Abstract
Theiler’s murine encephalomyelitis virus (TMEV) infection has been used as a viral model for multiple sclerosis (MS), as TMEV can induce chronic inflammatory demyelinating lesions with viral persistence in the spinal cord of SJL/J mice. In contrast, when C57BL/6 mice are infected with TMEV, the mice can clear the virus from the central nervous system (CNS), without viral persistence or demyelination, but develop seizures and hippocampal sclerosis, which has been used as a viral model for seizures/epilepsy. In the two TMEV-induced CNS disease models, not only viral infection, but also immune responses contribute to the pathogenesis. Interestingly, acquired immunity plays an effector role in the MS model, whereas innate immunity appears to contribute to the development of seizures. Recently, we have established the third TMEV-induced disease model, a mouse model for viral myocarditis, using C3H mice. TMEV-induced myocarditis is a triphasic disease, which mimics human myocarditis; phase I, mediated by viral replication in the heart and innate immunity; phase II, mediated by acquired immunity; and phase III, resulted from cardiac fibrosis. The genetic susceptibility to the aforementioned three models (MS, seizures and myocarditis) differs among mouse strains. We have compared and contrasted the three models induced by one single pathogen, TMEV, particularly in regard to the roles of T helper cells and natural killer T cells, which will give an insight into how interactions between the immune system and the host’s genetic background determine the tissue tropism of virus and the development of virus-induced organ-specific immunopathology.
Keywords: animal models, central nervous system demyelinating diseases, experimental autoimmune encephalomyelitis, Picornaviridae infections, Th17 cells
Introduction
Theiler’s murine encephalomyelitis virus
Theiler’s murine encephalomyelitis virus (TMEV) infection in mice has been used as a viral model for multiple sclerosis (MS).1–4 More recently, TMEV infection has also been used as a mouse model for seizures/epilepsy5 and myocarditis.6,7 While immune responses and genetic background of hosts have been shown to play key roles in all three models, the immune effector cells/molecules and susceptible mouse strain in each model differ among the models. In the present review, we will compare and contrast the three distinct disease models induced by one single pathogen, TMEV, which will give an insight into how the interactions between the immune system and the host’s genetic background determine the tissue tropism of TMEV, as well as the development of virus-induced, organ-specific, immune-mediated diseases. We also discuss the prevalence, viral involvement, and comorbidities of MS, seizures and myocarditis.
Virology of TMEV
TMEV is a positive-sense, single-stranded (ss) RNA virus that belongs to the genus Cardiovirus, family Picornaviridae, order Picornavirales (Table 1).8 The TMEV virion contains a capsid that is composed of four structural proteins: VP1, VP2 and VP3 form the shell, whereas VP4 lies on its inner surface.9 TMEV binds to a cell surface receptor (sialic acid is used, at least, in vitro), while the plausible receptor binding site is a depression around the fivefold axis of the virion called the pit,10 leading to virion uncoating11 and viral RNA genome delivery into the cytoplasm. The viral RNA genome, possessing a single long open reading frame, an internal ribosome entry site and a poly(A) tail, is infectious and has three roles: mRNA, template during replication and genetic material in the virion. The RNA genome is initially translated into a single protein, polyprotein, using an internal ribosome entry site.12 Then, polyprotein is processed proteolytically to form functional multiple viral proteins; that is, four capsid proteins and non-structural proteins. Newly synthesized virions are released from the cell by lysis (lytic infection).
Table 1.
Theiler’s murine encephalomyelitis virus
| Classification |
Theilovirus, the genus Cardiovirus, family Picornaviridae, order Picornavirales |
| Two subgroups: neurovirulent GDVII (GDVII, FA), demyelinating TO (BeAn, DA) |
|
| Genome (8.1 kb) |
Positive-sense ssRNA with IRES and poly(A) One ORF encoding non-structural proteins and four capsid proteins VP1-4 |
| Replication (8 h) |
Receptor binding → uncoating → polyprotein translation and processing → virion assembly → release by cell lysis |
GDVII, George’s disease 7; IRES, internal ribosome entry site; ORF, open reading frame; ssRNA, single-stranded RNA; TO, Theiler’s original.
TMEV is divided into two subgroups, George’s disease 7 (GDVII) and Theiler’s original (TO) based on different neurovirulence after intracerebral infection.9 Highly neurovirulent GDVII and FA strains belong to the GDVII subgroup, which causes acute fatal polioencephalomyelitis with high levels of viral replication in neurons,13 neuronal apoptosis14 and axonal degeneration15 in the central nervous system (CNS), with neither induction of antiviral immune responses nor demyelination; all infected mice die within 1 week postinfection (p.i.). The TO subgroup viral strains, including less neurovirulent BeAn 8386 (BeAn), Daniels (DA), TO and WW, can induce a biphasic CNS disease.16 During the acute phase, similar to GDVII virus, the TO subgroup viruses infect neurons and cause polioencephalitis. Although inflammation is severe in the gray matter of the brain with limited spinal cord involvement, infected mice usually have no obvious clinical signs and all mice survive. Then, during the chronic phase, the mice develop spastic paralysis clinically and inflammatory demyelination histologically with viral persistence in the white matter of the spinal cord.17 The susceptibility to the chronic demyelinating disease by the TO subgroup viruses depends on major histocompatibility complex (MHC) class I and is induced only in susceptible mouse strains, such as SJL/J mice, but not in resistant mouse strains, such as C57BL/6 (B6) or BALB/c mice, whereas acute neuronal infection in the gray matter of the brain (polioencephalitis) is caused by both GDVII and TO subgroup viruses in any mouse strains.18
TMEV-induced demyelinating disease
Roles of T helper cell subsets in viral infections and autoimmunity
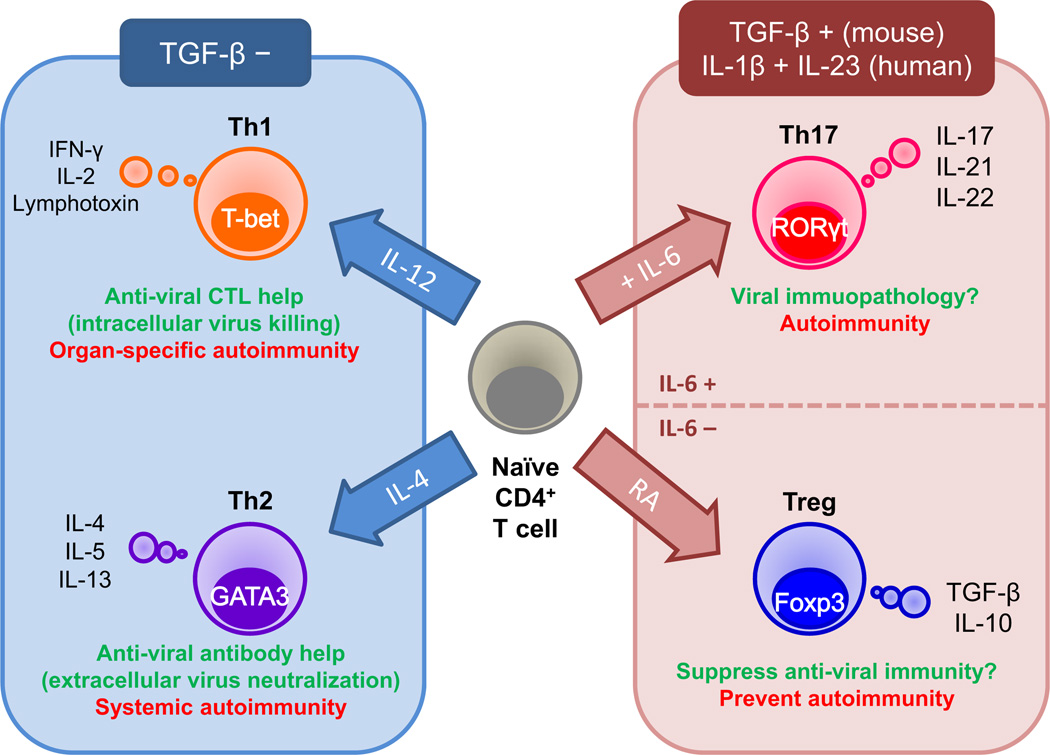
MS is an inflammatory demyelinating disease of the CNS,19,20 affecting more than 2 million people worldwide; the prevalence of MS in North America and Europe is 0.1–0.2% of the population.21 Although the precise etiology of MS is unknown, MS is believed to be an immune-mediated disease,22 occurring in genetically susceptible individuals,23 precipitated by one or more environmental agents,24 particularly viruses, including Epstein-Barr virus (human herpesvirus [HHV]-4),25 HHV-6, measles virus and retroviruses.21,26–28 CD4+ T helper (Th) cells have been proposed to play a key role in MS pathogenesis.29,30 CD4+ T cells can be divided into four subsets, Th1, Th2, Th17 and regulatory T cells (Tregs), based on their cytokine production: Th1 cells, interferon (IFN)-γ and interleukin (IL)-2; Th2 cells, IL-4, IL-5 and IL-13; Th17 cells, IL-17, IL-21 and IL-22; and Tregs, transforming growth factor (TGF)-β and IL-10 (Fig. 1).31–33 Specific transcription factor activation mediated by the cytokine milieu plays a central role in the differentiation toward distinct Th cell subsets: Th1 cells, T-box expressed in T cells (T-bet);34 Th2 cells, GATA-binding protein 3 (GATA3);35 Th17 cells, retinoic acid receptor-related orphan receptor-γt (RORγt);36 and Tregs, forkhead box P3 (Foxp3).
Figure 1.
Roles of T helper (Th) cell subsets in viral infections and autoimmunity. Th cell subset differentiation is influenced by the cytokine milieu and master transcription factor. Th1 cells are induced in the presence of interleukin (IL)-12 and the transcription factor, T-box expressed in T cells (T-bet). Th1 cells help induction of antiviral cytotoxic T lymphocytes (CTL) and activation of macrophages that eradicate intracellular virus, while Th1 cells can also be a pathogenic effector in organ-specific autoimmunity. Th2 cells are induced by IL-4 and the transcription factor, GATA-binding protein 3 (GATA3). Th2 cells help production of antiviral antibody that neutralizes extracellular virus, but can be detrimental to promote autoantibody production in systemic autoimmunity. Although Th1 and Th2 differentiation is inhibited by transforming growth factor (TGF)-β, both Th17 cells and regulatory T cells (Tregs) are induced by TGF-β. In addition, Th17 cell differentiation requires IL-6, whereas Treg differentiation requires retinoic acid (RA). Th17 cells express the transcription factor, retinoic acid receptor-related orphan receptor-γt (RORγt), secrete IL-17, 21 and 22, and might be involved in immunopathology in viral infections and autoimmunity. Tregs express the transcription factor, forkhead box P3 (Foxp3), and secrete the anti-inflammatory cytokines, TGF-β and IL-10, which can play a detrimental or protective role by suppressing antiviral immunity or autoimmunity, respectively.
In viral infections, Th1 cells help antiviral cellular immune responses by activation of macrophages and generation of CD8+ cytotoxic T lymphocytes (CTL), whereas Th2 cells help production of antiviral antibodies. The roles of Th17 cells and Tregs for viral infections are largely unknown.37 In autoimmune diseases, including an autoimmune model for MS, experimental autoimmune encephalomyelitis (EAE), Th1 and Th17 cells have been associated with organ-specific and cellular autoimmunities,38,39 whereas Th2 cells have been associated with systemic and humoral autoimmunities.40 Tregs can suppress such autoreactive Th1 and Th17 immune responses.41 Th1 cells have been shown to suppress Th2 and Th17 cells, and vice versa.42
TMEV-induced demyelinating disease, a viral model for MS
TMEV-induced demyelinating disease (TMEV-IDD) has been most widely used as a viral model for MS.43,44 Historically, although TMEV was first isolated and characterized by Max Theiler early in January 1933,45,46 TMEV-IDD was discovered by Daniels et al.47 in 1952, and started to be used as a viral model for MS after Howard L. Lipton48 awakened interest in TMEV-IDD in 1975. Although the precise pathomechanism is unclear, both viral persistence and immune responses play pathogenic roles in TMEV-IDD. Unlike EAE, a pure autoimmune model for MS, adoptive transfer of immune cells from TMEV-infected mice into naïve mice does not induce demyelination; immune cell alone cannot induce TMEV-IDD.49
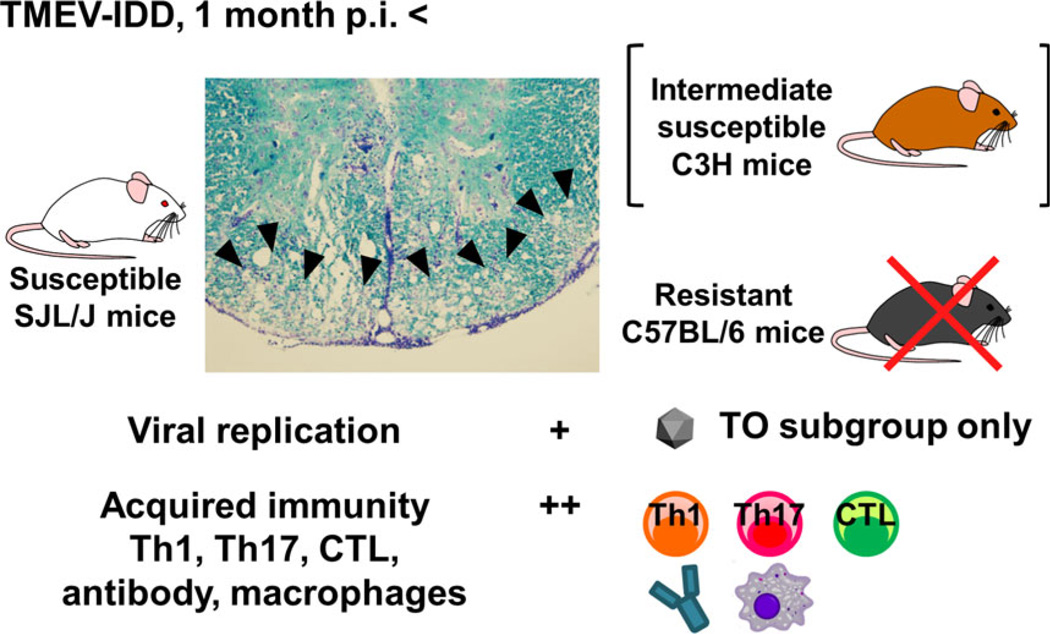
Intracerebral, but not peripheral, injection of TMEV can result in a biphasic disease in the CNS, depending on mouse strains. During the acute phase, 1 week p.i., the virus predominantly infects neurons in the gray matter of the brain, and induces polioencephalitis with neuronal apoptosis14 and axonal damage.15 Antiviral Th1, CTL and antibody responses contribute to viral clearance from the brain; here, resistant mouse strains, such as B6 and BALB/c mice, can eradicate the virus completely. In contrast, some mouse strains, including susceptible SJL/J mice and intermediate susceptible C3H mice, cannot clear the virus from the CNS;13,18 during the subclinical phase, 2–3 weeks p.i., although the virus is largely cleared from the brain, the virus is axonally transported to the white matter of the spinal cord,50,51 where axonal degeneration and microglia activation increase over time.52 Infected mice start to show obvious paralysis approximately 1 month p.i. during the chronic phase, when inflammatory demyelination is histologically present in the white matter of the spinal cord (Fig. 2); it should be noted that, in TMEV-IDD, axonal degeneration precedes demyelination (“Inside-Out model”).12,53 During the chronic phase, persistent viral infection can be detected in macrophages and glial cells, including oligodendrocytes,13,54,55 whereas TMEV-specific Th1 cells and CTL have been proposed to play pathogenic roles in demyelination.56,57 Anti-TMEV antibodies can cross-react with myelin antigens, further contributing to demyelination.58 Thereafter, the disease progresses continuously with no remission.
Figure 2.
Theiler’s murine encephalomyelitis virus-induced demyelinating disease (TMEV-IDD), a viral model for multiple sclerosis. During the chronic phase of TMEV infection, 1 month postinfection (p.i.), susceptible SJL/J mice develop severe inflammatory demyelination (arrowheads; Luxol fast blue stain) in the spinal cord, whereas no lesion is observed in the spinal cord of resistant C57BL/6 mice. As C3H mice develop mild demyelination in the spinal cord, C3H mice are intermediately susceptible to TMEV-IDD. The Theiler’s original (TO), but not GDVII, subgroup of TMEV can cause TMEV-IDD. Both viral pathology and immunopathology play key roles in TMEV-IDD. During the chronic phase, TMEV persistently infects glial cells and macrophages in the spinal cord at low levels; viral persistence is necessary for the pathogenesis of TMEV-IDD. Acquired immune responses, including Th1 and Th17 cells, CTL, antibodies and macrophages, also play a pathogenic role in TMEV-IDD.
Roles of Th17 cells and Tregs in TMEV-IDD
In EAE, Th17 cells can induce immune-mediated tissue damage (immunopathology) by production of pro-inflammatory cytokines,1,59–61 whereas Tregs can play a protective role by suppressing immunopathology; for example, by production of anti-inflammatory cytokines, such as IL-10 and TGF-β.62 Here, the roles of Th17 cells and Tregs had mainly been investigated in EAE, whose Th17/Treg responses were decreased by blocking antibodies, small interfering RNA or gene knockout (KO) mice (“loss-of-function” approach).38 This raised questions as to: (i) whether the Th17/Treg responses play the same role in TMEV-IDD as in EAE, as the roles of Th17 cells and Tregs in viral infections are largely unclear; and (ii) whether an increase in Th17/Treg responses affects inflammatory demyelinating diseases; this is a clinically relevant concern, as “gain-of-function” mutations have been shown to alter immune responses, including Th cells, and to increase the susceptibility to several inflammatory diseases and infections in humans.63,64
We determined the roles of Th17 cells and Tregs in TMEV-IDD.65,66 To clarify how increased Th17/Treg immune responses can influence TMEV-IDD, where both viral pathology (viral replication) and immunopathology play key roles, we have established two innovative “gain-of-function” approaches: (i) RORγt transgenic (Tg) B6 mice overexpressing RORγt, which have increased Th17 cells;66,67 and (ii) adoptive transfer of ex vivo expanded induced Tregs (iTregs).65,68,69
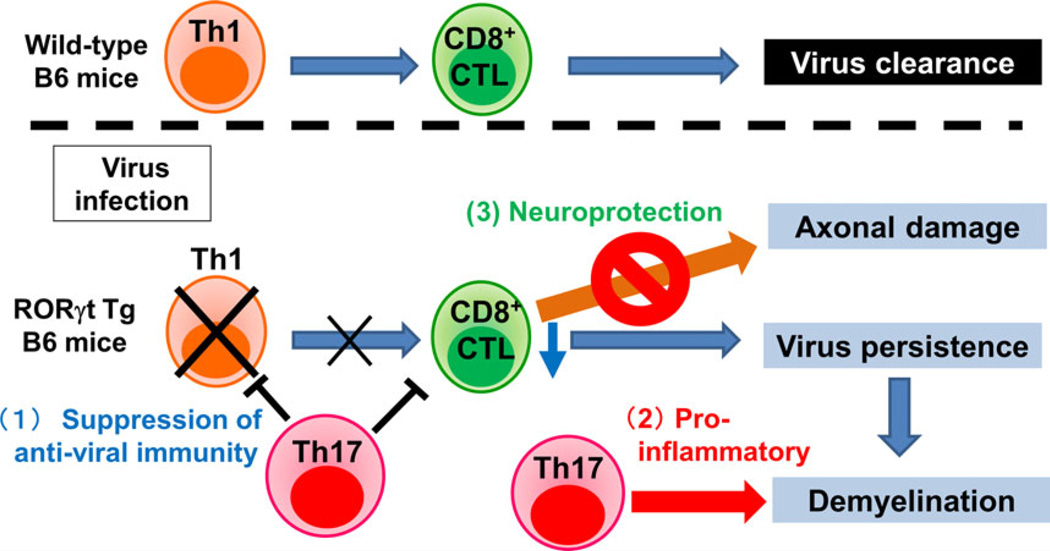
Although TMEV-infected wild-type B6 littermates, which are known to be resistant to TMEV-IDD,70 do not develop chronic demyelination in the CNS, TMEV-infected RORγt Tg B6 mice develop severe demyelinating lesions (with relative preservation of axons [see below]) and have viral persistence, higher levels of IL-17, lower levels of IFN-γ and fewer CD8+ T cells.66 Here, we have proposed three roles of Th17 cells in TMEV-IDD (Fig. 3). First, Th17 cells could impair antiviral CTL, promoting viral replication and persistence, which is consistent with a theory proposed by Hou et al.71 that IL-17 could inhibit antiviral CTL functions and might be responsible for susceptibility to TMEV-IDD. Second, increased Th17 cells in RORγt Tg B6 mice could play a role as a pro-inflammatory effector cells, contributing to inflammatory demyelination (immunopathology). Third, Th17 cells might impair anti-axonal CTL, resulting in suppression of axonal damage (neuroprotective role). In TMEV infection, although CTL are important for viral clearance, CTL have also been proposed to induce axonal damage as effector cells.72 Interestingly, although we observed severe inflammatory demyelinating lesions in the spinal cord of TMEV-infected RORγt Tg B6 mice comparable with those of infected SJL/J mice, RORγt Tg B6 mice did not develop paralysis clinically and had only minimum axonal damage histologically. Thus, viral persistence with relative preservation of axons in RORγt Tg B6 mice could be a result of inhibition of effector CTL functions by Th17 cells.
Figure 3.
Three roles of Th17 cells in TMEV infection. (Upper) Wild-type C57BL/6 (B6) mice mount an antiviral Th1 response, which helps the generation of CD8+ CTL, leading to viral clearance. (Lower) In RORγt transgenic (Tg) B6 mice, increased Th17 cells (1) suppress antiviral Th1 and CTL responses, resulting in viral persistence; and (2) secrete pro-inflammatory cytokines, causing inflammatory demyelination (detrimental role of Th17 cells). In contrast, Th17 cells can (3) suppress anti-axonal (autoreactive) CD8+ CTL, preventing axonal damage (neuroprotective role of Th17 cells).
iTregs can be expanded by our method to generate approximately 20 million iTregs from the spleen of one naive mouse.68,69 These iTregs express CD4, Foxp3 and CD25, and can suppress the proliferation of other CD4+ T cells in vitro as well as suppress inflammation in a mouse model of inflammatory bowel disease in B6 mice in vivo. We determined the role of iTregs during the time-course of TMEV infection by administering iTregs to TMEV-infected SJL/J mice either at day 0 p.i. (iTreg-early mice) or day 30 p.i. (iTreg-late mice).65 During the acute phase, iTreg-early mice showed more severe clinical signs compared with control mice (TMEV infection alone without iTreg injection): iTreg-early mice lost more weight with an impaired righting reflex, and had higher viral replication in the CNS than control mice. As less inflammation was observed in the CNS of the iTreg-early mice compared with control mice, this suggests that Tregs prevent antiviral immune cells from homing to the CNS, resulting in more active viral replication in iTreg-early mice. In contrast, during the chronic phase, less inflammatory demyelination with higher production of IL-10 was observed in iTreg-late mice, compared with controls, suggesting a protective role of Tregs. In summary, these results showed that: (i) Th17 cells could play a detrimental role in TMEV-IDD, to induce immunopathology with viral persistence, as well as a beneficial role to protect axonal degeneration; and (ii) Tregs could also play a detrimental role to suppress antiviral immune responses, favoring viral replication, as well as a beneficial role to inhibit immunopathology.
TMEV-induced myocarditis
Viral myocarditis
Myocarditis is inflammation of the heart muscle.73,74 The discovery of myocarditis in 1–9% of autopsies showed that myocarditis is an underdiagnosed cause of sudden death.73–77 For example, studies of military recruits found that 20–42% of sudden deaths were attributable to unsuspected myocarditis.78,79 Myocarditis most commonly results from infections with viruses (38–80%), including adenovirus, HHV-6, parvovirus B19, hepatitis C virus and picornaviruses (coxsackievirus B [CVB] and enterovirus).80–83
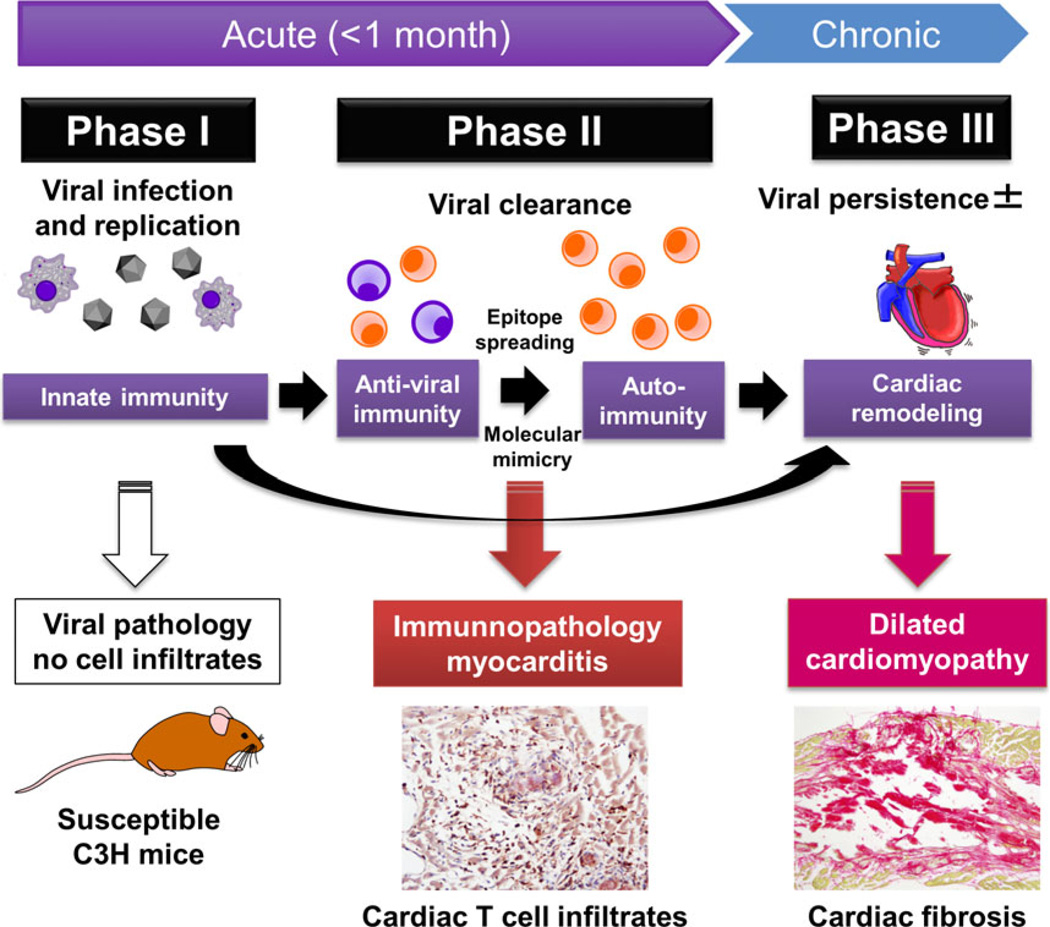
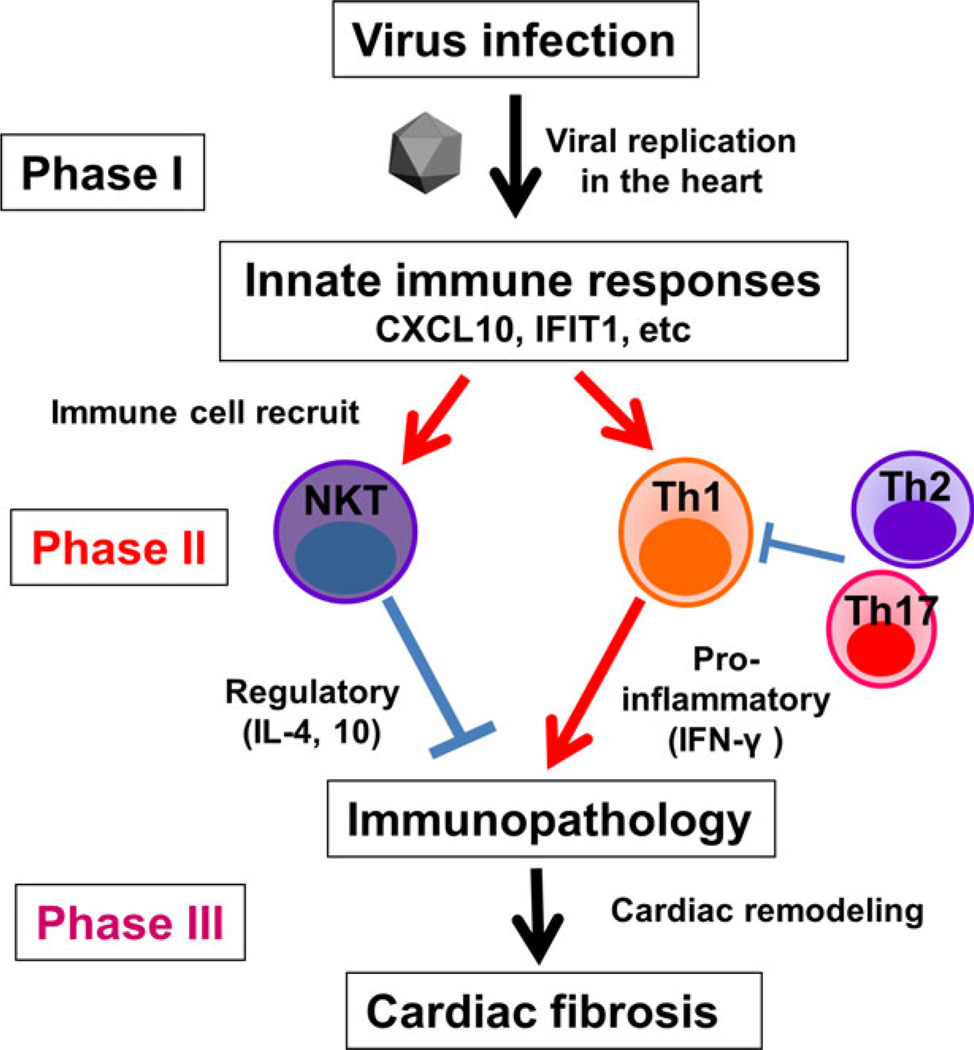
Viral myocarditis has been proposed to be a triphasic disease:37,75,77,81,84 in phase I, the disease is triggered by viral infection in the heart (viral pathology); in phase II, cardiomyocytes are damaged by uncontrolled antiviral immune and/or autoimmune responses that can be generated by molecular mimicry and determinant (or epitope) spreading (immunopathology);40 in phase III, as a result of phases I and II, cardiac fibrosis and remodeling lead to dilated cardiomyopathy (Fig. 4).85 Depending on pathogens and the host’s genetic background, pathophysiology of viral myocarditis might be different. Some viral infections only cause viral pathology (phase I) with no involvement of acquired immunity (e.g. only phase I in human parvovirus infections and a murine reovirus model, where no immune cell infiltrates in the heart).86
Figure 4.
Possible pathomechanisms in the three phases of viral myocarditis. In phase I, viral infection and replication in the heart cause myocardial damage directly (viral pathology). Innate immune responses to the virus play a key role in viral clearance, but might also damage cardiomyocytes. There are no immune cell infiltrates in the heart in phase I. In phase II, antiviral acquired immune responses, including Th1 cell (orange) and Th2 cells (purple), are induced to clear the virus. When antiviral immune responses are uncontrolled, T cells infiltrate in the heart (visualized by immunohistochemistry using anti-CD3 antibody), leading to myocardial damage in a bystander fashion or by generation of heart-specific autoimmunity through determinant (or epitope) spreading from viral antigens to cardiac antigens (immunopathology). In addition, if viral and cardiac antigens share immune epitopes, the “molecular mimicry” also results in immunopathology. When the myocardial damage is severe either in phase I or II, cardiac remodeling and fibrosis (visualized by picrosirius red staining) occur, resulting in dilated cardiomyopathy, regardless of the presence or absence of viral persistence in phase III.
In human viral myocarditis, the roles of Th subsets remain unclear, partly because only small numbers of immunological studies have been carried out in humans. Endomyocardial biopsies from patients with acute myocarditis and dilated cardiomyopathy showed the presence of several different cardiotropic viruses, and increased markers of Th1 cells, without changes in Th2 and Th17 markers.87 In the peripheral blood from viral myocarditis, high levels of Th1 cells were associated with early cardiomyocyte damage, whereas Th2 cells increased during the recovery phase.88 Although these findings need to be confirmed by analyses of larger cases, they are consistent with what has been observed in other immune-mediated diseases, where Th1 cells are proinflammatory, and Th2 cells are anti-inflammatory (Table 2).
Table 2.
Possible roles of T helper cell subsets and natural killer T cells in myocarditis
| Th1 | Th2 | Th17 | NKT | |
|---|---|---|---|---|
| Cytokines | IFN-γ | IL-4, IL-10 | IL-17 | IL-4, IFN-γ |
| Human cases | Cardiac damage | Recovery | No role | ? |
| CVB model | Protective | Inflammation | Inflammation | No role or Anti-viral |
| TMEV model | Detrimental | Protective | No role | Protective |
| Mutant mice† | T-bet Tg | GATA3 Tg | RORγt Tg | Jα18 KO |
CVB, coxsackievirus B; IFN, interferon; IL, interleukin; TMEV, Theiler’s murine encephalomyelitis virus.
Transgenic (Tg) and knockout (KO) mice used for myocarditis research. Protective role in italics. Detrimental role with bold. T-box expressed in T cells (T-bet) Tg, GATA-binding protein 3 (GATA3) Tg and retinoic acid receptor-related orphan receptor-γt (RORγt) Tg mice have increased numbers of T helper (Th)1, Th2, and Th17 cells, respectively, whereas Jα18KO mice are deficient in natural killer T (NKT) cells.
TMEV-induced myocarditis, a novel viral myocarditis model
During the acute phase, TMEV has been observed to cause viremia and infect various organs, including the skeletal muscle and the heart.89 Historically, although Gómez et al.7,90 described TMEV-induced cardiac pathology in 1976, TMEV was not used for myocarditis research until we used it in a mouse model for viral myocarditis. We showed that, depending on mouse strains, TMEV injection can lead to myocarditis; a high-dose intraperitoneal TMEV injection is the most efficient and consistent route for myocarditis induction, whereas an intracerebral injection of TMEV also induces myocarditis.6 The susceptibility to and progression of TMEV-induced myocarditis differ among mouse strains; C3H mice developed all three phases, whereas B6 mice had only mild cell infiltrates (phase II) without cardiac fibrosis. No SJL/J mice had immune cell infiltrates in the heart histologically; SJL/J mice developed only phase I cardiac pathology.
Using C3H mice, we have determined viral replication, cardiac damage (quantified by serum cardiac troponin), immunopathology and biomarkers in each phase of myocarditis, with multivariate data, such as virological and histological data, as well as a principal component analysis of microarray transcriptome data of the infected hearts. In phase I, although the heart appeared normal, we found active viral replication and upregulation of innate immune molecules, such as IFN-induced genes and chemokines, including IP-10/CXCL10 (IFN-γ-induced protein 10/C-X-C motif chemokine ligand 10). In phase II, the viral titer in the heart was lower than in phase I, whereas cardiomyocyte damage quantified by serum cardiac troponin was greater than in phase I. We found CD3+ T-cell infiltrates in the heart by immunohistochemistry, as well as upregulation of genes associated with CD3, pro-inflammatory IFN-γ pathway and MHC class II by microarray, suggesting involvement of MHC class II-restricted Th1 cells. In phase III, although we found only a small number of T cells with no viral replication in the heart, progressive cardiac fibrosis was observed by picrosirius red staining and Masson’s trichrome staining, which was consistent with upregulation of cardiac remodeling genes, such as matrix metallopeptidase 12.
We have characterized the role of immune cells, using T-bet, GATA3, or RORγt Tg mice, and NKT cell-deficient Jα18 KO mice.91 Among the Tg and KO mice infected with TMEV, Th1-biased T-bet Tg mice and NKT KO mice developed more severe myocarditis with lower ejection fraction in echocardiography than wild-type mice, whereas Th2-biased GATA3 Tg mice were resistant to TMEV-induced myocarditis.91 In summary, our studies suggest a pathogenic role of pro-inflammatory Th1 cells, and a protective role of Th2 cells and NKT cells, which were consistent with the findings in human viral myocarditis cases (Table 2). The working hypothesis of TMEV-induced myocarditis is as follows (Fig. 5): in phase I, viral infection and replication in the heart induce innate immune molecules, IFN and chemokines (e.g. IP-10/CXCL10), which recruit Th cell subsets and NKT cells.92 The balance between pro-inflammatory versus regulatory cytokines determines the extent of immune-mediated tissue damage (immunopathology) in phase II. Severe immunopathology can lead to cardiac fibrosis and remodeling in phase III.
Figure 5.
The working hypothesis of the mechanism of viral myocarditis. In phase I, viral infection and replication induce innate immune responses, upregulating chemokine and IFN-related genes (e.g. IFIT1) in the heart. In phase II, chemokines (e.g. CXCL10) recruit Th1 and natural killer T (NKT) cells to the heart. Th1 cells secrete pro-inflammatory IFN-γ, resluting in cardiac inflammation. In contrast, NKT cells produce IL-4 and IL-10, suppressing inflammation, while Th2 and Th17 cells might also suppress Th1 cells. The imbalance between pro-inflammatory versus anti-inflammatory immune responses causes immunopathology, followed by cardiac remodeling and fibrosis in phase III.
Unlike TMEV-induced myocarditis model, other current viral and autoimmune myocarditis models have not consistently reproduced the findings in human viral myocarditis. For example, in the CVB model and some other models, Th1 cells seemed to play a protective role, whereas Th2 and Th17 cells play a pathogenic role.93,94 This suggests that some of these models mimic human acute fulminant fatal myocarditis (animals succumb to acute myocarditis; thus, these models are inappropriate to study phase III), where viral pathology is the major cause of cardiac damage, whereas inflammation has beneficial effects to complete viral clearance.77,95
Among other immune cells, NKT cells are known as major effector cells that can produce both IFN-γ and IL-4.96 In other immune-mediated diseases, NKT cells can play a protective role in Th1/Th17-mediated diseases by production of IL-4.97 In CVB infection, however, the findings are not consistent: both wild-type and NKT KO mice developed severe myocarditis, suggesting that NKT cells might play no significant role,98 whereas a potential antiviral role has been suggested by others.99 The inconsistency in immunopathology between human viral myocarditis and current animal model systems can be due to several factors:85,100 (i) some viruses are used in their non-natural hosts (e.g. the natural host of CVB is humans, not mice, whereas TMEV is a natural pathogen of mice);101 (ii) the pathophysiology of human viral myocarditis is heterogeneous – one model cannot represent all cases (e.g. the reovirus model develops only phase I,102 whereas other models represent fulminant fatal myocarditis); (iii) some viral models are only inducible in neonatal animals; and (iv) an autoimmune model, experimental autoimmune myocarditis does not have microbial components.
TMEV-induced seizures/epilepsy
Seizures and epilepsy
A seizure is a clinical sign due to transient dysfunction of the brain, which is caused by an abnormal electrical discharge of neurons, while epilepsy is a chronic disorder with recurrence of seizures.103 Epidemiological studies of epilepsy are interested in active epileptic patients who have had at least one seizure during the past 5 years or are treated with anti-epileptic drugs. The prevalence of active epilepsy per 1000 population ranges from 6.2 to 7.6 in Europe and North America. Globally, a total of 43 million people have active epilepsy: 10 million children, 30 million adults and 3 million older adults.104
The three main pathologies from epilepsy surgery, 70–80% of which are for the treatment of temporal lobe epilepsy, are hippocampal sclerosis, cortical dysplasia and low-grade tumors. In hippocampal sclerosis (Ammon’s horn sclerosis), pyramidal neuronal loss and gliosis are identified mainly in the CA1 subfield and in the hiller region with sparing of the subiculum, and relative resistance of CA2 pyramidal neurons and dentate granule cells105 (the hippocampus proper has three subdivisions: CA3, CA2 and CA1; CA comes from cornu ammonis106).
Most cases of epilepsy result from interactions between genetic and non-genetic factors;107 risk factors for the development of seizures/epilepsy, including head trauma, brain tumors, CNS infection and inflammation, and cerebrovascular disease, vary with age, geographic location and other conditions.108 Clinically, several different viruses have been suggested to play a role in the development of seizures and epilepsy; HHV-6, herpes simplex virus type 1 (HSV-1 = HHV-1), Japanese encephalitis virus, Nipah virus and human immunodeficiency virus, as well as viruses in the family Picornaviridae, such as coxsackievirus, enterovirus and parechovirus, have been linked to seizures and epilepsy.5 Experimentally, although viral infections have been used to induce seizures in animals, some of them have not been used as an ideal animal model to study the roles of immune responses during and after infections, as infected animals in these models often died of acute encephalitis. In contrast, TMEV-induced seizures have been used as a unique animal model for seizures, as (i) all mice infected with the DA strain of TMEV survive after infection and (ii) host animals are inbred B6 mice, which have many advantages, such as a well-characterized immune system, and availabilities of KO and Tg mice, as well as immunomodulatory reagents.
TMEV-induced seizures, a viral model for seizures
Historically, TMEV-induced seizures were first observed inadvertently109 during experiments that aimed to see a role of axonal degeneration in TMEV infection, using C57BL/Wlds (C57/OlaHsd-Wld, Wallerian degeneration slow mutant [Wld]) mice.70 Wld mice are a substrain of B6 mice, and have prolonged survival of the distal stumps of transected axons. Because TMEV can use axons to disseminate in the CNS, slow axonal degeneration in Wld mice favors viral spread, compared with wild-type B6 mice, suggesting that axonal degeneration in B6 mice could be a beneficial mechanism that limits the viral spread.12,50 Unexpectedly, both Wld and B6 mice infected with TMEV developed seizures, 3–6 days p.i. TMEV-induced seizures in mice with a B6 genetic background were not recognized until the present study, as the B6 mice strain is resistant to TMEV-IDD, thus, not usually used in TMEV research.
In the aforementioned initial set of experiments,109 we found that, in TMEV-induced seizures: (i) both the GDVII and DA strains of TMEV induce seizures – the GDVII strain does not induce a TMEV-specific immune response,13 thus, acquired immunity has no role; (ii) unlike B6, other mouse strains resistant to TMEV-IDD, such as BALB/c,98 did not develop seizures; (iii) the incidence of seizures is associated with hippocampal CA1 lesions, such as neuronal loss and apoptosis;14 and (iv) axonal degeneration has no role, as both Wld and wild-type B6 mice develop seizures with similar severity and incidence.
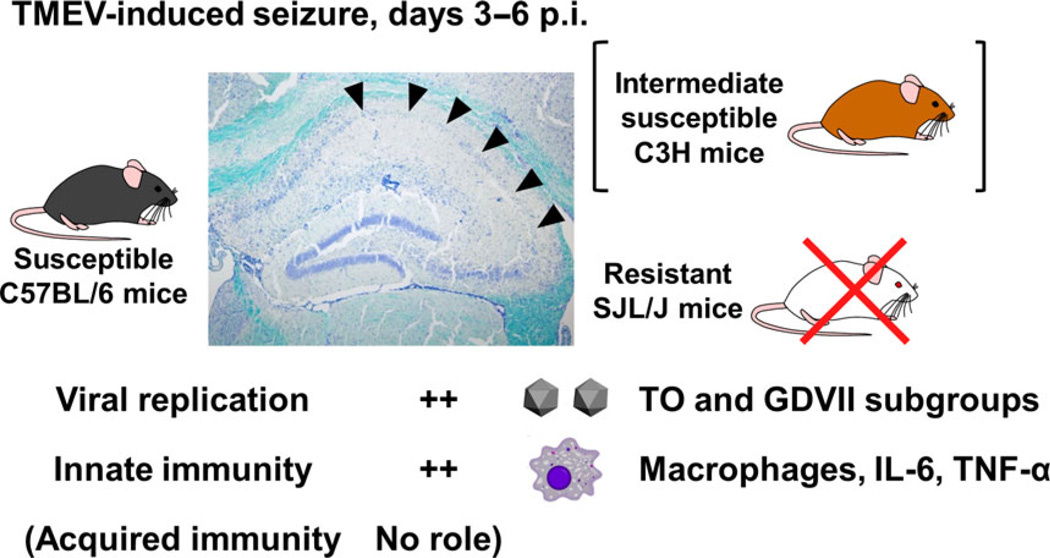
Subsequent studies have further characterized TMEV-induced seizures.5 Seizures were observed as early as on day 3, and peaked on day 6 after infection with any TMEV strains examined, including the GDVII strain, less neurovirulent TO subgroup viral strains (DA, BeAn, WW) and mutant strains (H10189 and DApBL2M110,111). The seizures lasted for 1– 2 min with a Rancine scale seizure score 3 (forelimb clonus), 4 (rearing) or 5 (rearing and falling).6,112,113 During the chronic phase, 2–7 months p.i., TMEV-infected B6 mice developed spontaneous epileptic seizures, monitored by video electroencephalogram, with hippocampal atrophy histologically.114 Innate immune responses, but not acquired immune responses, seem to contribute to seizures,115 as the number of infiltrating macrophages, but not lymphocytes, were associated with seizures (Fig. 6).116 Among molecules in the innate immune system, IL-6,117 tumor necrosis factor-α and complement component 3118 have been suggested to contribute to the development of seizures, but other factors, including polymorphonuclear cells, natural killer cells,117 NKT cells,91 IL-1, MyD88115 or toll-like receptor 4,6 were found to not contribute to the development of seizures in B6 mice.
Figure 6.
TMEV-induced seizures. During the early acute phase of TMEV infection, 3–6 days p.i., susceptible B6 mice develop seizures with severe neuronal loss in the pyramidal cell layer of the hippocampus (arrowheads), whereas no resistant SJL/J mice develop seizures. A small percentage of infected C3H mice also develop less severe seizures, compared with B6 mice; C3H mice are intermediately susceptible. High levels of viral replication in the brain have been detected in the brain of seized mice infected with both TO and GDVII subgroup viruses of TMEV. Innate immune responses, including macrophages, IL-6 and tumor necrosis factor-α, have been proposed to contribute to the development of TMEV-induced seizures, while acquired immunity has no role.
While the TMEV-induced seizure model is a rare viral model for seizures/epilepsy, further studies are required to clarify the pathomechanism. For example, although NBQX, a glutamate receptor antagonist, has been shown to suppress seizures in other animal models, it exacerbated TMEV-induced seizures.119 Furthermore, plausible contributing factors to seizures in B6 mice, such as macrophage infiltration and pro-inflammatory cytokine upregulation, have also been seen in the brain from TMEV-infected SJL/J mice that are resistant to TMEV-induced seizures.120
Prevalence, virus infections, and comorbidities in MS, myocarditis and seizures
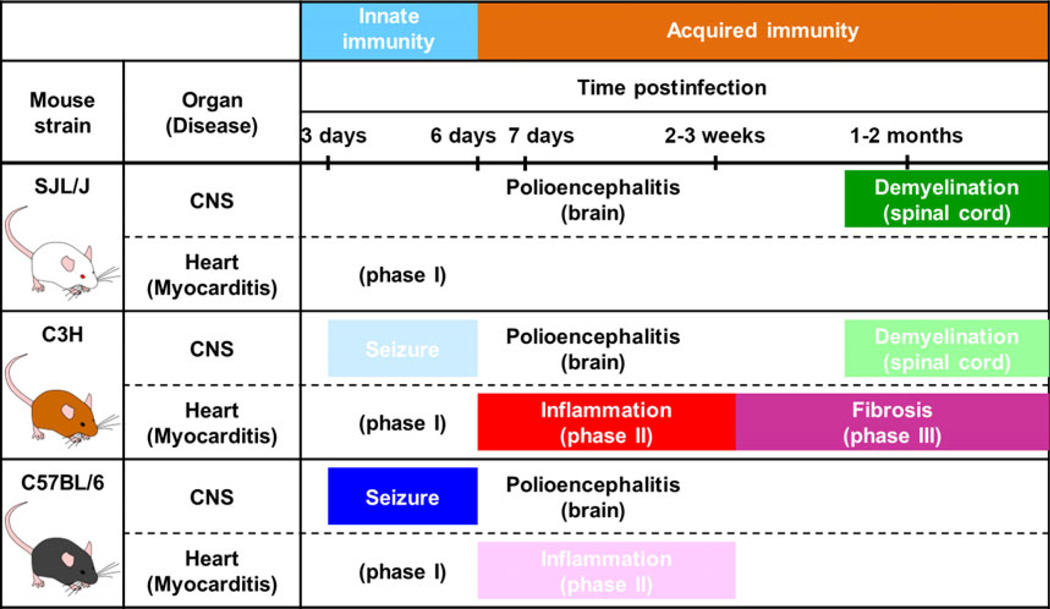
In TMEV infection, C3H mice develop all three immune-mediated diseases: (i) inflammatory demyelination in the spinal cord; (ii) myocarditis in the heart; and (iii) seizures triggered by the brain lesions, whereas SJL/J mice or C57BL/6 mice develop only demyelination or seizures, respectively (Fig. 7). This shows that one single virus can induce any three organ-specific immune-mediated diseases depending on the host’s genetic background.
Figure 7.
Susceptibility to three TMEV-induced immune-mediated disease models for multiple sclerosis (demyelination, green), myocarditis, (phases II and III, red and red-violet, respectively) and seizures (blue) among three mouse strains. In the central nervous system (CNS), 3–6 days p.i., TMEV induces innate immune responses, which cause severe seizures in C57BL/6 (B6) and mild seizures in C3H mice, but no clinical signs in SJL/J mice. Approximately 1 week p.i., TMEV infects neurons in the gray matter of the brain and causes inflammation (polioencephalitis) in all three mouse strains histologically without causing obvious clinical signs. Approximately 1 month p.i. (chronic phase), TMEV persistently infects the white matter of the spinal cord of SJL/J mice and C3H mice, inducing severe and mild inflammatory demyelination, respectively, whereas B6 mice eradicate the virus completely with no lesions in the spinal cord. In contrast, in the heart, although TMEV can infect the heart in all three mouse strains (phase I), only C3H mice develop substantial inflammation in phase II, 1–3 weeks p.i., leading to cardiac fibrosis in phase III, 1–2 months p.i. SJL/J mice develop no cardiac inflammation, whereas only a few B6 mice develop mild inflammation in phase II.
Although MS, myocarditis, and seizures/epilepsy are common diseases with their prevalence of 0.1– 0.2, 1–9 and 0.5–1.0%, respectively,104 the comorbidities among the three diseases have not been investigated, except between MS and epilepsy.121 The prevalence of epilepsy is 0.5–1.0% in the general population.104,122 Several MS cohort- and population-based studies reported that the prevalence of epilepsy in the MS population was two to three-fold higher (0.5–8.3%) than the general age-matched population.121,123–125 Marrie et al.123 showed that the comorbidity of epilepsy in the male MS population was higher than the female MS population, although the prevalence of epilepsy was not different between male and female matched populations. Although it has not been proven whether inflammatory demyelination in the brain can explain the increased frequency of seizures in MS,126 Calabrese et al.127 reported that relapsing–remitting MS patients with epilepsy have more extensive cortical inflammation than relapsing–remitting MS patients without epilepsy.
The brain and the heart have several histological similarities; for example, their major cell types, including neurons, glial cells and cardiomyocytes, are post-mitotic, and both organs are relatively immune-privileged sites. Several research groups have attempted to link some brain diseases, including epilepsy, with cardiac dysfunction through: (i) the autonomic nervous system; (ii) “neurocardiac” gene expression;128 or (iii) hemodynamics.129,130 However, no epidemiological studies seem to have been carried out to investigate the comorbidity between myocarditis and seizures/epilepsy. Based on the data from TMEV infection, here, we hypothesize that one single viral infection or virally induced-immune responses might lead to both myocarditis and epilepsy. This is consistent with clinical findings in humans: (i) some viral infections, such as herpesviruses and picornaviruses (e.g. coxsackievirus and enterovirus) can infect both the brain and the heart; and (ii) myocarditis and CNS involvement have been observed in several autoimmune diseases, including systemic lupus erythematosus.131
Although MS lesions are, in general, confined only in the CNS, increased comorbidities with other diseases in general organs, such as thyroiditis, have been reported. However, there are only a few reports that describe cardiac abnormalities in MS. Although most reports did not address the pathophysiological mechanism of cardiac dysfunction in MS, Akgül et al.132 speculated that a single microbial agent might induce myocarditis and CNS immunopathology in some MS patients. In MS, there is a case report describing one MS patient who died of acute eosinophilic myocarditis with cardiac histological findings.133 Although the authors speculated that myocarditis was likely drug-induced, there are several concerns on this case report; for example: (i) virological examination was incomplete;134 (ii) the drug responsible for hypersensitivity was unclear;135,136 and (iii) the association with MS was unclear (the patient had anti-nuclear antibodies, immunomodulatory treatment, and active CD4+ T-cell infiltration in the brain and the heart). Cosgrove et al.137 reported a case with neuromyelitis optica spectrum disorder with involvement of cardiac and skeletal muscle with sepsis. The authors speculated that this case represents a new spectrum disorder with myocarditis. However, the authors neither carried out histological examination (the only evidence for “myocarditis” was increased cardiac troponin) nor identified the microbe responsible for sepsis, while it is unclear which virological examination was carried out in that case.
In Table 3, we summarize the prevalence, associated viruses, and comorbidities between MS, myocarditis and seizures/epilepsy. Based on the high prevalence and common associated viruses, as well as data from TMEV studies, we propose that one single virus can induce or exacerbate any one of the aforementioned three immune-mediated conditions depending on the host’s genetic background. Clinical, epidemiological, and virological studies on the comorbidities between MS, myocarditis and seizures/epilepsy might lead to the discovery of a new spectrum of virally-mediated disorders, whose specific diagnosis and treatment can be beneficial for a subset of patients. In addition, experimental comparative studies among mouse strains to clarify the mechanism of different susceptibility to three different TMEV-induced organ-specific immune-mediated disease models (demyelinating disease, myocarditis and seizures) are useful not only to clarify the mechanism of human MS, viral myocarditis and seizures, but also to understand the organ-specific viral tropism and immunopathology, in general.6
Table 3.
Prevalence, associated viruses, and comorbidity of multiple sclerosis, myocarditis and seizures
| Comorbidity |
|||||
|---|---|---|---|---|---|
| Prevalence | Associated viruses | Multiple sclerosis | Myocarditis | Seizure | |
| Multiple sclerosis | 0.1–0.2% USA and Europe | EBV HHV-6 MeV retroviruses |
NA | ± case report |
+ |
| Myocarditis | 1–9% autopsy | Picornaviruses (CVB, enterovirus) parvovirus HHV-6 adenovirus, HCV |
± case report |
NA | ND |
| Seizures/epilepsy | 0.5–1.0% active epilepsy | HHV-6, HSV-1 JEV, HIV, picornaviruses (CVA, CVB, enterovirus, parechovirus) |
+ | ND | NA |
CVA, coxsackievirus A; CVB, coxsackievirus B; EBV, Epstein-Barr virus; HCV, hepatitis C virus; HHV-6, human herpesvirus type 6; HIV, human immunodeficiency virus; HSV-1, herpes simplex virus (type)-1; JEV, Japanese encephalitis virus; MeV, measles virus; NA, not applicable; ND, not determined.
Acknowledgments
This work was supported by grants from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (NIH, R21NS059724), the National Institute of General Medical Sciences COBRE Grant (P30-GM110703), and Japan Society for the Promotion of Science (Grants-in-Aid for Scientific Research-KAKENHI, 16H07356). We thank Dr. Eiichiro Kawai, MD, PhD for excellent technical assistance.
Footnotes
Conflict of interest
None declared.
References
- 1.Sato F, Omura S, Martinez NE, Tsunoda I. Animal models for multiple sclerosis. In: Minagar A, editor. Neuroinflammation. Burlington, MA, USA: Elsevier; 2011. pp. 55–79. [Google Scholar]
- 2.Didonna A. Preclinical models of multiple sclerosis: advantages and limitations Towards better therapies. Curr Med Chem. 2016;23:1442–1459. doi: 10.2174/0929867323666160406121218. [DOI] [PubMed] [Google Scholar]
- 3.Benner B, Martorell AJ, Mahadevan P, Najm FJ, Tesar PJ, Freundt EC. Depletion of Olig2 in oligodendrocyte progenitor cells infected by Theiler’s murine encephalomyelitis virus. J Neurovirol. 2016;22:336–348. doi: 10.1007/s13365-015-0402-7. [DOI] [PubMed] [Google Scholar]
- 4.Takizawa S, Kaneyama T, Tsugane S, et al. Role of the Programmed Death-1 (PD-1) pathway in regulation of Theiler’s murine encephalomyelitis virus-induced demyelinating disease. J Neuroimmunol. 2014;274:78–85. doi: 10.1016/j.jneuroim.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Libbey JE, Fujinami RS. Neurotropic viral infections leading to epilepsy: focus on Theiler’s murine encephalomyelitis virus. Future Virol. 2011;6:1339–1350. doi: 10.2217/fvl.11.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato F, Omura S, Kawai E, et al. Distinct kinetics of viral replication, T cell infiltration, and fibrosis in three phases of myocarditis following Theiler’s virus infection. Cell Immunol. 2014;292:85–93. doi: 10.1016/j.cellimm.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Omura S, Kawai E, Sato F, et al. Bioinformatics multivariate analysis determined a set of phase-specific biomarker candidates in a novel mouse model for viral myocarditis. Circ Cardiovasc Genet. 2014;7:444–454. doi: 10.1161/CIRCGENETICS.114.000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knowles NJ, Hovi T, Hyypia T, et al. Family Picornaviridae. In: King AMQ, Lefkowitz E, Adams MJ, Carstens EB, editors. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses. 1st. London, UK: Elsevier Inc.; 2012. pp. 855–880. [Google Scholar]
- 9.Tsunoda I, Fujinami RS. Theiler’s murine encephalomyelitis virus. In: Ahmed R, Chen ISY, editors. Persistent Viral Infections. Chichester, New York: John Wiley & Sons; 1999. pp. 517–536. [Google Scholar]
- 10.McCright IJ, Tsunoda I, Whitby FG, Fujinami RS. Theiler’s viruses with mutations in loop I of VP1 lead to altered tropism and pathogenesis. J Virol. 1999;73:2814–2824. doi: 10.1128/jvi.73.4.2814-2824.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCright IJ, Tsunoda I, Libbey JE, Fujinami RS. Mutation in loop I of VP1 of Theiler’s virus delays viral RNA release into cells and enhances antibody-mediated neutralization: a mechanism for the failure of persistence by the mutant virus. J Neurovirol. 2002;8:100–110. doi: 10.1080/13550280290049561. [DOI] [PubMed] [Google Scholar]
- 12.Sato F, Tanaka H, Hasanovic F, Tsunoda I. Theiler’s virus infection: pathophysiology of demyelination and neurodegeneration. Pathophysiology. 2011;18:31–41. doi: 10.1016/j.pathophys.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsunoda I, Iwasaki Y, Terunuma H, Sako K, Ohara Y. A comparative study of acute and chronic diseases induced by two subgroups of Theiler’s murine encephalomyelitis virus. Acta Neuropathol. 1996;91:595–602. doi: 10.1007/s004010050472. [DOI] [PubMed] [Google Scholar]
- 14.Tsunoda I, Kurtz CIB, Fujinami RS. Apoptosis in acute and chronic central nervous system disease induced by Theiler’s murine encephalomyelitis virus. Virology. 1997;228:388–393. doi: 10.1006/viro.1996.8382. [DOI] [PubMed] [Google Scholar]
- 15.Tsunoda I, Kuang L-Q, Libbey JE, Fujinami RS. Axonal injury heralds virus-induced demyelination. Am J Pathol. 2003;162:1259–1269. doi: 10.1016/S0002-9440(10)63922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kappel CA, Melvold RW, Kim BS. Influence of sex on susceptibility in the Theiler’s murine encephalomyelitis virus model for multiple sclerosis. J Neuroimmunol. 1990;29:15–19. doi: 10.1016/0165-5728(90)90143-b. [DOI] [PubMed] [Google Scholar]
- 17.Tsunoda I, Fujinami RS. Two models for multiple sclerosis: experimental allergic encephalomyelitis and Theiler’s murine encephalomyelitis virus. J Neuropathol Exp Neurol. 1996;55:673–686. doi: 10.1097/00005072-199606000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Lipton HL, Dal Canto MC. Susceptibility of inbred mice to chronic central nervous system infection by Theiler’s murine encephalomyelitis virus. Infect Immun. 1979;26:369–374. doi: 10.1128/iai.26.1.369-374.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Derfuss T. Personalized medicine in multiple sclerosis: hope or reality? BMC Med. 2012;10:116. doi: 10.1186/1741-7015-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura M, Matsuoka T, Chihara N, et al. Differential effects of fingolimod on B-cell populations in multiple sclerosis. Mult Scler. 2014;20:1371–1380. doi: 10.1177/1352458514523496. [DOI] [PubMed] [Google Scholar]
- 21.Penberthy WT, Tsunoda I. The importance of NAD in multiple sclerosis. Curr Pharm Des. 2009;15:64–99. doi: 10.2174/138161209787185751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kira J. Multiple sclerosis in the Japanese population. Lancet Neurol. 2003;2:117–127. doi: 10.1016/s1474-4422(03)00308-9. [DOI] [PubMed] [Google Scholar]
- 23.Saigoh K, Yoshimura S, Izumikawa T, et al. Chondroitin sulfate beta-1,4-N-acetylgalactosaminyltransferase-1 (ChGn-1) polymorphism: association with progression of multiple sclerosis. Neurosci Res. 2016;108:55–59. doi: 10.1016/j.neures.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Kira J. Genetic and environmental backgrounds responsible for the changes in the phenotype of MS in Japanese subjects. Mult Scler Relat Disord. 2012;1:188–195. doi: 10.1016/j.msard.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimura S, Isobe N, Matsushita T, et al. Genetic and infectious profiles influence cerebrospinal fluid IgG abnormality in Japanese multiple sclerosis patients. PLoS ONE. 2014;9:e95367. doi: 10.1371/journal.pone.0095367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson RT. Chronic Inflammatory and Demyelinating Diseases. Viral Infections of the Nervous System. 2nd. Philadelphia: Lippincott-Raven; 1998. pp. 227–263. [Google Scholar]
- 27.Derfuss T, Curtin F, Guebelin C, et al. A phase IIa randomized clinical study testing GNbAC1, a humanized monoclonal antibody against the envelope protein of multiple sclerosis associated endogenous retrovirus in multiple sclerosis patients - a twelve month follow-up. J Neuroimmunol. 2015;285:68–70. doi: 10.1016/j.jneuroim.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 28.Ricklin ME, Lorscheider J, Waschbisch A, et al. T-cell response against varicella-zoster virus in fingolimod-treated MS patients. Neurology. 2013;81:174–181. doi: 10.1212/WNL.0b013e31829a3311. [DOI] [PubMed] [Google Scholar]
- 29.O’Connor RA, Anderton SM. Foxp3+ regulatory T cells in the control of experimental CNS autoimmune disease. J Neuroimmunol. 2008;193:1–11. doi: 10.1016/j.jneuroim.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 30.Sato F, Omura S, Jaffe SL, Tsunoda I. Role of CD4+ T lymphocytes in pathophysiology of multiple sclerosis. In: Minagar A, editor. Multiple Sclerosis: a Mechanistic View. London, UK: Elsevier Inc.; 2016. pp. 41–69. [Google Scholar]
- 31.Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol. 2012;30:733–758. doi: 10.1146/annurev-immunol-020711-075043. [DOI] [PubMed] [Google Scholar]
- 32.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 33.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 35.Fernando V, Omura S, Sato F, et al. Regulation of an autoimmune model for multiple sclerosis in Th2-biased GATA3 transgenic mice. Int J Mol Sci. 2014;15:1700–1718. doi: 10.3390/ijms15021700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez NE, Sato F, Omura S, et al. RORγt, but not T-bet, overexpression exacerbates an autoimmune model for multiple sclerosis. J Neuroimmunol. 2014;276:142–149. doi: 10.1016/j.jneuroim.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez NE, Sato F, Kawai E, Omura S, Chervenak RP, Tsunoda I. Regulatory T cells and Th17 cells in viral infections: implications for multiple sclerosis and myocarditis. Future Virol. 2012;7:593–608. doi: 10.2217/fvl.12.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raveney BJ, Oki S, Hohjoh H, et al. Eomesodermin-expressing T-helper cells are essential for chronic neuroinflammation. Nat Commun. 2015;6:8437. doi: 10.1038/ncomms9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kadowaki A, Miyake S, Saga R, Chiba A, Mochizuki H, Yamamura T. Gut environment-induced intraepithelial autoreactive CD4+ T cells suppress central nervous system autoimmunity via LAG-3. Nat Commun. 2016;7:11639. doi: 10.1038/ncomms11639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shevach EM. Organ-specific autoimmunity. In: Paul WE, editor. Fundamental Immunology. 4th. Philadelphia: Lippincott-Raven; 1999. pp. 1089–1125. [Google Scholar]
- 41.Kimura K, Nakamura M, Sato W, et al. Disrupted balance of T cells under natalizumab treatment in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2016;3:e210. doi: 10.1212/NXI.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakae S, Iwakura Y, Suto H, Galli SJ. Phenotypic differences between Th1 and Th17 cells and negative regulation of Th1 cell differentiation by IL-17. J Leukoc Biol. 2007;81:1258–1268. doi: 10.1189/jlb.1006610. [DOI] [PubMed] [Google Scholar]
- 43.Tomiki H, Kaneyama T, Kobayashi K, et al. Therapeutic effect of anti-alphav integrin mAb on Theiler’s murine encephalomyelitis virus-induced demyelinating disease. J Neuroimmunol. 2014;268:25–34. doi: 10.1016/j.jneuroim.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 44.Shin T, Koh CS. Immunohistochemical detection of osteopontin in the spinal cords of mice with Theiler’s murine encephalomyelitis virus-induced demyelinating disease. Neurosci Lett. 2004;356:72–74. doi: 10.1016/j.neulet.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 45.Theiler M. Spontaneous encephalomyelitis of mice-a new virus disease. Science. 1934;80:122. doi: 10.1126/science.80.2066.122-a. [DOI] [PubMed] [Google Scholar]
- 46.Theiler M. Spontaneous encephalomyelitis of mice, a new virus disease. J Exp Med. 1937;65:705–719. doi: 10.1084/jem.65.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daniels JB, Pappenheimer AM, Richardson S. Observations on encephalomyelitis of mice (DA strain) J Exp Med. 1952;96:517–530. doi: 10.1084/jem.96.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lipton HL. Theiler’s virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975;11:1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gerety SJ, Rundell MK, Dal Canto MC, Miller SD. Class II-restricted T cell responses in Theiler’s murine encephalomyelitis virus-induced demyelinating disease. VI. Potentiation of demyelination with and characterization of an immunopathologic CD4+ T cell line specific for an immunodominant VP2 epitope. J Immunol. 1994;152:919–929. [PubMed] [Google Scholar]
- 50.Tsunoda I. Axonal degeneration as a self-destructive defense mechanism against neurotropic virus infection. Future Virol. 2008;3:579–593. doi: 10.2217/17460794.3.6.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsunoda I, Fujinami RS. Inside-Out versus Outside-In models for virus induced demyelination: axonal damage triggering demyelination. Springer Semin Immunopathol. 2002;24:105–125. doi: 10.1007/s00281-002-0105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sato F, Martinez NE, Stewart EC, Omura S, Alexander JS, Tsunoda I. “Microglial nodules” and “newly forming lesions” may be a Janus face of early MS lesions; implications from virus-induced demyelination, the Inside-Out model. BMC Neurol. 2015;15:219. doi: 10.1186/s12883-015-0478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsunoda I, Tanaka T, Saijoh Y, Fujinami RS. Targeting inflammatory demyelinating lesions to sites of Wallerian degeneration. Am J Pathol. 2007;171:1563–1575. doi: 10.2353/ajpath.2007.070147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodriguez M, Leibowitz JL, Lampert PW. Persistent infection of oligodendrocytes in Theiler’s virus-induced encephalomyelitis. Ann Neurol. 1983;13:426–433. doi: 10.1002/ana.410130409. [DOI] [PubMed] [Google Scholar]
- 55.Lipton HL, Twaddle G, Jelachich ML. The predominant virus antigen burden is present in macrophages in Theiler’s murine encephalomyelitis virus-induced demyelinating disease. J Virol. 1995;69:2525–2533. doi: 10.1128/jvi.69.4.2525-2533.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson AJ, Upshaw J, Pavelko KD, Rodriguez M, Pease LR. Preservation of motor function by inhibition of CD8+ virus peptide-specific T cells in Theiler’s virus infection. FASEB J. 2001;15:2760–2762. doi: 10.1096/fj.01-0373fje. [DOI] [PubMed] [Google Scholar]
- 57.Miller SD, Gerety SJ, Kennedy MK, et al. Class II-restricted T cell responses in Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease. III. Failure of neuroantigen-specific immune tolerance to affect the clinical course of demyelination. J Neuroimmunol. 1990;26:9–23. doi: 10.1016/0165-5728(90)90115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamada M, Zurbriggen A, Fujinami RS. Monoclonal antibody to Theiler’s murine encephalomyelitis virus defines a determinant on myelin and oligodendrocytes, and augments demyelination in experimental allergic encephalomyelitis. J Exp Med. 1990;171:1893–1907. doi: 10.1084/jem.171.6.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hofstetter HH, Ibrahim SM, Koczan D, et al. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237:123–130. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 60.Komiyama Y, Nakae S, Matsuki T, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 61.Kamimura D, Arima Y, Atsumi T, et al. Role of cytokine-mediated crosstalk between T cells and nonimmune cells in the pathophysiology of multiple sclerosis. In: Minagar A, editor. Multiple Sclerosis: a Mechanistic View. London, UK: Elsevier Inc.; 2016. pp. 101–126. [Google Scholar]
- 62.Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 63.Liu L, Okada S, Kong XF, et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med. 2011;208:1635–1648. doi: 10.1084/jem.20110958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Masters SL, Simon A, Aksentijevich I, Kastner DL. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease*. Annu Rev Immunol. 2009;27:621–668. doi: 10.1146/annurev.immunol.25.022106.141627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martinez NE, Karlsson F, Sato F, et al. Protective and detrimental roles for regulatory T cells in a viral model for multiple sclerosis. Brain Pathol. 2014;24:436–451. doi: 10.1111/bpa.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martinez NE, Sato F, Kawai E, et al. Th17-biased RORγt transgenic mice become susceptible to a viral model for multiple sclerosis. Brain Behav Immun. 2015;43:86–97. doi: 10.1016/j.bbi.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoh K, Morito N, Ojima M, et al. Overexpression of RORγt under control of the CD2 promoter induces polyclonal plasmacytosis and autoantibody production in transgenic mice. Eur J Immunol. 2012;42:1999–2009. doi: 10.1002/eji.201142250. [DOI] [PubMed] [Google Scholar]
- 68.Karlsson F, Robinson-Jackson SA, Gray L, Zhang S, Grisham MB. Ex vivo generation of regulatory T cells: characterization and therapeutic evaluation in a model of chronic colitis. Methods Mol Biol. 2011;677:47–61. doi: 10.1007/978-1-60761-869-0_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karlsson F, Martinez NE, Gray L, Zhang S, Tsunoda I, Grisham MB. Therapeutic evaluation of ex vivo-generated versus natural regulatory T-cells in a mouse model of chronic gut inflammation. Inflamm Bowel Dis. 2013;19:2282–2294. doi: 10.1097/MIB.0b013e31829c32dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsunoda I, Tanaka T, Terry EJ, Fujinami RS. Contrasting roles for axonal degeneration in an autoimmune versus viral model of multiple sclerosis: when can axonal injury be beneficial? Am J Pathol. 2007;170:214–226. doi: 10.2353/ajpath.2007.060683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hou W, Kang HS, Kim BS. Th17 cells enhance viral persistence and inhibit T cell cytotoxicity in a model of chronic virus infection. J Exp Med. 2009;206:313–328. doi: 10.1084/jem.20082030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsunoda I, Kuang L-Q, Fujinami RS. Induction of autoreactive CD8+ cytotoxic T cells during Theiler’s murine encephalomyelitis virus infection: implications for autoimmunity. J Virol. 2002;76:12834–12844. doi: 10.1128/JVI.76.24.12834-12844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cooper LT., Jr Myocarditis. N Engl J Med. 2009;360:1526–1538. doi: 10.1056/NEJMra0800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gravanis MB, Sternby NH. Incidence of myocarditis. A 10-year autopsy study from Malmö, Sweden. Arch Pathol Lab Med. 1991;115:390–392. [PubMed] [Google Scholar]
- 75.Pinney SP, Mancini DM. Myocarditis and specific cardiomyopathies. In: Fuster V, Walsh RA, Harrington RA, editors. Hurst’s The Heart. 13th. New York, NY: McGraw-Hill Companies; 2010. pp. 876–896. [Google Scholar]
- 76.Feldman AM, McNamara D. Myocarditis. N Engl J Med. 2000;343:1388–1398. doi: 10.1056/NEJM200011093431908. [DOI] [PubMed] [Google Scholar]
- 77.Sagar S, Liu PP, Cooper LT., Jr Myocarditis. Lancet. 2012;379:738–747. doi: 10.1016/S0140-6736(11)60648-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Phillips M, Robinowitz M, Higgins JR, Boran KJ, Reed T, Virmani R. Sudden cardiac death in Air Force recruits. A 20-year review. JAMA. 1986;256:2696–2699. [PubMed] [Google Scholar]
- 79.Eckart RE, Scoville SL, Campbell CL, et al. Sudden death in young adults: a 25-year review of autopsies in military recruits. Ann Intern Med. 2004;141:829–834. doi: 10.7326/0003-4819-141-11-200412070-00005. [DOI] [PubMed] [Google Scholar]
- 80.Bowles NE, Ni J, Kearney DL, et al. Detection of viruses in myocardial tissues by polymerase chain reaction. evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol. 2003;42:466–472. doi: 10.1016/s0735-1097(03)00648-x. [DOI] [PubMed] [Google Scholar]
- 81.Liu P, Baughman KL. Myocarditis. In: Bonow RO, Mann DL, Zipes DP, Libby P, editors. Braunwald’s Heart Disease: a Textbook of Cardiovascular Medicine. 9th. Philadelphia, PA: Elsevier Saunders; 2012. pp. 1595–1610. [Google Scholar]
- 82.Archard LC, Richardson PJ, Olsen EG, Dubowitz V, Sewry C, Bowles NE. The role of Coxsackie B viruses in the pathogenesis of myocarditis, dilated cardiomyopathy and inflammatory muscle disease. Biochem Soc Symp. 1987;53:51–62. [PubMed] [Google Scholar]
- 83.Kühl U, Pauschinger M, Seeberg B, et al. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation. 2005;112:1965–1970. doi: 10.1161/CIRCULATIONAHA.105.548156. [DOI] [PubMed] [Google Scholar]
- 84.Wiltshire SA, Leiva-Torres GA, Vidal SM. Quantitative trait locus analysis, pathway analysis, and consomic mapping show genetic variants of Tnni3k, Fpgt, or H28 control susceptibility to viral myocarditis. J Immunol. 2011;186:6398–6405. doi: 10.4049/jimmunol.1100159. [DOI] [PubMed] [Google Scholar]
- 85.Corsten MF, Schroen B, Heymans S. Inflammation in viral myocarditis: friend or foe? Trends Mol Med. 2012;18:426–437. doi: 10.1016/j.molmed.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 86.Dermody TS, Parker JS, Sherry B. Orthoreoviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 6th. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2013. pp. 1304–1346. [Google Scholar]
- 87.Noutsias M, Rohde M, Göldner K, et al. Expression of functional T-cell markers and T-cell receptor Vbeta repertoire in endomyocardial biopsies from patients presenting with acute myocarditis and dilated cardiomyopathy. Eur J Heart Fail. 2011;13:611–618. doi: 10.1093/eurjhf/hfr014. [DOI] [PubMed] [Google Scholar]
- 88.Fuse K, Kodama M, Aizawa Y, et al. Th1/Th2 balance alteration in the clinical course of a patient with acute viral myocarditis. Jpn Circ J. 2001;65:1082–1084. doi: 10.1253/jcj.65.1082. [DOI] [PubMed] [Google Scholar]
- 89.Tsunoda I, McCright IJ, Kuang L-Q, Zurbriggen A, Fujinami RS. Hydrocephalus in mice infected with a Theiler’s murine encephalomyelitis virus variant. J Neuropathol Exp Neurol. 1997;56:1302–1313. doi: 10.1097/00005072-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 90.Gomez RM, Rinehart JE, Wollmann R, Roos RP. Theiler’s murine encephalomyelitis virus-induced cardiac and skeletal muscle disease. J Virol. 1996;70:8926–8933. doi: 10.1128/jvi.70.12.8926-8933.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kawai E, Sato F, Omura S, et al. Organ-specific protective role of NKT cells in virus-induced inflammatory demyelination and myocarditis depends on mouse strain. J Neuroimmunol. 2015;278:174–184. doi: 10.1016/j.jneuroim.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tsunoda I, Lane TE, Blackett J, Fujinami RS. Distinct roles for IP-10/CXCL10 in three animal models, Theiler’s virus infection, EAE, and MHV infection, for multiple sclerosis: implication of differing roles for IP-10. Mult Scler. 2004;10:26–34. doi: 10.1191/1352458504ms982oa. [DOI] [PubMed] [Google Scholar]
- 93.Fairweather D, Stafford KA, Sung YK. Update on coxsackievirus B3 myocarditis. Curr Opin Rheumatol. 2012;24:401–407. doi: 10.1097/BOR.0b013e328353372d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yuan J, Yu M, Lin Q-W, et al. Th17 cells contribute to viral replication in coxsackievirus B3-induced acute viral myocarditis. J Immunol. 2010;185:4004–4010. doi: 10.4049/jimmunol.1001718. [DOI] [PubMed] [Google Scholar]
- 95.Nishii M, Inomata T, Takehana H, et al. Serum levels of interleukin-10 on admission as a prognostic predictor of human fulminant myocarditis. J Am Coll Cardiol. 2004;44:1292–1297. doi: 10.1016/j.jacc.2004.01.055. [DOI] [PubMed] [Google Scholar]
- 96.Tsunoda I, Tanaka T, Fujinami RS. Regulatory role of CD1d in neurotropic virus infection. J Virol. 2008;82:10279–10289. doi: 10.1128/JVI.00734-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sakuishi K, Miyake S, Yamamura T. Role of NK cells and invariant NKT cells in multiple sclerosis. Results Probl Cell Differ. 2010;51:127–147. doi: 10.1007/400_2009_11. [DOI] [PubMed] [Google Scholar]
- 98.Huber S, Sartini D, Exley M. Role of CD1d in coxsackievirus B3-induced myocarditis. J Immunol. 2003;170:3147–3153. doi: 10.4049/jimmunol.170.6.3147. [DOI] [PubMed] [Google Scholar]
- 99.Wu CY, Feng Y, Qian GC, et al. α-Galactosylceramide protects mice from lethal Coxsackievirus B3 infection and subsequent myocarditis. Clin Exp Immunol. 2010;162:178–187. doi: 10.1111/j.1365-2249.2010.04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Woodruff JF. Viral myocarditis. A review. Am J Pathol. 1980;101:425–484. [PMC free article] [PubMed] [Google Scholar]
- 101.Lipton HL, Kim BS, Yahikozawa H, Nadler CF. Serological evidence that Mus musculus is the natural host of Theiler’s murine encephalomyelitis virus. Virus Res. 2001;76:79–86. doi: 10.1016/s0168-1702(01)00256-8. [DOI] [PubMed] [Google Scholar]
- 102.Dermody TS, Parker JSL, Sherry B. Orthoreoviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 6th. Philadelphia, PA, USA: Lippincott Williams and Wilkins, a Wolsters Kluwer business; 2013. pp. 1304–1346. [Google Scholar]
- 103.Pedley TA, Baxil CW, Morrell MJ. Epilepsy. In: Rowland LP, editor. Merritt’s Neurology. 10th. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 813–833. [Google Scholar]
- 104.Forsgren L, Hesdorffer D. Epidemiology and prognosis of epilepsy. In: Shorvon SD, Perucca E, Engel J, editors. The Treatment of Epilepsy. 3rd. Chichester, UK; Hoboken, NJ: Wiley-Blackwell; 2009. pp. 21–31. [Google Scholar]
- 105.Walker M, Chan D, Thom M. Hippocampus and human disease. In: Andersen P, Morris R, Amaral D, Bliss T, O’Keefe J, editors. The Hippocampus Book. Oxford, New York: Oxford University Press; 2007. pp. 769–812. [Google Scholar]
- 106.Amaral D, Lavenex P. Hippocampal neuroanatomy. In: Andersen P, Morris R, Amaral D, Bliss T, O’Keefe J, editors. The Hippocampus Book. Oxford, New York: Oxford University Press; 2007. pp. 37–114. [Google Scholar]
- 107.Shorvon SD. Aetiology of epilepsy. In: Shorvon SD, Perucca E, Engel J, editors. The Treatment of Epilepsy. 3rd. Chichester, UK; Hoboken, NJ: Wiley-Blackwell; 2009. pp. 33–53. [Google Scholar]
- 108.Vezzani A, Fujinami RS, White HS, et al. Infections, inflammation and epilepsy. Acta Neuropathol. 2016;131:211–234. doi: 10.1007/s00401-015-1481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smith MCP, Tanaka T, Kirkman NJ, McCoy LL, Tsunoda I, Fujinami RS. A novel infections animal model for seizure disorders. Ann Neurol. 2005;58:MS8. [Google Scholar]
- 110.Tsunoda I, Wada Y, Libbey JE, Cannon TS, Whitby FG, Fujinami RS. Prolonged gray matter disease without demyelination caused by Theiler’s murine encephalomyelitis virus with a mutation in VP2 puff B. J Virol. 2001;75:7494–7505. doi: 10.1128/JVI.75.16.7494-7505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsunoda I, Libbey JE, Fujinami RS. TGF-β1 suppresses T cell infiltration and VP2 puff B mutation enhances apoptosis in acute polioencephalitis induced by Theiler’s virus. J Neuroimmunol. 2007;190:80–89. doi: 10.1016/j.jneuroim.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Libbey JE, Kirkman NJ, Smith MC, et al. Seizures following picornavirus infection. Epilepsia. 2008;49:1066–1074. doi: 10.1111/j.1528-1167.2008.01535.x. [DOI] [PubMed] [Google Scholar]
- 113.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 114.Stewart KA, Wilcox KS, Fujinami RS, White HS. Development of postinfection epilepsy after Theiler’s virus infection of C57BL/6 mice. J Neuropathol Exp Neurol. 2010;69:1210–1219. doi: 10.1097/NEN.0b013e3181ffc420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kirkman NJ, Libbey JE, Wilcox KS, White HS, Fujinami RS. Innate but not adaptive immune responses contribute to behavioral seizures following viral infection. Epilepsia. 2010;51:454–464. doi: 10.1111/j.1528-1167.2009.02390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cusick MF, Libbey JE, Patel DC, Doty DJ, Fujinami RS. Infiltrating macrophages are key to the development of seizures following virus infection. J Virol. 2013;87:1849–1860. doi: 10.1128/JVI.02747-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS. Interleukin-6, produced by resident cells of the central nervous system and infiltrating cells, contributes to the development of seizures following viral infection. J Virol. 2011;85:6913–6922. doi: 10.1128/JVI.00458-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Libbey JE, Kirkman NJ, Wilcox KS, White HS, Fujinami RS. Role for complement in the development of seizures following acute viral infection. J Virol. 2010;84:6452–6460. doi: 10.1128/JVI.00422-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Libbey JE, Hanak TJ, Doty DJ, Wilcox KS, Fujinami RS. NBQX, a highly selective competitive antagonist of AMPA and KA ionotropic glutamate receptors, increases seizures and mortality following picornavirus infection. Exp Neurol. 2016;280:89–96. doi: 10.1016/j.expneurol.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Theil DJ, Tsunoda I, Libbey JE, Derfuss TJ, Fujinami RS. Alterations in cytokine but not chemokine mRNA expression during three distinct Theiler’s virus infections. J Neuroimmunol. 2000;104:22–30. doi: 10.1016/s0165-5728(99)00251-9. [DOI] [PubMed] [Google Scholar]
- 121.Allen AN, Seminog OO, Goldacre MJ. Association between multiple sclerosis and epilepsy: large population-based record-linkage studies. BMC Neurol. 2013;13:189. doi: 10.1186/1471-2377-13-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Elger CE, Schmidt D. Modern management of epilepsy: a practical approach. Epilepsy Behav. 2008;12:501–539. doi: 10.1016/j.yebeh.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 123.Marrie RA, Patten SB, Tremlett H, et al. Sex differences in comorbidity at diagnosis of multiple sclerosis: a population-based study. Neurology. 2016;86:1279–1286. doi: 10.1212/WNL.0000000000002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Uribe-San-Martín R, Ciampi-Díaz E, Suarez-Hernández F, Vásquez-Torres M, Godoy-Fernández J, Cárcamo-Rodríguez C. Prevalence of epilepsy in a cohort of patients with multiple sclerosis. Seizure. 2014;23:81–83. doi: 10.1016/j.seizure.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 125.Koch M, Uyttenboogaart M, Polman S, De Keyser J. Seizures in multiple sclerosis. Epilepsia. 2008;49:948–953. doi: 10.1111/j.1528-1167.2008.01565.x. [DOI] [PubMed] [Google Scholar]
- 126.Kelley BJ, Rodriguez M. Seizures in patients with multiple sclerosis: epidemiology, pathophysiology and management. CNS Drugs. 2009;23:805–815. doi: 10.2165/11310900-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Calabrese M, De Stefano N, Atzori M, et al. Extensive cortical inflammation is associated with epilepsy in multiple sclerosis. J Neurol. 2008;255:581–586. doi: 10.1007/s00415-008-0752-7. [DOI] [PubMed] [Google Scholar]
- 128.Glasscock E. Genomic biomarkers of SUDEP in brain and heart. Epilepsy Behav. 2014;38:172–179. doi: 10.1016/j.yebeh.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ritz K, Denswil NP, Stam OCG, van Lieshout JJ, Daemen MJ. Cause and mechanisms of intracranial atherosclerosis. Circulation. 2014;130:1407–1414. doi: 10.1161/CIRCULATIONAHA.114.011147. [DOI] [PubMed] [Google Scholar]
- 130.van Buchem MA, Biessels GJ, Brunner la Rocca HP, et al. The heart-brain connection: a multidisciplinary approach targeting a missing link in the pathophysiology of vascular cognitive impairment. J Alzheimers Dis. 2014;42(Suppl 4):S443–S451. doi: 10.3233/JAD-141542. [DOI] [PubMed] [Google Scholar]
- 131.Buyon JP. Systemic lupus erythematosus: A. Clinical and laboratory features. In: Klippel JH, Crofford LJ, White PH, editors. Primer on the Rheumatic Diseases. 13th. New York, NY: Springer; 2008. pp. 303–338. [Google Scholar]
- 132.Akgül F, McLek I, Duman T, Seyfeli E, Seydaliyeva T, Yalcin F. Subclinical left ventricular dysfunction in multiple sclerosis. Acta Neurol Scand. 2006;114:114–118. doi: 10.1111/j.1600-0404.2006.00662.x. [DOI] [PubMed] [Google Scholar]
- 133.Sabatine MS, Poh K-K, Mega JL, Shepard J-A, Stone JR, Frosch MP. Case records of the Massachusetts General Hospital. Case 36-2007. A 31-year-old woman with rash, fever, and hypotension. N Engl J Med. 2007;357:2167–2178. doi: 10.1056/NEJMcpc079030. [DOI] [PubMed] [Google Scholar]
- 134.Descamps V, Joly P, Musette P. Case 36-2007: a woman with rash, fever, and hypotension. N Engl J Med. 2008;358:1406. doi: 10.1056/NEJMc073493. [DOI] [PubMed] [Google Scholar]
- 135.Ghei M. Case 36-2007: a woman with rash, fever, and hypotension. N Engl J Med. 2008;358:1405. doi: 10.1056/NEJMc073493. [DOI] [PubMed] [Google Scholar]
- 136.Fett JD. Case 36-2007: a woman with rash, fever, and hypotension. N Engl J Med. 2008;358:1405–1406. doi: 10.1056/NEJMc073493. [DOI] [PubMed] [Google Scholar]
- 137.Cosgrove J, Alli S, Ramadan H, Ford HL. Myocarditis and diffuse skeletal muscle oedema: new features of neuromyelitis optica spectrum disorder? A case report Mult Scler. 2014;20:120–122. doi: 10.1177/1352458513495939. [DOI] [PubMed] [Google Scholar]