Abstract
Varicella zoster virus (VZV), a human neurotropic alphaherpesvirus, becomes latent after primary infection and reactivates to produce zoster. To study VZV latency and reactivation, human trigeminal ganglia removed within 24 h after death were mechanically dissociated, randomly distributed into six-well tissue culture plates and incubated with reagents to inactivate nerve growth factor (NGF) or phosphoinositide 3-kinase (PI3-kinase) pathways. At 5 days, VZV DNA increased in control and PI3-kinase inhibitor-treated cultures to the same extent, but was significantly more abundant in anti-NGF-treated cultures (p = 0.001). Overall, VZV DNA replication is regulated in part by an NGF pathway that is PI3-kinase-independent.
Keywords: VZV, Human, Trigeminal ganglion, Latency, Nerve growth factor, Phosphoinositide-3-kinase
Introduction
Varicella zoster virus (VZV) is a ubiquitous human neurotropic alphaherpesvirus to which >95 % of the world’s population has been exposed (Virgin et al. 2009). During primary infection, VZV infects multiple cranial nerve ganglia, dorsal root ganglia, and autonomic and enteric ganglia (Steiner et al. 2007; Eshleman et al. 2011; Nagel et al. 2014; Gershon et al. 2012), where virus replication is blocked and latent infection ensues. VZV is latent in more than 90 % of human trigeminal ganglia (TG) (Mahalingam et al. 1992; Cohrs et al. 2000), where <10 copies of VZV DNA (Mahalingam et al. 1993; Wang et al. 2005) are present in neurons (Gilden et al. 1983, 1987; Kennedy et al. 1998) and from which a limited number of virus genes are transcribed (Kennedy et al. 1999; Cohrs and Gilden 2007). VZV reactivation typically causes zoster, frequently followed by postherpetic neuralgia. Zoster may be further complicated by meningoencephalitis, cerebellitis, cranial nerve palsies, myelopathy, and VZV vasculopathy (Gershon et al. 2015). Recently, giant cell arteritis was shown to be triggered by VZV in the temporal artery (Gilden 2015). Currently, there is no suitable animal model to study the molecular events of VZV reactivation (Shahzad et al. 2015).
VZV and human herpes simplex virus type 1 (HSV-1), members of the same alphaherpesvirus subfamily (Kennedy et al. 2015), can be latent in the same TG (Cohrs et al. 2005) and even in the same neuron (Theil et al. 2003). During latency, both virus genomes are circular (Efstathiou et al. 1986; Clarke et al. 1995) and are largely transcriptionally silent (Stevens et al. 1988; Ouwendijk et al. 2012). Latent HSV-1 can be recovered from about half of human TG explants 10–24 days in culture (Plummer 1973; Warren et al. 1977). Although VZV has never been isolated from latently infected human TG (Plotkin et al. 1977), virus DNA does replicate in human TG explants (Azarkh et al. 2012) and dissociated ganglia (Cohrs et al. 2016).
Alphaherpesvirus reactivation can be viewed as the culmination of a complex, multi-stage series of events with checkpoints that may block release of infectious virus (Roizman et al. 2011). Studies of HSV-1 in animal and tissue culture models of latency and reactivation indicate that DNA replication can be used to distinguish various stages of reactivation. Early-stage HSV-1 reactivation results in generalized deregulation of virus gene transcription, wherein virus genes are transcribed independent of their kinetic class (immediate-early, early, and late) and independent of virus DNA replication (Du et al. 2011; Kim et al. 2012). Late-stage virus reactivation includes virus DNA replication along with a full complement of virus gene transcription that ultimately results in assembly and release of infectious virions (Kobayashi et al. 2012). Since HSV-1 DNA replication is required for efficient virus reactivation (Kobayashi et al. 2012), in the current work VZV DNA replication was used as an indicator that VZV reactivation has been initiated, but not that the virus has reactivated to produce infectious virions. Using VZV DNA replication as a marker for the transition between early- and late-stage reactivation, we tested whether the nerve growth factor (NGF) and phosphoinositide 3-kinase (PI3-kinase) pathways, both involved in HSV-1 reactivation (Wilcox and Johnson 1988; Kobayashi et al. 2012), play a role in latent VZV DNA replication.
The left and right human TG were obtained within 24 h of death in accordance with approved protocols from the Colorado Multiple Institutional Review Board and Office for Human Research protections, US Department of Health and Human Services (http://www.hhs.gov/ohrp) and FDA (http://www.fda.gov/oc/ohrt/irbs/default.htm) guidelines. At autopsy, no subject showed cutaneous signs of herpesvirus reactivation and none were immunosuppressed before death (Table 1). The two TG from each subject were combined, washed with PBS, and mechanically dissociated into sizes sufficiently small to pass through the opening of a 1000-μl pipette tip. This critical step ensured that neurons were randomly distributed among samples to reduce sampling error upon assessment of VZV DNA content (Cohrs et al. 2016). While we did not count neurons or non-neuronal cells in the current experiments, human TG contain on average 27,400 ± 4800 neurons with approximately 100:1 non-neuronal cell/neuron ratio (Laguardia et al. 2000) and our dissociation protocol did not specifically eliminate non-neuronal cells or add antimitotic drugs to kill actively dividing cells. Dissociated ganglia were suspended in 20 % fetal bovine serum-supplemented Dulbecco’s modified Eagle medium containing antibiotics and antimycotics (Life Technologies, Waltham, MA) and cultured in six-well glass-bottom tissue culture plates (Cellvis, Burlington, Ontario, Canada) since cell density and support material are major contributors to HSV-1 reactivation efficiency (Warren et al. 1978; Lewis et al. 1982). Samples were harvested at 0, 5, and 10 days. Cell viability was determined by inclusion of red fluorescent dye (CellTracker; Thermo Fischer Scientific, Waltham, MA), while cell nuclei were identified by DAPI staining (Fig. 1). VZV DNA copy number was determined by TaqMan-based quantitative PCR of total DNA extracted from each sample (Cohrs et al. 2016).
Table 1.
Clinical features of humans from whom ganglia were obtained
| Subject | Age/gender | H postmortem | Cause of death |
|---|---|---|---|
| 1 | 49/F | 15 | Upper respiratory disease |
| 2 | 65/M | 21 | Sepsis |
| 3 | 31/F | 12 | Drug overdose |
| 4 | 61/F | 12 | Liver failure |
| 5 | 54/F | 13 | Unexpected |
| 6 | 61/M | 20 | Accident |
| 7 | 64/M | 21 | Pulmonary emphysema |
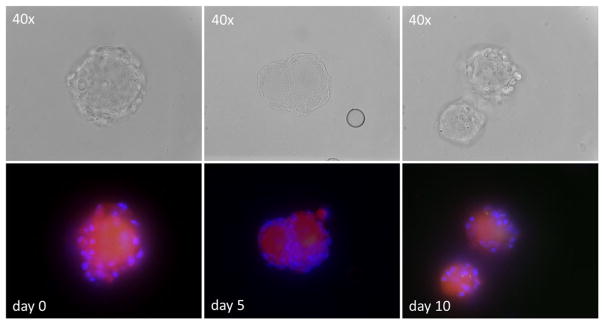
Fig. 1.
Viability and morphology of dissociated human trigeminal ganglia (TG) in culture. Mechanically dissociated human TG were cultured in six-well glass plates for the indicated days. Viable neurons incorporate the red-fluorescent vital dye with accompanying non-neuronal, probably satellite cell nuclei identified by DAPI (blue). Top row shows the same viable neurons seen in phase-contrast microscopy
Results and discussion
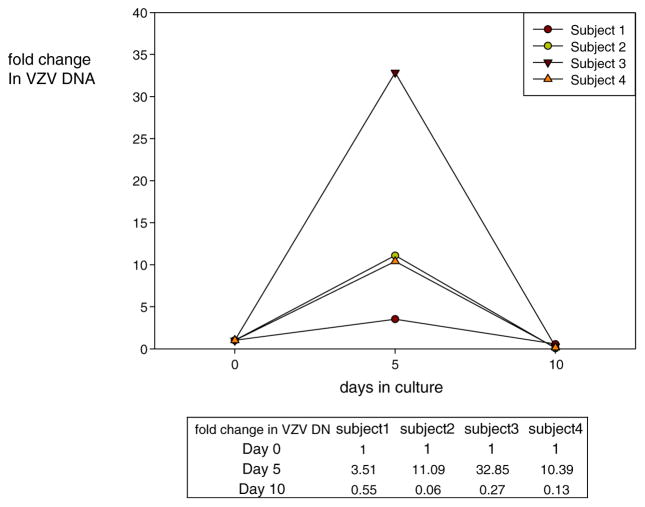
Dissociated human TG neurons with associated non-neuronal cells remained viable for 5 days in culture, although signs of neuronal stress (fainter viable staining) became evident at day 10 (Fig. 1). At no time was virus-induced cytopathology evident. At day 0, VZV DNA was present in the left and right TG from all four subjects. At day 5, VZV DNA abundance increased 3.5-, 11.1-, 32.8-, and 10.4-fold, respectively, in the four subjects (Fig. 2). The mean 14.4-fold increase in VZV DNA was significantly greater than the amount of VZV DNA present at day 0 (Wilconon signed-rank test; p = 0.0005) and was greater than the <3.5-fold increase at day 5 when dissociated individual human TG were cultured at higher density in 96-well tissue culture plates, although the abundance of VZV DNA in either culture condition was not increased at day 10 (Fig. 2; Cohrs et al. 2016).
Fig. 2.
Accumulation of VZV DNA in dissociated human trigeminal ganglia (TG) in tissue culture. DNA was extracted from mechanically dissociated human TG incubated in six-well glass plates, and VZV DNA was quantified by TaqMan-based PCR at the indicated days. Fold increase in VZV DNA was determined by ΔCt analysis: fold change at day 0 normalized to 1; fold change at day 5 = 2−(Ctday5 − Ctday 0); fold change at day 10 = 2−(Ctday10−Ctday 5)
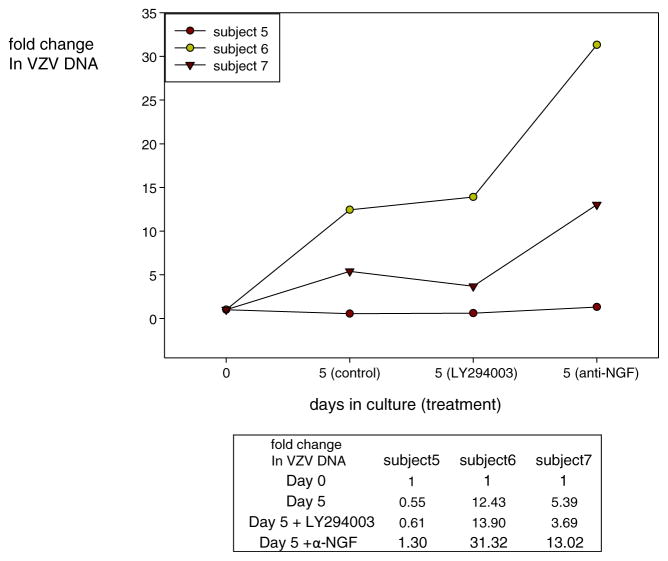
Using our modified culture conditions to optimize VZV DNA replication at day 5, we added the PI3-kinase inhibitor LY294003 (10 μM; Sigma-Aldrich, St Louis, MO) or rabbit polyclonal anti-NGF antibody (10 μg/ml) (Santa Cruz Antibodies, Santa Cruz, CA) to the medium and maintained the dissociated human TG cultures at 34 °C to facilitate VZV replication (Grose and Brunel 1978; Markus et al. 2015). VZV DNA abundance again increased after 5 days in culture, although the amount of VZV DNA increase was minimal for subject 5 (Fig. 3). Addition of LY29003 to cultures had no effect on the amount of VZV DNA at day 5 compared to untreated control cultures (p = 0.87). When anti-NGF antibody was added to the culture, total VZV DNA copies increased significantly by 1.3-, 31.0-, and 13.0-fold compared to untreated TG cultures (p = 0.001). While virus DNA replication is the simplest explanation for the observed increase in VZV DNA copy number, addition of acyclovir or phosphonoacetic acid would confirm that virus DNA replication was induced in the dissociated human TG cultures.
Fig. 3.
Accumulation of VZV DNA in dissociated trigeminal ganglia (TG) in tissue culture with or without PI3-kinase inhibitor (LY294003) and anti-NGF antibody. Human TG were mechanically dissociated and incubated in six-well glass plates before addition of PI3-kinase inhibitor (LY294003) or anti-NGF antibody at day 0. At days 0 and 5, DNA was extracted and VZV DNA was quantified by PCR. The VZV DNA fold change was calculated by ΔCt analysis and normalized to 1 at day 0. Fold change in cultures treated with PI3-kinase inhibitor (LY294003) or anti-NGF antibody at day 5 was calculated as 2−(Ctday 5 − Ctday0). VZV DNA abundance did not differ (p = 0.87) between untreated control and LY294003-treated TG cultures, but was significantly increased (p = 0.001) in TG cultures treated with anti-NGF antibody
The association between NGF and alphaherpesvirus was first noted based on clinical observations that HSV-1 reactivated after retrogasserian neurectomy (Cushing 1905; Carton and Kilbourne 1952) and is supported by in vitro experiments in which NGF deprivation induced HSV-1 reactivation in a murine model of HSV-1 latency (Wilcox and Johnson 1988), while addition of NGF to maintenance culture medium blocked HSV-1 reactivation in explants of latently infected mouse TG (Du et al. 2013, 2015; Shu et al. 2015). NGF provides continued PI3-kinase signaling through tropomyosin receptor kinase A (TrkA) to maintain HSV-1 latency (Camarena et al. 2010). VZV reactivation can occur after tympanomastoid (Vrabec 1999) or other orofacial surgery (Furuta et al. 2000), since these procedures might reduce dynein-dependent retrograde NGF transport from the axon to the cell body (Gluska et al. 2016). Treatment of VZV-infected human embryonic stem cell-derived neurons with neuronal and epidermal growth factors supports neuronal viability and suppresses lytic virus infection, while virus gene transcription is induced after growth factor removal (Markus et al. 2015; Sadaoka et al. 2016).
The association between PI3-kinase and alphaherpesviruses has been extensively studied. Binding of membrane-bound tyrosine kinases or G protein-coupled receptors by extracellular environmental signals (growth factors and cytokines) phosphorylates the p85 PI3-kinase regulatory subunit and activates the PI3-kinase p110 catalytic subunit. Activated pI3-kinase phosphorylates Akt kinase which is key to cell growth and survival, angiogenesis, and regulating cell metabolism (Manning and Cantley 2007). PI3-kinase activation is critical for efficient alphaherpesvirus replication (Liu and Cohen 2013). HSV-1 surface glycoprotein pB or gD induces PI3-kinase activity to facilitate virus entry (Tiwari and Shukla 2010), and virus replication is enhanced by PI3-kinase phosphorylation by HSV-1 UL11/12 or direct phosphorylation of PI3-kinase downstream substrates by HSV-1 US3-kinase (Eaton et al. 2014). VZValso uses PI3-kinase for efficient virus propagation. VZV ORF 12 protein binds the regulatory subunit of PI3-kinase resulting in activation of PI3-kinase, Akt phosphorylation, and induction of cell cycle cyclins, thereby increasing cell progression from G1 to M phase and virus production (Liu and Cohen 2013). PI3-kinase activation is also important in the course of HSV-1 reactivation. Continued PI3K-kinase activity through NGF is required to maintain HSV-1 latency, and its inhibition by LY29003 induces virus gene expression and release of infectious virions (Camarena et al. 2010).
Our results show that VZV DNA replication increases in NGF-depleted dissociated human TG cultures, most likely through a PI3-kinase-independent pathway. The induction of VZV DNA replication through NGF depletion provides an avenue to identify both viral and cellular transcripts that contribute to the transition of latent VZV from early- to late-stage reactivation.
Acknowledgments
This work was supported by Public Health Service grants NS082228 (R.J.C.), NS093716 (D.G.), and AG032958 (D.G., R.J.C.) from the National Institutes of Health. Hussain Badani was supported by training grant NS007321 to D.G. from the National Institutes of Health. We thank Marina Hoffman for editorial review and Cathy Allen for manuscript preparation.
Footnotes
This work is dedicated to the memory of Don Gilden, M.D.
Compliance with ethical standards The left and right human TG were obtained within 24 h of death in accordance with approved protocols from the Colorado Multiple Institutional Review Board and Office for Human Research protections, US Department of Health and Human Services ( http://www.hhs.gov/ohrp) and FDA (http://www.fda.gov/oc/ohrt/irbs/default.htm) guidelines.
Conflict of interest All authors declare that they have no conflict of interest.
References
- Azarkh Y, Bos N, Gilden D, Cohrs RJ. Human trigeminal ganglionic explants as a model to study alphaherpesvirus reactivation. J Neurovirol. 2012;18:456–461. doi: 10.1007/s13365-012-0123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarena V, Kobayashi M, Kim JY, Roehm P, Perez R, Gardner J, Wilson AC, Mohr I, Chao MV. Nature and duration of growth factor signaling through receptor tyrosine kinases regulates HSV-1 latency in neurons. Cell Host Microbe. 2010;8:320–330. doi: 10.1016/j.chom.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carton C, Kilbourne ED. Activation of latent herpes simplex by trigeminal sensory-root section. N Engl J Med. 1952;246:172–176. doi: 10.1056/NEJM195201312460503. [DOI] [PubMed] [Google Scholar]
- Clarke P, Beer T, Cohrs R, Gilden DH. Configuration of latent varicella-zoster virus DNA. J Virol. 1995;69:8151–8154. doi: 10.1128/jvi.69.12.8151-8154.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohrs RJ, Gilden DH. Prevalence and abundance of latently transcribed varicella-zoster virus genes in human ganglia. J Virol. 2007;81:2950–2956. doi: 10.1128/JVI.02745-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohrs RJ, Randall J, Smith J, Gilden DH, Dabrowski C, van Der KH, Tal-Singer R. Analysis of individual human trigeminal ganglia for latent herpes simplex virus type 1 and varicella-zoster virus nucleic acids using real-time PCR. J Virol. 2000;74:11464–11471. doi: 10.1128/jvi.74.24.11464-11471.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohrs RJ, Laguardia JJ, Gilden D. Distribution of latent herpes simplex virus type-1 and varicella zoster virus DNA in human trigeminal ganglia. Virus Genes. 2005;31:223–227. doi: 10.1007/s11262-005-1799-5. [DOI] [PubMed] [Google Scholar]
- Cohrs RJ, Badani H, Bos N, Scianna C, Hoskins I, Baird NL, Gilden D. Alphaherpesvirus DNA replication in dissociated human trigeminal ganglia. J Neurovirol. 2016 doi: 10.1007/s13365-016-0450-7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing H. Surgical aspects of major neuralgia of trigeminal nerve: report of 20 cases of operation upon the Gasserian ganglion with anatomic and physiologic notes on the consequences of its removal. J Am Med Assoc. 1905;44:3773–3379. [Google Scholar]
- Du T, Zhou G, Roizman B. HSV-1 gene expression from reactivated ganglia is disordered and concurrent with suppression of latency-associated transcript and miRNAs. Proc Natl Acad Sci U S A. 2011;108:18820–18824. doi: 10.1073/pnas.1117203108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du T, Zhou G, Roizman B. Modulation of reactivation of latent herpes simplex virus 1 in ganglionic organ cultures by p300/CBP and STAT3. Proc Natl Acad Sci U S A. 2013;110:E2621–E2628. doi: 10.1073/pnas.1309906110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du T, Han Z, Zhou G, Roizman B. Patterns of accumulation of miRNAs encoded by herpes simplex virus during productive infection, latency, and on reactivation. Proc Natl Acad Sci U S A. 2015;112:E49–E55. doi: 10.1073/pnas.1422657112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton HE, Saffran HA, Wu FW, Quach K, Smiley JR. Herpes simplex virus protein kinases US3 and UL13 modulate VP11/12 phosphorylation, virion packaging, and phosphatidylinositol 3-kinase/Akt signaling activity. J Virol. 2014;88:7379–7388. doi: 10.1128/JVI.00712-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstathiou S, Minson AC, Field HJ, Anderson JR, Wildy P. Detection of herpes simplex virus-specific DNA sequences in latently infected mice and in humans. J Virol. 1986;57:446–455. doi: 10.1128/jvi.57.2.446-455.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshleman E, Shahzad A, Cohrs RJ. Varicella zoster virus latency. Future Virol. 2011;6:341–355. doi: 10.2217/fvl.10.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Ohtani F, Fukuda S, Inuyama Y, Nagashima K. Reactivation of varicella-zoster virus in delayed facial palsy after dental treatment and orofacial surgery. J Med Virol. 2000;62:42–45. [PubMed] [Google Scholar]
- Gershon AA, Chen J, Davis L, Krinsky C, Cowles R, Reichard R, Gershon M. Latency of varicella zoster virus in dorsal root, cranial, and enteric ganglia in vaccinated children. Trans Am Clin Climatol Assoc. 2012;123:17–33. [PMC free article] [PubMed] [Google Scholar]
- Gershon AA, Breuer J, Cohen JI, Cohrs RJ, Gershon MD, Gilden D, Grose C, Hambleton S, Kennedy PG, Oxman MN, Seward JF, Yamanishi K. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;150(16):1–18. doi: 10.1038/nrdp.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden D. Varicella-zoster virus infections. Continuum (Minneapolis, MN) 2015;21:1692–1703. doi: 10.1212/CON.0000000000000246. [DOI] [PubMed] [Google Scholar]
- Gilden DH, Vafai A, Shtram Y, Becker Y, Devlin M, Wellish M. Varicella-zoster virus DNA in human sensory ganglia. Nature. 1983;306:478–480. doi: 10.1038/306478a0. [DOI] [PubMed] [Google Scholar]
- Gilden DH, Rozenman Y, Murray R, Devlin M, Vafai A. Detection of varicella zoster virus nucleic acid in neurons of normal human thoracic ganglia. Ann Neurol. 1987;22:377–280. doi: 10.1002/ana.410220315. [DOI] [PubMed] [Google Scholar]
- Gluska S, Chein M, Rotem N, Ionescu A, Perlson E. Tracking Quantum-Dot labeled neurotropic factors transport along primary neuronal axons in compartmental microfluidic chambers. Methods Cell Biol. 2016;131:365–387. doi: 10.1016/bs.mcb.2015.06.016. [DOI] [PubMed] [Google Scholar]
- Grose C, Brunel PA. Varicella-zoster virus: isolation and propagation in human melanoma cells at 36 and 32 degrees C. Infect Immun. 1978;19:199–203. doi: 10.1128/iai.19.1.199-203.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PG, Grinfeld E, Gow JW. Latent varicella-zoster virus is located predominantly in neurons in human trigeminal ganglia. Proc Natl Acad Sci U S A. 1998;95:4658–4662. doi: 10.1073/pnas.95.8.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PG, Grinfeld E, Gow JW. Latent Varicella-zoster virus in human dorsal root ganglia. Virology. 1999;258:451–454. doi: 10.1006/viro.1999.9745. [DOI] [PubMed] [Google Scholar]
- Kennedy PG, Rovnak J, Badani H, Cohrs RJ. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96:1581–1602. doi: 10.1099/vir.0.000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Mandarino A, Chao MV, Mohr I, Wilson AC. Transient reversal of episome silencing precedes VP16-dependent transcription during reactivation of latent HSV-1 in neurons. PLoS Pathog. 2012;8:e1002540. doi: 10.1371/journal.ppat.1002540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Wilson AC, Chao MV, Mohr I. Control of viral latency in neurons by axonal mTOR signaling and the 4E-BP translation repressor. Genes Dev. 2012;26:1527–1532. doi: 10.1101/gad.190157.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguardia JJ, Cohrs RJ, Gilden DH. Numbers of neurons and non-neuronal cells in human trigeminal ganglia. Neurol Res. 2000;22:565–566. doi: 10.1080/01616412.2000.11740719. [DOI] [PubMed] [Google Scholar]
- Lewis ME, Warren KG, Jeffrey VM, Shnitka TK. Factors affecting recovery of latent herpes simplex virus from human trigeminal ganglia. Can J Microbiol. 1982;28:123–129. doi: 10.1139/m82-013. [DOI] [PubMed] [Google Scholar]
- Liu X, Cohen JI. Varicella-zoster virus ORF12 protein activates the phosphatidylinositol 3-kinase/Akt pathway to regulate cell cycle progression. J Virol. 2013;87:1842–1848. doi: 10.1128/JVI.02395-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam R, Wellish MC, Dueland AN, Cohrs RJ, Gilden DH. Localization of herpes simplex virus and varicella zoster virus DNA in human ganglia. Ann Neurol. 1992;31:444–448. doi: 10.1002/ana.410310417. [DOI] [PubMed] [Google Scholar]
- Mahalingam R, Wellish M, Lederer D, Forghani B, Cohrs R, Gilden D. Quantitation of latent varicella-zoster virus DNA in human trigeminal ganglia by polymerase chain reaction. J Virol. 1993;67:2381–2384. doi: 10.1128/jvi.67.4.2381-2384.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus A, Lebenthal-Loinger I, Yang IH, Kinchington PR, Goldstein RS. An in vitro model of latency and reactivation of varicella zoster virus in human stem cell-derived neurons. PLoS Pathog. 2015;11:e1004885. doi: 10.1371/journal.ppat.1004885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel MA, Rempel A, Huntington J, Kim F, Choe A, Gilden D. Frequency and abundance of alphaherpesvirus DNA in human thoracic sympathetic ganglia. J Virol. 2014;88:8189–8192. doi: 10.1128/JVI.01070-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouwendijk WJD, Choe A, Nagel A, Gilden D, Osterhaus ADME, Cohrs RJ, Verjans GMGM. Restricted varicella zoster virus transcription in human trigeminal ganglia obtained soon after death. J Virol. 2012;86:10203–10206. doi: 10.1128/JVI.01331-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin SA, Stein S, Snyder M, Immesoete P. Attempts to recover varicella virus from ganglia. Ann Neurol. 1977;2:249. doi: 10.1002/ana.410020313. [DOI] [PubMed] [Google Scholar]
- Plummer G. Isolation of herpesviruses from trigeminal ganglia of man, monkeys, and cats. J Infect Dis. 1973;128:345–347. doi: 10.1093/infdis/128.3.345. [DOI] [PubMed] [Google Scholar]
- Roizman B, Zhou G, Du T. Checkpoints in productive and latent infections with herpes simplex virus 1: conceptualization of the issues. J Neurovirol. 2011;17:512–517. doi: 10.1007/s13365-011-0058-x. [DOI] [PubMed] [Google Scholar]
- Sadaoka T, Depledge DP, Rajbhandari L, Venkatesan A, Breuer J, Cohen JI. In vitro system using human neurons demonstrates that varicella-zoster vaccine virus is impaired for reactivation, but not latency. Proc Natl Acad Sci U S A. 2016;113:E2403–E2412. doi: 10.1073/pnas.1522575113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahzad A, Gilden D, Cohrs RJ. Translational medicine and varicella zoster virus: need for disease modeling. New Horiz Transl Med. 2015;2:89–91. doi: 10.1016/j.nhtm.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu M, Du T, Zhou G, Roizman B. Role of activating transcription factor 3 in the synthesis of latency-associated transcript and maintenance of herpes simplex virus 1 in latent state in ganglia. Proc Natl Acad Sci U S A. 2015;112:E5420–E5426. doi: 10.1073/pnas.1515369112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner I, Kennedy PG, Pachner AR. The neurotropic herpes viruses: herpes simplex and varicella-zoster. Lancet Neurol. 2007;6:1015–1028. doi: 10.1016/S1474-4422(07)70267-3. [DOI] [PubMed] [Google Scholar]
- Stevens JG, Haarr L, Porter DD, Cook ML, Wagner EK. Prominence of the herpes simplex virus latency-associated transcript in trigeminal ganglia from seropositive humans. J Infect Dis. 1988;158:117–123. doi: 10.1093/infdis/158.1.117. [DOI] [PubMed] [Google Scholar]
- Theil D, Paripovic I, Derfuss T, Herberger S, Strupp M, Arbusow V, Brandt T. Dually infected (HSV-1/VZV) single neurons in human trigeminal ganglia. Ann Neurol. 2003;54:678–682. doi: 10.1002/ana.10746. [DOI] [PubMed] [Google Scholar]
- Tiwari V, Shukla D. Phosphoinositide 3 kinase signalling may affect multiple steps during herpes simplex virus type-1 entry. J Gen Virol. 2010;91:3002–3009. doi: 10.1099/vir.0.024166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- Vrabec JT. Delayed facial palsy after tympanomastoid surgery. Am J Otol. 1999;20:26–30. [PubMed] [Google Scholar]
- Wang K, Lau TY, Morales M, Mont EK, Straus SE. Laser-capture microdissection: refining estimates of the quantity and distribution of latent herpes simplex virus 1 and varicella-zoster virus DNA in human trigeminal ganglia at the single-cell level. J Virol. 2005;79:14079–14087. doi: 10.1128/JVI.79.22.14079-14087.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren KG, Devlin M, Gilden DH, Wroblewska Z, Brown SM, Subak-Sharpe J, Koprowski H. Isolation of herpes simplex virus from human trigeminal ganglia, including ganglia from one patient with multiple sclerosis. Lancet. 1977;2:637–639. doi: 10.1016/s0140-6736(77)92501-6. [DOI] [PubMed] [Google Scholar]
- Warren KG, Brown SM, Wroblewska Z, Gilden DH, Koprowski H, Subak-Sharpe J. Isolation of latent herpes ximplex virus from the superior cervical and vagus ganglions of humans. N Engl J Med. 1978;298:1068–1069. doi: 10.1056/NEJM197805112981907. [DOI] [PubMed] [Google Scholar]
- Wilcox CL, Johnson EM., Jr Characterization of nerve growth factor-dependent herpes simplex virus latency in neurons in vitro. J Virol. 1988;62:393–399. doi: 10.1128/jvi.62.2.393-399.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]