Abstract
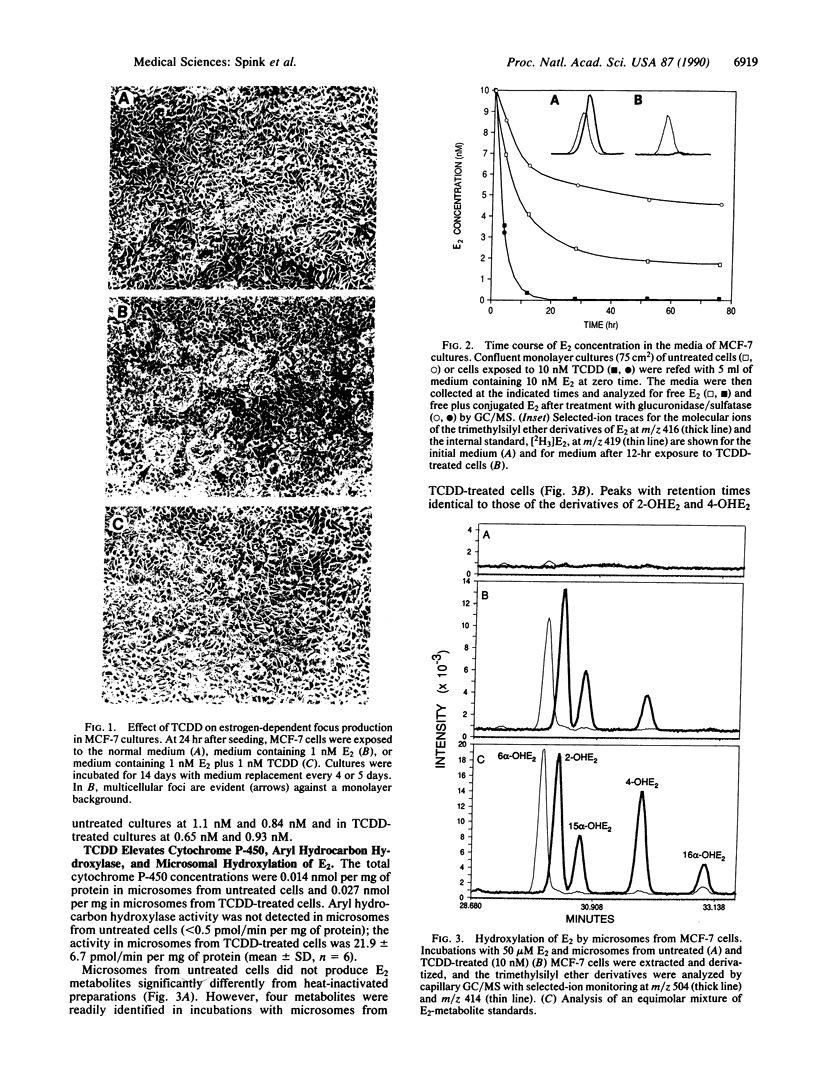
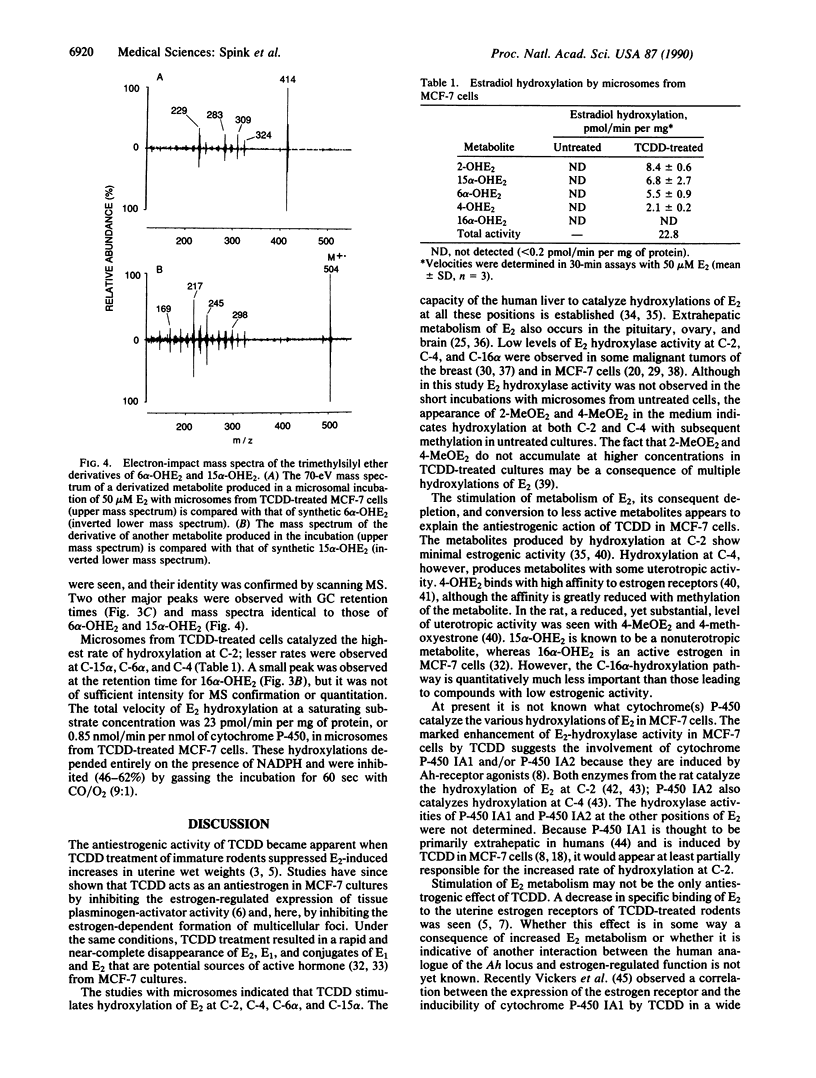
MCF-7 breast tumor cells form multicellular foci in vitro when supplemented with 17 beta-estradiol (E2). In the presence of E2 and the aryl hydrocarbon-receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), MCF-7 cells grow to confluence but do not form foci. To investigate the role of E2 metabolism in this antiestrogenic effect of TCDD, analyses were performed by capillary GC/MS. The results revealed that pretreatment of MCF-7 cultures with TCDD (10 nM) rapidly depletes E2. In untreated cultures supplemented with 10 nM E2, the concentration of free E2 decreased to 4 nM in the first 12 hr, followed by a slower rate of decline. After 3 days most E2 in the medium was in conjugated form(s); 1.7 nM was present as free E2, and 2.9 nM was released by treatment with glucuronidase/sulfatase. In TCDD-treated cultures, E2 declined to 290 pM in 12 hr and after 2 days was not detected (less than 100 pM) either as free steroid or after treatment with glucuronidase/sulfatase. Intracellular E2 and estrone were likewise depleted by pretreatment with TCDD. Microsomes from TCDD-treated cells showed highly elevated aryl hydrocarbon-hydroxylase activity and catalyzed hydroxylations of E2 at C-2, C-4, C-15 alpha, and C-6 alpha with a combined rate of 0.85 nmol/min per nmol of cytochrome P-450 at saturating E2. These results suggest that depletion of E2 by enhanced metabolism accounts for the antiestrogenic activity of TCDD in MCF-7 cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abul-Hajj Y. J., Thijssen J. H., Blankenstein M. A. Metabolism of estradiol by human breast cancer. Eur J Cancer Clin Oncol. 1988 Jul;24(7):1171–1178. doi: 10.1016/0277-5379(88)90124-1. [DOI] [PubMed] [Google Scholar]
- Adams D. J., Edwards D. P., McGuire W. L. Estrogen regulation of specific messinger RNA's in human breast cancer cells. Biochem Biophys Res Commun. 1980 Dec 31;97(4):1354–1361. doi: 10.1016/s0006-291x(80)80016-7. [DOI] [PubMed] [Google Scholar]
- Adams J. B., Phillips N. S., Hall R. Metabolic fate of estradiol in human mammary cancer cells in culture: estrogen sulfate formation and cooperativity exhibited by estrogen sulfotransferase. Mol Cell Endocrinol. 1988 Aug;58(2-3):231–242. doi: 10.1016/0303-7207(88)90159-1. [DOI] [PubMed] [Google Scholar]
- Astroff B., Safe S. Comparative antiestrogenic activities of 2,3,7,8-tetrachlorodibenzo-p-dioxin and 6-methyl-1,3,8-trichlorodibenzofuran in the female rat. Toxicol Appl Pharmacol. 1988 Sep 30;95(3):435–443. doi: 10.1016/0041-008x(88)90361-4. [DOI] [PubMed] [Google Scholar]
- Aström A., DePierre J. W. Metabolism of 2-acetylaminofluorene by eight different forms of cytochrome P-450 isolated from rat liver. Carcinogenesis. 1985 Jan;6(1):113–120. doi: 10.1093/carcin/6.1.113. [DOI] [PubMed] [Google Scholar]
- Ball P., Knuppen R. Formation of 2- and 4-hydroxyestrogens by brain, pituitary, and liver of the human fetus. J Clin Endocrinol Metab. 1978 Oct;47(4):732–737. doi: 10.1210/jcem-47-4-732. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Breuer H., Knuppen R., Haupt M. Metabolism of oestrone and oestradiol-17-beta in human liver in vitro. Nature. 1966 Oct 1;212(5057):76–76. doi: 10.1038/212076a0. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Jeltsch J. M., Roberts M., Chambon P. Activation of pS2 gene transcription is a primary response to estrogen in the human breast cancer cell line MCF-7. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6344–6348. doi: 10.1073/pnas.81.20.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavailles V., Augereau P., Garcia M., Rochefort H. Estrogens and growth factors induce the mRNA of the 52K-pro-cathepsin-D secreted by breast cancer cells. Nucleic Acids Res. 1988 Mar 25;16(5):1903–1919. doi: 10.1093/nar/16.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R., Brünner N., Katzenellenbogen B. S., Thompson E. W., Norman M. J., Koppi C., Paik S., Lippman M. E., Dickson R. B. Progression of human breast cancer cells from hormone-dependent to hormone-independent growth both in vitro and in vivo. Proc Natl Acad Sci U S A. 1989 May;86(10):3649–3653. doi: 10.1073/pnas.86.10.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes R. C., Goss P., Dowsett M., Gazet J. C., Brodie A. 4-Hydroxyandrostenedione in treatment of postmenopausal patients with advanced breast cancer. Lancet. 1984 Dec 1;2(8414):1237–1239. doi: 10.1016/s0140-6736(84)92795-8. [DOI] [PubMed] [Google Scholar]
- Dannan G. A., Porubek D. J., Nelson S. D., Waxman D. J., Guengerich F. P. 17 beta-estradiol 2- and 4-hydroxylation catalyzed by rat hepatic cytochrome P-450: roles of individual forms, inductive effects, developmental patterns, and alterations by gonadectomy and hormone replacement. Endocrinology. 1986 May;118(5):1952–1960. doi: 10.1210/endo-118-5-1952. [DOI] [PubMed] [Google Scholar]
- Dehennin L., Blacker C., Reiffsteck A., Scholler R. Estrogen 2-, 4-, 6- or 16-hydroxylation by human follicles shown by gas chromatography-mass spectrometry associated with stable isotope dilution. J Steroid Biochem. 1984 Jan;20(1):465–471. doi: 10.1016/0022-4731(84)90255-3. [DOI] [PubMed] [Google Scholar]
- Dickerman H. W., Martinez H. L., Seeger J. I., Kumar S. A. Estrogen regulation of human breast cancer cell line MCF-7 tissue plasminogen activator. Endocrinology. 1989 Jul;125(1):492–500. doi: 10.1210/endo-125-1-492. [DOI] [PubMed] [Google Scholar]
- Fisher B., Costantino J., Redmond C., Poisson R., Bowman D., Couture J., Dimitrov N. V., Wolmark N., Wickerham D. L., Fisher E. R. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989 Feb 23;320(8):479–484. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- Fishman J. Aromatic hydroxylation of estrogens. Annu Rev Physiol. 1983;45:61–72. doi: 10.1146/annurev.ph.45.030183.000425. [DOI] [PubMed] [Google Scholar]
- Gallo M. A., Hesse E. J., Macdonald G. J., Umbreit T. H. Interactive effects of estradiol and 2,3,7,8-tetrachlorodibenzo-p-dioxin on hepatic cytochrome P-450 and mouse uterus. Toxicol Lett. 1986 Jul-Aug;32(1-2):123–132. doi: 10.1016/0378-4274(86)90058-5. [DOI] [PubMed] [Google Scholar]
- Gierthy J. F., Lincoln D. W., 2nd Inhibition of postconfluent focus production in cultures of MCF-7 human breast cancer cells by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Breast Cancer Res Treat. 1988 Oct;12(2):227–233. doi: 10.1007/BF01805943. [DOI] [PubMed] [Google Scholar]
- Gierthy J. F., Lincoln D. W., 2nd, Kampcik S. J., Dickerman H. W., Bradlow H. L., Niwa T., Swaneck G. E. Enhancement of 2- and 16 alpha-estradiol hydroxylation in MCF-7 human breast cancer cells by 2,3,7,8-tetrachlorodibenzo-P-dioxin. Biochem Biophys Res Commun. 1988 Dec 15;157(2):515–520. doi: 10.1016/s0006-291x(88)80279-1. [DOI] [PubMed] [Google Scholar]
- Gierthy J. F., Lincoln D. W., Gillespie M. B., Seeger J. I., Martinez H. L., Dickerman H. W., Kumar S. A. Suppression of estrogen-regulated extracellular tissue plasminogen activator activity of MCF-7 cells by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Cancer Res. 1987 Dec 1;47(23):6198–6203. [PubMed] [Google Scholar]
- Guengerich F. P. Characterization of human microsomal cytochrome P-450 enzymes. Annu Rev Pharmacol Toxicol. 1989;29:241–264. doi: 10.1146/annurev.pa.29.040189.001325. [DOI] [PubMed] [Google Scholar]
- Guzelian P. S., Bissell D. M., Meyer U. A. Drug metabolism in adult rat hepatocytes in primary monolayer culture. Gastroenterology. 1977 Jun;72(6):1232–1239. [PubMed] [Google Scholar]
- Harris M., Piskorska-Pliszczynska J., Zacharewski T., Romkes M., Safe S. Structure-dependent induction of aryl hydrocarbon hydroxylase in human breast cancer cell lines and characterization of the Ah receptor. Cancer Res. 1989 Aug 15;49(16):4531–4535. [PubMed] [Google Scholar]
- Hoffman A. R., Paul S. M., Axelrod J. Catecholestrogen synthesis and metabolism by human breast tumors in vitro. Cancer Res. 1979 Nov;39(11):4584–4587. [PubMed] [Google Scholar]
- Ichimura K., Yamanaka H., Chiba K., Shinozuka T., Shiki Y., Saito K., Kusano S., Ameniya S., Oyama K., Nozaki Y. Simultaneous quantitative measurement of fourteen adrenal steroids by capillary column gas chromatography-mass spectrometry, and its clinical application. J Chromatogr. 1986 Jan 10;374(1):5–16. doi: 10.1016/s0378-4347(00)83247-8. [DOI] [PubMed] [Google Scholar]
- Jaiswal A. K., Gonzalez F. J., Nebert D. W. Human dioxin-inducible cytochrome P1-450: complementary DNA and amino acid sequence. Science. 1985 Apr 5;228(4695):80–83. doi: 10.1126/science.3838385. [DOI] [PubMed] [Google Scholar]
- Jellinck P. H., Fishman J. Activation and irreversible binding of regiospecifically labeled catechol estrogen by rat liver microsomes: evidence for differential cytochrome P-450 catalyzed oxidations. Biochemistry. 1988 Aug 9;27(16):6111–6116. doi: 10.1021/bi00416a042. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Kendra K. L., Norman M. J., Berthois Y. Proliferation, hormonal responsiveness, and estrogen receptor content of MCF-7 human breast cancer cells grown in the short-term and long-term absence of estrogens. Cancer Res. 1987 Aug 15;47(16):4355–4360. [PubMed] [Google Scholar]
- Kociba R. J., Keyes D. G., Beyer J. E., Carreon R. M., Wade C. E., Dittenber D. A., Kalnins R. P., Frauson L. E., Park C. N., Barnard S. D. Results of a two-year chronic toxicity and oncogenicity study of 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats. Toxicol Appl Pharmacol. 1978 Nov;46(2):279–303. doi: 10.1016/0041-008x(78)90075-3. [DOI] [PubMed] [Google Scholar]
- Lesko S. M., Rosenberg L., Kaufman D. W., Helmrich S. P., Miller D. R., Strom B., Schottenfeld D., Rosenshein N. B., Knapp R. C., Lewis J. Cigarette smoking and the risk of endometrial cancer. N Engl J Med. 1985 Sep 5;313(10):593–596. doi: 10.1056/NEJM198509053131001. [DOI] [PubMed] [Google Scholar]
- Martucci C. P., Fishman J. Impact of continuously administered catechol estrogens on uterine growth and luteinizing hormone secretion. Endocrinology. 1979 Dec;105(6):1288–1292. doi: 10.1210/endo-105-6-1288. [DOI] [PubMed] [Google Scholar]
- Matsubara T., Koike M., Touchi A., Tochino Y., Sugeno K. Quantitative determination of cytochrome P-450 in rat liver homogenate. Anal Biochem. 1976 Oct;75(2):596–603. doi: 10.1016/0003-2697(76)90114-7. [DOI] [PubMed] [Google Scholar]
- Michnovicz J. J., Hershcopf R. J., Naganuma H., Bradlow H. L., Fishman J. Increased 2-hydroxylation of estradiol as a possible mechanism for the anti-estrogenic effect of cigarette smoking. N Engl J Med. 1986 Nov 20;315(21):1305–1309. doi: 10.1056/NEJM198611203152101. [DOI] [PubMed] [Google Scholar]
- Muto H., Takizawa Y. Dioxins in cigarette smoke. Arch Environ Health. 1989 May-Jun;44(3):171–174. doi: 10.1080/00039896.1989.9935882. [DOI] [PubMed] [Google Scholar]
- Nebert D. W. Genetic differences in microsomal electron transport: the Ah locus. Methods Enzymol. 1978;52:226–240. doi: 10.1016/s0076-6879(78)52026-0. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Gonzalez F. J. P450 genes: structure, evolution, and regulation. Annu Rev Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- Niwa T., Bradlow H. L., Fishman J., Swaneck G. E. Determination of estradiol 2- and 16-alpha-hydroxylase activities in MCF-7 human breast cancer cells in culture using radiometric analysis. J Steroid Biochem. 1989 Aug;33(2):311–314. doi: 10.1016/0022-4731(89)90309-9. [DOI] [PubMed] [Google Scholar]
- Pasqualini J. R., Gelly C., Lecerf F. Biological effects and morphological responses to estriol, estriol-3-sulfate, estriol-17-sulfate and tamoxifen in a tamoxifen-resistant cell line (R-27) derived from MCF-7 human breast cancer cells. Eur J Cancer Clin Oncol. 1986 Dec;22(12):1495–1501. doi: 10.1016/0277-5379(86)90086-6. [DOI] [PubMed] [Google Scholar]
- Porubek D. J., Nelson S. D. A gas chromatographic/mass spectrometric assay for catechol estrogens in microsomal incubations: comparison with a radiometric assay. Biomed Environ Mass Spectrom. 1988 Feb 1;15(3):157–161. doi: 10.1002/bms.1200150307. [DOI] [PubMed] [Google Scholar]
- Purdy R. H., Moore P. H., Jr, Williams M. C., Goldzieher J. W., Paul S. M. Relative rates of 2- and 4-hydroxyestrogen synthesis are dependent on both substrate and tissue. FEBS Lett. 1982 Feb 8;138(1):40–44. doi: 10.1016/0014-5793(82)80390-6. [DOI] [PubMed] [Google Scholar]
- Robinson S. P., Jordan V. C. Antiestrogenic action of toremifene on hormone-dependent, -independent, and heterogeneous breast tumor growth in the athymic mouse. Cancer Res. 1989 Apr 1;49(7):1758–1762. [PubMed] [Google Scholar]
- Romkes M., Piskorska-Pliszczynska J., Safe S. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on hepatic and uterine estrogen receptor levels in rats. Toxicol Appl Pharmacol. 1987 Feb;87(2):306–314. doi: 10.1016/0041-008x(87)90292-4. [DOI] [PubMed] [Google Scholar]
- Ryan T. J., Seeger J. I., Kumar S. A., Dickerman H. W. Estradiol preferentially enhances extracellular tissue plasminogen activators of MCF-7 breast cancer cells. J Biol Chem. 1984 Dec 10;259(23):14324–14327. [PubMed] [Google Scholar]
- Schneider J., Huh M. M., Bradlow H. L., Fishman J. Antiestrogen action of 2-hydroxyestrone on MCF-7 human breast cancer cells. J Biol Chem. 1984 Apr 25;259(8):4840–4845. [PubMed] [Google Scholar]
- Van Aswegen C. H., Purdy R. H., Wittliff J. L. Binding of 2-hydroxyestradiol and 4-hydroxyestradiol to estrogen receptors from human breast cancers. J Steroid Biochem. 1989 Apr;32(4):485–492. doi: 10.1016/0022-4731(89)90380-4. [DOI] [PubMed] [Google Scholar]
- Vessey M., Baron J., Doll R., McPherson K., Yeates D. Oral contraceptives and breast cancer: final report of an epidemiological study. Br J Cancer. 1983 Apr;47(4):455–462. doi: 10.1038/bjc.1983.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers P. J., Dufresne M. J., Cowan K. H. Relation between cytochrome P450IA1 expression and estrogen receptor content of human breast cancer cells. Mol Endocrinol. 1989 Jan;3(1):157–164. doi: 10.1210/mend-3-1-157. [DOI] [PubMed] [Google Scholar]






