Abstract
Purpose
Misery perfusion is defined as marginally sufficient cerebral blood supply relative to cerebral metabolic demand. The aim of the present study was to determine the optimal brain 99mTc–ethyl cysteinate dimer (ECD) SPECT imaging and analysis to detect misery perfusion on 15O PET imaging in patients with chronic occlusive disease of unilateral internal carotid or middle cerebral artery (MCA).
Methods
For 97 patients, cerebral blood flow, cerebral metabolic rate of oxygen, and oxygen extraction fraction were measured using 15O PET; 99mTc-ECD SPECT was performed using dynamic scanning with a scan duration of 10 minutes each for 50 minutes after tracer administration. A region of interest was placed in the bilateral MCA territories and in the bilateral cerebellar hemispheres in all standardized images using a 3-dimensional stereotaxic region-of-interest template and affected-to-contralateral asymmetry ratio in the MCA territory (ARMCA) and contralateral-to-affected asymmetry ratio in the cerebellar hemisphere (ARcbl) were calculated.
Results
The ARMCA or ARcbl on 99mTc-ECD SPECT with a scan time of 20 to 30 minutes after tracer administration (ARMCA20–30 or ARcbl20–30) was correlated with ARMCA on PET cerebral blood flow (r = 0.654) or ARMCA on PET cerebral metabolic rate of oxygen (r = 0.576), respectively, more strongly than with other scan times. The area under the receiver operating characteristic curve for detecting abnormally elevated ARMCA on PET oxygen extraction fraction was significantly greater for ARcbl20–30/ARMCA20–30 (0.947) than for ARMCA20–30 alone (0.780) (difference between areas, 0.167; P = 0.0001) on 99mTc-ECD SPECT.
Conclusions
Combination of asymmetries in the cerebellar and cerebral hemispheres on 99mTc-ECD SPECT in a scan time of 20 to 30 minutes after tracer administration optimally detects misery perfusion in unilateral internal carotid artery or MCA occlusive disease.
Key Words: 99mTc-ECD, crossed cerebellar hypoperfusion, misery perfusion, SPECT
Misery perfusion is defined as marginally sufficient cerebral blood supply relative to cerebral metabolic demand.1 This condition occurs in patients with occlusive diseases of the major cerebral arteries (internal carotid artery [ICA] or middle cerebral artery [MCA]). An increased oxygen extraction fraction (OEF)1 as measured with PET indicates misery perfusion. A recent study demonstrated that increased OEF is still a predictor of a future stroke despite recent improvements in medical treatment for secondary prevention of stroke in patients with symptomatic chronic ICA or MCA occlusive diseases.2 In addition, preoperative increased OEF reportedly predicts development of adverse events including cerebral ischemia or cerebral hyperperfusion syndrome during or after carotid endarterectomy or carotid stenting for cervical ICA stenosis.3,4
A cerebral hemisphere with misery perfusion due to ipsilateral ICA or MCA occlusive disease essentially exhibits a reduction in cerebral blood flow (CBF)5 that can be detected using brain perfusion SPECT. However, misery perfusion cannot be strictly detected using brain perfusion SPECT because measurement of cerebral metabolic rate of oxygen (CMRO2) is necessary for calculation of OEF. Reduced blood flow in the cerebellar hemisphere contralateral to a supratentorial lesion is defined as crossed cerebellar hypoperfusion (CCH)6 and can be observed with brain perfusion SPECT.6–9 Crossed cerebellar hypoperfusion occurs because of transneural metabolic depression and functional deafferentation of 1 cerebellar hemisphere due to a decrease in activity in the opposite cerebral hemisphere via the cerebropontocerebellar pathway.8,9 In unilateral ICA occlusive disease, asymmetric blood flow in the contralateral-to-affected side of the cerebellar hemisphere indicates the cerebral metabolic rate of oxygen (CMRO2) of the affected cerebral hemisphere compared with the CMRO2 of the opposite cerebral hemisphere.10 Oxygen extraction fraction is a function of CMRO2/CBF. Therefore, the ratio of blood flow asymmetry on the contralateral-to-affected side in the cerebellar hemisphere to blood flow asymmetry on the affected-to-contralateral side in the cerebral hemisphere may indicate asymmetry in the affected-to-contralateral side OEF in the cerebral hemisphere. If so, misery perfusion could be observed with brain perfusion SPECT.11
99mTc-labeled ethyl cysteinate dimer (ECD), an accumulative radioligand, is used worldwide as a brain perfusion tracer for SPECT to investigate the pathophysiology of several neurological diseases. Several investigators have used 99mTc-ECD SPECT to image reduced blood flow in the cerebral hemisphere with unilateral ICA or MCA occlusive disease and have demonstrated that 99mTc-ECD SPECT images showed less affected-to-contralateral side asymmetry in the cerebral hemispheres than CBF PET images.12,13 Shimosegawa et al12 have also shown that such affected-to-contralateral side asymmetry on 99mTc-ECD SPECT images at 1 minute after tracer administration is mostly correlated with asymmetry on CBF PET images; 15 minutes later, the coefficient for the correlation decreases considerably. However, sample sizes of these studies were small (10–20 subjects), and SPECT imaging with such a short scanning duration (1 minute) is not clinically practical. Furthermore, whether the presence or absence of misery perfusion can be inferred on 99mTc-ECD SPECT images in patients with unilateral ICA or MCA occlusive disease remains uninvestigated.
The aim of the present study was to determine optimal brain 99mTc-ECD SPECT imaging and analysis parameters to detect misery perfusion on 15O PET imaging in patients with chronic occlusive disease of the unilateral ICA or MCA. We used a large sample size and performed 3 studies: (1) comparison of affected-to-contralateral side asymmetry in the cerebral hemispheres on CBF PET and asymmetry on 99mTc-ECD SPECT for every scan time after tracer administration, (2) comparison of affected-to-contralateral side asymmetry in the cerebral hemispheres on CMRO2 PET and contralateral-to-affected side asymmetry in the cerebellar hemispheres on 99mTc-ECD SPECT for every scan time after tracer administration, and (3) comparison of affected-to-contralateral side asymmetry in the cerebral hemispheres measured by OEF PET and asymmetry on 99mTc-ECD SPECT or the ratio of contralateral-to-affected side asymmetry in the cerebellar hemispheres to affected-to-contralateral side asymmetry in the cerebral hemispheres on 99mTc-ECD SPECT using the optimal scan time after tracer administration.
METHODS
Patients
Patients who met the following inclusion criteria were prospectively enrolled into the present study: presence of clinical symptoms suggesting ischemic episodes in the MCA or ICA territory within 3 months before visiting our institute; absence of clinical symptoms suggesting ischemic episodes in the vertebrobasilar territory; useful residual function (modified Rankin disability scale 0, 1, or 2); presence of unilateral MCA or ICA stenosis (>70% for the ICA, >50% for the MCA) or occlusion on cerebral angiography using arterial catheterization, computed tomography, or MRI; absence of occlusion or stenosis of greater than 50% in the contralateral ICA, contralateral MCA, basilar artery, and vertebral arteries; no infarct or only subcortical abnormalities (excluding infarcts involving the posterior limb of the internal capsule) in the MCA territory of the affected cerebral hemisphere on T1-, T2-, and diffusion-weighted MRI; absence of gross morphological alterations including ischemic lesions or atrophy in the cerebellum and brain stem on T1-, T2-, and diffusion-weighted MRI; and provision of written informed consent.
This protocol was reviewed and approved by our institutional ethics committee, and written informed consent was obtained from all patients or their next of kin.
Brain 99mTc-ECD SPECT Study
One to 3 months after the last ischemic episode, 99mTc-ECD SPECT studies were performed using a ring-type SPECT scanner (Headtome-SET080; Shimadzu Corp, Kyoto, Japan), which provides 31 tomographic images simultaneously. The spatial resolution of the scanner with a low-energy, all-purpose collimator was 13-mm full width at half maximum (FWHM) at the center of the field of view, and the slice thickness was 25-mm FWHM at the field-of-view center. Image slices were taken at 10-mm center-to-center spacing parallel to the orbitomeatal line. The images were reconstructed using the weighted-filtered backprojection technique, in which attenuation correction was made by detecting the edge of the object. An attenuation coefficient of 0.075 cm−1, a Butterworth filter (cutoff = 0.28 cycle/cm, order = 4), and a ramp filter were used for image reconstruction.
Each patient was given an intravenous bolus injection of 740 MBq 99mTc-ECD as a commercially supplied kit. Dynamic scanning with the previously mentioned collimator was started simultaneously and continued for a total of 50 minutes (50 scans) with a scanning duration of 1 minute. Next, the 50 dynamic scans obtained in each patient were combined with a scanning duration of 10 minutes as follows: 0 to 10 minutes, 10 to 20 minutes, 20 to 30 minutes, 30 to 40 minutes, and 40 to 50 minutes after tracer administration.
Brain 15O Gas PET Study
PET studies were performed 2 to 6 days before SPECT studies using a SET-3000GCT/M scanner (PET/CT; Shimadzu Corp).14 This modality uses gadolinium silica oxide detectors and provides 59 slices with 2.6-mm slice thickness. The axial field of view was 156 mm, and the spatial resolution was 3.5-mm FWHM at 1 cm in-plane and 4.2-mm FWHM at the center axially. The scanner was operated in a static scan mode with dual-energy window acquisition for scatter correction. The coincidence time window was set to 10 nanoseconds. A shield module consisting of 7-mm-thick lead plates was attached to the gantry bed and was used to cover the breast and shoulder of the subject to reduce the counting rate of random coincidence and scatter coincidence attributable to radioactivity outside the field of view.
Before the emission scan, a transmission scan (3 minutes) with a 137Cs point source was performed with a bismuth germanate transmission detector ring coaxially attached to the gadolinium silica oxide emission detector ring. Cerebral blood flow was determined while the subject continuously inhaled C15O2 through a mask. Measurements of CMRO2 and OEF were obtained during continuous inhalation of 15O2. Data were collected for 5 minutes. Cerebral blood flow, CMRO2, and OEF were calculated using the steady-state method.15 According to Lammertsma and Jones,16 these last 2 values are overestimated compared with values measured using arteriovenous difference techniques. This overestimation is theoretically due to the signal from nonextracted intravascular 15O.16 To correct for this intravascular component, patients took a single breath of C15O to measure cerebral blood volume, and CMRO2 and OEF were corrected according to cerebral blood volume.16
Data Analysis
All SPECT and PET images were transformed into the standard brain size and shape by linear and nonlinear transformation using SPM2 for anatomic standardization.17 Thus, brain images from all subjects had the same anatomic format. Three hundred eighteen constant regions of interest (ROIs) were automatically placed in both the cerebral and cerebellar hemispheres using a 3-dimensional stereotaxic ROI template.18 The ROIs were grouped into 10 segments (callosomarginal, pericallosal, precentral, central, parietal, angular, temporal, posterior, hippocampus, and cerebellar) in each hemisphere according to the arterial supply. Five (precentral, central, parietal, angular, and temporal) of these 10 segments were combined and defined as an ROI perfused by the MCA (Fig. 1). The mean value of radioactive counts on 99mTc-ECD SPECT images for each 10-minute scan time after tracer administration was measured in the bilateral MCA and cerebellar hemispheric ROIs; the mean values of CBF, CMRO2, and OEF on PET images were measured in the bilateral MCA ROIs. Then, for each patient, the asymmetry ratio in the MCA ROI (ARMCA) was calculated as follows: the value in the cerebral hemisphere ipsilateral to the side of the stenosed or occluded artery divided by the value in the contralateral cerebral hemisphere. The asymmetry ratio in the cerebellar hemispheric ROI (ARcbl) was calculated as follows: the value in the cerebellar hemisphere contralateral to the side of the stenosed or occluded artery divided by the value in the ipsilateral cerebellar hemisphere.
FIGURE 1.
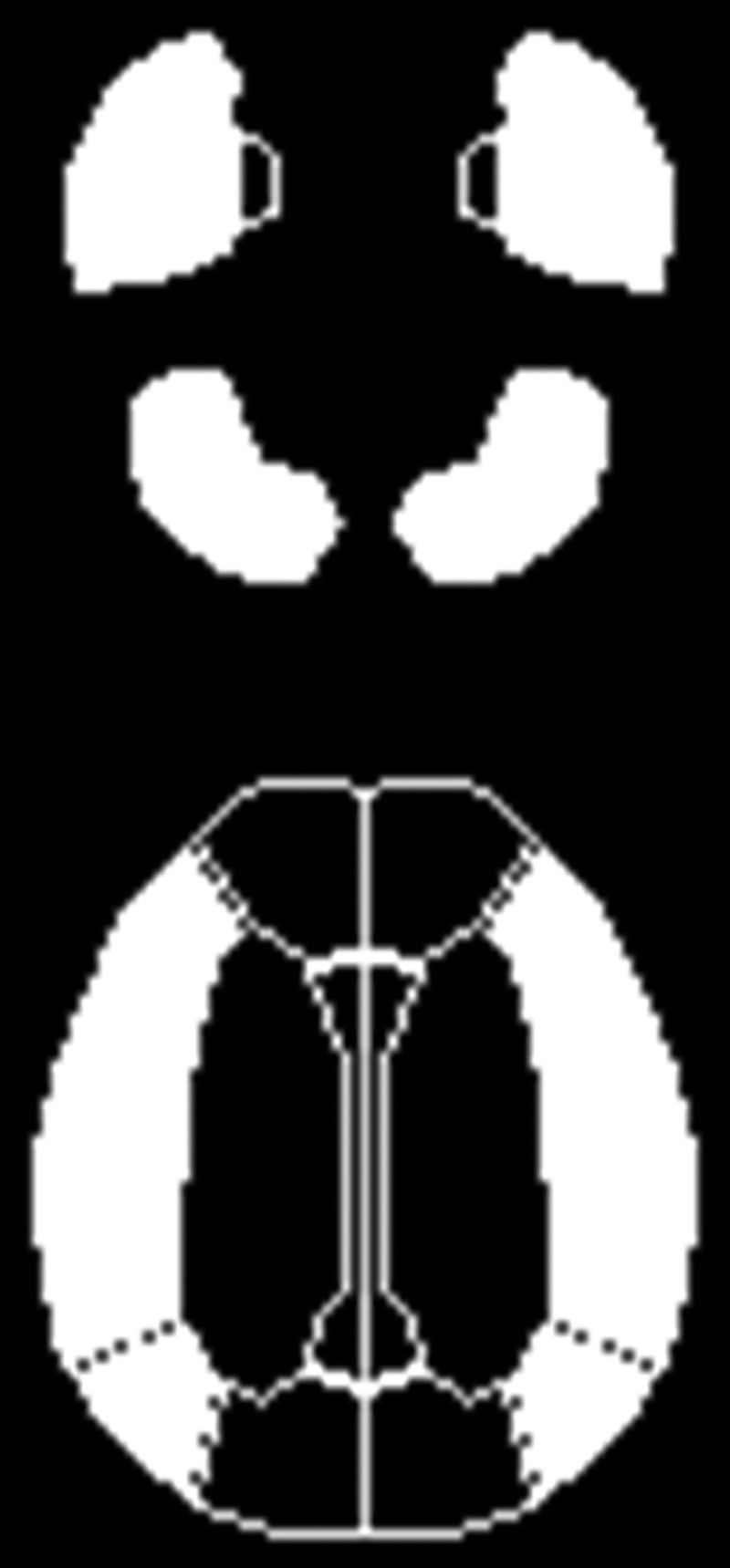
Diagrams showing some ROIs of a 3-dimensional stereotaxic ROI template on brain SPECT and PET. The white ROIs indicate the bilateral cerebellar hemispheres and cortical territories perfused by the bilateral MCAs.
Using the same method, 10 healthy subjects were studied to obtain control values: the mean and SD of ARMCA on OEF PET were 1.001 and 0.044, respectively.11 An abnormally elevated ARMCA on OEF PET was defined as a value greater than the mean ± 2 SDs (1.089) obtained in these healthy subjects.11
Statistical Analysis
Data are expressed as the mean ± SD. Correlations between various parameters were determined using linear regression analysis and by computing regression equations and correlation coefficients, and the function of better fit was determined. When 95% confidence intervals (CIs) of correlation coefficients did not overlap each other, these correlation coefficients were defined as statistically different. The accuracy of ARMCA or ARcbl/ARMCA on 99mTc-ECD SPECT to detect abnormally elevated ARMCA on OEF PET was determined by a receiver operating characteristic (ROC) curve. Pairwise comparisons between the area under the ROC curve for ARMCA on 99mTc-ECD SPECT and that for ARcbl/ARMCA on 99mTc-ECD SPECT were performed as proposed by Pepe and Longton.19 Statistical significance was set at the P < 0.05 level. Exact 95% CI of sensitivity, specificity, and positive and negative predictive values were computed using the binomial distributions. When the 95% CIs of the sensitivity, specificity, or positive or negative predictive value for detection of an abnormally elevated ARMCA on OEF PET did not overlap between ARMCA and ARcbl/ARMCA on 99mTc-ECD SPECT, each value was defined as statistically different.
RESULTS
A total of 97 patients met the inclusion criteria and were enrolled in the present study. The mean age of the 97 patients (31 women and 66 men) was 62 ± 10 years (range, 41–76 years). Forty and 57 patients had experienced only transient ischemic attacks and minor complete strokes with or without transient ischemic attacks, respectively. Fifty, 10, and 37 patients underwent cerebral angiography using arterial catheterization, computed tomography alone, and MRI alone, respectively. These angiography studies demonstrated ICA stenosis in 25 patients, ICA occlusion in 43 patients, MCA stenosis in 15 patients, and MCA occlusion in 14 patients.
The mean ± SD of ARMCA on CBF and CMRO2 PET in patients was 0.874 ± 0.107 (range, 0.619–1.086) and 0.905 ± 0.111 (range, 0.607–1.119), respectively. Table 1 shows correlation coefficients between values on 99mTc-ECD SPECT and those on PET in patients. Asymmetry ratio in the MCA ROI on 99mTc-ECD SPECT in all scan times after tracer administration significantly was significantly correlated with ARMCA on CBF PET. The correlation coefficient for ARMCA on 99mTc-ECD SPECT with a scan time of 20 to 30 minutes after tracer administration (ARMCA20–30) was greater than that for other scan times, but a significant difference among each correlation coefficient was not observed. In most patients, ARMCA20–30 on 99mTc-ECD SPECT was greater than ARMCA on CBF PET (Fig. 2, left graph). Asymmetry ratio in the cerebellar hemisphere on 99mTc-ECD SPECT for all scan times after tracer administration was significantly correlated with ARMCA on CMRO2 PET (Table 1). The correlation coefficient for ARcbl on 99mTc-ECD SPECT with a scan time of 20 to 30 minutes after tracer administration (ARcbl20–30) was greater than that for other scan times, but a significant difference among each correlation coefficient was not observed. In most patients, ARcbl20–30 on 99mTc-ECD SPECT was also greater than ARMCA on CMRO2 PET (Fig. 2, right graph).
TABLE 1.
Correlation Coefficients Between ECD SPECT and CBF or CMRO2 PET in Patients
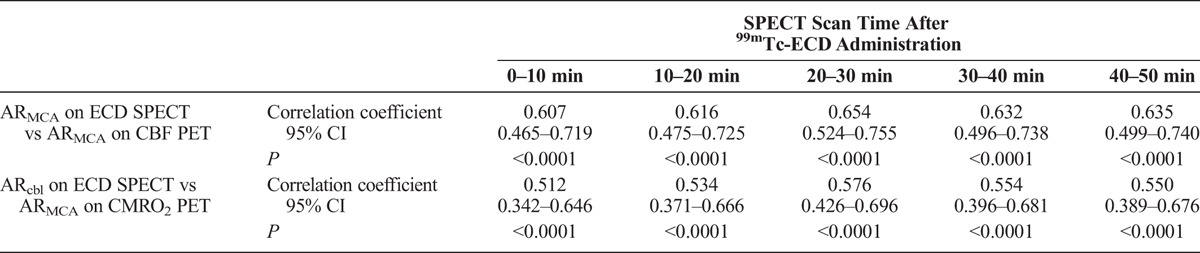
FIGURE 2.
Correlation between ARMCA on CBF PET and ARMCA on 99mTc-ECD SPECT with a scan time of 20 to 30 minutes after tracer administration (ARMCA20–30) in patients (A). Correlation between ARMCA on CMRO2 PET and the ARcbl on 99mTc-ECD SPECT with a scan time of 20 to 30 minutes after tracer administration (ARcbl20–30) in patients (B). The solid line (y = 0.803x ± 0.119 for left graph; y = 0.805x ± 0.130 for right graph) and dashed line are the regression line of patients and the line of identity, respectively. Each circle represents 1 patient.
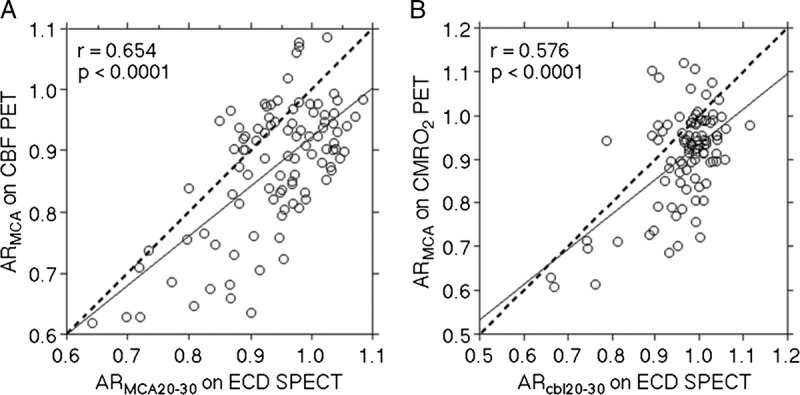
The mean ± SD of ARMCA on OEF PET in patients was 1.034 ± 0.083 (range, 0.924–1.475). Figure 3 shows a comparison of ARMCA on OEF PET and ARMCA20–30 and ARcbl20–30/ARMCA20–30 on99mTc- ECD SPECT, respectively, for each patient. The fit to the linear regression line of the values was significant for the ARMCA20–30 (r = −0.218 [95% CI, −0.400 to −0.020]; P = 0.0316) and the ARcbl20–30/ARMCA20–30 (r = 0.474 [95% CI, 0.303–0.615]; P < 0.0001). Of 97 patients studied, 17 were defined as having abnormally elevated ARMCA on OEF PET.
FIGURE 3.
Correlation between ARMCA on OEF PET and ARMCA20–30 (A) or ARcbl20–30/ARMCA20–30 (B) on 99mTc-ECD SPECT in patients. The horizontal line denotes mean ± 2 SDs for ARMCA on OEF PET obtained in healthy volunteers (a patient value greater than this line is defined as an abnormally elevated ARMCA on OEF PET). The vertical line denotes the cutoff point that is closest to the left upper corner of the ROC curve for detecting abnormally elevated ARMCA on OEF PET in patients. The solid line (y = −0.203x ± 1.226 for A; y = 0.584x ± 0.434 for B) denotes the regression line of patients. Each circle represents 1 patient.
Figure 4 shows a comparison of ROC curves for ARMCA20–30 and ARcbl20–30/ARMCA20–30 on 99mTc-ECD SPECT for detecting abnormally elevated ARMCA on OEF PET. The area under the ROC curve was significantly greater for ARcbl20–30/ARMCA20–30 (0.947 [95% CI, 0.882–0.982]) than for ARMCA20–30 (0.780 [95% CI, 0.685–0.858]) (difference between areas, 0.167; P = 0.0001).
FIGURE 4.
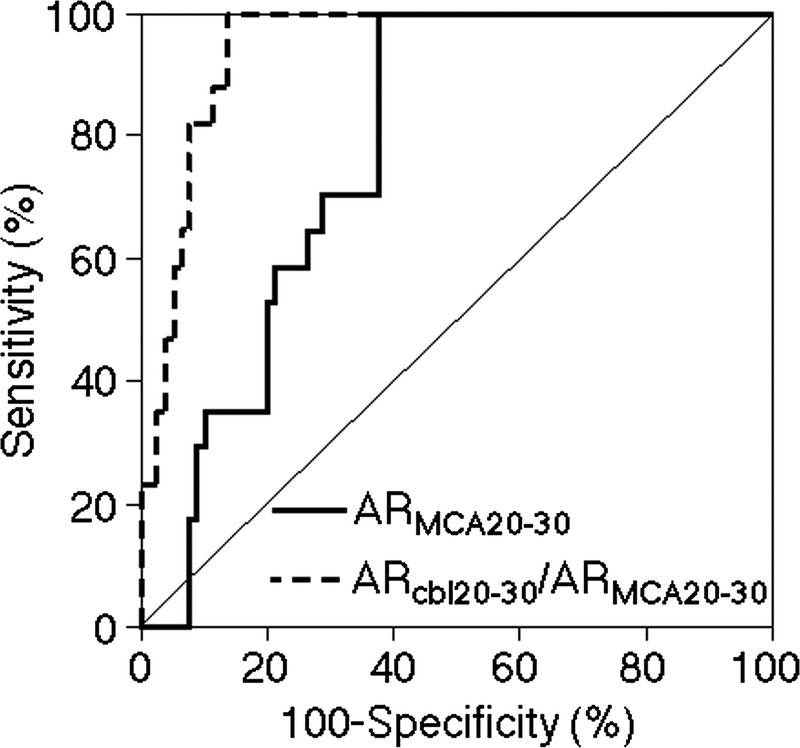
Receiver operating characteristic curves used to compare accuracy between ARMCA20–30 and ARcbl20–30/ARMCA20–30 on 99mTc-ECD SPECT for detecting abnormally elevated ARMCA on OEF PET in patients. The area under the ROC curve is significantly greater for ARcbl20–30/ARMCA20–30 than for ARMCA20–30 (difference between areas, 0.167; P = 0.0001).
Figure 3 and Table 2 show the sensitivity, specificity, and positive and negative predictive values for ARMCA20–30 and ARcbl20–30/ARMCA20–30 on 99mTc-ECD SPECT at the cutoff point (0.952 for ARMCA20–30; 1.062 for ARcbl20–30/ARMCA20–30) that was closest to the left upper corner of the ROC curve for detecting abnormally elevated ARMCA on OEF PET. The sensitivity and negative predictive value were 100% for both ARMCA20–30 and ARcbl20–30/ARMCA20–30. Although the positive predictive value was not significantly different between ARMCA20–30 and ARcbl20–30/ARMCA20–30, the specificity was significantly greater for the latter than for the former.
TABLE 2.
Sensitivity, Specificity, and Positive and Negative Predictive Values for ARMCA Versus ARcbl20–30/ARMCA20–30 or ARcbl20–30/ARMCA20–30 for Only Abnormally Reduced ARMCA20–30 on ECD SPECT to Detect Misery Perfusion
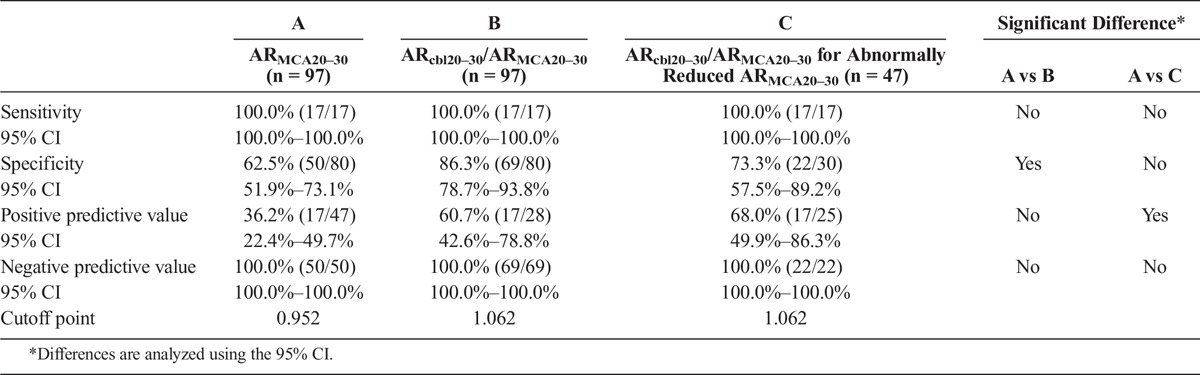
When the abnormally reduced ARMCA20–30 on 99mTc-ECD SPECT was defined as a value lower than the cutoff point, 0.952, obtained in the ROC analysis, 47 of 97 patients studied were defined as having abnormally reduced ARMCA20–30 on 99mTc-ECD SPECT. For these 47 patients, the sensitivity, specificity, and positive and negative predictive values for ARcbl20–30/ARMCA20–30 at the cutoff point that was closest to the left upper corner of the ROC curve for detecting abnormally elevated ARMCA on OEF PET are shown in Table 2. The positive predictive value was significantly greater for ARcbl20–30/ARMCA20–30 for patients with abnormally reduced ARMCA20–30 than for ARMCA20–30 alone.
Representative 15O gas PET and 99mTc-ECD SPECT images in a patient with unilateral ICA occlusion and abnormally elevated ARMCA on OEF PET are shown in Figure 5.
FIGURE 5.
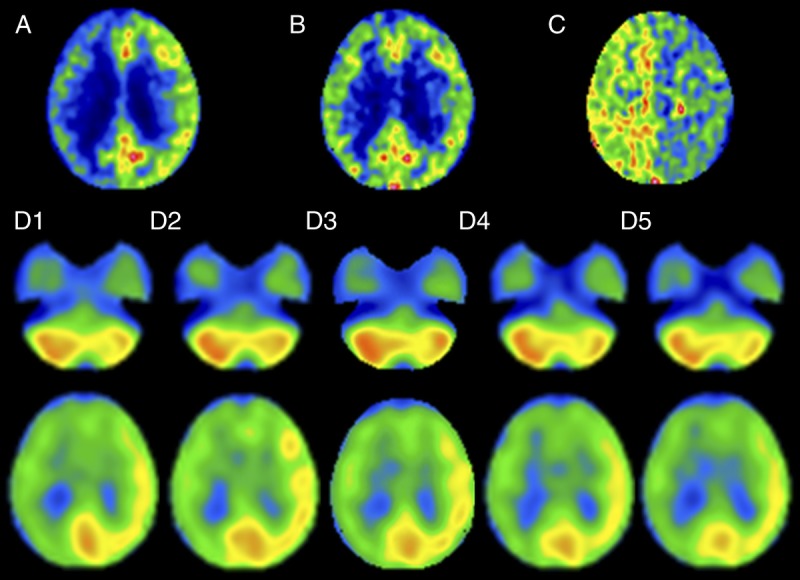
A 66-year-old man with symptomatic occlusion of the right ICA. Upper images, 15O gas PET images including CBF (A), CMRO2 (B), and OEF (C). Although CBF is lower in the right cerebral hemisphere than in the left cerebral hemisphere, the degree of asymmetry in CMRO2 is less, resulting in considerably greater OEF in the right cerebral hemisphere than in the left cerebral hemisphere. D1 to D5, 99mTc-ECD SPECT images at 0 to 10 minutes, 10 to 20 minutes, 20 to 30 minutes, 30 to 40 minutes, and 40 to 50 minutes (D1–D5) after tracer administration. Tracer uptake is lower in the right cerebral hemisphere than in the left cerebral hemisphere and in the left cerebellar hemisphere than in the right cerebellar hemisphere at all scan times after tracer administration. The degree of the asymmetry appears to be greater on images with a scan time of 10 to 20 minutes and 20 to 30 minutes after tracer administration than with other scan times.
DISCUSSION
The present study demonstrated that a combination of asymmetries in the cerebellar and cerebral hemispheres on 99mTc-ECD SPECT with a scan time of 20 to 30 minutes after tracer administration optimally detects misery perfusion in unilateral ICA or MCA occlusive disease.
To display CBF images using 99mTc-ECD SPECT in clinical settings, data acquisition is commonly started 5 to 60 minutes after tracer administration with a scanning duration of 10 to 20 minutes.13,20–26 Our data showed that the optimal scan time to display affected-to-contralateral side asymmetry of CBF in the cerebral hemispheres on 99mTc-ECD SPECT was 20 to 30 minutes after tracer administration. However, the affected-to-contralateral side asymmetry even with this scan time was underestimated in comparison with that on CBF PET in most patients, corresponding to previous findings that 99mTc-ECD accumulation has a nonlinear relationship with the true CBF.13,20–26 Limited extraction is likely important in this nonlinear relationship,13 which is present because of the fairly small permeability–surface area product.21,27 99mTc-ECD demonstrates not only rapid blood clearance, but also fast conversion of the original lipophilic compound to hydrophilic breakdown products in the blood and brain.28 For this reason, arterial input to the brain is small during the short amount of time after intravenous administration of the tracer. Limited extraction decreases radioactivity in regions with high blood flow.13
A previous study demonstrated that although contralateral-to-affected side asymmetry in the cerebellar hemispheres on CBF PET is correlated with affected-to-contralateral side asymmetry in the cerebral hemispheres on CMRO2 PET, the correlation coefficient is considerably higher when reanalyzed only in patients undergoing a PET study within 3 months after the last ischemic episode.11 Crossed cerebellar hypoperfusion is often not observed in patients with decreased CMRO2 in the cerebral hemisphere on the same side as occlusion of a major cerebral artery.29 Crossed cerebellar hypoperfusion often resolves 2 to 3 months after hypertensive putaminal hemorrhage.30 Thus, although CCH basically shows CMRO2 asymmetry in the cerebral hemisphere, this phenomenon may disappear over the course of several months after a supratentorial cerebrovascular event, even though CMRO2 asymmetry persists.11 Thus, our study included only patients undergoing PET and SPECT studies within 3 months after the last ischemic episode. As a result, contralateral-to-affected side asymmetry in the cerebellar hemispheres on 99mTc-ECD SPECT was correlated with affected-to-contralateral side asymmetry in the cerebral hemispheres on CMRO2 PET. This relationship corresponded with results comparing CBF and CMRO2 on PET.10,11 Our data also showed that the optimal scan time to infer affected-to-contralateral side asymmetry of CMRO2 in the cerebral hemispheres using 99mTc-ECD SPECT was 20 to 30 minutes after tracer administration. This scan time was conveniently identical to the optimal scan time required to display affected-to-contralateral side asymmetry of CBF in the cerebral hemispheres on 99mTc-ECD SPECT.
In the present study, abnormal elevation of OEF in the affected hemisphere relative to OEF in the contralateral hemisphere was defined as misery perfusion in the affected hemisphere, and abnormally elevated ARMCA on OEF PET was defined as a value greater than the mean ± 2 SDs (1.089) of the normal values. Patients showing ARMCA greater than 1.082 on OEF PET due to unilateral ICA steno-occlusive disease were considered to have misery perfusion and are at high risk of stroke.31 In our study, the cutoff point for ARMCA on OEF PET was similar to that of Grubb et al.31 Our study demonstrated that affected-to-contralateral side asymmetry in the cerebral hemispheres on 99mTc-ECD SPECT was correlated with asymmetry on OEF PET and detected misery perfusion with sensitivity and a negative predictive value of 100% at the cutoff point of 0.952. These findings corresponded to the principle that a cerebral hemisphere with misery perfusion due to unilateral ICA or MCA occlusive disease has reduced CBF in the affected hemisphere.5 Furthermore, the ratio of contralateral-to-affected side asymmetry in the cerebellar hemispheres to affected-to-contralateral side asymmetry in the cerebral hemispheres on 99mTc-ECD SPECT detected misery perfusion with high accuracy, with an area under the ROC curve of greater than 0.9. This accuracy was significantly higher for the ratio than for affected-to-contralateral side asymmetry in the cerebral hemispheres on 99mTc-ECD SPECT. These findings demonstrated that the combination of asymmetries in the cerebellar and cerebral hemispheres on 99mTc-ECD SPECT optimally detects misery perfusion.
Our study also showed that the additional calculation of cerebellar asymmetry for patients with strong cerebral asymmetry (ARMCA <0.952) on 99mTc-ECD SPECT significantly increased the positive predictive value for the detection of misery perfusion. Based on our data, we suggest the following clinical algorithm for optimal detection of misery perfusion using 99mTc-ECD SPECT: A patient with unilateral ICA or MCA occlusive disease undergoes 99mTc-ECD SPECT with a scan time of 20 to 30 minutes after tracer administration. If strong cerebral asymmetry is observed, cerebellar asymmetry is calculated next. A patient with a high ratio of cerebellar asymmetry to cerebral asymmetry (ARcbl/ARMCA >1.062) is finally determined to probably have misery perfusion in the affected hemisphere.
Our study has several limitations. First, patients with subcortical infarctions have not only primary ischemic cortical damage but also cerebral cortical hypometabolism because of transneuronal mechanisms and cerebrocerebellar tract damage, which lead to CCH.10 Deep infarcts injuring the internal capsule especially induce severe CCH.9 In patients with unilateral ICA or MCA steno-occlusive disorder in whom the infarction is restricted to the posterior limb of the internal capsule, CCH without hypometabolism in the ipsilateral cerebral cortex still occurs.10,32 We excluded patients with subcortical infarctions that affect the posterior limb of the internal capsule, and our results are not applicable to such patients. Second, as mentioned previously, our study included only patients undergoing PET and SPECT studies within 3 months after the last ischemic episode. Whether a combination of asymmetries in the cerebellar and cerebral hemispheres on 99mTc-ECD SPECT detects misery perfusion in patients in whom more than 3 months has elapsed after the last ischemic episode remains unknown.
CONCLUSIONS
The present study demonstrated that the combination of asymmetries in the cerebellar and cerebral hemispheres on 99mTc-ECD SPECT with a scan time of 20 to 30 minutes after tracer administration optimally detects misery perfusion in unilateral major cerebral artery occlusive disease.
Footnotes
Conflicts of interest and sources of funding: This work was partly supported by a Grant-in-Aid for Strategic Medical Science Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (S1491001) and Scientific Research from Japan Society for the Promotion of Science (JP15K10313).
REFERENCES
- 1.Baron JC, Bousser MG, Rey A, et al. Reversal of focal “misery-perfusion syndrome” by extra-intracranial arterial bypass in hemodynamic cerebral ischemia. A case study with 15O positron emission tomography. Stroke. 1981;12:454–459. [DOI] [PubMed] [Google Scholar]
- 2.Yamauchi H, Higashi T, Kagawa S, et al. Is misery perfusion still a predictor of stroke in symptomatic major cerebral artery disease? Brain. 2012;135:2515–2526. [DOI] [PubMed] [Google Scholar]
- 3.Sato Y, Ogasawara K, Kuroda H, et al. Preoperative central benzodiazepine receptor binding potential and cerebral blood flow images on SPECT predict development of new cerebral ischemic events and cerebral hyperperfusion after carotid endarterectomy. J Nucl Med. 2011;52:1400–1407. [DOI] [PubMed] [Google Scholar]
- 4.Kawai N, Hatakeyama T, Okauchi M, et al. Cerebral blood flow and oxygen metabolism measurements using positron emission tomography on the first day after carotid artery stenting. J Stroke Cerebrovasc Dis. 2014;23:e55–e64. [DOI] [PubMed] [Google Scholar]
- 5.Powers WJ. Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol. 1991;29:231–240. [DOI] [PubMed] [Google Scholar]
- 6.Komaba Y, Mishina M, Utsumi K, et al. Crossed cerebellar diaschisis in patients with cortical infarction: logistic regression analysis to control for confounding effects. Stroke. 2004;35:472–476. [DOI] [PubMed] [Google Scholar]
- 7.Takasawa M, Watanabe M, Yamamoto S, et al. Prognostic value of subacute crossed cerebellar diaschisis: single-photon emission CT study in patients with middle cerebral artery territory infarct. AJNR Am J Neuroradiol. 2002;23:189–193. [PMC free article] [PubMed] [Google Scholar]
- 8.Baron JC, Bousser MG, Comar D, et al. “Crossed cerebellar diaschisis”: a remote functional suppression secondary to supratentorial infarction in man. J Cereb Blood Flow Metab. 1981;1:s500. [Google Scholar]
- 9.Pantano P, Baron JC, Samson Y, et al. Crossed cerebellar diaschisis. Further studies. Brain. 1986;109:677–694. [DOI] [PubMed] [Google Scholar]
- 10.Yamauchi H, Fukuyama H, Yamaguchi S, et al. Crossed cerebellar hypoperfusion in unilateral major cerebral artery occlusive disorders. J Nucl Med. 1992;33:1637–1641. [PubMed] [Google Scholar]
- 11.Matsumoto Y, Ogasawara K, Saito H, et al. Detection of misery perfusion in the cerebral hemisphere with chronic unilateral major cerebral artery steno-occlusive disease using crossed cerebellar hypoperfusion: comparison of brain SPECT and PET imaging. Eur J Nucl Med Mol Imaging. 2013;40:1573–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimosegawa E, Hatazawa J, Aizawa Y, et al. Technetium-99m–ECD brain SPECT in misery perfusion. J Nucl Med. 1997;38:791–796. [PubMed] [Google Scholar]
- 13.Kado H, Iida H, Kimura H, et al. Brain perfusion SPECT study with 99mTc-bicisate: clinical pitfalls and improved diagnostic accuracy with a combination of linearization and scatter-attenuation correction. Ann Nucl Med. 2001;15:123–129. [DOI] [PubMed] [Google Scholar]
- 14.Ibaraki M, Miura S, Shimosegawa E, et al. Quantification of cerebral blood flow and oxygen metabolism with 3-dimensional PET and 15O: validation by comparison with 2-dimensional PET. J Nucl Med. 2008;49:50–59. [DOI] [PubMed] [Google Scholar]
- 15.Frackowiak RS, Lenzi GL, Jones T, et al. Quantitative measurement of regional cerebral blood flow and oxygen metabolism in man using 15O and positron emission tomography: theory, procedure, and normal values. J Comput Assist Tomogr. 1980;4:727–736. [DOI] [PubMed] [Google Scholar]
- 16.Lammertsma AA, Jones T. Correction for the presence of intravascular oxygen-15 in the steady-state technique for measuring regional oxygen extraction ratio in the brain: 1. description of the method. J Cereb Blood Flow Metab. 1983;3:416–424. [DOI] [PubMed] [Google Scholar]
- 17.Nishimiya M, Matsuda H, Imabayashi E, et al. Comparison of SPM and NEUROSTAT in voxelwise statistical analysis of brain SPECT and MRI at the early stage of Alzheimer’s disease. Ann Nucl Med. 2008;22:921–927. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi R, Matsuda H, Yoshioka K, et al. Cerebral blood flow SPET in transient global amnesia with automated ROI analysis by 3DSRT. Eur J Nucl Med Mol Imaging. 2004;31:578–589. [DOI] [PubMed] [Google Scholar]
- 19.Pepe MS, Longton G. Standardizing diagnostic markers to evaluate and compare their performance. Epidemiology. 2005;16:598–603. [DOI] [PubMed] [Google Scholar]
- 20.Yonekura Y, Ishizu K, Okazawa H, et al. Simplified quantification of regional cerebral blood flow with 99mTc-ECD SPECT and continuous arterial blood sampling. Ann Nucl Med. 1996;10:177–183. [DOI] [PubMed] [Google Scholar]
- 21.Yonekura Y, Tsuchida T, Sadato N, et al. Brain perfusion SPECT with 99mTc-bicisate: comparison with PET measurement and linearization based on permeability–surface area product model. J Cereb Blood Flow Metab. 1994;14:S58–S65. [PubMed] [Google Scholar]
- 22.Shishido F, Uemura K, Murakami M, et al. Cerebral uptake of 99mTc-bicisate in patients with cerebrovascular disease in comparison with CBF and CMRO2 measured by positron emission tomography. J Cereb Blood Flow Metab. 1994;14:S66–S75. [PubMed] [Google Scholar]
- 23.Ito H, Inoue K, Goto R, et al. Database of normal human cerebral blood flow measured by SPECT: I. Comparison between I-123-IMP, Tc-99m-HMPAO, and Tc-99m-ECD as referred with O-15 labeled water PET and voxel-based morphometry. Ann Nucl Med. 2006;20:131–138. [DOI] [PubMed] [Google Scholar]
- 24.Ishizu K, Yonekura Y, Magata Y, et al. Extraction and retention of technetium-99m-ECD in human brain: dynamic SPECT and oxygen-15-water PET studies. J Nucl Med. 1996;37:1600–1604. [PubMed] [Google Scholar]
- 25.Tsuchida T, Nishizawa S, Yonekura Y, et al. SPECT images of technetium-99m–ethyl cysteinate dimer in cerebrovascular diseases: comparison with other cerebral perfusion tracers and PET. J Nucl Med. 1994;35:27–31. [PubMed] [Google Scholar]
- 26.Ma J, Mehrkens JH, Holtmannspoetter M, et al. Perfusion MRI before and after acetazolamide administration for assessment of cerebrovascular reserve capacity in patients with symptomatic internal carotid artery (ICA) occlusion: comparison with 99mTc-ECD SPECT. Neuroradiology. 2007;49:317–326. [DOI] [PubMed] [Google Scholar]
- 27.Tsuchida T, Yonekura Y, Nishizawa S, et al. Nonlinearity correction of brain perfusion SPECT based on permeability–surface area product model. J Nucl Med. 1996;37:1237–1241. [PubMed] [Google Scholar]
- 28.Walovitch RC, Hill TC, Garrity ST, et al. Characterization of technetium-99m-l,l-ECD for brain perfusion imaging, part 1: pharmacology of technetium-99m ECD in nonhuman primates. J Nucl Med. 1989;30:1892–1901. [PubMed] [Google Scholar]
- 29.Martin WR, Raichle ME. Cerebellar blood flow and metabolism in cerebral hemisphere infarction. Ann Neurol. 1983;14:168–176. [DOI] [PubMed] [Google Scholar]
- 30.Ishikawa Y, Mukawa J, Kinjo T, et al. Crossed cerebellar diaschisis in putaminal hemorrhage—evaluation by the Xe-133 clearance method. No To Shinkei. 1994;46:335–340. [PubMed] [Google Scholar]
- 31.Grubb RL, Jr, Derdeyn CP, Fritsch SM, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA. 1998;280:1055–1060. [DOI] [PubMed] [Google Scholar]
- 32.Pappata S, Mazoyer B, Tran Dinh S, et al. Effects of capsular or thalamic stroke on metabolism in the cortex and cerebellum: a positron tomography study. Stroke. 1990;21:519–524. [DOI] [PubMed] [Google Scholar]


