Abstract
Organochlorine (OC) pesticides are synthetic pesticides widely used all over the world. They belong to the group of chlorinated hydrocarbon derivatives, which have vast application in the chemical industry and in agriculture. These compounds are known for their high toxicity, slow degradation and bioaccumulation. Even though many of the compounds which belong to OC were banned in developed countries, the use of these agents has been rising. This concerns particularly abuse of these chemicals which is in practice across the continents. Though pesticides have been developed with the concept of target organism toxicity, often non-target species are affected badly by their application. The purpose of this review is to list the major classes of pesticides, to understand organochlorine pesticides based on their activity and persistence, and also to understand their biochemical toxicity.
Keywords: LD50, pesticide persistence, biochemical toxicity, organochlorine (OC) pesticides
Introduction
Pesticides are a group of chemicals used for the destruction of insects, weeds, fungi, bacteria, etc. They are generally called insecticides, fungicides, bactericides, herbicides or rodenticides. Most of the pesticides have the ability to destroy a wide variety of pests or weeds, but some are developed against specific pests or pathogens. Most of these chemicals are designed in such a way as to disturb the physiological activities of the target organism, leading to dysfunction and reduced vitality. Pesticide residues may constitute a significant source of contamination of environmental factors such as air, water and soil. This phenomenon could become a continuous threat to the co-existence of plant and animal communities of the ecosystem. Problems caused by pest lead to loss of about one third of the world’s agricultural production every year, and that despite the fact that pesticide consumption comes up to more than two million tons. In India, the loss amounts to more than Rs 6,000 crores annually, by contributing factors such as weeds (33%), diseases (26%), insects (20%), birds (10%), rodents, and others (11%). Every year the magnitude of the problem increases by the appearance of newer pests and diseases (Rajendran, 2003).
The greater use of pesticides for high agricultural production has led to increased pollution of environmental compartments – soil, water and air. The characteristics of pesticides, such as high lipophilicity, bioaccumulation, long half-life and potential of long range transport, have increased the chances of contaminating the air, water and soil, even after many years of application. A study by Pimentel (1995) showed that only a small percentage (0.3%) of applied pesticides goes into the target pest while 99.7% go somewhere else into the environment. Application of a wide variety of pesticides has been advised to increase the crop productivity in tropical countries where crop loss is severe due to high temperature and humidity, which are conductive to rapid multiplication of pests (Kannan et al., 1993; Lakshmi, 1993). According to a World Health Organisation study, 80% of all pesticides are used by developing countries (Veil, 1990). Due to lack of proper legislation, improper market regulations and ignorance shown by people, agricultural workers from developing countries are prone to experience high levels of agricultural chemicals, including 1990) pesticides (Smith & Jong, 2001). Among agriculturalists of developing countries, pesticide exposure is the primary occupational hazard (Wasseling et al., 2001; Konradsen et al., 2003; Coronado et al., 2004) which leads to health issues and environmental contamination associated with pesticide use (Mancini, 2005; Remor et al., 2009). Although farmers are considered to be the main risk group, formulators, loaders, mixers, production workers and agricultural farm workers are all extremely susceptible groups. The non-occupational hazards may be due to pollution of the ecosystem or habitat as a whole. An estimate shows that deaths and chronic diseases due to pesticide poisoning amounts to about one million per year worldwide (Environews, 1999).
The overuse or misuse of pesticides is contributing adversely to the environmental health as well as to ecosystem services. Pesticides are reported to affect many aquatic and terrestrial species. Life in aquatic ecosystems such as microorganisms, invertebrates, plants and fish are badly affected by pesticides (Liess et al., 2005; Grande et al., 1994; De Lorenzo et al., 2001; Castillo et al., 2006; Frankart et al., 2003). In the Indian situation, massive use of pesticides has started since the 1960s when the “Green Revolution” was initiated and maximum agrochemicals were used to achieve high agricultural production.
Classification of pesticides
Classification of pesticides is mainly based on:
Chemical nature (organochlorines, organophosphates, etc).
Application requirement (agriculture, public health, domestic).
Target organism or targeted use (insecticide, herbicide, fungicide, etc).
Classification of pesticides based on chemical nature is given in Table 1.
Table 1.
Classification of pesticides based on their chemical nature.
| No | Chemical Group | Chemical names |
| 1 | Organochlorines | DDT,DDD, Dicofol, Eldrin, Dieldrin, Chlorobenziate, Lindane, BHC, Methoxychloro Aldrin, Chlordane, Heptaclor, Endosufan, Isodrin, Isobenzan, Toxaphene, Chloro propylate |
| 2 | Organophosphates | Dimefox, Mipafox, Methyl Parathion, Ronnel, enitrothion, Bidrin, Phorate, Fenthion, caumphos, Abate, Dichlorovas, Diptrex, Phosphomidon, Demetox, Oxydemeton-methyl, Malathion, Dimethoate, Trichlorofan |
| 3 | Carbamates |
Methyl Carbaryl, Carbanolate, Prupoxur, Dimethan, Dimetilan, Isolan, Carbofuran, Pyrolan, Aminocarb, Aldicarb Thio Vernolate, Pebulate, Diallate, Monilate, Butylate, Cycloate, Trillate, Thiourea Dithio Methan, Thiram, Ferban, Amoban, Naban, Zineb, Maneb, Ziram Polyran, Dithane M- 45 |
| 4 | Pyrethroids | Allethrin, Bonthrin, Dimethrin, Tetramethrin, Ptrethrin, Cyclethrin Furethrin, Fenevelerate, Alphamethrin, Decamethrin, Cypermethrin |
| 5 | Phenyl amides |
Carbanilates Barban, Carbetamide, Chlororprofan, Prophan, Phenyl Urea, Fenuron, Monuron, Diuron,Flumeturon, Chloroxuron, Neburon, Bromuron Acylanalide Propanil, Solan, Dicryl, Karsil, Propachlor, Alachlor, Butachlor Toluidines Trifluralin, Dipropanil, Benefin, Oryzalin, Isopropanil, Nitralin Acetamide Diphenamid |
| 6 | Phenoxyalkonates | 2,4-D(2,4 Dichloro phenoxy acetic acid) 2,4 5 T(2,4 5 Trichloro Phenoxy acetic acid) Dichloroprop, Mecoprop, Erbin, Sesone |
| 7 | Trazines | Atrazine, Simazine, Ametryn, Atratone, Chlorazine, Cynazine, Cyprazine, Metribuzin, Propazine, Turbutryn, Simetryn |
| 8 | Benzoic acid | Dicamba, Dichlorobenil, Chloroambin, Tricamba, Neptalan, Bromoxynil |
| 9 | Phtalimides | Captan, Diflotan, Folpet |
| 10 | Dipyrids | Paraquat, Diaquat |
| 11 | Others | Pentachlorophenol, Floroacetate, Phenyl mercuric acetate, Ethyl mercuric Phosphate,Methyl mercuric chloride, Sodium arsenate, Calcium arsenate, Lead arsenate, Cacodylic acid, Aluminium phosphide, Zinc phosphide |
Organochlorines
Organochlorines (OC) are a group of chlorinated compounds widely used as pesticides. These chemicals belong to the class of persistent organic pollutants (POPs) with high persistence in the environment. OC insecticides were earlier successfully used in control of malaria and typhus, yet they are banned in most of the advanced countries (Aktar et al., 2009). The review statistics on the use of different pesticides shows that 40% of all pesticides used belong to the organochlorine class of chemicals (Gupta, 2004; FAO, 2005). Due to their low cost and the need against various pests, organochlorine insecticides such as DDT, hexachlorocyclohexane (HCH), aldrin and dieldrin are among the most widely used pesticides in developing countries of Asia (FAO, 2005; Gupta, 2004; Lallas, 2001).
Organophosphates
Organophosphates (OP) are esters of phosphoric acid. The OP group of pesticides asserts its effects through irreversible inactivation of the enzyme acteylcholinesterase, which is essential for nerve function in humans, insects and many other animals. OP samples degrade rapidly by hydrolysis on exposure to light, air and soil, however small amounts are detected in food and drinking water.
Carbamates
Carbamates are organic compounds derived from carbamic acid (NH2COOH). The functional group present in carbamate insecticides are carbamate esters. Their mechanism of action is by reversible inactivation of the enzyme acteylcholinesterase. Carbamates break down in the environment within weeks or months (Goel & Aggarwal, 2007).
Pyrethroides
Pyrethroides and pyrethrins are similar organic compounds isolated from the flowers of pyrethrums (Chrysanthemum Coccineum and C. cinerariaefolium). The insecticidal properties of pyrethrins are derived from ketoalcoholic esters of chrysanthemic and pyrethroic acids (Reigert and Roberts, 1999). Pyrethroides affect the sodium channels and lead to paralysis of the organism. Pyrethroides have a comparatively slight level of mammalian toxicity and have a fast biodegradation capacity. Exposure to very high levels of the compounds in air, food or water may cause giddiness, headache, vomiting, muscle twitching, low energy, convulsions and loss of consciousness (Goel & Aggarwal, 2007).
Phenylamides
Phenylamide fungicides are systemic compounds that show potent eradicative anti-fungal activity (Schwinn & Staub, 1987). When added to the soil, they enhance plant growth and yield; in addition, these fungicides affect the homeostastis of the soil system (Monkiedje & Spiteller, 2002). These chemicals affect nutrient cycling and enter the food chain, and have thus been reported to affect higher organisms including humans. They affect nucleic acids by inhibiting the activity of RNA polymerase I system. They are known to impact mitosis and cell division in target fungi (Chao et al., 2011)
Phenoxyalkonates
Phenoxyalkonates are a widely used family of herbicides. These pesticides are mainly used to control weeds in agriculture. Nearly all compounds of this group are degraded by microorganisms (Viltos, 1952).
Triazines
The compounds that fall under this category are herbicidal pesticides. They include desmetryne, chlorazine, atriazine, propazine, etc. These compounds are known to have potential use as insect chemosterilants. Higher concentrations of these herbicides were found to inhibit plant catabolism pathway (Evan et al., 2007).
Benzoic acid
Benzoic acid herbicides include dicamba, dichlobenil, chlorambin, bromoxynil, ioxynil and naptalam. Little information is available regarding their degradation by soil microbes. Ioxynil is found to precipitate in acid soils (Zaki et al., 1967).
Phthalimide
Phthalimides include three fungicides, captan, folpet and captafol which together represent the second most important group of organic fungicides used in American agriculture. They represent about half the usage of the dithiocarbamates (NAS, 1975). The fungicides difolatan, captan and folpet react with thiols such as cysteine and glutathione at acidic pH levels of 4.0 to 5.0.
Dipyrids
The dipyridyl herbicides include paraquat and diquat. They are strongly adsorbed as organic cations in the soil (Funderburk, 1969; Funderburk & Bozarth, 1967). Microorganisms metabolize paraquat as the main source of nitrogen (Baldwin et al., 1966).
Others
There are many more pesticides used in agricultural practice. Heavy metals have found vast use as pesticides. Elements like iron, lead, sulphur, arsenic, mercury, zinc, tin, etc. have been used in inorganic or organic metal form. Methyl mercuric chloride, sodium arsenate, calcium arsenate, zinc phosphide are some of the compounds that fall under this category. Table 1 gives a comprehensive classification of pesticides based on their chemical nature. Among the various classes of pesticides, organochlorines and organophosphates are widely used. Organochlorines are known for their high persistence and toxicity characteristics. These pesticides cause neurological damage, endocrine disorders, and have acute and chronic health effects. Hence contamination of the environment with organochlorine pesticides drastically affects the ecosystem.
Organochlorine pesticides – chemistry, persistence and hazard classification
The basic characteristics of organochlorine pesticides are high persistence, low polarity, low aqueous solubility and high lipid solubility. Organochlorine pesticides can enter the environment after pesticide applications, polluted wastes discarded into landfills, and discharges from industrial units that synthesize these chemicals. They are volatile and stable; some can adhere to the soil and air, thus increasing the chances of high persistence in the environment, and are identified as agents of chronic exposure to animals and humans. Table 2 provides a comprehensive summary of major organochlorine pesticides with their chemical name, structure, toxicity, use and persistence in environmental medium.
Table 2.
Major organochlorine pesticides, their chemical structures, toxicity, use and persistence.
| No. | Chemical name | Structure | Toxicity LD50 | Use | Persistence in environment | WHO classification based on rat oral LD50 |
|---|---|---|---|---|---|---|
| 1 | Dichlorodiphenyltrichloroethane (DDT) C14H9Cl5 | 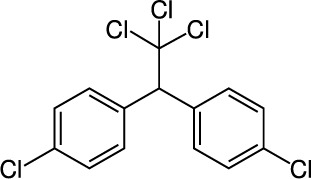 |
Rat Oral: 113–130 mg/kg Dermal: 2510 mg/kg Mice Oral: 150–300 mg/kg Gunia Pigs Oral: 300 mg/kg Rabbit Oral: 400 mg/kg |
Acaricide Insecticide | High Persistence Half life: 2–15 years | Moderately hazardous |
| 2 | 1,1-dichloro-2,2bis (p-chlorophenyl)ethane (DDD) | 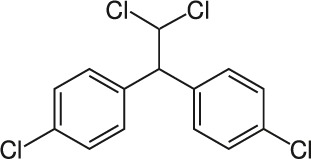 |
Rat Oral: 4000 mg/kg |
Insecticide | High Persistence Half life: 5–10 years | Acute hazard is unlikely |
| 3 | Dichloro diphenyl dichloroethane (DDE) | 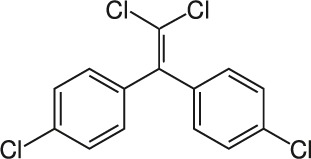 |
Rat Oral: 800–1240 mg/kg |
Insecticide | High Persistence Half life: 10 years | Slightly hazardous |
| 4 | Dicofol C14H9Cl5O | 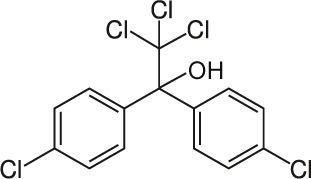 |
Rat Oral: 684–1495 mg/kg Rabbit Oral: 1810 mg/kg Dermal: 2.1 g/kg |
Acaricide | Moderate persistence Half life: 60 days | Moderately hazardous |
| 5 | Endrin C12H8Cl6O | 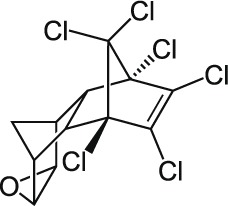 |
Rat Oral: 3 mg/kg Dermal: 15 mg/kg Mouse Oral: 1.37g/kg Intravenous: 2300 g/kg Goat Oral: 50 mg/kg Rabbit Oral: 60–94 mg/kg |
Avicide insecticide |
Moderate Persistence Half life: 1Day to 12 Years | Highly hazardous |
| 6 | Dieldrin C12H8Cl6O | 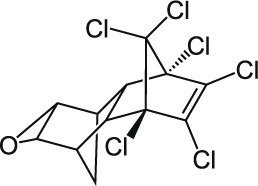 |
Rat Oral: 46 mg/kg Dermal: 50–120 mg/kg Mouse Oral: 38–77 mg/kg Dog Oral: 56–120 mg/kg Rabbit Oral: 45–50 mg/kg Cow Oral: 25 mg/kg Duck Oral: 381 mg/kg |
Insecticide | High Persistence Half life: 9 months | Highly hazardous |
| 7 | Methoxychlor C16H15Cl3O2 | 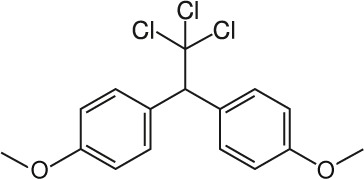 |
Rat Oral: 5000–6000 mg/kg Mice Oral: 2000 mg/kg Monkey Oral: 2500 mg/kg |
Insecticide | High Persistence Half life:< 120 Days | Acute hazard is unlikely |
| 8 | Chlordane C10H6Cl8 | 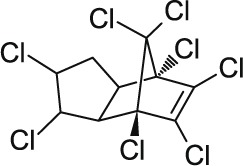 |
Rat Oral: 200 to 700 mg/kg Dermal: 530–690 mg/kg Mice Oral: 145–430 mg/kg Dermal: 153 mg/kg Rabbit Dermal: 780 mg/kg |
Insecticide | High Persistence Half life: 10 years | Moderately hazardous |
| 9 | Heptachlor C10H5Cl7 | 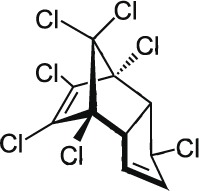 |
Rat Oral: 40–220 mg/kg Dermal: 119–320 mg/kg Mouse Oral: 30–68 mg/kg Guinea pigs Oral: 116 mg/kg Dermal: 1000 mg/kg Rabbit Dermal: 2000 mg/kg |
Insecticide | High Persistence Half life: 2 years | Highly – Moderately hazardous |
| 10 | Lindane C6H6Cl6 | 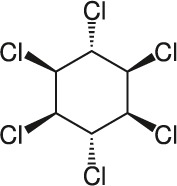 |
Rat Oral: 88 – 270 mg/kg Mouse Oral: 59–246 mg/kg |
Acaricide Insecticide Rodenticide |
High Persistence Half life: 15 months | Moderately hazardous |
| 11 | Endosulfan C9H6Cl6O3S | 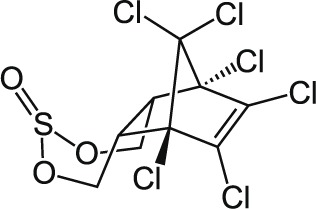 |
Rat Oral: 18 to 220 mg/kg Dermal: 74 mg/kg Rabbits Dermal: 200–359 mg/kg Ducks Oral: 33 mg/kg |
Insecticide | Moderate Persistence Half life Alpha Isomer:35days Beta Isomer:150days |
Highly hazardous |
| 12 | Isodrin C12H8Cl6 | 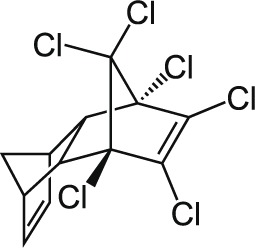 |
Rat Oral: 8.8 mg/kg |
Insecticide | High Persistence Half life: 0.5–6 years | Highly hazardous |
| 13 | Isobenzan C9H4Cl8O | 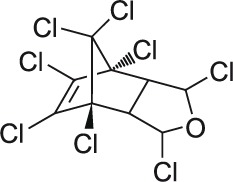 |
Rat Oral: 4.8 mg/kg Rabbit Dermal: 12 mg/kg Mouse Oral: 8.4 mg/kg |
Insecticide | High Persistence Half life: 2.8 years | Highly hazardous |
| 14 | Chloropropylate C17H16Cl2O3 | 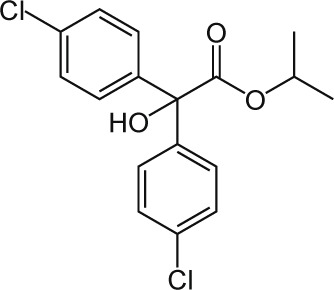 |
Rat Oral: 5000 mg/kg Birds Oral: 2500 mg/kg Rabbit Oral: 10200 mg/kg |
Insecticide Acaricide |
Moderate Persistence Half life: 50 days | Acute hazard is unlikely |
| 15 | Aldrin C12H8Cl6 | 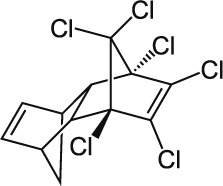 |
Oral: 39 to 60 mg/kg Dermal: 100 mg/kg Mouse Oral: 44 mg/kg Dog Oral: 65–95 mg/kg |
Insecticide | Moderate Persistence Half life: 4–7 years | Highly hazardous |
| 16 | 1,4-dichlorobenzene C6H4Cl2 | 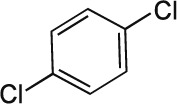 |
Rat Oral: 1516–2138 mg/kg |
Moderate Persistence Half life: < 50 days | Moderately hazardous | |
| 17 | Benzene hexachloride (BHC) C6H6Cl6 | 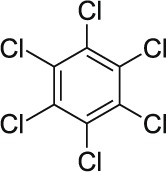 |
Rat Oral: 10,000 mg/kg Guinea pigs Oral: < 3000 mg/kg Rat Oral: 4000 mg/kg |
Acaricide Insecticide Rodenticide |
High Persistence Half life: 3 – 6 years | Acute hazard is unlikely |
| 18 | Mirex C10Cl12 | 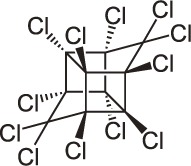 |
Rat Oral: 600–740 mg/kg |
Insecticide | High Persistence Half life: 10 years | Acute hazard is unlikely |
| 19 | Pentachlorophenol C6Cl5OH | 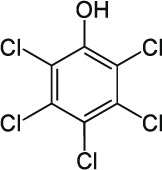 |
Rat Oral: 27–211 mg/kg Dermal: 96–330 mg/kg Mice Oral: 74–130 mg/kg Rabbit Oral: 70–300 mg/kg Dermal: < 100 mg/kg |
Fungicide Herbicide Insecticide |
Moderate Persistence Half life: 45 days | Highly – Moderately hazardous |
| 20 | Toxaphene (Camphechlor) C10H10Cl8 | 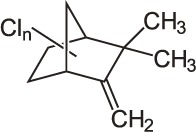 |
Rat Oral: 80–293 mg/kg Dogs: 25 mg/kg |
Acaricide Insecticide |
Moderate Persistence Half life 11 Years | Slightly hazardous |
They have a related chemical structure, showing chlorine substituted aliphatic or aromatic rings. Due to their structural resemblances, these compounds share certain physicochemical characteristics such as persistence, bioaccumulation and toxicity. One basic character that they share across the spectrum is persistence, where persistence is defined as half-life greater than two months in water or six months in soil sediment. The persistence of OC compounds varies from moderate persistence with half-life of approximately 60 days to high persistence with half-life up to 10–15 years. The most commonly used pesticide in agricultural practice is dichlorodiphenyltrichloroethane (DDT), which is moderately hazardous, with high persistence and a half-life of 2–15 years (Augustijn-Beckers et al., 1994). The use of DDT is now banned in many countries but it is illegally used in most of the developing countries. This applies also to endosulphan, an insecticide which is highly hazardous and has moderate persistence with a half-life of fifty days and is used in the production of cashew (Quijano, 2002).
Due to the high persistence and bioaccumulation potential, the Stockholm Convention has classified most of the OC compounds as environmental hazards and banned the use of many of them. However in many developing countries they are still in use making the ban ineffective.
Biochemical toxicity of organochlorines
Organochlorine toxicity is mainly due to stimulation of the central nervous system (Table 3). Cyclodines, such as the GABA antagonists endosulphan and lindane, inhibit the calcium ion influx and Ca- and Mg-ATPase causing release of neurotransmittors (Mathew, 2012). Epidemiological studies have exposed the etiological relationship between Parkinson’s disease and organochlorine pollutants.
Table 3.
Biochemical effects of major organochlorine pesticides.
| Sl.No | Chemical name | Organism | Biochemical effects | References |
|---|---|---|---|---|
| 1 | Aldrin and Dieldrin | Human | Neurotoxic, reproductive, developmental,immunological, genotoxic, tumerogenic effects, nausea, vomiting, muscle twitching and aplastic anemia | USEPA, 2003 |
| Mouse, rat, guniea pig, rabbit and dog | Convulsions, loss in body weight, depression, increased irritability, salivation, hyperexitability, prostration and death | |||
| 2 | Chlordane | Human | Convulsions, tremor, mental confusion and incoordination | ATSDR, 1997 |
| Mice | Reduced fertility, liver cancer | |||
| Seals | Cancer, trauma, meningocephalitis | Kajiwara et al., 2000 | ||
| 3 | BHC/ DDE | Human | Cyst in hands, itching, psoriasis, eczema, leucoderma, skin rashes | Subramaniam & Solomon, 2006 |
| 4 | DDT | Human | Prickling sensation of the mouth, nausea, dizziness, confusion, headache, lethargy, incoordination, vomiting, fatigue, tremors in the extremities, anorexia, anemia, muscular weakness, hyperexcitability, anxiety, and nervous tension | Klaassen et al., 1996 |
| Mice | Liver tumors, liver changes including hepatocellular hypertrophy, margination and formation of lipospheres. | WHO, 1979 | ||
| Birds | Egg shell thinning | USEPA, 1975 | ||
| Fish | Affects membrane function and enzymes | |||
| Salmons | Impaired behavioral development | |||
| 5 | Diazion | Rats | Neurotoxicity | USEPA, 2000 Peterson & Talcot, 2006 |
| Reptiles, fishes and Mammals | Lacrimation, salivation, anorexia, bradycardia, abdominal pain, hyperactivity, anxiety, depression and vomiting | |||
| Birds | Wing spasms, wing drop, hunched back, tenesmus, diarrhea, ptosis of eyelid, prostration, opisthotonos-like seizures or wing-beat convulsions. | |||
| Human | Dark or blurred vision, anxiety and restlessness, as well as psychiatric symptoms such as depression, memory loss, and confusion and acute pancreatitis. | Reigert & Roberts, 1999 Wagner, 1997 USEPA, 2000 |
||
| 6 | Dicofol | Rats | Decrease in body weight and acute neurotoxicity | Phang et al., 1996 |
| Dogs | Inhibition of ACTH (Adrenal cortical tropic hormone) | |||
| 7 | Endosulfan | Human | Decreases the white blood cell count and macrophage migration, adverse effects on humoral and cell-mediated immune system. Affects semen quality, sperm count, spermatogonial cells, sperm morphology and other defects in male sex hormones DNA damage and mutation | Pandey et al., 1990 Susan & Sania, 1999 Singh et al., 2007 |
| Rats | Immunosuppression, neurological disorders, congenital birth defects, chromosomal abnormalities, mental retardation, impaired learning and memory loss and glomerulonephritis | Stockholm Convention, 2009 | ||
| 8 | Lindane | Human | Damage human liver, kidney, neural and immune systems, and induces birth defects cancer, cause neurotoxicity, reproductive toxicity and hepatotoxicity | Sahoo et al., 2008, Bano & Bhatt, 2010 Vijaya Padma et al., 2011 |
| Rats | Alters gene expression of liver and hepatotoxicity | Sumida et al., 2007 Videla et al., 2004 |
||
| 9 | Methoxychlor | Sea Urchins | Fertilization and early development of eggs | Pesando et al., 2004 |
| Rats | Reduced fertility | Cummings & Gray, 1989 | ||
| 10 | Polychlorinated Biphenyls (PCB) | Human | Neurological disorders and short term memory | |
| Fishes, rats, monkeys and mice | Cancer, Hodgkins lymphoma, decreased birth weight and decreased size of thymus gland | Jacobson & Jacobson, 1996 USEPA, 1996 | ||
| 11 | Pentachlorophenol | Human | Inflammation of the upper respiratory tract and bronchitis, blood effects such as aplastic anemia, effects on the kidney and liver, immunological effects, and irritation of the eyes, nose, and skin | ATSDR, 1999 |
| Rats and Mice | Effects the cardiovascular system, blood, liver, immune system, and central nervous system (CNS) |
Effect in humans
Examination of effects of different classes of pesticides leads to the conclusion that many of them are responsible for hypertension, cardiovascular disorders and other health related problems in humans. Organochlorines act as endocrine disrupting chemicals (EDCs) by interfering with molecular circuitry and function of the endocrine system (Sohail et al., 2004). Farm workers, their families and those who pass through a region applied with pesticides can absorb a measurable quantity of pesticides. The presence of pesticide residues has been detected in blood plasma of workers in agricultural farms. Direct or indirect exposure to pesticides leads to neuromuscular disorders and stimulation of drug and steroid metabolism (Subramaniam and Solomon, 2006).
Another mode of exposure to these pesticides is through diet. Among food items, fatty food such as meat, fish, poultry, and dairy products serve as main causes (Rusiecki et al., 2008). Many of the organochlorine molecules are carcinogens and neurotoxic (Kaiser, 2000). The hazardous nature of organochlorines was explained by citing different examples. The menace caused by endosulfan is of great concern. Endosulfan remains in the environment for longer periods and bio-accumulates in plants and animals which leads to contamination of food consumed by humans (Briz et al., 2011). It affects mainly the central nervous system and was found to have higher acute inhalation toxicity than dermal toxicity. Gastrointestinal absorption of endosulfan is very high (USEPA, 2010).
Disproportion of thyroid hormones can lead to a variety of disorders. Serum concentrations of p-p’-DDE and HCB were found to be associated with abnormal thyroid hormone levels. p,p’-DDE was reported to increase free thyroxine (T4) and total triiodothyronine (T3) levels, and to be inversely associated with thyroid-stimulating hormone (TSH) (Meeker et al., 2007). On exposure to dioxinlike organochlorines, a dose-dependent decrease in total T4 was also reported (Turyk et al., 2006). Organochlorine pesticides were reported to increase the risk of hormone-related cancers including breast, prostate, stomach and lung cancer (Wolff et al., 1993). Recently dioxins have been found in human ovarian follicular fluid, which may lead to the development of endometriosis. Exposure to dioxins can cause several autoimmune diseases, including multiple sclerosis and eczema (Sinaii et al., 2002). Organochlorines can function as xenoestrogens and compounds such as TCDD, methoxychlor and alachlor were reported to exert effects on human and experimental animals due to inhibited synthesis and increased degradation of thyroid hormones.
Analysis of the National Health and Nutrition Examination Survey 1999–2004 studying the relation between organochlorine pesticides and prostate and breast cancers has shown that serum concentrations of b-HCH, trans-nonachlor, and dieldrin were significantly associated with prostate cancer prevalence (Xu et al., 2010). In children, exposure to dioxins showed significant positive associations with learning disability (LD) (Lee et al., 2007). Risk of attention deficit hyperactivity disorder (ADHD) at higher levels of p,p’-DDE and PCBs exposure was reported (Sagiv et al., 2010). Prenatal exposure to p,p’-DDE and its presence in cord serum was found to lead to disappearance of neuronal development after 12 months of infant age (Torres-Sánchez et al., 2009). Epidemiological studies have shown that exposure to persistent organic pollutants, mainly organochlorine pesticides, is strongly associated with type 2 diabetes. Some persistent organic pollutants, as highly chlorinated PCBs and trans-nonachlor, were associated with the incidence of type 2 diabetes in obese people (Lee et al., 2006).
Selected persistent organic pollutants are reported to induce divergent actions on blood pressure, suggesting a chemical structure based association of pesticides (Henríquez-Hernández et al., 2014). In a population based study, different persistent organic pollutants and pesticides were reported to be associated with liver dysfunction biomarkers such as bilirubin, ALT and ALP, suggesting that these environmental pollutants can cause adverse effects on liver functions (Kumar et al., 2014a). A study conducted in Costa Rica reported that occupational pesticide exposure to dialdrin could be partly responsible for the increased risk of Parkinson’s disease seen in the population (Steenland et al., 2014). Studies showed that the change of lipids over time, especially LDL-cholesterol, is linked to POP exposure (Penell et al., 2014). Increased oxidative stress markers in plasma were found to be associated with exposure of POPs and could be a causative agent for oxidative stress (Kumar et al., 2014b). Persistent organic pollutants were reported to influence the complement system, leading to activation of the immune system in humans (Kumar et al., 2014a). Detection of organochlorine pesticides from human breast milk was reported from many places in the world. In Croatia, p,p’-DDE was found to be the dominant organochlorine pesticide in human breast milk (Klinčić et al., 2014). Exposure of infants to chlordanes via breast milk was reported as a potential health risk in Korea (Lee et al., 2013). Another study from Korea also revealed the presence of organochlorine pesticides (OCPs) chlordanes, aldrin, dichlorodiphenyltrichloroethanes (DDTs), dieldrin, heptachlors, endrins, hexachlorocyclohexanes (HCHs), hexachlorobenzene (HCB), toxaphenes and mirex, in milk (Kim et al., 2013). Organochlorine pesticides HCB, β-HCH, pp’DDE, pp’DDT, pp’DDT, Σ-DDT were present in breast milk of the population in Guerrero, Mexico, proportionally to exposure (Chávez-Almazán et al., 2014).
A study conducted in China showed that prenatal exposure to DDT, β-BHC, HCB and mirex caused decrease in birth weight of infants (Guo et al., 2014). A number of studies were published on the effect of organochlorine pesticides on induction of diabetes mellitus in humans. A recent study reported that POP exposure is a risk factor contributing to insulin resistance (Arrebola et al., 2015). Chronic exposure to chlordecone was found to cause hypertensive disorders in pregnancy and gestational diabetes mellitus among French Caribbean women (Saunders et al., 2014). In a study conducted in Slovakia, highly increased blood levels of diabetes (fasting glucose and insulin) and obesity markers (BMI, triglyceride and cholesterol) were found in large groups of males and females in highly polluted areas. A significant decrease in testosterone level was also observed in males (Langer et al., 2014). Prevalence of type 2 diabetes and exposure to persistent organic pollutants has been established (Airaksinen et al., 2011). Recent studies on organochlorine pesticides have shown that β-HCH, HCB and DDT residues bio-accumulate in maternal and cord sera and from maternal blood they can be transferred through the placenta and affect thyroid hormone levels in the newborn (Li et al., 2014). OC pesticides have been suggested to affect the thyroid system through gender-specific mechanisms; the extent of the effect may differ among compounds (Freire et al., 2013). A report from Brazil had shown that OC compounds are reported to trigger anti-androgenic effects in men and estrogenic effects in women (Freire et al., 2014). OC pesticide heptachlor was reported to induce mitochondria-mediated cell death via impairing electron transport chain complex III, thus acting as a neurotoxicant with possible association with Parkinson’s disease (Hong et al., 2014)
Exposure to organochlorine pesticide residues was reported as a potential risk factor for gallstone disease in humans (Su et al., 2012). Potential neurotoxic effects of organochlorine compounds were reported on early psychomotor development even at low doses (Forns et al., 2012). A positive correlation was observed of exposure to some OC pesticides and vitamin D deficiency in humans (Yang et al., 2012). Early exposure to certain environmental chemicals, especially organochlorine compounds, with endocrine-disruption activity were reported to interfere with neonatal thyroid hormone status (Freire et al., 2011).
Toxic effect of pesticides in fauna
Wild birds are of great importance to the ecosystem. Decline in the bird community serves as an indicator of environmental pollution. Continuous use of pesticides is one of the major causes for the reduction of birds. In many cases the impact is not direct, however repetitive use of pesticides like DDT in soil is taken up by earthworms which are then ingested by birds and thus their accumulation may result in a large loss in bird population (Fry, 1995). Subsequent research has also identified other pesticides and industrial chemicals that cause mortality and reproductive impairment, which affects both embryos and adult birds. The effects on embryos include mortality or reduced hatchability, wasting syndrome and teratological effects that produce skeletal abnormalities and impaired differentiation of the reproductive and nervous systems through mechanisms of hormonal mimicking of estrogens. The range of chemical effects on adult birds covers acute mortality, sub-lethal stress, reduced fertility, suppression of egg formation, eggshell thinning and impaired incubation and chick rearing behaviors (Gilman et al., 1979). Pesticides cause extinction, behavioral changes, loss of safe habitat and population decline in several birds. Prolonged use of pesticides causes a drastic decrease in birds like the peregrine falcon, sparrow hawk and bald eagle (Mitra et al., 2011). The levels of organochlorines in seabird eggs were indicated by forming a deposit of pollutants in the body, thus serving as a useful indicator of environmental contamination (Pearce et al., 1989).
Toxic effect in farm animals
The prolonged use of pesticides in agriculture has caused serious health problems as these pesticides accumulate and affect the food chain. Organochlorine compounds are highly lipophilic and can accumulate in fat-rich food such as meat and milk (Hernandez et al., 1994). Pesticides are introduced into cattle mainly through fodder or contaminated water used for household and public purposes (Sabbah and Bouguerra 1997). Amphibians and insectivorous reptiles, like lizards, have an important function in linking invertebrates with vertebrates in the food chain. They serve as a food source for some organisms and are also a means by which chemical residues, especially residues of organochlorine pesticides taken in with contaminated prey, can enter food chains. Amphibians consume these pesticides by a number of ways, including inhalation, contact and through ingestion. Amphibians in open water bodies may also be exposed to pesticides due to run-off from adjacent agricultural land on which chemicals are used to control crop pests. Continuous exposure of honey bees to pesticides affects the quality of honey. The routes of honey contamination with pesticides are direct and indirect. The direct is treatment of beehives with pesticides (Tsipi et al., 1999). Wild animals, including the grasscutter (Thryonomys swinderianus), which are a good source of protein, are seriously affected by the use of pesticides. Grasscutters are a source of food for the people of Ghana in Africa (Sarah et al., 2011). As pesticides have high effect on the animal and bird community, ultimately humans also take up pesticides as meat, milk and crops derived from these animals and plants are consumed by humans.
Conclusion
The use of pesticides in order to improve agriculture has not only affected the crop, it has also altered the food chain and the ecosystem. These chemicals not only affect the crop, animals and birds in a specific area but also badly affect the ecosystem balance. Pesticides are causes of high morbidity and mortality. Hence the use of chemical pesticides should be controlled and more use of bio-pesticides should be employed. Many alternatives are available to reduce the effects of pesticides on the environment. Alternatives include manual removal, applying heat, covering weeds with plastic, placing traps and lures, removing pest breeding sites, maintaining healthy soils that breed healthy and more resistant plants, cropping native species that are naturally more resistant to native pests and supporting bio-control agents such as birds and other pest predators. Consumer awareness should be brought up among people in concern with the long-term harm caused by pesticides.
Acknowledgements
The authors thank Dr. B.S. Corrie, Director, KFRI and Kerala State Council for Science, Technology and Environment (KSCSTE), Govt. of Kerala, India for providing necessary facilities and encouragement
REFERENCES
- Agency for Toxic Substances and Disease Registry – Chlordane. In ATS-DRs Toxicological Profiles on CD-ROM [CD-ROM] Boca Raton, FL: Lewis Publishers; 1997. 1997. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Pentachlorophenol (Update) (Draft) Atlanta, GA: Public Health Service, U.S. Department of Health and Human Services; 1999. [Google Scholar]
- Airaksinen R, Rantakokko P, Eriksson JG, Blomstedt P, Kajantie E, Kiviranta H. Association between type 2 diabetes and exposure to persistent organic pollutants. Diabetes Care. 2011;34(9):1972–1979. doi: 10.2337/dc10-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aktar MW, Sengupta D, Chowdhury A. Impact of pesticides use in agriculture: their benefits and hazards. Interdisciplinary Toxicology. 2009;2(1):1–12. doi: 10.2478/v10102-009-0001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrebola JP, González-Jiménez A, Fornieles-González C, Artacho-Cordón F, Olea N, Escobar-Jiménez F, Fernández-Soto ML. Relationship between serum concentrations of persistent organic pollutants and markers of insulin resistance in a cohort of women with a history of gestational diabetes mellitus. Environmental Research. 2015;136:435–440. doi: 10.1016/j.envres.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Augustijn-Beckers PW, Hornsby AG, Wauchope RD. Additional Properties Reviews of Environmental Contamination and Toxicology. SCS/ARS/CES Pesticide Properties Database for Environmental Decisionmaking II. 1994:137. [PubMed] [Google Scholar]
- Baldwin BC, Bray MF, Geoghegan MJ. the microbial decomposition of paraquat. Biochemical Journal. 1966;101:15. [Google Scholar]
- Bano M, Bhatt DK. Ameliorative effect of a combination of vitamin-E, vitamin-C α-lipoic acid and stilbene resveratrol on lindane induced toxicity in mice olfactory lobe and cerebrum. Indian Journal of Experimental Biology. 2010;8:48–150. [PubMed] [Google Scholar]
- Briz V, Molina-Molina JM, Sánchez-Redondo S, Fernández MF, Grimalt JO, Olea N, Rodríguez-Farré E, Suñol C. Differential estrogenic effects of the persistent organochlorine pesticides dieldrin, endosulfan and lindane in primary neuronal cultures. Toxicological Sciences. 2011;120(2):413–27. doi: 10.1093/toxsci/kfr019. [DOI] [PubMed] [Google Scholar]
- Castillo LE, Martinez E, Ruepert C, Savage C, Gilek M, Pinnock M, Solis E. Water quality and macroinvertebrate community response following pesticide applications in a banana plantation, Limon, Costa Rica. Science of the Total Environment. 2006;367:418–32. doi: 10.1016/j.scitotenv.2006.02.052. [DOI] [PubMed] [Google Scholar]
- Chao Y, Hamel C, Vujanovic V, Gan Y. Fungicide: Mode of action and possible impact on non-target microorganisms. IRSN Ecology. 2011. Article ID 130239. http://dx.doi.org/10.5402/2011/130289.
- Chávez-Almazán LA, Diaz-Ortiz J, Alarcón-Romero M, Dávila-Vazquez G, Saldarriaga-Noreña H, Waliszewski SM. Organochlorine pesticide levels in breast milk in Guerrero, Mexico. Bulletin of Environmental Contamination and Toxicology. 2014;93(3):294–298. doi: 10.1007/s00128-014-1308-4. [DOI] [PubMed] [Google Scholar]
- Coronado GD, Thompson B, Strong L, Griffith WC, Islas I. Agricultural task and exposure to organophosphate pesticides among farmworkers. Environmental Health Perspectives. 2004;112:142–147. doi: 10.1289/ehp.6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings AM, Gray LE., Jr Antifertility effect of methoxychlor in female rats: dose- and time-dependent blockade of pregnancy. Toxicology and Applied Pharmacology. 1989;97(3):454–62. doi: 10.1016/0041-008x(89)90250-0. [DOI] [PubMed] [Google Scholar]
- De Lorenzo ME, Scott GI, Ross PE. Toxicity of pesticides to aquatic microorganisms: a review. Environmental Toxicology and Chemistry. 2001;20:84–98. doi: 10.1897/1551-5028(2001)020<0084:toptam>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Environews Forum. Killer environment. Environmental Health Perspectives. 1999;107:A62. doi: 10.1289/ehp.107-1566330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan H, Matt B, Kate J. The Fate and Effect of Gyphosphate on Amphibians. Final Report ENAC. 2007:202. [Google Scholar]
- FAO. Proceedings of the Asia Regional Workshop. Bangkok: Regional Office for Asia and the Pacific; 2005. [Google Scholar]
- Forns J, Lertxundi N, Aranbarri A, Murcia M, Gascon M, Martinez D, Grellier J, Lertxundi A, Julvez J, Fano E, Goñi F, Grimalt JO, Ballester F, Sunyer J, Ibarluzea J. Prenatal exposure to organochlorine compounds and neuropsychological development up to two years of life. Environment International. 2012;45:72–77. doi: 10.1016/j.envint.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Frankart C, Eullaffroy P, Vernet G. Comparative effects of four herbicides on non-photochemical fluorescence quenching in Lemna minor. Environmental and Experimental Botany. 2003;49:159–68. [Google Scholar]
- Freire C, Lopez-Espinosa MJ, Fernández M, Molina-Molina JM, Prada R, Olea N. Prenatal exposure to organochlorine pesticides and TSH status in newborns from Southern Spain. Science of the Total Environment. 2011;409(18):3281–3287. doi: 10.1016/j.scitotenv.2011.05.037. [DOI] [PubMed] [Google Scholar]
- Freire C, Koifman RJ, Sarcinelli PN, Simões Rosa AC, Clapauch R, Koifman S. Long-term exposure to organochlorine pesticides and thyroid status in adults in a heavily contaminated area in Brazil. Environmental Research. 2013;127:7–15. doi: 10.1016/j.envres.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Freire C, Koifman RJ, Sarcinelli PN, Rosa AC, Clapauch R, Koifman S. Association between serum levels of organochlorine pesticides and sex hormones in adults living in a heavily contaminated area in Brazil. International Journal of Hygiene and Environmental Health. 2014;217(2–3):370–378. doi: 10.1016/j.ijheh.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Fry DM. Reproductive effects in birds exposed to pesticides and industrial chemicals. Environmental Health Perspectives. 1995;103(Suppl 7):165–171. doi: 10.1289/ehp.95103s7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderburk HH. Diquit and paraquat. In: Kearney PC, Kaufman DD, editors. Degradation of herbicides. New York: Marcel Dekker; 1969. pp. 283–298. [Google Scholar]
- Funderburk HH, Jr, Bozarth GA. Review of the metabolism and decmpostion of diquat and paraquat. Journal of Agricultural and Food Chemistry. 1967;15:563–567. [Google Scholar]
- Gilman AP, Peakall DB, Hallett DJ, Fox GA, Norstrom RJ. Herring gulls (Larus argentatus) as monitors of contamination in the Great lakes. In: Peter F, Timmins P, Perry D, editors. Animals as monitors of environmental pollutants. Proceedings of the symposium on pathobiology of environmental pollutants: Animal models and wildlife as monitors. Washington DC: National Academy of Sciences; 1979. pp. 280–289. [Google Scholar]
- Goel A, Aggarwal P. Pesticide Poisoning. The National Medical Journal of India. 2007;20:182–191. [PubMed] [Google Scholar]
- Grande M, Andersen S, Berge D. Effects of pesticides on fish. Norwegian Journal of Agricultural Sciences. 1994;13:195–209. [Google Scholar]
- Guo H, Jin Y, Cheng Y, Leaderer B, Lin S, Holford TR, Qiu J, Zhang Y, Shi K, Zhu Y, Niu J, Bassig BA, Xu S, Zhang B, Li Y, Hu X, Chen Q, Zheng T. Prenatal exposure to organochlorine pesticides and infant birth weight in China. Chemosphere. 2014;110:1–7. doi: 10.1016/j.chemosphere.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PK. Pesticide exposure—Indian scene. Toxicology. 2004;198:83–90. doi: 10.1016/j.tox.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Henríquez-Hernández LA, Luzardo OP, Zumbado M, Camacho M, Serra-Majem L, Álvarez-León EE, Boada LD. Blood pressure in relation to contamination by polychlorobiphenyls and organochlorine pesticides: Results from a population-based study in the Canary Islands (Spain) Environmental Research. 2014;135:48–54. doi: 10.1016/j.envres.2014.05.036. [DOI] [PubMed] [Google Scholar]
- Hernández LM, Fernández MA, Jiménez B, Ma J, González, García JF. Organochlorine pollutants in meats and cow’s milk from Madrid (Spain) Bulletin of Environmental Contamination and Toxicology. 1994;52:246–253. doi: 10.1007/BF00198495. [DOI] [PubMed] [Google Scholar]
- Hong S, Hwang J, Kim JY, Shin KS, Kang SJ. Heptachlor induced nigral dopaminergic neuronal loss and Parkinsonism-like movement deficits in mice. Experimental & Molecular Medicine. 2014;46(2):e80. doi: 10.1038/emm.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Intellectual Impairment in Children Exposed to Polychlorinated Biphenyls in Utero. New England Journal of Medicine. 1996;335(11):783–789. doi: 10.1056/NEJM199609123351104. [DOI] [PubMed] [Google Scholar]
- Kaiser J. Endocrine disrupters: Panel cautiously confirms low-dose effects. Science. 2000;290:695–697. doi: 10.1126/science.290.5492.695. [DOI] [PubMed] [Google Scholar]
- Kannan K, Tanabe S, Borrell A, Aguilar A, Focardi S, Tatsukawa R. Isomer specific analysis and toxic evaluation of polychlorinated biphenyls in food stuffs from India and their implications on human dietary exposure. Journal of Agricultural Food Chemistry. 1993;40:158. [Google Scholar]
- Kajiwara N, Kannan K, Muraoka M, Watanabe M, Takahashi S, Gulland F, Olsen H, Blankenship AL, Jones PD, Tanabe S, Giesy JP. Organochlorine Pesticides, Polychlorinated Biphenyls, and Butyltin Compounds in Blubber and Livers of Stranded California Sea Lions, Elephant Seals, and Harbor Seals from Coastal California. Archives of Environmental Contamination and Toxicology. 2001;41(1):90–99. doi: 10.1007/s002440010224. [DOI] [PubMed] [Google Scholar]
- Kim D, Ryu HY, Lee JH, Lee JH, Lee YJ, Kim HK, Jang DD, Kim HS, Yoon HS. Organochlorine pesticides and polychlorinated biphenyls in Korean human milk: contamination levels and infant risk assessment. Journal of Environmental Science and Health, Part B. 2013;48(4):243–250. doi: 10.1080/03601234.2013.742413. [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Amdur MO, Doull J. The basic science of poisons. Fifth edition. New York: McGraw - Hill; 1996. Casarett & Doull’s toxicology. [Google Scholar]
- Klinčić D, Herceg Romanić S, Matek Sarić M, Grzunov J, Dukić B. Polychlorinated biphenyls and organochlorine pesticides in human milk samples from two regions in Croatia. Environmental Toxicology and Pharmacology. 2014;37(2):543–52. doi: 10.1016/j.etap.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Konradsen F, Van der Hoek, Cole W, Hutchinson DC, Daisley GH, Singh S, Eddleston M. Reducing acute poisoning in developing countries-options for restricting the availability of pesticides. Toxicology. 2003;192:249–261. doi: 10.1016/s0300-483x(03)00339-1. [DOI] [PubMed] [Google Scholar]
- Kumar J, Lind L, Salihovic S, van Bavel B, Ingelsson E, Lind PM. Persistent organic pollutants and liver dysfunction biomarkers in a population-based human sample of men and women. Environmental Research. 2014a;134:251–256. doi: 10.1016/j.envres.2014.07.023. [DOI] [PubMed] [Google Scholar]
- Kumar J, Monica Lind P, Salihovic S, van Bavel B, Lind L, Ingelsson E. Influence of persistent organic pollutants on oxidative stress in population-based samples. Chemosphere. 2014b;114:303–309. doi: 10.1016/j.chemosphere.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Lakshmi A. Pesticides in India: Risk assessment to aquatic ecosystem. Science of Environment. 1993;(1) [Google Scholar]
- Lallas P. Reproductive Effects in Birds Exposed to Pesticides and Industrial Chemicals. The Stockholm Convention on persistent organic pollutants. American Journal of International Law. 2001;95:692–708. [Google Scholar]
- Langer P, Ukropec J, Kocan A, Drobna B, Radikova Z, Huckova M, Imrich R, Gasperikova D, Klimes I, Trnovec T. Obesogenic and diabetogenic impact of high organochlorine levels (HCB, p,p’-DDE, PCBs) on inhabitants in the highly polluted Eastern Slovakia. Endocrine Regulations. 2014;48(1):17–24. doi: 10.4149/endo_2014_01_17. [DOI] [PubMed] [Google Scholar]
- Lee DH, Lee IK, Song K, Steffes M, Toscano W, Baker BA, Jacobs DR. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: Results from the National Health and Examination Survey, 1999–2002. Diabetes Care. 2006;29:1638–1644. doi: 10.2337/dc06-0543. [DOI] [PubMed] [Google Scholar]
- Lee D, Jacobs DR, Porta M. Association of serum concentrations of persistent organic pollutants with the prevalence of learning disability and attention deficit disorder. Journal of Epidemiology & Community Health. 2007;61:591–596. doi: 10.1136/jech.2006.054700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kim S, Lee HK, Lee IS, Park J, Kim HJ, Lee JJ, Choi G, Choi S, Kim S, Kim SY, Choi K, Kim S, Moon HB. Contamination of polychlorinated biphenyls and organochlorine pesticides in breast milk in Korea: time-course variation, influencing factors, and exposure assessment. Chemosphere. 2013;93(8):1578–1585. doi: 10.1016/j.chemosphere.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Li C, Cheng Y, Tang Q, Lin S, Li Y, Hu X, Nian J, Gu H, Lu Y, Tang H, Dai S, Zhang H, Jin C, Zhang H, Jin Y, Jin Y. The association between prenatal exposure to organochlorine pesticides and thyroid hormone levels in new borns in Yancheng, China. Environment International. 2014;129:47–51. doi: 10.1016/j.envres.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Liess M, Brown C, Dohmen P, Duquesne S, Heimbach F, Kreuger J. Effects of Pesticides in the Field—EPIF. Brussels, Belgium: SETAC Press; 2005. [Google Scholar]
- Mancini F. Acute pesticide poisoning among female and male cotton growers in India. International Journal of Occupational and Environmental Health. 2005;11:221–232. doi: 10.1179/107735205800246064. [DOI] [PubMed] [Google Scholar]
- Mathew LL. Organochloride Pesticide Toxicity. Drugs, Diseases and Procedures. Medscape References. 2012 [Google Scholar]
- Meeker JD, Altshul L, Hauser R. Serum PCBs, p,p’ -DDEand HCB predict thyroid hormone levels in men. Environmental Research. 2007;104:296–304. doi: 10.1016/j.envres.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A, Chatterjee C, Mandal FB. Synthetic Chemical Pesticides and Their Effects on Birds. Research Journal in Environmental Toxicology. 2011;5:81–96. [Google Scholar]
- Monkiedje A, Spiteller M. Effect of phenylamide fungicides, mefenoxam and metalaxyl on the microbiological properties of a sandy loam and a sandy clay soil. Biology and Fertility of Soils. 2002;35:393–398. [Google Scholar]
- National Academy of Sciences. Pest Control: An Assessment of Present and Alternative Technologies. Washington DC: 1975. 4 Volumes. [Google Scholar]
- Pandey N, Gundevia F, Prem AS, Ray PK. Studies on the genotoxicity of endosulfan, an organochlorine insecticide in mammalian germ cells. Mutation Research. 1990;242(1):1–7. doi: 10.1016/0165-1218(90)90093-h. [DOI] [PubMed] [Google Scholar]
- Pearce PA, Elliott JE, Peakall DB, Norstrom RJ. Organochlorine contaminants in eggs of seabirds in the northwest Atlantic, 1968–1984. Environmental Pollution. 1989;56:217–235. doi: 10.1016/0269-7491(89)90039-0. [DOI] [PubMed] [Google Scholar]
- Penell J, Lind L, Salihovic S, van Bavel B, Lind PM. Persistent organic pollutants are related to the change in circulating lipid levels during a 5 year follow-up. Environmental Research. 2014;134:190–197. doi: 10.1016/j.envres.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Pesando D, Robert S, Huitorel P, Gutknecht E, Pereira L, Girard JP, Ciapa B. Effects of methoxychlor, dieldrin and lindane on sea urchin fertilization and early development. Aquatic Toxicology. 2004;66(3):225–239. doi: 10.1016/j.aquatox.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Peterson ME, Talcot PA. Small Animal Toxicology. 2nd ed. St. Louis: Elsevier; 2006. pp. 526–527.pp. 941–949. 2006. [Google Scholar]
- Phang W, Funk S, Steinwand B, Leighton T. The Second Revised HED Chapter of the Reregistration Eligibility Decision Document (RED) for Dicofol USEPA. 1996 [Google Scholar]
- Pimentel D. Amounts of pesticides reaching target pests: Environmental impacts and ethics. J Agric Environ Ethics. 1995;8(1):17–29. [Google Scholar]
- Quijano R. Endosulfan poisoning in Kasaragod, Kerala, India, Report on a fact finding mission, PAN. 2002 [Google Scholar]
- Rajendran S. Environment and health aspects of pesticides use In Indian agriculture. Proceedings of the Third International Conference on Environment and Health; 15–17 December, 2003; Chennai, India. 2003. pp. 353–373. Martin J. Bunch, V. Madha Suresh and T. Vasantha Kumaran, eds. [Google Scholar]
- Reigert JR, Roberts JR. Organophosphate Insecticides. Recognition and Management of Pesticide Poisonings. U. S. Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances, Office of Pesticide Programs, U.S. Government Printing Office. (5th ed) 1999;5:34–40. [Google Scholar]
- Remor AP, Totti CC, Moreira DA, Dutra GP, Heuser VD, Boeira JM. Occupational exposure of farm workers to pesticides: biochemical parameters and evaluation of genotoxicity. Environment International. 2009;35:273–278. doi: 10.1016/j.envint.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Rusiecki JK, Baccarelli A, Bollati V, Tarantini L, Mooore LE, Bonefeld-Jorgenson EC. Global DNA hypomethylation is associated with high serum-persistent organic pollutants in Greenlandic unit. Environmental Health Perspectives. 2008;116:1547–1552. doi: 10.1289/ehp.11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbah S, Bouguerra M. Organochlorine Pesticides in cow milk from Tunisia. Fresenius Environmental Bulletin. 1997;6:359–364. [Google Scholar]
- Sagiv SK, Thurston SW, Bellinger DC, Tolbert PE, Altshul LM, Korrick SA. Prenatal organochlorine exposure and behaviours associated with attention deficit hyperactivity disorder in school-aged children. American Journal of Epidemiology. 2010;171(5):593–601. doi: 10.1093/aje/kwp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo DK, Roy A, Chainy GB. Protective effect of vitamin E and curcumin on L- thyroxine-induced rat testicular oxidative stress. Chemical and Biological Interactions. 2008;176:121–8. doi: 10.1016/j.cbi.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Sarah BA, Yeboah PO, Golow A. Levels of Organochlorine Pesticide Residues in Grasscutter (Thryonomys swinderianus) Tissues. Research Journal of Environmental and Earth Sciences. 2011;3(4):350–357. [Google Scholar]
- Saunders L, Kadhel P, Costet N, Rouget F, Monfort C, Thomé JP, Guldner L, Cordier S, Multigner L. Hypertensive disorders of pregnancy and gestational diabetes mellitus among French Caribbean women chronically exposed to chlordecone. Environment International. 2014;68:171–176. doi: 10.1016/j.envint.2014.03.024. [DOI] [PubMed] [Google Scholar]
- Schwinn FJ, Staub T. Phenylamides and other fungicides against Oomycetes. In: Lyr H, editor. In Modern Selective Fungicides. Jena: VEB Gustav Fischer; 1987. pp. 259–73. [Google Scholar]
- Sinaii N, Cleary SD, Ballweg ML, Niewman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic disease among women with endometriosis: A survey analysis. Human Reproduction. 2002;17(10):2715–2724. doi: 10.1093/humrep/17.10.2715. [DOI] [PubMed] [Google Scholar]
- Singh N, Sharma A, Dwivedi P, Patil R, Kumar M. Citrinin and endosulfan induced teratogenic effects in Wistar rats. Journal of Applied Toxicology. 2007;27(2):143–151. doi: 10.1002/jat.1185. [DOI] [PubMed] [Google Scholar]
- Smith A, Jong HM. Distribution of organochlorine pesticides in soils from South Korea. Chemosphere. 2001;43(2):137–140. doi: 10.1016/s0045-6535(00)00281-2. [DOI] [PubMed] [Google Scholar]
- Sohail E, Waseem A, Chae WL, Jong JL, Imitiaz H. Endocrine Disrupting Pesticides: A Leading Cause of Cancer among Rural People in Pakistan. Experimental Oncology. 2004;26(2):98–105. [PubMed] [Google Scholar]
- Susan S, Sania P. Endosulfan - A review of its toxicity and its effects on the endocrine system. WWF (World Wild Life Fund – Canada) 1999 [Google Scholar]
- Steenland K, Mora AM, Barr DB, Juncos J, Roman N, Wesseling C. Organochlorine chemicals and neurodegeneration among elderly subjects in Costa Rica. Environmental Research. 2014;134:205–209. doi: 10.1016/j.envres.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockholm Convention on Persistent Organic Pollutants. Report of the Persistent Organic Pollutants Review Committee on the work of its fifth meeting; 2009. UNEP/POPS/POPRC.5 /10/ Add.2. [Google Scholar]
- Subramaniam K, Solomon Jebakumar RD. Organochlorine pesticides BHC and DDE in human blood in and around Madurai, India. Indian Journal of Clinical Biochemistry. 2006;21(2):169–172. doi: 10.1007/BF02912936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Dai Y, Lin Y, Gao X, Han Y, Zhao B. Serum organochlorine pesticide residues and risk of gallstone disease: a case-control study in Xiamen. Annals of Epidemiology. 2012;22(8):592–597. doi: 10.1016/j.annepidem.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Sumida K, Saito K, Oeda K, Yakabe Y, Otsuka M, Matsumoto H, et al. A comparative study of gene expression profiles in rat liver after administration of alpha-hexachlorocyclohexane and lindane. Journal of Toxicological Sciences. 2007;32:261–88. doi: 10.2131/jts.32.261. [DOI] [PubMed] [Google Scholar]
- Torres-Sánchez L, Schnaas L, Cebrián ME, Hernández M. del C, Valencia EO, Hernández RMG, López-Carrillo L. Prenatal Dichlorodiphenyldichloroethylene (DDE) exposure and neurodevelopment: A follow-up from 12 to 30 months of age. Neurotoxicology. 2009;30(6):1162–1165. doi: 10.1016/j.neuro.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsipi D, Triantafyllou M, Hiskia A. Determination of organochlorine pesticides residues in honey applying solid-phase extraction with RP - C18 material. Analyst. 1999;124:473–475. doi: 10.1039/a809724k. [DOI] [PubMed] [Google Scholar]
- Turyk ME, Anderson HA, Freels S, Chatterton R, Jr, Needham LL, Patterson DG, Jr, Steenport DN, Knobeloch L, Imm P, Persky VW. Associations of-organochlorines with endogenous hormones in male Great Lakes fish consumers and nonconsumers. Environmental Research. 2006;102:299–307. doi: 10.1016/j.envres.2006.01.009. [DOI] [PubMed] [Google Scholar]
- USEPA. DDT- A review of scientific and economic aspects of the decision to ban its use as a pesticide ; EPA 540/1- 75-022; U. S. Environmental Protection Agency, Office of Pesticide Programs. Washington, DC: U. S. Government Printing Office; 1975. [Google Scholar]
- USEPA. EPA/600/P–96/001F September 1996 PCBs: Cancer Dose-Response Assessment and Application to Environmental Mixtures. 1996. http://www.epa.gov/epawaste/hazard/tsd/pcbs/pubs/pcb.pdf.
- USEPA. Environmental Risk Assessment for Diazinon ; U.S. Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances, Office of Pesticide Programs. Washington, DC: U.S. Government Printing Office; 2000. [Google Scholar]
- USEPA. Health Effects Support Document for Aldrin/Dieldrin, U.S. Environmental Protection Agency, Office of Water (4304T) Washington, DC 20460: Health and Ecological Criteria Division; 2003. [Google Scholar]
- USEPA. Endosulfan. The Health Effects Divion’s Human Health Risk Assessment. 2010. EPA DP Barcode: D372569. June 2010. Docket No.: EPA-HQ-OPP-2002-0262-0178; http://www.regulations.gov.
- Veil JF. Public health impact of pesticides used in agriculture, Geneva, Switzerland. WHO (World Health Organization); 1990. [Google Scholar]
- Videla LA, Tapia G, Varela P, Cornejo P, Guerrero J, Israel Y. Effects of acute gamma-hexachlorocyclohexane intoxication in relation to the redox regulation of nuclear factor-kappaB, cytokine gene expression, and liver injury in the rat. Antioxidant Redox Signaling. 2004;6:471–480. doi: 10.1089/152308604322899530. [DOI] [PubMed] [Google Scholar]
- Vijaya Padma V, Sowmya P, Arun FT, Baskaran R, Poornima P. Protective effect of gallic acid against lindane induced toxicity in experimental rats. Food and Chemical Toxicology. 2011;49:991–998. doi: 10.1016/j.fct.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Viltos AJ. Biological activation of sodium 2(2,4-dichlorophenoxy)ethylsulfate by Bacillus cereus var. mycoides, Contrb. Boyce Thompson Inst. 1952;16:435–438. [Google Scholar]
- Wagner SL. Diagnosis and treatment of organophosphate and carbamate intoxication. Human Health Effects of Pesticides. 1997;12:239–249. [PubMed] [Google Scholar]
- Wasseling C, Aragón A, Castillo L, Corriols M, Chaverri F, de la Cruz E, Keifer M, Monge P, Partanen TJ, Ruepert C, van Wendel de Joode B. Hazardous pesticides in Central America. International Journal of Occupational and Environmental Health. 2001;7(4):287–94. doi: 10.1179/107735201800339236. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Toniolo PG, Lee EW, Rivera M, Dubin N. Blood levels of organochlorine residues and risk of breast cancer. Journal of the National Cancer Institute. 1993;21:648–52. doi: 10.1093/jnci/85.8.648. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Environmental Health Criteria. Vol. 9. Geneva, Switzerland: 1979. DDT and its derivatives. 1979. [Google Scholar]
- Xu X, Dailey AB, Talbott EO, Ilacqua VA, Kearney G, Asal AR. Associations of serum concentrations of organochlorine pesticides with breast cancer and prostate cancer in U.S. adults. Environmental Health Perspectives. 2010;118:60–66. doi: 10.1289/ehp.0900919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JH, Lee YM, Bae SG, Jacobs DR, Jr, Lee DH. Associations between organochlorine pesticides and vitamin D deficiency in the U.S. population. PLoS One. 2012;7(1):e30093. doi: 10.1371/journal.pone.0030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki MA, Taylor HF, Wain RL. Studies with 35-Diiodo-4-hydroxybenzonitrile (ioxynil) and related compounds in soil and plants. Annals of Applied Biology. 1967;59:481–491. [Google Scholar]


