Abstract
Although regeneration through the reprogramming of one cell lineage to another occurs in fish and amphibians, it has not been observed in mammals. We discovered in the mouse that during wound healing, adipocytes regenerate from myofibroblasts, a cell type thought to be differentiated and nonadipogenic. Myofibroblast reprogramming required neogenic hair follicles, which triggered bone morphogenetic protein (BMP) signaling and then activation of adipocyte transcription factors expressed during development. Overexpression of the BMP antagonist Noggin in hair follicles or deletion of the BMP receptor in myofibroblasts prevented adipocyte formation. Adipocytes formed from human keloid fibroblasts either when treated with BMP or when placed with human hair follicles in vitro. Thus, we identify the myofibroblast as a plastic cell type that may be manipulated to treat scars in humans
Wound healing in adult humans and mice generally results in a scar with excess collagen and an absence of hair follicles and cutaneous fat. Recently, we and others discovered that a large skin wound in an adult mouse often regenerates hair follicles under the control of the Wnt and fibroblast growth factor (FGF) pathways (fig. S1) (1–6). We then noticed new adipocytes within healed wounds that are indistinguishable from normal cutaneous fat cells in terms of size, density, and depth from the skin surface (Fig. 1, A and B, and fig. S1C). The new adipocytes never form in the hairless part of the wound but develop exclusively around new hair follicles (Fig. 1B and fig. S1C). Regenerated hair follicles begin to form around 14 days postwounding, after reepithelialization (2). The first new adipocytes (orange) appear at 23 days, immediately adjacent to the growing hair follicle (blue), and their number and size increase over the next few days (Fig. 1A and fig. S2). The new adipocytes are classifiable as physiologically mature and metabolically active white adipose cells because they express fat tissue–specific hormones, resistin and adiponectin, detected as lacZ-positive cells in Retn-lacZ mice (7) (n = 9) (figs. S2 and S3A) and Adipoq-Cre;R26R mice (n = 8) (fig. S3B), respectively. Given the close spatial and temporal association between regenerated hair follicles and regenerated fat, we asked whether hair follicles are necessary to establish adipocyte precursors. To test for such precursors, we placed dermal cells from wounds in a culture that promotes adipocyte differentiation (8). Dermal cells from wounds with regenerated hair follicles differentiated into lipid-laden adipocytes, but dermal cells from wounds without hair follicles did not (Fig. 1C). Efficient in vitro differentiation and adipose gene up-regulation were consistently induced from dermal cells that originated from hair-bearing, but not hairless, wounds at different postwounding time points, as early as 22 days (Fig. 1C and fig. S4). To determine the cellular origin of the new adipocytes, we considered that during early wound repair, dermal wound tissue contains many myofibroblasts expressing smooth muscle actin. Myofibroblasts appear in large excisional wounds on day 5 and become abundant in the dermal scar tissue by day 12. These cells largely cease to express smooth muscle actin by day 17 (Fig. 2A). Cells expressing the adipogenic commitment factors ZFP423 (9, 10) (figs. S5B and S6A) and pCEBPb (figs. S5C and S6B) appear adjacent to the new hair follicles at days 21 and 24, respectively. This sequence suggests that myofibroblasts assume an alternative cell fate by converting to adipogenic precursors. To test this, we examined the lineage identity of new adipocytes in SM22-Cre;R26R and inducible SMA-CreERT2;R26R mice, in which Cre activity turns on in wound myofibroblasts (Fig. 2B and fig. S7, B to D). In normal depots of white fat, SM22-Cre and SMA-CreERT2 are not activated in adipocytes (Fig. 2C and fig. S7A) (11). However, in SM22-Cre;R26R (n = 12) and SMA-CreERT2;R26R mice induced during wound healing (n = 4), most new adipocytes in wounds expressed lacZ, indicating their origin from myofibroblasts (figs. S8 and S11). To functionally validate a myofibroblast-to adipocyte transformation, we generated the lossof-function SM22-Cre;Pparg flox/flox and inducible SMA-CreERT2;Pparg flox/flox mice. Wounds of SM22- Cre;Pparg flox/flox mice formed many new hair follicles but were nearly devoid of new adipocytes [n = 7; new adipocyte/follicle ratio, 0.62 ± 0.2, versus 24.1 ± 6.8 in control mice (n = 7)] (fig. S7F and materials and methods). The difference between experimental and control groups was nearly 40-fold (fig. S7E and table S1). Importantly, in SM22-Cre;Pparg f lox/f lox mice, depots of white fat outside the wound, both subcutaneous and elsewhere, were unaffected. Similarly, tamoxifen induction of SMA-CreERT2;Pparg flox/flox mice during early time points after wounding largely prevented regeneration of adipocytes [n = 6; new adipocyte/follicle ratio, 0.5 ± 0.07, versus 22.7 ± 5.1 in control mice (n = 6)] (Fig. 2, D and E). Taken together, our lineage tracing studies establish myofibroblasts as the source for new regenerating adipocytes (fig. S9F). To evaluate the possible contribution of other SM22- or SMA-positive cell populations—including vascular smooth muscle cells, panniculus carnosus muscle, and dermal papillae of new hair follicles— to fat regeneration, we traced the progeny of these cell populations by using relevant promoter systems. We found no contribution of these cell types to new adipocytes (figs. S9 and S10 and supplementary text S1). To comprehensively study the molecular nature of lineage reprograming of myofibroblasts to adipocytes in wounds in adult mice, we profiled the transcriptomes of wound myofibroblasts by RNA sequencing (Fig. 3, A to C; figs. S12 to S15; and supplementary text S2 to S6). Among 4120 differentially expressed genes (Fig. 3B), at the onset of adipocyte regeneration, several transcriptional regulators of the adipocyte lineage—including Zfp423, Crebl2, Stat5b, and Klf15—were up-regulated, whereas transcriptional regulators of chondrogenic and osteogenic lineages—including Sox9 and -11, Runx1 and -2, Fhl2, and Pitx1—were downregulated (fig. S14 and supplementary text S3). The reporter for the ZFP423 transcription factor, which drives commitment of mesenchymal progenitors to the adipocyte lineage during embryogenesis (9), was expressed by dermal cells juxtaposed to regenerated hair follicles starting on day 21 after wounding (figs. S17 and S18A). Then, the number of ZFP423-positive dermal cells increased, before diminishing by day 28, coincident with the increase in mature adipocytes (figs. S17 and S18, B and C). The temporal changes in ZFP423 expression suggest that wounding activates this embryonic pathway to facilitate adipocyte regeneration. Supporting this hypothesis, adult Zfp423 mutant mice (12) failed to regenerate fat completely, despite forming many new hair follicles after wounding [n = 9; new adipocyte/follicle ratio, 0.07 ± 0.06, versus 29.6 ± 5.4 in control mice (n = 9)] (Fig. 3D). The critical role of ZFP423 for reprogramming myofibroblasts during wound healing in the adult contrasts with its nonessential role for adipocyte development in the embryo because Zfp423 mutant mice possess skin adipocytes (fig. S16), likely owing to compensation by redundant pathways apparently available during development but not regeneration. To determine the molecular regulation of reprogramming, we considered that bone morphogenetic protein (BMP) signaling induces adipogenic commitment of cells in vitro (9, 13) and that actively growing hair follicles, which are critical for myofibroblast-to-adipocyte reprogramming, strongly express BMP2 and BMP4 (14). Our transcriptomic data also show that endogenous BMP ligands encoded by Bmp4 and Bmp7 are upregulated, whereas the soluble BMP antagonists encoded by Bambi andGrem1 are down-regulated in myofibroblasts by day 21 (fig. S14 and supplementary text S4). We also noted marked upregulation of expression of pSMAD1/5/8—indicators of active BMP signaling—in dermal cells next to regenerated hair follicles at the time of ZFP423 activation (day 21) (fig. S19A). To test whether BMP signaling modulates adipocyte regeneration, we studied K14-Noggin mice, which overexpress Noggin, a soluble BMP antagonist, in the epithelial cells of the hair follicle. After wounding, these mice failed to regenerate fat, despite forming normal-appearing hair follicles [n = 10; new adipocyte/follicle ratio, 0.2 ± 0.1, versus 30.6 ± 6.3 in control mice (n = 10)] (Fig. 3E). Similarly, treatment of mice during wound healing with a small-molecule inhibitor of SMAD1, -5, and -8 phosphorylation largely prevented new adipocyte regeneration in hair-bearing wounds (n = 7; new adipocyte/follicle ratio, 0.58 ± 0.35) (Fig. 3G). ZFP423 reporter activity was down-regulated in the Zfp423-lacZ;K14-Noggin (fig. S20) and inhibitor treated Zfp423-lacZ mice (fig. S21), indicating that BMP was activating ZFP423 in myofibroblasts. To specifically test whether BMP signaling in myofibroblasts is necessary for adipocyte regeneration, we deleted the BMP receptor BMPR1A in SMACreERT2;Bmpr1aflox/flox mice. This led to a lack of new adipocytes, despite the formation of many new hair follicles [n = 6; new adipocyte/follicle ratio, 0.38 ± 0.36, versus 23.9 ± 1.5 in control mice (n = 3)] (Fig. 3F). In addition, exposing myofibroblasts from early wounds to either BMP4 or BMP2 in vitro reprogrammed them toward an adipocyte fate (Fig. 4, A and B). Because Wnts are known inhibitors of adipocyte differentiation, we also examined K14-Wnt7a mice and discovered a lack of fat regeneration, despite an increased number of new hair follicles after wounding [n = 6; new adipocyte/follicle ratio, 0.6 ± 0.3, versus 28 ± 4.2 in control mice (n = 6)] (fig. S23). To determine the relevance of our findings to humans, we treated keloid scar cells in culture with BMP4 and induced their conversion to lipidladen adipocytes, as indicated by expression of adipocyte markers (n = 3) (Fig. 4, C and D). Coculture of human keloid fibroblasts with human scalp hair follicles also induced their adipogenic conversion (Fig. 4, E and F, and supplementary text S7). Taken together, our data suggest that new hair follicles in a wound reprogram myofibroblasts to an adipocyte fate by activation of the BMP-ZFP423 pathway (fig. S24). Myofibroblasts, which are characterized by contractile behavior, excessive collagen deposition, and secretion of profibrotic cytokines, are found in many tissues in response to injury and inflammation. Although the developmental origin of cutaneous myofibroblasts continues to be elucidated (15–18), at the transcriptome level, they are largely distinct from any well-characterized fibroblast populations in unwounded skin, including multipotent skin-derived precursors (fig. S15 and supplementary text S6), and their scar-promoting properties are thought to be maintained by epigenetic changes, including DNA hypermethylation (19). Therefore, myofibroblasts have not been considered capable of converting to another cell type, and their depletion has been viewed as a main antiscarring strategy. The observed conversion of myofibroblasts to adipocytes demonstrates lineage reprogramming in vivo in an adult mammal. Several recent studies have shown that tissue regeneration respects tissue boundaries: Epithelium regenerates from epithelium, dermis from dermis, and cartilage from cartilage (20–22). Our findings reveal the ability of wound myofibroblasts to convert to a completely different (adipocyte) lineage. The findings indicate a window of opportunity after wounding to influence regeneration rather than scarring of tissue by activating embryonic pathways and converting myofibroblasts to adipocytes. Our work shows that hair follicles grow independently of fat and that hair follicle regeneration is necessary and proximal to cutaneous fat regeneration. Our transcriptomic and functional data support a key role for BMP and indicate that strategies for regenerating hair follicles could ultimately benefit patients with disorders involving a lack of fat, such as acute scars, keloids, lipodystrophies, and aging.
Fig. 1.
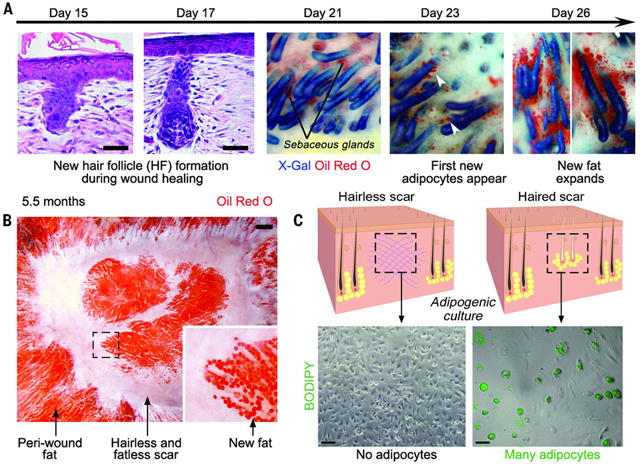
New adipocytes only regenerate around new hair follicles during wound healing. (A) Histological sections (left two panels) and whole-mount images (right three panels) of skin stained to detect follicular epithelium (blue) and adipocytes (orange) in K14-Cre;R26R mice at indicated postwounding days. New adipocytes (arrowheads) increase in number and size over several days. (B) Skin viewed from the undersurface. New adipocytes form and persist exclusively around regenerated hair follicles, which arise in the center of the wound. (C) Cultured dermal cells isolated from wounds with regenerated hair follicles differentiated into BODIPY-positive (green) adipocytes, whereas cultured dermal cells from wounds lacking follicles formed no adipocytes. Scale bars in (A) and (C), 20 mm; in (B), 1 mm.
Fig. 2.
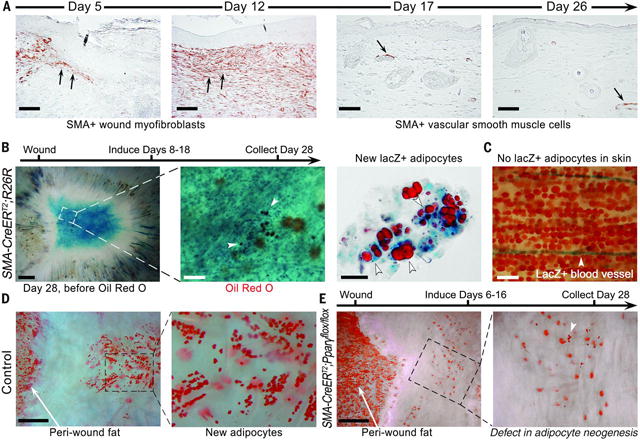
New adipocytes originate from wound myofibroblasts. (A) Smooth muscle actin (SMA)– positive myofibroblasts are present at the wound edge and then in the healing wound (arrows). By day 17, dermal wound cells express very little SMA, but vascular smooth muscle cells remain labeled (arrow). (B) Lineage tracing of myofibroblasts results in lacZ (blue)–expressing regenerated adipocytes (orange, white arrowheads). (C) Adipocytes in normal skin are not labeled. (D and E) Deletion of Pparg in myofibroblasts resulted in near-complete loss of new adipocytes, whereas normal cutaneous adipocytes at the wound edge remained intact. Scale bars in (A), 100 mm; in (B) (left), (D), and (E), 1 mm; in (B) (center), 200 mm; in (B) (right), 50 mm; in (C), 200 mm
Fig. 3.
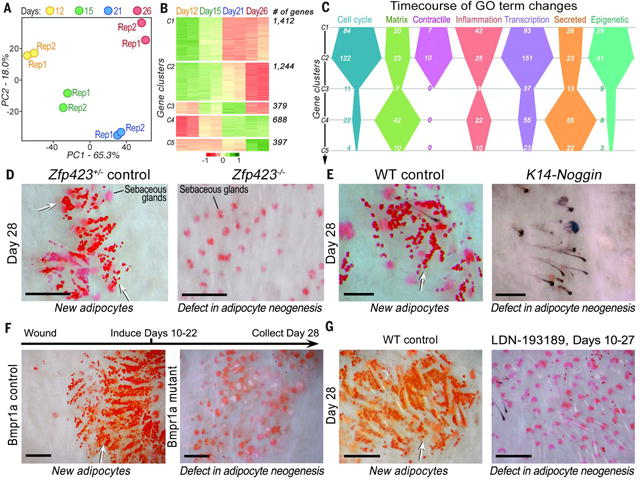
Molecular profiling and functional studies of adipocyte regeneration reveal that ZFP423 and BMP signaling are necessary for adipocyte regeneration.(A) Principal component analysis of the myofibroblast transcriptome reveals distinct changes across four postwounding time points. (B) Differentially expressed genes (4120 total) from myofibroblasts at days 12 to 26 group into five distinct clusters (table S5). (C) Differentially expressed genes in several gene ontologies (GOs) undergo distinct temporal changes in myofibroblasts. (D) Deletion of Zfp423, (E) overexpression of the soluble BMP antagonist Noggin in K14-Noggin mice, (F) SMA-CreERT2–driven deletion of BMPR1A, and (G) treatment with the BMP antagonist LDN-193189 (2 mg/kg) during wound healing all resulted in near-complete loss of regenerated adipocytes in wounds, despite normal regeneration of hair follicles. WT, wild type. Scale bars in (D) to (G), 100 mm.
Fig. 4.
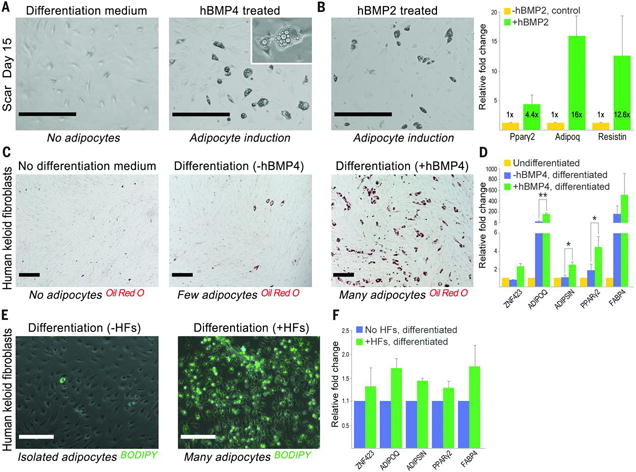
BMP drives reprogramming of mouse myofibroblasts and human keloid fibroblasts into adipocytes. (A and B) Treatment of cultured mouse dermal cells isolated from day-15 wounds with either human recombinant BMP4 or BMP2 induced their reprogramming into adipocytes and the activation of adipocyte-specific genes. Day-15 dermal cells cultured in pro-adipogenic differentiation media without BMP remained nonadipogenic. (C and D) Treatment of cultured human keloid scar cells with human recombinant BMP4 and adipocyte differentiation media induced their reprogramming into adipocytes with activation of adipocyte-specific genes. (E) In a coculture system, human scalp hair follicles induced adipogenic conversion of human keloid scar cells with (F) a concomitant increase in adipocyte genes. Scale bars in (A) and (B), 400 mm; in (C) and (E), 200 mm. Values in the graphs are means ± SEM. *P < 0.028; **P < 0.0002.
Supplementary Material
Acknowledgments
Funding is provided by U.S. NIH grant R01-AR055309, NIH Skin Diseases Research Core grant P30-AR057217, and the Edward and Fannie Gray Hall Center for Human Appearance. M.V.P. is supported by a pilot grant from the Diabetes and Endocrinology Research Center (University of Pennsylvania), a Dermatology Foundation research grant, an Edward Mallinckrodt Jr. Foundation grant, a Pew Charitable Trust grant, and NIH grants R01-AR067273 and R01-AR069653. M.A.L. is supported by NIH grant DK49210, M.I. by NIH grant R01-AR066022, S.E.M. by NIH grant R37-AR047709 and Penn Skin Biology and Diseases Resource-based Core grant P30-AR069589, W.S.P. by NIH grant R01-AI047833, R.K.G. by NIH grant R01-DK104789, T.-L.T. by NIH grant R01-GM095821, B.A.H. by NIH grant R01-NS05487, R.R. by California Institute for Regenerative Medicine training grant TG2- 01152, C.F.G.-J. by the NSF Graduate Research Fellowship Program (DGE-1321846) and a training grant from MBRS-IMSD (Initiative for Maximizing Student Development; GM055246), X.W. by a Canadian Institutes of Health Research postdoctoral fellowship (MFE-123724), J.W.O. by National Research Foundation of Korea grant 2016R1C1B1015211, C.H.L. by the Cutaneous Biology and Skin Disease training program (T32-AR064184), Y.R.L. by a NIH National Research Service Award F30 training grant and a Paul and Daisy Soros Fellowship for New Americans, H.-L.L. by NIH T32 training grant T32-CA009054-37, and M.S. by American Heart Association postdoctoral fellowship 16POST26420136. Retn-lacZ mice were generated with the Transgenic Mouse Core of the University of Pennsylvania Diabetes Research Center (NIH grant DK19525). We thank Y. Mishina for providing Bmpr1aflox mice, C.-M. Chuong for providing K14-Noggin mice, V. Scarfone and C. Tu for their assistance with fluorescence-activated cell sorting and tissue culture, Z. Yang for technical assistance, and P. Sterling for reviewing the manuscript. SMA-CreERT2 mice are available from P.C. under a material transfer agreement with the University of California, Irvine. P.C. and D.M. are inventors on patents EP 1 692 936 B1 and US 7112715 B2, held by GIE-CERBM (Centre Européen de Recherche en Biologie et Médecine), that cover the method for generating conditional DNA recombination in mice by using the Cre-ERT2 fusion protein. M.V.P., C.F.G.-J., and G.C. are co-inventors on a patent application filed through the U.S. Patent and Trademark Office by the University of Pennsylvania describing the BMP pathway as a target for promoting neogenic fat formation, among other claims.
REFERENCES AND NOTES
- 1.Gay D, et al. Fgf9 from dermal gammadelta T cells induces hair follicle neogenesis after wounding. Nature medicine. 2013;19:916–923. doi: 10.1038/nm.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ito M, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 3.Seifert AW, et al. Skin shedding and tissue regeneration in African spiny mice (Acomys) Nature. 2012;489:561–565. doi: 10.1038/nature11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, et al. Principles and mechanisms of regeneration in the mouse model for wound-induced hair follicle neogenesis. Regeneration (Oxf) 2015;2:169–181. doi: 10.1002/reg2.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breedis C. Regeneration of hair follicles and sebaceous glands from the epithelium of scars in the rabbit. Cancer Res. 1954;14:575–579. [PubMed] [Google Scholar]
- 6.Billingham RE, Russell PS. Incomplete wound contracture and the phenomenon of hair neogenesis in rabbits’ skin. Nature. 1956;177:791–792. doi: 10.1038/177791b0. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee RR, et al. Regulation of fasted blood glucose by resistin. Science. 2004;303:1195–1198. doi: 10.1126/science.1092341. [DOI] [PubMed] [Google Scholar]
- 8.Ishibashi J, et al. An Evi1-C/EBPbeta complex controls peroxisome proliferator-activated receptor gamma2 gene expression to initiate white fat cell differentiation. Mol Cell Biol. 2012;32:2289–2299. doi: 10.1128/MCB.06529-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta RK, et al. Transcriptional control of preadipocyte determination by Zfp423. Nature. 2010;464:619–623. doi: 10.1038/nature08816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang S, et al. Regulation of early adipose commitment by Zfp521. PLoS Biol. 2012;10:e1001433. doi: 10.1371/journal.pbio.1001433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang W, et al. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alcaraz WA, et al. Zfp423 controls proliferation and differentiation of neural precursors in cerebellar vermis formation. Proc Natl Acad Sci U S A. 2006;103:19424–19429. doi: 10.1073/pnas.0609184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammarstedt A, et al. WISP2 regulates preadipocyte commitment and PPARgamma activation by BMP4. Proc Natl Acad Sci U S A. 2013;110:2563–2568. doi: 10.1073/pnas.1211255110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulessa H, Turk G, Hogan BL. Inhibition of Bmp signaling affects growth and differentiation in the anagen hair follicle. The EMBO journal. 2000;19:6664–6674. doi: 10.1093/emboj/19.24.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Driskell RR, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277–281. doi: 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nature medicine. 2012;18:1262–1270. doi: 10.1038/nm.2848. [DOI] [PubMed] [Google Scholar]
- 17.Suga H, et al. Tracking the elusive fibrocyte: identification and characterization of collagen-producing hematopoietic lineage cells during murine wound healing. Stem Cells. 2014;32:1347–1360. doi: 10.1002/stem.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rinkevich Y, et al. Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science. 2015;348:aaa2151. doi: 10.1126/science.aaa2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plikus MV, Guerrero-Juarez CF, Treffeisen E, Gay DL. Epigenetic control of skin and hair regeneration after wounding. Exp Dermatol. 2015;24:167–170. doi: 10.1111/exd.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehoczky JA, Robert B, Tabin CJ. Mouse digit tip regeneration is mediated by fate-restricted progenitor cells. Proc Natl Acad Sci U S A. 2011;108:20609–20614. doi: 10.1073/pnas.1118017108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rinkevich Y, Lindau P, Ueno H, Longaker MT, Weissman IL. Germ-layer and lineage-restricted stem/progenitors regenerate the mouse digit tip. Nature. 2011;476:409–413. doi: 10.1038/nature10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung TH, Snyder ER, Liu Y, Wang J, Kim SK. A cellular, molecular, and pharmacological basis for appendage regeneration in mice. Genes Dev. 2015;29:2097–2107. doi: 10.1101/gad.267724.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


