Abstract
Background
In parallel with the obesity epidemic, liver transplantation for nonalcoholic steatohepatitis (NASH) is increasing dramatically in North America. Although survival outcomes are similar to other etiologies, liver transplantation in the NASH population has been associated with significantly increased resource utilization. We sought to compare outcomes between live donor liver transplantation (LDLT) and deceased donor liver transplantation (DDLT) at a high volume North American transplant center, with a particular focus on resource utilization.
Methods
The study population consists of primary liver transplants performed for NASH at Toronto General Hospital from 2000 to 2014. Recipient characteristics, perioperative outcomes, graft and patient survivals, and resource utilization were compared for LDLT versus DDLT.
Results
A total of 176 patients were included in the study (48 LDLT vs 128 DDLT). LDLT recipients had a lower model for end-stage liver disease score and were less frequently hospitalized prior to transplant. Estimated blood loss and early markers of graft injury were lower for LDLT. LDLT recipients had a significantly shorter hospitalization (intensive care unit, postoperative, and total hospitalization).
Conclusions
LDLT for NASH facilitates transplantation of patients at a less severe stage of disease, which appears to promote a faster postoperative recovery with less resource utilization.
The prevalence of nonalcoholic fatty liver disease (NAFLD) has increased dramatically in North America over the past 3 decades, in parallel with the obesity epidemic. NAFLD affects approximately 30% of US population and progresses to a more severe disease state termed nonalcoholic steatohepatitis (NASH) in approximately 20% of individuals.1-3 Approximately 20% of patients with NASH progress to cirrhosis, and approximately half of those individuals go on to develop decompensated liver failure.1,3,4 Histologically, the liver injury in NASH is characterized by hepatic steatosis, lobular hepatitis, cellular ballooning, focal necrosis, and fibrosis.1 Clinically, NASH is frequently associated with other manifestations of the metabolic syndrome, including obesity, type 2 diabetes, dyslipidemia, and hypertension. NASH is currently the fastest growing indication for liver transplantation (LT) in the United States and projects to overtake hepatitis C as the top indication for liver transplantation in the near future.5-7
Most studies examining outcomes of LT for NASH have demonstrated that graft and patient survival are comparable to other etiologies, despite the increased frequency of comorbidities, such as diabetes and obesity.7-15 In the current era, the true impact of these comorbidities may become more apparent when examining resource utilization. Several recent studies demonstrate increased resource utilization after LT in patients with diabetes and obesity.16-19 Agopian and colleagues15 at the University of California Los Angeles demonstrated that LT for NASH was associated with longer operative times, greater blood loss, and longer posttransplant hospitalization compared with other etiologies. The issue of resource utilization is of particular importance given the rapid rise of NASH as a leading indication for LT.
In the context of the severe shortage of deceased donor organs, live donor liver transplantation (LDLT) has proven to be a safe and effective alternative. At the University of Toronto, we have developed our living donor program over the past 15 years and have performed LDLT for a variety of indications across a spectrum of disease severity, including patients with hepatorenal syndrome and fulminant hepatic failure.20,21 We have demonstrated a survival advantage from time of listing for patients who undergo LDLT, reflecting diminished waitlist morbidity and mortality.22 More generally, we have observed that LDLT facilitates the transplantation of patients at an earlier stage of illness, which may have implications for speed of recovery and associated resource utilization.
Given the rapid rise of NASH in the setting of ongoing organ scarcity, we sought to examine our experience with LDLT in this population. There is a relative paucity of literature on this topic, and no studies have compared LDLT to the reference standard, deceased donor liver transplantation (DDLT). The purpose of this study was to compare outcomes between LDLT and DDLT for NASH, with particular attention to resource utilization at a high volume North American center.
MATERIALS AND METHODS
Study Cohort and Data Collection
The study population consists of primary liver transplants performed for NASH from 2000 to 2014 at Toronto General Hospital. The diagnosis of NASH was made by histopathologic data and/or clinical criteria including the absence of alcohol history, exclusion of all other forms of chronic liver disease by serologic testing, and the presence of at least 1 related comorbidity including obesity, type 2 diabetes mellitus (DM), hyperlipidemia, and hypertension. Patients with any coexisting diagnosis of chronic liver disease (viral hepatitis, alcohol, and so on) were excluded to ensure that NASH was the primary etiology in this cohort. Patients with associated hepatocellular carcinoma (HCC) were included in the study.
The following clinical and demographic data were retrospectively extracted from a prospectively collected electronic transplant database (Organ Transplant Tracking Record [OTTR]: Transplant Care Platform 6, OTTR Chronic Care Solutions, Omaha, NE): recipient characteristics (age, sex, BMI, presence of HCC, pretransplant DM, location at the time of transplant, creatinine, model for end-stage liver disease [MELD], and waiting time), perioperative outcomes (cold and warm ischemia times, estimated blood loss, biliary reconstruction, peak aspartate aminotransferase (AST)/alanine aminotransferase (ALT)/international normalized ratio (INR) at 48 hours, Dindo-Clavien score, biliary stricture, biliary leak, hepatic artery thrombosis, length of intensive care unit (ICU) and total hospitalization, 90-day graft loss, 90-day patient death, and need for retransplantation), graft/patient survival, and living donor characteristics (relationship to recipient, donor length of hospitalization, and complication rate).
Data Analysis and Statistics
Comparisons were made between LDLT and DDLT for the above parameters. For continuous variables, data are reported as mean (standard deviation) or median [interquartile range] as dictated by the data distribution. Categorical variables are reported as number (percentage). Continuous variables were compared using the Student t test or Mann-Whitney test as dictated by the data distribution. Categorical variables were compared using χ2 or Fischer exact test.
Linear regression analysis was performed to examine factors contributing to posttransplant length of hospitalization. First, a series of univariable analyses were performed to assess variables hypothesized to contribute to posttransplant length of hospitalization. Those variables found to be significant on univariable analysis (P < 0.05) were then included in a multivariable analysis.
Survival analyses were performed using Kaplan-Meier methodology, and comparisons between LDLT and DDLT were made using the log-rank test. Graft survival was calculated in uncensored fashion, with failure events including death with a functioning graft and retransplantation.
LDLT Practice at the University of Toronto
At our institution, the decision to list a patient for LT is made by a multidisciplinary team including transplant surgeons, hepatologists, and mental health/social work professionals, independent of the availability of a live donor. Once a patient has been activated on the waitlist, they are encouraged to consider LDLT, irrespective of disease etiology or severity. Potential live donors and recipients are advised of the risks and benefits of this approach, including discussion of our program’s historical outcomes.23-26 In our program, adult-to-adult LDLT is performed using right lobe grafts in the vast majority of cases. The decision regarding whether to include the middle hepatic vein in the graft is made on a case-by-case basis after considering anatomic parameters (size of segment 5/8 outflow veins) and CT volumetry, with a desired graft recipient-body weight ratio (GRWR) of 0.8% or greater.
RESULTS
From 2000 to 2014, a total of 176 liver transplants were performed for a primary diagnosis of NASH. During the study period, an increasing percentage of liver transplants were performed for NASH (Figure 1). Among the 176 transplants performed for NASH, there were 48 LDLT and 128 DDLT, with a median follow-up of 52.9 months. All 48 LDLTs were performed with right lobe grafts, with 11 (22.9%) including the middle hepatic vein.
FIGURE 1.
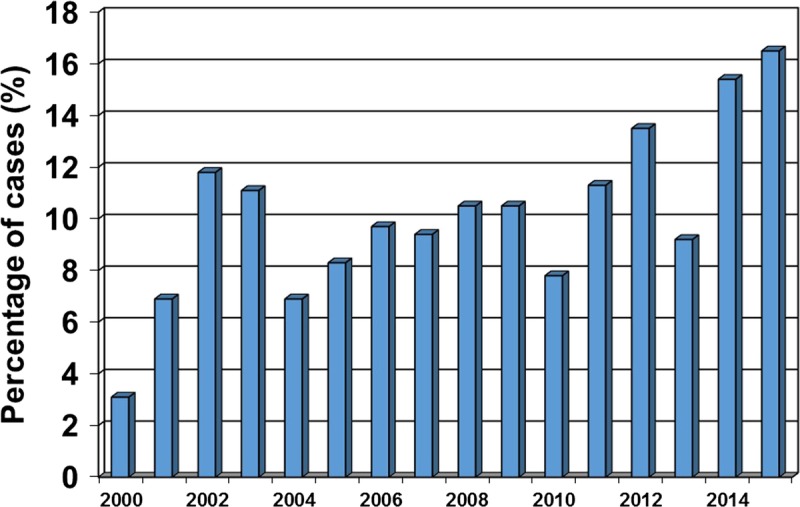
Percentage of LTs performed for NASH by year, Toronto General Hospital.
Recipient Characteristics
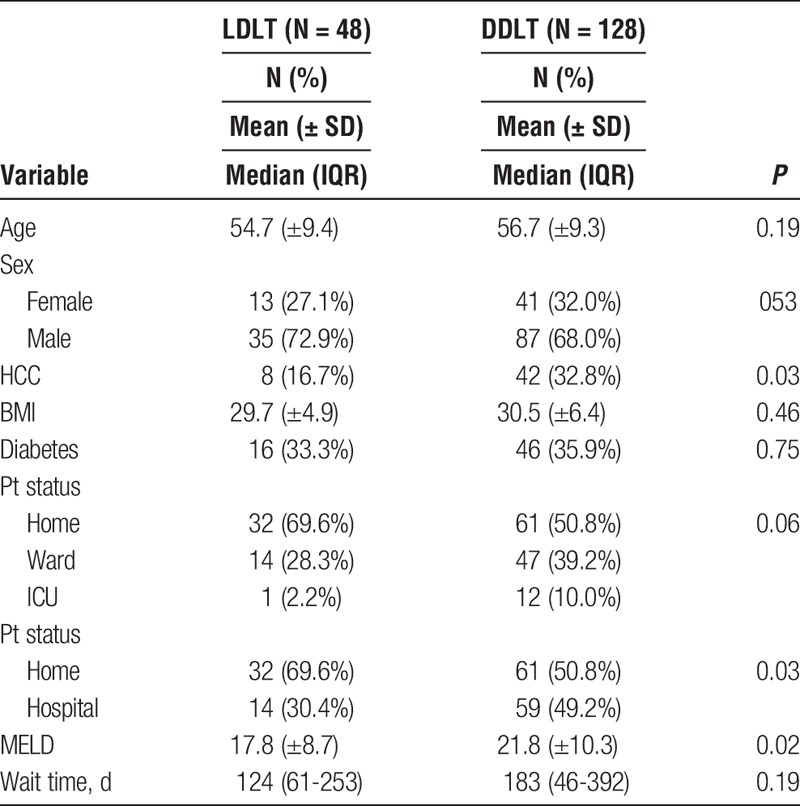
Recipient characteristics are shown in Table 1. Recipient age and sex distribution were similar between groups. There was a lower prevalence of concomitant HCC in the LDLT group compared with DDLT (16.7% vs 32.8%; P = 0.03). BMI was similar between groups (29.7 ± 4.9 vs 30.5 ± 6.4; P = 0.46). The rate of pretransplant DM was similar between groups (33.3% vs 35.9%; P = 0.75). A lower proportion of the LDLT patients was hospitalized before transplant (30.4% vs 49.2%; P = 0.03). Preoperative creatinine was lower in the LDLT group (78 mmol/L [67-117] vs 115 mmol/L [79-163]; P = 0.002). The mean MELD score was lower for LDLT than DDLT (17.8 ± 8.7 vs 21.8 ± 10.3; P = 0.02). The median waiting time prior to transplant was similar between groups (124 days [61-253] vs 183 days [46-392]; P = 0.19).
TABLE 1.
Recipient characteristics
Operative Parameters and Early Markers of Graft Injury/Function
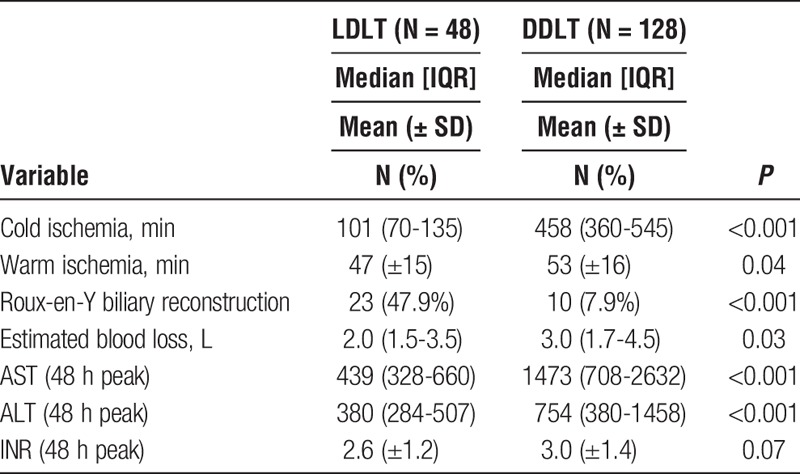
Operative parameters and early markers of graft injury and function are shown in Table 2. Both cold ischemia time (101 minutes [70-135] vs 458 minutes [360-545]; P <0.001) and warm ischemia time (47 ± 15 minutes vs. 53 ± 16 minutes, P = 0.04) were shorter for LDLT. There was a significantly higher rate of roux-en-Y biliary reconstruction in the LDLT group (48.0% vs 7.9%; P <0.001). Estimated blood loss was significantly lower for LDLT than DDLT (2.0 L [1.5-3.5] vs. 3.0 L [1.7-4.5]; P = 0.03). Peak AST (439 [328-660] vs 1473 [708-2632]; P <0.001) and peak ALT (380 [284-507] vs. 754 [380-1458]; P < 0.001) in the first 48 hours were significantly lower for LDLT than DDLT. There was a trend toward lower peak INR for LDLT, but this did not reach statistical significance (2.6 ± 1.2 vs 3.0 ± 1.4; P = 0.07).
TABLE 2.
Operative parameters and early markers of graft function
Postoperative Recipient Outcomes
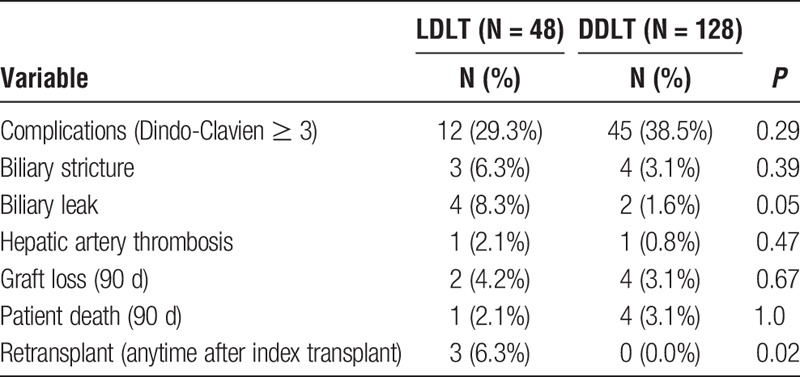
Postoperative outcomes are shown in Table 3. The rate of severe complications (Dindo-Clavien score ≥ 3) was similar between groups (29.3% vs 38.5%, P = 0.29). The rate of hepatic artery thrombosis was similar between groups (2.1% vs 0.8%, P = 0.47). There was no difference in the rate of biliary stricture between groups (6.3% vs 3.1%, P = 0.39), but there was a significantly increased rate of biliary leak in the LDLT group (8.3% vs 1.6%, P = 0.05). Ninety-day graft loss (4.2% vs 3.1%, P = 0.67) and 90-day patient death (2.1% vs 3.1%, P = 1.0) were similar between groups.
TABLE 3.
Postoperative outcomes
Resource Utilization
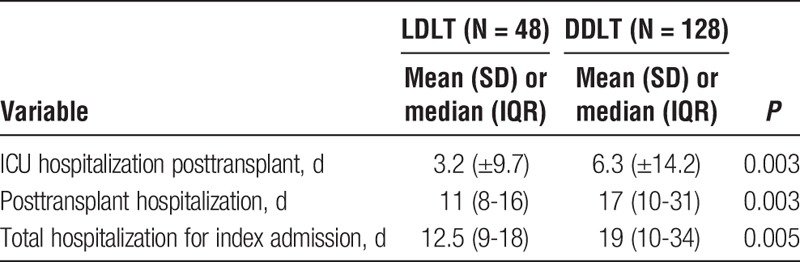
We used length of hospitalization as a surrogate for overall resource utilization, as has been previously established15,27 (Table 4). LDLT recipients had a significantly shorter mean ICU hospitalization (3.2 ± 9.7 days vs 6.3 ± 14.2 days, P = 0.003). Median posttransplant hospitalization was also significantly shorter for LDLT than DDLT (11 days [8-16] vs 17 days [10-31], P = 0.003). Finally, median total hospitalization for the index admission was significantly shorter for LDLT (12.5 days [9-18] vs 19 days [10-34], P = 0.005), reflecting the decreased need for pretransplant hospitalization and faster posttransplant recovery in this population.
TABLE 4.
Duration of hospitalization, a surrogate for resource utilization
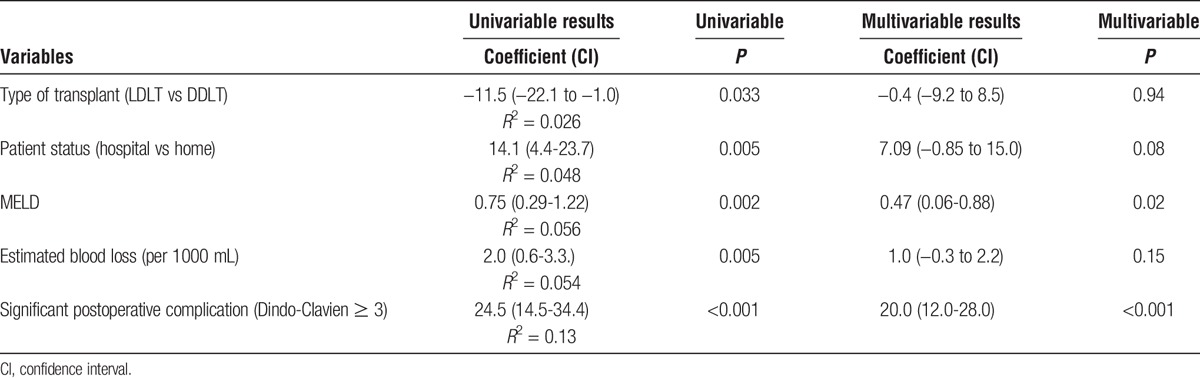
To assess the underlying factors contributing to these observations, we performed a linear regression analysis of variables that were hypothesized to contribute to length of posttransplant hospitalization. Variables tested included era of transplant, recipient age, sex, BMI, presence of diabetes, patient location pretransplant (hospital vs. home), MELD score, type of transplant (LDLT vs DDLT), estimated blood loss, and occurrence of severe posttransplant complication (Dindo-Clavien ≥ 3). The variables that were significant on univariable analysis (hospitalization pretransplant, MELD score, type of transplant, estimated blood loss, and occurrence of a severe postoperative complication; all P <0.05) were then included in a multivariable analysis (Table 5). In the multivariable model, the 2 factors that were found to be independently associated with posttransplant length of hospitalization were MELD score and the occurrence of a significant complication (Dindo-Clavien score ≥ 3).
TABLE 5.
Linear regression analysis of factors contributing to length of posttransplant hospitalization
Long-Term Survival Analyses and Retransplantation
Uncensored graft survival was similar between groups (Figure 2, P = 0.47). One-, 3-, and 5-year graft survival was 90%, 87%, and 84% for LDLT versus 91%, 86%, and 81% for DDLT. Patient survival was also similar between groups (Figure 3, P = 0.94). One-, 3-, and 5-year patient survival was 92%, 92%, and 88% for LDLT versus 91%, 86%, and 81% for DDLT. The overall retransplant rate (anytime after the index transplant) was significantly higher for LDLT (6.3% vs 0%, P = 0.02). The reasons for retransplantation in the LDLT group included hepatic artery thrombosis (1 of 3), biliary complications (1 of 3), and chronic rejection (1 of 3).
FIGURE 2.
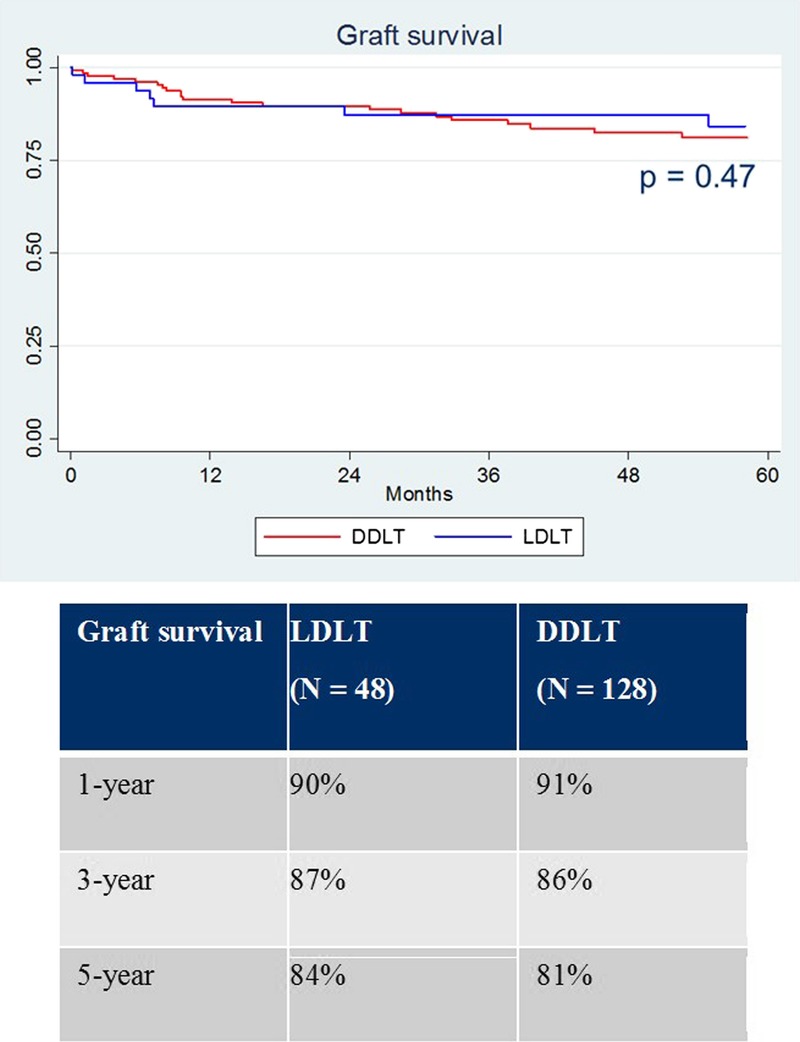
Graft survival.
FIGURE 3.
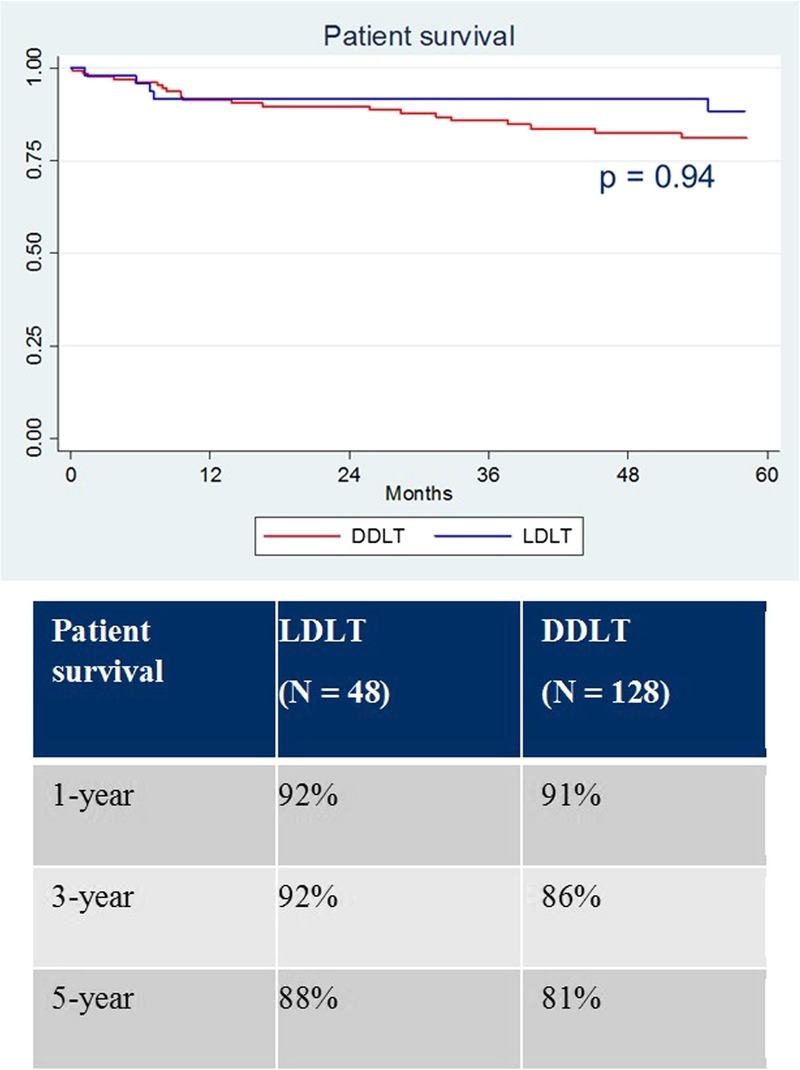
Patient survival.
Living Donor Characteristics and Outcomes
The mean GRWR in the study was 0.88% (±0.38%). The median GRWR (interquartile range) was 0.93% (0.75%-1.07%). Of the 48 total living donors in the study, 28 (58.3%) were genetically related to the recipient. There were no differences in perioperative complications based on genetic relationship (Dindo-Clavien score ≥ 3, biliary stricture, biliary leak, hepatic artery thrombosis, 90-day graft loss, 90-day patient death, need for retransplantation; all P > 0.05). Similarly, there was no difference in graft or patient survival observed based on genetic relationship (log-rank test P = 0.19 and 0.12, respectively). The mean donor length of hospitalization was 6.4 days (±1.4). No living donors required ICU hospitalization. The overall incidence of donor complications at 30 days was 16.7%. Severe complications (Dindo-Clavien ≥ 3B) occurred in 4 (8.3%) patients and included subphrenic abscess requiring percutaneous drainage, wound dehiscence requiring return to the operating room, and postoperative hemorrhage requiring return to the operating room (in 2 patients).
DISCUSSION
The growing prevalence of NAFLD and NASH in North America in the context of the obesity epidemic is a significant public health concern. In the United States, NASH is projected to overtake hepatitis C as the dominant indication for LT in the near future.5-7 Although both registry and single-center studies demonstrate that survival outcomes for NASH appear to be comparable to other indications,7-15 the medical complexity of this patient population can lead to significantly increased resource utilization both before and after LT.15
Very few studies have specifically examined outcomes after LDLT for NASH, but the limited data available suggest it is safe and effective. Tanaka and colleagues28 from Japan reported their experience with 7 patients who underwent LDLT for NASH with 100% patient and graft survivals at a median follow up of 5 years. Malik and colleagues9 included their experience with 13 LDLTs among 98 LTs performed for NASH, and found similar patient survival compared to LT performed for other etiologies, although a focused analysis of LDLT was not performed.
Our study is the largest series examining outcomes of LDLT for NASH, and the North American study population makes it highly relevant to the burgeoning NASH epidemic. Our study demonstrates that LDLT appears to be safe and effective for NASH, with similar perioperative outcomes and graft and patient survival in comparison to DDLT. In our estimation, the key finding from this study is that LDLT facilitates earlier transplantation of NASH patients at a less decompensated state. LDLT patients had a shorter wait time, lower MELD score, and were less frequently in-hospital at the time of transplant. The difference in patient acuity between the groups had a direct impact on posttransplant resource utilization, because DDLT patients experienced a more prolonged recovery after LT with significantly longer ICU and posttransplant hospitalization. Our multivariable analysis of factors contributing to posttransplant hospitalization demonstrated the 2 highest impact variables were MELD score and the occurrence of a significant complication. This finding supports the intuition that transplantation at an earlier stage of disease is advantageous, regardless of surgical approach. The natural history of patients with decompensated NASH cirrhosis suggests that medical management is unlikely to improve their condition enough to avoid transplant.4,29 In light of this progressive natural history and the findings in our study, it appears reasonable to consider LDLT early in the course of NASH to promote a faster recovery and decreased resource utilization posttransplant.
There are key limitations of this study that should be recognized, primarily related to its retrospective single center study design. A subject of interest in NASH is the recurrence rate after LT, as patients still have the same metabolic risk factors predisposing to NASH posttransplant.30-33 Most of the literature on NASH recurrence has come from centers who perform protocol liver biopsies at defined time points posttransplant, which we do not perform at our center. However, our study does suggest that recurrent NASH is unlikely to be a common cause of graft loss. Of the 3 patients who required retransplantation in this study, 1 was for hepatic artery thrombosis, 1 was for biliary complications, and 1 was for chronic rejection. An additional limitation to note is that we have not assessed actual hospital costs in this analysis, instead using length of hospitalization as a surrogate for resource utilization, similar to what has been previously published.15,27 A cost analysis may be useful in the future for a detailed comparison of the financial impact of longer hospitalization.
In conclusion, the rising prevalence of NAFLD and NASH in North America requires attention by the transplant community. LDLT appears to be safe and effective in this population, but the real advantage of this strategy may lie in transplanting patients at a less decompensated state, thereby reducing waitlist morbidity and facilitating a smoother recovery posttransplant. In the current environment with an increased emphasis on providing high quality yet cost-effective health care, considering LDLT for this growing patient population may help stem the tide of the upcoming NASH epidemic.
Footnotes
Published online 10 May, 2017.
The authors declare no funding or conflicts of interest.
A.B. and N.G. participated in the collection of data. A.B. and N.G. participated in the data analysis. A.B., N.G., A.A., G.S., and D.G. participated in the drafting of the article. M.D., D.A., L.L., E.R., N.S., M.B., M.C., A.G., I.M., M.S., and P.G. participated in the critical review and revision.
REFERENCES
- 1.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. Jama. 2015;313:2263–2273. [DOI] [PubMed] [Google Scholar]
- 2.Zezos P, Renner EL. Liver transplantation and non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:15532–15538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. [DOI] [PubMed] [Google Scholar]
- 4.Sanyal AJ, Banas C, Sargeant C, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006;43:682–689. [DOI] [PubMed] [Google Scholar]
- 5.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188–2195. [DOI] [PubMed] [Google Scholar]
- 6.Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. [DOI] [PubMed] [Google Scholar]
- 7.Singal AK, Guturu P, Hmoud B, et al. Evolving frequency and outcomes of liver transplantation based on etiology of liver disease. Transplantation. 2013;95:755–760. [DOI] [PubMed] [Google Scholar]
- 8.Wong RJ, Chou C, Bonham CA, et al. Improved survival outcomes in patients with non-alcoholic steatohepatitis and alcoholic liver disease following liver transplantation: an analysis of 2002–2012 United Network for Organ Sharing data. Clin Transplant. 2014;28:713–721. [DOI] [PubMed] [Google Scholar]
- 9.Malik SM, deVera ME, Fontes P, et al. Outcome after liver transplantation for NASH cirrhosis. Am J Transplant. 2009;9:782–793. [DOI] [PubMed] [Google Scholar]
- 10.Charlton MR, Burns JM, Pedersen RA, et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249–1253. [DOI] [PubMed] [Google Scholar]
- 11.Bhagat V, Mindikoglu AL, Nudo CG, et al. Outcomes of liver transplantation in patients with cirrhosis due to nonalcoholic steatohepatitis versus patients with cirrhosis due to alcoholic liver disease. Liver Transpl. 2009;15:1814–1820. [DOI] [PubMed] [Google Scholar]
- 12.Barritt AS, 4th, Dellon ES, Kozlowski T, et al. The influence of nonalcoholic fatty liver disease and its associated comorbidities on liver transplant outcomes. J Clin Gastroenterol. 2011;45:372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Afzali A, Berry K, Ioannou GN. Excellent posttransplant survival for patients with nonalcoholic steatohepatitis in the United States. Liver Transpl. 2012;18:29–37. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy C, Redden D, Gray S, et al. Equivalent survival following liver transplantation in patients with non-alcoholic steatohepatitis compared with patients with other liver diseases. HPB (Oxford). 2012;14:625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agopian VG, Kaldas FM, Hong JC, et al. Liver transplantation for nonalcoholic steatohepatitis: the new epidemic. Ann Surg. 2012;256:624–633. [DOI] [PubMed] [Google Scholar]
- 16.Singhal A, Wilson GC, Wima K, et al. Impact of recipient morbid obesity on outcomes after liver transplantation. Transpl Int. 2015;28:148–155. [DOI] [PubMed] [Google Scholar]
- 17.Hoehn RS, Singhal A, Wima K, et al. Effect of pretransplant diabetes on short-term outcomes after liver transplantation: a national cohort study. Liver Int. 2015;35:1902–1909. [DOI] [PubMed] [Google Scholar]
- 18.LaMattina JC, Foley DP, Fernandez LA, et al. Complications associated with liver transplantation in the obese recipient. Clin Transplant. 2012;26:910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reichman TW, Therapondos G, Serrano MS, et al. “Weighing the risk”: Obesity and outcomes following liver transplantation. World J Hepatol. 2015;7:1484–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldaracena N, Spetzler VN, Marquez M, et al. Live donor liver transplantation: a valid alternative for critically ill patients suffering from acute liver failure. Am J Transplant. 2015;15:1591–1597. [DOI] [PubMed] [Google Scholar]
- 21.Goldaracena N, Marquez M, Selzner N, et al. Living vs. deceased donor liver transplantation provides comparable recovery of renal function in patients with hepatorenal syndrome: a matched case-control study. Am J Transplant. 2014;14:2788–2795. [DOI] [PubMed] [Google Scholar]
- 22.Shah SA, Levy GA, Greig PD, et al. Reduced mortality with right-lobe living donor compared to deceased-donor liver transplantation when analyzed from the time of listing. Am J Transplant. 2007;7:998–1002. [DOI] [PubMed] [Google Scholar]
- 23.Reichman TW, Katchman H, Tanaka T, et al. Living donor versus deceased donor liver transplantation: a surgeon-matched comparison of recipient morbidity and outcomes. Transpl Int. 2013;26:780–787. [DOI] [PubMed] [Google Scholar]
- 24.Cattral MS, Molinari M, Vollmer CM, Jr, et al. Living-donor right hepatectomy with or without inclusion of middle hepatic vein: comparison of morbidity and outcome in 56 patients. Am J Transplant. 2004;4:751–757. [DOI] [PubMed] [Google Scholar]
- 25.Shah SA, Grant DR, Greig PD, et al. Analysis and outcomes of right lobe hepatectomy in 101 consecutive living donors. Am J Transplant. 2005;5:2764–2769. [DOI] [PubMed] [Google Scholar]
- 26.Adcock L, Macleod C, Dubay D, et al. Adult living liver donors have excellent long-term medical outcomes: the University of Toronto liver transplant experience. Am J Transplant. 2010;10:364–371. [DOI] [PubMed] [Google Scholar]
- 27.Hosein Shokouh-Amiri M, Osama Gaber A, Bagous WA, et al. Choice of surgical technique influences perioperative outcomes in liver transplantation. Ann Surg. 2000;231:814–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka T, Sugawara Y, Tamura S, et al. Living donor liver transplantation for non-alcoholic steatohepatitis: a single center experience. Hepatol Res. 2014;44:E3–E10. [DOI] [PubMed] [Google Scholar]
- 29.Marengo A, Jouness RI, Bugianesi E. Progression and natural history of nonalcoholic fatty liver disease in adults. Clin Liver Dis. 2016;20:313–324. [DOI] [PubMed] [Google Scholar]
- 30.Patil DT, Yerian LM. Evolution of nonalcoholic fatty liver disease recurrence after liver transplantation. Liver Transpl. 2012;18:1147–1153. [DOI] [PubMed] [Google Scholar]
- 31.Merola J, Liapakis A, Mulligan DC, et al. Non-alcoholic fatty liver disease following liver transplantation: a clinical review. Clin Transplant. 2015;29:728–737. [DOI] [PubMed] [Google Scholar]
- 32.El Atrache MM, Abouljoud MS, Divine G, et al. Recurrence of non-alcoholic steatohepatitis and cryptogenic cirrhosis following orthotopic liver transplantation in the context of the metabolic syndrome. Clin Transplant. 2012;26:E505–E512. [DOI] [PubMed] [Google Scholar]
- 33.Yalamanchili K, Saadeh S, Klintmalm GB, et al. Nonalcoholic fatty liver disease after liver transplantation for cryptogenic cirrhosis or nonalcoholic fatty liver disease. Liver Transpl. 2010;16:431–439. [DOI] [PubMed] [Google Scholar]


