Supplemental digital content is available in the text.
Key Words: gadolinium-based contrast agents, gadoterate, gadobutrol, gadodiamide, gadopentetate, gadobenate, dentate nucleus, globus pallidus, gadolinium brain retention, brain tissue extraction, chromatography
Abstract
Objective
Multiple clinical and preclinical studies have reported a signal intensity increase and the presence of gadolinium (Gd) in the brain after repeated administration of Gd-based contrast agents (GBCAs). This bioanalytical study in rat brain tissue was initiated to investigate whether the residual Gd is present as intact GBCA or in other chemical forms by using tissue fractionation and chromatography.
Materials and Methods
Rats were divided randomly in 6 groups of 10 animals each. They received 10 daily injections of 2.5 mmol/kg bodyweight of 1 of 5 different GBCAs: linear GBCAs such as gadodiamide (Omniscan; GE Healthcare), gadopentetate dimeglumine (Gd-DTPA, Magnevist; Bayer), or gadobenate dimeglumine (Multihance; Bracco) and macrocyclic GBCAs such as gadobutrol (Gadovist; Bayer) and gadoterate meglumine (Gd-DOTA, Dotarem; Guerbet) or saline. On days 3 and 24 after the last injection (p.i.), 5 randomly chosen animals of each group were killed by exsanguination, and their brains were excised and divided into cerebrum, pons, and cerebellum. The brain sections were homogenized by sonication in ice-cold buffer at pH 7.4. Soluble and insoluble fractions were separated by centrifugation, and the soluble fractions were further separated by gel permeation chromatography (GPC). The Gd concentration in all tissue fractions and in the GPC eluate was measured by inductively coupled plasma–mass spectrometry. In a recovery control experiment, all GBCAs were spiked to blank brain tissue and more than 94% recovery of Gd in the tissue fractions was demonstrated.
Results
Only traces of the administered Gd were found in the rat brain tissue on day 3 and day 24 p.i. In the animals treated with macrocyclic GBCAs, Gd was found only in the soluble brain fraction and was present solely as low molecular weight molecules, most likely the intact GBCA. In the animals treated with linear GBCAs Gd was found to a large extent in the insoluble tissue fraction. The Gd concentration in the soluble fraction was comparable to the macrocyclic agents. According to GPC, a smaller portion of the Gd in the soluble fraction of the linear GBCAs groups was bound to macromolecules larger than 250 to 300 kDa. The nature of the Gd-containing macromolecules and the insoluble species were not determined, but they appeared to be saturable with Gd. The excretion of the soluble Gd species in the linear and macrocyclic GBCA groups was still ongoing between days 3 and 24 p.i. This was also observed for the macromolecular Gd species in the linear GBCA groups, but at a slower rate.
Conclusions
The residual Gd found in the rat brain after repeated administration of all 3 linear GBCAs was present in at least 3 distinctive forms—soluble small molecules, including the intact GBCA, soluble macromolecules, and to a large extent in insoluble form. The latter 2 are most likely responsible for the prolonged signal intensity enhancement in brain structures observed in magnetic resonance imaging. No relevant differences between the 3 linear GBCAs were observed. The Gd concentrations in the brain after administration of macrocyclic GBCAs are lower, and the Gd is only present in soluble small molecules, which were slowly excreted. This underlines the crucial importance of the kinetic inertness of macrocyclic agents in the prevention of potential retention of Gd in the brain compared with the 3 linear, kinetically less restricted GBCAs.
Gadolinium-based contrast agents (GBCAs) are generally considered to have an excellent safety profile.1–3 The discovery of a possible link between some of the clinically used GBCAs and nephrogenic systemic fibrosis (NSF) in renally impaired patients about 10 years ago4–6 and recent findings of increased signal intensity (SI) in the brain, specifically in the dentate nucleus and the globus pallidus, on T1-weighted images in patients with normal kidney function after multiple injections of primarily linear GBCAs7–11 have focused inquiry on the potential retention of GBCAs in the human organism. Nevertheless, the mechanism of NSF and how GBCAs contribute to its development is still not well understood, although many theories are discussed.12 It is common knowledge that all clinically used GBCAs are extracellular agents, meaning that they passively distribute in the extracellular fluid and do not enter cells (with the exception of gadoxetate and gadobenate, which are in part taken up by hepatocytes by specific transport mechanisms) and are not thought to penetrate the intact blood-brain barrier. The route by which small portions of the GBCA dose can nevertheless enter the brain tissue in the absence of known blood-brain barrier defects is not yet fully understood, but recent studies have demonstrated that it very likely occurs through the penetration of the blood–cerebrospinal fluid barrier.13,14
In a multitude of studies during the last few decades, it has been shown that all GBCAs are excreted renally and some to a certain extent also via the hepatobiliary pathway. The renal excretion follows the glomerular filtration with an elimination half-life of ca. 1.5 h. By using contemporary analytical methods, these studies have also shown that GBCAs are not metabolized.15–18 In general, GBCAs are recognized as inert compounds that do not interact with biological systems (except for the targeted hepatobiliary excretion and plasma protein binding of some agents). The excretion of close to 100% of the administered GBCA dose within several days, as it was observed during the phase I studies in healthy volunteers,16,18–20 was considered to be sufficiently safe.
More recent studies in animals21–23 and patients24–29 have demonstrated that a very small fraction of the administered GBCA was retained in the body for a prolonged period and that this Gd retention was inversely correlated with the thermodynamic stability and/or kinetic inertness of the injected GBCA. Although some of the tissue deposits have been identified as Gd and phosphorous-containing particles by electron microscopy28,30 or as intact GBCA (gadoteridol,26 or gadobenate and Gd-DTPA29), very little is known about other chemical species of the retained gadolinium (Gd). The fraction of Gd that is responsible for the observed T1-signal enhancement of certain brain areas must be in a form that has free access to the surrounding water, as a soluble or immobilized molecule, and it must have a very high relaxivity to generate a visible SI enhancement in magnetic resonance imaging (MRI) at very low Gd concentration. Because the physiological environment in which the GBCA is present is chemically very diverse, a large range of different potential Gd species can be expected. This bioanalytical study was initiated to shed some light on the potential Gd species in brain tissue by using tissue fractionation and chromatography.
MATERIALS AND METHODS
Contrast agents were used as supplied by the manufacturer: linear GBCAs such as gadodiamide (Omniscan; GE Healthcare Buchler, Braunschweig, Germany), gadopentetate dimeglumine (Gd-DTPA, Magnevist; Bayer-Vital, Leverkusen, Germany), and gadobenate dimeglumine (Multihance; Bracco, Konstanz, Germany) as well as macrocyclic GBCAs such as gadobutrol (Gadovist; Bayer-Vital) and gadoterate meglumine (Gd-DOTA, Dotarem; Guerbet, Sulzbach/Taunus, Germany). All reagents used were of the highest purity. Deionized water was obtained from a Milli-Q system (Waters, Eschborn, Germany).
Animal Study
This report is based on an animal study that has been published recently22 and which focused on the SI increase on unenhanced T1-weighted MRIs in the rat brain after repeated, extended doses of different GBCAs. The samples and results presented here were obtained from the same animals, after the MRI study was finished. In brief, healthy Han-Wistar rats (200–225 g, age ~2 months; Charles River, Sulzfeld, Germany) were divided randomly in 6 groups of 10 animals each. In each group, the animals were injected intravenously (~0.8 mL/min) with one of the GBCAs at a dose of 2.5 mmol/kg (about 4-fold the human standard dose, based upon body surface area normalization), or with the equal volume of saline (control group). The injection was repeated daily to achieve a total of 10 injections (5 per week) per animal. The injection was performed under short anesthesia (4% isoflurane for less than 5 minutes, Baxter, Unterschleißheim, Germany). On day 3 after the last injection (p.i.), 5 randomly chosen animals of each group obtained a T1-weighted whole-brain MRI (results in Jost et al22) and were then killed by exsanguination. The brain was excised and divided into cerebrum, cerebellum, and pons as outlined in Figure 1. The tissues were frozen at −80°C until further analysis. On day 24 p.i., the remaining 5 animals of each group were treated in the same way. The regulations of the German Animal Protection Law were followed in all animal studies.
FIGURE 1.
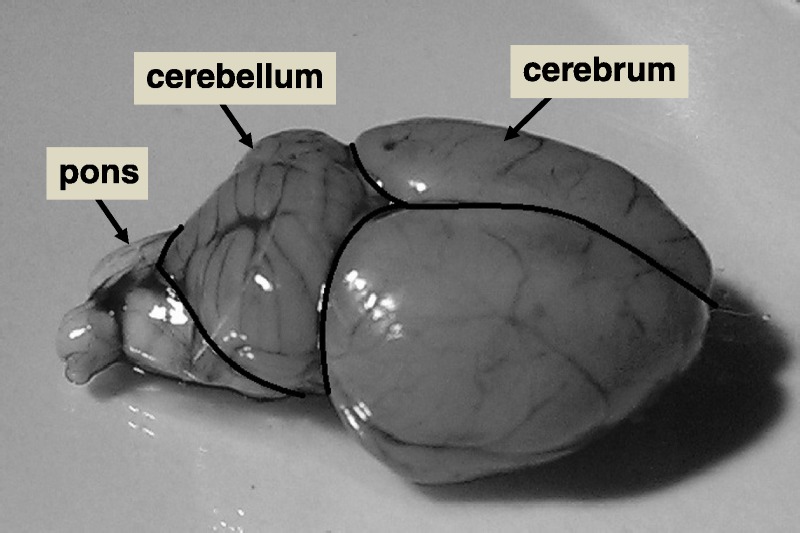
Rat brain with an outline of the sections cerebrum, cerebellum, and pons.
Tissue Fractionation
The resected brain sections cerebrum, cerebellum, and pons were homogenized in 10 mM Tris–HCl buffer pH 7.4 (100 mg tissue + 400 μL buffer) using ultrasound (Sonifier, Branson) in an ice bath. The tissue homogenate was centrifuged at 21,000g at 4°C, and the supernatant was separated from the pellet. The pellet was washed 3 times with buffer, and all supernatants were combined to give the soluble fraction of the tissue homogenate. The washed pellet was not separated any further. The combined supernatant was analyzed by gel permeation chromatography (GPC) to investigate the molecular weight distribution of all Gd species in the soluble fraction. Much care was taken to avoid cross-contamination between samples, and all instruments were thoroughly rinsed before the next samples were processed. Aliquots were taken at each step to determine the Gd concentration in all fractions by inductively coupled plasma–mass spectrometry (ICP-MS). The samples were kept on ice or at 4°C during the whole procedure and were stored at −20°C until analysis (see scheme in Fig. 2).
FIGURE 2.
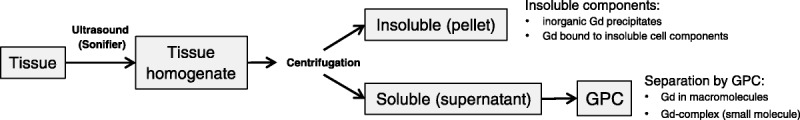
Flow diagram of the brain tissue fractionation and expected Gd species in the separated fractions.
Gadolinium Concentration
Aliquots (n = 3) of 10 μL from each fraction were solubilized by pressurized digestion in 50 μL concentrated nitric acid and 30 μL hydrogen peroxide at 120°C. Terbium was added as internal standard to obtain 5 nmol/L final concentration. The clear solutions were then appropriately diluted with 1% nitric acid containing 0.01% Triton-X100. The Gd concentration was determined by ICP-MS (7900; Agilent, Waldbronn, Germany). The quantification range of the method was 0.1 to 1000 nmol Gd/L in solutions, corresponding to a lower limit of quantification (LOQ) of 0.01 nmol Gd/g in wet tissue. For all fractions, the measured Gd concentrations were converted to tissue concentrations (nmol Gd/g wet tissue) taking all dilutions and separation steps into account.
Gel Permeation Chromatography
An Agilent 1290 HPLC system, equipped with a Superdex 75 HR column (10 × 300 mm, Pharmacia) and an ICP-MS (Agilent 7900) as element-specific detector for Gd, was used. The mobile phase was an aqueous buffer containing 10 mM Tris and 40 mM ammonium formate at pH 7.4. A flow rate of 0.9 mL/min, an injection volume of 20 μL, and a run time of 25 minutes were used.
The GPC allows separation of the analytes according to their molecular weight. The highly hydrophilic column material is based on cross-linked dextran, and the used aqueous buffer at pH 7.4 provides an ambient environment for most biological macromolecules such as proteins or carbohydrates and for Gd complexes. The column was connected to the ICP-MS to obtain the 158Gd-specific chromatograms. The molecular weight distribution was determined by proteins of known molecular weight. Details of the calibration are available in the Supplementary Figure 1, Supplemental Digital Content 1, http://links.lww.com/RLI/A308. To eliminate system background peaks, samples from the control animals were run between the samples from the GBCA groups and their chromatograms were subtracted before peak integration was performed. The chromatograms were manually integrated, and the peak areas of identified peaks were tabulated. The individual peak area ratios were converted to tissue concentration (nmol Gd/g wet tissue) of the individual components identified by chromatography, taking the measured Gd concentrations into account.
Recovery of Gadolinium
The recovery of Gd in the different fractions of the fractionation and separation process was investigated. Parts of the cerebrum of untreated rats (650 mg, n = 2 for each GBCA or saline) were spiked with the different GBCAs to obtain control samples with 10 nmol Gd/g tissue in the homogenate, which was in the range of the samples from treated animals. These control samples were run through the entire fractionation process and the subsequent chromatographic procedure. Detailed results of the recovery control study are available in the Supplementary Figures 2 and 3, Supplemental Digital Content 1, http://links.lww.com/RLI/A308.
RESULTS
Gadolinium Concentration in Brain Tissue Fractions
Tissue Homogenate
For all GBCAs, the total Gd concentrations in the 3 brain sections, cerebrum, cerebellum, and pons, were well above the LOQ on day 3 and on day 24 p.i. and were below or around the LOQ in the saline group (Fig. 3A). The highest concentrations were found in animals 3 days p.i., which received the linear agents gadodiamide and Gd-DTPA. The much higher hepatobiliary excretion of gadobenate in rats compared with humans31 results in a much faster excretion and hence in lower Gd concentrations. The tissue concentrations of the 2 macrocyclic agents were about 50% lower than for Gd-DTPA or gadodiamide on day 3 p.i. The Gd concentrations in the 3 brain sections showed a clear trend for lower concentrations in the pons, especially for the linear agents. The differences between cerebellum and cerebrum were minimal for all agents. A clear decrease in the tissue concentrations between day 3 and day 24 p.i. was observed for all GBCAs, but it was more pronounced for the macrocyclic agents (−62% to −72%) than for the linear agents (−23% to -47%). For cerebellum and pons, the washout was at the upper end of this range (occurred faster) and for cerebrum at the lower end (slower washout).
FIGURE 3.
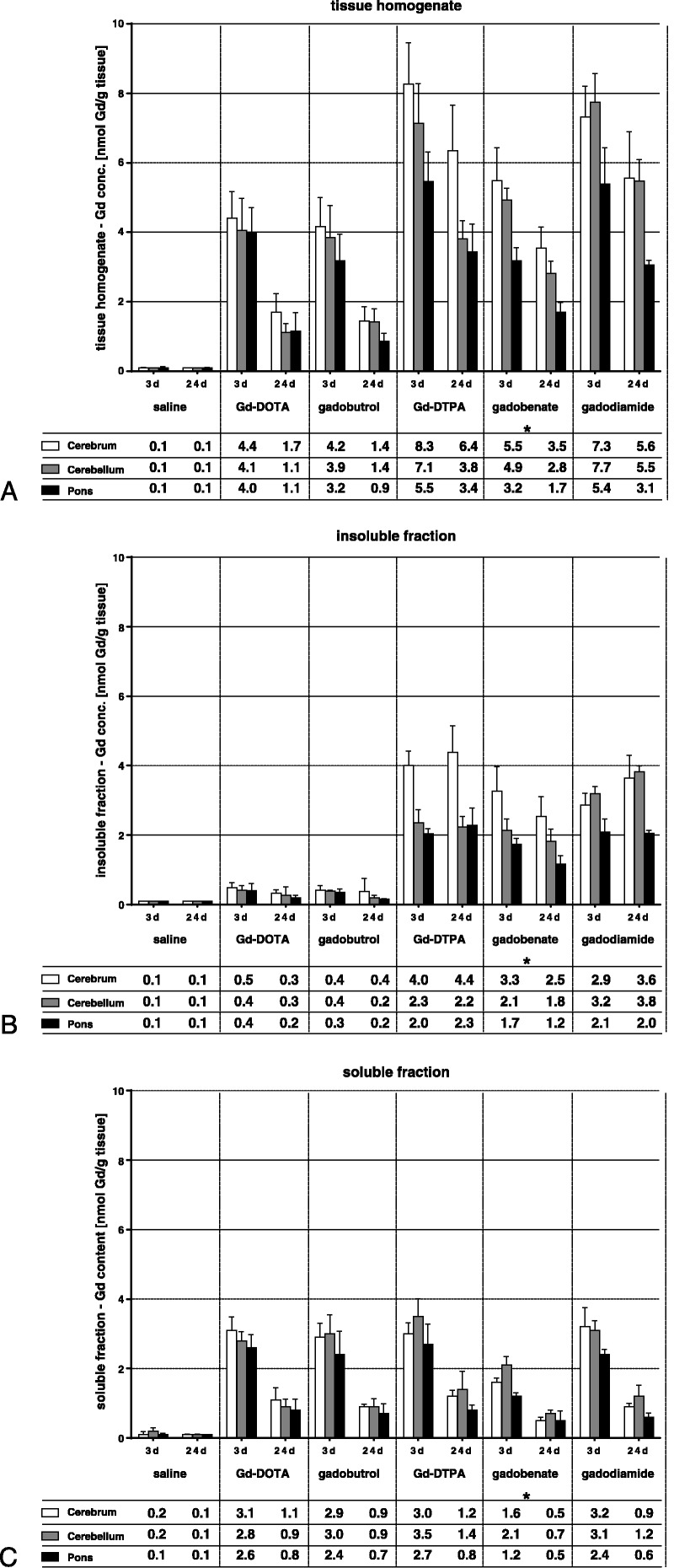
Total gadolinium concentrations (in nmol Gd/g tissue) in the tissue homogenates (A) of the 3 brain, sections cerebrum, cerebellum, and pons, measured 3 and 24 days after the last injection and in the insoluble (B) and soluble (C) fractions. The tables show the mean of 5 animals. The SD is shown in the error bars. Asterisk indicates faster elimination of gadobenate likely due to additional hepatobiliary excretion of ~50% in rats, which accounts for only 3% to 5% in humans.31,32
The first step to separate different Gd species that might be present in the brain tissue was a simple centrifugation step of the tissue homogenates, which separated the soluble fractions (supernatant) from the insoluble fractions (pellet). The pellets were washed with buffer 3 times to remove as much as possible of the soluble components. The Gd concentrations measured in both fractions are summarized in the tables in Figure 3, B and C.
Insoluble Fraction
The Gd concentrations in the insoluble fractions of the brain sections were considerably lower for the macrocyclic GBCAs compared with the linear GBCAs. For the macrocyclic agents, the low concentrations in the insoluble fractions on days 3 and 24 (0.2–0.5 nmol Gd/g tissue) were almost identical to the concentrations found in the recovery control study (0.4–0.7 nmol Gd/g tissue, please refer to Supplementary Fig. 3, Supplemental Digital Content 1, http://links.lww.com/RLI/A308). Only minor differences were observed between the different sections of the brain. In contrast to the macrocyclic agents, for all linear agents, the Gd concentrations in the insoluble fractions were much higher with 1.7 to 4.4 nmol Gd/g tissue on day 3 p.i. This concentration did not change much from day 3 to day 24 p.i. for all 3 linear agents in all 3 brain sections. On day 3 p.i., the measured Gd concentrations in the insoluble fractions corresponded to 33% to 60% of the Gd in the homogenates, and this portion increased for all 3 agents to 63% to 83% on day 24 p.i.
Soluble Fraction
The Gd concentrations in the soluble fractions of the brain tissue homogenates were almost identical for both macrocyclic and linear agents on day 3 and on day 24, with the exception of gadobenate, which is excreted also by the liver. The differences observed between the brain sections were small, with a trend toward lower concentrations in the pons. The washout from day 3 p.i. to day 24 p.i. was in the range from −60% to −73%, which was also very similar for all investigated GBCAs and for the 3 brain sections. The recovery of Gd from the tissue of treated animals was calculated as the sum of the measured Gd concentrations of the insoluble and soluble fractions divided by the concentration in the homogenates. It was in the range of 87% ± 12% for all tissues from all treated animals. This is comparable to the recovery in the control experiment with spiked blank cerebrum where 94% to 96% of the added Gd was found in the different fractions (see Supplementary Fig. 3, Supplemental Digital Content 1, http://links.lww.com/RLI/A308, for details).
Gel Permeation Chromatography of the Soluble Brain Tissue Fractions
The separation of the components of the soluble fractions by GPC is based on differences in molecular weight. Large molecules travel faster through the column than small molecules. Examples of chromatograms of the soluble fractions of cerebellum from each GBCAs and from day 3 p.i and day 24 p.i. are presented in Figure 4. The GBCAs with molecular weights in the range from 547 to 668 Dalton (Da) eluted at about 18 to 20 minutes, whereas macromolecules eluted earlier, after 8 to 14 minutes, depending on their molecular weight (see Supplementary Table 1 and Fig. 2, Supplemental Digital Content 1, http://links.lww.com/RLI/A308, for details). A substantial difference in the detection of early and late peaks was seen when comparing the chromatograms of brain tissue sections from animals administered with linear versus macrocyclic agents. While Gd was solely detected in a late eluting large peak with an identical retention time as the intact GBCA in chromatograms of brain tissue sections from animals receiving macrocyclic GBCAs, early eluting high molecular weight Gd peaks appeared with a retention time of 8 to 9 minutes only in the chromatograms from the linear agents groups. According to the calibration of the column, the observed retention times correspond to macromolecules with a molecular weight of more than 250 to 300 kDa.
FIGURE 4.
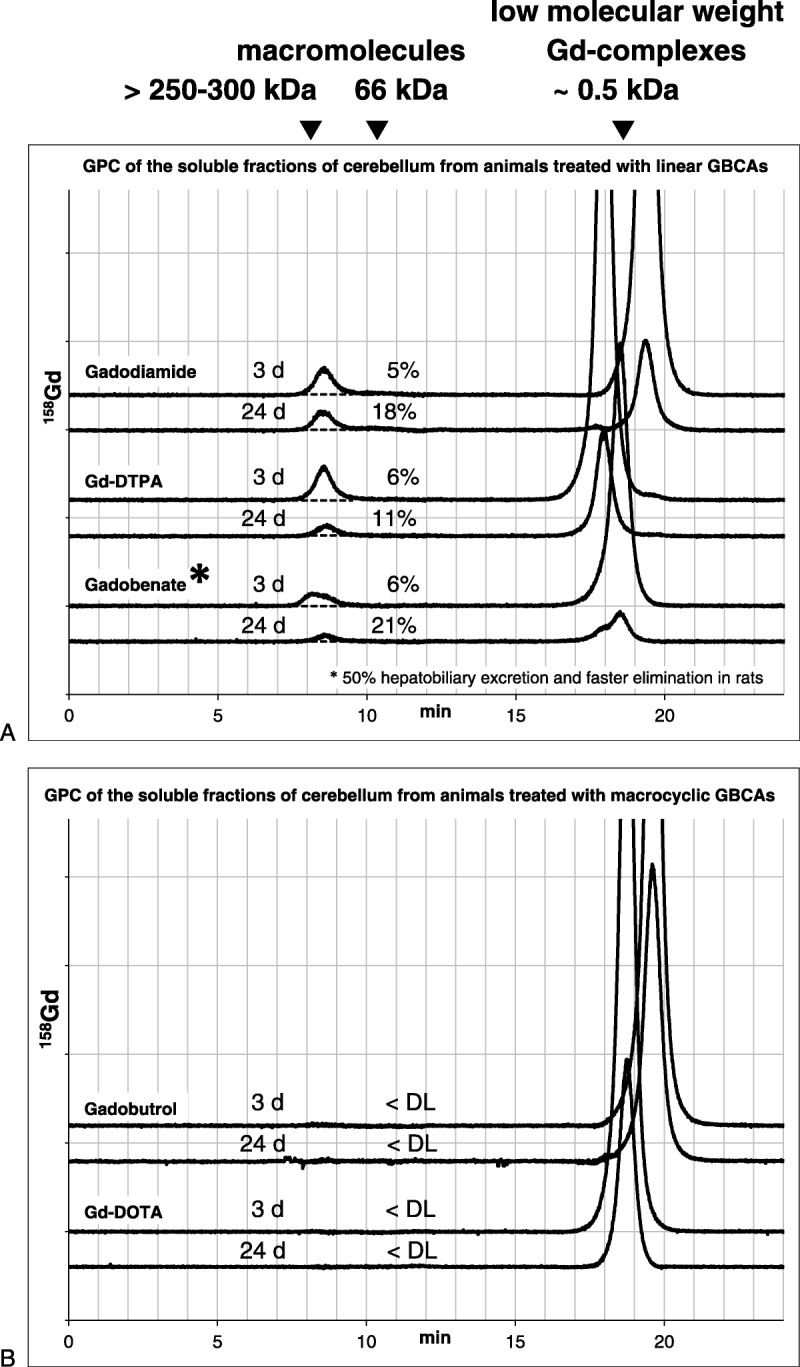
Examples of Gd-specific gel permeation chromatograms of the soluble fractions of cerebellum homogenates. For the linear (A) and macrocyclic GBCAs (B), chromatograms from day 3 and 24 days p.i. are presented. The relative portion of the Gd bound to macromolecules is given as percentage of the total Gd in the respective sample. <DL indicates no peak detectable; asterisk indicates faster elimination of gadobenate likely due to additional hepatobiliary excretion of ~50% in rats, which accounts for only 3% to 5% in humans.31,32
On day 3 p.i., the total peak area in the gadobenate group was slightly smaller than in the Gd-DTPA and gadodiamide groups, which was probably a result of the very efficient additional hepatic excretion pathway of gadobenate in rats (~50%) and which is only 3% to 5% in humans31 (Fig. 4). The total peak area reflects the total Gd concentration in the soluble fraction (Fig. 3C). For all 3 linear agents and in all 3 tissue sections, approximately 5% to 7% of the soluble Gd fraction was present in macromolecular structures on day 3. There was a trend for the highest concentration (0.12–0.22 nmol Gd/g tissue) in the cerebellum and the lowest (0.07–0.15 nmol Gd/g tissue) in pons for all 3 linear agents. For Gd-DTPA and gadodiamide, a clear decrease of the Gd concentration from day 3 to day 24 p.i. was observed in all 3 brain sections (−30% to −50%), whereas for gadobenate, a slight increase was found in cerebellum and pons (Fig. 5). The relative portion of Gd bound to macromolecular structures increased from day 3 p.i. to day 24 p.i. to about 8% to 27% of the soluble Gd fraction. This was observed for all 3 linear agents, but was most prominent for gadobenate. All calculated tissue concentrations of Gd-carrying macromolecules as well as their relative portions in the soluble fraction are summarized in Figure 5 as the mean of all 5 animals in each group of GBCAs.
FIGURE 5.
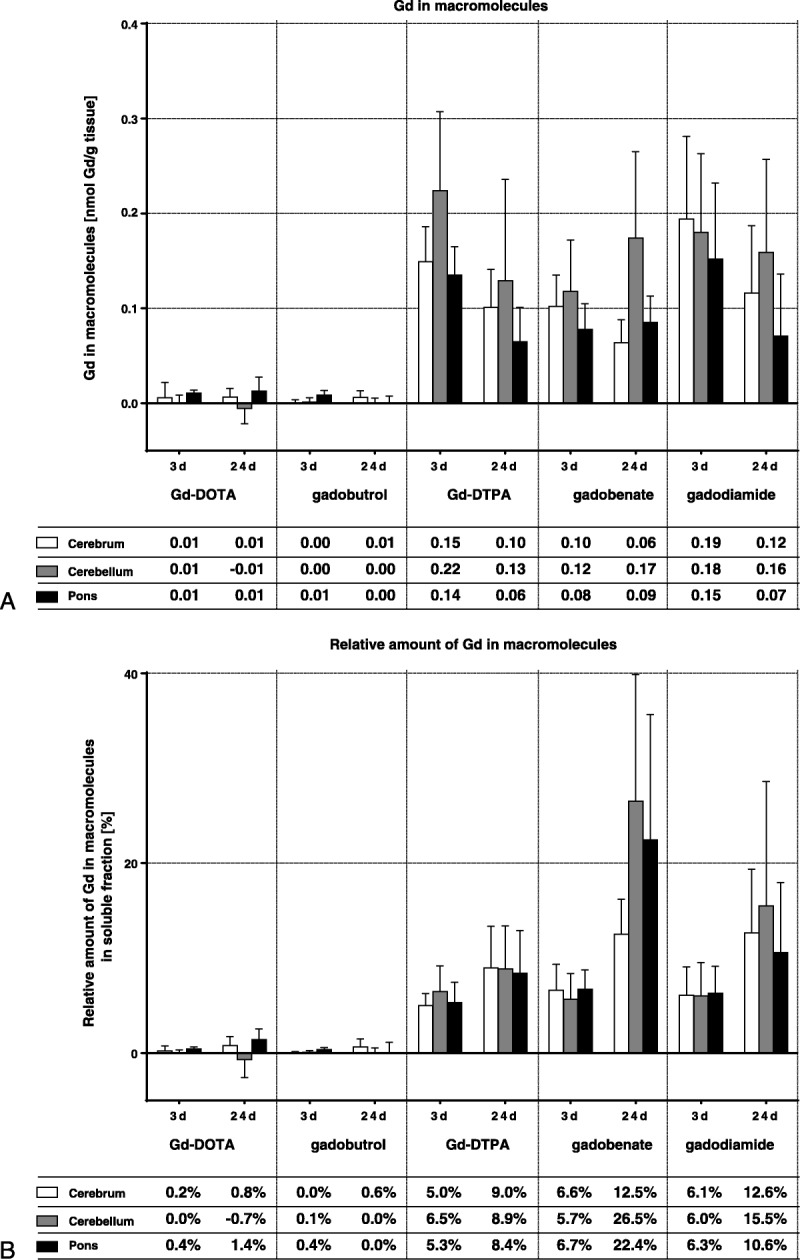
Total amount of gadolinium (in nmol Gd/g tissue, A) and relative portion of the soluble fractions that was bound to macromolecular structures according to gel permeation chromatogram analysis (B). The tables show the mean of 5 animals. The SD is shown in the error bars.
DISCUSSION
This bioanalytical study is a continuation of a previously published study in rats,22 in which an SI increase in the cerebellar nuclei on unenhanced T1-weighted MRI was observed after repeated injection of linear GBCAs, similar to findings in many human studies in the dentate nuclei.7,9,11 In several human27,28 and animal studies,23,33,34 the concentration of Gd in these brain structures has been shown to be significantly above background values. These observations raised the question about the underlying mechanism of Gd retention in the brain and the chemical species of the present Gd. The currently used methods to detect and quantify Gd in tissue, such as ICP-MS, electron microscopy, or energy-dispersive x-ray spectroscopy28 are highly sensitive and specific. However, these techniques measure or detect only the elemental composition and do not reveal any molecular information. In this study, mild conditions of tissue fractionation, extraction, and chromatography were used to retrieve the Gd in its chemically bound form. As the Gd3+ ion is not covalently bound to a molecule but through ionic interactions, conditions have to be chosen to maintain the delicate equilibrium and to prevent the dissociation and formation of potential new artificial Gd species. The properties and stabilities of these Gd species are unknown, and thus mild conditions that resemble the physiological environment were essential. To demonstrate that all Gd present in the brain was captured and analyzed in this study and did not remain undetected in the tissue, a control experiment was conducted, in which the used GBCAs were spiked into blank brain tissue. The spiked samples were then analyzed in the same way as the samples from treated animals. Such controls were necessary, because other, comparable studies were able to extract GBCAs and other potential Gd species with only very limited recovery. Only 1% to 2% of the spiked Gd was recovered from human skin29 or 50% to 70% from human kidney.35 In the current study, nearly complete (94%–96%) recovery of the spiked Gd was obtained in the control experiment. The recovery from all samples from treated animals was only slightly less (87% ± 12%) than in the control experiment (see Supplementary Fig. 4, Supplemental Digital Content 1, http://links.lww.com/RLI/A308), indicating that nearly all of the Gd present in the tissues was captured in the analyzed tissue fractions. Differences in the recovery between the various GBCAs were not observed.
The fractionation of the 3 major brain sections, cerebrum, cerebellum, and pons (Fig. 1) started with the ultrasonic homogenization of the tissue in buffer at pH 7.4 in an ice bath. This method destroys most cells by mechanical forces and allowed extraction of all soluble components whether located intracellularly or extracellularly. The Gd concentration in the homogenates was between 3 and 8 nmol Gd/g wet tissue for all agents on day 3 p.i. The total amount of Gd still present in the whole brain (2–3 g) was only a trace (ca. 0.0005%) of the injected dose (about 5 mmol Gd per 200 g animal). All percentages in this study refer as a basis to this very low Gd concentration in the brain tissues.
Increased Gd concentrations have been observed before in rat tissues in a study addressing NSF using a similar dosing regimen.36 However, a fundamental difference was observed between the Gd concentrations measured in skin (in the NSF study by Sieber et al36) and in the brains of the current study. In the former NSF study, multiple injections of gadodiamide led to an 8-fold higher Gd concentration in skin than Gd-DTPA and more than 30-fold higher than macrocyclic agents such as gadobutrol or Gd-DOTA. By contrast, the presence of Gd in the brain homogenates in the current study was similar after the injection of all 3 linear agents and was only slightly higher than for the macrocyclic GBCAs.
This finding was reflected in the Gd concentrations of the soluble brain tissue fractions from all animal groups, which were almost identical with the exception of gadobenate. The additional hepatobiliary excretion pathway of gadobenate accounts for about 50% of the excretion in rats, but only for about 3% to 5% in humans.31 The additional excretion pathway leads to a significantly reduced elimination half-time of 14 minutes,32 compared with 18 to 20 minutes for other linear GBCAs37,38 and thus to faster body elimination in rats. In humans, the elimination rate of gadobenate is not different to the other GBCAs.31 The presence of all GBCAs in the soluble fractions probably reflects their passive infiltration into the brain, which is dependent on neither the chemical structure, the Gd complex stability, nor the ionicity of the agents. The Gd concentration of the soluble fractions from all agents showed a clear washout between days 3 and 24 p.i., indicating that the elimination from brain was still ongoing, but occurred at a much slower rate than from other tissues (in rats the typical elimination half-life is about 20 minutes37,38).
A large fraction of the tissue remained insoluble after the sonication and was separated by centrifugation and large portions (33%–60% on day 3 p.i. and 59%–71% on day 24 p.i.) of the Gd from all 3 linear agents were found in these insoluble fractions.
Insoluble Gd-containing deposits have been observed before in human brain28 and skin25,30 samples by scanning electron microscopy/energy-dispersive x-ray spectroscopy and in the skin of rats in a study addressing NSF.39 Calcium and phosphorous were co-located in the same deposits, but their chemical composition was not fully elucidated. The pellets may contain inorganic precipitates, such as Gd phosphate, carbonate, or hydroxide,40 which are all highly insoluble species as well as cell debris and denatured macromolecules, which may also bind Gd3+ ions, or entrap GBCAs. For the time being, no attempt was made to further investigate the insoluble fractions due to the lack of suitably mild methods to solubilize the pellets without compromising the nature of the contained Gd species. To get at least some information on the Gd in the pellets, the relaxation times at 1.41 T (MiniSpec mq60, Bruker) were measured in some of the resuspended pellets and also in the soluble fractions. This could have provided information about whether the Gd was present in a high-relaxivity state. However, no difference between the various GBCA groups and the saline group was observed (data not shown). There are 2 possible reasons why this method failed—first, because the Gd species in the insoluble fraction are inorganic precipitates with no relevant T1 relaxivity or, second, the low Gd concentration (<1–5 nmol Gd/g tissue). The clear SI enhancement of the deep cerebellar nuclei seen in the preceding MRI of the extracted brain samples22 would have predicted a considerable shortening of the T1-time; however, this enhancement was restricted to small tissue structures within the entire cerebellum, which became diluted during the tissue homogenization.
In the macrocyclic GBCA groups, the Gd concentrations in the insoluble fractions of the brain tissue sections were very low and clearly different to those in the linear GBCA groups on days 3 and 24 p.i. Because the composition of the insoluble fraction was not further determined in this study, the results were compared with the recovery control experiment, where blank tissue was spiked with comparable amounts of macrocyclic GBCAs. The Gd concentrations found in this control study were in the same low range (see Supplementary Fig. 3, Supplemental Digital Content 1, http://links.lww.com/RLI/A308). Because the macrocyclic GBCAs are expected not to dissociate during the short time interval of the separation procedure, this indicates that the low Gd concentrations found in the control study and in the samples from treated animals were due to an incomplete washout and incomplete separation of the soluble and insoluble fractions.
Although the total Gd concentrations in the brain homogenate did not strictly follow the complex stability of the different GBCAs,41 it was evident that the kinetically inert macrocyclic agents did not form insoluble Gd deposits after 3 or 24 days p.i., whereas the kinetically unrestricted linear agents were found in this fraction to a large extent. However, the extent did not reflect the large differences in the thermodynamic stabilities of the linear agents. A possible explanation for this finding could be the hindered access to the brain and the limited availability of potential binding partners for Gd in the brain which may have become saturated.
The composition of the soluble fractions was further analyzed using GPC. This method used a dextran-based column material and aqueous buffer at pH 7.4 as solvent. These mild conditions are well tolerated by most water-soluble biological macromolecules and should not interfere with Gd complexes. Gel permeation chromatography separates fast-eluting macromolecules from slow-eluting small molecules such as the GBCAs. The soluble fractions from animals receiving macrocyclic GBCAs contained only small Gd-containing molecules, most probably the intact GBCA, because the peaks matched the respective retention times. The soluble fractions from animals receiving linear agents contained a relevant portion (5%–7% on day 3 p.i. and 8%–27% on day 24 p.i.) of Gd bound to macromolecules with a size larger than 250–300 kDa. The differences of the relative portion of macromolecular Gd and its absolute amount between days 3 and 24 p.i. suggest that these species were also excreted or transferred the bound Gd to other ligands over time, but at a slower rate than the soluble fraction as a whole. As observed in the insoluble fractions, the formation of Gd-containing macromolecules did not reflect the differences in complex stability of the linear GBCAs. The extent of their formation was comparable for all 3 linear GBCAs. As discussed previously, this might be due to saturable binding sites for Gd in brain tissue. The relative portion of Gd in macromolecules slightly increased from day 3 to day 24 p.i. (Fig. 5B).
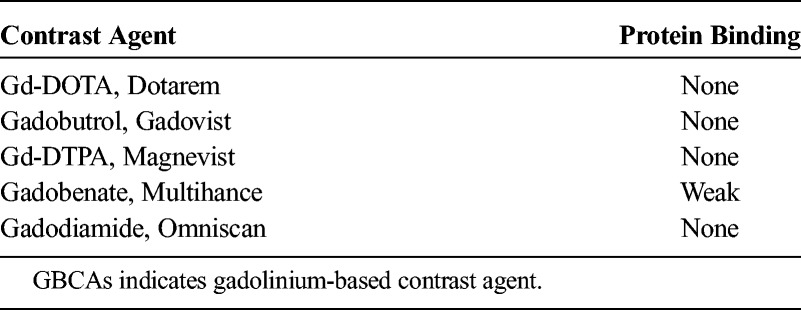
The GPC separation did not allow identification of the chemical nature of the macromolecule and whether it had bound the intact GBCA or the transmetallated Gd3+ ion. However, it is very unlikely that the intact GBCAs were bound to a macromolecule as their binding to plasma proteins is very low or negligible (Table 1). Several structural classes have been suggested as potential hosts for the Gd3+ ion such as Ca2+-binding proteins,42–46 which constitute one of the largest class of metalloproteins present in the extracellular and intracellular space, glucosaminoglycans,47 apotransferrin,48 or neuromelanins.49 Some of them are relatively small macromolecules such as parvalbumin or calmodulin with molecular sizes of about 10 to 20 kDa, whereas others have sizes of several hundred kilodaltons or may be an integral part of the cell membrane. The relaxivity of Gd complexes increases with the molecular weight or immobilization due to much longer tumbling rates. Interestingly, even the relatively small Gd-carrying derivatives of parvalbumin exhibit very high relaxivities,42,46 which generate a visible MRI signal enhancement at concentrations of 0.5 to 1 μmol Gd/L.46 This concentration is in the range of the measured Gd concentration in the homogenized brain sections (1–8 nmol Gd/g tissue, which is roughly equivalent to μmol Gd/L), whereas the local Gd concentration in regions of accumulation such as the cerebellar nuclei may be even much higher.
TABLE 1.
Protein Binding of GBCAs to Human Blood Plasma (According to the Package Inserts and Kirchin et al31)
In this study, 3 distinct chemical forms of Gd were detected in brain tissue after the repeated injection of GBCAs—soluble small molecules, soluble macromolecules, and insoluble forms. Gadolinium or Gd complexes attached to large macromolecular structures with slow tumbling rates provide high T1-relaxivities. These are necessary to achieve sufficient T1-time shortening at the observed, very low Gd concentrations. Depending on the so far unknown identity of the Gd species in the insoluble fraction, they will exhibit high relaxivity only if they have free access to water. For example, Gd bound to insoluble cell fragments or membranes will exhibit slow tumbling rates and high relaxivities. On the other hand, inorganic Gd precipitates such as Gd phosphate have no relevant relaxivity. The macromolecular and to some extent the insoluble fraction therefore most likely contributed most to the persisting SI enhancement seen in MRI. The Gd present in the soluble fraction, on the other hand, was slowly excreted, even though this occurred over a time interval of several weeks.
It has to be noted that during the recovery control study, when gadodiamide was spiked to blank brain tissue, degradation was observed in both samples, which were prepared in independent duplicates (see Supplementary Fig. 2, Supplemental Digital Content 1, http://links.lww.com/RLI/A308). The portion of Gd that was found in the insoluble fraction in the control study was low and not different to what was observed for the other GBCAs used. However, on GPC, about 14% of the Gd was found in the macromolecular peak. The whole fractionation procedure lasted about 4 to 6 hours, and the samples were kept on ice the entire time. The extent of degradation was surprising because a similar extent of degradation was observed in blood plasma at 37°C only after 1 to 2 weeks of incubation.41 Such degradation was not observed with any of the other linear or macrocyclic GBCAs, which appeared to be stable (>99%) during the fractionation process. The results for gadodiamide in the treated animals may be biased by this instability as it may have also occurred during the workup of the tissues from treated animals. However, the results obtained with gadodiamide-treated animals were not much different from those treated with Gd-DTPA, so the effect on these samples may have been limited.
This observation also indicates how “hostile” the physiological environment can be for Gd complexes and that some are more vulnerable than others. In addition, the Gd species being present in vivo may appear and disappear in a highly dynamic fashion, as they are in rapid equilibrium with each other and the multitude of available free ligands may change from one tissue compartment to another and the individual capacity to bind Gd may be limited as discussed previously. It is also an indication that the conditions (solvent, buffer substance, pH, ion strength, additives, etc), which are needed to obtain samples that can be analyzed with highly specific methods (usually in solution), have a large influence on the results. All of that make the identification of in vivo Gd species a highly demanding puzzle.
CONCLUSIONS
The repeated injection of kinetically inert macrocyclic or kinetically less restricted linear GBCAs at very high dosages into rats resulted in very low Gd concentrations in brain tissues and in some remarkable differences in the observed chemical Gd species. A large portion of the Gd from the linear agents was found in the insoluble fractions and to a lesser degree bound to soluble macromolecules. The Gd in these 2 fractions is most likely responsible for the observed prolonged SI enhancement in some brain segments in MRI. Despite their differences in thermodynamic complex stability, no relevant differences were observed between the 3 linear agents, indicating saturation of Gd deposition in the brain. The Gd from macrocyclic GBCAs was exclusively present in the soluble fraction, and no Gd bound to macromolecules was detected. This underlines the paramount importance of kinetic inertness of GBCAs to prevent potential deposition of Gd in the brain. The decrease of Gd in the soluble brain tissue fractions indicated ongoing excretion between days 3 and 24 p.i. at a slow rate for all GBCAs. This study was an initial attempt to assess some of the potential Gd species with prolonged residence times in the brain. Further work is needed to identify and characterize the components of the insoluble fraction and the nature of the Gd-binding macromolecules and also to look at the small molecules in more detail to confirm either the presence of intact GBCA or other low molecular weight Gd complexes.
Supplementary Material
Footnotes
The authors from Bayer AG are all employees of Bayer AG.
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.investigativeradiology.com).
REFERENCES
- 1.Haneder S, Kucharczyk W, Schoenberg SO, et al. Safety of magnetic resonance contrast media: a review with special focus on nephrogenic systemic fibrosis. Top Magn Reson Imaging. 2015;24:57–65. [DOI] [PubMed] [Google Scholar]
- 2.Matsumura T, Hayakawa M, Shimada F, et al. Safety of gadopentetate dimeglumine after 120 million administrations over 25 years of clinical use. Magn Reson Med Sci. 2013;12:297–304. [DOI] [PubMed] [Google Scholar]
- 3.Voth M, Rosenberg M, Breuer J. Safety of gadobutrol, a new generation of contrast agents: experience from clinical trials and postmarketing surveillance. Invest Radiol. 2011;46:663–671. [DOI] [PubMed] [Google Scholar]
- 4.Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104–1108. [DOI] [PubMed] [Google Scholar]
- 5.Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17:2359–2362. [DOI] [PubMed] [Google Scholar]
- 6.Thomsen HS. Nephrogenic systemic fibrosis and gadolinium-based contrast media. In: Thomsen HS, Webb JAW, eds. Contrast Media. Safety Issues and ESUR Guidelines. Berlin, Heidelberg, Germany: Springer; 2014:207–217. [Google Scholar]
- 7.Kanda T, Ishii K, Kawaguchi H, et al. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270:834–841. [DOI] [PubMed] [Google Scholar]
- 8.Malayeri AA, Brooks KM, Bryant LH, et al. National Institutes of Health perspective on reports of gadolinium deposition in the brain. J Am Coll Radiol. 2016;13:237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanda T, Nakai Y, Oba H, et al. Gadolinium deposition in the brain. Magn Reson Imaging. 2016;34:1346–1350. [DOI] [PubMed] [Google Scholar]
- 10.Radbruch A. Are some agents less likely to deposit gadolinium in the brain? Magn Reson Imaging. 2016;34:1351–1354. [DOI] [PubMed] [Google Scholar]
- 11.Runge VM. Safety of the gadolinium-based contrast agents for magnetic resonance imaging, focusing in part on their accumulation in the brain and especially the dentate nucleus. Invest Radiol. 2016;51:273–279. [DOI] [PubMed] [Google Scholar]
- 12.Idée JM, Fretellier N, Robic C, et al. The role of gadolinium chelates in the mechanism of nephrogenic systemic fibrosis: a critical update. Crit Rev Toxicol. 2014;44:895–913. [DOI] [PubMed] [Google Scholar]
- 13.Jost G, Frenzel T, Lohrke J, et al. Penetration and distribution of gadolinium-based contrast agents into the cerebrospinal fluid in healthy rats: a potential pathway of entry into the brain tissue. Eur Radiol. 2016. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Öner AY, Barutcu B, Aykol Ş, et al. Intrathecal contrast-enhanced magnetic resonance imaging-related brain signal changes: residual gadolinium deposition? Invest Radiol. 2016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15.Gries H. Extracellular MRI contrast agents based on gadolinium. Contrast Agents I Top Curr Chem. 2002;221:1–24. [Google Scholar]
- 16.Staks T, Schuhmann-Giampieri G, Frenzel T, et al. Pharmacokinetics, dose proportionality, and tolerability of gadobutrol after single intravenous injection in healthy volunteers. Invest Radiol. 1994;29:709–715. [DOI] [PubMed] [Google Scholar]
- 17.Hamm B, Staks T, Mühler A, et al. Phase I clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: safety, pharmacokinetics, and MR imaging. Radiology. 1995;195:785–792. [DOI] [PubMed] [Google Scholar]
- 18.Weinmann HJ, Laniado M, Mützel W. Pharmacokinetics of GdDTPA/dimeglumine after intravenous injection into healthy volunteers. Physiol Chem Phys Med NMR. 1984;16:167–172. [PubMed] [Google Scholar]
- 19.Le Mignon MM, Chambon C, Warrington S, et al. Gd-DOTA. Pharmacokinetics and tolerability after intravenous injection into healthy volunteers. Invest Radiol. 1990;25:933–937. [PubMed] [Google Scholar]
- 20.Van Wagoner M, Worah D. Gadodiamide injection. First human experience with the nonionic magnetic resonance imaging enhancement agent. Invest Radiol. 1993;28(suppl 1):S44–S48. [PubMed] [Google Scholar]
- 21.Sieber MA, Steger-Hartmann T, Lengsfeld P, et al. Gadolinium-based contrast agents and NSF: evidence from animal experience. J Magn Reson Imaging. 2009;30:1268–1276. [DOI] [PubMed] [Google Scholar]
- 22.Jost G, Lenhard DC, Sieber MA, et al. Signal increase on unenhanced T1-weighted images in the rat brain after repeated, extended doses of gadolinium-based contrast agents: comparison of linear and macrocyclic agents. Invest Radiol. 2016;51:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robert P, Violas X, Grand S, et al. Linear gadolinium-based contrast agents are associated with brain gadolinium retention in healthy rats. Invest Radiol. 2016;51:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White GW, Gibby WA, Tweedle MF. Comparison of Gd (DTPA-BMA)(Omniscan) versus Gd (HP-DO3A)(ProHance) relative to gadolinium retention in human bone tissue by inductively coupled plasma mass spectroscopy. Invest Radiol. 2006;41:272–278. [DOI] [PubMed] [Google Scholar]
- 25.Abraham JL, Thakral C, Skov L, et al. Dermal inorganic gadolinium concentrations: evidence for in vivo transmetallation and long-term persistence in nephrogenic systemic fibrosis. Br J Dermatol. 2008;158:273–280. [DOI] [PubMed] [Google Scholar]
- 26.Birka M, Wentker KS, Lusmöller E, et al. Diagnosis of nephrogenic systemic fibrosis by means of elemental bioimaging and speciation analysis. Anal Chem. 2015;87:3321–3328. [DOI] [PubMed] [Google Scholar]
- 27.Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology. 2015;276:228–232. [DOI] [PubMed] [Google Scholar]
- 28.McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology. 2015;275:772–782. [DOI] [PubMed] [Google Scholar]
- 29.Roberts DR, Lindhorst SM, Welsh CT, et al. High levels of gadolinium deposition in the skin of a patient with normal renal function. Invest Radiol. 2016;51:280–289. [DOI] [PubMed] [Google Scholar]
- 30.Thakral C, Abraham JL. Gadolinium-induced nephrogenic systemic fibrosis is associated with insoluble Gd deposits in tissues: in vivo transmetallation confirmed by microanalysis. J Cutan Pathol. 2009;36:1244–1254. [DOI] [PubMed] [Google Scholar]
- 31.Kirchin MA, Pirovano GP, Spinazzi A. Gadobenate dimeglumine (Gd-BOPTA). An overview. Invest Radiol. 1998;33:798–809. [DOI] [PubMed] [Google Scholar]
- 32.de Haën C, Lorusso V, Tirone P. Hepatic transport of gadobenate dimeglumine in TR− rats. Acad Radiol. 1996;3:S452–S454. [DOI] [PubMed] [Google Scholar]
- 33.Robert P, Lehericy S, Grand S, et al. T1-weighted hypersignal in the deep cerebellar nuclei after repeated administrations of gadolinium-based contrast agents in healthy rats: difference between linear and macrocyclic agents. Invest Radiol. 2015;50:473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kartamihardja AAP, Nakajima T, Kameo S, et al. Distribution and clearance of retained gadolinium in the brain: differences between linear and macrocyclic gadolinium-based contrast agents in a mouse model. Br J Radiol. 2016;89:20160509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kahakachchi CL, Moore DA. Identification and characterization of gadolinium (III) complexes in biological tissue extracts. Metallomics. 2010;2:490–497. [DOI] [PubMed] [Google Scholar]
- 36.Sieber MA, Lengsfeld P, Frenzel T, et al. Preclinical investigation to compare different gadolinium-based contrast agents regarding their propensity to release gadolinium in vivo and to trigger nephrogenic systemic fibrosis-like lesions. Eur Radiol. 2008;18:2164–2173. [DOI] [PubMed] [Google Scholar]
- 37.Weinmann H, Brasch R, Press W, et al. Characteristics of gadolinium-DTPA complex: a potential NMR contrast agent. AJR Am J Roentgenol. 1984;142:619–624. [DOI] [PubMed] [Google Scholar]
- 38.Harpur ES, Worah D, Hals PA, et al. Preclinical safety assessment and pharmacokinetics of gadodiamide injection, a new magnetic resonance imaging contrast agent. Invest Radiol. 1993;28:S28–S43. [DOI] [PubMed] [Google Scholar]
- 39.Sieber MA, Pietsch H, Walter J, et al. A preclinical study to investigate the development of nephrogenic systemic fibrosis: a possible role for gadolinium-based contrast media. Invest Radiol. 2008;43:65–75. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Zhang H, Yang K, et al. Computer simulation of Gd(III) speciation in human interstitial fluid. Biometals. 2004;17:599–603. [DOI] [PubMed] [Google Scholar]
- 41.Frenzel T, Lengsfeld P, Schirmer H, et al. Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37 degrees C. Invest Radiol. 2008;43:817–828. [DOI] [PubMed] [Google Scholar]
- 42.Xue S, Yang H, Qiao J, et al. Protein MRI contrast agent with unprecedented metal selectivity and sensitivity for liver cancer imaging. Proc Natl Acad Sci U S A. 2015;112:6607–6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Angst BD, Marcozzi C, Magee AI. The cadherin superfamily: diversity in form and function. J Cell Sci. 2001;114(pt 4):629–641. [DOI] [PubMed] [Google Scholar]
- 44.Bertini I, Gelis I, Katsaros N, et al. Tuning the affinity for lanthanides of calcium binding proteins. Biochemistry. 2003;42:8011–8021. [DOI] [PubMed] [Google Scholar]
- 45.Hu J, Jia X, Li Q, et al. Binding of La3+ to calmodulin and its effects on the interaction between calmodulin and calmodulin binding peptide, polistes mastoparan. Biochemistry. 2004;43:2688–2698. [DOI] [PubMed] [Google Scholar]
- 46.Grum D, Franke S, Kraff O, et al. Design of a modular protein-based MRI contrast agent for targeted application. PLoS One. 2013;8:e65346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taupitz M, Stolzenburg N, Ebert M, et al. Gadolinium‐containing magnetic resonance contrast media: investigation on the possible transchelation of Gd3+ to the glycosaminoglycan heparin. Contrast Med Mol Imaging. 2013;8:108–116. [DOI] [PubMed] [Google Scholar]
- 48.Du XL, Zhang TL, Yuan L, et al. Complexation of ytterbium to human transferrin and its uptake by K562 cells. Eur J Biochem. 2002;269:6082–6090. [DOI] [PubMed] [Google Scholar]
- 49.Zecca L, Bellei C, Costi P, et al. New melanic pigments in the human brain that accumulate in aging and block environmental toxic metals. Proc Natl Acad Sci U S A. 2008;105:17567–17572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


