Abstract
The hypothesis that chronic feeding of the triglycerides of octanoate (trioctanoin) and decanoate (tridecanoin) in “a regular non-ketogenic diet” is anticonvulsant was tested and possible mechanisms of actions were subsequently investigated. Chronic feeding of 35E% of calories from tridecanoin, but not trioctanoin, was reproducibly anticonvulsant in two acute CD1 mouse seizure models. The levels of beta-hydroxybutyrate in plasma and brain were not significantly increased by either treatment relative to control diet. The respective decanoate and octanoate levels are 76 µM and 33 µM in plasma and 1.17 and 2.88 nmol/g in brain. Tridecanoin treatment did not alter the maximal activities of several glycolytic enzymes, suggesting that there is no reduction in glycolysis contributing to anticonvulsant effects. In cultured astrocytes, 200 µM of octanoic and decanoic acids increased basal respiration and ATP turnover, suggesting that both medium chain fatty acids are used as fuel. Only decanoic acid increased mitochondrial proton leak which may reduce oxidative stress. In mitochondria isolated from hippocampal formations, tridecanoin increased respiration linked to ATP synthesis, indicating that mitochondrial metabolic functions are improved. In addition, tridecanoin increased the plasma antioxidant capacity and hippocampal mRNA levels of heme oxygenase 1, and FoxO1.
Keywords: Antioxidant, anti-seizure, decanoate, mitochondrial function, octanoate
Introduction
The ketogenic diet is a high fat, low carbohydrate and low protein diet that was first introduced in the 1920 s for the treatment of epilepsy, particularly in children before the development of anticonvulsant drugs. The classic ketogenic diet consists mainly of long-chain triglycerides and very few carbohydrates and protein. Alternatively, the ketogenic medium-chain triglycerides (MCTs) containing the triglycerides of eight-carbon octanoate (55% trioctanoin) and 10-carbon decanoate (45% tridecanoin) can be used to provide 50–60% of caloric intake,1 allowing higher amounts of protein and carbohydrates to be consumed.
In addition to elevated levels of ketone bodies1 (β-hydroxybutyrate, BHB and acetoacetate), a MCT ketogenic diet also leads to increases in octanoate and decanoate levels in the plasma,2 suggesting that both octanoate and decanoate might have direct anticonvulsant and neuroprotective effects. Indeed, acute administration of decanoate and sometimes octanoate was found to exert anticonvulsant effects in rodent seizure models. Pre-treatment with sodium decanoate, but not sodium octanoate (1 mmol/kg; i.p.), increased the latency to clonic seizure onset and survival time in picrotoxin- and pentylenetetrazole (PTZ)-induced seizures, respectively, in mice.3 Wlaź et al.4 showed that high amounts of decanoic acid (1.7–8.6 g/kg; p.o.) increased seizure thresholds in the 6 Hz and maximal electroshock test in mouse models.4 In another study, Wlaź et al.5 also found that oral administration of high amounts of octanoic acid (1.4–4.3 g/kg) increased seizure thresholds dose-dependently when given 30 min prior to 6 Hz and PTZ seizure tests.5 In contrast, only decanoic (1 mM) but not octanoic acids (1 mM) reduced the frequency of epileptiform discharges in a PTZ hippocampal slice model.6 With regard to chronic administration, our laboratory previously found anticonvulsant effects in the 6 Hz model after feeding the triglyceride of octanoate,7 but decanoate had not been tested. In summary, there is a lack of knowledge about the extent to which chronic administration of the medium chain fatty acids (MCFAs) is anticonvulsant, which was addressed in this study.
Previous work has shown that MCFAs can alter brain metabolism. For example, we found that chronic trioctanoin feeding increased the levels of brain glucose-6-phosphate and fructose-6-phopshate, but lowered the amounts of downstream glycolytic metabolites, indicating reduced glycolysis.7 Further to these effects, a number of studies suggested that MCFAs exert anti-inflammatory and antioxidant effects. In in vitro enzyme activity assays, both octanoate and decanoate (100 µg/mL) inhibited cyclooxygenase 1, but only decanoate inhibited cyclooxygenase 2 activity.8 In cultured osteoclasts, high concentrations of decanoate (1 mM) reduced lipopolysaccharide-induced nitric oxide production in conjunction with reduced protein levels of cyclooxygenase 2 and inducible nitric oxide synthase.9 A more recent study showed that treatment with decanoic acid (250 μM) but not octanoic acid for six days increased the activities of citrate synthase, complex I and catalase, in addition to an increased mitochondrial number in a neuronal cell line,10 suggestive of distinct mechanisms of action for octanoic and decanoic acids. In vivo, when decanoate was used to substitute 20% of long-chain fatty acids in native mustard oil, it enhanced the activities of several antioxidant enzymes including catalase, superoxide dismutase and glutathione peroxidase in both liver and brain tissues of rats when given as 20% of the total diet for 30 days.11
Both octanoic and decanoic acids are unsuitable for chronic administration, due to their acidity. Alternative administration approaches, such as the respective triglycerides, are essential to avoid acid or sodium overload especially for long-term treatment. Here, we investigated the anticonvulsant effects of trioctanoin or tridecanoin in two acute mouse seizure models. We then quantified the levels of octanoate and decanoate in plasma and brain and investigated potential alterations in several glycolytic enzymes, mitochondrial function as well as antioxidant effects in mice. Additionally, changes in oxygen consumption rate which reflect mitochondrial function were evaluated following incubation with octanoate and decanoate in cultured astrocytes.
Methods and materials
All chemicals and reagents were obtained from Sigma Aldrich (St. Louis, MO, USA) unless stated otherwise.
Animals
Male CD1 mice (Animal Resources Centre, Perth, WA, Australia) aged 7–8 weeks were housed individually under a 12-h light–dark cycle with free access to food and water. All experiments involving animals were conducted during the dark cycle from 6.00 p.m. to 9.00 p.m., except for the isolation of mitochondria from mouse hippocampal formations, which was conducted from 9.00 a.m. to 11.00 a.m. All experiments were approved by the animal ethics committee of the University of Queensland (SBMS/128/14) and followed the guidelines of the Queensland Animal Care and Protection Act 2001 to minimize the suffering of the animals. All work was performed according to the ARRIVE guidelines. Please note that we used only one outbred mouse strain, and therefore the results may not be representative for inbred or other outbred strains of mice.
Dietary treatment
Mice were randomly assigned to three diet groups and were given control, 35E% trioctanoin or 35E% tridecanoin diet for 10 days prior to acute seizure tests since we previously found that at least seven days of feeding was necessary to observe the anticonvulsant effects of both triheptanoin and trioctanoin.7 The compositions of all diets (Specialty Feeds, Perth, WA, Australia) were as previously described, except trioctanoin (Neobee 895) or tridecanoin (Neobee 1095; both gifted by Stepan Lipid Nutrition, Maywood, NJ, USA) was added just prior to diet preparation to provide 35% of calories, instead of triheptanoin.12 All diets were similar in the amounts of protein, vitamins and minerals relative to caloric density. Body weight changes of mice were monitored throughout the dietary treatment.
6 Hz and fluorothyl seizure tests
The 6 Hz seizure test was performed 10 days after treatment initiation as previously described.12 Briefly, 0.5% tetracaine was administered as topical anaesthetic to the corneas 15 min prior to seizure test. Mice were given a 3 s stimulation with 0.2 ms-duration pulses at 6 Hz using a constant current unit (ECT Unit 57800, Ugo Basile, Lombardy, Italy) and seizures were characterized by rearing, forelimb clonus and falling over. The “up and down” method with 2 mA interval was used, as previously described, to determine CC50 which is the current needed to induce seizures in 50% of the mice tested.
Similarly, fluorothyl seizure tests were conducted 10 days after treatment initiation. Fluorothyl, a GABAA receptor antagonist, was dripped onto a filter paper near the top of a clear closed container (12 cm × 22 cm × 29 cm) at a constant flow rate of 20 µL/min. The time taken from the start of fluorothyl drip to the first generalized seizure and final tonic extension of the hind limbs was recorded and mice were euthanized immediately after via cervical dislocation.
Measurement of glucose, BHB, octanoate and decanoate levels in brain and plasma
Brain and blood sample collection
After 20 days of dietary treatment, blood and forebrain samples were collected from mice that had undergone 3 s 6 Hz stimulation 10 days earlier, decapitated under light isoflurane anaesthesia to assess the levels of glucose, BHB, octanoic and decanoic acids in brain and plasma. Blood (0.5 ml) was collected using heparinized syringes and centrifuged at 2000 g for 10 min at 4℃ for plasma collection. Brains were extracted and frozen on dry ice immediately. Both brain and plasma samples were stored at −80℃ until needed.
Sample extraction for GC-MS
Plasma and brain samples were processed as previously described for GC-MS measurement.13 Briefly, one half of the forebrain was homogenized using an Ultra Turrax in 400 µL of MilliQ water with 20 nmol of D13-heptanoic acid (Cambridge Isotope Laboratory, MA, USA) as an internal standard while 20 nmol of D13-heptanoic acid was added to 40 µL of plasma sample. All samples were deproteinized using acetonitrile and methanol (7:3; vol/vol), vortexed and centrifuged at 4000 g for 10 min at 4℃. The supernatant was then transferred to glass vials and evaporated on a heating plate at 80℃ and stored at 4℃ until analyzed.
GC-MS protocol
The levels of octanoic acid, decanoic acid and BHB in brain and plasma were measured using a mass selective detector (model 5973 N, Agilent) equipped with Agilent gas chromatography system (model 6890). The protocol used was as previously described14,15 with the following modifications. Briefly, the samples were chemically derivatized using 70 µL of N-methyl-N-(tert-butyldimethylsilyl) trifluoroacetamide + 1% tert-butyldimetheylchlorosilane (Regis Technologies, Inc. Morton Grove, IL, USA) and reacted at 75℃ for 40 min. The GC-MS was equipped with a DB-17MS capillary column (30 m × 0.25 mm × 0.25 µm) and a helium flow of 1 mL/min was used. The starting oven temperature was set to 80℃ with pressure of 14.82 psi and flow velocity of 45 cm/s. Temperature was then increased linearly to 220℃ and held for 1 min. The derivatized samples were injected into the GC-MS in split mode (1:40) and analyzed in selected ion monitoring mode using electron impact ionization for ions reflecting fragments of d13 C7:0 (m/z = 200), C8:0 (m/z = 201), C10:0 (m/z = 229) and BHB (m/z = 159). Regression analysis was used to quantify the concentrations of MCFAs and BHB present in each sample against the added internal standard.
Colorimetric assays
Plasma levels of glucose were measured using a commercial colorimetric assay (Cayman Chemical, MI, USA) according to the manufacturer’s instructions.
Enzyme activity assays
Mitochondrial isolation
Reduction in glycolysis has been proposed to be anticonvulsant (reviewed by Bough and Rho16) and our previous findings showed that trioctanoin reduced the levels of downstream glycolytic intermediates.7 Here, we investigated alterations in the maximal activity of several glycolytic enzymes after trioctanoin and tridecanoin feeding as potential mechanism of action. Mice were decapitated under light isoflurane anaesthesia after 10 days of control, 35E% trioctanoin or tridecanoin feeding. The bilateral hippocampal formations from each mouse were dissected and stored at −80℃ until further processed. Samples were immersed in 10 mL/g tissue of ice-cold mitochondrial isolation buffer containing 0.32 M sucrose, 1 mM EDTA, 10 mM Tris HCl and 1 µL of protease inhibitor cocktail (Sigma cat no. P8340; pH 7.4). Samples were homogenized with a glass-teflon homogenizer, and centrifuged at 1000 g for 10 min at 4℃. The supernatant was removed and centrifuged at 12,000 g for 10 min at 4℃. The supernatant (cytosolic fraction) was removed and the pellet (mitochondrial fraction) was resuspended in 1 mL of mitochondrial isolation buffer. Both fractions were stored at −80℃ until analyzed.
Continuous spectrophotometric assays
The activities of specific enzymes were measured through continuous spectrophotometric assays using the Spectramax 190 Microplate Reader (Molecular Devices, CA, USA). Briefly, 15–20 µg of cytosolic (for glycolytic enzymes) or mitochondrial (citrate synthase) protein from the extracts described above was added to reaction mix (Supplementary Table 1). All enzymes were purchased from Sigma Aldrich and product codes are listed in Supplementary Table 1. Reactions were started just prior to measurement by the addition of the initiating substrate. The rate of change in absorbance was measured over the linear portion of the reaction and the activity of each enzyme was calculated by the equation
The specific activity for each enzyme was calculated by normalizing to protein content, measured with Pierce BCA Protein Assay Kit (Thermo Scientific, IL, USA).
Cortical astrocyte cultures
Previous studies showed that fatty acid oxidation occurs mainly in astrocytes.17 Therefore, we investigated whether octanoate and decanoate can be oxidized directly and change mitochondrial function parameters in cultured astrocytes following incubation with octanoate or decanoate. All culture media and reagents were obtained from Life Technologies (CA, USA) unless stated otherwise. The cultures were prepared as previously described18 except that after at least two weeks, microglial cells were shaken off and astrocytes were replated at high density (150,000 cells/well) in polyethylenimine-coated XFe96 Cell Culture Microplates (Seahorse Bioscience) and cultured for three days prior to mitochondrial function assessment.
Mitochondrial function assessment in cultured astrocytes
Confluent astrocyte cultures were pre-treated with either 1 mM sodium pyruvate, 0.2 mM octanoic or decanoic acid in XF Assay Medium Modified DMEM (Seahorse Bioscience) containing physiological concentrations of glucose (2 mM),19 lactic acid (0.8 mM)19 and glutamine (0.4 mM)20 for 2 h prior to mitochondrial function assessment. Mitochondrial function parameters were evaluated using Seahorse XFe96 Analyzer and XF Cell Mito Stress Kit (Seahorse Bioscience) based on oxygen consumption rate (OCR) at 37℃. Briefly, OCR was measured after sequential addition of 1.5 µM oligomycin, 1 µM FCCP and 1 µM each of antimycin A/rotenone to stimulate different states of mitochondrial respiration. Basal respiration, maximal respiration, proton leak, RCRu, coupling efficiency (ATP turnover/basal respiration) and ATP turnover of astrocytes in the presence of different substrates were calculated as previously described21 and expressed as percentage relative to 1 mM sodium pyruvate.
Mitochondrial function in mouse hippocampal formation
Mitochondrial preparations
A high capacity to produce ATP is important in maintaining stable neuronal membrane potentials which when altered may contribute to seizure generation. The rapid oxidation of MCFAs is likely to improve mitochondrial function thereby beneficial in the treatment of epilepsy. Mice were euthanized under light isoflurane anaesthesia via decapitation after 10 days of control or 35E% tridecanoin feeding. Mitochondria were prepared and isolated as previously described.21 All buffer solutions used were chilled on ice and centrifugation was done at 4℃. Briefly, freshly isolated bilateral hippocampal formations from each mouse were homogenized separately in 25 volumes of chilled MSHE buffer (70 mM sucrose, 210 mM mannitol and 5 mM HEPES, 1 mM EGTA and 0.2% (w/v) fatty-acid free BSA, pH 7.2) using a Teflon dounce homogenizer for 10 strokes on ice. After centrifugation, the supernatant was collected and centrifuged at 12,000 g for 10 min. The supernatant (cytosolic fraction) was removed and the pellet containing mitochondria was washed, centrifuged at 10,000 g for 10 min and resuspended in MSHE buffer without BSA for protein content measurement using Pierce BCA Protein Assay Kit (Thermo Scientific). The total weight of both sides of the hippocampal formations was 27.7 ± 2.1 mg (n = 24). Mitochondria were kept on ice and used fresh for mitochondrial function analysis.
Mitochondrial coupling assay
The degree of coupling between electron transport chain, oxidative phosphorylation machinery and ATP production in the mitochondria was evaluated using the extracellular flux XFe96 Analyzer at 37℃ (Seahorse Bioscience, MA, USA) as previously described.21 Mitochondrial function parameters were then calculated as previously described.22 To stimulate different states of mitochondrial respiration using 10 mM succinate as a substrate, OCR was measured after sequential addition of 3 mM ADP (to calculate state 3 ADP), 2.5 µg/ml oligomycin (to determine state 4o), 4 µM FCCP (to estimate maximal respiration) and 4 µM of antimycin A (to quantify the non-mitochondrial respiration). The contribution of the non-mitochondrial respiration (calculated after the addition of antimycin A) to OCR measured was subtracted from every mitochondrial function parameter. Subsequently, respiration linked to ATP synthesis (state 3 ADP minus state 4o), coupling efficiency ((state 3 ADP minus state 4o)/state 3 ADP), proton leak (state 4o minus non-mitochondrial respiration) and respiratory control ratio uncoupler-stimulated (RCRu; state 3 u/state 4o) were calculated. All mitochondrial function parameters were normalized to protein content and are shown as a percentage OCR relative to baseline values except for coupling efficiency and RCRu.
Ferric reducing ability of plasma (FRAP)
Some studies suggested that MCFAs exert antioxidant effects. An increase in ATP production inevitably leads to an increase in generation of free radicals, resulting in oxidative stress. Therefore, a FRAP assay was used to determine the antioxidant power in the plasma of mice given dietary treatment by measuring the ability of the plasma to reduce colourless ferric tripyridyltriazine (TPTZ) adduct to ferrous form.23 Briefly, diluted plasma (1:3 in MilliQ water) was added to acetate buffer (300 mM; pH 3.6) containing FeCl3–6H2O (20 mM) and TPTZ (10 mM). The absorbance of the mixture was measured at 560 nm after 30-min incubation at 37℃. The antioxidant power was calculated using a standard curve generated from FeSO4 at various concentrations (0–600 µM) and expressed as µmol of FeSO4/L plasma.
Quantitative real-time PCR (qPCR)
The gene expression of various antioxidant enzymes was measured using qPCR to further assess the antioxidant effects of tridecanoin. Mice were anesthetized using an overdose of sodium pentobarbitone (240 mg/kg; i.p.; Provet) before being subjected to transcardial perfusion with cold 0.9% saline containing 1% of the vasodilator sodium nitrite. Hippocampal formations from both hemispheres were dissected, frozen on dry ice immediately and stored at −80℃ until needed. Each hippocampal sample was pulverized under liquid nitrogen and extracted using the RNeasy Mini Kit (Qiagen, NRW, Germany). After DNase treatment (RQ1 RNase-free DNase kit, Promega, WI, USA), reverse transcription was carried out using the Tetro cDNA Synthesis Kit (Bioline, NSW, Australia) according to the manufacturer’s instructions.
The primer efficiency of each primer pair was assessed by measuring the cycle threshold (Ct) values from five fivefold dilutions of two cDNA samples in triplicate reactions as previously described.24 SYBR Green PCR Master Mix (Applied Biosystems, CA, USA) and gene-specific primers (Integrated DNA Technologies, IA, USA; Supplementary Table 2) were used for amplification using the QuantStudio 7 Flex Real-Time PCR System (Applied Biosystems) under the following cycling conditions: initial hot start of 95℃ for 10 min and 40 cycles of 95℃ for 30 s, 60℃ for 1 min and 72℃ for 30 s. The relative fold expression of gene of interest was calculated relative to the geometric mean of two housekeeping genes (Tbp and Hmbs) with the efficiency of the primer pairs taken into consideration.
Catalase and superoxide dismutase activity
The cytosolic and mitochondrial fractions of mouse hippocampal formations were isolated as described above (in “Mitochondrial preparations” section) after 10 days of control or 35E% tridecanoin feeding. The activity of catalase in cytosolic fraction and superoxide dismutase (SOD) in both cytosolic and mitochondrial fractions were measured using Catalase Assay Kit and Superoxide Dismutase Assay Kit, respectively (both from Cayman Chemical) according to the manufacturer’s instructions.
Statistical analysis
All statistical comparisons were conducted using unpaired, two-tailed Student’s t-test or one-way analysis of variance (ANOVA; p-values in text) followed by Bonferroni’s post hoc test at significance level of p < 0.05 except for body weight changes, which were analyzed using two-way ANOVA. Analyses were performed using GraphPad Prism 6.0 (GraphPad Software, CA, USA) and all data were depicted as mean ± SEM. The number of stars in the figures indicates the significance in Bonferroni’s post hoc tests and Student’s t-tests. Based on a recent study showing that reasonable test power cannot be maintained with small sample sizes and statistical significance smaller than p < 0.05 is not justified,25 we indicate all statistical significances as *p < 0.05, despite higher significance levels found in several of our experiments. To compensate for small sample size and to maximize reproducibility of results, most experiments were repeated independently and only reproducible results are discussed.
Results
Anticonvulsant effects and levels of octanoate and decanoate in the brain and plasma
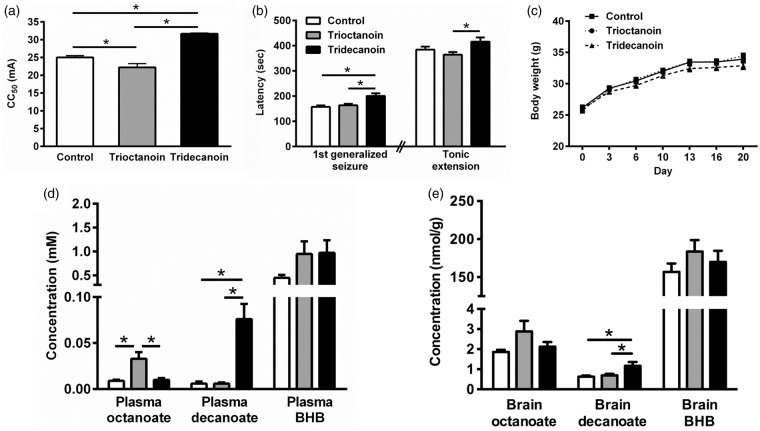
Tridecanoin but not trioctanoin feeding increased the seizure thresholds in 6 Hz seizure tests by 27% (Figure 1(a)) and 13% in two independent experiments compared to control diet (both p < 0.05). In contrast, mice fed trioctanoin showed lowered seizure thresholds by 11% (Figure 1(a)) and 14% in the two experiments (both p < 0.05, one-way ANOVAs; n = 14–15 mice/group each). In the fluorothyl model, tridecanoin increased the latency to reach the first generalized seizure by 27% (Figure 1(b)) and 20% in two repeated experiments (both p < 0.05, one-way ANOVAs; n = 13–14 mice/group each) despite a lack of protection in the latency to tonic extension in both experiments. No significant differences in body weight were observed between diet groups throughout the dietary treatment (two-way ANOVA; n = 15 mice/group; Figure 1(c)).
Figure 1.
Anticonvulsant effects of tridecanoin and levels of octanoate and decanoate in the plasma and brain of mice given control, trioctanoin or tridecanoin treatment. Seizure thresholds were assessed after 10 days of control, 35E% trioctanoin or tridecanoin feeding. (a) Tridecanoin but not trioctanoin feeding increased the seizure threshold of mice in the 6 Hz model, expressed as critical current needed to induce seizures in 50% of the mice (CC50; n = 14–15 mice/group). (b) Tridecanoin also increased the latency to the first generalized seizures in the fluorothyl model compared to control-fed mice (n = 13–14 mice/group). (c) Body weights were monitored and were not altered between any diet groups throughout the treatment phase (two-way ANOVA; n = 15 mice/group). (d) Plasma octanoate and decanoate levels were elevated in trioctanoin- and tridecanoin-fed groups respectively relative to the control-fed group, but plasma BHB levels remained unaltered (n = 7–8 mice/group). (e) In the brain, BHB amounts were similar in all groups and only decanoate levels were increased in tridecanoin-fed mice (n = 7–9 mice/group). (a, b, d, e) All one-way ANOVAs followed by Bonferroni’s post hoc test, stars indicate Bonferroni’s post hoc test significances.
We investigated brain and plasma levels of octanoate and decanoate shortly after eating started (around 6–8 p.m.) in mice that had undergone 6 Hz stimulations 10 days earlier. Plasma glucose levels were similar in all diet groups (control: 11.3 ± 1.2, trioctanoin: 11.5 ± 2.4 and tridecanoin: 11.3 ± 2.6 mM; p = 0.96, one-way ANOVA; n = 13–14 mice/group). Both plasma (p = 0.16; n = 7–8 mice/group; Figure 1(d)) and brain BHB levels were not increased in any diet group (p = 0.39, both one-way ANOVAs; n = 7–9 mice/group; Figure 1(e)). Trioctanoin and tridecanoin feeding increased plasma octanoate and decanoate levels, respectively, to 33 µM and 76 µM (both p < 0.05, one-way ANOVAs; n = 7–8 mice/group; Figure 1(d)). In the brain, only decanoate levels were elevated in the tridecanoin-fed group, namely 1.9-fold to 1.17 nmol/g (p = 0.05, one-way ANOVA; n = 8–9 mice/group; Figure 1(e)) while octanoate levels were in the 1–3 nmol/g range in all treatment groups.
Metabolic changes in the brain: glycolysis
Previous work indicated that reduction in glycolysis could be anticonvulsant and is one of the proposed mechanisms of action of the ketogenic diet.16 Here we tested the hypothesis that tridecanoin feeding reduces glycolysis by altering maximal activity of enzymes involved in the pathway, as well as lactate dehydrogenase and citrate synthase. Mostly the activities were found to be similar in all diet groups, except for the maximal activity of phosphofructokinase, which was reduced by 23% in the trioctanoin compared to the control diet-fed group (p = 0.05, one-way ANOVA; n = 9–10 mice/group; Table 1).
Table 1.
Activities of glycolytic enzymes, lactate dehydrogenase and citrate synthase after trioctanoin and tridecanoin feeding in mice.
| Enzymes | Activity – µmol/min/mg protein (Mean ± SD) |
p-Values | ||
|---|---|---|---|---|
| Control | Trioctanoin | Tridecanoin | ||
| Hexokinase | 13.1 ± 1.1 | 12.9 ± 1.2 | 12.9 ± 1.1 | 0.95 |
| Phosphoglucose isomerase | 423.2 ± 25.2 | 420.4 ± 21.0 | 418.0 ± 27.3 | 0.91 |
| Glucose-6-phosphate dehydrogenase | 1.99 ± 0.26 | 1.95 ± 0.28 | 1.98 ± 0.24 | 0.94 |
| Phosphofructokinase | 28.8 ± 3.2 | 22.3 ± 5.8a | 25.6 ± 2.7 | 0.05 |
| Pyruvate kinase | 70.6 ± 16.8 | 64.7 ± 13.3 | 74.4 ± 22.4 | 0.52 |
| Lactate dehydrogenase | 49.9 ± 6.1 | 52.9 ± 8.3 | 48.5 ± 4.9 | 0.38 |
| Citrate synthase | 26.1 ± 4.5 | 26.3 ± 3.2 | 24.3 ± 3.2 | 0.47 |
Note: Hippocampal extracts of cytosol were prepared from mice fed different MCTs, and maximal enzyme activities were quantified. One-way ANOVA P values are shown.
P < 0.05 compared to control diet group by Bonferroni’s post hoc test; n = 9–10 mice/group.
Effects of octanoic and decanoic acids on mitochondrial function in cultured astrocytes
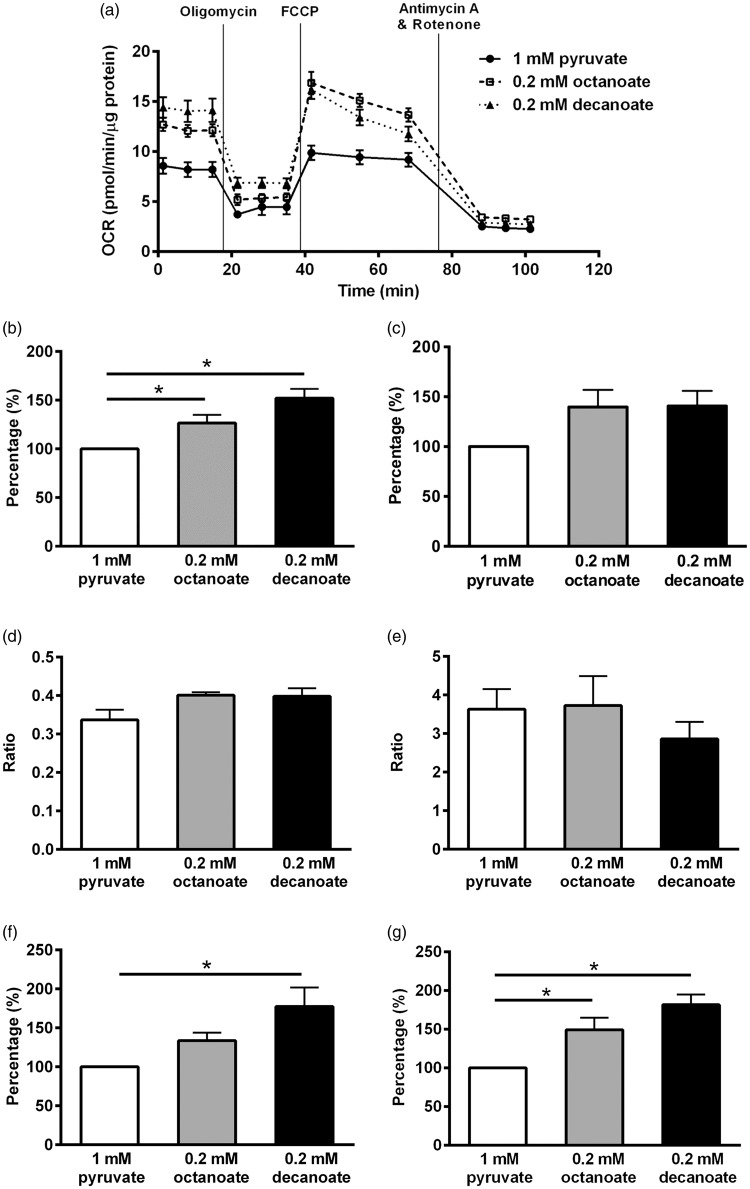
It has previously been shown that fatty acid oxidation occurs mainly in astrocytes.17 To further elucidate the metabolic effects of MCFAs, mitochondrial function parameters in cultured astrocytes were assessed following pre-treatment with 0.2 mM octanoic and decanoic acids for 2 h in media containing other substrates at concentrations similar to those found in mouse brain extracellular fluid (specifically 2 mM glucose,19 0.4 mM glutamine20 and 0.8 mM lactic acid19). The concentration of MCFAs was chosen based on their amounts found in plasma of children on MCT ketogenic diet.2 An example of the different stages of mitochondrial respiration measured by the Extracellular Flux Analyzer in one astrocytic culture preparation is shown in Figure 2(a). The experiment was repeated five more times and the basal respiration with 1 mM pyruvate was 7.4 ± 0.4 pmol oxygen/min/µg protein (n = 6 cultures). The bar graphs show the average results from the six different astrocyte cultures relative to the results with pyruvate. The increased basal respiration by 27% and 52% with octanoate and decanoate, respectively, relative to 1 mM pyruvate (p < 0.05; Figure 2(b)) indicates that both MCFAs are oxidized. Although maximal respiration of the mitochondria increased by approximately 40% in the presence of octanoate or decanoate, this was not statistically significant (p = 0.08; Figure 2(c)). Supplementation with octanoate and decanoate neither altered the coupling efficiency (p = 0.08; Figure 2(d)) nor the RCRu (p = 0.54; Figure 2(e)) of astrocytic mitochondria. Only decanoate resulted in an increase in proton leak of mitochondria (p < 0.05; Figure 2(f)) despite increases in ATP turnover by both octanoate and decanoate (p < 0.05, all one-way ANOVAs; n = 6 independent culture preparations; Figure 2(g)).
Figure 2.
Effects of octanoate and decanoate on mitochondrial functions in cultured astrocytes. Using an extracellular flux analyzer, mitochondrial functions in cultured astrocytes were determined following a two-hour preincubation with 0.2 mM octanoate, 0.2 mM decanoate or 1 mM pyruvate in media with limited substrates (2 mM glucose, 0.8 mM lactic acid and 0.4 mM glutamine) and expressed as percentage relative to 1 mM sodium pyruvate. (a) An example of the stages of mitochondrial respiration from one culture preparation (n = 5–6 wells). Please note that the OCR is normalized to the total protein content per well. (b to f) The bar graphs show the effects found in six independent astrocyte culture preparations. Both 0.2 mM octanoate and decanoate enhanced (b) the basal respiration, but no significant differences among the three compounds were found in (c) the maximal respiration, (d) the coupling efficiency or (e) the respiratory control ratio (uncoupled). (f) Only decanoate resulted in an increase in proton leak although (g) ATP turnover was higher with octanoate and decanoate treatments. Taken together, these data show that the medium chain fats are utilized as substrates in cultured astrocytes. (b to g) All one-way ANOVAs followed by Bonferroni’s post hoc test, stars indicate Bonferroni’s post hoc test significances (all n = 6 per group).
Tridecanoin improves mitochondrial function in mouse hippocampal formations
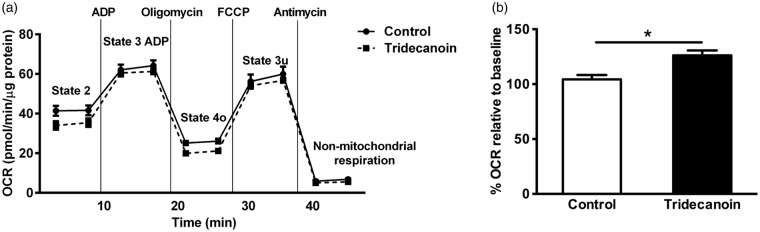
To determine the effects of tridecanoin on mitochondrial function, various functional parameters were assessed using mitochondria isolated from the hippocampal formations of mice fed control or tridecanoin diet. Two independent experiments with n = 4 mice/group and n = 5–6 mice/group were conducted and results from the first experiment are shown here (Figure 3(a)). Similar to previous studies, all mitochondrial function parameters were expressed as percentage of baseline (state 2) values to account for differences in OCR due to experimental alterations during the mitochondrial isolation procedures.26,27 In both independent experiments, respiration linked to ATP synthesis was consistently increased by 21%, when we normalized it to the baseline (both p < 0.05; Figure 3(b); all unpaired, two-tailed Student’s t-tests). Other parameters, including state 3 u, proton leak and the ratios of coupling efficiency and RCRu were not consistently changed in both experiments and are thus not shown.
Figure 3.
Tridecanoin improved mitochondrial functions in mouse hippocampal formations. Mitochondrial function in the presence of 10 mM succinate as a substrate was assessed in isolated mitochondria from the hippocampal formations of mice given control or tridecanoin diet for 10 days using an extracellular flux analyzer. (a) Oxygen consumption rate (OCR) of different stages of mitochondrial respiration from the first experiment is shown. (b) Respiration linked to ATP production (state 3 ADP – state 4o; n = 4 mice/group each) is expressed as percentage relative to baseline values (state 2) and was also increased in the second experiment when normalized to the baseline values (n = 6 mice/group each; two-tailed Student’s t-tests).
Antioxidant effects of tridecanoin
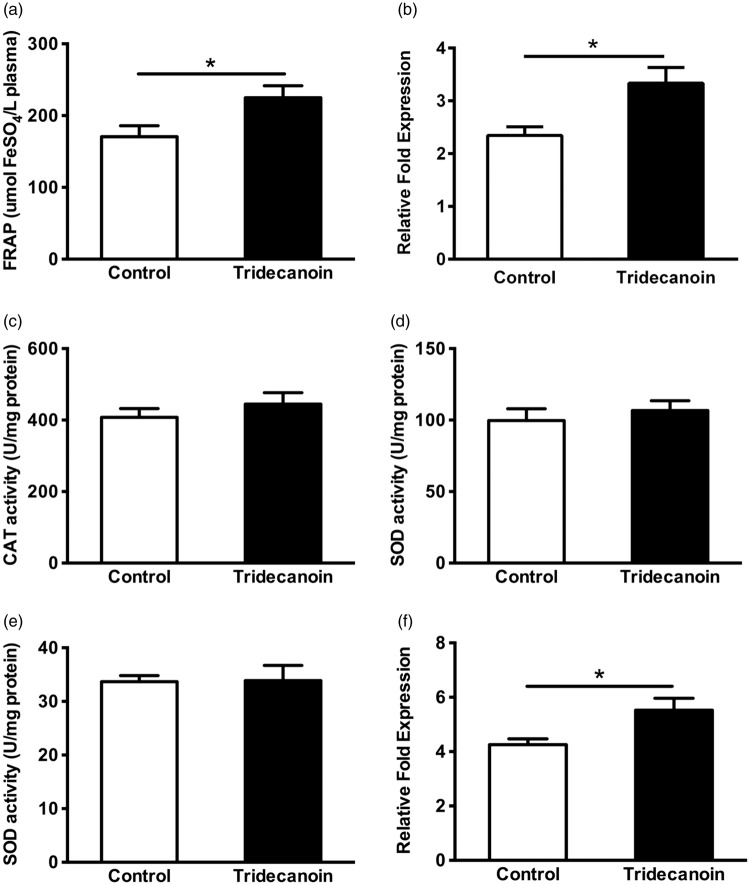
The antioxidant power of plasma following dietary treatment was measured using a FRAP assay. As shown in Figure 4(a), plasma from tridecanoin-fed mice showed 32% higher reducing ability (p = 0.05, Student’s t-test; n = 8–9 mice/group) compared with control-fed mice. The effect was comparable to that found after a five-day treatment with sulforaphane (5 mg/kg daily, i.p.), a known antioxidant (28% increase compared to vehicle treatment).21
Figure 4.
Antioxidant effects of tridecanoin. The antioxidant effects of tridecanoin in plasma and hippocampal formations in control or tridecanoin-fed mice were evaluated as potential mechanisms of action underlying the anticonvulsant effects observed. After 10 days of feeding, tridecanoin (a) increased the antioxidant capacity in the plasma in a FRAP assay (n = 8–9 mice/group) and the relative fold expression of (b) heme oxygenase 1 mRNA levels (n = 7–9 mice/group). The enzyme activities of (c) catalase (CAT) and both the (d) cytosolic and (e) mitochondrial fractions of superoxide dismutase (SOD; all n = 11–12 mice/group) were unaltered. (f) The relative fold expression of forkhead box protein O1 (FoxO1) was increased in hippocampal formations (n = 7–9 mice/ group). (a to f) All unpaired, two-tailed Student’s t-tests, stars indicate t-test significances.
Since tridecanoin was antioxidant in the FRAP assay (Figure 4(a)), hippocampal mRNA levels of several antioxidant enzymes were measured relative to two housekeeping genes. Tridecanoin feeding resulted in increased mRNA levels of heme oxygenase 1 (Hmox1) by 42% in comparison to the control diet group (p = 0.05; n = 7–9 mice/group; Figure 4(b)). The relative expression of other antioxidant enzymes (n = 7–9 mice/group; Table 2) including NAD(P)H:quinone oxidoreductase 1 (Nqo1), glutamate cysteine ligase catalytic subunit (Gclc), glutamate cysteine ligase modifier subunit (Gclm), superoxide dismutase 1 (Sod1), superoxide dismutase 2 (Sod2) and glutathione peroxidase 1 (Gpx1) was not significantly altered after tridecanoin feeding. In addition, the enzyme activities of catalase (p = 0.37; Figure 4(c)) and cytosolic (p = 0.52; Figure 4(d)) as well as mitochondrial SOD (p = 0.95; Figure 4(e); all n = 11–12 mice/group; all unpaired, two-tailed Student’s t-tests) were not changed.
Table 2.
Lack of gene expression changes by tridecanoin.
| Genes | Relative fold expression (mean ± SD) |
p-Values | |
|---|---|---|---|
| Control | Tridecanoin | ||
| Nqo1 | 0.93 ± 0.37 | 0.81 ± 0.35 | 0.52 |
| Gclc | 2.16 ± 0.5 | 2.10 ± 1.0 | 0.88 |
| Gclm | 7.31 ± 2.0 | 7.43 ± 3.3 | 0.93 |
| Sod1 | 5.27 ± 2.3 | 6.60 ± 2.4 | 0.32 |
| Sod2 | 34.6 ± 5.7 | 29.7 ± 8.2 | 0.18 |
| Gpx1 | 47.3 ± 12.7 | 49.1 ± 20.3 | 0.84 |
| FoxO3 | 11.0 ± 2.8 | 14.2 ± 5.1 | 0.12 |
| FoxO6 | 2.22 ± 0.7 | 2.91 ± 0.7 | 0.08 |
| Sirt1 | 3.62 ± 0.8 | 3.24 ± 1.3 | 0.52 |
| Ucp2 | 6.66 ± 1.8 | 8.18 ± 2.7 | 0.19 |
| Ucp3 | 0.28 ± 0.06 | 0.33 ± 0.08 | 0.20 |
| Ucp4 | 5.63 ± 0.8 | 5.55 ± 1.6 | 0.89 |
| Ucp5 | 8.23 ± 1.6 | 7.35 ± 2.0 | 0.34 |
p-Values of unpaired, two-tailed Student’s t-tests are shown; n = 6–9 mice/group.
The relative expression of the transcription factors forkhead box protein O1 (FoxO1), FoxO3 and FoxO6, which have been suggested to play an important role in regulating the expression of antioxidant enzymes, was also investigated. Tridecanoin increased the hippocampal mRNA levels of FoxO1 by 30% (p = 0.05, unpaired, two-tailed Student’s t-test; n = 7–9 mice/group; Figure 4(f)) relative to the control group. The relative expression of FoxO3, FoxO6 and Sirt1, a post-translational regulator of FoxOs, remained unaltered (n = 6–9 mice/group; Table 2).
Changes in mRNA levels of uncoupling proteins after tridecanoin feeding
Previous studies suggested that fatty acids are able to activate uncoupling proteins (UCPs) and can alter the expression of UCPs.16 However, tridecanoin did not alter the mRNA levels of Ucp2, Ucp3, Ucp4 and Ucp5 compared with control-fed mice (n = 6–9 mice/group; Table 2).
Discussion
Previous studies focused mainly on the neuroprotective and acute anticonvulsant properties of the MCFAs octanoic and decanoic acids. Chronic administration of pure fatty acids is impractical, but is feasible in the form of triglycerides, which avoids sodium and/or acid overloads of the body. This is evident from the MCT ketogenic diet and our previous studies showing anticonvulsant effects of triheptanoin, the triglyceride of the seven-carbon heptanoic acid, in mouse epilepsy models7,12,28 and in clinical trial.29 Therefore, the anticonvulsant properties of octanoic and decanoic acids following chronic administration in the form of triglycerides as well as mechanisms of action remained to be elucidated.
We found that tridecanoin, but not trioctanoin, was consistently anticonvulsant in two acute mouse seizure models. We then determined the possible anticonvulsant mechanisms in vivo and in vitro. The main findings of this study are summarized as follow: (1) anticonvulsant effects of tridecanoin, but not trioctanoin, were consistently found in the 6 Hz model and a chemical seizure model using fluorothyl, a GABAA receptor blocker. (2) Both trioctanoin and tridecanoin feeding did not alter glucose levels in plasma and BHB levels in both brain and plasma, indicating that BHB levels alone are not responsible for the anticonvulsant effects of tridecanoin. Only trioctanoin reduced the maximal activity of phosphofructokinase, suggesting that reduced glycolysis is also unlikely to explain the effects of tridecanoin. (3) The decanoate and octanoate levels in plasma and brain are very low in mice, indicating fast metabolism. This is consistent with our findings that in cultured astrocytes, both 0.2 mM octanoate and decanoate increased basal respiration and enhanced ATP turnover to a greater extent than 1 mM pyruvate, indicating oxidation of these MCFAs. (4) Mitochondria isolated from the hippocampal formations of mice fed tridecanoin showed increases in respiration linked to ATP synthesis consistently in two independent experiments, suggestive of improvement in mitochondrial function. (5) Tridecanoin enhanced the plasma antioxidant capacity and hippocampal Hmox1 mRNA levels. In addition, the increased mitochondrial proton leak specifically found with decanoate in cultured astrocytes may also reduce oxidative stress, which may contribute to the anticonvulsant effects of decanoate. (6) The mRNA levels of the transcription factor FoxO1 were increased by tridecanoin and may be involved in the increased antioxidant gene expression.
Decanoate and octanoate as fuel for the brain
The decanoate and octanoate levels in plasma and brain are very low in mice; however, they are higher in children given MCTs orally.2 The seven-fold higher whole body energy metabolism (based on mass-specific rate) in mice compared to humans is one possible explanation for the differences in plasma concentrations. On the other hand, it is also possible that at least some of the triglycerides are metabolized to glucose by the liver. Our new data demonstrate that in cultured astrocytes, incubation with either 0.2 mM octanoate or decanoate resulted in higher basal respiration and ATP turnover relative to 1 mM pyruvate, indicating that both MCFAs can be metabolized directly by brain cells. Previous reports showing that octanoate30,31 and heptanoate32 are metabolized in the brain corroborate this finding. Bolus injection of 14C-octanoate and decanoate lead to brain uptake of 94% and 88%, respectively.33 In addition, infusion of 13C-octanoate shows that octanoate readily crossed the blood brain barrier and when given in high amounts can contribute approximately 20% to total brain oxidative energy production.31 This metabolism appears to occur mostly in astrocytes.17,32 These studies indicate that the brain is able to utilize medium-chain fatty acids as substrates, which may contribute to the anticonvulsant, antioxidant and metabolic effects found in our work. However, given the low MCFA plasma levels found, glucose is still likely to be the major fuel for the brain under our experimental conditions.
Furthermore, both tridecanoin and trioctanoin did not elevate the levels of plasma BHB, which has been shown to be a fuel for the brain34 and have antioxidant properties.35 However, the similar low BHB levels with the two MCTs do not correlate with the difference in anticonvulsant effects found here. These findings are consistent with the anticonvulsant mechanisms discussed for the ketogenic diet, where elevated BHB alone appears not to be fully responsible for the effects of the diet. In conclusion, our data that both octanoate and decanoate can be used directly as fuel and both did not alter the amounts of BHB significantly, suggest that other mechanisms of action underlie the consistent anticonvulsant effects of tridecanoin. Indeed, a recent study found that decanoic but not octanoic acid can act as a non-competitive antagonist of AMPA receptors through direct binding,36 further corroborating this notion.
Reduction and inhibition of glycolysis have been found to be anticonvulsant in various animal models (reviewed by Bough and Rho16) and appear to contribute to protection against seizures by the ketogenic diet. However, only trioctanoin was found to lower the maximal activity of phosphofructokinase (Table 1), one of the regulatory steps in glycolysis. This is consistent with our earlier results showing that trioctanoin feeding decreased the levels of downstream glycolytic intermediates.7 The implications and possible mechanisms of this inhibition are discussed in our previous work.7 Further studies are necessary to understand the effects of decanoate on brain glucose metabolism.
ATP is important to keep neuronal membrane potentials stable, which is thought to avoid seizure generation. The mitochondrial electron transport chain is the largest generator of ATP in aerobic metabolism. Isolated mitochondria from the hippocampal formations of mice fed tridecanoin diet showed an increased respiration linked to ATP production. In addition, incubation with octanoate and decanoate as substrates in cultured astrocytes enhanced basal respiration and ATP turnover, suggesting that MCFAs are capable of improving mitochondrial function, consistent with the observations found after triheptanoin treatment.37
An increase in mitochondrial activity can lead to exacerbated generation of free radicals, resulting in oxidative stress. Therefore, if ATP generation is enhanced it is important to keep free radicals at bay in order to prevent oxidative stress, which can be achieved by increasing proton leakage over the mitochondrial membrane. Our findings that decanoate increases ATP turnover and proton leak in cultured astrocytes suggest that decanoate is likely to protect the brain from seizures and damage. We do not understand the mechanism underlying the increased proton leakage in vitro since we found no significant increases in proton leak or levels of various uncoupling protein transcripts in vivo. However, increased UCP protein levels are still possible since ketogenic diet was found to increase the protein levels of UCP2 and UCP4 in hippocampal mitochondria (reviewed by Bough and Rho16). Also, two models have been proposed to explain the activation of UCPs by fatty acids, namely the “proton-buffering model” and “fatty-acid-cycling model”. In the “proton-buffering model”, the fatty acid carboxyl group is presumed to be a prosthetic group which accepts protons in the intermembrane space and transports protons back into mitochondrial matrix38 while the “fatty-acid-cycling model” proposes that UCPs transport fatty acid anions across mitochondrial matrix into the intermembrane space where they then accept protons that are in that space. Upon accepting protons, the fatty acids are able to flip-flop across the membrane into the mitochondrial matrix and the protons are released.39 The small increases in free decanoate levels found in the brain appear to favor the first model, where decanoate would be bound and act as a prosthetic group.
Other effects of decanoate: reduction of oxidative stress
Some studies suggested that MCFAs exert antioxidant effects. Therefore, in addition to improvement in mitochondrial function, we propose that decanoate protects the brain by reducing oxidative stress, potentially by increasing the expression of antioxidant enzymes as direct scavenging of reactive oxygen or nitrogen species by MCFAs does not seem plausible based on their structure. Apart from an increased proton leakage in cultured astrocytes, tridecanoin feeding increased the overall plasma antioxidant capacity and hippocampal Hmox1 and FoxO1 mRNA levels, which are likely to contribute to the anticonvulsant effects observed although the activity and protein levels remained to be investigated. Hmox1 is an important phase II antioxidant enzymes which catalyzes the breakdown of heme into free iron, carbon monoxide and biliverdin and is usually upregulated during oxidative stress in glial cells as a redox homeostatic defence. The expression of Hmox1 can also be induced by phytochemicals from plants40 or metalloporphyrins.41 It has been found in numerous studies that the expression of Hmox1 is regulated by nuclear factor erythroid 2-related factor 2 (Nrf2) through direct binding to the antioxidant response element of Hmox1 promoter.42 However, in the hippocampus of mice fed tridecanoin, the mRNA levels of other antioxidant enzymes that are known to be regulated by Nrf2 via antioxidant response element, including Nqo1, Gclc, Gclm, Sod and Gpx were not altered, suggestive of an Nrf2-independent regulation of Hmox1 expression. Indeed, other studies have shown that Hmox1 can be regulated by transcription factor FoxO1, which is expressed by dentate granule cells and ventral posterior CA pyramidal cell layers.41,43 In addition, Nrf2-independent regulation of Hmox1 expression by FoxO1 is evident in Nrf2 knockout mice where Hmox1 mRNA levels remained upregulated in skeletal muscles.44 Therefore, it is possible that tridecanoin upregulates Hmox1 mRNA levels through a FoxO1-dependent pathway. Nonetheless, the activity and protein levels of Hmox1 and FoxO1 as well as their cellular origin and the post-translational modifications of FoxO1 including phosphorylation and acetylation remain to be determined to further elucidate the pathways involved in the anticonvulsant and antioxidant effects of tridecanoin.
Conclusion
Here, we investigated the anticonvulsant effects of the triglycerides of octanoate and decanoate and found that tridecanoin, but not trioctanoin, was consistently anticonvulsant in two acute CD1 mouse seizure models. We then measured the mitochondrial function in isolated mitochondria and cultured astrocyte as well as the antioxidant effects as potential mechanisms of action underlying the anticonvulsant effects of tridecanoin. Both MCFAs did not alter BHB levels significantly in brain and plasma and were oxidized well by cultured astrocytes, evidenced by increased ATP turnover. After tridecanoin feeding, mitochondria isolated from mouse hippocampal formations showed increased respiration linked to ATP production. In addition, tridecanoin increased plasma antioxidant capacity, hippocampal Hmox1 and FoxO1 mRNA levels in vivo and incubation with decanoate enhanced mitochondrial proton leak in cultured astrocytes. Taken together, although both MCFAs were oxidized and improved mitochondrial function, the antioxidant effects of tridecanoin could contribute to the differences in anticonvulsant effects observed following trioctanoin or tridecanoin feeding.
Supplementary Material
Acknowledgement
We are grateful to Stepan Lipid Nutrition for gifting both trioctanoin and tridecanoin for research purposes.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We are grateful for funding from NHMRC (project grant 1044007 to KB), Fondecyt Initiation into Research (Grant 11130232 to CC), Case MMPC (U24 DK76174 to MP) and UQ scholarships (KT and TM). We thank Dr Melissa Benson for cortical astrocyte culture preparation.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
KT, CC and KB planned and designed the experiments. KT, CC, TM and MP performed the experiments and analyzed the data. KT and KB drafted the manuscript.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Huttenlocher PR. Ketonemia and seizures: metabolic and anticonvulsant effects of two ketogenic diets in childhood epilepsy. Pediatr Res 1976; 10: 536–540. [DOI] [PubMed] [Google Scholar]
- 2.Haidukewych D, Forsythe WI, Sills M. Monitoring octanoic and decanoic acids in plasma from children with intractable epilepsy treated with medium-chain triglyceride diet. Clin Chem 1982; 28: 642–645. [PubMed] [Google Scholar]
- 3.Nakamura J, Koh N, Sakakibara F, et al. Polyol pathway hyperactivity is closely related to carnitine deficiency in the pathogenesis of diabetic neuropathy of streptozotocin-diabetic rats. J Pharmacol Exp Therapeut 1998; 287: 897–902. [PubMed] [Google Scholar]
- 4.Wlaź P, Socała K, Nieoczym D, et al. Acute anticonvulsant effects of capric acid in seizure tests in mice. Prog Neuropsychopharmacol Biol Psychiatr 2014; 57 c: 110–116. [DOI] [PubMed] [Google Scholar]
- 5.Wlaź P, Socała K, Nieoczym D, et al. Anticonvulsant profile of caprylic acid, a main constituent of the medium-chain triglyceride (MCT) ketogenic diet, in mice. Neuropharmacology 2012; 62: 1882–1889. [DOI] [PubMed] [Google Scholar]
- 6.Chang P, Terbach N, Plant N, et al. Seizure control by ketogenic diet-associated medium chain fatty acids. Neuropharmacology 2013; 69: 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald TS, Tan KN, Hodson MP, et al. Alterations of hippocampal glucose metabolism by even versus uneven medium chain triglycerides. J Cereb Blood Flow Metab 2014; 34: 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henry GE, Momin RA, Nair MG, et al. Antioxidant and cyclooxygenase activities of fatty acids found in food. J Agric Food Chem 2002; 50: 2231–2234. [DOI] [PubMed] [Google Scholar]
- 9.Park EJ, Kim SA, Choi YM, et al. Capric acid inhibits NO production and STAT3 activation during LPS-induced osteoclastogenesis. PLoS One 2011; 6: e27739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes SD, Kanabus M, Anderson G, et al. The ketogenic diet component decanoic acid increases mitochondrial citrate synthase and complex I activity in neuronal cells. J Neurochem 2014; 129: 426–433. [DOI] [PubMed] [Google Scholar]
- 11.Sengupta A, Ghosh M. Comparison of native and capric acid-enriched mustard oil effects on oxidative stress and antioxidant protection in rats. Br J Nutr 2012; 107: 845–849. [DOI] [PubMed] [Google Scholar]
- 12.Thomas NK, Willis S, Sweetman L, et al. Triheptanoin in acute mouse seizure models. Epilepsy Res 2012; 99: 312–317. [DOI] [PubMed] [Google Scholar]
- 13.Gu L, Zhang GF, Kombu RS, et al. Parenteral and enteral metabolism of anaplerotic triheptanoin in normal rats. II. Effects on lipolysis, glucose production, and liver acyl-CoA profile. Am J Physiol Endocrinol Metab 2010; 298: E362–E371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bederman IR, Foy S, Chandramouli V, et al. Triglyceride synthesis in epididymal adipose tissue: contribution of glucose and non-glucose carbon sources. J Biol Chem 2009; 284: 6101–6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Zhang S, Marin-Valencia I, et al. Decreased carbon shunting from glucose toward oxidative metabolism in diet-induced ketotic rat brain. J Neurochem 2015; 132: 301–312. [DOI] [PMC free article] [PubMed]
- 16.Bough KJ, Rho JM. Anticonvulsant mechanisms of the ketogenic diet. Epilepsia 2007; 48: 43–58. [DOI] [PubMed] [Google Scholar]
- 17.Edmond J, Robbins R, Bergstrom J, et al. Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes, and oligodendrocytes from developing brain in primary culture. J Neurosci Res 1987; 18: 551–561. [DOI] [PubMed] [Google Scholar]
- 18.Benson MJ, Thomas NK, Talwar S, et al. A novel anticonvulsant mechanism via inhibition of complement receptor C5ar1 in murine epilepsy models. Neurobiol Dis 2015; 76: 87–97. [DOI] [PubMed] [Google Scholar]
- 19.Samala R, Klein J, Borges K. The ketogenic diet changes metabolite levels in hippocampal extracellular fluid. Neurochem Int 2011; 58: 5–8. [DOI] [PubMed] [Google Scholar]
- 20.Alexander GM, Deitch JS, Seeburger JL, et al. Elevated cortical extracellular fluid glutamate in transgenic mice expressing human mutant (G93A) Cu/Zn superoxide dismutase. J Neurochem 2000; 74: 1666–1673. [DOI] [PubMed] [Google Scholar]
- 21.Carrasco-Pozo C, Tan KN, Borges K. Sulforaphane is anticonvulsant and improves mitochondrial function. J Neurochem 2015; 135: 932–942. [DOI] [PubMed] [Google Scholar]
- 22.Rogers GW, Brand MD, Petrosyan S, et al. High throughput microplate respiratory measurements using minimal quantities of isolated mitochondria. PLoS One 2011; 6: e21746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 1996; 239: 70–76. [DOI] [PubMed] [Google Scholar]
- 24.Hadera MG, Smeland OB, McDonald TS, et al. Triheptanoin partially restores levels of tricarboxylic acid cycle intermediates in the mouse pilocarpine model of epilepsy. J Neurochem 2014; 129: 107–119. [DOI] [PubMed] [Google Scholar]
- 25.Button KS, Ioannidis JP, Mokrysz C, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 2013; 14: 365–376. [DOI] [PubMed] [Google Scholar]
- 26.Mailloux RJ, Adjeitey CN, Xuan JY, et al. Crucial yet divergent roles of mitochondrial redox state in skeletal muscle vs. brown adipose tissue energetics. FASEB J 2012; 26: 363–375. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Nuebel E, Wisidagama DR, et al. Measuring energy metabolism in cultured cells, including human pluripotent stem cells and differentiated cells. Nat Protoc 2012; 7: 1068–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willis S, Stoll J, Sweetman L, et al. Anticonvulsant effects of a triheptanoin diet in two mouse chronic seizure models. Neurobiol Dis 2010; 40: 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pascual JM, Liu P, Mao D, et al. Triheptanoin for glucose transporter type I deficiency (G1D): modulation of human ictogenesis, cerebral metabolic rate, and cognitive indices by a food supplement. JAMA Neurol 2014; 71: 1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cremer JE, Teal HM, Heath DF, et al. The Influence of portocaval anastomosis on the metabolism of labeled octanoate, butyrate and leucine in rat brain. J Neurochem 1977; 28: 215–222. [DOI] [PubMed] [Google Scholar]
- 31.Ebert D, Haller RG, Walton ME. Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. J Neurosci 2003; 23: 5928–5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marin-Valencia I, Good LB, Ma Q, et al. Heptanoate as a neural fuel: energetic and neurotransmitter precursors in normal and glucose transporter I-deficient (G1D) brain. J Cereb Blood Flow Metab 2013; 33: 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oldendorf WH. Carrier-mediated blood-brain barrier transport of short-chain monocarboxylic organic acids. Am J Physiol 1973; 224: 1450–1453. [DOI] [PubMed] [Google Scholar]
- 34.Jiang L, Mason GF, Rothman DL, et al. Cortical substrate oxidation during hyperketonemia in the fasted anesthetized rat in vivo. J Cereb Blood Flow Metab 2011; 31: 2313–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim DY, Davis LM, Sullivan PG, et al. Ketone bodies are protective against oxidative stress in neocortical neurons. J Neurochem 2007; 101: 1316–1326. [DOI] [PubMed] [Google Scholar]
- 36.Chang P, Augustin K, Boddum K, et al. Seizure control by decanoic acid through direct AMPA receptor inhibition. Brain 2016; 139(Pt 2): 431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwarzkopf TM, Koch K, Klein J. Reduced severity of ischemic stroke and improvement of mitochondrial function after dietary treatment with the anaplerotic substance triheptanoin. Neuroscience 2015; 300: 201–209. [DOI] [PubMed] [Google Scholar]
- 38.Winkler E, Klingenberg M. Effect of fatty acids on H + transport activity of the reconstituted uncoupling protein. J Biol Chem 1994; 269: 2508–2515. [PubMed] [Google Scholar]
- 39.Garlid KD, Orosz DE, Modriansky M, et al. On the mechanism of fatty acid-induced proton transport by mitochondrial uncoupling protein. J Biol Chem 1996; 271: 2615–2620. [DOI] [PubMed] [Google Scholar]
- 40.Keum YS, Yu S, Chang PP, et al. Mechanism of action of sulforaphane: inhibition of p38 mitogen-activated protein kinase isoforms contributing to the induction of antioxidant response element-mediated heme oxygenase-1 in human hepatoma HepG2 cells. Cancer Res 2006; 66: 8804–8813. [DOI] [PubMed] [Google Scholar]
- 41.Liu X, Cui Y, Li M, et al. Cobalt protoporphyrin induces HO-1 expression mediated partially by FOXO1 and reduces mitochondria-derived reactive oxygen species production. PLoS One 2013; 8: e80521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin D, Rojo AI, Salinas M, et al. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J Biol Chem 2004; 279: 8919–8929. [DOI] [PubMed] [Google Scholar]
- 43.Hoekman MF, Jacobs FM, Smidt MP, et al. Spatial and temporal expression of FoxO transcription factors in the developing and adult murine brain. Gene Expr Patterns 2006; 6: 134–140. [DOI] [PubMed] [Google Scholar]
- 44.Kang J, Jeong MG, Oh S, et al. A FoxO1-dependent, but NRF2-independent induction of heme oxygenase-1 during muscle atrophy. FEBS Lett 2014; 588: 79–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.