Abstract
Although early diffusion lesion reversal after recanalization treatment of acute ischaemic stroke has been observed in clinical settings, the reversibility of lesions observed by diffusion-weighted imaging remains controversial. Here, we present consistent observations of sustained diffusion lesion reversal after transient middle cerebral artery occlusion in a monkey stroke model. Seven rhesus macaques were subjected to endovascular transient middle cerebral artery occlusion with in-bore reperfusion confirmed by repeated prospective diffusion-weighted imaging. Early diffusion lesion reversal was defined as lesion reversal at 3 h after reperfusion. Sustained diffusion lesion reversal was defined as the difference between the ADC-derived pre-reperfusion maximal ischemic lesion volume (ADCD-P Match) and the lesion on 4-week follow-up FLAIR magnetic resonance imaging. Diffusion lesions were spatiotemporally assessed using a 3-D voxel-based quantitative technique. The ADCD-P Match was 9.7 ± 6.0% (mean ± SD) and the final infarct was 1.2–6.0% of the volume of the ipsilateral hemisphere. Early diffusion lesion reversal and sustained diffusion lesion reversal were observed in all seven animals, and the calculated percentages compared with their ADCD-P Match ranged from 8.3 to 51.9% (mean ± SD, 26.9 ± 15.3%) and 41.7–77.8% (mean ± SD, 65.4 ± 12.2%), respectively. Substantial sustained diffusion lesion reversal and early reversal were observed in all animals in this monkey model of transient focal cerebral ischaemia.
Keywords: Animal model, brain ischaemia, brain imaging, brain recovery, diffusion-weighted magnetic resonance imaging
Introduction
Recent randomized prospective multicentre clinical trials (MR CLEAN, ESCAPE, EXTEND-IA, SWIFT PRIME and REVASCAT) have indicated that rapid intraarterial (IA) treatment benefits functional recovery, with statistically significant volume differences in final infarcts (19 ml in the MR CLEAN and 24.4 ml in the EXTEND-IA trials) between the control group (use of IV alteplase only) and intervention group.1–3 The trials revealed a positive relationship between IA treatment and clinical outcome and suggested recovery of ischaemic penumbra. However, they did not present evidence of salvaged tissue or diffusion lesion reversal (DLR).
Diffusion-weighted MR imaging (DWI) is widely used to identify the infarct core in acute ischaemic stroke patients,4,5 and diffusion–perfusion mismatch is considered to indicate ischaemic penumbra or salvageable brain tissue.6–9 DLR after recanalization treatment has been documented in both clinical practice10–21 and experimental settings.22–24 Early abnormalities on DWI thus overestimate the infarct core by including part of the penumbra, and the actual ‘salvageable brain tissue’ includes the D-P mismatch area and DLR.
Because substantial DLRs have been reported in clinical practice, it is widely accepted that DLR in the early stage is quite frequent in early recanalized or reperfused acute ischaemic stroke patients.11,12,14,16 However, whether DLR is sustained in the long-term remains controversial, and several studies have reported that most DLRs are miscalculated due to inaccurate coregistration and infarct shrinkage over time, apparent early diffusion lesion reversal (eDLR) is transient, and permanent DLR is uncommon and typically involves only a small volume of tissue.12,17,18
However, there are limitations to achieving actual DLR in clinical practice because most IA treatments require 1 h or more to recanalize occluded arteries and pre-IA treatment magnetic resonance images (MRIs) do not reflect pre-reperfusion maximal diffusion lesions. Furthermore, there is no standard definition for sustained diffusion lesion reversal (sDLR). Some researchers have analysed sDLR with only 5 days of follow-up MRI studies after IA treatment, even though MRI studies during that period cannot accurately depict sDLR because severe brain swelling is still present at the infarction site during this period.
We hypothesized that an acute DWI positive lesion could be salvaged by reperfusion, and the salvageable tissue might be sustained 4 weeks later. In this study, to evaluate the extent of sDLR, we used a monkey stroke model with a prospective in-bore reperfusion technique and serial follow-up MRIs for 4 weeks.
Materials and methods
Experimental animals
Healthy rhesus macaques of both sexes (n = 9, 4.6–6.7 kg) were subjected to transient endovascular middle cerebral artery occlusion (MCAO) and focal cerebral ischaemia. Two animals died during the post-reperfusion follow-up period. The presumed causes of death were severe brain oedema due to large territorial infarction (n = 1) and anaesthesia side effects (n = 1). A total of seven animals were included and analysed in this study. The monkeys were housed in indoor individual cages and fed commercial monkey chow (Harlan, USA) supplemented with various fruits daily and water ad libitum. The environmental conditions were controlled to provide a temperature of 24 ± 2℃, a relative humidity of 50 ± 5%, 100% fresh air at a rate of ≥12 room changes per hour, and a 12:12 h light:dark cycle. Animals were fasted for 12 h prior to anaesthesia and MCAO. All housing conditions and experimental procedures were conducted according to the guidelines set by the Korea Research Institute of Bioscience and Biotechnology Institutional Animal Care and approved by the Korea Research Institute of Bioscience and Biotechnology Animal Care Committee. Reporting of the work complies with ARRIVE guidelines.
Endovascular MCAO and veterinary care
Animals were ventilated and anaesthetized with ketamine induction and 1.5–2% isoflurane in medical air. O2 saturation (SpO2), heart rate, respiration rate, and blood pressure were continuously monitored and maintained within normal ranges.25 The animals were covered with blankets and warm saline bags to maintain body temperature (37 ± 0.5℃) during the endovascular procedures and MRIs. The whole body of the animal was fixed using a custom semi-hard plastic harness with Velcro-taped bands that did not disturb fluoroscopy, angiography or MRI, and the whole body of the animal was covered with disposable draping for paediatric angiography. A previously reported endovascular MCAO technique26 was modified and applied. The right common femoral artery was aseptically approached using the Seldinger technique after a small skin incision. A 5 French arterial sheath (Sungwon Medical, Cheongju, Korea) was inserted using a 5 F micropuncture set (Merit Medical, South Jordan, UT). The right proximal internal carotid artery was selectively catheterized using a 0.035″ guide wire (Terumo, Tokyo, Japan) and a 5 F catheter (Weinberg catheter, A&A Medical, Seongnam, Korea), and selective angiography was performed to obtain anterior–posterior and lateral views using a digital angiography unit (Allura Xper FD20, Philips Medical System, the Netherlands). To prevent thromboembolic complications during the endovascular intervention, an IV bolus of 500 units of heparin was injected after insertion of a femoral arterial sheath, and heparinized normal saline (3000 units in 1000 ml) was continuously flushed through the 5 F guiding catheter. Using double-fluoroscopic road map images, an appropriate endovascular occluder was advanced over the microguidewire (Synchro 14, Boston Scientific, Fremont, CA) and wedged in the distal M1 or superior M2 division branch of the right MCA. Contrast media (Iversense 240, Accuzen, Seoul, Korea) was carefully injected through the microcatheter, and flow arrest/stasis of the contrast media in the distal vasculature indicated successful MCAO (Supplementary Figure 1).
Repeated diffusion MRIs and in-bore reperfusion
Baseline MRI was performed in all experimental animals with anaesthesia at 8 days before MCAO procedure to evaluate any underlying brain disease and to serve as a standard for comparison with the post-infarct status. Immediately after MCAO, the animal was transported to the neighbouring 3.0 T MRI (Achieva 3.0 T, Philips Medical Systems, Best, the Netherlands) suite while maintaining the endovascular MCAO. At 15 min after the MCAO procedure, post-occlusion diffusion-weighted MR images were acquired (DWI parameters of b = 1000; FOV, 120 × 120; matrix, 100 × 100; TR/TE, 6937/56.3; FA, 90; NEX, 3.0; slice thickness, 1.8 mm/no gap; receiving coil, dual coil), and MRI was repeated every 10 ∼ 15 min thereafter (animals 1 ∼ 2, every 10 min; animals 3 ∼ 7, every 15 min) (Figure 1). Right MCAO was maintained with stable vital signs to ensure the safety of the experimental animals under general anaesthesia. The microcatheter was removed to achieve reperfusion in the MRI scanner (in-bore reperfusion) after various durations of MCAO when the ADC-derived volume of the focal ischaemia had plateaued. Plateau was defined when the ischaemic lesions did not change for three consecutive MRIs and was determined by the pixel-based real-time volume analysis technique using image analysis software (ImageJ 1.46, NIH, Bethesda, MD, USA) and consensus of three experienced stroke neuroradiologists (S-H. Cha, C-H. Choi, K.S. Yi) (Supplementary Figure 2). DSC perfusion MRI was performed immediately prior to reperfusion (DSC perfusion parameters: FOV, 120 × 120; matrix, 64 × 63; TR/TE, 1600/35; FA, 63; NEX, 1.0; slice thickness, 3.0 mm/no gap).
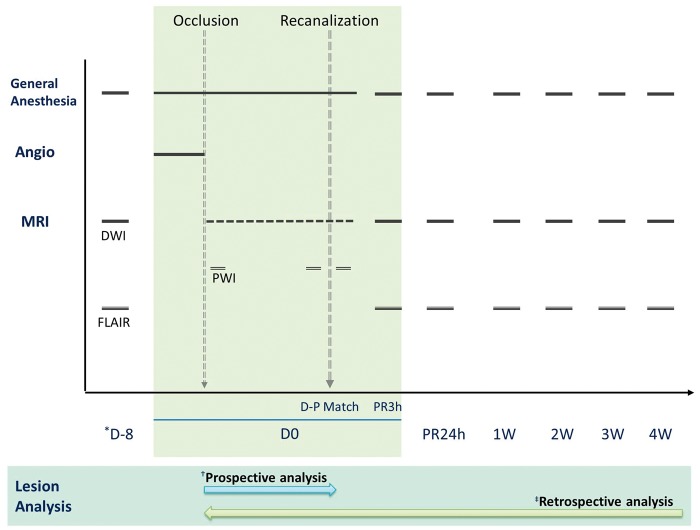
Figure 1.
Timeline of monkey stroke model using prospective repeated MRI and in-bore reperfusion. Baseline MRI was performed in all experimental animals with anaesthesia at 8 days before MCAO procedure to evaluate any underlying brain disease and to serve as a standard for comparison with the post-infarct status. After endovascular occlusion of the middle cerebral artery (MCAO), the experimental animals were transported to the MRI suite while maintaining endovascular MCAO. Post-occlusion diffusion MRIs (DWIs) were repeated every 10 ∼ 15 min thereafter (animals 1 ∼ 2, every 10 min; animals 3 ∼ 7, every 15 min). Transient right MCAO was maintained until the ADC-derived volume of the focal ischaemia had plateaued with variable duration to each animal (plateau was defined when the ischaemic lesions did not change for three consecutive DWI MRIs). Immediately prior to reperfusion, DSC perfusion MRI was performed to confirm diffusion–perfusion matching. Follow-up MRIs were performed at 3-h post-reperfusion (PR3h), 24 h and then every week for 4 weeks. (*D-8, 8 days before MCAO; †Prospective analysis was performed using ImageJ software for real-time determination of an acute phase diffusion lesion after MCAO to determine the time point for arterial recanalization; ‡Retrospective analysis was performed by a sophisticated processing method for precise lesional evaluation).
Post-operative care and follow-up MRI scans
After the in-bore reperfusion and immediate post-reperfusion MRI scan, all interventional devices were removed, and postoperative veterinary care was provided to the post-stroke animals. Follow-up MR imaging was performed at 3 h (PR3h) after reperfusion to evaluate eDLR and then every week for 4 weeks (Figure 1).
MR data processing and analysis
Two different analysis techniques (prospective real-time analysis and retrospective analysis) were used in this study. The prospective real-time analysis technique (pixel-based analysis by ImageJ) used an absolute ADC cutoff value (650 × 10−6 mm2/s) for segmentation of an acute diffusion lesion.27 It has advantages in the process speed, but it is less reliable than a relative threshold method. Therefore, a prospective real-time analysis technique was used only for rapid real-time assessment of the acute phase diffusion lesion after MCAO to determine the time point for arterial recanalization with the consensus of three experienced stroke neuroradiologists. Retrospective analysis was performed on all MR images of the entire time series for precise lesion analysis after the 4-week follow-up period. All data sets in digital imaging and communication (DICOM) format were transferred from the MR scanner to a personal desktop (Intel Carework How-4100, i7 quad core CPU, Ubuntu 12.04 LTS). All data sets were converted to three-dimensional NIFTI format using dcm2nii (MRIcron, http://www.mccauslandcenter.sc.edu/mricro/mricron/). All data were processed using the FSL software library (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/v5.0) and AFNI software package (http://afni.nimh.nih.gov/afni/). The following three processing steps were implemented (Figure 2): (1) affine registration of the entire time series of ADC maps and FLAIRs to baseline FLAIR (normal brain parenchyma at 8 days before MCAO) using FLIRT in FSL; (2) lesion segmentation on ADC maps (after MCAO ∼ PR3h) and FLAIRs (PR24h ∼ 4 weeks) using ITKsnap software (http://www.itksnap.org/pmwiki/pmwiki.php). (a) Conversion to relative ADC (rADC) map compared with the mean normal contralateral hemisphere. (b) Automatic thresholding (first step): lesion included voxels with value of rADC less than 80.28–30 (c) Semi-automatic thresholding (second step): fine adjustment compared with pre-reperfusion ADC map to each animal with the consensus of three neuroradiologists. The results of adjustment ranged 78 ∼ 84 (Supplementary Table 1); and (3) lesion volume measurements using fslstats in FSL. DSC MR Image processing and colour-mapping were performed by an academic perfusion analysis program (Perfusion Mismatch Analyzer [PMA], Acute Stroke Imaging Standardization Group [ASIST], Japan, http://asist.umin.jp/index-e.htm). Diffusion–perfusion status was determined by consensus of three neuroradiologists. The final infarction was segmented into segments of sufficient size to include the adjacent CSF space and cystic parenchymal defect area, as in previous studies.17,18 DLRs at 2 weeks (2WDLR, n = 7) and 3 weeks (3WDLR, n = 6) were also calculated to compare with the results of e- and sDLRs.
Figure 2.
Steps of MR data processing and lesion segmentation (representative demonstration in animal 4). The following three processing steps were implemented for MR imaging processing. (1) Affine registration of the entire time series of ADC maps and FLAIRs to baseline FLAIR (normal brain parenchyma at 8 days before MCAO) using FLIRT in FSL; (2) lesion segmentation on ADC maps and FLAIRs using ITKsnap software (http://www.itksnap.org/pmwiki/pmwiki.php). (a) Conversion to relative ADC (rADC) map compared with the mean normal contralateral hemisphere. (b) Automatic thresholding (first step): lesion included voxels with value of rADC less than 80 (the colour-coded image shows a step of speed image generation in ITKsnap). (c) Semi-automatic thresholding (second step): fine adjustment (rADC = 78 in animal 4) compared with pre-reperfusion ADC map.
DLR
We used ADC-derived 3D voxel-based lesion analysis in retrospective analysis to evaluate diffusion lesions with greater objectivity and accuracy than can be obtained by visual determination and manual outlining of the DWI lesion. To evaluate the effect of recanalization in the acute and chronic stages, eDLR and sDLR were examined, respectively. eDLR was defined as the ADC-derived hemispheric lesion difference between the pre-reperfusion maximal ischemic lesion volume (ADCD-P Match) and the lesion volume at post-reperfusion 3 h (ADCPR3h). sDLR was defined as the volume difference between ADCD-P Match and the lesion volume on FLAIR at the 4-week follow-up (FLAIR4w) (Figure 3). 2WDLR and 3WDLR were also calculated similarly to sDLR as the volume differences between ADCD-P Match and the lesion volumes on FLAIR at the 2- and 3-week follow-up. The following ratios were also calculated: eDLR/ADCD-P Match (Ratio of eDLR), sDLR/ADCD-P Match (Ratio of sDLR), 2WDLR/ADCD-P Match (Ratio of 2WDLR) and 3WDLR/ADCD-P Match (Ratio of 3WDLR).
Figure 3.
Coregistered serial MR images of experimental animals subjected to endovascular MCAO and in-bore reperfusion. Early DLR (eDLR) was defined as the ADC-derived hemispheric lesion volume (HLV) difference between the pre-reperfusion lesion (ADCD-P Match) and the lesion volume at 3 h after reperfusion (ADCPR3h), and sustained DLR (sDLR) was the volume difference between the ADCD-P Match and the lesion on the 4-week follow-up FLAIR MRI (FLAIR4w) after the procedure. Affine registration was applied to all images for intrasubject coregistration.
Results
Temporal evolution of focal ischemia/infarct lesion volumes
Representative serial MR images and temporal changes in lesion volumes are presented in Figures 3 and 4. The ADCD-P Match were 12.5 ± 9.6% (mean ± SD). The ADCPR3h were decreased to 7.6 ± 4.5% (mean ± SD), and the FLAIR4w ranged from 0.9 to 20.8% (Table 1).
Figure 4.

Temporal changes in mean abnormal lesion volumes of all experimental animals during 4 weeks of follow-up. Error bars present standard deviation of the mean; %HLV, percentage of the hemispheric lesion volume.
Table 1.
ADC-derived lesion volumes and diffusion lesion reversal in monkey stroke models.
| Animal | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Age/sex | 4/M | 5/M | 5/M | 6/M | 6/M | 8/F | 5/F |
| BW (kg) | 5.9 | 5.2 | 4.6 | 6.2 | 6.7 | 5.7 | 5.3 |
| Duration of occlusion (min) | 240 | 160 | 135 | 165 | 145 | 150 | 110 |
| ADCD-P Match (%) | 14.5 | 19.6 | 5.4 | 7.9 | 12.0 | 2.4 | 5.9 |
| FLAIR4w/ ADCD-P Match (%) | 34.5 (5.0/14.5) | 30.6 (6.0/19.6) | 22.2 (1.2/5.4) | 24.1 (1.9/7.9) | 31.6 (3.8/12.0) | 58.3 (1.4/2.4) | 40.7 (2.4/5.9) |
| Ratio of eDLR (%) | 29.7 (4.3/14.5) | 34.7 (6.8/19.6) | 51.9 (2.8/5.4) | 34.2 (2.7/7.9) | 17.5 (2.1/12.0) | 8.3 (0.2/2.4) | 11.9 (0.7/5.9) |
| Ratio of sDLR (%) | 65.5 (9.5/14.5) | 69.4 (9.5/19.6) | 77.8 (4.2/5.4) | 75.9 (6.0/7.9) | 68.3 (8.2/12.0) | 41.7 (1.0/2.4) | 59.3 (3.5/5.9) |
MCAO: middle cerebral artery occlusion; ADCD-P Match: ADC-derived hemispheric lesion volume at D-P match; FLAIR 4w: hemispheric lesions volume on FLAIR at 4 week; eDLR: early diffusion lesion reversal; sDLR: sustained diffusion lesion reversal; Ratio of eDLR, eDLR/ADCD-P Match; Ratio of sDLR, sDLR/ADCD-P Match.
DLRs
eDLR was observed in all seven animals (0.2 ∼ 6.8% hemispheric lesion volume (HLV)), and the ratio of eDLR ranged from 8.3 to 51.9% (mean ± SD, 26.9 ± 15.3%). sDLRs were detected in all animals (6.6 ± 4.2%, mean ± SD), and the ratio of sDLR ranged from 41.7 to 77.8% (mean ± SD, 65.4 ± 12.2%) (Figure 5). The ratios of 2WDLR and 3WDLR were 35.0 ± 21.6% and 41.3 ± 26.2%, respectively (Supplementary >Figure 3).
Figure 5.

Diffusion lesion reversals in experimental animals. Ratio of eDLR and ratio of sDLR are presented (ratio of eDLR=eDLR/ADCD-P Match; ratio of sDLR=sDLR/ADCD-P Match).
Diffusion–perfusion status at the time of recanalization
DSC perfusion MRI was obtained in four animals (animals 1, 4, 5 and 6) immediately prior to reperfusion. Perfusion MRIs (CBV and CBF maps) were compared with pre-reperfusion diffusion MRIs, demonstrating a diffusion–perfusion match (Figure 6).
Figure 6.
Diffusion and perfusion MR images at the pre-reperfusion time point (animals 1, 4, 5, and 6). Representative MR images of ADC maps and DSC perfusion MRIs (CBV/CBF maps) showing diffusion–perfusion match for all animals.
Discussion
We induced transient ischaemic stroke in nonhuman primates using an endovascular technique. The reperfusion time point for our transient ischaemic stroke model was determined using prospective and repeated diffusion MRIs, and reperfusion was achieved using the in-bore reperfusion technique. Reperfusion was performed when the lesion volume had plateaued to ensure that the lesions had a diffusion–perfusion match; thus, the infarct durations differed among the animals. MRI follow-up was performed for 4 weeks after reperfusion, and the lesion was evaluated by 3D-voxel-based analysis to achieve enhanced accuracy and objectivity. In summary, we observed sDLR and early reversal in all animals.
Choice of experimental animals
We used macaques for the endovascular MCAO model because they have gyrencephalic brains and a vascular anatomy more similar to that of humans, with fewer vascular collaterals, than baboons.26,31 According to the updated STAIR recommendations,32 efficacy studies should be performed in both male and female animals; therefore, we used animals of both sexes in this study.
Endovascular MCAO
Several MCAO methods have been developed and surgical and endovascular methods are most frequently used in nonhuman primates. An advantage of surgical methods is the ability to produce reproducible infarcts, but invasiveness is a limitation of surgery. Head injury is not involved in the natural pathophysiological process of human stroke and could thus be a major confounding factor in infarct animal models generated using surgical methods.33 Furthermore, for infarcts generated via surgical methods, repeated/prospective in-bore MRIs are not suitable because the images are susceptible to artefacts caused by the surgical materials. Endovascular methods of MCAO are less invasive and, like surgical methods, can produce reproducible infarcts. Earlier endovascular models used balloons, glue or thrombus injection and often produced large infarcts with variable locations and extents.26 We used a modified version of the endovascular MCAO technique described by de Crespigny et al.26 to generate smaller and reproducible infarctions. MR-compatible microcatheters of various sizes with non-magnetic tips were used for the first time in this study to generate MCA occlusions of different vascular sizes with the aim of occluding an M2 branch of the MCA. This method resulted in reduced morbidity/mortality and allowed us to obtain hyperacute infarction and perform repeated/prospective in-bore MRI.
Timing of recanalization and variable duration of MCAO
The time from onset to completion of ischaemic stroke varies among individuals depending on the site of vessel occlusion, degree of collateral blood supply, systemic blood pressure, blood volume, serum glucose and other factors.34,35 We assumed that an MCAO model with a fixed duration of arterial occlusion would not guarantee similar ischaemic damages in all experimental animals and would limit the ability to evaluate the effect of recanalization. In our study, the acute ischaemic lesions expanded at different rates in each animal and eventually plateaued on prospective repeated diffusion MRIs. Then, we recanalized the occluded arteries and our endovascular model had variable duration of MCAO. When the diffusion lesion reached to the maximum, it is supposed to be a diffusion–perfusion match36 and the ischaemic lesions exhibited very little diffusion–perfusion mismatch of our qualitative analysis at this time point.
Assessment of focal cerebral ischaemia on ADC map
To evaluate sDLR, we used the 3D voxel-based analysis technique with ADC maps rather than DWIs. Previous studies of DLR in humans, including EPITHET, DEFUSE and DEFUSE 2,12,17–19 used visual determination and two-dimensional manual outlining of the DWI lesion because quantitative ADC thresholds tend to be inaccurate.17 The ADC thresholding technique is limited by heterogeneity among individuals and the overlap of different time series and ADC between the normal parenchyma and infarct core. However, ischaemic lesion determination and delineation by ADC analysis has advantages in terms of interrater agreement and accuracy compared with the DWI outlining technique.37 Furthermore, we defined ADC-derived lesions using relative ADC values in reference to those of contralateral normal brain parenchyma rather than absolute ADC values because relative ADC values more accurately indicate tissue viability.38,39
eDLR and sDLR
There are two main differences in our study when compared with previous clinical studies: the eDLR/sDLR was always present in all animals, and the sDLR was larger than the eDLR. Prior clinical studies have shown that eDLR is quite frequent (7−50% incidence and 0.9−17.5 ml volume) in early recanalized or reperfused acute ischaemic stroke patients.11,12,14,16 However, we found eDLR in all of our study animals. This suggests that the diffusion lesion volumes on the initial MRIs (i.e. baseline MRI) of acute stroke patients do not reflect the ‘immediate pre-reperfusion’ ischaemic lesion volume in clinical settings. IA or IV treatment requires time to recanalize occluded arteries and may explain why diffusion lesion volume on follow-up MRIs (immediately or 1 day after the procedure) was frequently larger when compared with the initial MRI, even when recanalization was successful and clinical symptoms improved. We used the in-bore reperfusion technique to obtain a true ‘immediate pre-reperfusion’ lesion volume similar to the baseline lesion volume.
Previous clinical studies reported that sDLR is infrequent and often transient.12,17,18 Furthermore, Inoue et al. used initial DWI MRIs and 5-day follow-up FLAIR MRIs to calculate sDLR; therefore, these data cannot be directly compared with our study. When compared with other studies,17,18 our most unique findings is an sDLR larger than the eDLR. According to preliminary research on the correlation of FLAIR MRI and histopathology in one animal (animal 7) at the 4-week follow-up period, sDLR regions that had normal signal intensities on the FLAIR MRI showed prominent astrocytosis and neuronal damage despite a normal signal intensity on the MRI (Supplementary Figure 4). The histopathological findings in the ischaemic penumbra or peri-infarct region have varying degrees of scattered neuronal injury, i.e. ‘incomplete infarction’.40–42 Few studies have described the histopathological findings of eDLR regions. These data showed an incomplete infarction, including selective neuronal necrosis in 10- or 20-min-transient MCAOs in rats.24,43 We found histopathological characteristics in the sDLR region that contrast with these studies; therefore, FLAIR images are unable to fully depict micro-ischaemic pathological abnormalities of the brain, and this discrepancy may result from the spatial resolution difference between MRI and histology to detect tissue damage.24,43,44
Animals 5 through 7 had relatively small eDLRs when compared with the other animals. Animals 5 through 7 had similar infarct characteristics, including relatively small infarct volumes and the involvement of mainly cortical areas. This effect resulted from an attempt to occlude the M2 distal branch selectively to decrease mortality. This result might be explained by a recent report that white matter is more prone to DLR than the cortex.10
Clinical implications of sDLR
According to recent randomized, multicentre clinical trials (MR CLEAN, ESCAPE, EXTEND-IA, SWIFT PRIME and REVASCAT) exploring the efficacy and safety of treatments for acute ischaemic stroke patients, IA treatment is related to positive clinical outcomes.1–3,45,46 The treatment options for stroke patients with large artery occlusions have consequently been modified. In those clinical trials, the final infarct volume was relatively small in the IA treatment groups compared with the non-intervention groups,1,3,45 reflecting the salvage of ischaemic tissue by early recanalization. Those trials focused on clinical outcomes rather than the amount of salvageable tissue or DLR and there were no imaging evidences to prove direct influence of arterial recanalization. We presumed that DLR is a part of the salvageable tissues in those clinical trials, but could not compare the clinical status of the animals studied here before and after reperfusion because of an in-bore reperfusion technique under general anaesthesia. Further studies of the clinical implications of DLR would facilitate the proper evaluation of DLR.
Limitations
The main limitation of our study is that the number of experimental animals was small. However, sDLR was consistently observed in this study, and this finding may not be altered by the studies with larger experimental populations. The second limitation is the unsatisfactory correction of acute brain swelling and chronic atrophy. Despite our best efforts to compensate for the effect of local brain shrinkage for global registration compared with baseline FLAIRs, we were not able to compensate for local anatomic differences due to brain shift from swelling or atrophy. Shift of water content in acute brain swelling and chronic atrophy may distort results of sDLR lesion and sDLR in our study might be overestimated because of the effect of enlargement of the anatomic area of a pre-reperfusion maximal lesion (ADCD-P Match). To overcome ‘pseudo’ reversal effect of diffusion lesion, we calculated the volume differences between ADCPR3h and FLAIR4w (Supplementary Figure 5), because ADCPR3h had decreased effect of the enlargement of anatomic area. Also substantial were DLRs at 2 and 3 weeks after MCAO (2WDLR and 3WDLR), time points at which no or less brain shrinkage is observed (Supplementary Figure 3). Thirdly, the animals were young adults according to the lifespan of the rhesus monkey; therefore, it is unclear whether these results can be generalized, further studies using older animals are needed. Finally, although the use of ADC map and 4-week FLAIR is well accepted in the previous clinical literatures for the analysis of the sustained DLR,17,18 the use of different MR imaging methods of lesion definition in acute and chronic stages might be a limitation of this study.
Conclusion
Substantial sDLR and early reversal were observed in all animals of the nonhuman primate model of transient focal cerebral ischaemia with diffusion–perfusion matching. Our results show that ischaemic brain tissue could be salvaged after recanalization of the occluded artery, and might be imaging evidences for the better clinical outcomes of the recent clinical trials of IA recanalization treatment in acute stroke patients. Future studies should explore the histopathological correlations and clinical implications of DLR.
Supplementary Material
Acknowledgements
The authors are grateful for the efforts of Ms. Mihee Song, Messrs. Sang-Yeol Lee, Hyung-Jun Cho, Gi-Seon Lim, Sang-Il Jeong, Gwang-Hun Ko, Sang-Hui Yun, Chang-Yeop Jeon and Hyeon-Gu Yeo.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: A research grant from the Chungcheongbuk-do, the Korea Research Institute of Bioscience and Biotechnology (KRIBB) Research Initiative Program KGM4611613, and the Bio & Medical Technology Development Program of the NRF funded by the Korean government, MSIP (NRF-2016M3A9B6902954, NRF-2016M3A9B6903268), Republic of Korea.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
KSY, CHC, SRL, HJL, KTC and SHC contributed to the study design. KSY, SRL, YJL, KJJ and SHC performed the experiments. CHC, JWH, KSY and SHC contributed to data acquisition and analysis. KSY, CHC, SRL, KTC and SHC contributed to preparation of the manuscript.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 3.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 4.Guadagno JV, Warburton EA, Jones PS, et al. The diffusion-weighted lesion in acute stroke: heterogeneous patterns of flow/metabolism uncoupling as assessed by quantitative positron emission tomography. Cerebrovasc Dis 2005; 19: 239–246. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez RG, Copen WA, Schaefer PW, et al. The Massachusetts General Hospital acute stroke imaging algorithm: an experience and evidence based approach. J Neurointerv Surg 2013; 5 Suppl 1: i7–i12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neumann-Haefelin T, Wittsack HJ, Wenserski F, et al. Diffusion- and perfusion-weighted MRI. The DWI/PWI mismatch region in acute stroke. Stroke 1999; 30: 1591–1597. [DOI] [PubMed] [Google Scholar]
- 7.Karonen JO, Vanninen RL, Liu Y, et al. Combined diffusion and perfusion MRI with correlation to single-photon emission CT in acute ischemic stroke. Ischemic penumbra predicts infarct growth. Stroke 1999; 30: 1583–1590. [DOI] [PubMed] [Google Scholar]
- 8.Albers GW. Expanding the window for thrombolytic therapy in acute stroke. The potential role of acute MRI for patient selection. Stroke 1999; 30: 2230–2237. [DOI] [PubMed] [Google Scholar]
- 9.Baird AE, Benfield A, Schlaug G, et al. Enlargement of human cerebral ischemic lesion volumes measured by diffusion-weighted magnetic resonance imaging. Ann Neurol 1997; 41: 581–589. [DOI] [PubMed] [Google Scholar]
- 10.Tisserand M, Malherbe C, Turc G, et al. Is white matter more prone to diffusion lesion reversal after thrombolysis? Stroke 2014; 45: 1167–1169. [DOI] [PubMed] [Google Scholar]
- 11.Luby M, Warach SJ, Nadareishvili Z, et al. Immediate changes in stroke lesion volumes post thrombolysis predict clinical outcome. Stroke 2014; 45: 3275–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inoue M, Mlynash M, Christensen S, et al. Early diffusion-weighted imaging reversal after endovascular reperfusion is typically transient in patients imaged 3 to 6 hours after onset. Stroke 2014; 45: 1024–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asdaghi N, Campbell BC, Butcher KS, et al. DWI reversal is associated with small infarct volume in patients with TIA and minor stroke. AJNR Am J Neuroradiol 2014; 35: 660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakamoto Y, Kimura K, Shibazaki K, et al. Early ischaemic diffusion lesion reduction in patients treated with intravenous tissue plasminogen activator: infrequent, but significantly associated with recanalization. Int J Stroke 2013; 8: 321–326. [DOI] [PubMed] [Google Scholar]
- 15.Albach FN, Brunecker P, Usnich T, et al. Complete early reversal of diffusion-weighted imaging hyperintensities after ischemic stroke is mainly limited to small embolic lesions. Stroke 2013; 44: 1043–1048. [DOI] [PubMed] [Google Scholar]
- 16.Labeyrie MA, Turc G, Hess A, et al. Diffusion lesion reversal after thrombolysis: a MR correlate of early neurological improvement. Stroke 2012; 43: 2986–2991. [DOI] [PubMed] [Google Scholar]
- 17.Campbell BC, Purushotham A, Christensen S, et al. The infarct core is well represented by the acute diffusion lesion: sustained reversal is infrequent. J Cereb Blood Flow Metab 2012; 32: 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chemmanam T, Campbell BC, Christensen S, et al. Ischemic diffusion lesion reversal is uncommon and rarely alters perfusion-diffusion mismatch. Neurology 2010; 75: 1040–1047. [DOI] [PubMed] [Google Scholar]
- 19.Olivot JM, Mlynash M, Thijs VN, et al. Relationships between cerebral perfusion and reversibility of acute diffusion lesions in DEFUSE: insights from RADAR. Stroke 2009; 40: 1692–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chalela JA, Ezzeddine M, Latour L, et al. Reversal of perfusion and diffusion abnormalities after intravenous thrombolysis for a lacunar infarction. J Neuroimaging 2003; 13: 152–154. [PubMed] [Google Scholar]
- 21.Ringer TM, Neumann-Haefelin T, Sobel RA, et al. Reversal of early diffusion-weighted magnetic resonance imaging abnormalities does not necessarily reflect tissue salvage in experimental cerebral ischemia. Stroke 2001; 32: 2362–2369. [DOI] [PubMed] [Google Scholar]
- 22.Lo EH, Matsumoto K, Pierce AR, et al. Pharmacologic reversal of acute changes in diffusion-weighted magnetic resonance imaging in focal cerebral ischemia. J Cereb Blood Flow Metab 1994; 14: 597–603. [DOI] [PubMed] [Google Scholar]
- 23.van Lookeren Campagne M, Verheul JB, Nicolay K, et al. Early evolution and recovery from excitotoxic injury in the neonatal rat brain: a study combining magnetic resonance imaging, electrical impedance, and histology. J Cereb Blood Flow Metab 1994; 14: 1011–1023. [DOI] [PubMed] [Google Scholar]
- 24.Li F, Liu KF, Silva MD, et al. Transient and permanent resolution of ischemic lesions on diffusion-weighted imaging after brief periods of focal ischemia in rats: correlation with histopathology. Stroke 2000; 31: 946–954. [DOI] [PubMed] [Google Scholar]
- 25.Li CX, Patel S, Auerbach EJ, et al. Dose-dependent effect of isoflurane on regional cerebral blood flow in anesthetized macaque monkeys. Neurosci Lett 2013; 541: 58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Crespigny AJ, D’Arceuil HE, Maynard KI, et al. Acute studies of a new primate model of reversible middle cerebral artery occlusion. J Stroke Cerebrovasc Dis 2005; 14: 80–87. [DOI] [PubMed] [Google Scholar]
- 27.Na DG, Thijs VN, Albers GW, et al. Diffusion-weighted MR imaging in acute ischemia: value of apparent diffusion coefficient and signal intensity thresholds in predicting tissue at risk and final infarct size. AJNR Am J Neuroradiol 2004; 25: 1331–1336. [PMC free article] [PubMed] [Google Scholar]
- 28.Meng X, Fisher M, Shen Q, et al. Characterizing the diffusion/perfusion mismatch in experimental focal cerebral ischemia. Ann Neurol 2004; 55: 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petty MA, Neumann-Haefelin C, Kalisch J, et al. In vivo neuroprotective effects of ACEA 1021 confirmed by magnetic resonance imaging in ischemic stroke. Eur J Pharmacol 2003; 474: 53–62. [DOI] [PubMed] [Google Scholar]
- 30.Hoehn-Berlage M, Norris DG, Kohno K, et al. Evolution of regional changes in apparent diffusion coefficient during focal ischemia of rat brain: the relationship of quantitative diffusion NMR imaging to reduction in cerebral blood flow and metabolic disturbances. J Cereb Blood Flow Metab 1995; 15: 1002–1011. [DOI] [PubMed] [Google Scholar]
- 31.Cook DJ, Teves L, Tymianski M. Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic primate brain. Nature 2012; 483: 213–217. [DOI] [PubMed] [Google Scholar]
- 32.Fisher M, Feuerstein G, Howells DW, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 2009; 40: 2244–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao B, Shang G, Chen J, et al. A more consistent intraluminal rhesus monkey model of ischemic stroke. Neural Regen Res 2014; 9: 2087–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kidwell CS, Alger JR, Saver JL. Beyond mismatch: evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke 2003; 34: 2729–2735. [DOI] [PubMed] [Google Scholar]
- 35.Saver JL. Time is brain–quantified. Stroke 2006; 37: 263–266. [DOI] [PubMed] [Google Scholar]
- 36.Shen Q, Meng X, Fisher M, et al. Pixel-by-pixel spatiotemporal progression of focal ischemia derived using quantitative perfusion and diffusion imaging. J Cereb Blood Flow Metab 2003; 23: 1479–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bratane BT, Bastan B, Fisher M, et al. Ischemic lesion volume determination on diffusion weighted images vs. apparent diffusion coefficient maps. Brain Res 2009; 1279: 182–188. [DOI] [PubMed] [Google Scholar]
- 38.Oppenheim C, Grandin C, Samson Y, et al. Is there an apparent diffusion coefficient threshold in predicting tissue viability in hyperacute stroke? Stroke 2001; 32: 2486–2491. [DOI] [PubMed] [Google Scholar]
- 39.Rosso C, Hevia-Montiel N, Deltour S, et al. Prediction of infarct growth based on apparent diffusion coefficients: penumbral assessment without intravenous contrast material. Radiology 2009; 250: 184–192. [DOI] [PubMed] [Google Scholar]
- 40.Back T, Ginsberg MD, Dietrich WD, et al. Induction of spreading depression in the ischemic hemisphere following experimental middle cerebral artery occlusion: effect on infarct morphology. J Cereb Blood Flow Metab 1996; 16: 202–213. [DOI] [PubMed] [Google Scholar]
- 41.Back T. Pathophysiology of the ischemic penumbra–revision of a concept. Cell Mol Neurobiol 1998; 18: 621–638. [DOI] [PubMed] [Google Scholar]
- 42.Lassen NA, Vorstrup S. Ischemic penumbra results in incomplete infarction: is the sleeping beauty dead? Stroke 1984; 15: 755–758. [DOI] [PubMed] [Google Scholar]
- 43.Sicard KM, Henninger N, Fisher M, et al. Long-term changes of functional MRI-based brain function, behavioral status, and histopathology after transient focal cerebral ischemia in rats. Stroke 2006; 37: 2593–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ejaz S, Williamson DJ, Ahmed T, et al. Characterizing infarction and selective neuronal loss following temporary focal cerebral ischemia in the rat: a multi-modality imaging study. Neurobiol Dis 2013; 51: 120–132. [DOI] [PubMed] [Google Scholar]
- 45.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 46.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.