Abstract
Diphenhydramine, a sedative histamine H1-receptor (H1R) antagonist, was evaluated as a probe to measure drug/H+-antiporter function at the blood–brain barrier. In situ brain perfusion experiments in mice and rats showed that diphenhydramine transport at the blood–brain barrier was saturable, following Michaelis–Menten kinetics with a Km = 2.99 mM and Vmax = 179.5 nmol s−1 g−1. In the pharmacological plasma concentration range the carrier-mediated component accounted for 77% of diphenhydramine influx while passive diffusion accounted for only 23%. [14C]Diphenhydramine blood–brain barrier transport was proton and clonidine sensitive but was influenced by neither tetraethylammonium, a MATE1 (SLC47A1), and OCT/OCTN (SLC22A1-5) modulator, nor P-gp/Bcrp (ABCB1a/1b/ABCG2) deficiency. Brain and plasma kinetics of [11C]diphenhydramine were measured by positron emission tomography imaging in rats. [11C]Diphenhydramine kinetics in different brain regions were not influenced by displacement with 1 mg kg−1 unlabeled diphenhydramine, indicating the specificity of the brain positron emission tomography signal for blood–brain barrier transport activity over binding to any central nervous system target in vivo. [11C]Diphenhydramine radiometabolites were not detected in the brain 15 min after injection, allowing for the reliable calculation of [11C]diphenhydramine brain uptake clearance (Clup = 0.99 ± 0.18 mL min−1 cm−3). Diphenhydramine is a selective and specific H+-antiporter substrate. [11C]Diphenhydramine positron emission tomography imaging offers a reliable and noninvasive method to evaluate H+-antiporter function at the blood–brain barrier.
Keywords: Biological transporter, blood–brain barrier, proton antiporter, positron emission tomography, solute carrier
Introduction
For many years, passive diffusion was thought to be the sole mechanism controlling drug permeation through the blood–brain barrier (BBB) and the subsequent exposure of the central nervous system (CNS) to the drug. In the 1990s, the discovery of unidirectional membrane transporters from the ATP-binding cassette (ABC) family at the BBB challenged this assumption.1 P-glycoprotein (P-gp/ABCB1) and breast cancer resistance protein (BCRP/ABCG2) are the main ABC transporters at the BBB.2,3 They mediate the exit of substrates across the luminal BBB membrane back to the blood compartment, with a substantial impact on their CNS distribution and effects.2 Considerable efforts are being made to assess the functional properties of transporters expressed at the BBB and predict their implications for CNS pharmacokinetics and pharmacodynamics.2 Positron emission tomography (PET) imaging using radiolabeled transporter substrates is an appealing approach for the noninvasive study of transporter function at the BBB in animals and humans, in health and disease.4 It provides a unique tool to evaluate the risk of drug–drug interactions (DDI) involving transporter substrates and inhibitors and their impact on brain exposure to the drug in vivo,5 in particular the impact of ABC-transporter function at the BBB.6,7
Several studies have revealed that the brain uptake of many psychoactive drugs is saturable, further challenging the picture of passive diffusion as the exclusive regulator of adequate brain exposure. Diphenhydramine (2-(diphenylmethoxy)-N,N-dimethylethanamine) is a clinically approved histamine H1-receptor (H1R) antagonist with known sedative effects.8 In situ brain perfusion experiments have shown a saturable uptake transport mechanism for diphenhydramine at the rat BBB.9 Recent brain microdialysis experiments have also suggested the importance of diphenhydramine-influx transport at the BBB in different species including rats, dogs, and nonhuman primates.10 The functional characterization of this transport remains to be established in vivo.
Several in vivo/in situ studies have provided evidence of a novel and molecularly unknown drug transporter that controls the permeation of many CNS compounds across the mouse BBB.11–13 This specific transport has also been observed in vitro using rodent and human immortalized brain endothelium or intestinal cell lines.14–17 Notably, these studies have elucidated the functional properties of the transporter involved, characterizing it as a cationic drug/proton (H+)-antiporter. This so-called drug/H+-antiporter function does not correspond to any of the other solute carrier (SLC) drug transporters identified at the molecular level: OCT1-3 (SLC22A1-3), OCTN (SLC22A4-5), or MATE1 (SLC47A1).14–17 So far, identified H+-antiporter substrates mainly include cationic tertiary/secondary amines with CNS activity, such as cocaine,12 clonidine,11 nicotine,13 oxycodone,17 and ecstasy.16 Some H+-antiporter function inhibitor drugs have been screened and identified using in vitro BBB models14 or in situ brain perfusion11; saturation or inhibition of H+-antiporter function using known inhibitors occurs at blood concentrations too toxic or unreachable for in vivo studies.14
The identification of this H+-antiporter function at the BBB raises questions as to its impact on the variability of the response to CNS substrate drugs, which remains a major issue in neuropharmacology. Thus, probes are needed to selectively investigate the pathophysiological parameters that modulate H+-antiporter function at the BBB. In the present study, we used in situ brain perfusion in rats to investigate the qualitative and quantitative contributions of passive diffusion and the H+-antiporter function to diphenhydramine transport at the BBB, and thus consider diphenhydramine as a specific and selective probe to study H+-antiporter function at the BBB. The pharmacokinetic properties of carbon-11 radiolabeled diphenhydramine were evaluated to validate its use as a PET probe for the noninvasive study of drug/H+-antiporter function in vivo.
Materials and methods
Animals
Experiments were performed with male Sprague Dawley rats (285 ± 40 g; 7–9 weeks; Janvier, Genest, France). The 90 rats were housed in a controlled environment (22 ± 3℃; 55 ± 10% relative humidity) and a 12 h dark/light cycle, with access to food and tap water ad libitum. All studies involving animals are reported in accordance with ARRIVE guidelines for reporting experiments and complied with the ethical rules of the European directive (210/63/EU) for experimentation with laboratory animals; they were approved by the ethics review committee of Paris Descartes University (approvals no. 12-183/12-186/2012).
Chemicals and radiochemicals
Diphenhydramine hydrochloride and tetraethylammonium hydrochloride (TEA) were purchased from Inresa (Strasbourg, France) and Sigma (St Quentin Fallavier, France), respectively. All other chemicals were purchased from Sigma. [14C]Diphenhydramine (26 × 1010 Bq mmol−1) and 3H-inulin (48 × 1010 Bq mmol−1) was purchased from Moravek (La Brea, CA, US) and Perkin Elmer Life Sciences (Courtaboeuf, France), respectively. Ready to inject [11C]-diphenhydramine was synthesized from the corresponding desmethyl precursor (N-desmethyl-diphenhydramine; 1 mg) using [11C]methyl-triflate in a TRACERlab™ FX CPro module. Radiomethylation was carried out in acetone (400 µl) in the presence of 3 M sodium hydroxide (7 µl) at 120℃ for 2 min. Semi-preparative HPLC purification (Agilent Zorbax® C18, 250 × 9.4 mm, 5 µm; CH3CN/H2O/TFA 30/70/0.1 v/v/v) followed by formulation afforded radiochemically pure [11C]diphenhydramine (1.5-3 GBq) within 45 min in 15% decay corrected yield (n = 11).
BBB transport kinetics using in situ brain perfusion
Experimental procedure
The qualitative and quantitative transport properties of [14C]diphenhydramine were assessed in vivo at the BBB using the in situ brain perfusion method in rats.18 The in situ carotid method replaces blood by an artificial perfusate fluid so that [14C]diphenhydramine did not undergo peripheral metabolism. In this condition, the radiochemical purity of [14C]diphenhydramine in the perfused brain was previously assessed.9 The composition of the perfusate can be set up to study the effect of inhibitors, substrate concentrations, or specific electrolytic conditions that cannot be reached physiologically in vivo. Therefore, the transport properties and functional/biochemical features of the diffusion processes through the living BBB can be characterized regardless of the peripheral kinetics or toxicity of tested conditions.
Once anesthetized with a mixture of xylazine and ketamine at 4–80 mg kg−1, briefly the right carotid arteries were exposed and a catheter was inserted into the right common carotid artery after ligation of the appropriate vessels.19 The catheter was connected to a syringe containing the perfusion fluid. The thorax was then opened, the heart was cut and perfusion started immediately at a flow rate of 10 ml min−1 in rats.18 At this time, the perfusion fluid replaces all the blood in the right brain vasculature. After 60 s perfusion, animals were decapitated and the brain removed from the skull and dissected on ice. Carbon-14 and tritium radioactivity were counted in the whole or selected regions of the right brain hemisphere, and perfusate aliquots using a Tri-Carb 2810TR counter (Perkin Elmer).
Each animal was perfused with protein-free Krebs carbonate buffer physiological saline containing (mM): 128 NaCl, 24 NaHCO3, 4.2 KCl, 2.4 NaH2PO4, 1.5 CaCl2, 0.9 MgSO4, and 9 d-glucose. The perfusion fluid pH (pHe) was adjusted and checked with a digital pH meter (± 0.05 pH units) immediately before perfusion. The proton dependency of diphenhydramine transport was assessed under conditions of nonphysiological vascular or intracellular pH modifications. Hydrochloric acid was added to the gassed perfusion fluid to bring the pH of the perfusion fluid to 6.40. In some experiments, NH4Cl (30 mM) was added in the perfusion fluid (final pH of 7.40) to modify intracellular pH (pHi). The perfusion fluid was gassed with 95% O2/5% CO2 for pH control (7.40), unless otherwise specified, and warmed to 37℃. [14C]Diphenhydramine (4 × 103 Bq ml−1; ∼1.4 µmol l−1) and [3H]inulin (11 × 103 Bq ml−1), a vascular integrity marker, were finally added to the solution, immediately before the start of perfusion.
Cis-inhibition experiments were carried out to confirm the involvement of the drug H+/antiporter function. The concentrations were selected to obtain a modulation rather than to reflect the relevance of in vivo DDI, as these unbound concentrations selected cannot be produced by systemic administration. TEA (20 mM) a known prototypical inhibitor of OCT, OCTN, and MATE was shown not to interact with H+/antiporter function.11,12,16,17 Clonidine (10 mM) was used as a competitive inhibitor of H+/antiporter function at the BBB.11
Calculation of kinetic parameters
Calculations were done as previously described.19 The brain vascular volume was estimated using the vascular marker [3H]inulin distribution volume (Vv; µl g−1)
| (1) |
where X* (dpm g−1) is the tritium amount in the tissue sample and Cperf* (dpm µl−1) its concentration in the perfusion fluid. The data for any animal whose Vv was above the normal value18 was excluded from the study.
The apparent tissue distribution volume (Vbrain, µl g−1) was calculated as
| (2) |
where Xtissue (dpm g−1) is the calculated amount of [14C]diphenhydramine from the right brain and Cperf (dpm µl−1) its concentration in the perfusion fluid. This total activity was corrected for “vascular” contamination using the Vv subtraction
| (3) |
where Xtot (dpm g−1) is the total quantity of [14C]diphenhydramine measured in the sample tissue.
The initial transport expressed as a Kin (µl s−1 g−1) was calculated from
| (4) |
where T is the perfusion time (60 s).
The diphenhydramine BBB flux () is composed of both a saturable (Michaelis–Menten term) and passive (unsaturable) components
| (5) |
where Ctot (mmol l−1) is the total (unbound) diphenhydramine concentration in the perfusate (ranging from 1.4 µM to 30 mM), Vmax (nmol s−1 g−1) is the maximal velocity of transport, Km (mM) represents the concentration at the half-maximal carrier velocity. Kpassive (µl s−1 g−1) is an unsaturable component, which represents the rate of transport by passive diffusion. The data were fitted using nonlinear regression analysis.
A relative value for the permeability of a compound into the tissue was obtained from the extraction parameter (E, %), where F is the vascular fluid flow rate (42 µl s−1 g−1) of the whole right hemisphere measured using [3H]diazepam, a free passively diffusible totally extracted compound in mice and rats,18 calculated as
| (6) |
In situ brain perfusion in mice
Additional in situ brain perfusion experiments were performed in mice in order to compare diphenhydramine kinetic parameters between mice and rats and investigate the influence of P-gp and Bcrp function at the BBB on diphenhydramine flux through the BBB using P-gp/Bcrp (Abcb1a–/–, Abcb1b–/–, Abcg2-/-) triple knock-out mice. Methods and results of mice experiments are described in a supplementary information (available on the JCBFM website; see supplementary information link at the top of the online article).
[11C]Diphenhydramine PET imaging
MicroPET scans were performed using an Inveon® microPET system (Siemens, Germany) in rats. Anesthesia was induced and thereafter maintained using 3 and 1.5–2.5% isoflurane in O2, respectively. Sixty-minute dynamic acquisitions were performed, starting from i.v. injection of a bolus [11C]diphenhydramine (45.3 ± 5.6 MBq; 109.8 ± 51.7 ng) in a catheter inserted in the caudal lateral vein. Baseline scans were performed in four different rats. Displacement experiments were performed in three other rats using the same acquisition protocol and the injection of 1 mg/kg i.v. (3.9 µmol kg−1) of unlabeled diphenhydramine at 30 min after [11C]diphenhydramine injection.
Images were reconstructed with the FORE+OSEM2D algorithm including normalization, attenuation, scatter, and random corrections. Image analysis and quantification of radioactivity uptake were performed using Pmod® software (version 3.7, Switzerland). The Schiffer rat brain atlas was used to delineate brain regions and generate the corresponding time-activity curves (TACs). Radioactivity was corrected for carbon-11 decay, injected dose and animal weight to express the measurements in standardized uptake values (SUV). Tmax was defined as the time at which the maximum of the TAC curve (SUVmax) occurred.
PET-based estimation of [11C]diphenhydramine brain uptake clearance was performed using the integration plot analysis from 0 to 2 min20 considering the mean metabolite-corrected arterial input function measured in three additional rats. To that end, a catheter was inserted in the femoral artery of anesthetized rats. After [11C]diphenhydramine injection, arterial blood samples were withdrawn, centrifuged, and counted. Plasma samples withdrawn at 2, 5, 15, 30, and 60 min were used to estimate the fraction of parent [11C]diphenhydramine in plasma using radio-HPLC analysis. Radio-HPLC system consisted of a quaternary gradient pump, an ASI100T autosampler, and a UVD170U UV–vis detector (Thermo Scientific, France) online with an LB-509 radioisotope detector (Berthold, France). [11C]diphenhydramine and its radiometabolites were detected and separated using an Atlantis preparative T3 10 µm, 10 × 250 mm at 25℃ (Waters, France). The mobile phases consisted of 10 mM ammonium acetate in purified water (A) and acetonitrile (B). A linear gradient from 30 to 75% of B in 9 min was applied to the column at a flow rate of 5 ml min−1. In these conditions, the retention time of diphenhydramine and N-desmethyl-diphenhydramine was 8.5 and 7.5 min, respectively. Representative chromatograms are shown in a supplementary information (available on the JCBFM website; see supplementary information link at the top of the online article).
Additional rats were used to investigate the presence of radiometabolites in the brain. [11C]Diphenhydramine (60.7 ± 7.6 MBq) was injected and rats were decapitated after either 15 or 60 min (n = 3 in each condition). Brains were added 200 µl purified water before sonication at 4℃. Brain homogenates were mixed with 700 µl acetonitrile. The extracts (400 µl) were injected onto the radio-HPLC system described above.
Statistical analysis
In situ brain perfusion data are presented as means ± SD. One way ANOVA and post hoc test (Dunnett) or Student’s two-tailed unpaired t-test were used to identify significant differences and statistical significance was set at p < 0.05. The transport parameters (Km, Vmax, Kpassive) were estimated by plotting drug flux data against total concentration using equation (5) and nonlinear regression with WinNonlin® software. The errors associated with these parameters are asymptotic standard errors returned by the nonlinear regression routine.
Results
Characterization of diphenhydramine transport across the luminal BBB
Passive and carrier-mediated transport components
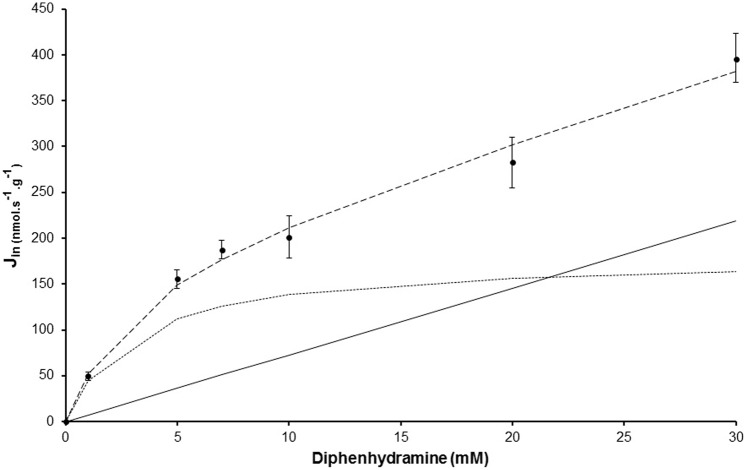
Figure 1 displays the concentration dependency of [14C]diphenhydramine BBB flux (Jin) measured at pHe 7.40. Carrier-mediated flux was calculated by subtracting unsaturated diphenhydramine BBB flux from the total flux and was plotted against total diphenhydramine (unbound) vascular concentration. The regression analysis of the carrier-mediated flux was best fitted with a Hill coefficient of 1. The carrier-mediated flux model yielded an apparent Km of 2.98 ± 1.4 mM and a Vmax of 179.5 ± 44.5 nmol s−1 g−1. The total BBB passive diffusion component at pH 7.40 yielded a Kpassive of 7.28 ± 1.58 µl s−1 g−1 for diphenhydramine, equivalent to a brain extraction Epassive of 17.2%. A comparison of the in vivo passive (∼7.3 µl s−1 g−1) and carrier-mediated (∼24.5 µl s−1 g−1) transport rates suggests that the carrier-mediated influx of diphenhydramine through the BBB is ∼3.36 times greater than its passive diffusion when the concentration range is less than the apparent carrier-mediated Km. The global (passive and carrier-mediated) brain transport rate for diphenhydramine under unsaturated conditions was 31.8 µl s−1 g−1, which corresponds to an extraction Etotal,brain of 75.2%. The carrier-mediated flux was higher than the passive diffusion flux for diphenhydramine concentrations below 21.6 mM, the concentration at which the fluxes were equal (equation (5)), as also shown graphically by the curve intercept (Figure 1).
Figure 1.
Passive and carrier-mediated flux of diphenhydramine at the rat luminal BBB. Total flux (Jin; nmol s−1 g−1; dashed line), reported as mean ± SD, measured in the rat right brain hemisphere (n = 4–5 animals per concentration) and fitted to total diphenhydramine concentrations in Krebs carbonate perfusion fluid at pH 7.40. The straight solid line represents the passive diffusion flux for diphenhydramine. The dotted line represents data fitted to the carrier-mediated Michaelis–Menten equation by nonlinear least-squares regression obtained by subtracting the passive flux from the total flux (dashed line).
Proton dependency of [14C]diphenhydramine BBB transport
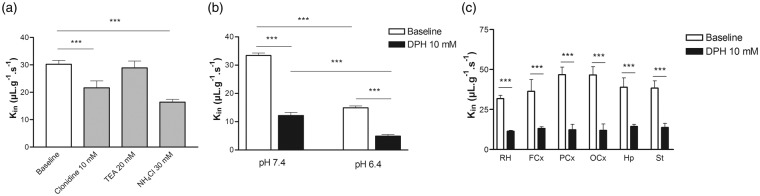
The pHi or pHe was manipulated to obtain biochemical information for the characterization of the proton effect on [14C]diphenhydramine transport across the BBB. The proportion of neutral and cationic species of diphenhydramine depends on the vascular pHe, which can be estimated using a basic pKa value of 8.98. Diphenhydramine is 0.26% neutral at pHe 6.40 and 2.6% neutral at pHe 7.40. Transport (Kin) of [14C]diphenhydramine measured without adding unlabeled diphenhydramine increased significantly (274%) between pHe 6.40 and 7.40 (Figure 2(a)). Adding unlabeled diphenhydramine (10 mM) significantly decreased the [14C]diphenhydramine Kin at pH 6.40 and 7.40 by 2.5 - and 2.3-fold, respectively, suggesting the involvement of a saturable carrier-mediated system (Figure 2). To limit the confounding effect of the proportion of neutral [14C]diphenhydramine in the perfusate and thus more clearly reveal the effect of protons on transporter activity, [14C]diphenhydramine transport (pHe 7.40) was evaluated using a protocol that increased only the intracellular pHi.11 [14C]Diphenhydramine was perfused with or without NH4Cl (30 mM) in the perfusate, adjusted to a pHe of 7.40. Modulation of the pHi induced a significant twofold reduction in the BBB transport of [14C]diphenhydramine, suggesting a role for the H+-antiporter function (Figure 2).
Figure 2.
Modulation of [14C]diphenhydramine transport across the rat luminal blood–brain barrier (BBB) measured by in situ rat brain perfusion. (a) Effect of cis-inhibition using clonidine 10 mM or tetraethylammonium (TEA) 20 mM as well as of the increase of the BBB pHi (NH4Cl; 30 mM) on [14C]diphenhydramine transport (Kin; µl s−1 g−1; n = 4–5). (b) Effect of Krebs carbonate perfusion buffer at a pHe of 6.40 or 7.40 on [14C]diphenhydramine brain transport, with (black column; n = 4) or without (white column; n = 4) coperfusion with unlabeled diphenhydramine (DPH; 10 mM). (c) [14C]Diphenhydramine brain transport in the whole right brain hemisphere (RH, n = 6) and five right brain regions including the frontal cortex (FCx), parietal cortex (PCx), occipital cortex (OCx), hippocampus (Hp), and striatum (St), with (black column; n = 4) or without (white column; n = 4) coperfusion with unlabeled diphenhydramine (DPH; 10 mM), measured by in situ rat brain perfusion for 60 s. Data represent means ± SD. ***P < 0.001.
Cis-inhibition experiments
The sensitivity and selectivity of the transport were evaluated with TEA and clonidine, two known drugs that interact with different ranges of cationic transporters. TEA (20 mM) did not significantly affect [14C]diphenhydramine transport (Figure 2(c)), suggesting the lack of both the OCTN and MATE H+-antiporters and OCT transporter functions. Clonidine (10 mM), a validated H+-antiporter substrate and inhibitor, induced a significant 1.4-fold reduction (p < 0.001) in [14C]diphenhydramine BBB transport (Figure 2). The effect of unlabeled diphenhydramine (10 mM) coperfusion on [14C]diphenhydramine transport was measured in different brain regions. The decrease in [14C]diphenhydramine transport ranged from 2.8 - to 4.0-fold and was significant in all brain regions (Figure 2(c); P < 0.001).
[11C]Diphenhydramine PET imaging
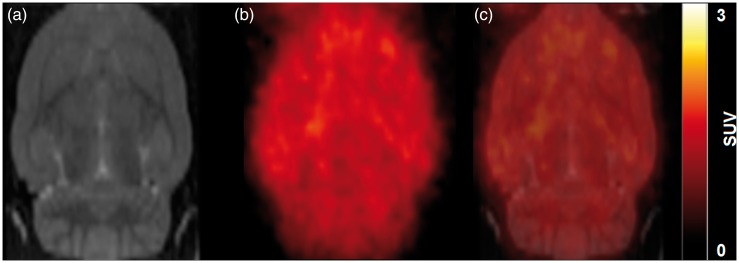
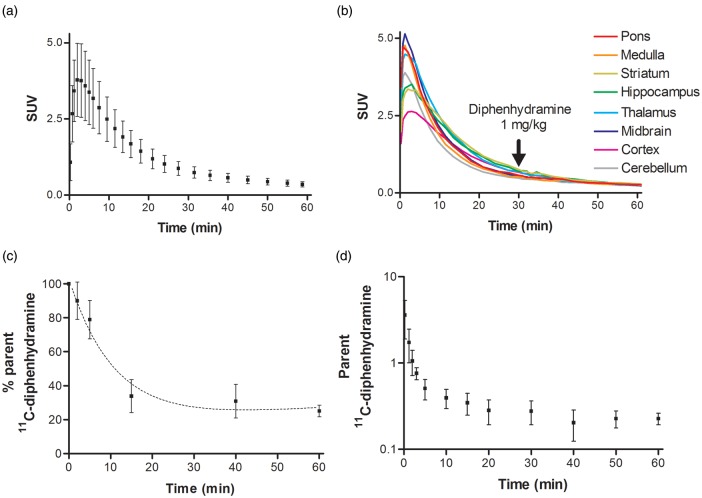
Figure 3 shows the accumulation and distribution of radioactivity in the rat brain after [11C]diphenhydramine. Radioactivity was homogeneously distributed within the brain parenchyma. Figure 4 displays the mean TACs obtained in the brain after [11C]diphenhydramine injection. Radioactivity peaked rapidly (Tmax ∼ 2 min) followed by a fast washout. Regional analysis showed the absence of retention in specific brain regions, suggesting nonspecific binding to the brain (Figure 4). The nonspecific nature of the binding was confirmed by displacement experiments using 1 mg kg−1 unlabeled diphenhydramine. Displacement had no detectable impact on [11C]diphenhydramine brain kinetics in any brain region (Figure 4).
Figure 3.
[11C]Diphenhydramine brain distribution in rat. Rats are injected with [11C]diphenhydramine i.v. followed by PET acquisition for 60 min. (a) shows the rat MRI template used for delineation of the different brain regions. (b) shows a representative SUV-normalized summed [11C]diphenhydramine PET images from 0 to 60 min). Overlaid images are shown in (c).
Figure 4.
[11C]Diphenhydramine PET kinetics in rats. (a) Mean time–activity curve (TAC, SUV value versus time; n = 4) for the rat brain after injection of [11C]diphenhydramine. (b) Representative TACs obtained from different brain regions (pons, medulla, striatum, hippocampus, thalamus, midbrain, cortex, and cerebellum) during displacement experiments: rats were injected with unlabeled diphenhydramine 1 mg kg−1 i.v., 30 min after [11C]diphenhydramine injection. (c) Percentage of parent (unmetabolized) [11C]diphenhydramine in plasma versus time. (d) Mean TAC of unmetabolized [11C]diphenhydramine (metabolite-corrected input function) in plasma.
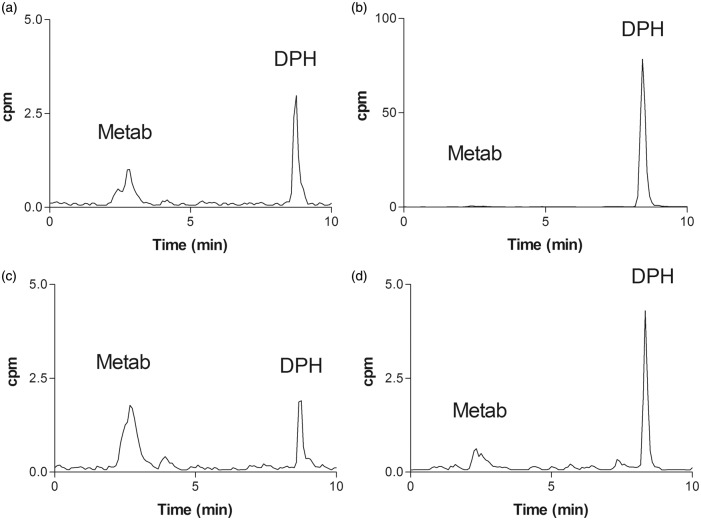
The study of the [11C]diphenhydramine arterial input function showed that [11C]diphenhydramine was metabolized, with unmetabolized [11C]diphenhydramine accounting for 25 ± 3.4% of total radioactivity in the plasma at 60 min postinjection (Figures 4 and 5). The resulting TACs of unmetabolized [11C]diphenhydramine in the plasma are shown in Figure 4. [11C]diphenhydramine brain uptake clearance (Cluptake), estimated from 0 to 2 min of acquisition, was 0.99 ± 0.18 ml min−1 cm−3. No radiometabolites could be detected in the brain at 15 min, confirming the radiochemical purity of brain radioactivity in the distribution phase. Radiometabolite(s) accounted for only 25% of brain radioactivity at 60 min, suggesting poor BBB permeation of radiometabolite(s) compared to unmetabolized [11C]diphenhydramine. Radio-HPLC analysis, based on the retention time of detected radiometabolite(s) (Rt = 2.3 min) indicated that these radiometabolite(s) did not correspond to N-desmethyl-diphenhydramine (Rt = 7.5 min) (Figure 5, Figure 3S).
Figure 5.
Representative radio-HPLC chromatograms obtained from the plasma and the brain after [11C]diphenhydramine injection in rats. Data are chromatograms obtained from the plasma and the brain at 15 and 60 min. Peaks corresponding to parent (unmetabolized) [11C]diphenhydramine (DPH) and radiometabolite(s) (Metab) are shown on each chromatogram. Cpm: counts per minute. (a) Plasma (15 min), (b) brain (15 min), (c) plasma (60 min), and (d) brain (60 min).
Discussion
In this study, we used in situ brain perfusion experiments to show that a pH-sensitive carrier-mediated flux, rather than passive diffusion, controls diphenhydramine transport across the BBB. This TEA-insensitive transport corresponds to known features of the H+-antiporter function at the BBB, in accordance with previous in vivo studies in mice.11,12 These results validate diphenhydramine as a substrate probe for the study of H+-antiporter function at the BBB.
Diphenhydramine transport has an apparent BBB Km of 3.0 mM in rats and 4.4 mM in mice, which are similar to the properties of the H+-antiporter-mediated transport of diphenhydramine (Km 4.1 mM) measured at the mouse blood–retina barrier.21 These relatively high apparent Km values with regard to the blood concentrations suggest that diphenhydramine is transported by a “low-affinity” and high-capacity transporter. Therefore, the saturation of diphenhydramine transport is not likely to occur in vivo. The highest total (bound and unbound) pharmacological/toxicological concentrations of diphenhydramine reported in the plasma in rodents and humans are lower than 40 µM.22 These concentrations are several orders of magnitude lower than the Km and match the linear/proportional BBB kinetics (first-order kinetics) of transporter flux. Thus, toxicological concentrations are still in this proportional activity range of the transporter and far from the maximum velocity (zero-order kinetics reached above the Km) of this transport system in vivo. Quantitative analysis of the passive and active transport components confirms that active influx controls 77% of total diphenhydramine uptake by the brain for concentrations lower than the Km, which include the usual micromolar pharmacological concentrations.
The diphenhydramine influx component reported here in rats has previously been demonstrated in sheep,23 dogs, and nonhuman primates,10 using the invasive brain microdialysis approach. These findings suggest the conservation of a carrier-mediated influx function at the BBB across animal species, possibly involving drug/H+-antiporter function, as recently illustrated in the human hCMEC/D3 BBB cell line.12,14
So far, H+-antiporter function at the BBB has been investigated using invasive and terminal animal experiments. However, PET imaging using radiolabeled H+-antiporter substrates is an appealing approach for the noninvasive study of this specific transport system function at the BBB of animals and humans. The characterization of the H+-antiporter substrate property of [14C]diphenhydramine at the BBB, investigated using in situ brain perfusion, and the possibility of using carbon-11 radiolabeling, prompted us to develop [11C]diphenhydramine as a PET probe. Using in vivo PET imaging, we show here that [11C]diphenhydramine readily enters the brain although its distribution does not correspond to the known distribution of H1R in the rat brain.24 In humans, 30 mg diphenhydramine administered orally displaced the brain binding of [11C]doxepin, a dedicated H1R PET probe.25 In vitro studies suggested that diphenhydramine binding may not restrict to H1R but may involve CNS off-targets such as the dopamine transporter (SLC6A3), the sodium channel SCN10A, or the M3 muscarinic receptor.26 The nonspecific (nondisplaceable) nature of the binding of [11C]diphenhydramine was therefore assessed using diphenhydramine 1 mg kg−1 rather than specific H1R ligands. Displacement did not impact regional TACs, even in H1R-rich regions such as the cortex.
Inhibition of H+-antiporter function at the BBB in vivo remains a major challenge.14 Sadiq et al. failed to inhibit the carrier-mediated transport of diphenhydramine at the rat BBB using oxycodone as an inhibitor.11,27 They concluded that the plasma concentrations that could be attained in vivo, without causing adverse effects, were far below the inhibitory concentrations found in vitro.27 In the absence of safe and efficient inhibitor for in vivo PET imaging, the regional response to transport saturation was estimated from in situ brain perfusion data (Figure 2(c)). The results showed that H+-antiporter function exists in all brain regions, indicating that the whole brain can serve as a unique tissue region to estimate [11C]diphenhydramine uptake clearance which reflects H+-antiporter function in vivo. Radio-HPLC analysis showed that the brain entry of [11C]diphenhydramine radiometabolites was poor compared to unmetabolized [11C]diphenhydramine. The brain radioactive signal was composed of unmetabolized [11C]diphenhydramine up to 15 min after injection, allowing for a reliable estimation of [11C]diphenhydramine brain uptake clearance from 0 to 2 min (Cluptake = 0.99 ± 0.18 ml min−1 cm−3). This value is on the same order of magnitude as baseline [14C]diphenhydramine uptake clearance measured using in situ brain perfusion (1.9 ± 0.1 ml min−1 g−1) in the same rat strain. It could be hypothesized that any differences observed are due to [11C]diphenhydramine binding to plasma proteins following i.v. injection, whereas for [14C]diphenhydramine, the in situ brain perfusate does not contain any proteins.
Discrepancies in the sedative properties of H1R antagonists have been attributed to differences in their ability to cross the BBB.28 Although it has been clearly shown that H1R affinity and lipophilicity (e.g. passive diffusion) do not account for these differences, the P-gp-mediated BBB efflux of anti-H1R drugs lacking sedative properties has been more convincingly established.28,29 Diphenhydramine influx does not differ between wild-type and P-gp/Bcrp knockout mice (see supplementary information), showing that diphenhydramine is not a substrate for the major efflux ABC transporters expressed at the BBB.30 Our studies suggest that the H+-antiporter function may govern diphenhydramine access to the brain and represents a critical determinant of its CNS effects.
Conclusion
Diphenhydramine is a selective and specific probe to study H+-antiporter function at the BBB in vivo. The brain distribution of this compound is mainly controlled by H+-antiporter activity and is not influenced by diphenhydramine target availability in vivo. An increasing number of drugs of neuropharmacological interest have been shown to interact with this drug/H+ antiporter at the BBB. [11C]Diphenhydramine PET imaging offers a reliable and noninvasive method to evaluate pharmacological strategies to inhibit H+-antiporter function in vivo and investigate its contribution to variations in the pharmacodynamics response to CNS drugs in animals and humans.
Supplementary Material
Acknowledgements
We thank Dr Alfred H. Schinkel (The Netherlands Cancer Institute Amsterdam, The Netherlands) for supplying the knockout mice. We thank Dr S. Rasika for editing the English text. We thank the animal platform of UMS US025 INSERM, 3612 CNRS, CRP2, Faculté de Pharmacie, Université Paris Descartes (Paris, France).
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Sylvain Auvity received a public grant overseen by the French National research Agency (ANR) as part of the “Investissement d’Avenir” program, through the “Lidex-PIM” project funded by the IDEX Paris-Saclay, ANR-11-IDEX-0003-02. We thank the Paris Descartes University foundation and Servier Technologies for the financial support granted to Hélène Chapy. Salvatore Cisternino received a financial support from the Commissariat à l’énergie atomique et aux énergies alternatives (CEA) and l’Assistance publique – Hôpitaux de Paris (AP-HP): “Postes d’accueil CEA/AP-HP.”
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author’s contribution
NT and SC contributed equally to this work. NT and SC designed the research. SA, HC, SG, BH and MS performed the majority of the experiments. FC developed and provided crucial reagents (radiochemistry). SA, NT, HC, SC analyzed the data. SA, HC, SC and NT interpreted the results. SA wrote the paper and SC, SG and NT helped write and revise the manuscript. SA, HC, SG, FC, BH, MS, XD, NT and SC approved the manuscript.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Abbott NJ, Patabendige AAK, Dolman DEM, et al. Structure and function of the blood-brain barrier. Neurobiol Dis 2010; 37: 13–25. [DOI] [PubMed] [Google Scholar]
- 2.König J, Müller F, Fromm MF. Transporters and drug-drug interactions: important determinants of drug disposition and effects. Pharmacol Rev 2013; 65: 944–966. [DOI] [PubMed] [Google Scholar]
- 3.Shawahna R, Uchida Y, Declèves X, et al. Transcriptomic and quantitative proteomic analysis of transporters and drug metabolizing enzymes in freshly isolated human brain microvessels. Mol Pharm 2011; 8: 1332–1341. [DOI] [PubMed] [Google Scholar]
- 4.Kusuhara H. Imaging in the study of membrane transporters. Clin Pharmacol Ther 2013; 94: 33–36. [DOI] [PubMed] [Google Scholar]
- 5.Wulkersdorfer B, Wanek T, Bauer M, et al. Using positron emission tomography to study transporter-mediated drug-drug interactions in tissues. Clin Pharmacol Ther 2014; 96: 206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pottier G, Marie S, Goutal S, et al. Imaging the impact of the P-glycoprotein (ABCB1) function on the brain kinetics of metoclopramide. J Nucl Med 2016; 57: 309–314. [DOI] [PubMed] [Google Scholar]
- 7.Wanek T, Mairinger S, Langer O. Radioligands targeting P-glycoprotein and other drug efflux proteins at the blood-brain barrier. J Label Compd Radiopharm 2013; 56: 68–77. [DOI] [PubMed] [Google Scholar]
- 8.Richardson GS, Roehrs TA, Rosenthal L, et al. Tolerance to daytime sedative effects of H1 antihistamines. J Clin Psychopharmacol 2002; 22: 511–515. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg MJ, Spector R, Chiang CK. Transport of diphenhydramine in the central nervous system. J Pharmacol Exp Ther 1987; 240: 717–722. [PubMed] [Google Scholar]
- 10.Shaffer CL, Osgood SM, Mancuso JY, et al. Diphenhydramine has similar interspecies net active influx at the blood-brain barrier. J Pharm Sci 2014; 103: 1557–1562. [DOI] [PubMed] [Google Scholar]
- 11.André P, Debray M, Scherrmann J-M, et al. Clonidine transport at the mouse blood-brain barrier by a new H+ antiporter that interacts with addictive drugs. J Cereb Blood Flow Metab 2009; 29: 1293–1304. [DOI] [PubMed] [Google Scholar]
- 12.Chapy H, Smirnova M, André P, et al. Carrier-mediated cocaine transport at the blood-brain barrier as a putative mechanism in addiction liability. Int J Neuropsychopharmacol 2014; 18(1).: doi:10.1093/ijnp/pyu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cisternino S, Chapy H, André P, et al. Coexistence of passive and proton antiporter-mediated processes in nicotine transport at the mouse blood-brain barrier. AAPS J 2013; 15: 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapy H, Goracci L, Vayer P, et al. Pharmacophore-based discovery of inhibitors of a novel drug/proton antiporter in human brain endothelial hCMEC/D3 cell line. Br J Pharmacol 2015; 172: 4888–4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer W, Metzner L, Hoffmann K, et al. Substrate specificity and mechanism of the intestinal clonidine uptake by Caco-2 cells. Pharm Res 2006; 23: 131–137. [DOI] [PubMed] [Google Scholar]
- 16.Kuwayama K, Inoue H, Kanamori T, et al. Uptake of 3,4-methylenedioxymethamphetamine and its related compounds by a proton-coupled transport system in Caco-2 cells. Biochim Biophys Acta 2008; 1778: 42–50. [DOI] [PubMed] [Google Scholar]
- 17.Okura T, Hattori A, Takano Y, et al. Involvement of the pyrilamine transporter, a putative organic cation transporter, in blood-brain barrier transport of oxycodone. Drug Metab Dispos Biol Fate Chem 2008; 36: 2005–2013. [DOI] [PubMed] [Google Scholar]
- 18.Takasato Y, Rapoport SI, Smith QR. An in situ brain perfusion technique to study cerebrovascular transport in the rat. Am J Physiol 1984; 247: H484–H493. [DOI] [PubMed] [Google Scholar]
- 19.Cisternino S, Rousselle C, Debray M, et al. In vivo saturation of the transport of vinblastine and colchicine by P-glycoprotein at the rat blood-brain barrier. Pharm Res 2003; 20: 1607–1611. [DOI] [PubMed] [Google Scholar]
- 20.Traxl A, Wanek T, Mairinger S, et al. Breast cancer resistance protein and P-glycoprotein influence in vivo disposition of 11C-Erlotinib. J Nucl Med 2015; 56: 1930–1936. [DOI] [PubMed] [Google Scholar]
- 21.Chapy H, André P, Declèves X, et al. A polyspecific drug/proton antiporter mediates diphenhydramine and clonidine transport at the mouse blood-retinal barrier. Br J Pharmacol 2015; 172: 4714–4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pragst F, Herre S, Bakdash A. Poisonings with diphenhydramine—a survey of 68 clinical and 55 death cases. Forensic Sci Int 2006; 161: 189–197. [DOI] [PubMed] [Google Scholar]
- 23.Au-Yeung SCS, Rurak DW, Gruber N, et al. A pharmacokinetic study of diphenhydramine transport across the blood-brain barrier in adult sheep: potential involvement of a carrier-mediated mechanism. Drug Metab Dispos 2006; 34: 955–960. [DOI] [PubMed] [Google Scholar]
- 24.Ishiwata K, Kawamura K, Wang W-F, et al. Evaluation of in vivo selective binding of [11C]doxepin to histamine H1 receptors in five animal species. Nucl Med Biol 2004; 31: 493–502. [DOI] [PubMed] [Google Scholar]
- 25.Tashiro M, Duan X, Kato M, et al. Brain histamine H1 receptor occupancy of orally administered antihistamines, bepotastine and diphenhydramine, measured by PET with 11C-doxepin. Br J Clin Pharmacol 2008; 65: 811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lounkine E, Keiser MJ, Whitebread S, et al. Large-scale prediction and testing of drug activity on side-effect targets. Nature 2012; 486: 361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadiq MW, Borgs A, Okura T, et al. Diphenhydramine active uptake at the blood-brain barrier and its interaction with oxycodone in vitro and in vivo. J Pharm Sci 2011; 100: 3912–3923. [DOI] [PubMed] [Google Scholar]
- 28.Broccatelli F, Carosati E, Cruciani G, et al. Transporter-mediated efflux influences CNS side effects: ABCB1, from antitarget to target. Mol Inform 2010; 29: 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chishty M, Reichel A, Siva J, et al. Affinity for the P-glycoprotein efflux pump at the blood-brain barrier may explain the lack of CNS side-effects of modern antihistamines. J Drug Target 2001; 9: 223–228. [DOI] [PubMed] [Google Scholar]
- 30.Chen C, Hanson E, Watson JW, et al. P-glycoprotein limits the brain penetration of nonsedating but not sedating H1-antagonists. Drug Metab Dispos 2003; 31: 312–318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.