78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- Ricketts M. H., Levinson A. D. High-level expression of c-H-ras1 fails to fully transform rat-1 cells. Mol Cell Biol. 1988 Apr;8(4):1460–1468. doi: 10.1128/mcb.8.4.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roop D. R., Lowy D. R., Tambourin P. E., Strickland J., Harper J. R., Balaschak M., Spangler E. F., Yuspa S. H. An activated Harvey ras oncogene produces benign tumours on mouse epidermal tissue. 1986 Oct 30-Nov 5Nature. 323(6091):822–824. doi: 10.1038/323822a0. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. Biological implications of consistent chromosome rearrangements in leukemia and lymphoma. Cancer Res. 1984 Aug;44(8):3159–3168. [PubMed] [Google Scholar]
- Spandidos A., Wilkie N. M. The normal human H-ras1 gene can act as an onco-suppressor. Br J Cancer Suppl. 1988 Dec;9:67–71. [PMC free article] [PubMed] [Google Scholar]
- Tabin C. J., Weinberg R. A. Analysis of viral and somatic activations of the cHa-ras gene. J Virol. 1985 Jan;53(1):260–265. doi: 10.1128/jvi.53.1.260-265.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Wirschubsky Z., Tsichlis P., Klein G., Sumegi J. Rearrangement of c-myc, pim-1 and Mlvi-1 and trisomy of chromosome 15 in MCF- and Moloney-MuLV-induced murine T-cell leukemias. Int J Cancer. 1986 Nov 15;38(5):739–745. doi: 10.1002/ijc.2910380518. [DOI] [PubMed] [Google Scholar]