Abstract
Changes in P-glycoprotein and ABCG2 densities may play a role in amyloid-beta accumulation in Alzheimer’s disease. However, previous studies report conflicting results from different brain regions, without correcting for changes in vessel density. We developed an automated method to measure transporter density exclusively within the vascular space, thereby correcting for vessel density. We then examined variability in transporter density across brain regions, matter, and disease using two cohorts of post-mortem brains from Alzheimer’s disease patients and age-matched controls. Changes in transporter density were also investigated in capillaries near plaques and on the mRNA level. P-glycoprotein density varied with brain region and matter, whereas ABCG2 density varied with brain matter. In temporal cortex, P-glycoprotein density was 53% lower in Alzheimer’s disease samples than in controls, and was reduced by 35% in capillaries near plaque deposits within Alzheimer’s disease samples. ABCG2 density was unaffected in Alzheimer’s disease. No differences were detected at the transcript level. Our study indicates that region-specific changes in transporter densities can occur globally and locally near amyloid-beta deposits in Alzheimer’s disease, providing an explanation for conflicting results in the literature. When differences in region and matter are accounted for, changes in density can be reproducibly measured using our automated method.
Keywords: ABC transporters, blood–brain barrier, density, Alzheimer’s disease, amyloid-beta plaque
Introduction
Alzheimer disease (AD) is a neurodegenerative disorder that is pathologically characterized by excess levels of amyloid-beta (Aβ) plaques in cortical regions of the brain. Although Aβ is normally exported from the brain into the bloodstream, it may accumulate and form plaques in the AD brain in part due to insufficient removal by transporters located at the blood–brain barrier. This “Aβ clearance hypothesis” suggests that changes in the function and/or density of transporters such as P-glycoprotein (P-gp, encoded by ABCB1) and ABCG2 (encoded by ABCG2) may play an important role in the pathogenesis of AD.1 Two lines of experimental evidence are necessary to support this hypothesis: (1) that Aβ peptide is transported by P-gp and/or ABCG2, and (2) that changes in P-gp and ABCG2 function and/or density exist in AD patients.
While a number of studies have shown that P-gp facilitates transmembrane movement of Aβ analogs,2–5 studies performed in post-mortem brains from humans have reported conflicting results on the relationship between the densities of P-gp and ABCG2 in brain capillaries and Aβ deposition. Vogelgesang et al.6,7 manually scored P-gp-immunoreactive (or positive) capillaries in 243 elderly subjects without dementia and reported an inverse correlation between P-gp and Aβ levels.6,7 A more recent study used a similar semi-quantitative method and reported a reduction in P-gp-positive capillaries of AD subjects compared to controls.8 Wijesuriya et al.9 measured P-gp and ABCG2 fluorescence in individual capillaries and found a reduction in P-gp but not ABCG2 in the endothelial cell layer of capillaries in AD; however, mRNA and protein levels of P-gp and ABCG2 in these samples were not altered.9 In contrast to these results, Carrano et al.10 reported a reduction in ABCG2-positive, but not P-gp-positive, capillaries in the AD brain parenchyma,10 whereas Xiong et al.11 reported an increase in ABCG2 expression at the mRNA and protein levels.
One explanation for the different results could be the variability in the mode of analysis used to quantify transporter density in these studies. Some used immunohistochemical staining of P-gp and ABCG2 to score the number of capillaries in fields of view,6–8 while others measured the fluorescence intensity of P-gp and ABCG2 immunoreactivity within individual capillaries,9,11 or as a percentage of immunofluorescence staining with respect to a stain for brain endothelial cells.10 Most of the analysis strategies used in these studies could be interpreted as a simple count of capillaries or a percent area of staining, instead of a measure of the expression levels of efflux transporter protein, because they do not quantify the actual intensity and distribution of staining. Furthermore, these studies measured transporter density in a small number of capillaries (10–20) from different regions: four studies measured P-gp and/or ABCG2 density in the hippocampus or other parts of the medial temporal cortex,6–9 while two measured density in the occipital lobe.10,11
We hypothesized that absolute densities of P-gp and ABCG2 in capillaries, rather than the area of positive staining for P-gp and ABCG2, would give insight into the variability of transporter density between regions and whether P-gp and ABCG2 densities correlated with AD status. To this end, we sought to develop an analysis strategy based on immunofluorescence images from post-mortem human brain that would measure the magnitude of protein expression within vessels (and thus correct for capillary density). The method was subsequently applied to two cohorts of post-mortem brains from AD patients and matched controls: one cohort to examine the variability of transporter density (measured from ≥60 capillaries total) across brain region and matter, and the other cohort to determine whether P-gp and ABCG2 densities correlated with AD status. Finally, we used super-resolution, structured illumination microscopy to investigate local changes in the density and distribution of P-gp and ABCG2 in capillaries located in proximity to amyloid plaques.
Materials and methods
Brain tissues
Two independent sets of post-mortem brain samples from Alzheimer patients and age-matched controls were obtained from the Netherlands Brain Bank (Netherlands Institute for Neuroscience, Netherlands). Set 1 was used to assess the variability in transporter density across brain regions and matter, while set 2 was used to assess whether P-gp and ABCG2 densities correlated with AD (Supplementary Tables 1 and 2). Neuropathologic criteria were used to diagnose AD, and the groups were matched with respect to hemisphere, sex and origin of tissue, age, and post-mortem interval (see Supplementary Information for further details). All experiments were performed in accordance with The Act (2003:460) Concerning Ethical Review of Research Involving Humans (Sweden), and were approved by the Central Ethical Review Board in Sweden (Etikprövningsnämnderna) and the Swedish Board of Social Welfare (ethical permission number: EPN 2013/474-31/2).
Immunofluorescence staining
Triple-labeling immunofluorescence was carried out on post-mortem sections to visualize the distribution of glucose transporter-1 (glut-1), P-gp or ABCG2, and Aβ. Dual-labeling of post-mortem sections using two vascular markers, glut-1 and CD31, was carried out to validate the glut-1 vessel mask (Supplementary Tables 3 and 4; see Supplementary Information for further details). Sudan Black counterstaining was used to distinguish white and gray matter, and to quench autofluorescence from lipofuscin.12 The specificity of our detection system was verified by omission of the primary antibody, and the specificity of antibodies against P-gp and ABCG2 was assessed by comparing results obtained with different antibodies against these targets (Supplementary Figure 1).
Image acquisition
To determine whether transporter density changes on a “global” level, fluorescence of glut-1, P-gp and ABCG2, and plaque stainings was measured from entire tissue sections using an automated scanning system (VSlide Scanning Platform, MetaSystems GmbH). Once sample names were blinded, entire tissue sections from sets 1 and 2 were scanned (see Supplementary Information for further details). For each sample and brain region, two regions of interest were randomly chosen and exported as uncompressed 8-bit files in tiff format for analysis. Each region of interest contained at least two fields of view, and each field of view contained ≥ 30 capillaries. In set 1, the sizes (mean ± SD) of the regions were 8.9 ± 9.0 mm2 (gray matter) and 1.4 ± 3.8 mm2 (white matter) for P-gp slides, and 8.8 ± 4.5 mm2 (gray matter), and 2.4 ± 2.9 mm2 (white matter) for ABCG2 slides. In set 2, the size of the regions was 0.61 mm2 (gray matter).
To investigate the relationship between Aβ plaques and P-gp and ABCG2 distributions in proximal capillaries, we used structured illumination microscopy to measure fluorescence within gray matter capillaries from temporal cortex samples of AD cases (n = 5). For each case, staining of P-gp and ABCG2 and glut-1 was measured in three capillaries located near plaques and in three capillaries not near plaques (see Supplementary Information for further details). Multichannel images were exported for analysis.
Quantification of protein density and area coverage
Transporter density and blood vessel coverage were measured using algorithms written in Matlab R2011b (v.7.13.0.564, MathWorks Inc.). Vessel masks were generated in a two-part method. In the first part, a median filter of 10 × 10 was applied to the glut-1 image to remove electronic noise before vessel edges were detected using the Sobel method (which measures the maximum rate of change in pixel intensity). The detected edges were dilated using a 2D convolution between the edge mask and a “smoothing” factor (corresponding to the width of the Gaussian kernel) to generate a binary mask.
In the second part, the original glut-1 image was thresholded using a user-defined value to remove pixels outside of the capillary. The thresholded glut-1 image was then multiplied by the binary mask to obtain the final vessel mask. We tested eight different smoothing factors (ranging from 4 to 18) and seven values for threshold (ranging from 30 to 60) on thalamus regions from both the P-gp and ABCG2 slides to determine which values resulted in minimal variability in the percent area coverage of blood vessels; the final values were 15 for smoothing factor, and 45 (P-gp) and 35 (ABCG2) for threshold (Supplementary Figure 2). Once the final vessel mask was generated, it was overlaid onto the original glut-1 or P-gp and ABCG2 images to quantify the average fluorescence intensity (which is proportional to protein concentration) within the capillary space. This value is reported as the density of each transporter. To prevent user bias, sample names were broken only after all values were calculated.
The same edge detection and smoothing operators were applied to quantify the amounts of plaque and vessel coverage. Plaque load was defined as the percent of pixels within the plaque mask divided by the total pixel number in the image. Percent area covered by vessels was defined as the percent of pixels constituting the glut-1-derived vessel mask divided by the total pixel number in the image. Vessel masks generated from glut-1 staining were validated using a second vessel marker, CD31 (see Supplementary Information for further details).
Fluorescence intensity and distribution of P-gp and ABCG2 in capillaries proximal to Aβ plaques were analyzed using ImageJ (v1.47 h, National Institutes of Health). Capillaries with visible amyloid deposits within the walls were excluded. The endothelial cell layer was selected based on glut-1 staining. Size, intensity, and distribution of P-gp and ABCG2 clusters (i.e., groups of pixels) were determined using particle analysis with a cut-off size of 0.01 µm2 and circularity between 0.4 and 1.0. P-gp and ABCG2 coverage were determined as the area covered by P-gp and ABCG2 clusters divided by the total area measured (glut-1), and the normalized integrated optical densities of P-gp and ABCG2 were calculated by dividing the total density by the area measured (glut-1). Cluster size and P-gp signal intensities were measured from 2324 ± 274 P-gp clusters per capillary and from 746 ± 49 ABCG2 clusters per capillary.
Quantitative PCR on human temporal cortex micro-punch samples
Pieces of gray matter or white matter were punched using a micro-punch from unfixed fresh frozen samples of temporal cortex from set 2. Total RNA was extracted, verified for quality (mean ± SD; gray matter: 2.03 ± 0.09; white matter: 1.94 ± 0.13), and reverse transcribed as previously described.13 To measure gene expression, quantitative reverse transcription PCR was performed on a 384-well plate using custom-designed primers (Invitrogen, Supplementary Table 5). Expression was measured for two target genes (ABCB1, ABCG2), and four reference genes (CD31, CD105, OLIG1, TUBULIN). Changes in expression were calculated using the comparative reference method (2−ΔΔCT), where CT is the cycle number at which the signal crosses the reaction threshold and ΔΔCT = ΔCT of target mRNA – ΔCT of control mRNA. The amount of OLIG1 (marker for white matter) was normalized to TUBULIN as a reference gene, and the amounts of ABCB1 and ABCG2 (target genes) were normalized to CD31 and CD105 (markers for endothelial cells) as reference genes.
Statistical analysis
Details on statistical analysis are provided in the text and in Supplementary Information.
Results
Development of automated method to measure protein density and coverage
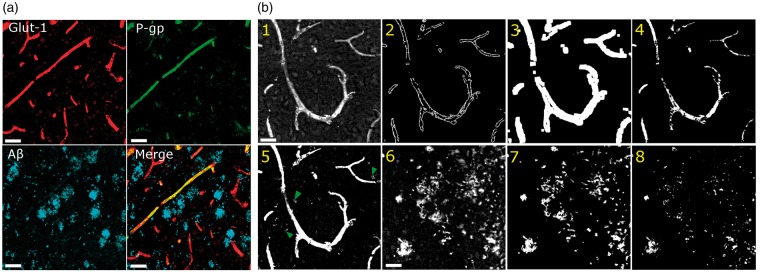
We developed an automated method to quantify the capillary densities of P-gp and ABCG2 in post-mortem sections stained simultaneously with antibodies against glucose transporter-1 (glut-1), P-gp or ABCG2, and Aβ (Figure 1(a), Supplementary Figure 1). A crucial step in the development of the automated method was the generation of a vessel mask from a capillary marker (i.e. glut-1), which was included in our study as a positive marker of vessels independent of P-gp or ABCG2 presence. First, edges of each glut-1-positive region were detected and subsequently smoothed using convolution, and these regions were defined as capillaries (Figure 1(b), panels 1–3). Second, the original glut-1 image was thresholded to remove background pixels before being multiplied by the smoothed edge mask to generate the final vessel mask (Figure 1(b), panel 4). Values for smoothing factor and threshold were chosen such that the measured variability in area coverage values from thalamus was minimal (Supplementary Figure 2). We observed that the vessel mask generated by the automated method included fewer artifacts than that generated by a user-defined threshold (green arrows, Figure 1(b), panel 5). Vessel masks generated by glut-1 staining detected at least 80% of all vessels jointly detected by glut-1 and a second endothelial marker, CD31.
Figure 1.
Quantification method of immunofluorescence staining in post-mortem sections of human brain. (a) Representative images of staining for glucose transporter-1 (glut-1, red), P-glycoprotein (P-gp, green), and amyloid-beta Aβ17-24 (A-beta, blue). (b) Images illustrating the automatic generation of masks used to quantify fluorescence intensity and percent coverage of blood vessels and plaque. Glut-1 staining (1) is used to identify the edges of the blood vessels (2), which is then convolved with a factor to smooth the edges (3). Background pixels outside the capillary are removed using a user-defined threshold to provide the final mask (4), which is then overlaid onto the original images to obtain integrated optical density within the vessel mask. The method can also be applied to images of Aβ staining (6) to obtain the plaque mask (7). In contrast to the semi-automatic method, simple thresholding either identifies background artifacts (green arrows, 5) or detects too few pixels in the mask (8). Scale bars = 50 µm.
We also tested whether the automated method based on edge detection and smoothing could be applied to measure the percent tissue area covered by Aβ plaque. Plaque masks were generated either by the automated method, or by simple thresholding of plaque-positive pixels using a user-defined value. The automated method included more plaque-positive pixels within the mask than did the method using a user-defined threshold (Figure 1(b), panels 6–8).
Automated method distinguishes AD and control cases based on Aβ plaque staining
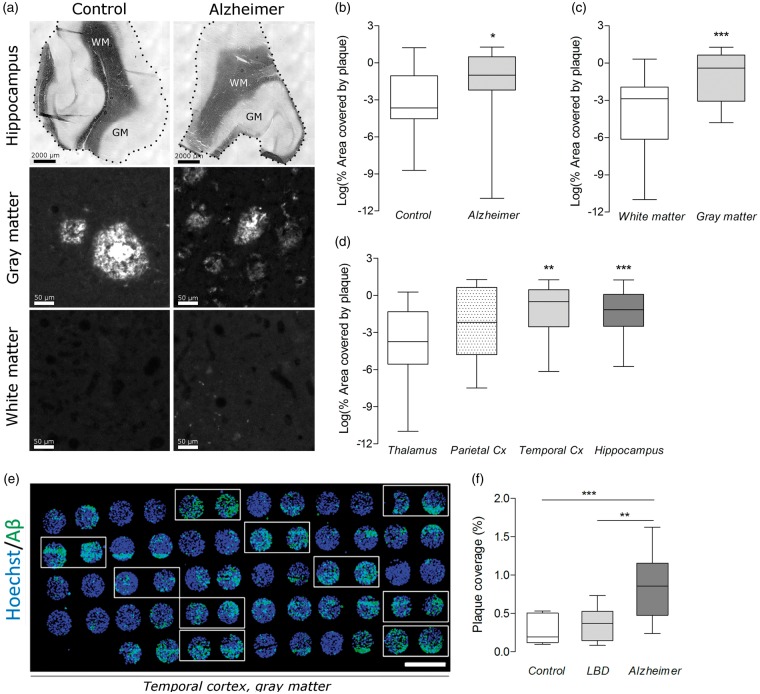
Since a hallmark of AD is higher deposition of Aβ plaque in gray matter cortical regions,14 we first assessed whether the automated method could detect higher plaque load/coverage in gray matter regions of AD patients than in those of controls (Figure 2). Plaque coverage was measured in gray and white matter areas (≥1 mm2, Figure 2(a)) from four different brain regions of AD patients (Braak stage 5/6, n = 5) and age-matched controls (Braak stage 1/2, n = 5; Supplementary Table 1, henceforth referred to as set 1). Values were then analyzed using a linear mixed-effects model to determine whether plaque expression varied with case status, brain matter, and brain region (Table 1, equation (1)). Plaque load (median value of percent area covered) was 62% higher in AD cases (0.37) than in healthy controls (0.02, Figure 2(b)), 87% higher in gray matter regions (0.67) than in white matter regions (0.05, Figure 2(c)), and at least 75% higher in cortical regions (hippocampus: 0.31, temporal cortex: 0.60) than in the control region (thalamus: 0.02, Figure 2(d)); plaque coverage was not significantly higher in parietal cortex (0.11, Figure 2(d)). The model, however, did not reveal evidence for any interaction effect between AD case status and gray matter (or any other case status and matter), possibly due to small sample size in set 1. We found that sample variability accounted for 16% of the residual variation (Table 1, equation (1), variance of bi0).
Figure 2.
Plaque coverage (or plaque load) measured by the automated method across several brain regions, brain matter, and cases. (a) Representative images of hippocampal sections from set 1 stained with Sudan Black to distinguish white and gray matters (first row) and stained against Aβ (second and third rows). Scale bars = 2000 µm (top row) and 50 µm (for rest). Dots delineate the outer edge of the tissue from the slide. (b–d) A linear regression model found that plaque expression (dependent variable, log-transformed) is elevated: (b) in Alzheimer cases compared to controls, (c) in gray matter compared to white matter, and (d) in temporal cortex and hippocampus compared to thalamus. Data are shown as box and whisker plots, indicating 75th percentile, median, and 25th percentile (boxes), and minimum and maximum values (whiskers). Asterisks in b–d designate raw P-values (not adjusted for multiple testing) to provide an indication of a parameter’s influence on the dependent variable. (e) Image of tissue microarray (set 2) showing plaque staining in gray matter sections (circles) from temporal cortex of all samples (two sections/case). AD cases are indicated with white boxes. Scale bar = 2000 µm. (f) In set 2, plaque coverage is 77% higher in AD cases than in controls, and 57% higher in AD cases than in Lewy-Body Dementia cases. Data are shown as box and whisker plots, indicating 75th percentile, median, and 25th percentile (boxes), and minimum and maximum values (whiskers). ***P < 0.001 and ** P < 0.01 by one-way ANOVA followed by Dunnett’s t-test for multiple comparisons (one-sided, α = 0.05).
Table 1.
Equations of the fitted linear regression models and their parameters for plaque coverage (1), blood vessel area (2), P-gp density (3), and ABCG2 density (4).
| Variable | Value | SE | t-value | P |
|---|---|---|---|---|
| (1) Log (plaque)ij = β0 + β1Matterij + β2Statusij + β3Regionij + bi0 + ɛij | ||||
| (Intercept) | −5.866 | 0.577 | −10.16 | 1.69e-14 |
| Matter gray | 2.802 | 0.376 | 7.46 | 4.93e-10 |
| Status AD | 2.095 | 0.628 | 3.34 | 1.03e-02 |
| Region hippocampus | 2.021 | 0.522 | 3.87 | 2.76e-04 |
| Region parietal Cx | 0.831 | 0.512 | 1.62 | 1.10e-01 |
| Region temporal Cx | 1.462 | 0.540 | 2.71 | 8.86e-03 |
| Variance of bi0 | 0.445 | 3.3e-02 | ||
| (2) Blood vessel areaij = β0 + β1Matterij + β2Statusij + β3 IOD(glut-1)ij + β4Matter:Statusij + bi0 + ɛij | ||||
| (Intercept) | −2.399 | 1.159 | −2.07 | 4.27e-02 |
| Matter gray | 1.190 | 0.256 | 4.64 | 1.92e-05 |
| Status AD | −0.161 | 0.347 | −0.46 | 6.55e-01 |
| IOD (glut-1) | 0.070 | 0.018 | 4.00 | 1.76e-04 |
| Matter gray: status AD | 0.803 | 0.340 | 2.36 | 2.15e-02 |
| Variance of bi0 | 0.140 | 2.1e-02 | ||
| (3) Log (IOD P-gp)ij = β0 + β1Matterij + β2Regionij + β3log(Plaque)ij + β4IGray (Matterij)log(Plaque)ij + bi0 + ɛij | ||||
| (Intercept) | 4.067 | 0.049 | 82.43 | 5.56e-61 |
| Matter gray | −0.171 | 0.038 | −4.53 | 3.03e-05 |
| Region hippocampus | −0.202 | 0.037 | −5.47 | 1.03e-06 |
| Region parietal Cx | −0.202 | 0.033 | −6.04 | 1.26e-07 |
| Region temporal Cx | −0.162 | 0.036 | −4.45 | 4.11e-05 |
| log (plaque) | 0.018 | 0.008 | 2.30 | 2.52e-02 |
| Matter gray: log (plaque) | −0.034 | 0.011 | −2.98 | 4.18e-03 |
| Variance of bi0 | 0.004 | 3.0e-03 | ||
| (4) Log(IOD ABCG2)ij = β0 + β1 Matterij + β2log(glut-1)ij + bi0 + ɛij | ||||
| (Intercept) | −1.080 | 2.660 | −0.41 | 6.86e-01 |
| Matter gray | 7.187 | 1.300 | 5.53 | 7.39e-07 |
| IOD (glut-1) | 1.134 | 0.105 | 10.78 | 1.17e-15 |
| Variance of bi0 | 18.15 | 1.0e-03 | ||
where Log(plaque)ij (or BloodVesselAreaij, Log (IOD P-gp)ij, Log (IOD ABCG2)ij) is the jth measurement in sample i, β0 is the intercept, bi0 is the random-effect coefficient for sample i in samples from a normal distribution with zero mean, and IGray(Matterij) is an indicator variable that is 1 if Matter is Gray and 0 otherwise. Statistical significance was evaluated using t-tests, but P-values were not corrected for multiple testing due to the model search based on the Bayesian information criterion. Non-adjusted P-values are reported only to provide an indication of the degree of influence a certain parameter has on the dependent variable.
IOD: integrated optical density; Cx: cortex; glut-1: glucose transporter 1
After determining that plaque expression varied with brain matter and region, we measured whether plaque expression varied with case status by using an independent set of post-mortem samples constructed as a tissue microarray13,15 (Figure 2(e)). This set contained only gray matter areas of temporal cortex from controls (Braak stage 0/1, n = 9), patients with AD (Braak stage 5/6, n = 10), and patients with Lewy-Body Dementia (Braak stage 0/1, n = 10; Supplementary Table 2, henceforth referred to as set 2). Plaque load (mean percent area covered ± SD) was 77% higher in AD cases (0.83 ± 0.43) than in healthy controls (0.27 ± 0.19; P < 0.001), and 57% higher in AD cases than in Lewy-Body Dementia cases (0.36 ± 0.20, P < 0.01; Figure 2(f)).
Automated method detects higher vessel coverage in gray matter than in white matter
As a second internal control, we assessed whether the automated method could detect differences in blood vessel coverage between gray and white matters because previous studies have shown that capillary density in gray matter is almost double than that found in white matter.16 Blood vessel coverage, determined by the percent area of each image covered by glut-1-positive pixels, was measured in the same fields of view extracted for analysis of plaque coverage from set 1. Using a linear regression model (Table 1, equation (2)), we found that blood vessel area was 41% higher in gray matter than in white matter. Within gray matter, coverage was 23% higher in Alzheimer cases than in controls. No significant differences in blood vessel area within the white matter areas were found between patients and control (Supplementary Figure 3). Of the residual variance, sample variability accounted for 21% of the values (Table 1, equation (2), variance of bi0).
P-gp and ABCG2 density vary with brain matter and/or brain region
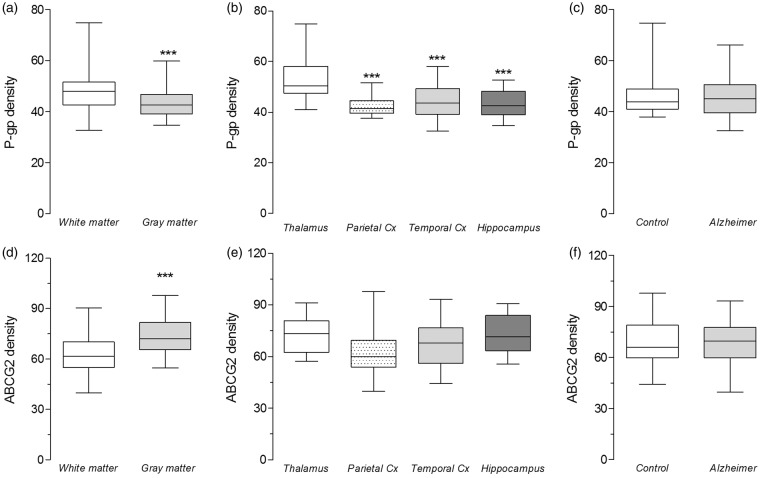
After verifying that the automated method detects known differences in plaque and vessel coverage, we used the method to examine whether the densities P-gp and ABCG2 in capillaries varied with AD status, brain matter, and brain region using set 1 of post-mortem brains. P-gp density was measured as the integrated optical density averaged from two regions of interest (≥ 1 mm2) for each sample, where each region contained ≥ 60 capillaries. Analysis using a linear regression model (Table 1, equation (3)) provided strong evidence that P-gp density was 9% lower in gray matter than in white matter (Figure 3(a)) and 15–19% lower in cortical regions than in the non-cortical region, thalamus (Figure 3(b)). An interaction effect was found between gray matter and log plaque, suggesting that reduction in P-gp density is correlated with plaque expression in gray matter (Table 1, equation (3)). The model could not find evidence that AD status or glut-1 density contributed to variation in P-gp density (Figure 3(c)) because adding these variables into the mixed-effect model adversely increased the Bayesian information criterion. Sample variability accounted for 25% of residual variation (Table 1, equation (3), variance of bi0).
Figure 3.
On a “global” level, P-gp and ABCG2 densities vary substantially with brain matter and/or region, but not case status. Density was calculated from the average fluorescence intensity within capillary pixels. (a–c) Analysis using a linear regression model on log-transformed data provided evidence that P-gp expression: (a) is 9% lower in gray matter than in white matter, (b) is 19% lower in parietal cortex, 15% lower in temporal cortex, and 17% lower in hippocampus. (c) The model did not provide any evidence for differences in P-gp expression between the AD and control groups. (d–f) Linear regression model performed on ABCG2 data indicated that ABCG2 expression is: (d) 33% higher in gray matter than in white matter, (e) but is not different between regions or (f) between the AD and control groups. Data are shown as box and whisker plots, indicating 75th percentile, median, and 25th percentile (boxes), and minimum and maximum values (whiskers). Asterisks designate raw P-values (not adjusted for multiple testing) to provide an indication of a parameter’s influence on the dependent variable.
The density of ABCG2 was found to vary with brain matter, but not with brain region or with AD status (Figure 3(d) to (f)). Linear regression analysis provided strong evidence that ABCG2 density was 33% higher in gray matter than in white matter (Figure 3(d)) and correlated with glut-1 density (Table 1, equation (4)), but was not affected by brain region (Figure 3(e)) or AD status (Figure 3(f)). Sample variability accounted for 39% of the residual variation (Table 1, equation (4), variance of bi0).
P-gp density decreased in capillaries of cortical regions on a global and a local level
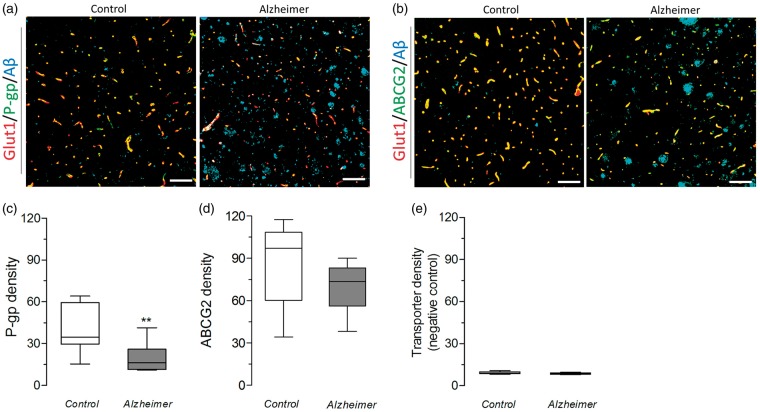
Given that P-gp and ABCG2 density varied substantially with brain region, matter, and sample, we calculated that 10 samples per group would be needed (using error rates of 0.05 for Type I and 0.2 for Type II) to measure the previously reported ∼25% decrease in transporter density in AD.9 Transporter density was then examined within capillaries of gray matter regions (0.61 mm2) from temporal cortex regions of 10 AD cases and 9 controls (set 2, Figure 4(a)). P-gp density (mean ± SD) was 53% lower in AD cases (19.5 ± 9.9) than in controls (40.0 ± 16.8, P < 0.01; Figure 4(b)), but ABCG2 density did not change between AD cases (70.0 ± 16.4) and controls (84.4 ± 28.8, P = 0.42; Figure 4(c)). When the primary antibodies were omitted, the automated method detected no signal (Figure 4(d)). Since glut-1 expression has been shown to be downregulated in AD,17 we verified that changes in transporter density were not a result of missing vessels in glut-1 staining. Vessel masks generated from glut-1 staining identified 80 ± 5% (in control samples) and 75 ± 5% (in AD samples) of all vessels jointly identified by glut-1 and CD31, and were not significantly different between the two groups (P = 0.10, Supplementary Figure 4).
Figure 4.
In gray matter areas of temporal cortex of an independent, larger cohort of post-mortem samples, capillary density of P-gp but not ABCG2 is lower in AD cases than in controls. (a–b) Representative images of sections from set 2 stained with glut-1 (red), P-gp and ABCG2 (green), and Aβ (blue). Scale bars = 100 µm. (c) P-gp density is 53% lower in AD cases than in controls, (d) but ABCG2 density is not significantly different between AD cases and controls. (e) The automated method detected no signal in transporter density when a negative control slide was used. Data are shown as box and whisker plots, indicating 75th percentile, median, and 25th percentile (boxes), and minimum and maximum values (whiskers). ** P < 0.01 by Student’s t-test with Welch’s correction (unpaired, two-sided, α = 0.05) followed by Bonferroni correction for multiple Welch corrected t-tests.
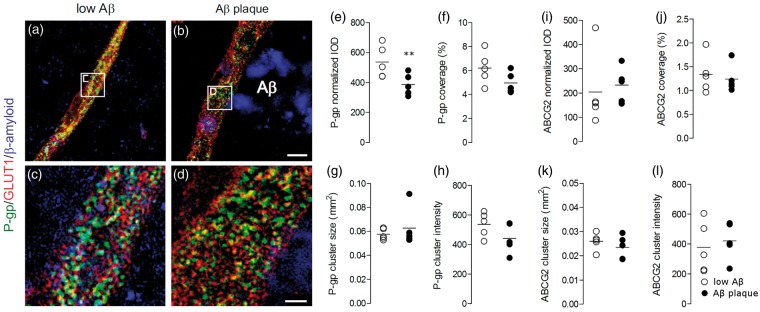
We also investigated whether P-gp and ABCG2 densities and distribution decreased locally in capillaries situated proximally to Aβ plaques, irrespective of disease pathology. Using structured illumination microscopy to measure P-gp and ABCG2 in gray matter capillaries of temporal cortex regions of only AD patients (Figure 5(a) to (d)), we found that P-gp density (mean ± SD) was 35% lower in capillaries near Aβ deposits (382.0 ± 51.2) than in those that were not near deposits (588.2 ± 123.0, P < 0.01, Figure 5(e)). P-gp distribution, measured as area covered above threshold, was not significantly lower in high plaque areas (4.9 ± 0.8 µm2) than in low plaque ones (6.1 ± 1.3 µm2, P = 0.05, Figure 5(f)). The cluster size of P-gp immunoreactive structures (P = 0.78, Figure 5(g)) and cluster intensity (P = 0.16, Figure 5(h)) were not altered near Aβ deposits. Similarly, ABCG2 density (P = 0.72, Figure 5(i)), distribution (P = 0.67, Figure 5(j)), cluster size (P = 0.39, Figure 5(k)), and cluster intensity (P = 0.65, Figure 5(l)) were not altered near Aβ deposits.
Figure 5.
In gray matter areas of temporal cortex from AD cases, the density of P-gp but not ABCG2 is lower in capillaries located near Aβ plaque than in those that are not. (a–d) Structured illumination microscopy was used to identify clusters of P-gp (green) within capillaries (glut-1 staining, red) located near high and low amounts of surrounding Aβ plaque (blue). (e) The integrated optical density (IOD) of P-gp, normalized to the staining of glut-1, is 35% lower in capillaries near plaque than in those that are not near visible plaques. (f) Percent coverage of P-gp within the capillary, (g) cluster size and (h) intensity were not significantly reduced near Aβ deposits. (i) ABCG2 density, (j) distribution, (k) cluster size, and (l) cluster intensity were not altered near Aβ deposits. Data points from each sample are plotted along with the mean value for each group. ** P < 0.01 by a Student’s t-test (one-sided) followed by Bonferroni correction for multiple t-tests (α = 0.01).
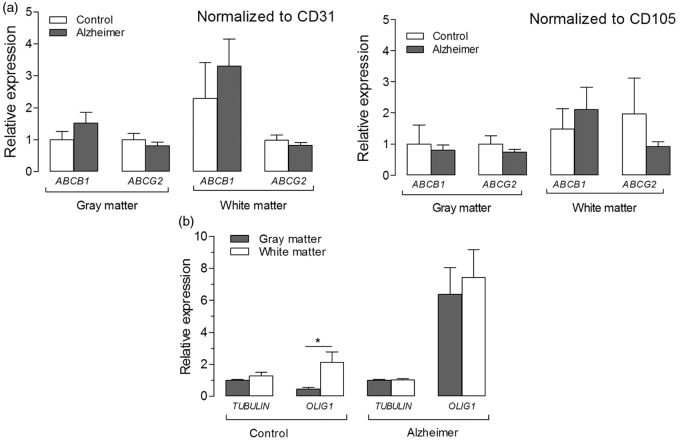
Finally, to determine whether these changes are also evident on the transcript level, we measured mRNA expression of ABCB1 and ABCG2 in gray and white matter regions from micro-punch samples taken from fresh frozen temporal cortex of set 2. When normalized to CD31 and CD105 as reference genes, no significant changes in transcript levels of ABCB1 or ABCG2 were measured between AD cases and controls, or between gray and white matters (Figure 6(a)). Expression of OLIG1, a marker for oligodendrocytes present in white matter, was 4.7-fold higher in white matter than in gray matter of controls (P < 0.05, Figure 6(b)). However, OLIG1 expression was not significantly different between white and gray matter samples of AD cases (P = 0.40, Figure 6(b)).
Figure 6.
Expression of ABCB1 and ABCG2 in gray and white matter regions of temporal cortex from AD cases and controls. (a) Relative expression was calculated using the comparative CT method (2−ΔΔCT) method, with cycle threshold (CT) values normalized to those of reference genes CD31 and CD105 (markers for endothelial cells), and then to the average of controls (gray matter). In gray and white matters, neither ABCB1 nor ABCG2 expression is significantly different between AD cases and controls. (b) Expression of OLIG1 (a marker for white matter) is roughly five-fold higher in white matter than in gray matter of controls, but is not significantly different between white and gray matter regions of AD cases. TUBULIN, which was used as reference gene for OLIG1, was not significantly different between AD cases and controls. Data are expressed as mean ± SD. ***P < 0.001 and *P < 0.05 by two-way ANOVA followed by Bonferroni corrected t-tests (two-sided, α = 0.05).
Discussion
Automated method to measure protein staining in post-mortem brain sections
In this study, we developed an image analysis method to quantify the variability of P-gp and ABCG2 densities in capillaries from different brain regions of post-mortem sections from AD and matched controls. Because AD has a complex pathophysiology with altered protein expression in many brain cells,18 we aimed to minimize the effect of these changes on the measurement of transporter densities. Our method therefore relies upon the use of a vessel mask, which is generated from the staining of a protein marker (i.e. glut-1) independent of the one being measured. Once generated, the mask is “smoothed” using convolution and then applied onto images of transporter staining to measure density within the vascular space. Since the mask is generated automatically using change in pixel intensity rather than intensity itself, it not only excludes staining artifacts present outside the capillary, but also includes protein-positive pixels that may have otherwise been ignored due to variations in intensity. This effect was evidenced by visually comparing the mask generated by the automatic method to that generated by a single intensity threshold.
The image analysis strategy developed here can also be used to identify other structures based on contrast with background. When a similar algorithm was used to measure plaque staining, it detected reported differences19–21 in the post-mortem samples from both tissue sets. In set 1, plaque coverage was 60–87% higher in gray matter regions (versus white matter), in cortical regions (versus thalamus, a non-cortical region), and in AD patient samples (versus healthy) than in those of the respective controls. The lack of interaction effect between AD case status and gray matter/cortical regions in our samples was likely due to the low number of samples in set 1. One caveat of such comparisons among regions is that plaque load is not evenly distributed among layers of cortex, as superficial layers tend to have greater plaque levels than deep layers. However, in our study, we used large pieces of tissue that covered both superficial and deep layers to enable relative comparisons among regions. Furthermore, in an independent, larger set of post-mortem samples (set 2) containing only gray matter areas of temporal cortex, the automated method distinguished AD cases from controls, as well as AD cases from patients with a different type of dementia.
Similarly, when the algorithm was used to measure vessel area (as a percentage of the field of view), it detected reported differences in the post-mortem samples. Vessel coverage was almost twice as high in gray matter than in white matter, was 23% higher within gray matter areas of AD cases than in controls, and was not different within white matters of the two groups, similar to previous findings.22 While some reports have observed increased vascular density in AD samples,23 possibly due to angiogenesis,9,24 other studies have shown that deterioration of gray matter regions in AD may account for measured increases in vessel density.22,25,26 We cannot ascertain which explanation may be most likely in our study without further information on angiogenic markers or cortical thicknesses from the samples. Another factor that may have impacted measures of vessel density is the vascular risk profile of each case in our study, but this information is not known and may be a potential confound in our analysis.
Advantages and limitations of automated method to quantify transporter densities
The automated method developed in this study is valuable for measuring proteins in post-mortem brain tissue because it overcomes limitations of previous modes of analysis in three ways. First, the automated method inherently normalizes for vessel density, avoiding any changes in density that may result from different brain regions and disease status. Second, it allows the user to rapidly measure a large number of capillaries (≥60 capillaries) across several regions, reducing user bias and time. Third, it can be used to analyze any protein, not just those based in capillaries. Because the quantification is contrast-based, it is less sensitive to staining artifacts, which are a common problem in immunofluorescence analysis of proteins in pathological brain samples. In contrast, previous methods suffer from one or multiple problems. They either do not measure density exclusively in vessels, do not account for changes in capillary density, and/or only measure density in a small number of capillaries. For example, the strategies of counting the number of transporter-positive capillaries in the field of view or the number of transporter-positive pixels have been used as a measure of transporter density in AD6,8 and in other brain disorders, such as epilepsy.27 However, such analysis does not truly measure transporter density because the results can be interpreted as simply a count of capillaries or as the area covered by protein-positive pixels. These two methods also do not account for any changes in capillary density that may occur between samples, regions, and/or cases,7,27 and were performed on 10–20 capillaries for each sample, precluding a more “global” assessment of density from various brain regions.
One potential limitation of the automated method, however, might occur in a scenario in which some capillaries do not express glut-1 at all—a possibility given that glut-1 expression may be downregulated in AD.17 Because the vessel mask relies on detection of vessels to generate a mask, missing vessels could potentially bias the outcome, depending on the relationship between glut-1 and the transporter of interest. If the proteins were not correlated, then the missing vessels would not affect the measurements: all sampled capillaries would be representative of the population, and a reliable estimate of the sample mean can be obtained by measuring a sufficient number of vessels. If the proteins were positively or negatively correlated, then the measurements of transporter density would be biased in one direction. Statistical power to detect differences would be reduced, but the false positive rate (Type I error) would remain unchanged. In our study, missing vessels were unlikely to affect measures of P-gp density because: (1) glut-1 detected > 75% of all vessel pixels identified using two endothelial markers in both control and AD samples, and (2) glut-1 did not correlate with P-gp density. In contrast, missing vessels may have led to an overestimate of ABCG2 values because glut-1 and ABCG2 were positively correlated. As a result, differences between samples may have been underestimated. Future studies might benefit from including two vascular markers to generate a capillary mask and reduce the bias resulting from missing vessels because protein expression of many capillary markers is reported to change in AD.18,28
Relationship of P-gp density and Aβ plaque
We found that P-gp density in capillaries varies substantially with plaque expression, brain matter, and brain region. In set 1, the automated method detected a decrease in P-gp density only within gray matter and cortical regions—the same regions in which plaque coverage was highest. However, no statistical difference in density was found between AD and controls. This result was probably due to low sample size. When we controlled for brain region and matter, as well as for effect size/power in set 2, the automated method found that capillary P-gp density was 53% lower in AD than in controls. This value is larger than previously reported (20–25%),8,9 and the effect size detected in our study is likely a result of measuring P-gp levels per capillary unit, normalizing for brain region and matter, and sampling a larger number of capillaries (60 as opposed to 10–20) from each case. In addition to this global decrease in P-gp density in AD, we also found that P-gp density was decreased by 35% in capillaries near detectable Aβ deposits of AD samples, as evidenced by structured illumination microscopy. This local decrease in AD samples, which to our knowledge has not been reported before, was likely due to a reduction in the number of P-gp clusters, as we did not measure a change in the size or intensity of P-gp clusters within the capillary. If fibrillary forms of soluble Aβ plaque are indeed transported by P-gp, then such local decreases in the density of P-gp located proximal to Aβ deposits would exacerbate pathological accumulation of Aβ.
The differences measured in protein expression did not occur at the transcript level, as we did not find any change in mRNA levels of ABCB1 between AD and controls, or between gray and white matters. Wijesuriya et al.9 reported similar findings in AD samples, but did not account for gray vs. white matter. A methodological explanation for the discrepancy between the protein and transcript results is that mRNA levels were measured in whole brain homogenates. As such, any potential changes in mRNA expression of ABCB1 within endothelial cells may have been diluted by transcripts extracted from whole brain. A biological explanation might be that expression levels are not altered at the transcript level, but rather that P-gp degradation is increased in AD through the ubiquitin-proteasome pathway, resulting in reduced availability.29
Our results are consistent with some, but not all, of the previous studies investigating the relationship between Aβ plaque and P-gp density in the human brain. Similar to our findings, some studies have reported that reduced P-gp density is associated with Aβ plaque deposition, in non-demented healthy subjects,6 in subjects with cerebral amyloid angiopathy7,10 (a condition characterized by Aβ deposits exclusively in capillaries), and in AD cases.8,9 In contrast, one study found that P-gp density was not reduced in AD subjects.10
Comparisons among studies are limited in scope, however, because of differences in analysis methods, analyzed brain regions, and selection of post-mortem samples. As discussed before, previous methods have some limitations and some may not even measure density effectively. Differences in the investigated brain regions must also be considered, since we have shown that P-gp density varies with both brain region and matter. For example, studies showing a decrease in P-gp density (including ours) used temporal cortex samples,8,9 whereas the study showing no difference in P-gp density used occipital cortex samples.10 Other important factors, such as the diversity in age and Braak staging of the post-mortem samples, may also influence density. One study, for example, used a control group whose mean age was 17 ± 5 years lower than that of the AD group,8 confounding the results because P-gp density decreases with age. The range in Braak staging of the samples—from unreported9 to stages one to six for AD cases8,10—is also a potential confounding factor, as pathological changes in P-gp density may only occur at certain points in disease progression. Our study highlights the importance of controlling several factors that influence density, including method of analysis, brain region, brain matter, and age.
Relationship of ABCG2 density and Aβ plaque
We found that ABCG2 density varies with matter but not with AD status. This was evidenced by the results from the automated analysis on multiple regions: ABCG2 density was a third higher in gray matter than in white matter regions, but not different between regions or between AD and control cases. Analysis of ABCG2 density in an independent, larger set confirmed this result. However, protein differences were not detected at the transcript level. These results are consistent with some but not all reports on the relationship between ABCG2 and Aβ: one group reported no change in ABCG2 density in AD,9 one reported an increase,11 while another reported a decrease.10 Once again, variations in methods and samples used to quantify ABCG2 density in these studies could account for the conflicting results. Our study reproduced the same result in two separate sets of post-mortem brains. ABCG2 density was also found to be highest in the same areas, where P-gp density was lowest (i.e. gray matter), and subsequent studies may investigate whether ABCG2 density could be up-regulated in regions of high plaque deposition to compensate for the decrease in P-gp density.
In summary, we developed an automated method that quantifies transporter density from post-mortem sections of human brain. By using vessel masks, the method measures density in the vascular space, which inherently accounts for changes in capillary density that may occur between regions and in pathological conditions. In addition to this methodological advance, our study reveals important biological findings. First, P-gp density varies with brain region and with matter at the protein but not transcript level—a finding that, to our knowledge, has not previously been reported. We also confirmed that P-gp density was reduced in AD, and found that P-gp levels reduce locally by 35% in capillaries proximal to plaque within AD cases. Second, we found that ABCG2 density changes with brain matter, but does not change in AD—a finding that was reproduced using two independent cohorts of post-mortem brains. These findings highlight that variations in method, region, age, and staging can affect measures of transporter density in pathological conditions, and that previous studies may have underestimated the extent of changes in the blood–brain barrier by not accounting for these variables. In fact, by accounting for such variations, we found a 53% reduction in P-gp density as compared to previously reported values of 20–25%. The automated method should facilitate future studies of transporter density, by allowing simultaneous measurement of density in several brain regions and samples, minimizing user bias, and increasing reproducibility.
Supplementary Material
Acknowledgements
We thank Siv Eriksson for assistance with the post-mortem tissue, George Leiman for editorial assistance, and Dr. Daniel Warren for helpful discussions.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by Alzheimerfonden grant (AF-554531), Stockholm Brain Institute, and the Intramural Research Program of the National Institutes of Mental Health (project #ZIA-MH0022795) and the National Cancer Institute (#Z01-BC-005598) at the National Institutes of Health. WWK is funded by the Wellcome Trust (WT097307).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
PK, MS, BG, MMG, RBI, MDH, and JM conceived and designed experiments. PK, LW, NM, HGB, and JM conducted experiments. PK, MS, WWK, MDH, and JM performed data and statistical analysis. All authors contributed to data interpretation, and to the writing and editing of this manuscript.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Wolf A, Bauer B, Hartz AMS. ABC transporters and the Alzheimer’s disease enigma. Front Psychiatry 2012; 3: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartz AMS, Miller DS, Bauer B. Restoring blood-brain barrier P-glycoprotein reduces brain amyloid-beta in a mouse model of Alzheimer’s disease. Mol Pharmacol 2010; 77: 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lam FC, Liu R, Lu P, et al. Beta-amyloid efflux mediated by P-glycoprotein. J Neurochem 2001; 76: 1121–1128. [DOI] [PubMed] [Google Scholar]
- 4.Kuhnke D, Jedlitschky G, Grube M, et al. MDR1-P-Glycoprotein (ABCB1) mediates transport of Alzheimer’s amyloid-beta peptides – implications for the mechanisms of Abeta clearance at the blood-brain barrier. Brain Path 2007; 17: 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cirrito J, Deane R, Fagan A, et al. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-β deposition in an Alzheimer disease mouse model. J Clin Invest 2005; 115: 3285–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogelgesang S, Cascorbi I, Schroeder E, et al. Deposition of Alzheimer’s B-amyloid is inversely correlated with P-glycoprotein expression in the brains of elderly non-demented humans. Pharmacogenetics 2002; 12: 535–541. [DOI] [PubMed] [Google Scholar]
- 7.Vogelgesang S, Warzok R, Cascorbi I, et al. The Role of P-glycoprotein in Cerebral amyloid angiopathy; implications for the early pathogenesis of Alzheimer’s disease. Curr Alzheimer Res 2004; 1: 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeynes B, Provias J. An investigation into the role of P-glycoprotein in Alzheimer’s disease lesion pathogenesis. Neurosci Lett 2011; 487: 389–93. [DOI] [PubMed] [Google Scholar]
- 9.Wijesuriya HC, Bullock JY, Faull RLM, et al. ABC efflux transporters in brain vasculature of Alzheimer’s subjects. Brain Res 2010; 1358: 228–38. [DOI] [PubMed] [Google Scholar]
- 10.Carrano A, Snkhchyan H, Kooij G, et al. ATP-binding cassette transporters P-glycoprotein and breast cancer related protein are reduced in capillary cerebral amyloid angiopathy. Neurobiol Aging 2014; 35: 565–75. [DOI] [PubMed] [Google Scholar]
- 11.Xiong H, Callaghan D, Jones A, et al. ABCG2 is upregulated in Alzheimer’s brain with cerebral amyloid angiopathy and may act as a gatekeeper at the blood-brain barrier for Abeta(1-40) peptides. J Neurosci 2009; 29: 5463–5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulder J, Zilberter M, Pasquaré SJ, et al. Molecular reorganization of endocannabinoid signalling in Alzheimer’s disease. Brain 2011; 134: 1041–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ádori C, Glück L, Barde S, et al. Critical role of somatostatin receptor 2 in the vulnerability of the central noradrenergic system: new aspects on Alzheimer’s disease. Acta Neuropathol 2015; 129: 541–563. [DOI] [PubMed] [Google Scholar]
- 14.Thal DR, Attems J, Ewers M. Spreading of amyloid, tau, and microvascular pathology in Alzheimer’s disease: findings from neuropathological and neuroimaging studies. J Alzheimers Dis 2014; 42: S421–S429. [DOI] [PubMed] [Google Scholar]
- 15.Kampf C, Olsson I, Ryberg U, et al. Production of tissue microarrays, immunohistochemistry staining and digitalization within the human protein atlas. J Vis Exp 2012; 63: e3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ballabh P, Braun A, Nedergaard M. Anatomic analysis of blood vessels in germinal matrix, cerebral cortex, and white matter in developing infants. Pediatr Res 2004; 56: 117–124. [DOI] [PubMed] [Google Scholar]
- 17.Winkler EA, Nishida Y, Sagare AP, et al. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat Neurosci 2015; 18: 521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lepelletier F-X, Mann DMA, Robinson AC, et al. Early changes in extracellular matrix in Alzheimer’s disease. Neuropathol Appl Neurobiol. 2015. Epub ahead of print 6 November 2015. DOI: 10.1111/nan.12295. [DOI] [PubMed] [Google Scholar]
- 19.Zhan SS, Veerhuis R, Kamphorst W, et al. Distribution of beta amyloid associated proteins in plaques in Alzheimer’s disease and in the non-demented elderly. Neurodegeneration 1995; 4: 291–297. [DOI] [PubMed] [Google Scholar]
- 20.Vlassenko AG, Benzinger TLS, Morris JC. PET amyloid-beta imaging in preclinical Alzheimer’s disease. Biochim Biophys Acta 2012; 1822: 370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shukla C, Bridges LR. Regional distribution of tau, beta-amyloid and beta-amyloid precursor protein in the Alzheimer’s brain: a quantitative immunolabelling study. Neuroreport 1999; 10: 3785–3789. [DOI] [PubMed] [Google Scholar]
- 22.Hunter JM, Kwan J, Malek-Ahmadi M, et al. Morphological and pathological evolution of the brain microcirculation in aging and Alzheimer’s disease. PLoS One 2012; 7: e36893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perlmutter LS, Barrón E, Saperia D, et al. Association between vascular basement membrane components and the lesions of Alzheimer’s disease. J Neurosci Res 1991; 30: 673–681. [DOI] [PubMed] [Google Scholar]
- 24.Desai BS, Schneider JA, Li J-L, et al. Evidence of angiogenic vessels in Alzheimer’s disease. J Neural Transm 2009; 116: 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buée L, Hof PR, Bouras C, et al. Pathological alterations of the cerebral microvasculature in Alzheimer’s disease and related dementing disorders. Acta Neuropathol 1994; 87: 469–480. [DOI] [PubMed] [Google Scholar]
- 26.Fischer VW, Siddiqi A, Yusufaly Y. Altered angioarchitecture in selected areas of brains with Alzheimer’s disease. Acta Neuropathol 1990; 79: 672–679. [DOI] [PubMed] [Google Scholar]
- 27.Feldmann M, Asselin M-C, Liu J, et al. P-glycoprotein expression and function in patients with temporal lobe epilepsy: a case-control study. Lancet 2013; 12: 777–785. [DOI] [PubMed] [Google Scholar]
- 28.Hagnelius N-O, Boman K, Nilsson TK. Fibrinolysis and von Willebrand factor in Alzheimer’s disease and vascular dementia – a case-referent study. Thromb Res 2010; 126: 35–38. [DOI] [PubMed] [Google Scholar]
- 29.Hartz AMS, Zhong Y, Wolf A, et al. Aβ40 Reduces P-Glycoprotein at the blood-brain barrier through the ubiquitin-proteasome pathway. J Neurosci 2016; 36: 1930–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.