Abstract
The inability to quantify cerebral blood flow and changes in macrocirculation cross-sectional area in all brain regions impedes robust insight into hypoxic cerebral blood flow control. We applied four-dimensional flow magnetic resonance imaging to quantify cerebral blood flow (ml • min−1) and cross-sectional area (mm2) simultaneously in 11 arteries. In healthy adults, blood pressure, O2 Saturation (SpO2), and end-tidal CO2 were measured at baseline and steady-state hypoxia (FiO2 = 0.11). We investigated left and right: internal carotid, vertebral, middle, anterior, posterior cerebral arteries, and basilar artery. Hypoxia (SpO2 = 80±2%) increased total cerebral blood flow from 621±38 to 742±50 ml • min−1 (p < 0.05). Hypoxia increased cerebral blood flow, except in the right posterior cerebral arteries. Hypoxia increased cross-sectional area in the anterior arteries (left and right internal carotid arteries, left and right middle, p < 0.05; left and right anterior p = 0.08) but only the right vertebral artery of the posterior circulation. Nonetheless, relative cerebral blood flow distribution and vascular reactivity (Δ%cerebral blood flow • ΔSpO2−1) were not different between arteries. Collectively, moderate hypoxia: (1) increased cerebral blood flow, but relative distribution remains similar to normoxia, (2) evokes similar vascular reactivity between 11 arteries, and (3) increased cross-sectional area primarily in the anterior arteries. This study provides the first wide-ranging, quantitative, functional and structural data regarding intracranial arteries during hypoxia in humans, highlighting cerebral blood flow regulation of microcirculation and macrocirculation differs between anterior and posterior circulation.
Keywords: Cerebral blood flow, cerebral blood flow measurement, magnetic resonance imaging, physiology, vascular biology
Introduction
The human brain has a critical requirement for cerebral blood flow (CBF) to meet constantly high metabolic demands. The inability to maintain appropriate CBF is linked to many neurologic and cerebrovascular diseases, such that cerebrovascular health remains a substantial concern for the American population.1,2 These diseases are commonly expressed heterogeneously across brain regions, such that, non-uniform vascular reactivity or CBF distribution may serve to prevent, or promote, pathologic progression.
With the prevalence of disorders like sleep apnea, as well as human visitation to high altitude, cerebrovascular responses to hypoxia have increased in relevance.3,4 Evidence in both animal5,6 and human studies7–9 suggest the CBF response to hypoxia may be regionally specific, but conclusions are limited due to limited number of arteries studied, as well the use of Doppler methods which primarily provide blood velocity but not volumetric flow. For example, during moderate hypoxia (SpO2 above 80% associated with sleep apnea), changes in blood velocity were observed in the cerebral arteries, while no changes in CBF were observed in extracerebral vessels.7 Interestingly, the posterior cerebral circulation (vertebral artery) displayed greater reactivity compared to the anterior circulation (internal carotids and middle cerebral arteries) during severe hypoxia (SpO2 = 70%), suggesting CBF responses to hypoxia are non-uniform.7 However, recent discovery of middle cerebral artery dilation during hypoxic stress10,11 has highlighted the difficulty in interpreting physiological significance of changes in blood velocity alone, without providing volumetric flow measures that incorporate vessel area changes. In fact, quantifying CBF in cerebral arteries is rarely accomplished due to poor ultrasound access to intracranial vessels. Taken together, comprehensive and quantitative examination of the conduit cerebral arterial structural and functional responses to hypoxia have not yet been attained in humans. Consequently, mechanistic insight into where and how CBF is regulated during systemic hypoxia has been hindered.
Therefore, the purpose of this experiment was to quantify total and regional CBF, cerebral arterial cross-sectional area (CSA), and CBF distribution during systemic hypoxia in conscious humans, to comprehensively test whether hypoxic cerebrovascular reactivity differs between brain regions. This investigation was possible due to recent advances in magnetic resonance imaging (MRI) methodology with a 4D MR Flow12 approach with high spatial resolution called Phase Contrast Vastly undersampled Isotropic Projection Reconstruction (PC VIPR).13,14 This methodology allows non-invasive measurement of human cerebral vascular CSA and flow over a large imaging volume within a single acquisition, without use of a contrast agent.15–22 Simultaneous measurement of volumetric flow and CSAs of 11 major cerebral arteries offers extensive new insight into whether hypoxic cerebral vasodilation varies between extracerebral versus cerebral, or anterior versus posterior arteries, in addition to whether macrocirculation contributes to change in vascular resistance that are observed.
Methods
Subjects and informed consent
Twelve healthy young volunteers (six females, 30 ± 2 years, Table 1) were enrolled in the study. Subjects were lean (BMI < 25 kg • m−2), normotensive, non-smoking, and not currently on any medications. Subjects were instructed to arrive on the study day fasted (≥ 4 h) and to refrain from caffeine, exercise, alcohol, over the counter medications, supplements, and nonsteroidal anti-inflammatory drugs for a minimum of 24 h prior to study day. Women were studied during the early follicular phase of the menstrual cycle (days 1–5) and were not pregnant (urine pregnancy test). Procedures were approved by the Institutional Review Board of the University of Wisconsin – Madison and conformed to the standards set by the Declaration of Helsinki. Written informed consent was obtained before study participation.
Table 1.
Subject characteristics.
| Characteristic | Mean ± SE |
|---|---|
| Age (years) | 30 ± 2 |
| Height (cm) | 174 ± 2 |
| Mass (kg) | 70 ± 3 |
| Body mass index (kg • m−2) | 23 ± 0.5 |
| Waist circumference (cm) | 81 ± 2 |
| Hip circumference (cm) | 99 ± 1 |
| Fasting glucose (mg • dL−1) | 70 ± 2 |
| Total cholesterol (mg • dL−1) | 155 ± 9 |
| High-density lipoprotein (mg • dL−1) | 62 ± 5 |
| Low-density lipoprotein (mg • dL−1) | 78 ± 14 |
| Triglycerides (mg • dL−1) | 83 ± 14 |
| Systolic blood pressure (mmHg) | 119 ± 2 |
| Diastolic blood pressure (mmHg) | 75 ± 2 |
| Mean arterial pressure (mmHg) | 90 ± 2 |
Screening visit
Before participation in the study, all subjects completed a laboratory visit to determine eligibility and safety before completing the experimental procedures. During this visit, height, weight, hip and waist circumference, fasting blood glucose and lipids, as well as blood pressure were measured.
Instrumentation
After the screening visit, the imaging study was completed on a clinical 3 T MRI system (Discovery MR750, GE Healthcare, Waukesha, WI, USA) using an eight-channel head coil. Heart rate (HR, pulse rate via pulse oximeter), blood pressure (MAP, by automated sphygmomanometry), arterial oxygen saturation (SpO2, pulse oximeter), and breath-by-breath end-tidal CO2 (PETCO2)7 were collected continuously throughout the imaging study and acquired with an MRI compatible monitor (Medrad Veris MR Vital Signs Patient Monitor, Bayer Healthcare, Whippany, NJ, USA). In addition, the subject wore a two-way non-rebreathing Hans-Rudolph valve (2630 series, Hans Rudolph Inc., Shawnee, KS) and nose clip to experimentally control gas concentrations of inhaled air.
Hypoxia
Initially, a 3-min scout scan was conducted to prescribe the 4D Flow MR imaging volume over the Circle of Willis. After the scout scan, subjects rested quietly while breathing room air for 2 min before the acquisition of baseline PC VIPR flow MRI scans. After normoxia baseline scans, subjects breathed a hypoxic gas mixture (11% O2, and balance nitrogen). To maintain isocapnia, breath-by-breath PETCO2 was monitored, and CO2 (100% CO2) was titrated into the gas mixture to maintain a steady level of baseline PETCO2. Once a steady level of oxygen saturation and end-tidal CO2 was reached (no change in % SpO2 and PETCO2 for > 1 min), typically within 5 min, CBF was measured with PC VIPR MRI.
Flow imaging
4D Flow MRI measurements were obtained with PC VIPR, which has been shown to provide accurate measurement of pulsatile blood flow in cerebral arteries.15 The scan parameters used were: imaging volume = 22 × 22 × 22 cm3, (0.69 mm)3 acquired isotropic resolution, scan time = 5 min 30 s, velocity encoding (Venc) = 100 cm/s, flip angle = 20°, TR/TE = 6.7/2.8 ms, 20 reconstructed cardiac time frames using retrospective cardiac gating and temporal view sharing.17,23
Post-processing
All PC VIPR flow MRI processing was completed using an in-house tool developed in commercial software (Matlab, The Mathworks, Natick, MA, USA). This tool has been show to be a reliable, fast, and user-independent method for the assessment of intracranial arteries from 4D flow MRI.16,17 With a centerline-processing scheme, a user can interactively select a vessel position and an underlying algorithm conducts the segmentation of a vessel plane that is perpendicular to the local vessel path and one voxel width thick (0.69 mm) to simultaneously provide the corresponding velocity, CSA, and flow measures.
Flow and CSA parameters were averaged over five consecutive cross sections to produce a 0.69 mm × 5 = 3.45 mm long segment in each artery of interest. This averaging of cross sections provides a more robust estimate of the flow parameters,16 especially in the presence of complex flow near branches. Eleven arteries of interest were assessed for each subject as shown in Figure 1: left and right vertebral arteries (VA), the inferior basilar artery (BA), left and right internal carotid arteries (ICA), left and right middle (MCA), left and right anterior (ACA), and left and right posterior cerebral arteries (PCA). VA flow was measured 4–5 mm from the junction with the basilar artery, and basilar flow was assessed in the most inferior portion near this junction. PCA measurements were taken 4–5 mm from the junction with the BA. ICA measurements were performed in the straight portion of the ICA C4 segment.24 ACA (A1 segment) and MCA (M1 segment) measurements were performed 4–5 mm from their junction with the ICA. Total CBF was calculated as the sum of ICAs and BA as all the flow into the brain will have derived from these arteries (flow from VA feed the BA).25 Intracerebral blood flow was calculated as the sum of ACAs, MCAs, and PCAs. Therefore, ICAs, VAs, and BA were referred to as extracerebral arteries, and ACAs, MCAs, and PCAs were referred to as intracerebral arteries. Vascular reactivity was calculated as the Δ in blood flow relative to the Δ in SpO2 from normoxia to hypoxia (Δ ml • min−1 • Δ SpO2−1). Relative vascular reactivity was calculated as the %Δ in blood flow relative to the Δ in SpO2 (Δ% ml • min−1 • Δ SpO2−1). Flow distribution was calculated as the flow through the given artery divided by CBF, multiplied by 100%.25
Figure 1.
Four-dimensional MRI- (PC VIPR) derived angiograms from a representative subject without contrast agent. Blue squares indicate cut-planes for 3.45 mm long measurements within a given vessel. (a) Coronal view, (b) Axial view, (c) Sagittal view of intracranial arteries segmented from the PC VIPR angiogram. Angiograms with overlaid velocity tracings along measurement points (d) Coronal view, (e) Axial view, and (f) Sagittal view. Arteries examined: ICA: right (r) and left (l) internal carotid artery; VA: right (r) and left (l) vertebral artery; BA: basilar artery; MCA: right (r) and left (l) middle cerebral artery; ACA: right (r) and left (l) anterior cerebral artery; PCA: right (r) and left (l) posterior cerebral artery.
Statistical analysis
Statistical analysis was completed using SigmaPlot Version 12.0 (Systat Software, Inc.). Not all arterial responses passed in Shaprio–Wilk tests of normality. Therefore, non-parametric statistical tests were used as a more conservative statistical approach for all variables. Subject characteristics and hemodynamic values were analyzed using a Wilcoxon Signed Rank Test to determine differences between normoxia and hypoxia. Changes in CSA, diameter, and blood flow from baseline (Δ) were tested with One-Sample Wilcoxon Signed Rank Test to determine if the values are different than zero (zero would represent no change from baseline). Differences between reactivity of vessels were examined with Kruskal-Wallis One Way ANOVA. Multiple comparisons were examined with Tukey test. All data are presented as mean ± standard error, and significance was determined a priori at p < 0.05.
Results
Figure 1 illustrates representative images generated from the PC VIPR data. The centerline maps of the entire arterial circulation facilitated artery selection and quantification of key variables in all major cerebral arteries simultaneously. Figure 1(a) to (c) identifies the locations for five single voxel slices used for quantification of blood flow and vessel characteristics. All subjects had normal angiograms with only one presenting P1 hypoplasia and a fetal type left PCA, which is prevalent in 15%–32% of healthy humans.26 Additionally, one subject had no visible left VA, and a separate volunteer had no visible right VA. Therefore, statistical analysis of variables derived from each of those arteries was conducted with an n = 11.
Systemic hemodynamic responses are summarized in Table 2. Hypoxia increased HR and blood pressure (Table 2) (p < 0.05), and reduced arterial saturation from 98±1% to 80±2% (Table 2). PETCO2 decreased slightly during hypoxia (∼1 mmHg, p = 0.03), despite monitoring PETCO2 and titration of CO2 into gas mixture to maintain isocapnia.
Table 2.
Hemodynamic parameters during normoxia (baseline) and hypoxia.
| Normoxia | Hypoxia | |
|---|---|---|
| Heart rate (bpm) | 66 ± 2 | 82 ± 2* |
| SpO2 (%) | 98 ± 0.4 | 80 ± 2* |
| MAP (mmHg) | 98 ± 3 | 101 ± 3* |
| Systolic BP (mmHg) | 132 ± 4 | 133 ± 4 |
| Diastolic BP (mmHg) | 82 ± 3 | 85 ± 3* |
| PETCO2 (mmHg) | 40 ± 0.6 | 39 ± 0.8* |
Data are presented as mean ± SE. Hypoxia increased HR, MAP, DBP, and reduced SpO2 and PET CO2.
MAP: mean arterial pressure; BP: blood pressure.
Significantly different between hypoxia and normoxic baseline (P < 0.05).
Hypoxia increased total CBF from 626 ± 37 to 742 ± 50 ml • min−1 representing a 19 ± 3% increase from baseline (p < 0.05). Similar increases in CBF were observed when calculating extracerebral (BA and both ICAs, Δ 120 ± 24 ml • min−1) or intracerebral flow (2 MCAs + 2 ACA + 2 PCAs, Δ 113 ± 26 ml • min−1). The intracerebral CBF was quantitatively lower than the total CBF due to contributions to smaller unexamined vessels.16,25
Blood flow increased in all arteries examined, except the right PCA (Figure 2(a)). Interestingly, the relative distribution of flow throughout the brain was unaltered by hypoxia (Figure 2(b)). When expressed as relative increase in CBF, to account for differences in normoxic baseline CBF between different arteries, the increase in CBF was similar across all 11 arteries (Figure 2(c), p > 0.05).
Figure 2.
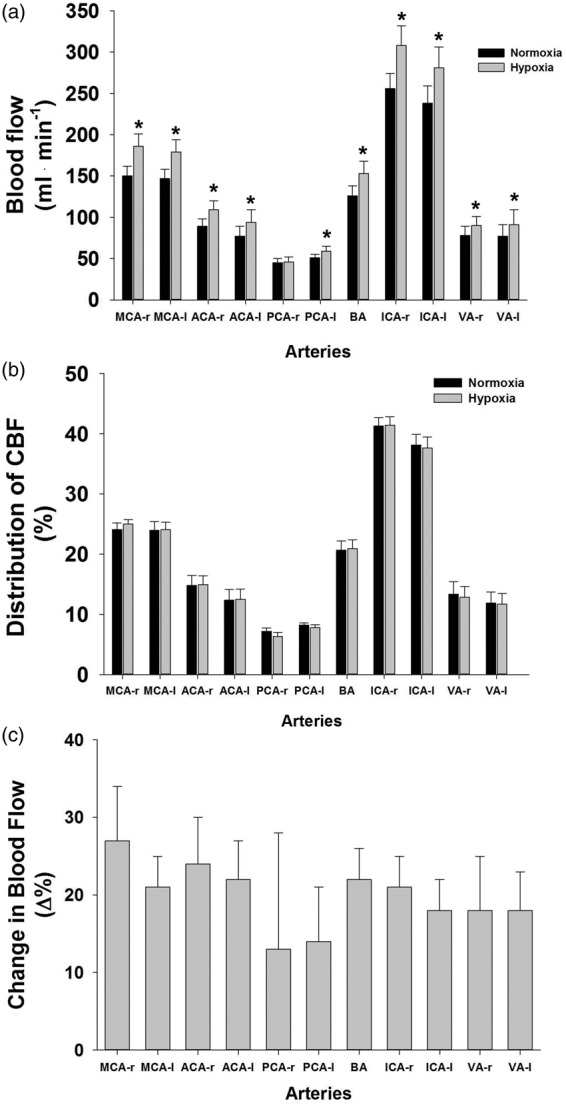
Cerebral blood flow quantified in 11 arteries supplying the brain. (a) absolute blood flow in each artery at baseline and hypoxia, (b) percent distribution of blood flow in each artery at baseline and hypoxia, and (c) percent change in blood flow from baseline. Data are mean ± SE, * indicates statistical significance from normoxia, p < 0.05. VA—r and VA—l n = 11.
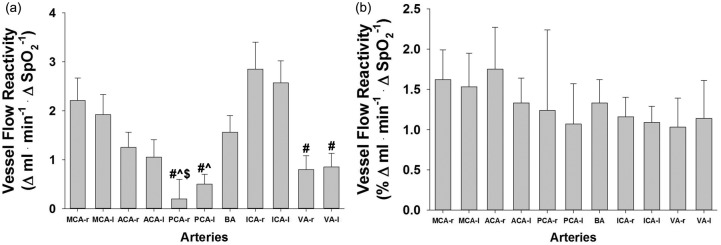
To assess if hypoxic sensitivity differed between vessels, flow reactivity was calculated (Figure 3). In terms of absolute flow (Δ ml • min−1 • Δ SpO2−1, Figure 3(a)), vessels of the posterior circulation (PCAs and VAs) were found to be significantly less reactive than the ICAs. However, when compared on a relative CBF basis, all vessels were found to have a similar vascular reactivity to the hypoxia ranging between 1% and 1.6%Δ ml • min−1 • Δ SpO2−1 (Figure 3(b)).
Figure 3.
Hypoxic vascular reactivity in 11 arteries supplying the brain. (a) Absolute blood flow reactivity and (b) Relative blood flow reactivity. Data are means ± SE, #, significantly different than ICA—r,, significantly different than ICA—l, $, significantly different than MCA—r, p < 0.05. VA (r and l) n = 11.
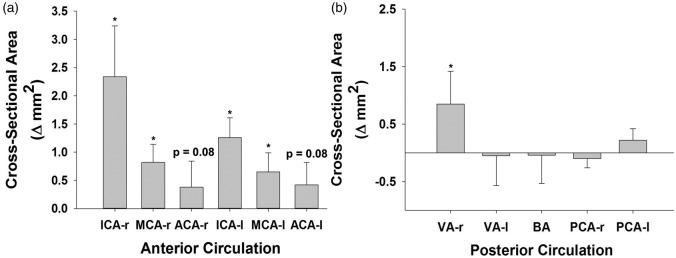
CSA and arterial diameter increased in response to hypoxia in several arteries (Figure 4, Table 3). Most arteries associated with the anterior cerebral circulation dilated. Specifically, both right and left ICAs and MCAs were observed to dilate (∼15 ± 5% and 11 ± 4%, p < 0.05), and ACA vessels trended to increase (∼ Δ 8 ± 7%, p = 0.08). In contrast, CSA of most arteries of the posterior circulation (BA, PCA, VA) remained unchanged to hypoxia, with the exception of right VA dilation (Figure 4(b)).
Figure 4.
Hypoxia-induced change in cross-sectional area (CSA) from baseline. (a) Arteries of the anterior circulation and (b) Arteries of the posterior circulation. Data ± SE, * indicates Δ statistically significant p < 0.05. VA (r–l) n = 11.
Table 3.
Artery cross-sectional area and diameter at normoxia (baseline) and during systemic hypoxia.
| CSA (mm2) |
Diameter (mm) |
||||
|---|---|---|---|---|---|
| Artery | Normoxia | Hypoxia | Normoxia | Hypoxia | |
| ICA | Right | 13.5 ± 1.3 | 15.8 ± 1.6* | 4.1 ± 0.2 | 4.4 ± 0.2* |
| Left | 13.0 ± 1.5 | 14.3 ± 1.7* | 4.0 ± 0.2 | 4.2 ± 0.2* | |
| MCA | Right | 6.7 ± 0.4 | 7.5 ± 0.5* | 2.9 ± 0.1 | 3.1 ± 0.1* |
| Left | 6.9 ± 0.4 | 7.5 ± 0.6* | 2.9 ± 0.1 | 3.1 ± 0.1* | |
| ACA | Right | 5.2 ± 0.4 | 5.6 ± 0.4 | 2.6 ± 0.1 | 2.7 ± 0.1 |
| Left | 4.9 ± 0.4 | 5.3 ± 0.7 | 2.5 ± 0.1 | 2.6 ± 0.2 | |
| VA | Right | 5.6 ± 0.7 | 6.4 ± 1.0* | 2.5 ± 0.3 | 2.7 ± 0.3* |
| Left | 6.4 ± 1.0 | 6.3 ± 1.0 | 2.4 ± 0.4 | 2.4 ± 0.3 | |
| BA | 7.4 ± 0.7 | 7.3 ± 0.6 | 2.4 ± 0.2 | 2.3 ± 0.1 | |
| PCA | Right | 3.8 ± 0.2 | 3.7 ± 0.2 | 4.2 ± 0.2 | 4.5 ± 0.3 |
| Left | 2.2 ± 0.1 | 2.2 ± 0.1 | 2.3 ± 0.1 | 2.4 ± 0.1 | |
Data are presented as mean ± SE. Anterior circulation is the top portion of table, and bottom portion of the table is the posterior circulation. ICA: internal carotid arteries; MCA: middle cerebral arteries; ACA: anterior cerebral arteries; VA: vertebral arteries; BA: basilar artery; PCA: posterior cerebral arteries; CSA: cross-sectional area.
Significantly different between hypoxia and normoxic baseline (p < 0.05).
Discussion
To our knowledge, this is the first study in humans to simultaneously quantify regional cerebrovascular functional responses to hypoxic exposure, in all medium to large cerebral arteries. The novel findings of this study were: (1) CBF increased in 10 of the 11 vessels studied, (2) CBF distribution in intracerebral arteries remained similar to normoxia; (3) relative vascular reactivity to hypoxia is similar between extracerebral and intracerebral arteries; and (4) anterior conduit arteries dilate in response to hypoxia more so than posterior arteries. Collectively, the results of this study show that regional blood flow responses and relative vascular reactivity to moderate hypoxia are fairly uniform throughout the cerebral circulation. However, CSA changes in arteries feeding anterior brain regions suggest regulation of arterial resistance differs between the anterior and posterior circulations.
CBF and distribution response to hypoxia
Absolute CBF during resting normoxia (626 ± 37 ml • min−1) is similar to other human studies using ultrasound or MRI technologies.17,27 By measuring total and regional CBF, our data allow the opportunity to better understand distribution of CBF under both normoxic and hypoxic conditions. The first observation is that during normoxia, the relative CBF (% of total) through the arteries examined was similar to those observed at rest using similar MRI scanning techniques.25 The absolute increases in CBF in response to hypoxia in each vessel varied widely (Figure 2(a)), with the greatest increases in ICA, since they provide CBF to several intracerebral arteries. Surprisingly, the relative CBF distribution was unchanged during hypoxia (Figure 2(b)), offering the first comprehensive data supporting that at moderate levels of hypoxia, each macrovascular region responds similarly to the reduction in arterial oxygen content.
Reactivity to hypoxia
Due to differences in severity of systemic isocapnic hypoxia in the literature, comparing reactivity (both total and vessel specific) appears to be the most appropriate (% Δ ml • min−1 • Δ SpO2−1). The ∼1%–1.6% increase per % decrease in SpO2 observed in the current study (Figure 3(b)) fits within the range of 0.5%–2.5% increase per decrease of SpO2 reported in the literature using various CBF measurements and degrees of isocapnic hypoxia.7,10,11,28–34 Regionally, specific vascular hypoxic reactivity to SpO2 ∼80% was quite similar across 11 arteries (Figure 3(b)). Thus, our work verifies previous studies suggesting hypoxic reactivity is similar in ICA and VA during mild to moderate hypoxia, however, do not support the concept of regional differences in hypoxic reactivity.7 Importantly, new findings extend this observation to seven additional arteries, including intracerebral vessels that are difficult to access. Taken together, this study provides the first data to quantify CBF responses in MCA, ACA, PCA, and BA, which collectively indicate a largely uniform hypoxic vascular reactivity across all medium to large cerebral arteries.
Literature directly comparing regional CBF responses to hypoxia is sparse. In the most comprehensive prior study, Willie et al.7 quantified blood flow responses in the left ICA and right VA (echo and Doppler ultrasound), as well as blood velocity responses in a MCA and a PCA (trans-cranial Doppler, TCD) to graded isocapnic hypoxia (∼SpO2 70%, 80%, 90%). Interestingly, Willie et al. found CBF was unchanged in the neck vessels at 80% SpO2 despite increases in blood velocity in the PCA and MCA. In fact, CBF only increased in the extracerebral vessels at 70% SpO2,7 when the VA demonstrated 50% greater reactivity than ICA, suggesting flow increases to a greater extent in the VA supplying blood to the brainstem.7
The present data are the first to use absolute measures of CBF to reflect true reactivity in a comprehensive approach across the cerebral artery circulation. Previous work in both animals and humans has provided indirect evidence that hypoxic vascular reactivity is similar between regions.28,35,36 Physiologic insight has been limited in these studies due to lack of fully quantified CBF responses and/or a limited number of arteries studied. Despite these limitations, previous work largely argues regional reactivity is uniform. Specifically, our results confirm prior reports from Willie et al.7,28 that VA and ICA exhibit similar reactivity at ∼80% hypoxia. Only at more severe hypoxia (70%) did the data suggest relative vascular reactivity to hypoxia is higher in VA,28 which to our knowledge are the only data from humans suggesting extracerebral vascular reactivity may differentiate at more severe desaturation.
The report of greater reactivity in VA versus ICA at SpO2 = 70% suggests more severe hypoxia may uncover heterogeneous vascular reactivity or regional CBF distribution. Our choice of ∼80% SpO2 was aimed toward applied relevance since most mountaineering activities and even clinically relevant sleep disorders are experienced above 80% SpO2.28,37 Other potential differences in reactivity may be due to differences in experimental protocols. It is plausible that brainstem blood flow is sensitive to duration (≥ 30 min) and/or severity of hypoxia.7,27 The current protocol used less than 10 min exposure. Additionally, technical differences between MRI and ultrasound may have contributed to the small differences between studies.
A final consideration is the segment of artery examined differs between studies. Specifically, the imaging volume for PC VIPR scans was centered over the Circle of Willis, and therefore measurements of ICA and VA flow were performed superior (anatomically) to those made in previous studies using ultrasound.7,30 However, this is unlikely to explain any differences in results, since these data add to two independent studies suggesting ICA and VA hypoxia reactivity is similar above a SpO2 of 80%.7,30 Thus, three reports suggest the majority of the segments of ICAs, and VAs exhibit similar hypoxic reactivity to moderate systemic hypoxia. Collectively, the most likely explanation for greater VA reactivity in the previous study7 is more severe hemoglobin desaturation.
Taken together with the relative distribution of CBF during 80% hypoxia (Figure 2), our data strongly support the conclusion that during moderate hypoxia, the overarching, integrated hypoxic vascular reactivity does not differ between the posterior and anterior cerebral circulations, or between intracerebral and extracerebral arteries.
Contribution of CSA to CBF
Table 3 and Figure 4 summarize changes observed in CSA and diameter. These data support previous studies demonstrating changes in MCA,10,11, ICA,27,28 and VA27 diameter contribute to regulation of cerebrovascular resistance during hypoxia.10,11 This study provides new data on intracranial artery CSA (2 × ACA, 2 × PCA, and BA), which provides novel insight into cerebrovascular control that was previously unattainable. These new data demonstrate arteries of the anterior circulation vasodilate (2 × ICA, 2 × MCA, trend for 2 × ACA, Figure 4(b)), whereas, posterior arteries (PCA, BA) do not dilate with the exception of the right VA (Figure 4(b)). These data have noteworthy implications for understanding hypoxic vascular control. First, studies which do not account for increases in arterial diameter38 may severely underestimate CBF responses, and therefore limit insight into the contribution of conduit arteries to CBF regulation. Second, despite similar integrative hypoxic vascular reactivity (Figure 3(b)) between anterior and posterior cerebral arteries, the anatomical sites where cerebrovascular resistance changes with hypoxia are not uniform throughout the brain (Figure 4). In other words, anterior and posterior arteries achieve increased CBF differently from one another. The absence of an increase in CSA in most of the posterior circulation (VAs, BA, PCAs) indicates downstream pial arteries are the primary sites of vascular control in response to 80% SpO2 hypoxia. Conversely, CSA increases in ICAs, MCAs, and a strong trend for ACAs (Table 3) indicate that in addition to pial arteries, the macrocirculation plays an important role in hypoxic CBF regulation in the anterior circulation.
Experimental considerations
There are a few important limitations that should be noted. Despite the attempt to maintain isocapnia by titration of CO2, we observed a slight decrease of ∼1 mmHg in PETCO2. Therefore, it is possible that our values slightly underestimate the hypoxic response, as it is well documented that hypocapnia lowers hypoxic increases in CBF.39,40 Blood pressure during screening visit indicated healthy levels, but blood pressure was higher during scanning procedures. We speculate the elevated blood pressure is due to the stress of being placed inside the MRI bore, with a mouthpiece and nose clip inside of a very confining head coil. Importantly, blood pressure did not change drastically with hypoxia, and expressing data relative to perfusion pressure (vascular conductance, data not shown) did not alter conclusions. Additionally, we did not examine greater severity of hypoxia, where regional differences in integrated hypoxic reactivity, or distribution of flow, may be uncovered.7 Finally, we have no direct measures of arterial blood gases. We substituted measures of breath-by-breath PETCO2 as surrogate measures of arterial CO2 considering PETCO2 has shown to be strongly related to direct measures of arterial CO2 (r2 = 0.98).7
An additional consideration is the MRI sequence used in the current study, PC VIPR. PC VIPR is not a commercially available scanning sequence, however, it has been validated extensively for anatomical and hemodynamic assessment. In vivo validation of flow measures is challenging for the lack of a gold standard. Prior work with traditional manual placement of analysis planes and vessels contours includes: (1) vessel area comparisons with contrast-enhanced MRA in the abdomen,41 (2) pressure measurements compared with catheter-based invasive measures in a canine neck aneurysm model,42 a canine carotid artery stenosis model,43 and a porcine study of renal artery stenosis,44 (3) flow comparisons with 2D PC MRI in the chest,45 abdomen,46 and for intracranial vessels,15 and (4) flow comparisons with TCD and 2D PC MRI for intracranial vessels.20 Good correlations were found for vessel area measurements across various diameters41 as well as flow measures obtained with 2D PC and TCD with systematically lower peak flow for 2D and 4D PC MRI compared to TCD.20 Processing with the recently introduced automated processing procedure used here was validated in a cranial phantom and in vivo study and ensures full reproducibility by eliminating user input in the segmentation/contour processing.16 Measures are established at five consecutive planes generated automatically perpendicular to the centerline across a 3.5 mm segment of each artery, to reduce the impact of any potential measurement errors.17 While even minor patient motion can impact high spatial resolution scans such as this one, radial acquisitions have been shown to suffer much less benign artifacts in form of averaging effects rather than ghosting artifacts seen in traditional spinwarp imaging.47 This property has found widespread use in PROPELLER-type diffusion brain imaging48 that also uses motions sensitizing bipolar gradients like PC VIPR.
Conclusions
The purpose of this experiment was to quantify CBF, distribution of CBF, and arterial CSA, in order to comprehensively assess the existence of regional differences within the cerebrovascular network during systemic hypoxia in humans. The ability to use advanced MRI methodology to simultaneously quantify CSA and volumetric flow through 11 cerebral arteries adds new quantitative structural and functional insight into control of CBF during hypoxia. Our results indicate during moderate hypoxia: (1) CBF increases in 10 of 11 arteries, however, distribution of CBF remains similar to normoxia, (2) integrated hypoxic vascular reactivity was similar between arteries of anterior and posterior circulation as well as between extracerebral and intracerebral arteries, (3) increases in artery CSA highlight the potential for regulation of CBF at the level of macrocirculation, and (4) increases in CSA occur predominantly in the anterior circulation, suggesting a differential regulation of vascular resistance in anterior compared to posterior cerebral arteries. This study provides the first detailed, quantitative, functional, and structural data of intracranial vessels during hypoxia in humans. Considering human travel to altitudes and nocturnal desaturations during sleep apnea, regional differences in vascular control observed in this study hold important implications for deeper understanding of cerebrovascular health and disease.
Acknowledgments
We would like to thank the subjects for their time and effort. We would also like to acknowledge Cameron Rousseau, Sara John, and Jenelle Fuller for their help in data collection.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This experiment was funded by the American Diabetes Association ADA 1-12-IN-39. J. Mikhail Kellawan is supported by the American Heart Association, AHA-15POST23100020.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
JMK: Acquisition of data, data analysis and interpretation, drafted the article, approved the version to be published. JWH: Contribution to concept and design, acquisition of data, revised it critically for important intellectual content, approved the version to be published. ARA: Contribution to concept and design, acquisition of data, revised it critically for important intellectual content, approved the version to be published. OW: Contribution to concept and design, acquisition of data, revised it critically for important intellectual content, approved the version to be published. WGS: Contribution to concept and design, acquisition of data, revised it critically for important intellectual content, approved the version to be published.
References
- 1.Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement 2015; 11: 332–384. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 2014; 129: e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kety SS, Schmidt CF. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J Clin Invest 2004; 27: 484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ainslie PN, Ashmead JC, Ide K, et al. Differential responses to CO2 and sympathetic stimulation in the cerebral and femoral circulations in humans. J Physiol 2005; 566: 613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nolan WF, Davis DG. Effect of craniotomy on regional brain blood flow and ECF pH changes during isocapnic hypoxia. Brain Res 1982; 241: 388–392. [DOI] [PubMed] [Google Scholar]
- 6.Neubauer JA, Edelman NH. Nonuniform brain blood flow response to hypoxia in unanesthetized cats. J Appl Physiol Respir Environ Exerc Physiol 1984; 57: 1803–1808. [DOI] [PubMed] [Google Scholar]
- 7.Willie CK, Macleod DB, Shaw AD, et al. Regional brain blood flow in man during acute changes in arterial blood gases. J Physiol 2012; 590: 3261–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jansen GFA, Kagenaar DA, Basnyat B, et al. Basilar artery blood flow velocity and the ventilatory response to acute hypoxia in mountaineers. Respir Physiol Neurobiol 2002; 133: 65–74. [DOI] [PubMed] [Google Scholar]
- 9.Binks AP, Cunningham VJ, Adams L, et al. Gray matter blood flow change is unevenly distributed during moderate isocapnic hypoxia in humans. J Appl Physiol 2008; 104: 212–217. [DOI] [PubMed] [Google Scholar]
- 10.Wilson MH, Edsell MEG, Davagnanam I, et al. Cerebral artery dilatation maintains cerebral oxygenation at extreme altitude and in acute hypoxia—an ultrasound and MRI study. J Cerebr Blood Flow Metabol 2011; 31: 2019–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imray C, Chan C, Stubbings A, et al. Time course variations in the mechanisms by which cerebral oxygen delivery is maintained on exposure to hypoxia/altitude. High Alt Med Biol 2014; 15: 21–27. [DOI] [PubMed] [Google Scholar]
- 12.Markl M, Frydrychowicz A, Kozerke S, et al. 4D flow MRI. J Magn Reson Imaging 2012; 36: 1015–1036. [DOI] [PubMed] [Google Scholar]
- 13.Gu T, Korosec FR, Block WF, et al. PC VIPR: a high-speed 3D phase-contrast method for flow quantification and high-resolution angiography. AJNR Am J Neuroradiol 2005; 26: 743–749. [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson KM, Lum DP, Turski PA, et al. Improved 3D phase contrast MRI with off-resonance corrected dual echo VIPR. Magn Reson Med 2008; 60: 1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wåhlin A, Ambarki K, Birgander R, et al. Measuring pulsatile flow in cerebral arteries using 4D phase-contrast MR imaging. Am J Neuroradiol 2013; 34: 1740–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schrauben E, Wåhlin A, Ambarki K, et al. Fast 4D flow MRI intracranial segmentation and quantification in tortuous arteries. J Magn Reson Imaging 2015; 42: 1458–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kellawan JM, Harrell JW, Schrauben EM, et al. Quantitative cerebrovascular 4D flow MRI at rest and during hypercapnia challenge. Magn Reson Imaging 2016; 34: 422–428. [DOI] [PubMed] [Google Scholar]
- 18.Rivera-Rivera LA, Turski P, Johnson KM, et al. 4D flow MRI for intracranial hemodynamics assessment in Alzheimer’s disease. J Cerebr Blood Flow Metabol 2015. DOI: 10.1177/0271678X15617171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schrauben EM, Johnson KM, Huston J, et al. Reproducibility of cerebrospinal venous blood flow and vessel anatomy with the use of phase contrast-vastly undersampled isotropic projection reconstruction and contrast-enhanced MRA. Am J Neuroradiol 2014; 35: 999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang W, Landgraf B, Johnson KM, et al. Velocity measurements in the middle cerebral arteries of healthy volunteers using 3D radial phase-contrast HYPRFlow: comparison with transcranial Doppler sonography and 2D phase-contrast MR imaging. Am J Neuroradiol 2011; 32: 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edjlali M, Roca P, Gentric JC, et al. Advanced technologies applied to physiopathological analysis of central nervous system aneurysms and vascular malformations. Diagn Interv Imaging 2014; 95: 1187–1193. [DOI] [PubMed] [Google Scholar]
- 22.Berman SE, Rivera-Rivera LA, Clark LR, et al. Intracranial arterial four-dimensional flow is associated with metrics of brain health and Alzheimer’s disease. Alzheimer’s Dement 2015; 1: 420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Redmond MJ, Brodsky EK, et al. Generation and visualization of four-dimensional MR angiography data using an undersampled 3-D projection trajectory. IEEE Trans Med Imaging 2006; 25: 148–157. [DOI] [PubMed] [Google Scholar]
- 24.Bouthillier A, van Loveren HR, Keller JT. Segments of the internal carotid artery: a new classification. Neurosurgery 1996; 38: 425–432. Discussion 432–433. [DOI] [PubMed] [Google Scholar]
- 25.Zarrinkoob L, Ambarki K, Wåhlin A, et al. Blood flow distribution in cerebral arteries. J Cerebr Blood Flow Metabol 2015; 35: 648–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaban A, Albright KC, Boehme AK, et al. Circle of Willis variants: fetal PCA. Stroke Res Treat 2013; 2013: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis NCS, Messinger L, Monteleone B, et al. Effect of acute hypoxia on regional cerebral blood flow: effect of sympathetic nerve activity. J Appl Physiol 2014; 116: 1189–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willie CK, Smith KJ, Day TA, et al. Regional cerebral blood flow in humans at high altitude: gradual ascent and 2 wk at 5,050 m. J Appl Physiol 2014; 116: 905–910. [DOI] [PubMed] [Google Scholar]
- 29.Hoiland RL, Bain AR, Rieger MG, et al. Hypoxemia, oxygen content, and the regulation of cerebral blood flow. AJP Regul, Integr Comp Physiol 2016; 310: R398–R413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogoh S, Sato K, Nakahara H, et al. Effect of acute hypoxia on blood flow in vertebral and internal carotid arteries. Exp Physiol 2013; 98: 692–698. [DOI] [PubMed] [Google Scholar]
- 31.Reichmuth KJ, Dopp JM, Barczi SR, et al. Impaired vascular regulation in patients with obstructive sleep apnea. Am J Respir Crit Care Med 2009; 180: 1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen JB, Sperling B, Severinghaus JW, et al. Augmented hypoxic cerebral vasodilation in men during 5 days at 3,810 m altitude. J Appl Physiol 1996; 80: 1214–1218. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro W, Wasserman AJ, Baker JP, et al. Cerebrovascular response to acute hypocapnic and eucapnic hypoxia in normal man. J Clin Invest 1970; 49: 2362–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Querido JS, Godwin JB, Sheel AW. Intermittent hypoxia reduces cerebrovascular sensitivity to isocapnic hypoxia in humans. Respir Physiol Neurobiol 2008; 161: 1–9. [DOI] [PubMed] [Google Scholar]
- 35.Julien-Dolbec C, Tropres I, Montigon O, et al. Regional response of cerebral blood volume to graded hypoxic hypoxia in rat brain. Br J Anaesth 2002; 89: 287–293. [DOI] [PubMed] [Google Scholar]
- 36.Dyer EAW, Hopkins SR, Perthen JE, et al. Regional cerebral blood flow during acute hypoxia in individuals susceptible to acute mountain sickness. Respir Physiol Neurobiol 2008; 160: 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dempsey JA, Veasey SC, Morgan BJ, et al. Pathophysiology of sleep apnea. Physiol Rev 2010; 90: 47–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coverdale NS, Gati JS, Opalevych O, et al. Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. J Appl Physiol 2014; 117: 1090–1096. [DOI] [PubMed] [Google Scholar]
- 39.Ainslie PN, Poulin MJ. Ventilatory, cerebrovascular, and cardiovascular interactions in acute hypoxia: regulation by carbon dioxide. J Appl Physiol 2004; 97: 149–159. [DOI] [PubMed] [Google Scholar]
- 40.Mardimae A, Balaban DY, Machina MA, et al. The interaction of carbon dioxide and hypoxia in the control of cerebral blood flow. Pflugers Arch – Eur J Physiol 2012; 464: 345–351. [DOI] [PubMed] [Google Scholar]
- 41.François CJ, Lum DP, Johnson KM, et al. Renal arteries: isotropic, high-spatial-resolution, unenhanced MR angiography with three-dimensional radial phase contrast. Radiology 2011; 258: 254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moftakhar R, Aagaard-Kienitz B, Johnson K, et al. Noninvasive measurement of intra-aneurysmal pressure and flow pattern using phase contrast with vastly undersampled isotropic projection imaging. Am J Neuroradiol 2007; 28: 1710–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turk AS, Johnson KM, Lum D, et al. Physiologic and anatomic assessment of a canine carotid artery stenosis model utilizing phase contrast with vastly undersampled isotropic projection imaging. AJNR Am J Neuroradiol 2007; 28: 111–115. [PMC free article] [PubMed] [Google Scholar]
- 44.Bley TA, Johnson KM, François CJ, et al. Noninvasive assessment of transstenotic pressure gradients in porcine renal artery stenoses by using vastly undersampled phase-contrast MR angiography. Radiology 2011; 261: 266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frydrychowicz A, Wieben O, Niespodzany E, et al. Quantification of thoracic blood flow using volumetric magnetic resonance imaging with radial velocity encoding: in vivo validation. Invest Radiol 2013. DOI: 10.1097/RLI.0b013e31829a4f2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wentland AL, Grist TM, Wieben O. Repeatability and internal consistency of abdominal 2D and 4D phase contrast MR flow measurements. Acad Radiol 2013; 20: 699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glover GH, Pauly JM. Projection reconstruction techniques for reduction of motion effects in MRI. Magn Reson Med 1992; 28: 275–289. [DOI] [PubMed] [Google Scholar]
- 48.Pipe JG. Motion correction with PROPELLER MRI: application to head motion and free-breathing cardiac imaging. Magn Reson Med 1999; 42: 963–969. [DOI] [PubMed] [Google Scholar]