Abstract
Background
While cocaine use is an established risk factor for acute cardiovascular complications, associations between cocaine use and markers of cardiac injury outside of acute hospital presentation remain poorly characterized. We leveraged advances in cardiac troponin (cTnI) testing to assess low but clinically meaningful levels of cardiac injury among cocaine users and non-users.
Methods
We conducted a case control study comparing cTnI levels by the presence of cocaine among patients presenting for non-cardiac care in an urban safety net hospital. Samples were chosen sequentially among those for which urine drug screens were ordered by providers hospital-wide.
Results
During 2015, 14% of all hospital drug screens ordered were cocaine-positive. Among unique persons providing cocaine-positive (N=100) and cocaine-negative (N=100) samples, 37% were female, 45% were African-American and the median age was 51. Detectable cTnI (>0.02 ng/mL) was observed in 21 samples (11%). It was more common in subjects using cocaine (Adjusted OR=2.81; 95% CI=1.03–7.65), but not other drugs. Moreover, there was a significant correlation between concentrations of cTnI and the cocaine metabolite, benzoylecgonine (Spearman Correlation=0.34, p<0.01).
Conclusions
Among urban safety net hospital patients, 11% had detectable cTnI, and cTnI concentration was significantly correlated with benzoylecgonine concentration. While these preliminary results require additional confirmation, they suggest the potential utility of considering cocaine use as more than just an episodic exposure leading to acute cardiac events. The consideration of cocaine use as an ongoing chronic exposure leading to subclinical cardiac injury may improve risk-stratification and patient outcomes in populations where cocaine use is high.
Keywords: cocaine, benzoylecgonine, cardiac injury, troponin, cTnI
1.0 INTRODUCTION
Cocaine use leads to 64,000 acute health care visits in the US annually (Maraj et al., 2010), and with a 42% increase in the total number of cocaine-related deaths between 2001 and 2014, it resulted in 5,415 deaths in 2015 alone (National Institutes of Health, (2015). We recently reported a 3% annual mortality rate among homeless and unstably housed adult women living in San Francisco, California (Riley et al., 2013), which is roughly 10 times higher than women of the same age range (ages 45–54) in the general US population (Xu et al., 2010). While rates of co-morbidity were high in this cohort, existing health conditions did not account for the disproportionately high level of mortality. Instead, the most common cause of death was acute intoxication in which cocaine was detected at autopsy. This increase in mortality follows a national trend as deaths due to illegal drug use have increased across the US over the past decade (Miech et al., 2013).
As cocaine is a highly addictive substance, its use is highly prevalent, frequent and sustained over years in some populations, which leads to substantial health risks (Marasco et al., 2014; Spronk et al., 2013; Wolf, 2010). Specifically, the prevalence of crack cocaine use is approximately 50–60% among impoverished women (Riley et al., 2015; Torchalla et al., 2011). Between 30% and 40% of all cocaine users from communities with a high prevalence of poverty report daily use (Hayashi et al., 2016; Kuo et al., 2014), and the median number of years used among active cocaine users is as high as nine (Parker and Anthony, 2014). In addition, less than 50% of users from low-income communities have ever been in treatment (Booth et al., 2014).
According to the San Francisco Medical Examiner, at least one-third of cocaine-related deaths among San Francisco residents are directly linked to a cardiovascular event (CA Electronic Death Record System, data extracted 8/15/2012), which is consistent with existing research (Maraj et al., 2010; Phillips et al., 2009). Cocaine is a known risk factor for cardiovascular dysfunction (Grund et al., 2010; Lange and Hillis, 2001), and has been linked to a variety of complications, including thrombus formation (Stenberg et al., 1989), enhanced platelet activation (Kugelmass et al., 1993; Rezkalla et al., 1993; Rinder et al., 1994), premature atherosclerosis (Kolodgie et al., 1991), endothelial dysfunction (Gan et al., 1999), hypertrophy (Brickner et al., 1991) and aortic dissection (Hsue et al., 2002). It also exacerbates the effects of other drugs to increase cardiac complications (Tacker et al., 2006; Tacker and Okorodudu, 2004). Life-threatening arrhythmias and sudden death caused by arrhythmia related to cocaine use occur with and without cardiac risk factors (Hollander et al., 1994; Hollander et al., 1997; Hsue et al., 2007; Lange and Hillis, 2001; Minor et al., 1991).
Advances in biomarker testing are creating new opportunities to improve existing knowledge regarding cardiac risks, particularly subclinical conditions. Newer tests with higher levels of sensitivity are expanding the use of cardiac troponins I (cTnI) and T (cTnT), which are current gold standard biomarkers for detecting myocardial infarction (MI), to now detect lower levels of cardiac injury. These advances have led to the establishment of a dose-response association between troponin concentration and degree of cardiac damage (Wu, 1999). Lower concentrations of troponin can now be used to risk-stratify patients suspected of suffering an acute coronary syndrome (Apple and Collinson, 2012; Karras and Kane, 2001), and predict clinical outcomes including death among cardiac surgery patients and those with suspected acute MI (Hochholzer et al., 2011; Lurati Buse et al., 2009). The use of higher-sensitivity assays has led to the understanding that patients with detectable values far below the traditional 99th percentile cutoff, required for the diagnosis of an MI, are at increased risk for morbidity (Zethelius et al., 2006) and mortality (Jaffe, 2006; Waxman et al., 2006). Moreover, cTnI predicts clinical outcomes including death and a first coronary heart disease (CHD) event in individuals free from cardiovascular disease at baseline, indicating the importance of subclinical cardiac damage in the development of CHD and mortality (Zethelius et al., 2006).
More recently, the use of higher-sensitivity troponin has been tested, not only among hospitalized persons or patients receiving cardiac care, but also in the general population. Population-based cohort studies across the United States and Europe report that troponin is a predictor of cardiovascular events (McKie et al., 2014; Zeller et al., 2014), as well as all-cause mortality among individuals aged 30–65 (de Lemos et al., 2010). The addition of troponin to variables of established risk models improves prediction of cardiovascular death and cardiovascular disease in the general population (Blankenberg et al., 2010; Neumann et al., 2014), even in individuals free of cardiovascular disease at baseline (Blankenberg et al., 2016).
While prior work has used cardiovascular magnetic resonance (CMR) examination to indicate a high prevalence of cardiac damage in asymptomatic cocaine users (Aquaro et al., 2011), simpler and lower-cost methods of identifying cardiac injury, such as measuring peripheral cardiac troponin, have received little attention. Given that higher sensitivity troponin is now recognized as a strong predictor of future cardiac events in the general population, significant associations between cocaine and troponin could be used, not only for the treatment of existing cardiac conditions, but for prevention efforts to deter serious future dysfunction. Few studies have compared troponin concentrations by cocaine use outside of acute MI, and those that have, found no significant difference (Epelde et al., 2000; Hollander et al., 1998). However, previous studies evaluating this association are limited by small sample sizes and older, less sensitive troponin assays. Further work in this area has the potential to influence risk assessment and risk stratification in populations that include a high proportion of cocaine users.
To test the hypotheses that cocaine use is associated with detectable concentrations of cTnI, and higher concentrations of cocaine are correlated with higher levels of troponin, we conducted a case-control study among patients using both inpatient and outpatient health care, but not currently receiving care for cardiac complications, at an urban safety net hospital.
2.0 MATERIAL AND METHODS
We conducted cTnI testing among remnant serum from patients receiving lab services in health care units throughout San Francisco General Hospital. During two six-week periods occurring from June 1st, 2015 through July 9th, 2015 and December 14th, 2015 through January 25th, 2016, specimens were sequentially obtained among those for which urine drug testing was ordered by a health care provider and accompanied by a serum sample. Drug screens throughout all hospital units, including inpatient and outpatient settings, were processed by a single lab, thus specimens for the current study came from patients presenting for care hospital-wide.
To isolate the effect of cocaine, we restricted samples to those negative for amphetamines. This was done to minimize synergistic effects from commonly used stimulants other than cocaine, which are often difficult to analyze and understand in small cross-sectional studies. Given that effects from depressants oppose those of stimulants, and would therefore not likely have synergistic effects with cocaine, depressants were considered as potential confounders and no restrictions on depressant use were imposed during recruitment. The first 100 specimens to test positive for cocaine and/or the cocaine metabolite, benzoylecgonine (cocaine/benzoylecgonine), and negative for amphetamines, were considered cases; the first 100 specimens to test negative for cocaine, benzoylecgonine and amphetamines were considered controls. Samples were de-identified and linked to patient age, race and gender. Based on medical chart review of sequential eligible specimens, patients receiving care for cardiac complications were excluded, and only the first sample provided by any patient was included. Serum samples were batch tested for cTnI after all case and control specimens were collected. The study was considered to have minimal risk to human subjects as data were limited to de-identified retrospective medical records and testing of existing biological specimens. The study protocol was approved by the Institutional Review Board at the University of California, San Francisco, USA.
The primary outcome of the study was cardiac injury measured as cardiac troponin I (cTnI) (Siemens Healthcare Diagnostics, Inc.) >0.02 ng/mL. Measurements were conducted with Siemens ADVIA Centaur TnI Ultra®, a contemporary three-site sandwich immunoassay using direct chemiluminometric technology. Exposure covariates included age, race and gender, each obtained from the electronic medical record, as well as drug use (cocaine, benzoylecgonine, benzodiazepine, methadone, opiates, and oxycodone), which was directly measured by standard competitive immunoassays. Cocaine and benzoylecgonine concentrations were measured using a clinically validated liquid chromatography tandem mass spectrometry method. Due to direct effects of cocaine on heart, kidney and renal function (Gitman and Singhal, 2004; Grund et al., 2010; Hoefsloot et al., 2009; Jaffe and Kimmel, 2006; Lange and Hillis, 2001; Nzerue et al., 2000; Volcy et al., 2000), placing them in the causal pathway between cocaine use and cardiac injury, we did not adjust for these conditions in this case control study as doing so would violate analytic assumptions of independence among covariates.
We used proportional odds models to assess the association of each drug exposure with an ordinal measure of troponin concentration, categorized as low (≤0.02 ng/mL), intermediate (>0.02–0.04 ng/mL), and high (>0.04 ng/mL). In addition, we used Fisher’s exact test to assess the associations with a dichotomous indicator or troponin levels >0.02 ng/mL. Log-transformation was used to account for skewed data when comparing drug and troponin concentrations as continuous variables. Comparisons were accomplished with Kruskal-Wallis tests and analyses were conducted in Stata Version 14.1 (Stata Corp, College Station, TX). Given that this is one of the first studies to consider associations between illegal drug use and troponin, we used more inclusive methods advised by Rothman and did not adjust for multiple comparisons (Rothman, 1990).
3. 0 RESULTS
Cumulative records showed an average of 1,307 drug screens ordered monthly by San Francisco General Hospital providers during 2015, 14% of which were cocaine/benzoylecgonine-positive. During the study period, 100 case and 100 control specimens were obtained. Specimens came from a variety of hospital clinics and departments, the most common being emergency medicine (46%), general medicine primary care (31%), inpatient locations (12%), HIV primary care (5%) and psychiatry (4%). The final sample consisted of specimens from 200 individuals, of whom 71 (36%) were female, 47 (24%) were Latino/a, and 90 (45%) were African-American (Table 1). The median patient age was 51 years (Inter-Quartile Rage: 37–59). Comparing cases and controls, a higher proportion of cases were African-American and a lower proportion were Caucasian (p<0.01).
Table 1.
Characteristics of urban safety net hospital patients using non-cardiology clinics between 6/1/15–7/9/15 and 12/14/15–1/25/16 (N=200)
| Total (N=200) N (%) |
Cases (n=100) n (%) |
Controls (n=100) n (%) |
p-value* | |
|---|---|---|---|---|
| Age | Median=51 (IQR: 37–59) | Median=52 (IQR:40–57) | Median=51 (IQR: 33–60) | 0.82 |
| Female sex | 71 (36%) | 36 (36%) | 35 (35%) | 1.00 |
| Race/Ethnicity | ||||
| Caucasian | 51 (26%) | 13 (13%) | 38 (38%) | <0.01* |
| Asian/Pacific Islander | 9 (4%) | 3 (3%) | 6 (6%) | 0.31 |
| African American | 90 (45%) | 62 (62%) | 28 (28%) | <0.01* |
| Latino/a | 47 (24%) | 19 (19%) | 28 (28%) | 0.14 |
| Other | 3 (1%) | 3 (3%) | 0 | 0.16 |
| Toxicology results | ||||
| Cocaine/Benzoylecgonine (+) | 100 (50%) | 100 (100%) | 0 (0%) | -- |
| Amphetamine (+) | 0 | 0 | 0 | -- |
| Benzodiazapine (+) | 50 (25%) | 25 (25%) | 25 (25%) | 1.00 |
| Methadone (+) | 24 (12%) | 17 (17%) | 7 (7%) | 0.05* |
| Opiates (+) | 58 (29%) | 32 (32%) | 26 (26%) | 0.44 |
| Oxycodone (+)** | 29 (15%) | 12 (12%) | 17 (17%) | 0.42 |
| Number of drugs indicated by toxicology | ||||
| 0 | 53 (27%) | 0 | 53 (53%) | -- |
| 1 | 65 (32%) | 40 (40%) | 25 (25%) | 0.02* |
| >1 | 82 (41%) | 60 (60%) | 22 (22%) | <0.01* |
p<0.05
Persons who were positive for Oxycodone were a subset of persons positive for opiates
--Included in sampling criteria
Cardiac, kidney and renal dysfunction, as well as associated conditions, are directly affected by cocaine and therefore in the causal pathway between cocaine and cardiac dysfunction. For this reason, these conditions were not included in this cross sectional adjusted analysis; however, their description provides context for understanding results presented below. Based on medical chart review of these conditions, no significant differences (p>0.05) were observed between cases and controls regarding median concentrations (mg/dL) of sodium (141 vs. 140), potassium (4.2 vs. 4.1), calcium (9.0 v.s 9.1), chloride (106 vs. 106), carbon dioxide (28 vs. 28) and blood urea nitrogen (14 vs. 15). Conversely, median creatinine concentration was significantly higher in cases compared to controls (0.94 vs. 0.81, p<0.01), and glucose concentration was significantly lower (92 mg/dL vs. 100 mg/dL, p=0.04).
All controls were toxicology negative for cocaine and amphetamines, but almost half (47%) tested positive for at least one other drug. A higher proportion of cases tested positive for methadone (p=0.05), as well as multiple drugs (polydrug use) (p<0.01) (Table 1).
Among all patient samples, 21 (11%) were positive for detectable troponin. Intermediate troponin levels (0.02–0.04 ng/mL) were observed among 8 individuals (4%) and high troponin levels (>0.04 ng/mL), representing the 99th percentile and thus high enough to diagnose MI, among 13 (7%). Tests for detectable troponin levels exceeding 0.02 ng/mL and tests for trend in greater concentrations of troponin were non-significant for all drugs except cocaine/benzoylecgonine. The presence of cocaine/benzoylecgonine was significantly associated with both detectable troponin (>0.02 ng/mL) (p=0.04) and higher concentrations of troponin (p for trend=0.04) (Table 2).
Table 2.
Troponin level by presence of drug type among patients using a non-cardiology urban safety net hospital clinics between 6/1/15–7/9/15 and 12/14/15–1/25/16 (N=200)
| Troponin Level (ng/mL) | p-value | |||
|---|---|---|---|---|
| <0.02 n (row%) |
0.02–0.04 n (row%) |
>0.04 n (row%) |
||
| Cocaine/Benzoylecgonine (+) | 84 (85) | 6 (6) | 9 (9) | ptrend=0.04* ptroponin>0.02=0.04* |
| (−) | 94 (94) | 2 (2) | 4 (4) | |
| Amphetamine (+) | 0 | 0 | 0 | ptrend= -- ptroponin>0.02= -- |
| (−) | 178 (89) | 8 (4) | 13 (7) | |
| Benzodiazapine (+) | 45 (90) | 2 (4) | 3 (6) | ptrend=0.89 ptroponin>0.02=0.89 |
| (−) | 134 (89) | 6 (4) | 10 (7) | |
| Methadone (+) | 23 (96) | 1 (4) | 0 | ptrend=0.28 ptroponin>0.02=0.30 |
| (−) | 156 (89) | 7 (4) | 13 (7) | |
| Opiates (+) | 53 (91) | 2 (4) | 3 (5) | ptrend=0.58 ptroponin>0.02=0.58 |
| (−) | 126 (89) | 6 (4) | 10 (7) | |
| Oxycodone** (+) | 27 (93) | 0 | 2 (7) | ptrend=0.53 ptroponin>0.02=0.50 |
| (−) | 152 (89) | 8 (5) | 11 (6) | |
| # drugs detected | ptrend=0.63 | |||
p<0.05
Persons who were positive for Oxycodone were a subset of persons positive for opiat
The proportion of persons with detectable troponin among polydrug users was not significantly different from the proportion among those who tested positive for one drug only. In addition, there were no significant pairwise interactions between drugs. The only significant effect modification found by age, race or sex was a sex effect on the presence of benzodiazepines. Specifically, detectable troponin levels were significantly higher among persons negative for benzodiazepines, but this effect only approached statistical significance for men (p=.048). Adjusting for the interaction between male sex and benzodiazepine use resulted in a negligible increase in the association between the presence of cocaine and detectable troponin (Adjusted ORcocaine =2.81; 95% CI=1.03–7.65).
Among cocaine-users only, the log-transformed concentration of cocaine by itself (i.e., without benzoylecgonine) was not significantly correlated with the log-transformed concentration of troponin (Spearman Correlation=0.06, .5% change per SD [95% CI=−31.3% to 46.9%, p=0.98]; Figure 1). However, the concentration of benzoylecgonine by itself was (Spearman Correlation=0.34, 108% change per SD [95% CI=46.7% to 194.9%, p<0.01]; Figure 2).
Figure 1.
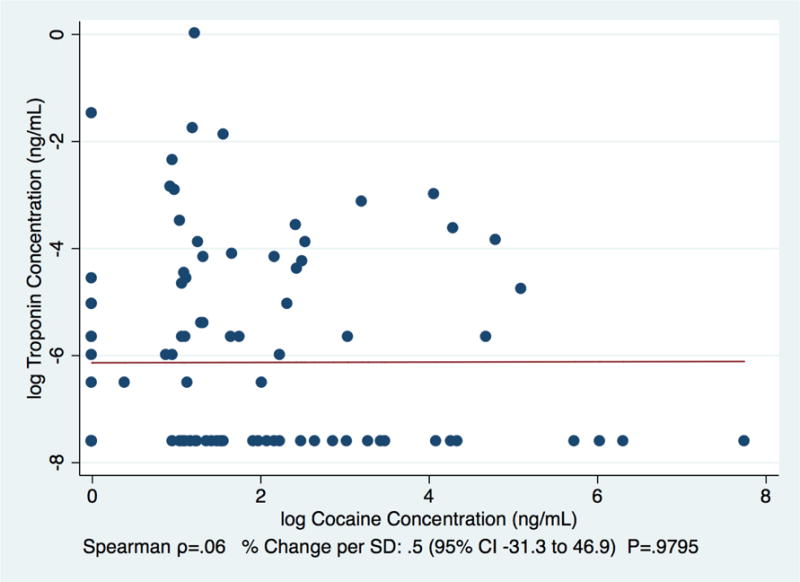
Correlation between concentrations of cocaine and troponin among patients using a non-cardiology urban safety net hospital clinics between 6/1/15–7/9/15 and 12/14/15–1/25/16 (N=100)
Figure 2.
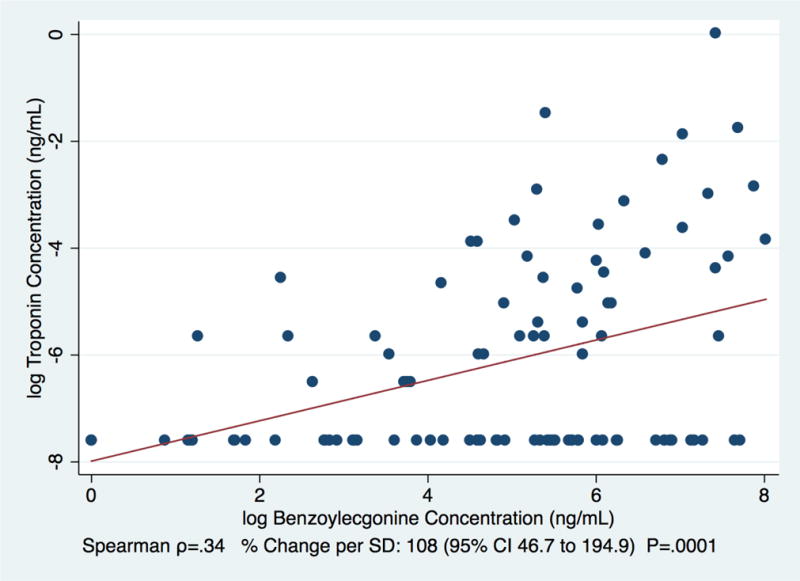
Correlation between concentrations of the cocaine metabolite, benzoylecgonine and troponin among patients using a non-cardiology urban safety net hospital clinics between 6/1/15–7/9/15 and 12/14/15–1/25/16 (N=100)
4.0 DISCUSSION
In this modest sample of urban safety net hospital patients, 11% were observed to have detectable troponin, indicating cardiac injury and elevated risk for cardiac complications. Moreover, no patient was currently being seen for cardiac care, suggesting high levels of subclinical cardiac damage. Given that 14% of approximately 15,600 drug screens ordered by hospital providers in 2015 were positive for cocaine, results presented here suggest that subclinical cardiac injury, which is known to predict cardiovascular events and all-cause mortality in the general population (Blankenberg et al., 2016; Blankenberg et al., 2010; de Lemos et al., 2010; McKie et al., 2014; Neumann et al., 2014; Zeller et al., 2014), may be pertinent for a substantial portion of patients receiving care at urban safety net hospitals.
Detectable troponin was significantly associated with cocaine/benzoylecgonine (Tables 1; Table 2). In addition, the concentration of benzoylecgonine was positively correlated with the concentration of troponin (Figure 2), while the concentration of cocaine without benzoylecgonine did not reach a level of significance (Figure 1), which is consistent with the shorter half-life of cocaine compared to benzoylecgonine. Given the previously established dose-response association between troponin concentration and degree of cardiac damage (Wu, 1999), and prior associations between cocaine use and severe cardiac conditions, as well as acute cardiac events (Hollander et al., 1994; Hollander et al., 1997; Hsue et al., 2007; Lange and Hillis, 2001; Minor et al., 1991), these results reinforce the importance of subclinical cardiac injury caused by cocaine. In particular, they highlight the potential utility of contemporary troponin testing to use detectable cTnI as a prevention-screening tool in cardiac risk assessment for populations with high proportions of cocaine users.
Outside of reports addressing acute MI, this is the first study of which we are aware to report significantly higher troponin concentrations in cocaine-using patients compared to patients who do not use cocaine. Two prior studies included patients evaluated for chest pain without acute MI and did not find significant differences in troponin by cocaine use (Epelde et al., 2000; Hollander et al., 1998). Potential reasons for non-significant findings in prior studies include smaller sample sizes (<100 patients), which limited statistical power, and older, less sensitive troponin assays. In addition, while we were not able to evaluate duration of drug use, a higher proportion of safety net hospital patients included in the current study may have been chronic cocaine users compared to previous studies, with more time to develop subclinical cardiovascular disease. Combined with previous reports showing that frequent cocaine use is highly prevalent and sustained over years in some populations (Hayashi et al., 2016; Kuo et al., 2014; Parker and Anthony, 2014; Riley et al., 2015; Torchalla et al., 2011), results presented here suggest the importance of considering cocaine use not only as an episodic phenomenon leading to acute health events, but also as a chronic condition leading to subclinical cardiac injury.
Large studies, such as the Framingham Heart Study (Pencina et al., 2009), have established assessment tools for predicting cardiovascular disease, which have been incorporated into national guidelines for assessing cardiovascular risk (Goff et al., 2014). Risk assessment tools include age, race, sex, total cholesterol, high-density lipoprotein (HDL) cholesterol, systolic blood pressure, diastolic blood pressure, treatment for high blood pressure, diabetes, smoking, and a prior heart attack or stroke. Drug use, cocaine use in particular, is not commonly considered in standard risk assessment tools. Although further research is needed, these findings suggest that evaluating for cocaine use may improve the sensitivity of cardiovascular risk assessment, particularly in hospitals and clinics caring for populations with high proportions of cocaine-using patients.
Results presented here should be considered in the context of several limitations. First, remnant biological specimens were obtained from patients whose health care provider ordered a drug test and accompanied the order with a serum sample. Such orders suggest a priori concern of drug influences, which may have overrepresented higher-risk patients among those analyzed as negative for cocaine (controls). However, this limitation would have biased results toward the null, indicating that the association between cocaine and cardiac injury is at least as strong as that reported here. Also, as information was abstracted from medical records, we did not have consistent data on duration of cocaine use or other cardiac risk factors such as smoking status and family history of heart disease. In addition, while the sample was larger than prior studies considering associations between cocaine and troponin (Epelde et al., 2000; Hollander et al., 1998), it was still relatively small, providing only limited statistical power to detect significant associations between troponin and drugs other than cocaine. For example, all individuals but one who were positive for methadone had a non-detectable troponin concentration, which may suggest a role for future larger studies in examining a potentially protective effect from methadone. In addition, due to direct effects of cocaine on heart, kidney and renal function (Gitman and Singhal, 2004; Grund et al., 2010; Hoefsloot et al., 2009; Jaffe and Kimmel, 2006; Lange and Hillis, 2001; Nzerue et al., 2000; Volcy et al., 2000), placing them in the causal pathway between cocaine use and cardiac injury, we did not adjust for these conditions in this case control analysis. Descriptive analyses confirmed higher concentrations of creatinine in cases compared to controls, suggesting mediation of cocaine’s effects on troponin by creatinine. Future longitudinal studies will be able to more accurately quantify cocaine’s direct and indirect effects on cardiac injury. Finally, to isolate the effect of cocaine, and rule out the possibility of misattributing synergistic effects from other commonly used stimulants to cocaine, we restricted samples to those negative for amphetamines. In addition, we did not test for additional lesser-used stimulants, thus results presented here cannot confirm whether multiple stimulant intake has differential or combined effects on cardiac troponin concentration. Larger prospective studies are needed to clearly delineate independent and possibly interactive effects from all stimulants. In addition, the consideration of additional drug byproducts known to have negative health consequences (e.g., cocaethylene, which results from the use of cocaine and alcohol) will help to more clearly delineate independent effects of cocaine on cardiac injury.
5.0 CONCLUSION
The association of cocaine/benzoylecgonine-positivity with elevated troponin, and the positive correlation between benzoylecgonine and troponin concentration in patients not seeking cardiac care, suggest that cocaine use results in subclinical cardiac injury, which is known to predict future cardiac events in the general population. While these preliminary results require additional confirmation, they suggest that the consideration of cocaine use as not just an episodic exposure leading to acute cardiac events, but also as an ongoing chronic exposure leading to subclinical cardiac injury, may improve cardiac risk-stratification and patient outcomes.
Highlights.
We determined associations between cocaine use and cardiac injury
Detectable cardiac troponin (cTnI) was higher in cocaine-positive subjects
Detectable cTnI was not higher in subjects positive for other drugs
Considering cocaine use as a chronic exposure may improve cardiac risk stratification
Acknowledgments
The authors thank Ryk Sheppard for his laboratory work and medical record data collection.
This study was supported by the National Institute of Drug Abuse R01 DA037012 and K24 DA039780.
Role of funding source
The National Institutes of Health and the National Institute on Drug Abuse had no role in the design or conduct of the study; the collection, management, analysis or interpretation of the data; or the preparation, review or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
E.D. Riley led the development of hypotheses, study design, interpretation of data analysis and manuscript preparation. P.Y. Hsue contributed to research conception, study design and manuscript preparation. E. Vittinghoff contributed to study design and manuscript preparation; he conducted all data analysis and created all figures. A.H.B. Wu contributed to research conception, study design, interpretation of data analysis and manuscript preparation. P.O. Coffin contributed to research conception, study design, interpretation of data analysis and manuscript preparation. P.K. Moore contributed to interpretation of data analysis and manuscript preparation. K.L. Lynch contributed to research conception, study design, interpretation of data analysis and manuscript preparation. All authors have reviewed and approved of the final manuscript before submission.
Conflict of Interest
No conflict declared.
References
- Apple FS, Collinson PO. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem. 2012;58:54–61. doi: 10.1373/clinchem.2011.165795. [DOI] [PubMed] [Google Scholar]
- Aquaro GD, Gabutti A, Meini M, Prontera C, Pasanisi E, Passino C, Emdin M, Lombardi M. Silent myocardial damage in cocaine addicts. Heart. 2011;97:2056–62. doi: 10.1136/hrt.2011.226977. [DOI] [PubMed] [Google Scholar]
- Blankenberg S, Salomaa V, Makarova N, Ojeda F, Wild P, Lackner KJ, Jorgensen T, Thorand B, Peters A, Nauck M, Petersmann A, Vartiainen E, Veronesi G, Brambilla P, Costanzo S, Iacoviello L, Linden G, Yarnell J, Patterson CC, Everett BM, Ridker PM, Kontto J, Schnabel RB, Koenig W, Kee F, Zeller T, Kuulasmaa K, BiomarCa REI. Troponin I and cardiovascular risk prediction in the general population: The BiomarCaRE consortium. Eur Heart J. 2016;37:2428–2437. doi: 10.1093/eurheartj/ehw172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenberg S, Zeller T, Saarela O, Havulinna AS, Kee F, Tunstall-Pedoe H, Kuulasmaa K, Yarnell J, Schnabel RB, Wild PS, Munzel TF, Lackner KJ, Tiret L, Evans A, Salomaa V, Project M. Contribution of 30 biomarkers to 10-year cardiovascular risk estimation in 2 population cohorts: The MONICA, risk, genetics, archiving, and monograph (MORGAM) biomarker project. Circulation. 2010;121:2388–2397. doi: 10.1161/CIRCULATIONAHA.109.901413. [DOI] [PubMed] [Google Scholar]
- Booth BM, Stewart KE, Curran GM, Cheney AM, Borders TF. Beliefs and attitudes regarding drug treatment: Application of the theory of planned behavior in African-American cocaine users. Addict Behav. 2014;39:1441–1446. doi: 10.1016/j.addbeh.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickner ME, Willard JE, Eichhorn EJ, Black J, Grayburn PA. Left ventricular hypertrophy associated with chronic cocaine abuse. Circulation. 1991;84:1130–1135. doi: 10.1161/01.cir.84.3.1130. [DOI] [PubMed] [Google Scholar]
- de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelde F, del Rio M, Argilaga R, Tomas S. Myocardial necrosis is not observed during acute cocaine abuse detectable by the serum levels of troponin T. An Med Interna. 2000;17:59–61. [PubMed] [Google Scholar]
- Gan X, Zhang L, Berger O, Stins MF, Way D, Taub DD, Chang SL, Kim KS, House SD, Weinand M, Witte M, Graves MC, Fiala M. Cocaine enhances brain endothelial adhesion molecules and leukocyte migration. Clin Immunol. 1999;91:68–76. doi: 10.1006/clim.1998.4683. [DOI] [PubMed] [Google Scholar]
- Gitman MD, Singhal PC. Cocaine-induced renal disease. Expert Opin Drug Saf. 2004;3:441–448. doi: 10.1517/14740338.3.5.441. [DOI] [PubMed] [Google Scholar]
- Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF, American College of Cardiology/American Heart Association Task Force on Practice, G 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- Grund JPC, Coffin P, Jaufreet-Roustide M, Dijkstra M, de Bruin D, Blanken P. The fast and furious —cocaine, amphetamines and harm reduction. In: Rhodes T, Hedrich D, editors. Mongraph 10: Harm Reduction: evidence, impacts and challenges. European Monitoring Centre for Drugs and Drug Addiction; Lison, Portugal: 2010. pp. 191–232. [Google Scholar]
- Hayashi K, Wood E, Kerr T, Dong H, Nguyen P, Puskas CM, Guillemi S, Montaner JS, Milloy MJ. Factors associated with optimal pharmacy refill adherence for antiretroviral medications and plasma HIV RNA non-detectability among HIV-positive crack cocaine users: A prospective cohort study. BMC Infect Dis. 2016;16:455. doi: 10.1186/s12879-016-1749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochholzer W, Reichlin T, Twerenbold R, Stelzig C, Hochholzer K, Meissner J, Haaf P, Schaub N, Steuer S, Bassetti S, Reiter M, Roost K, Freidank H, Winkler K, Mueller C. Incremental value of high-sensitivity cardiac troponin T for risk prediction in patients with suspected acute myocardial infarction. Clin Chem. 2011;57:1318–1326. doi: 10.1373/clinchem.2011.162073. [DOI] [PubMed] [Google Scholar]
- Hoefsloot W, de Vries RA, Bruijnen R, Bosch FH. Renal infarction after cocaine abuse: A case report and review. Clin Nephrol. 2009;72:234–236. doi: 10.5414/cnp72234. [DOI] [PubMed] [Google Scholar]
- Hollander JE, Hoffman RS, Gennis P, Fairweather P, DiSano MJ, Schumb DA, Feldman JA, Fish SS, Dyer S, Wax P, et al. Prospective multicenter evaluation of cocaine-associated chest pain. Cocaine Associated Chest Pain (COCHPA) Study Group. Acad Emerg Med. 1994;1:330–339. doi: 10.1111/j.1553-2712.1994.tb02639.x. [DOI] [PubMed] [Google Scholar]
- Hollander JE, Levitt MA, Young GP, Briglia E, Wetli CV, Gawad Y. Effect of recent cocaine use on the specificity of cardiac markers for diagnosis of acute myocardial infarction. Am Heart J. 1998;135:245–252. doi: 10.1016/s0002-8703(98)70089-4. [DOI] [PubMed] [Google Scholar]
- Hollander JE, Vignona L, Burstein J. Predictors of underlying coronary artery disease in cocaine associated myocardial infarction: a meta-analysis of case reports. Vet Hum Toxicol. 1997;39:276–280. [PubMed] [Google Scholar]
- Hsue PY, McManus D, Selby V, Ren X, Pillutla P, Younes N, Goldschlager N, Waters DD. Cardiac arrest in patients who smoke crack cocaine. Am J Cardiol. 2007;99:822–824. doi: 10.1016/j.amjcard.2006.10.044. [DOI] [PubMed] [Google Scholar]
- Hsue PY, Salinas CL, Bolger AF, Benowitz NL, Waters DD. Acute aortic dissection related to crack cocaine. Circulation. 2002;105:1592–1595. doi: 10.1161/01.cir.0000012524.44897.3a. [DOI] [PubMed] [Google Scholar]
- Jaffe AS. Chasing troponin: how low can you go if you can see the rise? J Am Coll Cardiol. 2006;48:1763–1764. doi: 10.1016/j.jacc.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Jaffe JA, Kimmel PL. Chronic nephropathies of cocaine and heroin abuse: A critical review. Clin J Am Soc Nephrol. 2006;1:655–667. doi: 10.2215/CJN.00300106. [DOI] [PubMed] [Google Scholar]
- Karras DJ, Kane DL. Serum markers in the emergency department diagnosis of acute myocardial infarction. Emerg Med Clin North Am. 2001;19:321–337. doi: 10.1016/s0733-8627(05)70186-3. [DOI] [PubMed] [Google Scholar]
- Kolodgie FD, Virmani R, Cornhill JF, Herderick EE, Smialek J. Increase in atherosclerosis and adventitial mast cells in cocaine abusers: an alternative mechanism of cocaine-associated coronary vasospasm and thrombosis. J Am Coll Cardiol. 1991;17:1553–1560. doi: 10.1016/0735-1097(91)90646-q. [DOI] [PubMed] [Google Scholar]
- Kugelmass AD, Oda A, Monahan K, Cabral C, Ware JA. Activation of human platelets by cocaine. Circulation. 1993;88:876–883. doi: 10.1161/01.cir.88.3.876. [DOI] [PubMed] [Google Scholar]
- Kuo I, Golin CE, Wang J, Haley DF, Hughes J, Mannheimer S, Justman J, Rompalo A, Frew PM, Adimora AA, Soto-Torres L, Hodder S, Team, H.S. Substance use patterns and factors associated with changes over time in a cohort of heterosexual women at risk for HIV acquisition in the United States. Drug Alcohol Depend. 2014;139:93–99. doi: 10.1016/j.drugalcdep.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange RA, Hillis LD. Cardiovascular complications of cocaine use. N Engl J Med. 2001;345:351–358. doi: 10.1056/NEJM200108023450507. [DOI] [PubMed] [Google Scholar]
- Lurati Buse GA, Koller MT, Grapow M, Bruni CM, Kasper J, Seeberger MD, Filipovic M. 12-month outcome after cardiac surgery: prediction by troponin T in combination with the European system for cardiac operative risk evaluation. Ann Thorac Surg. 2009;88:1806–1812. doi: 10.1016/j.athoracsur.2009.07.080. [DOI] [PubMed] [Google Scholar]
- Maraj S, Figueredo VM, Lynn Morris D. Cocaine and the heart. Clin Cardiol. 2010;33:264–269. doi: 10.1002/clc.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasco CC, Goodwin CR, Winder DG, Schramm-Sapyta NL, McLean JA, Wikswo JP. Systems-level view of cocaine addiction: The interconnection of the immune and nervous systems. Exp Biol Med (Maywood) 2014;239:1433–1442. doi: 10.1177/1535370214537747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKie PM, AbouEzzeddine OF, Scott CG, Mehta R, Rodeheffer RJ, Redfield MM, Burnett JC, Jr, Jaffe AS. High-sensitivity troponin I and amino-terminal pro–B-type natriuretic peptide predict heart failure and mortality in the general population. Clin Chem. 2014;60:1225–1233. doi: 10.1373/clinchem.2014.222778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miech R, Koester S, Dorsey Holliman B. Towards an Explanation of the Recent Increase in Drug-Related Mortality. Springer; New York: 2013. [Google Scholar]
- Minor RL, Jr, Scott BD, Brown DD, Winniford MD. Cocaine-induced myocardial infarction in patients with normal coronary arteries. Ann Intern Med. 1991;115:797–806. doi: 10.7326/0003-4819-115-10-797. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse, N. National Overdose Death Rates: Number of Deaths from Cocaine 2015 [Google Scholar]
- Neumann JT, Havulinna AS, Zeller T, Appelbaum S, Kunnas T, Nikkari S, Jousilahti P, Blankenberg S, Sydow K, Salomaa V. Comparison of three troponins as predictors of future cardiovascular events–prospective results from the FINRISK and BiomaCaRE studies. PLoS One. 2014;9:e90063. doi: 10.1371/journal.pone.0090063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nzerue CM, Hewan-Lowe K, Riley LJ., Jr Cocaine and the kidney: A synthesis of pathophysiologic and clinical perspectives. Am J Kidney Dis. 2000;35:783–795. doi: 10.1016/s0272-6386(00)70246-0. [DOI] [PubMed] [Google Scholar]
- Parker MA, Anthony JC. Should anyone be riding to glory on the now-descending limb of the crack-cocaine epidemic curve in the United States? Drug Alcohol Depend. 2014;138:225–228. doi: 10.1016/j.drugalcdep.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pencina MJ, D’Agostino RB, Sr, Larson MG, Massaro JM, Vasan RS. Predicting the 30-year risk of cardiovascular disease: the framingham heart study. Circulation. 2009;119:3078–3084. doi: 10.1161/CIRCULATIONAHA.108.816694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips K, Luk A, Soor GS, Abraham JR, Leong S, Butany J. Cocaine cardiotoxicity: A review of the pathophysiology, pathology, and treatment options. Am J Cardiovasc Drugs. 2009;9:177–196. doi: 10.2165/00129784-200909030-00005. [DOI] [PubMed] [Google Scholar]
- Rezkalla SH, Mazza JJ, Kloner RA, Tillema V, Chang SH. Effects of cocaine on human platelets in healthy subjects. Am J Cardiol. 1993;72:243–246. doi: 10.1016/0002-9149(93)90173-a. [DOI] [PubMed] [Google Scholar]
- Riley ED, Cohen J, Shumway M. Overdose fatality and surveillance as a method for understanding mortality trends in homeless populations. JAMA Intern Med. 2013;173:1264. doi: 10.1001/jamainternmed.2013.6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley ED, Shumway M, Knight KR, Guzman D, Cohen J, Weiser SD. Risk factors for stimulant use among homeless and unstably housed adult women. Drug Alcohol Depend. 2015;153:173–179. doi: 10.1016/j.drugalcdep.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinder HM, Ault KA, Jatlow PI, Kosten TR, Smith BR. Platelet alpha-granule release in cocaine users. Circulation. 1994;90:1162–1167. doi: 10.1161/01.cir.90.3.1162. [DOI] [PubMed] [Google Scholar]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- Spronk DB, van Wel JH, Ramaekers JG, Verkes RJ. Characterizing the cognitive effects of cocaine: A comprehensive review. Neurosci Biobehav Rev. 2013;37:1838–1859. doi: 10.1016/j.neubiorev.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Stenberg RG, Winniford MD, Hillis LD, Dowling GP, Buja LM. Simultaneous acute thrombosis of two major coronary arteries following intravenous cocaine use. Arch Pathol Lab Med. 1989;113:521–524. [PubMed] [Google Scholar]
- Tacker DH, Herzog NK, Okorodudu AO. Cocaethylene affects human microvascular endothelial cell p38 mitogen-activated protein kinase activation and nuclear factor-kappaB DNA-binding activity. Clin Chem. 2006;52:1926–1933. doi: 10.1373/clinchem.2005.065250. [DOI] [PubMed] [Google Scholar]
- Tacker DH, Okorodudu AO. Evidence for injurious effect of cocaethylene in human microvascular endothelial cells. Clin Chim Acta. 2004;345:69–77. doi: 10.1016/j.cccn.2004.02.031. [DOI] [PubMed] [Google Scholar]
- Torchalla I, Strehlau V, Li K, Krausz M. Substance use and predictors of substance dependence in homeless women. Drug Alcohol Depend. 2011;118:173–179. doi: 10.1016/j.drugalcdep.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Volcy J, Nzerue CM, Oderinde A, Hewan-Iowe K. Cocaine-induced acute renal failure, hemolysis, and thrombocytopenia mimicking thrombotic thrombocytopenic purpura. Am J Kidney Dis. 2000;35:E3. doi: 10.1016/S0272-6386(00)70321-0. [DOI] [PubMed] [Google Scholar]
- Waxman DA, Hecht S, Schappert J, Husk G. A model for troponin I as a quantitative predictor of in-hospital mortality. J Am Coll Cardiol. 2006;48:1755–1762. doi: 10.1016/j.jacc.2006.05.075. [DOI] [PubMed] [Google Scholar]
- Wolf ME. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci. 2010;33:391–398. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AH. A comparison of cardiac troponin T and cardiac troponin I in patients with acute coronary syndromes. Coron Artery Dis. 1999;10:69–74. [PubMed] [Google Scholar]
- Xu J, Kochanek KD, Murphy SL, Tejada-Vera B. 2007 National Vital Statistics. 2010. (National Vital Statistics Report 58). [PubMed] [Google Scholar]
- Zeller T, Tunstall-Pedoe H, Saarela O, Ojeda F, Schnabel RB, Tuovinen T, Woodward M, Struthers A, Hughes M, Kee F, Salomaa V, Kuulasmaa K, Blankenberg S, Investigators, M High population prevalence of cardiac troponin I measured by a high-sensitivity assay and cardiovascular risk estimation: The MORGAM Biomarker Project Scottish Cohort. Eur Heart J. 2014;35:271–281. doi: 10.1093/eurheartj/eht406. [DOI] [PubMed] [Google Scholar]
- Zethelius B, Johnston N, Venge P. Troponin I as a predictor of coronary heart disease and mortality in 70-year-old men: A community-based cohort study. Circulation. 2006;113:1071–1078. doi: 10.1161/CIRCULATIONAHA.105.570762. [DOI] [PubMed] [Google Scholar]


