Abstract
Heterogeneity is a fundamental property of biological systems at all scales that must be addressed in a wide range of biomedical applications including basic biomedical research, drug discovery, diagnostics and the implementation of precision medicine. There are a number of published approaches to characterizing heterogeneity in cells in vitro and in tissue sections. However, there are no generally accepted approaches for the detection and quantitation of heterogeneity that can be applied in a relatively high throughput workflow. This review and perspective emphasizes the experimental methods that capture multiplexed cell level data, as well as the need for standard metrics of the spatial, temporal and population components of heterogeneity. A recommendation is made for the adoption of a set of three heterogeneity indices that can be implemented in any high throughput workflow to optimize the decision-making process. In addition, a pairwise mutual information method is suggested as an approach to characterizing the spatial features of heterogeneity, especially in tissue-based imaging. Furthermore, metrics for temporal heterogeneity are in the early stages of development. Example studies indicate that the analysis of functional phenotypic heterogeneity can be exploited to guide decisions in the interpretation of biomedical experiments, drug discovery, diagnostics and the design of optimal therapeutic strategies for individual patients.
Keywords: Heterogeneity, High Content Screening, flow cytometry, drug discovery, cellular models, organs-on-chips, computational pathology, systems biology, quantitative systems pharmacology, precision medicine
Introduction: Biological heterogeneity is a fundamental property of life
Heterogeneity is a fundamental property of biological systems that contributes to development,1 differentiation,2, 3 immune-mediated responses,1 and many other cellular, tissue, organ, and organism functions1 as well as diseases and disease progression.4–6 Figure 1 illustrates the different scales or levels of biological systems exhibiting heterogeneity, that can be measured with the appropriate methods. This perspective will focus primarily on heterogeneity in populations of cells in vitro and in tissue sections, but much of the discussion, especially with reference to the need for standard metrics and their application to biomedical research, drug discovery, and diagnostics, can also be applied to populations at all scales.
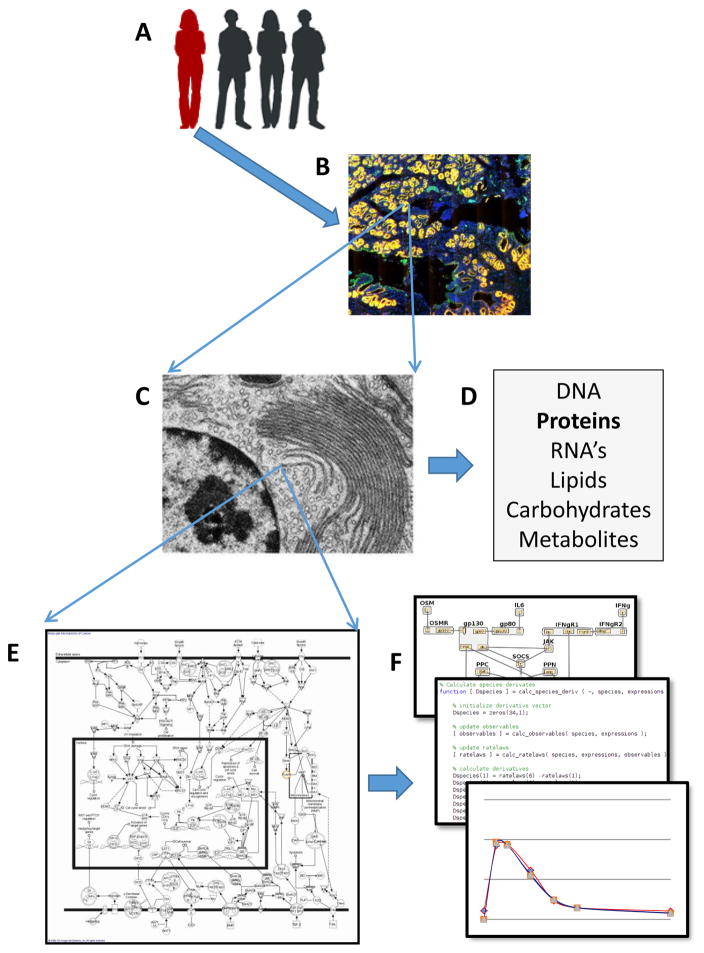
Figure 1. The multiple scales of biological heterogeneity detected in a population of organisms, as well as within organs, tissues, cells, molecules, pathways and networks.
A. Individuals in a population exhibit heterogeneity in a variety of genomic and phenotypic measures. Heterogeneity can be detected B. between and within organs and tissues, C. between cells in terms of expression levels, genomics and functions, and within cells in terms of D. cellular constituents. E. Combinations of molecules interact in time and space within and between cells as part of biological pathways that result in normal and abnormal cellular functions. F. Computational or mathematical models of “systems” including cellular pathways, organ, multi-organ and organism can be generated and used to predict responses that must incorporate heterogeneity of components in the models.
Heterogeneity results from genetic variation,7 non-genetic characteristics,1 or a combination of these (Figure 2). Non-genetic heterogeneity can be driven by extrinsic factors (e.g., tissue microenvironment) and intrinsic factors (e.g., variation in protein expression).1 Although heterogeneity is sometimes referred to as ‘noise’ or as arising from ‘noise’ in cellular networks, the presence of noise hinders information transfer, while the presence of heterogeneity provides information.
Figure 2.
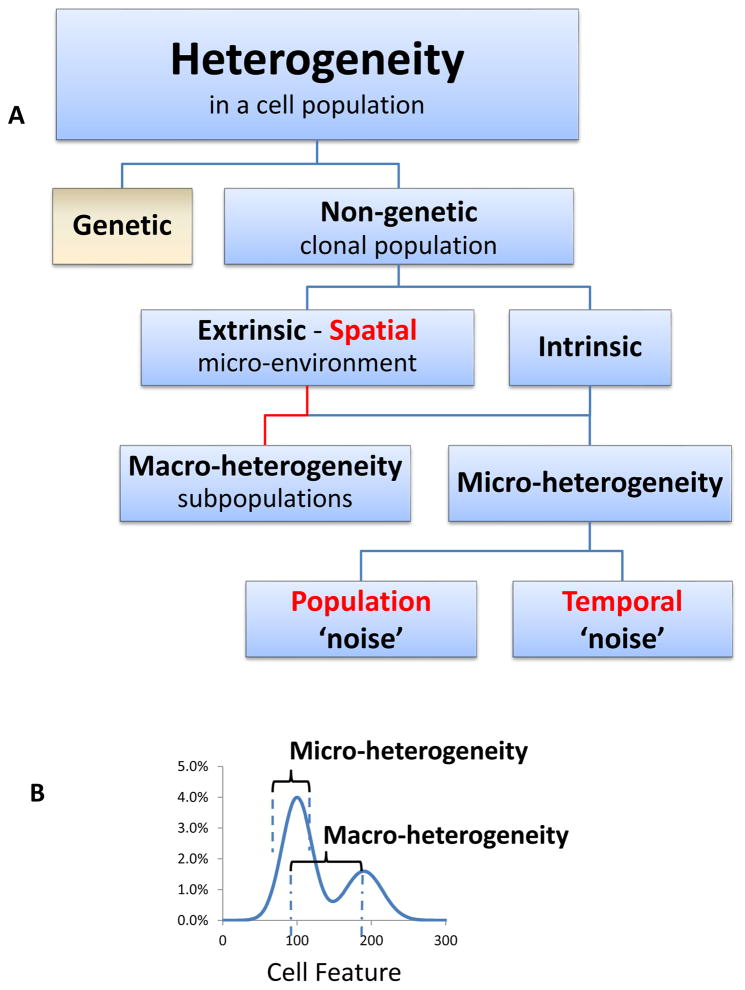
Classification of the types of heterogeneity that can be exhibited by a population of cells (adapted from Huang 1). A. Heterogeneity can be the result of genetic variations, and/or non-genetic factors even in a clonal population. Non-genetic heterogeneity, also called phenotypic heterogeneity, can be driven by extrinsic factors, such as the microenvironment in a tissue that can influence for example, the protein expression levels in surrounding cells. Extrinsic factors drive Spatial heterogeneity often exhibited as macro-heterogeneity. Intrinsic heterogeneity can be detected even in a uniform environment and has been classified as macro-or micro-heterogeneity depending on the characteristics of the distribution. B. Macro-heterogeneity refers to variations in one or more cellular traits that results in discrete phenotypes or sub-populations of cells, and can be driven by both extrinsic and intrinsic factors. Micro-heterogeneity refers to random variations within a single phenotype that can include population ‘noise’ resulting from variations in regulatory networks, for example, or temporal ‘noise’ such as variation in protein synthesis over time. Highlighted in red are three important measurable components of the distribution of a cell feature.
Analysis of heterogeneity is expected to inform a wide range of biological applications, from biomedical research to medical diagnostics. Whether developing an assay for drug discovery, a therapy for cancer, or optimizing a protocol for stem cell differentiation, the prevalence of heterogeneity in biological systems suggests that more can be learned through analysis of the population distribution than merely evaluating the population average. In contrast, most cell experimentation currently assumes a normal distribution of data and uses the population average for the sake of speed and simplicity. However, it is becoming clear that heterogeneity is the rule rather than the exception, such that homogeneity in population data cannot be assumed when analyzing and interpreting data.
Measurement of heterogeneity most often involves methods with single-cell resolution (Figure 3), although population-based methods have also been used to detect heterogeneity. For example, experiments by Luria and Delbruck 8 on populations of bacteria demonstrated in the 1940s that bacteria spontaneously mutated, forming a heterogeneous population in which predisposed subpopulations, harboring virus-resistance mutations, were selected as a result of viral infection. More commonly, though, heterogeneity is detected through examination of the phenotypes of the individuals in the population and is characterized by quantitation of the distributions of those phenotypes. In studies where cellular heterogeneity has been characterized, the methods and metrics have varied (Table 1).2, 4, 9–17 The lack of an accepted standard for measuring and reporting cellular heterogeneity makes it difficult to compare the degree of heterogeneity in different studies and biological systems. Therefore, at the present time, only the methods and metrics can be compared. However, we will make suggestions on the application of metrics.
Figure 3.
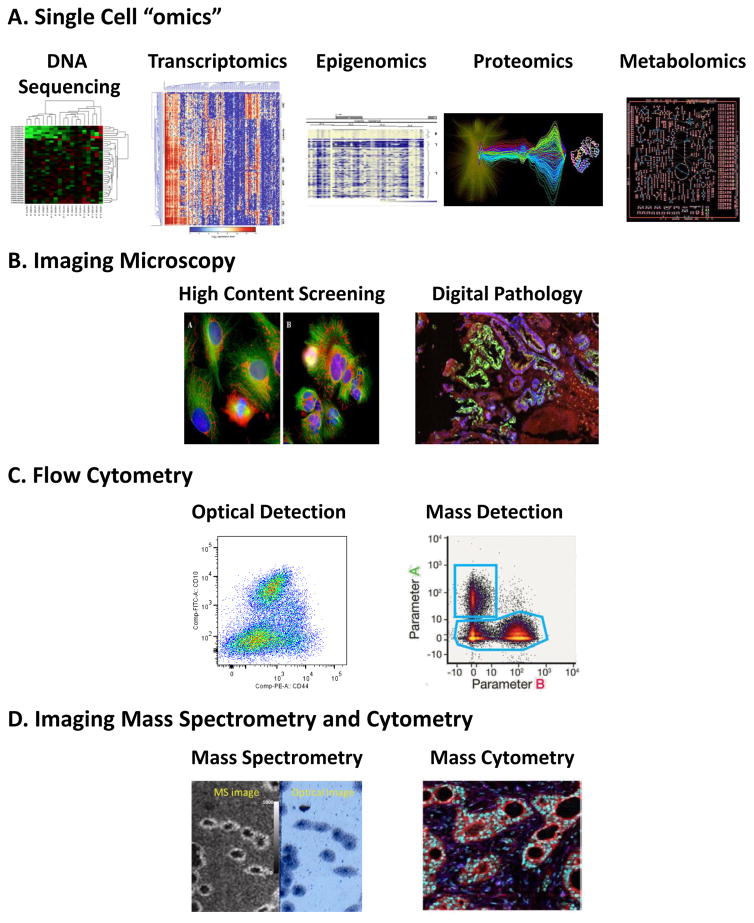
Heterogeneity in populations of cells can be quantified by a variety of methods that permit cell-by-cell measurements. A. Single cell genomics, epigenomics, proteomics and metabolomics (reprinted with permission from Spagnolo et al.21), and/or transcriptomics (from Saadatpour et al.235) to study heterogeneity use either ground up tissue samples or single cells and can provide a comprehensive analysis of heterogeneity for a large number of cells. B. High Content Screening and Digital Pathology21 employs multiple fluorescent probes to capture a broad range of information including expression levels and subcellular localization of molecules within and across individual cells. C. Optical (from Hines et al.114) and mass cytometry (from Spitzer and Nolan105) can provide information on expression levels of several molecules simultaneously as well as some morphological information in large populations but do not report spatial heterogeneity. D. Mass Spectrometry readouts expand the range of molecules that can be simultaneously detected in flow cytometry (mass cytometry) and can be used to image tissues and cells in imaging Mass Cytometry (from Giesen et al.93) and Imaging Mass Spectrometry (from Zavalin et al.100).
Table 1.
Example Approaches to Quantifying Heterogeneity
| Approach | Examples | Characteristics |
|---|---|---|
| Univariate, Gaussian statistics | mean,230 standard deviation,230 z-score,24 skew,231 kurtosis,231 moment230 | Assumes normal distribution, insensitive to subpopulations, no information on type of heterogeneity |
| Entropy | Quadratic,4, 76, 134 Shannon,232 Simpson,232 Renyi233 | Established measures of diversity and information content, only established for univariate data |
| Non-parametric statistics | KS statistic14, 145 | can improve accuracy of results, no assumptions on distribution, no information on distribution shape |
| Model functions | Gaussian mixture models61, 88 | Assumes there is some number of normally distributed subpopulations, can be applied to multivariate data, normal model may not be appropriate |
| Combined Metrics | PHI4, 37 | Model independent, descriptive of heterogeneity |
| Spatial methods | fractal dimension,233 Pointwise Mutual Information (PMI)21 | No assumption of distribution, leverages spatial interactions, applies to multivariate data |
| Temporal methods | Temporal distance between robust centers of mass of 2 feature sets,13,234 | Applies to multivariate data, Method developed based on genomic data |
Biologically relevant heterogeneity can be divided into three categories: population heterogeneity, spatial heterogeneity, and temporal heterogeneity (Table 2). In each category, the heterogeneity can be characterized as micro- or macro-heterogeneity, depending on the nature of the distribution (Figure 2). Micro-heterogeneity refers to heterogeneity within an apparently uniform population (i.e., the variance of a single bell-shaped distribution) whereas macro-heterogeneity refers to the presence of distinct populations (i.e., multi-modal)1 Establishing standardized terminology, methods, and metrics will be essential to the routine extraction and communication of insights from biological heterogeneity.
Table 2.
Selected Definitions
| Term | Definition |
|---|---|
| Biologically Relevant Heterogeneity | General term for heterogeneity detected or measured at some scale (level) of a biological system (molecule, cell, tissue, organ, organism) after correcting for any instrumental “systems response” variations, as well as sample preparation. |
| Population Heterogeneity | Variation in some phenotype(s) among individuals in a population at a single time point. Requires measurements of many individuals in a population. |
| Spatial Heterogeneity | Variation in some variable(s) at different spatial locations within a sample. Requires a set of measurements at different spatial locations |
| Temporal Heterogeneity | Variation in some variable(s) measured as a function of time. Requires a set of measurements at different time points. |
| Quantitative Systems Pharmacology (QSP) | Determining the mechanism(s) of disease progression and mechanism(s) of action of drugs on multi-scale systems through iterative and integrated computational and experimental methods to optimize the development of therapeutic strategies. |
| Precision Medicine | Development and use of individual or combinations of features that tell clinicians about risk of disease, selection of the best treatment, and likely disease course, including response to treatment for a specific patient. |
| Pseudotime | Quantitative measures of biological progression127 |
| Heteroscedasticity | Unequal variance in the distribution (with respect to an independent variable) |
Detection of biologically relevant heterogeneity
Biologically relevant heterogeneity can be detected and quantified with a variety of methods, provided they have sufficient fidelity over the population. One of the earliest indications of biological heterogeneity was in tumors, where morphological variations were noted by pathologists who examined fixed sections of animal and human cancers.18 However, manual cell-by-cell scoring limits the size of the regions and number of cells that can be analyzed as well as the objectivity of the analysis. Digital pathology now enables a more comprehensive and objective assessment of cellular phenotypes in tissues, allowing analysis of population and spatial heterogeneity of biomarkers and microenvironment components such as immune cells.19–23 The detection of heterogeneity is currently most advanced in isolated cell systems, where automated microscope imaging, e.g., High Content Screening (HCS), is used to extract multiple phenotypic features from many, relatively large populations of adherent cells,24–26 flow cytometry is used for bacterial27, 28 and suspension cell analysis,29–32 and other single cell methods, such as recent developments in single cell genomics and proteomics33–36 that are being used with progress toward in situ analysis (Figure 3).
Distinguishing between biologically relevant heterogeneity and ‘system variability’ resulting from sample preparation, data acquisition and/or data processing requires a well-founded understanding of the sources of noise in the measurements, achieved by calibration and characterization of the systems response using appropriate standards or reference measures.4, 37–39 The importance of minimizing the ‘system variability’ is critical to achieving consistent, quantitative measurements, as has been discussed in detail for high content imaging and flow cytometry.40–42 Flow cytometry has a long history and mature process for system calibration, characterization and standardization, including published protocols43 and an array of reference standards.44–46 As a result, data can be generated and compared between different systems and in different labs. However, manual gating and segmentation of populations of cells can still be a source of variation in the results.47 Recent progress on automated segmentation of cell populations shows some promise in addressing this source of variability.48–51 Establishing standard methods and metrics for the characterization of system reproducibility is a key to more reliable detection and quantitation of biological heterogeneity.
The need for methods/metrics to detect, quantify and characterize heterogeneity in biological systems
Historically, ’population average’ metrics have dominated the measurement and interpretation of cellular data. Most cellular assays rely on whole-well measurements, such as total enzyme activity or total fluorescence intensity per well, making contributions from subpopulations or extreme outliers impossible to parse from the average response. This well average approach has also extended to High Content cellular assays where the standard methods generally assume a normal data distribution to “save time” and to “simplify analysis” by producing a single value that is easily understood, even if not fully representative of the biology. In fact, when computational models based on these assumptions are employed and fail to explain observations, or give variable results, investigators will often discover biologically relevant heterogeneity in the system they are studying.8
Population average measures are routinely used as assay readouts and to assess assay performance in chemical or biological library screens or structure activity relationship (SAR) campaigns. Standard assay performance metrics such as the Z’-Factor52 or the Strictly Standardized Mean Difference (SSMD)53 only measure the degree of separation of the positive and negative control wells, based on the average and standard deviations of the assay readouts. The assumptions are that there is a normal distribution of the assay readouts across wells and that the assay readout adequately represents the biology in the well. However, population average metrics don’t adequately reflect the distribution of the biology within the wells which can lead to misinterpretation of assay consistency. This was recently illustrated by Gough et al.37 in a retrospective analysis of a high content assay where there was heterogeneity in the IL-6 activation of STAT3. They showed that even though the Z’-prime indicated a robust assay at the well level (Z’≥0.5) across all the plates, the fundamental biology on several plates was found to be quite different. Thus, in order to reliably assess the biology, it is necessary to establish quality control (QC) metrics for the distribution of the cell population within each well.
Meaningful quantitation of heterogeneity requires selecting an appropriate set of metrics, while interpretation of heterogeneity requires a strategy for dissecting the inherent complexity of cellular distributions (Figure 2). In one approach, the distinction between homogeneous and heterogeneous data is defined by a measure of diversity in the sample. In a sample that exhibits heterogeneity, micro-heterogeneity is indicated by a normal distribution, and macro-heterogeneity, 1 by the degree of non-normality, using a metric such as the Kolmogorov-Smirnov (KS) statistic.4, 54 Macro-heterogeneity requires the use of analytics that can characterize, visually or using model functions, as consisting of a number of discrete subpopulations (sometimes referred to as modality), a continuous and potentially complex distribution, or some combination.14
In addition to population heterogeneity, it is also important to consider spatial heterogeneity. The detection and interpretation of spatial heterogeneity, using methods such as pointwise mutual information (PMI) or computational modeling, can be used to identify patterns of phenotypic heterogeneity that may be correlated with the microenvironment or potentially the result of intrinsic factors.21 The analysis of temporal heterogeneity is also important and presents some unique challenges, including deconvolution of cell cycle effects (which may also be a source of heterogeneity) and avoiding artifacts in monitoring cells over time.55 However, there are examples of live cell studies which have addressed these challenges, collecting large cell level data sets to analyze and model the temporal changes and heterogeneity in live cell phenotypes.55–57 A systematic approach to the detection, quantitation and characterization of heterogeneity will make it a source of insight, rather than simply an added burden to investigators.
Even though researchers are more frequently detecting and investigating heterogeneity, additional attention must be given to the practical need for robust generally applicable tools that can be implemented in high throughput production environments, rather than continuing to introduce custom solutions that are intrinsically too narrow in scope to support integration of datasets. Ultimately we need commonly understood metrics for heterogeneity, just as we use statistical concepts like mean and standard deviation for normal distributions.
Potential insights from the analysis of heterogeneity in biology and drug discovery
Whether heterogeneity is inherent to a population of cells,58 induced by the microenvironment,59, 60 or induced by compound or reagent treatment,4, 61–63 analysis of phenotypically similar cell subpopulations, derived from the analysis of heterogeneity, is expected to: improve the accuracy of cellular measurements; better support the interpretation of the data; provide insights into the regulation of cellular networks; guide the computational modeling of the networks; guide the prioritization of compounds for development in drug discovery; optimize the development of diagnostics for precision medicine and further basic biological knowledge.
Cell-to-cell variability is believed to be the result of deterministic molecular regulatory mechanisms that remain largely uncharacterized.1, 64, 65 Subpopulations of cells with distinct phenotypes isolated from a macro-heterogeneous population have been demonstrated to revert to the original macro-heterogeneous phenotype distribution over time,66, 67 indicating that heterogeneity is a persistent characteristic of a population, reflecting transitions among distinct metastable cell states induced by cell autonomous and non-cell autonomous signaling in contrast to simply noise.66 A recent study suggests that heterogeneity can be decomposed into groups of biomarkers that are consistent with known signaling pathways, also implying a mechanistic basis for the cell-to-cell variation.9 In other studies it has been shown that patterns of signaling heterogeneity can distinguish cellular subpopulations with different drug sensitivities.4, 68 The differential sensitivity to drug treatment of subpopulations of cells may well provide an indication of compound mechanism(s) of action.1, 5, 9, 64, 65, 68, 69 Differential sensitivity measurements in vitro also provide insights into how effective a therapy might be in vivo. For example, if the half-maximal response represents all cells showing 50% inhibition, then treatment cycles in vivo may produce a different response rate than if the half maximal response is a result of 100% inhibition in half of the cells. In the latter case, a significant survivor population among the unaffected cells may result in a treatment with poor efficacy in the clinic, despite apparently good efficacy in cell assays. In addition, cells treated with drugs of similar mechanism of action exhibited similar heterogeneity.61 Taken together, these findings suggest that there is an integral link between phenotypes, networks, drug sensitivity and patterns of heterogeneity. The analysis of heterogeneity therefore provides a basis for the generation of hypotheses regarding regulatory networks, such as that suggested by Gascoigne and Taylor 62 that the heterogeneity induced by drugs was the result of interacting networks.
Implications of heterogeneity for precision medicine
Because there is heterogeneity amongst individual patients, the challenges associated with improving the success rate in developing therapies may seem daunting. However, the solution may be in the development of precision therapies that address the heterogeneity exhibited in sub-populations of patients, as discussed by Stern, et al. 6 in a perspective on quantitative systems pharmacology. There is growing evidence that some heterogeneity enables physiological and evolutionary adaptation.70, 71 The association between cellular heterogeneity and adaptation suggests that ignoring heterogeneity in the in vitro cellular response to candidate therapeutics may lead to the selection of compounds to which cells will readily adapt, leading to a loss of efficacy.72, 73 On the other hand, an understanding of inter-clonal interactions that can lead to disease-specific phenotypic traits could provide novel therapeutic opportunities.72, 74
When heterogeneity is associated with dysregulated genetic-based and/or non-genetic-based functions, it can play a critical role in the progression of complex diseases such as cancer,75 where intra-tumor heterogeneity poses a formidable challenge to the development of therapeutics,5, 65 as well as diagnostics.5, 21, 22, 76 Thus identifying, quantifying and characterizing heterogeneity in patient samples and disease relevant models using validated cell-by-cell analysis methods,5, 21, 73, 75–78 addresses an important unmet need.
Methods for single cell evaluation in cell populations
There are many systems and methods for the evaluation of single cells in the context of a population, including: high content imaging methods such as high content screening (HCS) and Digital Pathology; Imaging Mass Spectrometry (IMS); Imaging Mass Cytometry (IMC); Flow Cytometry; Mass Cytometry (MC); and single cell “omics” (Figure 3). In general, each of these approaches delivers information with enough signal-to-noise at the single cell level, and sufficient throughput at the population level, to characterize the heterogeneity in cellular phenotypes. The metrics discussed below can be applied to all of these methods.
Optical high content imaging/digital pathology
High content imaging, such as HCS or digital pathology, when applied to multiple labeled targets, can provide data from large numbers of cells in large numbers of samples. HCS is commonly used to measure fixed or live cells in up to 5 dimensions (3D plus time and wavelength) using expressed fluorescent protein biosensors, a wide range of fluorescent probes and transmitted light methods.24, 79 Digital pathology typically uses stains for transmitted light imaging and fluorescent antibodies and nucleic acid probes to label specific biomarkers in formalin fixed paraffin embedded (FFPE) tissue sections. Both applications benefit from capturing a broad range of information about the population, including spatial distributions of tissue structures and molecules within each cell, within cellular compartments and spatial relationships between cells. Live cell imaging also provides temporal and direct functional readouts such as cell motility and division.80–82 Light microscopic approaches range from low magnification, large area images that contain hundreds to thousands of cells that are analyzed individually, to one-by-one serial evaluation of tens to hundreds of cells with high magnification, including super-resolution.83–85 In addition to HCS applications,37, 86 a wide range of automated microscopy analyses are routinely used in research4, 9, 68, 87, 88 and digital pathology.20, 21, 23, 69, 76, 89
Several light microscope imaging platforms have been developed to acquire multivariate information from images of large area tissue sections and tissue microarrays (TMAs) using DNA, RNA and protein biomarkers.22, 90, 91 Although typically limited to 1–6 labels per cell due to spectral overlap, recent technological advances have now enabled imaging of highly multiplexed (‘hyperplexed’) biomarkers (> 60) in many individual cells in situ in fixed tissues, with subcellular resolution that captures the spatial arrangement of many discrete cellular phenotypes (i.e. spatial heterogeneity).73, 77, 92–94 It is now possible to “map” the location of specific cell types, cell activation states and cell biomarker expression levels, as well as extracellular constituents, in tissue sections and TMAs. The determination of spatial heterogeneity at subcellular resolution is still nascent, but it promises to help elucidate the cellular networks, and their cell-autonomous and heterotypic signaling interactions, involved in the regulation of both normal and disease processes. The importance of understanding the dynamic regulation of cellular heterogeneity is discussed below (Sections 6 & 7.4).
Imaging Mass Spectrometry (IMS) and Imaging Mass Cytometry (IMC)
The application of Mass Spectrometry (MS) to image analysis has enabled a higher degree of multiplexing of a wider range of analytes that can be simultaneously imaged in cell and tissue samples at the single cell level. There are basically three approaches to imaging that utilize MS, a label free method, IMS, and two epitope tagging methods: IMC and Multiplex Ion Beam Imaging (MIBI).
IMS is a label-free method that allows the visualization of ionizable species within a given mass range while retaining spatial information.95 The technique can measure a range of molecular species from small molecule drugs to full length proteins in samples ranging from whole animals to single cells.95–97 There are three basic ionization approaches for IMS: Matrix Assisted Laser Desorption Ionization (MALDI), Secondary Ion Mass Spectroscopy (SIMS), and Desorption Electrospray Ionization (DESI).98 Each approach has advantages in terms of types of analytes that can be measured and the spatial resolution. Lipids, peptides and small molecules can be detected by all three, with MALDI also capable of measuring full length proteins with molecular mass of ~50 kDa. The spatial resolution of IMS typically ranges from 100 μm for DESI, 30 – 50μm for MALDI and 0.5 to 1 μm for SIMS,95 although advances in MALDI technology has enabled subcellular resolution.97, 99, 100 Furthermore, IMS can report molecular distributions in 3D volumes thereby extending the spatial environment.101
Both IMC93 and MIBI102 utilize antibodies that are tagged with non-biological, unique rare earth metal reporters that are easily identified in MS. Samples are ionized either with a laser or ion beam, the metal tags are quantified, and then the images are computationally reconstructed based on known raster positions of the laser or ion beams.93, 102, 103 These approaches are still developing, but have already enabled quantification of >40 parameters at the single cell level,103, 104,105 and have been used to detect heterogeneity in breast cancer tissues93, 106
The power of IMS lies in its ability to quantitatively measure hundreds of analytes simultaneously enabling the discernment of novel molecular species involved in specific biological contexts. IMS can be used in a targeted mode, looking at known molecular entities, or in a discovery mode which requires no prior knowledge of the biology. This aspect has been successful in identifying intratumor heterogeneity at the molecular level in otherwise histomorphologically homogenous tumor regions in primary gastric cancers.11 In a more targeted approach, Mao et al.107 used air flow assisted ionization mass spectrometry to image the distribution of lipids in breast cancer tissues and demonstrated that various histological grades of invasive ductal carcinoma and ductal carcinoma in situ can be distinguished by the lipid profile. Other studies have reported the application of IMS to studying intra tumor heterogeneity and differentiation of tumor/tissue types108, 109 as well as heterogeneous distribution of drugs in tissues.110 The ability of IMS to quantify metabolites enables a functional assessment of the biology not seen by other methods and enables a deeper understanding of the disease state as well as mechanisms of action of drugs.96
Flow Cytometry
Flow Cytometry is a standard method that rapidly evaluates many cells (up to ~10,000 cells/s) in a population one at a time. The application of flow cytometry to the analysis of heterogeneity in cellular systems is certainly not new.27 Like the microscopy methods described above, cells can be labeled using expressed fluorescent proteins as well as with a wide range of fluorescent probes and antibodies. Highly multiplexed flow cytometry allows up to 17 fluorescent markers111 per cell using photodetection or more than 36 mass markers per cell using mass cytometry detection.112
In flow cytometry, individual biomarkers are most often used for binary classification of cells, using either manual or automated gating to distinguish positive from negative cells, but the data collected from the samples includes the distribution of the intensity of the labels, and therefore can be used to identify and characterize the heterogeneity of the cells.113 Because cells must be suspended to be measured, flow cytometry is most often used for non-adherent cells, but can be used for any cells that can be isolated and suspended in media.30 By suspending cells in media, the spatial context of the cell is lost, as well as some of the subcellular spatial context, however, the cells can be sorted based on the signal intensity from one or more markers, allowing the selection of live subpopulations of cells for further experiment. Sample preparation, especially when isolating cells from tissue, can lead to significant differences between samples and laboratories and therefore needs to be carefully controlled.114
Single Cell “Omics”
Multidisciplinary technological advances in experimental design and computational analysis have now made it possible to measure global gene expression in thousands of individual cells in a single experiment, to infer biochemical and genetic regulatory mechanisms.115 Single cell RNA-seq (scRNAseq)116, 117 and its complementary single cell-based platforms for epigenome (i.e., bisulfite sequencing118, 119 and DNAse I hypersensitivity120–122), proteome93, 104, 123 and metabolome124 analyses have begun to provide an unprecedented view of cellular heterogeneity.115 The power of defining the spatial and temporal relationships among distinct subpopulations of cells circumvents the limitations of averaged readouts intrinsic to bulk analyses125 enabling the determination of the dynamics and regulation of cellular processes such as differentiation, tissue homeostasis, and complex disease progression.115 However, the single-cell measurements are often quite variable requiring that novel normalization strategies be introduced into the experimental design to distinguish technical variability from genuine biological variability.126 Furthermore, while variation in measurements (i.e., gene expression) linked to the cell cycle can provide important biological insights, this variation could also obscure more physiologically important differences among cells.36 To address the potential confounding effects of cell cycle asynchrony and more generally discriminate among different sources of biological heterogeneity, single cell latent variable models have been introduced.36 This computational approach for analyzing cell-to-cell heterogeneity has enabled the identification of otherwise undetectable subpopulations of cells that, for example, have provided insights into the differentiation of naive T-cells into T-helper cells.36 Normalized single cell data for which sources of heterogeneity have been addressed can be processed using unsupervised clustering algorithms to identify cell types, define stable states and reconstruct transition paths (i.e., trajectories) between these stable states.115 Quantitative measures of biological progression (i.e. pseudotime, Table 2) through complex processes such as differentiation and oncogenic transformation can be generated using these algorithms that in turn provide valuable mechanistic insights.127 For instance, Monocle has been designed to work with scRNAseq and by analogy Wanderlust104 with high dimensional cytometry for proteomic measures of pseudotime. We expect, for example, that the mechanistic insights gained from comprehensive network-based single cell analysis of heterogeneity will be applied to circulating tumor cells for the early detection of rare resistant subpopulations to inform precision therapeutic strategies.59, 128
Need for a standard set of heterogeneity metrics
There have been many methods and metrics applied to the analysis of heterogeneity. Table 1 lists some of the major classes of metrics with their key characteristics. The majority of the metrics are focused on characterization of population heterogeneity, while relatively few methods address the important spatial aspect of heterogeneity, and temporal heterogeneity56,57 remains to be addressed.
Value in establishing a standard set of metrics
Although a single set of standard metrics for heterogeneity may not be optimal in all situations, it would provide a number of advantages. First, it would encourage integration into software packages like Spotfire (Tibco Software, Boston, MA), R129 and HCS and flow cytometry analysis packages. Second, it would facilitate communication and enable comparison of heterogeneity between systems and assays. Third, only after a method has been established through a peer-reviewed, transparent approach, can it be routinely used in a scope beyond the focus of the investigator who developed it. As the formal quantification and analysis of heterogeneity becomes more common there is a need both for tools that can be applied efficiently, but also tools that provide some insights into the system under study.
The most important characteristics of an optimal set of heterogeneity metrics are to facilitate interpretation of the biology and to produce clear communication of the results of the analysis. Heterogeneity measures need to describe the shape of the population distribution (see 3.2 below), and should be as simple and clear as describing a normal (unimodal) distribution by the “mean”, “median”, “mode” and “standard deviation”. A second key aspect of optimal metrics is a clear understanding of where they can be applied and why they are appropriate for a particular situation. Optimal metrics for heterogeneity, as they gain acceptance, will have more general or more specific applications.
Comparison of published metrics for heterogeneity
Several types of metrics have been applied to the identification of heterogeneity in cell populations. Generally, the metrics characterize 3 aspects of the distribution, the overall extent or diversity, the shape or modality, and the tails. As a first pass, graphical methods, including histograms and the Q-Q plot can be useful for visualization and detection of modality.14 Nonparametric statistics, such as interquartile range (IQR),130 %outliers,131 the KS statistic,54 Shannon index,132 Simpson index133 and Quadratic entropy134 have been used to describe the distribution of a population. Extent measures include the IQR and entropy measures. The IQR, defined to be the 1st quartile subtracted from the 3rd quartile, is a measure of statistical dispersion130 that can be applied to any distribution, however, half the data falls outside the range and therefore the IQR is only sensitive to the central portion of the distribution. The Shannon entropy and Simpson indices have been used to describe the diversity of species in the ecological sciences. The disadvantage of both Shannon and Simpson indices is that they ignore the magnitude of the difference between species. The quadratic entropy incorporates a distance matrix to create a more robust measure of diversity including the magnitude of the differences. Quadratic entropy has been applied to describe the diversity in cell populations.4,37, 76, 134 Shape measures often use a normal distribution as a reference and make a qualitative or quantitative comparison with the data.
The KS statistic is a well-known method for quantifying the difference between two distributions. This can be used for example as a normality test when a sample distribution is compared to a normal distribution,4, 14, 37 or as a QC test to track the shape of the distribution in controls (Section 3.4). Other statistical tests of normality, such as Anderson-Darling, also compare a sample distribution to a normal distribution returning a numerical measure of the goodness-of-fit.135 In selecting a test, it is important to consider the sample size, as some tests of normality work best for sample sizes of 10–1000. Cellular assays may contain data from hundreds to many thousands of cells, and such tests may be too sensitive for these large populations and thus may overestimate the significance of small differences in heterogeneity. Finally, the tail of the distribution can be characterized by the outliers. The percent outliers in the population4, 37 can indicate whether the population has a normal or more heavy-tailed distribution.131
A simple pair of metrics to indicate a non-homogeneous response is the measure of maximum effect (efficacy, Emax) and the Hill Slope (HS) which can be observed even in population averaged measurements, but only in a dose-response format. Maximal effects that plateau below 100% could be indicative of differential response to treatment by subpopulations which should be investigated further. In a study looking at the response of panel of breast cancer cell lines to various anticancer compounds with different mechanisms of action, Fallahi-Sichani et al.136 suggest that during drug development where the aim is to understand variability in patient response, Emax and HS are more informative than simply looking at potency. A shallow Hill slopes in the concentration response curve was shown to be correlated with high cell-to-cell variability in target inhibition. This variability could be the result of fluctuations of target amount, activity or other interactions of the target in different cells. Interestingly, in that study, it was noted that inhibitors of the mTOR pathway, which is subject to complex feedback regulation and potentially a high degree of heterogeneity, had the lowest HS values. While Emax and HS may be useful as indicators of heterogeneity, alone they provide no specific information about the nature of the heterogeneity.
Another common approach to characterizing heterogeneity is the use of principal components analysis (PCA) to reduce the dimensionality of multiparameter data followed by segmentation of the population using a Gaussian mixture model (GMM).61, 68, 88 When there are clear subpopulations, GMM can be a powerful approach to quantifying the relative size of subpopulations, and the movement of cells between subpopulations in response to treatment. However, this approach is not conducive to automated, high throughput applications.
An alternative is the direct analysis of the shape of the distributions of cellular phenotypes, without assuming some number of discrete subpopulations. In this method the distributions are characterized and compared using three indices that describe the diversity, normality and percent outliers in the distribution. Together, referred to as the Pittsburgh Heterogeneity Indices (PHI), the quadratic entropy, the norm-KS test, and the percent outliers can be used to quantify heterogeneity.4, 37 This approach is broadly applicable, can be used to compare data between laboratories and methods, can be incorporated in existing cell analysis software packages and is able to identify differential sensitivity of individual cells to compound exposure. The University of Pittsburgh is presently working with one of the suppliers of data analysis packages to incorporate the PHI as a standard approach to the quantitation of population heterogeneity and will also provide an R-script to calculate the PHI on the UPDDI website.137
QC metrics for characterizing the reproducibility of population distributions
An important question in the analysis of heterogeneity is reproducibility from day-to-day, week-to-week or even month-to-month. Analysis of heterogeneity in large scale biology and drug discovery projects requires methods for validation of consistent cell-to-cell variability,4, 37–39 and establishment of a quality control procedure to monitor reproducibility.37 It is important to note that metrics such as the Z’-factor, or the SSMD give no information about the consistency of the distributions in the wells.37 Figure 4 illustrates a workflow for heterogeneity analysis that addresses the need for metrics and quality control. The suggested procedure follows the same principles used for quality control in screening and therefore integrates well with a standard screening protocol. The procedure adopts a new metric, the QC-KS (Figure 4, steps 2 & 3) that uses the KS statistic to compare the distributions in the control wells on each plate to a set of reference distributions established during validation.37 The QC-KS metric ensures that the shape of the control distributions is consistent throughout the project.
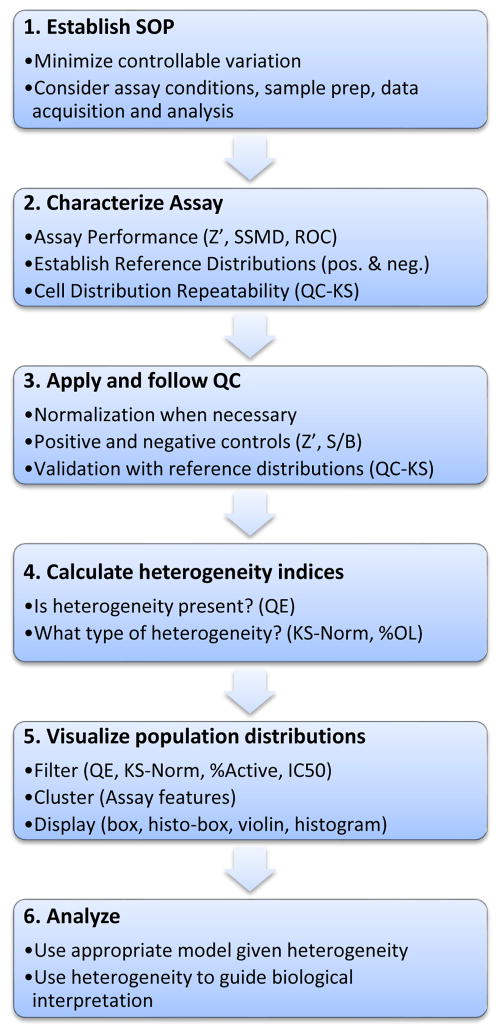
Figure 4. A workflow for quantitation of heterogeneity.
The quantitative analysis of biological heterogeneity requires assay validation and quality control similar to a screen, but with the addition of quality control methods and metrics for ensuring the reproducibility of the population distributions. After establishing the assay SOP (1), one approach is to establish a reference distribution while characterizing assay performance (2). The reference distribution is used throughout the project (3) to track the population distributions in the control wells. Once the consistency of the assay has been established, heterogeneity metrics can be applied to dissect the heterogeneity (4), and interactive analysis and visualization tools used to examine filtered or clustered distributions (5). Selected distributions can then be analyzed with various models and used to guide interpretations or drive the next experiments (6).
Informatics tools for evaluating, visualizing and comparing population distributions in biological data
The analysis of heterogeneity presents a major opportunity to enhance our understanding of biological systems. Extracting insights from the heterogeneity in cell based experiments requires informatics tools to support visualization and analysis of population distributions. Visualization of the distribution of data is most often the initial evidence of heterogeneity in a set of measurements. However, the application of heterogeneity metrics is expected to be a more reliable, quantitative and objective indication of heterogeneity. Selection of the optimal visualization tools often depends on the type of data or the data distribution. For example, histograms are useful for univariate data while scatter plot matrices or density plots are more useful for multivariate data.138 Visualization not only provides some immediate understanding of the nature of the variation in phenotypes, but guides the selection of analysis approaches. Informatics tools for heterogeneity analysis can be categorized as: interactive visualization tools for ‘drilling down’ into distributions; modeling tools for clustering, classification and pathway modeling; general purpose tools that combine visualization and modeling; and application specific tools that are customized to the specific data source.
Drilling down into the distributions
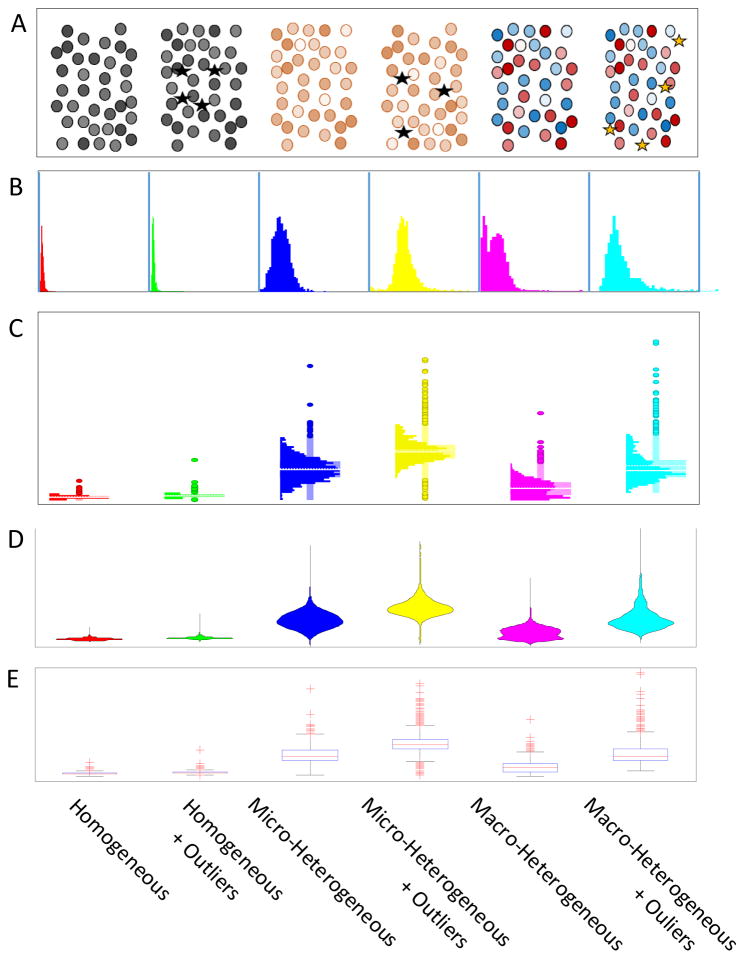
Whatever the initial method for detecting heterogeneity, there is a need for data exploration tools that provide general mathematical and statistical functions along with interactive visualization. Optimally, these tools would also provide a means to incorporate heterogeneity metrics. Figure 5 illustrates how 6 different patterns of heterogeneity for a single phenotype might appear in some standard visualizations. Figure 5A illustrates the 6 patterns as they might appear in an image, where color saturation or pseudocolor could be used to indicate variations in the phenotype, and where a few outlier cells (depicted as stars) might exhibit a more extreme phenotype. While heterogeneity can be directly observed in images, it is difficult to assess and compare the extent of the heterogeneity or the presence or absence of outliers, except perhaps for a few extremes. As an initial evaluation of a distribution, a histogram like the ones in Figure 5B might be used. However, although the overall shape of the distributions is clear, and it is fairly easy to see whether the distribution is uni- or multi-modal, and whether it is reasonably normal (micro-heterogeneous) or more complex (macro-heterogeneous), the presence and distribution of outliers is not easy to see. Figure 5C–D illustrates two plot types, the histo-box plot4 and the violin plot,139 respectively, that combine the features of a histogram with a display of outliers similar to a box plot (Figure 5E). Combining the histogram with the distribution of outliers provides a more detailed view of the heterogeneity in the sample data. Note that in the images and standard box plot it is generally not possible to distinguish between micro- and macro-heterogeneity. Multidimensional scatterplots or density plots are also commonly used to visualize heterogeneity. It is relatively easy to visually pick out a cluster that represents a sub population in a scatterplot. Software tools for detailed analysis of distributions are available in a wide range of statistical and data visualization packages, including commercial and open-source packages described below.
Figure 5.
Visualization of patterns of heterogeneity in population samples. Patterns are described based on six general classes of heterogeneity on the horizontal axis. A. Depiction of the various types of heterogeneity among cells as they might appear in an image. B. Histograms with outliers depicted as individual points based on a standard box plot (“Histo-box plot”4). C. Traditional histograms. D. “Violin plots”,139 essentially double sided histograms. E. A standard box plot.
General purpose informatics tools
Currently there are many general purpose data analysis tools that can be used to implement metrics and visualizations for heterogeneity analysis. Commercial software like Matlab (Mathworks, Natick MA) and open source software like R are programmable and provide large archives of user contributed functions. In addition, some commercial programs like Spotfire, primarily a data visualization tool with some statistical analysis functions, provide an interface for incorporating R or Matlab scripts into the analysis.4, 37 Commercial statistical analysis packages such as SAS/JMP (SAS Institute, Cary, NC), SPSS (IBM, Armonk, NY), and Minitab (Minitab, State College, PA) all have many functions to characterize and visualize distributions of data.
Defining the development of tools for assessing heterogeneity is based on two needs. The first is that the resources described above are very powerful and flexible, but generally require some training before using them. This makes for a high cost to adopt (in terms of effort required to analyze data), therefore limiting acceptance and general use by researchers. Second, they also become highly individualized solutions, resulting in numerous methods for quantifying heterogeneity, making comparisons across systems or studies difficult. In this regard, some universal definitions of heterogeneity and standard practices, such as the workflow in Figure 4, will help develop a general appreciation of and consensus on when heterogeneity analysis is suggested or even required for interpreting an experiment.
Machine learning: Clustering data and classifying subpopulations
Although implicit in much of the discussion above, it becomes important at this point to recognize that heterogeneity results from multiple signaling or metabolic effects.140 Generally, these may be measured at the same time, thus providing some opportunity to explore the complex influence of heterogeneity and interactions between networks and signals. In this regard, combining multiparameter experimental and computational methods with detailed analysis of heterogeneity is necessary in order to understand the highly dynamic mechanisms that control cell plasticity and fate.141 Much of the work in this area incorporates methods for clustering and classifying multiparametric flow cytometry, HCS and transcriptional profiling data, as well as general methods for machine learning derived from ecology, business intelligence and other fields.142
Statistical measures such as KS distance can be used to quantitatively compare distributions of a single biomarker, for example with respect to a reference distribution.143–145 Although each cell can be simply described using the levels of one or more biomolecules, the abundance of data collected from phenotyping experiments allow much more detailed descriptions. Often biomarker levels are transformed into derived features, thereby amplifying the separation between distinct sub-populations that are identified using machine learning approaches. Image data allow calculating higher moments (variance, skewness, etc.) of intracellular biomarker levels, as well as morphological features including shapes of cellular compartments or standard texture features such as Haralick or Zernike features.146 A cell that is imaged using 3-channel IF can easily be described as a vector of hundreds of derived features,61, 68, 147 and this space can be reduced using PCA, t-Distributed Stochastic Neighbor Embedding (T-SNE) or other methods.61, 147–149 Sub-populations within the selected feature space can be identified by clustering using standard methods like K-means150 or hierarchical agglomerative144, 151 clustering, or by fitting the data to distributions of known form, such as GMMs.61, 88 Quantitatively defined cellular phenotypes are useful for training classifiers15 and represent the first step toward constructing mechanistic models to explore the biochemical origins of heterogeneity.152–154
Application specific tools
Many data acquisition systems such as flow cytometry, mass cytometry and HCS, come with advanced, but proprietary tools for visualization and analysis of the data. In some applications third parties provide additional commercial and open source software tools. The establishment of standard metrics for heterogeneity would encourage manufacturers to incorporate those metrics into their proprietary software tools, facilitating the analysis. Meanwhile, open source software presents the most immediate opportunity for integration of heterogeneity metrics. For flow cytometry open source data analysis tools include the BioConductor155 packages iFlow156 and OpenCyto,157 as well as FlowCytometryTools,158 a python package. For HCS data analysis open source options include Cell Profiler Analyst,159 HCS-Analyzer,160 KNIME161 and OMERO.162 High dimensional data, such as that produced by Mass Cytometry and hyperplexed fluorescence imaging, presents some unique challenges for visualization and heterogeneity analysis, for which tools are being developed including viSNE163 which has been integrated into a workflow for discovery and characterization of cell subsets.164
Current application of heterogeneity analysis in drug discovery
Drug discovery and development
The development of disease relevant models and assays begins with the analysis of disease and normal patient samples to identify suitable biomarkers and assay readouts, and also to characterize the organization and heterogeneity profiles of the selected biomarkers. Physiologically relevant models of the disease state, such as 3D tissue models and organs-on-chips, should recapitulate the architecture of the normal and disease tissues, including multiple cell types, which optimally will also recapitulate the tissue heterogeneity.6
In a screening campaign to identify compounds for drug development, heterogeneity indices (HI’s) would then be reported alongside the compound potency and assay performance statistics, including a heterogeneity QC metric, flagging compound concentrations that exceed thresholds established during assay development, indicating significant heterogeneity in the response. In drug development, compounds exhibiting macro-heterogeneity would need to be further studied, perhaps starting with histo-box plots for the dose series. Compounds exhibiting heterogeneity within a defined population (e.g., subpopulation of cells targeted for therapy development) present two options: (1) deprioritize in favor of compounds that modulate the cell population more uniformly; or (2) select the compounds with complementary efficacy in subpopulations for use in a combination therapy strategy. The objective of monitoring heterogeneity in secondary assays should be to make more informed decisions in selecting compounds to advance through drug development by identifying potential differences in mechanism of action (MOA) among lead compounds. To the latter point, the distribution of cell responses impacts the interpretation of drug activity.
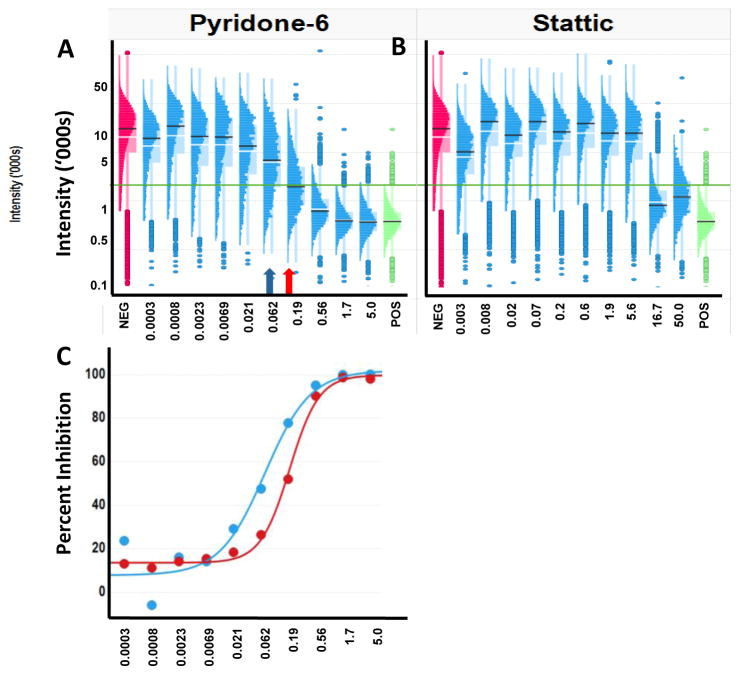
The objective of phenotypic drug discovery is to identify compounds that can revert the disease phenotype to the normal phenotype. These clinical phenotypes are represented in the assay by the negative and positive control samples, respectively. Profiling the changes in the distributions with compound treatment in a screen provides insight into the MOA. This is illustrated in Figure 6A & 6B where the concentration response profiles for the inhibition of STAT3 activation by pyridone-6 (a pan kinase inhibitor) and Stattic (an SH2 binding domain inhibitor) are different, consistent with their different MOA.
Figure 6. The shape of a dose-response curve can be influenced by the underlying distributions of measurements at each dose.
The distinctive transitions in the populations which may indicate different biological processes. A. Histo-box plots of Pyridone-6 inhibition of IL-6 activated STAT3 shows a gradual inhibition with increasing concentration indicating differential sensitivity of the cells. The mean (white bar) and median (black bar) are shown on the distributions. The negative control (red) and the positive control (green) are shown for reference. The green horizontal line is the 3 standard deviations above the mean of the positive control representing the cut-off between cells with and without activated STAT3. The blue arrow indicates the conventional IC50, while the red arrow indicates the concentration at which 50% of the cells are inhibited. B. Histo-box plots of the inhibition by Stattic shows a much steeper inhibition, indicating a more uniform population response, even though the cells at each dose show a variable sensitivity. C. Dose-response curves for Pyridone-6 inhibition calculated based on the population average (blue) or the percent of cells that were inhibited (red).
Analysis of the distributions in response is also important in establishing an optimal assessment of compound activity. If the goal of the screen is to identify compounds that bring the population to a state equivalent to the positive control, then the distribution of the positive control should be used to establish relevant criteria for identifying cells that have reached that state. For example, cells within 3 standard deviations of the mean positive control response could be classified as positive. It is usually assumed that the IC50 derived from the population average measures indicates the concentration at which the population has been induced (or inhibited) half way to the positive control state. IC50s calculated on well- averaged data represent the point at which the signal drops 50% between the negative and positive controls. This calculation does not indicate if the signal in all of the cells was reduced by 50% (which would be a homogeneous response) or, for example, if all of the signal in only half of the cells was reduced (which would be a heterogeneous response). Cell level analysis allows for the detection of heterogeneity and an assessment of when 50% of the cells have reverted to the positive control state (such as within 3 SD of the positive control population). The blue arrow in Figure 6A indicates the IC50 calculated using the well-averaged signal and the red arrow indicates the point at which 50% of the cells have reached the positive control state. This calculation considers heterogeneity in cell response. As shown in Figure 6C, analysis of the pyridone-6 dose-dependence of the distribution of cells revealed that the concentration required to induce half of the cells into the positive control state (red curve), which may be a more relevant measure of the IC50, is 2–10 fold higher than the population average IC50 (blue curve). Furthermore, the degree of rightward shift from the population averaged IC50 can vary depending on the complexity of the transition profile. In the case of Stattic, the steep dose-response leads to similar results for the average and the percent inhibited, while shallower curves result in a significantly greater differential.
Finally, it is important to follow the heterogeneity profile while investigating SAR in the lead optimization stage to ensure that changes in the compound structure do not introduce additional or undesirable heterogeneity in the response, implying altered mechanisms of action. Furthermore, the heterogeneity profile can provide a more sensitive determination of changes in compound potency, and therefore be used in combination with traditional measures of potency to help drive the SAR of a lead series towards a “normal” profile.4
Insights from heterogeneity analysis on basic biomedical research and drug discovery
Cellular heterogeneity arises from biological networks, and therefore provides insights into the network connectivity that can be used to guide selection of biomarkers.9, 165 Observations of individual cell behavior also provide information about the role of heterogeneity in cell differentiation, an essential component of tumor evolution,166 as well as the transition from normal to disease cellular states.88, 166 Neglecting cell heterogeneity can lead to errors in disease classification.166 When combined with computational models, the analysis of cell heterogeneity can be used to predict the responses of subpopulations of cells to drugs (e.g., cancer therapies).9, 61, 65, 167 For example, Johnston et al.168 demonstrated using HCS that patient specific and cell type specific differences in the response of primary breast epithelial cell subpopulations to ionizing radiation were correlated with gene function. Furthermore, an analysis of fluctuations in the disease proteome, together with targeting of the proteins which contributed the most to the heterogeneity within a population, has been used to design combination therapy strategies.169 These and other insights gained from heterogeneity analysis are expected to lead to a better understanding of the biology of disease and the design of more effective therapies.
Current applications of Heterogeneity analysis for Computational Pathology
Digital pathology enables quantitative analysis of heterogeneity
Digital pathology typically uses transmitted light and/or fluorescence imaging for a comprehensive assessment of heterogeneity in tissues at the cellular and subcellular levels.20, 21,91 Recently however, there has been increasing application of IMS to imaging tissue sections.98,107, 110 Subcellular resolution permits the identification of the activation state of specific biomarkers, such as translocation of transcription factors into the nucleus.5 In one study the Quadratic Entropy was used as a measure of diversity, called the HetMap, based on the pathologist’s scoring of individual cells in regions of interest in the tissue.76 The HetMap was shown to be correlated with discordant scoring between pathologists, and therefore useful to identify more complex tissues that required more detailed analysis. However, the dependence on manual cell scoring limited the extent of the regions that could be analyzed and the objectivity of the analysis. Digital pathology enables a more objective and comprehensive assessment of heterogeneity in the tissues,20 and has been used to identify population and spatial heterogeneity in the overall abundance or activation of biomarkers,170 and various microenvironment components including immune cells.19
Importance of the spatial aspect of heterogeneity in tissue
For many malignancies, molecular and cellular heterogeneity is a prominent feature among tumors from different patients, between different sites of neoplasia in a single patient and within a single tumor.171 Intratumor heterogeneity involves phenotypically distinct clonal cell subpopulations and distinct cell types that comprise the tumor microenvironment (TME) or “tumor tissue system” including: local and bone marrow derived stromal stem and progenitor cells; subclasses of immune inflammatory cells that are either tumor promoting or tumor-killing; cancer associated fibroblasts; endothelial cells; and pericytes.4, 22, 172, 173 The TME can be viewed as an evolving ecosystem where cancer cells engage in heterotypic interactions with these other cell types and use available resources to proliferate and survive.72, 74 Consistent with this perspective, the spatial relationships among the cell types within the TME (i.e., spatial heterogeneity) appear to be one of the main drivers of disease progression and therapy resistance.73, 75, 174 Thus, it is imperative to define the spatial heterogeneity within the TME to properly diagnose the specific disease sub-type and identify the optimal course of therapy for individual patients.
Intratumor heterogeneity has been explored using three major approaches. The first approach is to take multiple core samples from specific regions of tumors and measure population heterogeneity within each core and spatial heterogeneity among the cores. The specific analyses include whole exome sequencing,175–179 epigenetics,180 proteomics,11, 181 and metabolomics.11 The second approach involves “single cell analyses” using the above methods,182, 183 RNASeq,33 microscope imaging57 or flow cytometry184 following separation of the cells from the tissue. The third approach uses the spatial resolution of light microscope imaging or IMS, coupled with molecular-specific labels, to capture the spatial context of biomarkers in the cells.21, 22, 185, 186
Heterogeneity and application of image statistics
A major challenge in digital pathology is to develop algorithms that quantify key spatial relationships (interactions or lack thereof) within the TME, based on images of panels of biomarkers. Figure 7A illustrates the spatial heterogeneity of cancer cells and stromal cells, including the migratory immune cells, within a tumor. Indeed, the spatial organization of cancer and non-cancer cells in the TME has been hypothesized to be an important diagnostic187 in addition to the expression level of the selected biomarkers.
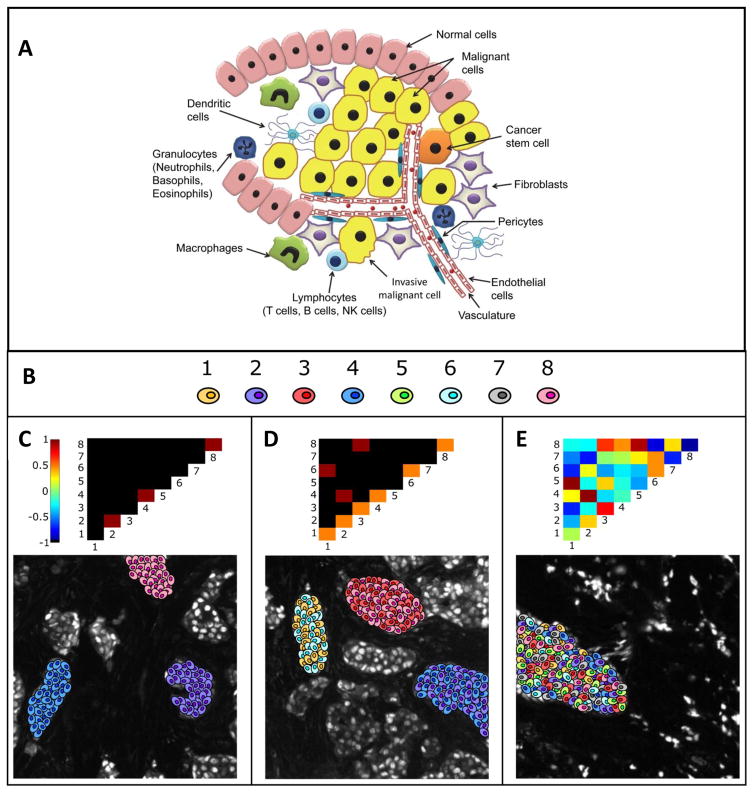
Fig. 7. Canonical pointwise mutual information (PMI) maps depicting various forms of spatial ITH.
A. Illustration of the heterogeneity in a tumor. B. Cartoon representation of 8 different cellular phenotypes based on high-dimensional biomarker intensity patterns acquired via pattern recognition algorithms C. A PMI map with strong diagonal entries and weak off-diagonal entries describes a globally heterogeneous but locally homogeneous tumor. In this example, the PMI map highlights locally homogeneous tumor microdomains containing cells of only one type each, phenotypes 2, 4, and 8 respectively. D. On the contrary, a PMI map with strong off-diagonal entries describes a tumor that is locally heterogeneous. In this example, locally heterogeneous tumor microdomains exist, as portrayed by the off-diagonal entries. One domain contains phenotypes 1 and 5, another contains phenotypes 2 and 4, and yet another containing phenotypes 3 and 8. E. PMI maps can also portray anti-associations (e.g., if phenotype 1 never occurs spatially near phenotype 3). The ensemble of associations and anti-associations of varying intensities along or off the diagonal represent the true complexity of tumor images in a format that can be summarized and interrogated. In this example, changing the distance threshold used in the PMI calculations have minor effects on the results. While Increasing the distance tends to promote positive associations and decreasing the distance tends to increase negative associations, the effects are not significant and the overall conclusions regarding the heterogeneity remain the same. Figures B–E reprinted with permission from Spagnolo et al.21
To address this challenge, a method was developed to quantify intra-tumor spatial heterogeneity (Figure 7B–E) of a single biomarker, as well as multiplexed or hyperplexed biomarkers. The method learns a set of dominant biomarker intensity patterns and maps the spatial distribution of the patterns with a network. The pairwise association statistics for the patterns are described using pointwise mutual information (PMI)188, 189 and visually represented as a two-dimensional heat map. PMI is generalizable to spatial data from other in situ methods such as FISSEQ190 and CyTOF93 that sample multiple markers within the TME.
Other methods applied to the characterization of heterogeneity in tumors have: used region of interest sampling but without a network-based approach or taking advantage of multiplexed data;76 have characterized multiplexed cell phenotype associations within the tumor but not the underlying spatial organization;9 or have analyzed linear relationships between biomarkers in multiplexed/hyperplexed IF data without considering nonlinear associations or spatial information.191 The PMI method uses both the expression and spatial information of an entire tumor tissue section and/or spot in a TMA to characterize spatial associations of both major and minor subpopulations as a two-dimensional heterogeneity map. The characterization of intra-tumor spatial heterogeneity by the PMI is expected to become an important diagnostic biomarker for cancer progression, proliferation, and response to therapy, and to uncover key interactions in the TME that contribute to disease proliferation and progression.21
Insights from spatial heterogeneity analysis in pathology samples
Non-cell-autonomous interactions often govern cell fate decisions and consequently play a major role in complex biological processes.72, 74 Spatial heterogeneity reflects these heterotypic signaling and extracellular matrix reorganization networks. Given the role that TME interactions have in tumorigenesis and metastasis, it may well be expected that spatial genetic heterogeneity can be correlated with poor long-term patient outcome as exemplified in HER-2 positive breast cancer.73 Several groups have developed computational strategies to infer spatial reconstruction of single cell RNAseq data from dissociated cells by integrating single cell expression data with in situ RNA patterns in developing mouse and zebrafish embryos.192–194 By integrating these computational strategies with combinatorial fluorescence in situ hybridization approaches such as SeqFISH and MERFISH,195, 196 it may be possible to spatially reconstruct single cell data derived from tissues such as tumors where, in contrast to embryos, there is no guarantee of reproducible spatial patterning.
Despite the valuable information that can be generated from these powerful approaches focused on single cell analysis, they cannot account for perturbation of the signaling state of an individual cell or a biased recovery of specific cell types during single cell dissociation from bulk tissue. In addition, analysis of cell lysates precludes resolution of subcellular spatial heterogeneity of RNAs and proteins and their associated complexes and networks. Platforms that integrate optical or mass spec imaging with subcellular resolution have great potential to connect spatial and population heterogeneity with cell state, function, and communication.77, 92,93, 197 These in situ approaches are compatible with FFPE biopsies and represent transformative computational pathology platforms aimed at optimizing diagnosis and treatment for individual patients.
Outlook for heterogeneity analysis in biomedical research
Basic biology
The presence of heterogeneity in biological systems has been demonstrated and discussed in many publications, however the functional roles for heterogeneity are just beginning to be elucidated. As an example, recent technological advances in lineage tracing and specific subpopulation ablation, using inducible genetic labeling198, 199 in conjunction with in vivo imaging,200 have provided evidence for the role of dynamic cell population heterogeneity in the regulation of cell fate decisions intrinsic to processes including differentiation, proliferation, and tumorigenesis.3, 17, 71, 72 Hyperplexed measurements with single cell resolution using flow cytometry (e.g., transcriptome profiling, mass cytometry201) coupled with machine learning algorithms have been used to: circumvent averaging artifacts of bulk population measurements (i.e., Simpson’s paradox125); enable the reconstruction of complex cellular hierarchies of differentiation; reveal rare cell states; and identify novel regulators.113, 127
Pluripotent stem cells are a platform with tremendous potential for development of patient specific disease models, for modeling biological development, and for regenerative medicine. However, stem cells exhibit heterogeneity on several levels: in the functional capacity to differentiate; in mRNA expression profiles; and in epigenetic and genetic states.202 Studies of differentiating stem cells have found that heterogeneity reflects the presence of an evolving mixture of phenotypically distinct subpopulations, consistent with a hypothesis that differentiating cells transit through multiple robust and discrete phenotypic states.66, 88, 203 Improved understanding and manipulation of the differentiation of stem cells requires tools to reliably characterize and monitor the evolution of these subpopulations and their associated phenotypes.
The maintenance and repair of cycling adult tissues usually relies on the turnover of a small population of adult stem cells that possess the ability to self-renew, giving rise to differentiated progeny while maintaining their number.3, 204, 205 Tissue homeostasis can be achieved only when the rates of stem cell proliferation and differentiation are balanced. Fate asymmetry can occur at the level of a single stem cell involving asymmetric segregation of fate determinants during cell division, leading one cell to follow a differentiation pathway and the other to stay in the stem cell compartment.3, 204 Alternatively, fate asymmetry can be achieved at the population level where differentiation of one stem cell is compensated for by the symmetric division of a neighboring stem cell.3, 204 In this case it is only the population that persists, whereas the life span of any individual stem cell is not defined. Although either of these alternative models can be induced by intrinsic and extrinsic factors, each nevertheless suggests distinct regulatory mechanisms and therefore a need to identify and monitor these subpopulations of cells. 3, 205
Recent studies of intestinal maintenance,206 mammalian spermatogenesis,207 and hair follicle cycling208 employing genetic lineage tracing and in vivo imaging suggest a more flexible organization in which long-term self-renewal potential, fate, and proliferative activity may be modulated by location within specific microenvironments (e.g., stem cell niche) and by dynamic changes in transcriptional activity that are often induced epigenetically.3, 198, 205, 209, 210 In this model, stem cells form a dynamically heterogeneous pool in which cells may transfer reversibly among states of variable survival and fate potential.3, 205 In addition, progenitors that are normally committed to differentiation may re-acquire (through dedifferentiation) long-term self-renewal potential following exposure to niche factors. Such flexibility may strengthen the resilience of tissues to crisis and injury enabling the population of differentiating progeny to function as a stem cell reserve.3, 205 Thus the heterotypic signaling between stem cells and the niche, likely to be symbiotic,204 indicates the important regulatory role of spatial heterogeneity in tissue homeostasis.205 Several studies also suggest a reversible transfer of stem cells between an active and quiescent state.3 This manifestation of dynamic heterogeneity may provide a robust mechanism to maintain a stem cell pool such that the overall turnover rate of the tissue is steady but slow, particularly in the context of aging.3 Perhaps equally important, a dormant state within a cycling tissue may provide an insurance mechanism to protect the wider population from the stressful demands of active cell cycling, insuring the long-term integrity of the tissue.3 Such behavior would mirror the strategy of phenotypic switching observed in bacterial populations.211
Study of cell signaling networks/pathways
Throughout this perspective a recurring theme has been that heterogeneity both reflects and influences cellular networks and therefore encodes a wealth of basic biological information that can be extracted with systems modeling techniques. Only recently has there been a definitive push to understand phenotypic heterogeneity through systems modeling, revealing the role of ‘noise’ and cell-to-cell variability in cellular systems organization.212, 213 Mechanistic models214 that represent the chemical underpinnings of the cell are easy to interpret in terms of basic molecular principles, but the tradeoff for these insights is the effort required to assemble and parameterize them.215, 216 Simply identifying the correct network topology poses a challenge, as network topology may vary by cell type217 and inconsistencies exist among curated databases of molecular interactions.218
Computational modeling studies have shown that phenotypic heterogeneity in apoptosis is more dependent on extrinsic factors, rather than from intrinsic differences in cells.213, 219, 220 Modeling also suggests that spatial heterogeneity influences tumor aggression. Heterogeneous environments may provide safe havens within which resistant tumors can flourish,221 and spatial heterogeneity promotes immunosuppressive signaling in the TME.222 Incorporating heterogeneity into models of cell signaling networks will be a key to understanding the details of how specific pathway activity drives cellular heterogeneity, and how heterogeneity impacts the regulation of the network.
Drug Discovery-Example of cancer
Darwinian-like clonal evolution in tumors, significantly contributes to the observed phenotypic diversity,7, 75, 173 as do epigenetic changes7, 75, 153 and heterotypic signaling in the TME.173, 223 This diversity and plasticity present a major challenge to the development of therapeutic regimens, as the targeting of a predominant tumor subpopulation often only provides transient benefit that will inevitably result in the emergence of resistant populations and relapse.224 However, recent studies suggest that knowledge of the tumor composition and the response of heterogeneous subpopulations to single drugs, in conjunction with computational and experimental modeling, can identify drug combinations that minimize the outgrowth of resistant subpopulations in tumors, while enhancing tumor free survival in mice.225, 226 Importantly, the experimentally validated simulations demonstrated that the prediction of the optimal drug combination required the analysis of multiple tumor subpopulations, not just a particular subpopulation. Over time, the role of these models in developing treatments will increase and the “one target one drug” paradigm will be replaced by strategies driven by Quantitative Systems Pharmacology (QSP) where development is focused on rationally designed drug combinations.6, 227
Precision medicine-Example of cancer
Intratumor genetic heterogeneity171 and its region-specific diversity,175, 179 reflecting genetic instability as an acquired hallmark of cancer,173 have been well studied. Darwinian forces of evolution, however, act on heritable phenotypes and not genotypes per se.72 Although historically challenging to study in patients, recent studies employing genetic models and novel in situ single cell imaging methods have begun to shed light on phenotypic heterogeneity that arises from environmental selection pressures in the tumor.73, 75 Positive interactions among distinct clonal subpopulations have been observed and can be thought of as one of the major drivers of persistent intratumor heterogeneity.74, 228 These types of collaborative interactions support the possibility that instead of a clonal population accumulating all the necessary mutations that enable it to acquire the hallmarks of cancer, a time consuming and inefficient process, several cooperating partially transformed subclones may circumvent full transformation and thus accelerate tumor progression.72, 229 In situ single cell analysis of primary tumors of HER-2 positive breast cancer patients receiving neoadjuvant chemotherapy indicated a therapy-induced spatial heterogeneity among clones that was associated with shorter disease-free survival following adjuvant therapy with trastuzumab.73 In contrast, no such association was evident when the fraction of cells harboring resistance-conferring mutations (i.e., PIK3CA) or the overall cellular diversity changes before and after neoadjuvant treatment was considered.73 Because long-term survival is largely defined by progression to metastatic disease, these results imply a potential role for spatial heterogeneity in the TME in selecting treatment-resistant cancer cells capable of migration and metastatic dissemination.73
The results of the above study suggest that neoadjuvant chemotherapy prior to HER-2 targeted therapy may be contraindicated as it might promote treatment resistance. Furthermore, this study suggests the potential benefit of implementing in situ single cell hyperplexing technologies with subcellular resolution77, 92, 93 in conjunction with machine learning algorithms as a powerful diagnostic platform to identify targetable tumor dependencies resulting from heterotypic signaling networks (e.g., positively cooperating subclonal populations).73 Thus, knowledge of functional phenotypic heterogeneity, in contrast to simply genetic heterogeneity, could be exploited to guide the design of precision therapeutic strategies.
Acknowledgments
The authors would like to acknowledge the discussions with members of their laboratories, as well as other colleagues that influenced our thinking about heterogeneity. This work was supported in part by funding from the University of Pittsburgh Cancer Institute (2P30 CA047904), The State of Pennsylvania (4100068731), and the National Institutes of Health (5UH3TR000503).
References
- 1.Huang S. Non-genetic heterogeneity of cells in development: more than just noise. Development. 2009;136:3853–3862. doi: 10.1242/dev.035139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gingold JA, Coakley ES, Su J, et al. Distribution Analyzer, a methodology for identifying and clustering outlier conditions from single-cell distributions, and its application to a Nanog reporter RNAi screen. BMC Bioinformatics. 2015;16:225. doi: 10.1186/s12859-015-0636-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krieger T, Simons BD. Dynamic stem cell heterogeneity. Development. 2015;142:1396–1406. doi: 10.1242/dev.101063. [DOI] [PubMed] [Google Scholar]
- 4.Gough AH, Chen N, Shun TY, et al. Identifying and Quantifying Heterogeneity in High Content Analysis: Application of Heterogeneity Indices to Drug Discovery. PLoS One. 2014;9:e102678. doi: 10.1371/journal.pone.0102678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gough A, Lezon T, Faeder JR, et al. High-Content Analysis with Cellular and Tissue Systems Biology: a Bridge between Cancer Cell Biology and Tissue-Based Diagnostics. In: Mendelsohn J, Howley PM, Israel MA, et al., editors. The molecular basis of cancer. 4. Chapter 25. Saunders/Elsevier; Philadelphia, PA: 2015. pp. 369–392. [Google Scholar]
- 6.Stern AM, Schurdak ME, Bahar I, et al. A Perspective on Implementing a Quantitative Systems Pharmacology Platform for Drug Discovery and the Advancement of Personalized Medicine. J Biomol Screen. 2016;21:521–534. doi: 10.1177/1087057116635818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer. 2012;12:323–334. doi: 10.1038/nrc3261. [DOI] [PubMed] [Google Scholar]
- 8.Luria SE, Delbruck M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943;28:491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steininger RJ, Rajaram S, Girard L, et al. On comparing heterogeneity across biomarkers. Cytometry A. 2015;87:558–567. doi: 10.1002/cyto.a.22599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruiz C, Li J, Luttgen MS, et al. Limited genomic heterogeneity of circulating melanoma cells in advanced stage patients. Phys Biol. 2015;12:016008. doi: 10.1088/1478-3975/12/1/016008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balluff B, Frese CK, Maier SK, et al. De novo discovery of phenotypic intratumour heterogeneity using imaging mass spectrometry. J Pathol. 2015;235:3–13. doi: 10.1002/path.4436. [DOI] [PubMed] [Google Scholar]
- 12.Shalek AK, Satija R, Shuga J, et al. Single-cell RNA-seq reveals dynamic paracrine control of cellular variation. Nature. 2014;510:363–369. doi: 10.1038/nature13437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarz RF, Trinh A, Sipos B, et al. Phylogenetic quantification of intra-tumour heterogeneity. PLoS Comput Biol. 2014;10:e1003535. doi: 10.1371/journal.pcbi.1003535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haney SA. Rapid Assessment and Visualization of Normality in High-Content and Other Cell-Level Data and Its Impact on the Interpretation of Experimental Results. J Biomol Screen. 2014 doi: 10.1177/1087057114526432. [DOI] [PubMed] [Google Scholar]
- 15.Loo LH, Lin HJ, Steininger RJ, 3rd, et al. An approach for extensibly profiling the molecular states of cellular subpopulations. Nat Methods. 2009;6:759–765. doi: 10.1038/nmeth.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spudich JL, Koshland DE., Jr Non-genetic individuality: chance in the single cell. Nature. 1976;262:467–471. doi: 10.1038/262467a0. [DOI] [PubMed] [Google Scholar]
- 17.Bhang HE, Ruddy DA, Krishnamurthy Radhakrishna V, et al. Studying clonal dynamics in response to cancer therapy using high-complexity barcoding. Nat Med. 2015;21:440–448. doi: 10.1038/nm.3841. [DOI] [PubMed] [Google Scholar]
- 18.Rubin H. Early origin and pervasiveness of cellular heterogeneity in some malignant transformations. Proc Natl Acad Sci U S A. 1984;81:5121–5125. doi: 10.1073/pnas.81.16.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan KL, Scott DW, Hong F, et al. Tumor-associated macrophages predict inferior outcomes in classic Hodgkin lymphoma: a correlative study from the E2496 Intergroup trial. Blood. 2012;120:3280–3287. doi: 10.1182/blood-2012-04-421057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heindl A, Nawaz S, Yuan Y. Mapping spatial heterogeneity in the tumor microenvironment: a new era for digital pathology. Lab Invest. 2015;95:377–384. doi: 10.1038/labinvest.2014.155. [DOI] [PubMed] [Google Scholar]
- 21.Spagnolo DM, Gyanchandani R, Al-Kofahi Y, et al. Pointwise Mutual Information Quantifies Intra-Tumor Heterogeneity in Tissue Sections Labeled with Multiple Fluorescent Biomarkers. J Pathol Inform. 2016 doi: 10.4103/2153-3539.194839. In press. [DOI] [PMC free article] [PubMed]
- 22.Critchley-Thorne RJ, Miller SM, Taylor DL, et al. Applications of Cellular Systems Biology in Breast Cancer Patient Stratification and Diagnostics. Combinatorial Chemistry and High Throughput Screening. 2009;12:860–869. doi: 10.2174/138620709789383222. [DOI] [PubMed] [Google Scholar]
- 23.Prichard JW, Davison JM, Campbell BB, et al. TissueCypher(™): A systems biology approach to anatomic pathology. J Pathol Inform. 2015;6:48. doi: 10.4103/2153-3539.163987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchison TJ. Small-molecule screening and profiling by using automated microscopy. Chembiochem. 2005;6:33–39. doi: 10.1002/cbic.200400272. [DOI] [PubMed] [Google Scholar]
- 25.Giuliano KA, DeBiasio RL, Dunlay RT, et al. High-Content Screening: A New Approach to Easing Key Bottlenecks in the Drug Discovery Process. J Biomol Screen. 1997;2:249–259. [Google Scholar]
- 26.Abraham VC, Taylor DL, Haskins JR. High content screening applied to large-scale cell biology. Trends Biotechnol. 2004;22:15–22. doi: 10.1016/j.tibtech.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Kell DB, Ryder HM, Kaprelyants AS, et al. Quantifying heterogeneity: flow cytometry of bacterial cultures. Antonie van Leeuwenhoek. 1991;60:145–158. doi: 10.1007/BF00430362. [DOI] [PubMed] [Google Scholar]
- 28.Davey HM, Kell DB. Flow cytometry and cell sorting of heterogeneous microbial populations: the importance of single-cell analyses. Microbiol Rev. 1996;60:641–696. doi: 10.1128/mr.60.4.641-696.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards BS, Sklar LA. Flow Cytometry: Impact on Early Drug Discovery. J Biomol Screen. 2015;20:689–707. doi: 10.1177/1087057115578273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keller PJ, Lin AF, Arendt LM, et al. Mapping the cellular and molecular heterogeneity of normal and malignant breast tissues and cultured cell lines. Breast Cancer Res. 2010;12:R87. doi: 10.1186/bcr2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan IA, Lupi M, Campbell L, et al. Interoperability of time series cytometric data: a cross platform approach for modeling tumor heterogeneity. Cytometry A. 2011;79:214–226. doi: 10.1002/cyto.a.21023. [DOI] [PubMed] [Google Scholar]
- 32.Ambriz-Avina V, Contreras-Garduno JA, Pedraza-Reyes M. Applications of flow cytometry to characterize bacterial physiological responses. Biomed Res Int. 2014;2014:461941. doi: 10.1155/2014/461941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel AP, Tirosh I, Trombetta JJ, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344:1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang D, Bodovitz S. Single cell analysis: the new frontier in ‘omics’. Trends Biotechnol. 2010;28:281–290. doi: 10.1016/j.tibtech.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diercks A, Kostner H, Ozinsky A. Resolving cell population heterogeneity: real-time PCR for simultaneous multiplexed gene detection in multiple single-cell samples. PLoS One. 2009;4:e6326. doi: 10.1371/journal.pone.0006326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buettner F, Natarajan KN, Casale FP, et al. Computational analysis of cell-to-cell heterogeneity in single-cell RNA-sequencing data reveals hidden subpopulations of cells. Nat Biotech. 2015;33:155–160. doi: 10.1038/nbt.3102. [DOI] [PubMed] [Google Scholar]
- 37.Gough A, Shun TY, Lansing Taylor D, et al. A metric and workflow for quality control in the analysis of heterogeneity in phenotypic profiles and screens. Methods. 2016;96:12–26. doi: 10.1016/j.ymeth.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh S, Bray MA, Jones TR, et al. Pipeline for illumination correction of images for high-throughput microscopy. J Microsc. 2014;256:231–236. doi: 10.1111/jmi.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bray MA, Fraser AN, Hasaka TP, et al. Workflow and Metrics for Image Quality Control in Large-Scale High-Content Screens. J Biomol Screen. 2012;17:266–274. doi: 10.1177/1087057111420292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y-l, Taylor DL. Flourescence Microscopy of Living Cells in Culture, Part B: Quantitaive Flourescence Microscopy-Imaging and Spectroscopy. Vol. 30. Academic Press; San Diego: 1989. p. 503. [Google Scholar]
- 41.Wang Y-l, Taylor DL. Flourescence Microscopy of Living Cells in Culture, Part A: Fluorescent Analogs, Labeling Cells, and Basic Microscopy. Vol. 29. Academic Press; San Diego: 1988. p. 333. [Google Scholar]
- 42.Chakravarty A, Bowman D, Ecsedy JA, et al. High Content Screening. John Wiley & Sons, Inc; 2007. Developing Robust High Content Assays; pp. 85–109. [Google Scholar]
- 43.Hoffman RA. Standardization, calibration, and control in flow cytometry. Curr Protoc Cytom. 2005;Chapter 1(Unit 13) doi: 10.1002/0471142956.cy0103s32. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz A, Marti GE, Poon R, et al. Standardizing flow cytometry: a classification system of fluorescence standards used for flow cytometry. Cytometry. 1998;33:106–114. [PubMed] [Google Scholar]
- 45.Mittag A, Tarnok A. Basics of standardization and calibration in cytometry--a review. J Biophotonics. 2009;2:470–481. doi: 10.1002/jbio.200910033. [DOI] [PubMed] [Google Scholar]
- 46.Alvarez DF, Helm K, Degregori J, et al. Publishing flow cytometry data. Am J Physiol Lung Cell Mol Physiol. 2010;298:L127–130. doi: 10.1152/ajplung.00313.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aghaeepour N, Finak G, Hoos H, et al. Critical assessment of automated flow cytometry data analysis techniques. Nat Methods. 2013;10:228–238. doi: 10.1038/nmeth.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aghaeepour N, Jalali A, O’Neill K, et al. RchyOptimyx: cellular hierarchy optimization for flow cytometry. Cytometry A. 2012;81:1022–1030. doi: 10.1002/cyto.a.22209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Neill K, Jalali A, Aghaeepour N, et al. Enhanced flowType/RchyOptimyx: a BioConductor pipeline for discovery in high-dimensional cytometry data. Bioinformatics. 2014;30:1329–1330. doi: 10.1093/bioinformatics/btt770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brinkman RR, Aghaeepour N, Finak G, et al. Automated analysis of flow cytometry data comes of age. Cytometry A. 2016;89:13–15. doi: 10.1002/cyto.a.22810. [DOI] [PubMed] [Google Scholar]
- 51.Van Gassen S, Vens C, Dhaene T, et al. FloReMi: Flow density survival regression using minimal feature redundancy. Cytometry A. 2016;89:22–29. doi: 10.1002/cyto.a.22734. [DOI] [PubMed] [Google Scholar]
- 52.Zhang JH, Chung TDY, Oldenburg KR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 53.Zhang XD. A pair of new statistical parameters for quality control in RNA interference high-throughput screening assays. Genomics. 2007;89:552–561. doi: 10.1016/j.ygeno.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 54.Lilliefors HW. On the Kolmogorov-Smirnov Test for Normality with Mean and Variance Unknown. J Am Stat Assoc. 1967;62:399–402. [Google Scholar]
- 55.Basak S, Behar M, Hoffmann A. Lessons from mathematically modeling the NF-κB pathway. Immunological Reviews. 2012;246:221–238. doi: 10.1111/j.1600-065X.2011.01092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee Robin EC, Walker Sarah R, Savery K, et al. Fold Change of Nuclear NF-κB Determines TNF-Induced Transcription in Single Cells. Mol Cell. 2014;53:867–879. doi: 10.1016/j.molcel.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cohen AA, Geva-Zatorsky N, Eden E, et al. Dynamic proteomics of individual cancer cells in response to a drug. Science. 2008;322:1511–1516. doi: 10.1126/science.1160165. [DOI] [PubMed] [Google Scholar]
- 58.Sisan DR, Halter M, Hubbard JB, et al. Predicting rates of cell state change caused by stochastic fluctuations using a data-driven landscape model. Proc Natl Acad Sci U S A. 2012;109:19262–19267. doi: 10.1073/pnas.1207544109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen F, Zhuang X, Lin L, et al. New horizons in tumor microenvironment biology: challenges and opportunities. BMC Med. 2015;13:45. doi: 10.1186/s12916-015-0278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lloyd MC, Cunningham JJ, Bui MM, et al. Darwinian Dynamics of Intratumoral Heterogeneity: Not Solely Random Mutations but Also Variable Environmental Selection Forces. Cancer Res. 2016;76:3136–3144. doi: 10.1158/0008-5472.CAN-15-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Slack MD, Martinez ED, Wu LF, et al. Characterizing heterogeneous cellular responses to perturbations. Proc Natl Acad Sci U S A. 2008;105:19306–19311. doi: 10.1073/pnas.0807038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gascoigne KE, Taylor SS. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell. 2008;14:111–122. doi: 10.1016/j.ccr.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 63.Toriello NM, Douglas ES, Thaitrong N, et al. Integrated microfluidic bioprocessor for single-cell gene expression analysis. Proc Natl Acad Sci U S A. 2008;105:20173–20178. doi: 10.1073/pnas.0806355106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Snijder B, Pelkmans L. Origins of regulated cell-to-cell variability. Nat Rev Mol Cell Biol. 2011;12:119–125. doi: 10.1038/nrm3044. [DOI] [PubMed] [Google Scholar]
- 65.Altschuler SJ, Wu LF. Cellular heterogeneity: when do differences make a difference? Cell. 2010;141:559–563. doi: 10.1016/j.cell.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chang HH, Hemberg M, Barahona M, et al. Transcriptome-wide noise controls lineage choice in mammalian progenitor cells. Nature. 2008;453:544–547. doi: 10.1038/nature06965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu J, Tzanakakis ES. Deconstructing stem cell population heterogeneity: single-cell analysis and modeling approaches. Biotechnol Adv. 2013;31:1047–1062. doi: 10.1016/j.biotechadv.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singh DK, Ku CJ, Wichaidit C, et al. Patterns of basal signaling heterogeneity can distinguish cellular populations with different drug sensitivities. Mol Syst Biol. 2010;6:369. doi: 10.1038/msb.2010.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gerdes MJ, Sood A, Sevinsky C, et al. Emerging understanding of multiscale tumor heterogeneity. Front Oncol. 2014;4:366. doi: 10.3389/fonc.2014.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tawfik DS. Messy biology and the origins of evolutionary innovations. Nat Chem Biol. 2010;6:692–696. doi: 10.1038/nchembio.441. [DOI] [PubMed] [Google Scholar]
- 71.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tabassum DP, Polyak K. Tumorigenesis: it takes a village. Nat Rev Cancer. 2015;15:473–483. doi: 10.1038/nrc3971. [DOI] [PubMed] [Google Scholar]
- 73.Janiszewska M, Liu L, Almendro V, et al. In situ single-cell analysis identifies heterogeneity for PIK3CA mutation and HER2 amplification in HER2-positive breast cancer. Nat Genet. 2015;47:1212–1219. doi: 10.1038/ng.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marusyk A, Tabassum DP, Altrock PM, et al. Non-cell-autonomous driving of tumour growth supports sub-clonal heterogeneity. Nature. 2014;514:54–58. doi: 10.1038/nature13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Almendro V, Cheng YK, Randles A, et al. Inference of Tumor Evolution during Chemotherapy by Computational Modeling and In Situ Analysis of Genetic and Phenotypic Cellular Diversity. Cell Rep. 2014;6:514–527. doi: 10.1016/j.celrep.2013.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Potts SJ, Krueger JS, Landis ND, et al. Evaluating tumor heterogeneity in immunohistochemistry-stained breast cancer tissue. Lab Invest. 2012;92:1342–1357. doi: 10.1038/labinvest.2012.91. [DOI] [PubMed] [Google Scholar]
- 77.Gerdes MJ, Sevinsky CJ, Sood A, et al. Highly multiplexed single-cell analysis of formalin-fixed, paraffin-embedded cancer tissue. Proc Natl Acad Sci U S A. 2013;110:11982–11987. doi: 10.1073/pnas.1300136110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lawson DA, Bhakta NR, Kessenbrock K, et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature. 2015;526:131–135. doi: 10.1038/nature15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abraham VC, Samson B, Lapets O, et al. Automated Classification of Individual Cellular Responses Across Multiple Targets. Preclinica. 2004;2:349–355. [Google Scholar]
- 80.Stilwell JL, Guan Y, Neve RM, et al. Systems biology in cancer research: genomics to cellomics. Methods Mol Biol. 2007;356:353–365. doi: 10.1385/1-59745-217-3:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shi J, Orth JD, Mitchison T. Cell type variation in responses to antimitotic drugs that target microtubules and kinesin-5. Cancer Res. 2008;68:3269–3276. doi: 10.1158/0008-5472.CAN-07-6699. [DOI] [PubMed] [Google Scholar]
- 82.McCann T. Live cell imaging: an industrial perspective. Methods Mol Biol. 2010;591:47–66. doi: 10.1007/978-1-60761-404-3_3. [DOI] [PubMed] [Google Scholar]
- 83.Pereira PM, Almada P, Henriques R. Chapter 7 - High-content 3D multicolor super-resolution localization microscopy. In: Ewa KP, editor. Methods in Cell Biology. Academic Press; 2015. pp. 95–117. [DOI] [PubMed] [Google Scholar]
- 84.Legant WR, Shao L, Grimm JB, et al. High-density three-dimensional localization microscopy across large volumes. Nat Methods. 2016;13:359–365. doi: 10.1038/nmeth.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taylor DL. Past, present, and future of high content screening and the field of cellomics. Methods Mol Biol. 2007;356:3–18. doi: 10.1385/1-59745-217-3:3. [DOI] [PubMed] [Google Scholar]
- 86.LaPan P, Zhang J, Pan J, et al. Single cell cytometry of protein function in RNAi treated cells and in native populations. BMC Cell Biol. 2008;9:43. doi: 10.1186/1471-2121-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bright GR, Whitaker JE, Haugland RP, et al. Heterogeneity of the changes in cytoplasmic pH upon serum stimulation of quiescent fibroblasts. J Cell Physiol. 1989;141:410–419. doi: 10.1002/jcp.1041410223. [DOI] [PubMed] [Google Scholar]
- 88.Loo LH, Lin HJ, Singh DK, et al. Heterogeneity in the physiological states and pharmacological responses of differentiating 3T3-L1 preadipocytes. J Cell Biol. 2009;187:375–384. doi: 10.1083/jcb.200904140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Racoceanu D, Belhomme P. Breakthrough technologies in digital pathology. Comput Med Imaging Graph. 2015;42:1. doi: 10.1016/j.compmedimag.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 90.Nederlof M, Watanabe S, Burnip B, et al. High-throughput profiling of tissue and tissue model microarrays: Combined transmitted light and 3-color fluorescence digital pathology. J Pathol Inform. 2011;2:50. doi: 10.4103/2153-3539.89849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McCabe A, Dolled-Filhart M, Camp RL, et al. Automated quantitative analysis (AQUA) of in situ protein expression, antibody concentration, and prognosis. J Natl Cancer Inst. 2005;97:1808–1815. doi: 10.1093/jnci/dji427. [DOI] [PubMed] [Google Scholar]
- 92.Lee JH, Daugharthy ER, Scheiman J, et al. Highly multiplexed subcellular RNA sequencing in situ. Science. 2014;343:1360–1363. doi: 10.1126/science.1250212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Giesen C, Wang HA, Schapiro D, et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat Methods. 2014;11:417–422. doi: 10.1038/nmeth.2869. [DOI] [PubMed] [Google Scholar]
- 94.Lin JR, Fallahi-Sichani M, Sorger PK. Highly multiplexed imaging of single cells using a high-throughput cyclic immunofluorescence method. Nat Commun. 2015;6:8390. doi: 10.1038/ncomms9390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weaver EM, Hummon AB. Imaging mass spectrometry: from tissue sections to cell cultures. Adv Drug Deliv Rev. 2013;65:1039–1055. doi: 10.1016/j.addr.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 96.Aichler M, Walch A. MALDI Imaging mass spectrometry: current frontiers and perspectives in pathology research and practice. Lab Invest. 2015;95:422–431. doi: 10.1038/labinvest.2014.156. [DOI] [PubMed] [Google Scholar]
- 97.Lanni EJ, Rubakhin SS, Sweedler JV. Mass spectrometry imaging and profiling of single cells. J Proteomics. 2012;75:5036–5051. doi: 10.1016/j.jprot.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bodzon-Kulakowska A, Suder P. Imaging mass spectrometry: Instrumentation, applications, and combination with other visualization techniques. Mass Spectrom Rev. 2016;35:147–169. doi: 10.1002/mas.21468. [DOI] [PubMed] [Google Scholar]
- 99.Passarelli MK, Ewing AG. Single-cell imaging mass spectrometry. Curr Opin Chem Biol. 2013;17:854–859. doi: 10.1016/j.cbpa.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zavalin A, Todd EM, Rawhouser PD, et al. Direct imaging of single cells and tissue at subcellular spatial resolution using transmission geometry MALDI MS. J Mass Spectrom. 2012;47:i. doi: 10.1002/jms.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Seeley EH, Caprioli RM. 3D imaging by mass spectrometry: a new frontier. Anal Chem. 2012;84:2105–2110. doi: 10.1021/ac2032707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Angelo M, Bendall SC, Finck R, et al. Multiplexed ion beam imaging of human breast tumors. Nat Med. 2014;20:436–442. doi: 10.1038/nm.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bodenmiller B. Multiplexed Epitope-Based Tissue Imaging for Discovery and Healthcare Applications. Cell Syst. 2016;2:225–238. doi: 10.1016/j.cels.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 104.Bendall SC, Davis KL, Amir el AD, et al. Single-cell trajectory detection uncovers progression and regulatory coordination in human B cell development. Cell. 2014;157:714–725. doi: 10.1016/j.cell.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Spitzer MH, Nolan GP. Mass Cytometry: Single Cells, Many Features. Cell. 2016;165:780–791. doi: 10.1016/j.cell.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Levenson RM, Borowsky AD, Angelo M. Immunohistochemistry and mass spectrometry for highly multiplexed cellular molecular imaging. Lab Invest. 2015;95:397–405. doi: 10.1038/labinvest.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mao X, He J, Li T, et al. Application of imaging mass spectrometry for the molecular diagnosis of human breast tumors. Sci Rep. 2016;6:21043. doi: 10.1038/srep21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jones EA, van Remoortere A, van Zeijl RJM, et al. Multiple Statistical Analysis Techniques Corroborate Intratumor Heterogeneity in Imaging Mass Spectrometry Datasets of Myxofibrosarcoma. PLoS ONE. 2011;6:e24913. doi: 10.1371/journal.pone.0024913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tata A, Zheng J, Ginsberg HJ, et al. Contrast Agent Mass Spectrometry Imaging Reveals Tumor Heterogeneity. Anal Chem. 2015;87:7683–7689. doi: 10.1021/acs.analchem.5b01992. [DOI] [PubMed] [Google Scholar]
- 110.Thompson CG, Bokhart MT, Sykes C, et al. Mass spectrometry imaging reveals heterogeneous efavirenz distribution within putative HIV reservoirs. Antimicrob Agents Chemother. 2015;59:2944–2948. doi: 10.1128/AAC.04952-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Perfetto SP, Chattopadhyay PK, Roederer M. Seventeen-colour flow cytometry: unravelling the immune system. Nat Rev Immunol. 2004;4:648–655. doi: 10.1038/nri1416. [DOI] [PubMed] [Google Scholar]
- 112.Bendall SC, Nolan GP, Roederer M, et al. A deep profiler’s guide to cytometry. Trends Immunol. 2012;33:323–332. doi: 10.1016/j.it.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bodenmiller B, Zunder ER, Finck R, et al. Multiplexed mass cytometry profiling of cellular states perturbed by small-molecule regulators. Nat Biotechnol. 2012;30:858–867. doi: 10.1038/nbt.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hines WC, Su Y, Kuhn I, et al. Sorting out the FACS: a devil in the details. Cell Rep. 2014;6:779–781. doi: 10.1016/j.celrep.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 115.Trapnell C. Defining cell types and states with single-cell genomics. Genome Res. 2015;25:1491–1498. doi: 10.1101/gr.190595.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nakamura T, Yabuta Y, Okamoto I, et al. SC3-seq: a method for highly parallel and quantitative measurement of single-cell gene expression. Nucleic Acids Res. 2015;43:e60. doi: 10.1093/nar/gkv134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Macosko EZ, Basu A, Satija R, et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell. 2015:161. doi: 10.1016/j.cell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guo H, Zhu P, Wu X, et al. Single-cell methylome landscapes of mouse embryonic stem cells and early embryos analyzed using reduced representation bisulfite sequencing. Genome Res. 2013:23. doi: 10.1101/gr.161679.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Smallwood SA, Lee HJ, Angermueller C, et al. Single-cell genome-wide bisulfite sequencing for assessing epigenetic heterogeneity. Nat Methods. 2014:11. doi: 10.1038/nmeth.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jin W, Tang Q, Wan M, et al. Genome-wide detection of DNase I hypersensitive sites in single cells and FFPE tissue samples. Nature. 2015:528. doi: 10.1038/nature15740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cusanovich DA, Daza R, Adey A, et al. Epigenetics. Multiplex single-cell profiling of chromatin accessibility by combinatorial cellular indexing. Science. 2015:348. doi: 10.1126/science.aab1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Buenrostro JD, Wu B, Litzenburger UM, et al. Single-cell chromatin accessibility reveals principles of regulatory variation. Nature. 2015:523. doi: 10.1038/nature14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lu Y, Xue Q, Eisele MR, et al. Highly multiplexed profiling of single-cell effector functions reveals deep functional heterogeneity in response to pathogenic ligands. Proc Natl Acad Sci U S A. 2015;112:E607–615. doi: 10.1073/pnas.1416756112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Onjiko RM, Moody SA, Nemes P. Single-cell mass spectrometry reveals small molecules that affect cell fates in the 16-cell embryo. Proc Natl Acad Sci U S A. 2015;112:6545–6550. doi: 10.1073/pnas.1423682112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Simpson EH. The Interpretation of Interaction in Contingency Tables. J Roy Stat Soc B. 1951;13:238–241. [Google Scholar]
- 126.Stegle O, Teichmann SA, Marioni JC. Computational and analytical challenges in single-cell transcriptomics. Nat Rev Genet. 2015:16. doi: 10.1038/nrg3833. [DOI] [PubMed] [Google Scholar]
- 127.Trapnell C, Cacchiarelli D, Grimsby J, et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat Biotechnol. 2014;32:381–386. doi: 10.1038/nbt.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schissler AG, Li Q, Chen JL, et al. Analysis of aggregated cell-cell statistical distances within pathways unveils therapeutic-resistance mechanisms in circulating tumor cells. Bioinformatics. 2016;32:i80–i89. doi: 10.1093/bioinformatics/btw248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. [Google Scholar]
- 130.Yule GU. An Introduction to the Theory of Statistics. Charles Griffin and Company; 1911. [Google Scholar]
- 131.Barnett VLT. Outliers in Statistical Data. 3. Wiley; 1994. [Google Scholar]
- 132.Shannon CE, Weaver W. The mathematical theory of communication. University of Illinois Press; Urbana: 1949. p. v.p. vii.p. 117. [Google Scholar]
- 133.Simpson EH. Measurement of Diversity. Nature. 1949;163:688–688. [Google Scholar]
- 134.Rao CR. Diversity and Dissimilarity Coefficients - a Unified Approach. Theor Popul Biol. 1982;21:24–43. [Google Scholar]
- 135.Razali NM, Wah YB. Power Comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors and Anderson-Darling Tests. Journal of Statistical Modeling and Analytics. 2011;2:13. [Google Scholar]
- 136.Fallahi-Sichani M, Honarnejad S, Heiser LM, et al. Metrics other than potency reveal systematic variation in responses to cancer drugs. Nat Chem Biol. 2013;9:708–714. doi: 10.1038/nchembio.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.UPDDI. [Accessed July 8, 2016];University of Pittsburgh Drug Discovery Institute. upddi.pitt.edu.
- 138.Dinov ID. Methodological challenges and analytic opportunities for modeling and interpreting Big Healthcare Data. Gigascience. 2016;5:12. doi: 10.1186/s13742-016-0117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hintze JL, Nelson RD. Violin plots: A box plot-density trace synergism. Am Stat. 1998;52:181–184. [Google Scholar]
- 140.Kiviet DJ, Nghe P, Walker N, et al. Stochasticity of metabolism and growth at the single-cell level. Nature. 2014;514:376–379. doi: 10.1038/nature13582. [DOI] [PubMed] [Google Scholar]
- 141.Spiller DG, Wood CD, Rand DA, et al. Measurement of single-cell dynamics. Nature. 2010;465:736–745. doi: 10.1038/nature09232. [DOI] [PubMed] [Google Scholar]
- 142.Haney SA. An Introduction to High Content Screening. John Wiley & Sons, Inc; 2014. Factoring and Clustering High Content Data; pp. 211–229. [Google Scholar]
- 143.Giuliano KA, Chen YT, Taylor DL. High-Content Screening with siRNA Optimizes a Cell Biological Approach to Drug Discovery: Defining the Role of P53 Activation in the Cellular Response to Anticancer Drugs. J Biomol Screen. 2004;9:557–568. doi: 10.1177/1087057104265387. [DOI] [PubMed] [Google Scholar]
- 144.Giuliano KA, Cheung WS, Curran DP, et al. Systems Cell Biology Knowledge Created from High Content Screening. Assay Drug Dev Technol. 2005;3:501–514. doi: 10.1089/adt.2005.3.501. [DOI] [PubMed] [Google Scholar]
- 145.Perlman ZE, Slack MD, Feng Y, et al. Multidimensional drug profiling by automated microscopy. Science. 2004;306:1194–1198. doi: 10.1126/science.1100709. [DOI] [PubMed] [Google Scholar]
- 146.Boland MV, Murphy RF. A neural network classifier capable of recognizing the patterns of all major subcellular structures in fluorescence microscope images of HeLa cells. Bioinformatics. 2001;17:1213–1223. doi: 10.1093/bioinformatics/17.12.1213. [DOI] [PubMed] [Google Scholar]
- 147.Loo LH, Wu LF, Altschuler SJ. Image-based multivariate profiling of drug responses from single cells. Nat Methods. 2007;4:445–453. doi: 10.1038/nmeth1032. [DOI] [PubMed] [Google Scholar]
- 148.van der Maaten L, Hinton G. Visualizing Data using t-SNE. J Mach Learn Res. 2008;9:2579–2605. [Google Scholar]
- 149.van der Maaten L. Accelerating t-SNE using Tree-Based Algorithms. J Mach Learn Res. 2014;15:3221–3245. [Google Scholar]
- 150.Low J, Huang S, Blosser W, et al. High-content imaging characterization of cell cycle therapeutics through in vitro and in vivo subpopulation analysis. Mol Cancer Ther. 2008;7:2455–2463. doi: 10.1158/1535-7163.MCT-08-0328. [DOI] [PubMed] [Google Scholar]
- 151.Naik AW, Kangas JD, Sullivan DP, et al. Active machine learning-driven experimentation to determine compound effects on protein patterns. Elife. 2016:5. doi: 10.7554/eLife.10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Harrison PM, Badel L, Wall MJ, et al. Experimentally Verified Parameter Sets for Modelling Heterogeneous Neocortical Pyramidal-Cell Populations. PLoS Comput Biol. 2015;11:e1004165. doi: 10.1371/journal.pcbi.1004165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gupta PB, Fillmore CM, Jiang G, et al. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146:633–644. doi: 10.1016/j.cell.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 154.Hasenauer J, Heinrich J, Doszczak M, et al. A visual analytics approach for models of heterogeneous cell populations. EURASIP J Bioinform Syst Biol. 2012;2012:4. doi: 10.1186/1687-4153-2012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gentleman RC, Carey VJ, Bates DM, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lee K, Hahne F, Sarkar D, et al. iFlow: A Graphical User Interface for Flow Cytometry Tools in Bioconductor. Adv Bioinformatics. 2009:103839. doi: 10.1155/2009/103839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Finak G, Frelinger J, Jiang W, et al. OpenCyto: An Open Source Infrastructure for Scalable, Robust, Reproducible, and Automated, End-to-End Flow Cytometry Data Analysis. PLoS Comput Biol. 2014;10:e1003806. doi: 10.1371/journal.pcbi.1003806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Friedman J, Yurtsev E. [Accessed June 14, 2016];FlowCytometryTools v0.4.5, a python package for visualization and analysis of high-throughput flow cytometry data. http://eyurtsev.github.io/FlowCytometryTools/
- 159.Jones TR, Kang IH, Wheeler DB, et al. CellProfiler Analyst: data exploration and analysis software for complex image-based screens. BMC Bioinformatics. 2008;9:1–16. doi: 10.1186/1471-2105-9-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ogier A, Dorval T. HCS-Analyzer: open source software for high-content screening data correction and analysis. Bioinformatics. 2012;28:1945–1946. doi: 10.1093/bioinformatics/bts288. [DOI] [PubMed] [Google Scholar]
- 161.Stoter M, Niederlein A, Barsacchi R, et al. CellProfiler and KNIME: open source tools for high content screening. Methods Mol Biol. 2013;986:105–122. doi: 10.1007/978-1-62703-311-4_8. [DOI] [PubMed] [Google Scholar]
- 162.Allan C, Burel JM, Moore J, et al. OMERO: flexible, model-driven data management for experimental biology. Nat Methods. 2012;9:245–253. doi: 10.1038/nmeth.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Amir E-aD, Davis KL, Tadmor MD, et al. viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat Biotech. 2013;31:545–552. doi: 10.1038/nbt.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Diggins KE, Ferrell PB, Jr, Irish JM. Methods for discovery and characterization of cell subsets in high dimensional mass cytometry data. Methods. 2015;82:55–63. doi: 10.1016/j.ymeth.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Burrell RA, McGranahan N, Bartek J, et al. The causes and consequences of genetic heterogeneity in cancer evolution. Nature. 2013;501:338–345. doi: 10.1038/nature12625. [DOI] [PubMed] [Google Scholar]
- 166.Batchelor E, Kann MG, Przytycka TM, et al. Modeling cell heterogeneity: from single-cell variations to mixed cells. Pac Symp Biocomput. 2013:445–450. [PubMed] [Google Scholar]
- 167.Caie PD, Walls RE, Ingleston-Orme A, et al. High-content phenotypic profiling of drug response signatures across distinct cancer cells. Mol Cancer Ther. 2010;9:1913–1926. doi: 10.1158/1535-7163.MCT-09-1148. [DOI] [PubMed] [Google Scholar]
- 168.Johnston RL, Wockner L, McCart Reed AE, et al. High content screening application for cell-type specific behaviour in heterogeneous primary breast epithelial subpopulations. Breast Cancer Res. 2016;18:18. doi: 10.1186/s13058-016-0681-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Niepel M, Spencer SL, Sorger PK. Non-genetic cell-to-cell variability and the consequences for pharmacology. Curr Opin Chem Biol. 2009;13:556–561. doi: 10.1016/j.cbpa.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chung GG, Zerkowski MP, Ghosh S, et al. Quantitative analysis of estrogen receptor heterogeneity in breast cancer. Lab Invest. 2007;87:662–669. doi: 10.1038/labinvest.3700543. [DOI] [PubMed] [Google Scholar]
- 171.Alizadeh AA, Aranda V, Bardelli A, et al. Toward understanding and exploiting tumor heterogeneity. Nat Med. 2015;21:846–853. doi: 10.1038/nm.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;125:5591–5596. doi: 10.1242/jcs.116392. [DOI] [PubMed] [Google Scholar]
- 173.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 174.Waclaw B, Bozic I, Pittman ME, et al. A spatial model predicts that dispersal and cell turnover limit intratumour heterogeneity. Nature. 2015;525:261–264. doi: 10.1038/nature14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kumar A, Boyle EA, Tokita M, et al. Deep sequencing of multiple regions of glial tumors reveals spatial heterogeneity for mutations in clinically relevant genes. Genome Biol. 2014;15:530. doi: 10.1186/s13059-014-0530-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Govindan R. Cancer. Attack of the clones. Science. 2014;346:169–170. doi: 10.1126/science.1259926. [DOI] [PubMed] [Google Scholar]
- 178.Bashashati A, Ha G, Tone A, et al. Distinct evolutionary trajectories of primary high-grade serous ovarian cancers revealed through spatial mutational profiling. J Pathol. 2013;231:21–34. doi: 10.1002/path.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yates LR, Gerstung M, Knappskog S, et al. Subclonal diversification of primary breast cancer revealed by multiregion sequencing. Nat Med. 2015;21:751–759. doi: 10.1038/nm.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rivenbark AG, O’Connor SM, Coleman WB. Molecular and cellular heterogeneity in breast cancer: challenges for personalized medicine. Am J Pathol. 2013;183:1113–1124. doi: 10.1016/j.ajpath.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sugihara Y, Taniguchi H, Kushima R, et al. Laser microdissection and two-dimensional difference gel electrophoresis reveal proteomic intra-tumor heterogeneity in colorectal cancer. J Proteomics. 2013;78:134–147. doi: 10.1016/j.jprot.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 182.Navin N, Kendall J, Troge J, et al. Tumour evolution inferred by single-cell sequencing. Nature. 2011;472:90–94. doi: 10.1038/nature09807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wang Y, Waters J, Leung ML, et al. Clonal evolution in breast cancer revealed by single nucleus genome sequencing. Nature. 2014;512:155–160. doi: 10.1038/nature13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Irish JM, Hovland R, Krutzik PO, et al. Single cell profiling of potentiated phospho-protein networks in cancer cells. Cell. 2004;118:217–228. doi: 10.1016/j.cell.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 185.Camp RL, Chung GG, Rimm DL. Automated subcellular localization and quantification of protein expression in tissue microarrays. Nat Med. 2002;8:1323–1328. doi: 10.1038/nm791. [DOI] [PubMed] [Google Scholar]
- 186.Chung GG, Zerkowski MP, Ghosh S, et al. Quantitative analysis of estrogen receptor heterogeneity in breast cancer. Laboratory investigation. 2007;87:662–669. doi: 10.1038/labinvest.3700543. [DOI] [PubMed] [Google Scholar]
- 187.Salo T, Vered M, Bello IO, et al. Insights into the role of components of the tumor microenvironment in oral carcinoma call for new therapeutic approaches. Exp Cell Res. 2014;325:58–64. doi: 10.1016/j.yexcr.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 188.Church KH, Word P. Association Norms Mutual Information, and Lexicography. Computational Linguistics. 1990;16:22– 29. [Google Scholar]
- 189.Role FNM. Handling the Impact of Low frequency Events on Co-occurrence-based Measures of Word Similarity:A Case Study of Pointwise Mutual Information. International Conference on Knowledge Discovery and Information Retrieval Paris, France; Paris, France. 2011. [Google Scholar]
- 190.Lee JH, Daugharthy ER, Scheiman J, et al. Fluorescent in situ sequencing (FISSEQ) of RNA for gene expression profiling in intact cells and tissues. Nat Protoc. 2015;10:442–458. doi: 10.1038/nprot.2014.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Clarke GM, Zubovits JT, Shaikh KA, et al. A novel, automated technology for multiplex biomarker imaging and application to breast cancer. Histopathology. 2014;64:242–255. doi: 10.1111/his.12240. [DOI] [PubMed] [Google Scholar]
- 192.Durruthy-Durruthy R, Gottlieb A, Hartman BH, et al. Reconstruction of the mouse otocyst and early neuroblast lineage at single-cell resolution. Cell. 2014;157:964–978. doi: 10.1016/j.cell.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Achim K, Pettit JB, Saraiva LR, et al. High-throughput spatial mapping of single-cell RNAseq data to tissue of origin. Nat Biotechnol. 2015;33:503–509. doi: 10.1038/nbt.3209. [DOI] [PubMed] [Google Scholar]
- 194.Satija R, Farrell JA, Gennert D, et al. Spatial reconstruction of single-cell gene expression data. Nat Biotechnol. 2015:33. doi: 10.1038/nbt.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lubeck E, Cai L. Single-cell systems biology by super-resolution imaging and combinatorial labeling. Nat Methods. 2012;9:743–U159. doi: 10.1038/nmeth.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chen KH, Boettiger AN, Moffitt JR, et al. RNA imaging. Spatially resolved, highly multiplexed RNA profiling in single cells. Science. 2015:348. doi: 10.1126/science.aaa6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Stahl PL, Salmen F, Vickovic S, et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science. 2016;353:78–82. doi: 10.1126/science.aaf2403. [DOI] [PubMed] [Google Scholar]
- 198.Tetteh PW, Farin HF, Clevers H. Plasticity within stem cell hierarchies in mammalian epithelia. Trends Cell Biol. 2015;25:100–108. doi: 10.1016/j.tcb.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 199.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 200.Ritsma L, Ellenbroek SI, Zomer A, et al. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature. 2014;507:362–365. doi: 10.1038/nature12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bendall SC, Nolan GP. From single cells to deep phenotypes in cancer. Nat Biotechnol. 2012;30:639–647. doi: 10.1038/nbt.2283. [DOI] [PubMed] [Google Scholar]
- 202.Cahan P, Daley GQ. Origins and implications of pluripotent stem cell variability and heterogeneity. Nat Rev Mol Cell Biol. 2013;14:357–368. doi: 10.1038/nrm3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Huang S, Ingber DE. A non-genetic basis for cancer progression and metastasis: self-organizing attractors in cell regulatory networks. Breast Dis. 2006;26:27–54. doi: 10.3233/bd-2007-26104. [DOI] [PubMed] [Google Scholar]
- 204.Simons BD, Clevers H. Strategies for homeostatic stem cell self-renewal in adult tissues. Cell. 2011;145:851–862. doi: 10.1016/j.cell.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 205.Greulich P, Simons BD. Dynamic heterogeneity as a strategy of stem cell self-renewal. Proc Natl Acad Sci U S A. 2016;113:7509–7514. doi: 10.1073/pnas.1602779113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–284. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 207.Hara K, Nakagawa T, Enomoto H, et al. Mouse spermatogenic stem cells continually interconvert between equipotent singly isolated and syncytial states. Cell Stem Cell. 2014;14:658–672. doi: 10.1016/j.stem.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rompolas P, Greco V. Stem cell dynamics in the hair follicle niche. Semin Cell Dev Biol. 2014;25–26:34–42. doi: 10.1016/j.semcdb.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 210.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 211.Balaban NQ, Merrin J, Chait R, et al. Bacterial persistence as a phenotypic switch. Science. 2004;305:1622–1625. doi: 10.1126/science.1099390. [DOI] [PubMed] [Google Scholar]
- 212.Munsky B, Neuert G, van Oudenaarden A. Using gene expression noise to understand gene regulation. Science. 2012;336:183–187. doi: 10.1126/science.1216379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ooi HK, Ma L. Modeling heterogeneous responsiveness of intrinsic apoptosis pathway. BMC Syst Biol. 2013;7:65. doi: 10.1186/1752-0509-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.De Smet R, Marchal K. Advantages and limitations of current network inference methods. Nat Rev Microbiol. 2010;8:717–729. doi: 10.1038/nrmicro2419. [DOI] [PubMed] [Google Scholar]
- 215.Borisov NM, Markevich NI, Hoek JB, et al. Signaling through receptors and scaffolds: independent interactions reduce combinatorial complexity. Biophys J. 2005;89:951–966. doi: 10.1529/biophysj.105.060533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Schaber J, Klipp E. Model-based inference of biochemical parameters and dynamic properties of microbial signal transduction networks. Curr Opin Biotechnol. 2011;22:109–116. doi: 10.1016/j.copbio.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 217.Taylor IW, Linding R, Warde-Farley D, et al. Dynamic modularity in protein interaction networks predicts breast cancer outcome. Nat Biotechnol. 2009;27:199–204. doi: 10.1038/nbt.1522. [DOI] [PubMed] [Google Scholar]
- 218.Kirouac DC, Saez-Rodriguez J, Swantek J, et al. Creating and analyzing pathway and protein interaction compendia for modelling signal transduction networks. BMC Syst Biol. 2012;6:29. doi: 10.1186/1752-0509-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Eydgahi H, Chen WW, Muhlich JL, et al. Properties of cell death models calibrated and compared using Bayesian approaches. Mol Syst Biol. 2013;9:644. doi: 10.1038/msb.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Spencer SL, Gaudet S, Albeck JG, et al. Non-genetic origins of cell-to-cell variability in TRAIL-induced apoptosis. Nature. 2009;459:428–432. doi: 10.1038/nature08012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Fu F, Nowak MA, Bonhoeffer S. Spatial heterogeneity in drug concentrations can facilitate the emergence of resistance to cancer therapy. PLoS Comput Biol. 2015;11:e1004142. doi: 10.1371/journal.pcbi.1004142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wells DK, Chuang Y, Knapp LM, et al. Spatial and functional heterogeneities shape collective behavior of tumor-immune networks. PLoS Comput Biol. 2015;11:e1004181. doi: 10.1371/journal.pcbi.1004181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li HJ, Reinhardt F, Herschman HR, et al. Cancer-stimulated mesenchymal stem cells create a carcinoma stem cell niche via prostaglandin E2 signaling. Cancer Discov. 2012;2:840–855. doi: 10.1158/2159-8290.CD-12-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Garraway LA, Janne PA. Circumventing cancer drug resistance in the era of personalized medicine. Cancer Discov. 2012;2:214–226. doi: 10.1158/2159-8290.CD-12-0012. [DOI] [PubMed] [Google Scholar]
- 225.Zhao B, Pritchard JR, Lauffenburger DA, et al. Addressing Genetic Tumor Heterogeneity through Computationally Predictive Combination Therapy. Cancer Discov. 2014;4:166–174. doi: 10.1158/2159-8290.CD-13-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Pritchard JR, Bruno PM, Gilbert LA, et al. Defining principles of combination drug mechanisms of action. Proc Natl Acad Sci U S A. 2013;110:E170–179. doi: 10.1073/pnas.1210419110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Dawson JC, Carragher NO. Quantitative phenotypic and pathway profiling guides rational drug combination strategies. Front Pharmacol. 2014;5:118. doi: 10.3389/fphar.2014.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Inda MM, Bonavia R, Mukasa A, et al. Tumor heterogeneity is an active process maintained by a mutant EGFR-induced cytokine circuit in glioblastoma. Genes Dev. 2010;24:1731–1745. doi: 10.1101/gad.1890510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Eirew P, Steif A, Khattra J, et al. Dynamics of genomic clones in breast cancer patient xenografts at single-cell resolution. Nature. 2015;518:422–426. doi: 10.1038/nature13952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.de Smith M. [Accessed September 17, 2016];Statistical Analysis Handbook. http://www.statsref.com/HTML/index.html.
- 231.NIST/SEMATECH e-Handbook of Statistical Methods. [Accessed September 17, 2016]; http://www.itl.nist.gov/div898/handbook/eda/section3/eda35b.htm.
- 232.Almendro V, Kim HJ, Cheng YK, et al. Genetic and phenotypic diversity in breast tumor metastases. Cancer Res. 2014;74:1338–1348. doi: 10.1158/0008-5472.CAN-13-2357-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Rose CJ, Mills SJ, O’Connor JP, et al. Quantifying spatial heterogeneity in dynamic contrast-enhanced MRI parameter maps. Magn Reson Med. 2009;62:488–499. doi: 10.1002/mrm.22003. [DOI] [PubMed] [Google Scholar]
- 234.Schwarz RF, Ng CK, Cooke SL, et al. Spatial and temporal heterogeneity in high-grade serous ovarian cancer: a phylogenetic analysis. PLoS Med. 2015;12:e1001789. doi: 10.1371/journal.pmed.1001789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Saadatpour A, Guo G, Orkin SH, et al. Characterizing heterogeneity in leukemic cells using single-cell gene expression analysis. Genome Biol. 2014;15:525. doi: 10.1186/s13059-014-0525-9. [DOI] [PMC free article] [PubMed] [Google Scholar]