Abstract
Objective
The incidence of epilepsy is highest in the elderly and the prevalence of epilepsy is higher in nursing home residents than in other cohorts. Co-medications that act in the central nervous system (CNS) are frequently prescribed in this population. The objective was to identify the most commonly prescribed antiseizure drugs (ASDs) and determine the frequency of use of antipsychotic and antidepressant medications in elderly nursing home residents receiving ASDs.
Methods
Data were obtained from a pharmacy database serving 18,752 patients in Minnesota and Wisconsin nursing homes. Prescribing information was available on ASD, antidepressant and antipsychotic drugs on one day in October 2013. The frequency distribution by age, formulation, trademarked/generic drugs, route of administration, and multiple drug combinations were determined.
Results
Overall, 66.8% of 18,752 residents received at least one CNS-active drug as classified by the Generic Product Identifier classification system. For those 65 years and older, ASDs were prescribed for 14.3% residents. Gabapentin comprised 7.3%; valproate 3.0%; levetiracetam 1.8%; and phenytoin 0.9%. An antidepressant was used in 64.2% of persons prescribed an ASD. Antidepressant use varied for specific ASDs and ranged from 50 to 75%. An antipsychotic medication was used in 30% of persons prescribed an ASD and ranged from 16.8 to 54.2% for specific ASDs. Both antidepressant and antipsychotic use occurred in 22.2% of persons prescribed an ASD, respectively.
Significance
The pattern of CNS-active drug use has changed from previous years in this geographic region. Use of phenytoin has declined markedly, but antidepressant use has increased substantially. The CNS side effect profile of these medications and the possible long-term consequences in this population can greatly complicate therapy in these individuals.
Keywords: Epilepsy, Elderly, Nursing Home, Antiseizure drug, Antidepressant, Antipsychotic
1.0 Introduction
The prevalence of epilepsy exhibits a U-shaped curve, with high rates in children and even higher rates in the elderly [1]. The number of elderly patients in the United States is rapidly increasing and is projected to be 71 million by 2030 [2]. The prevalence of epilepsy is higher in elderly nursing home residents than in any other cohort, with the point prevalence of an ICD-9 epilepsy/seizure code present in 7.8% of all elderly home nursing residents in the USA during 2007 [3]. Information regarding the patterns of antiseizure drug (ASD) use in this population is limited, and most of the available data is from studies reported more than a decade ago. The earliest report from a large nursing home population was by Schachter et al. in 1998 that reported phenytoin to be the most commonly used ASD [4]. Other studies also indicated phenytoin to be the most commonly prescribed ASD in this setting [5–7].
The use of ASDs parallels the prevalence of epilepsy, and use of CNS co-medications such as antidepressants and antipsychotics is also high [5]. All three classes of drugs affect brain function and have the potential for pharmacodynamic as well as pharmacokinetic interactions. Recent information regarding the patterns of use of ASD and other CNS-active drugs in nursing homes is not readily available. This report presents information regarding use of CNS drugs at one time point during 2013 in a region previously reported and discusses how patterns of prescribing have changed.
2.0 Methods
2.1 Subjects
The data for this study were obtained from a pharmacy database serving Minnesota and Wisconsin nursing home residents and included information on residents who were receiving at least one ASD, antipsychotic, or antidepressant medication. Each drug was classified into one of the three groups (ASD, antipsychotic, or antidepressant) based on the Generic Product Identifier (GPI) classification system. The data extracted included age, formulation type, trademarked/generic drugs, and route of administration. Data were a cross section on a single day in October 2013. Diagnostic codes associated with drug use were not available.
2.2 Data analysis
Evidence shows that incidence and prevalence rates of epilepsy increase with age (across the entire age span) in the community-dwelling population [1, 8]. It may not be appropriate to equate “elderly” who are 65 years of age with those who are 85 years of age in terms of functional or cognitive abilities. In addition, elderly with 85 years of age are more likely to develop chronic illness, be disabled, and be more dependent on others for assistance with daily activities [9]. Therefore, elderly patients were divided into one (≥65 years) and four age groups: 65–74, 75–84, 85–94, and 95+ years. Descriptive statistics were performed (RStudio, Inc.) to determine the distribution of drugs prescribed by age, formulation type, trademarked/generic drugs, and route of administration. The percentage of residents taking a particular drug was calculated as the ratio of number of residents receiving the particular drug to the total number of residents receiving all the drugs in the particular drug type. The number and percentage of residents receiving ASDs in combination with antipsychotic and antidepressant medications (ASD + antidepressants, or ASD + antipsychotics, or ASD + antipsychotics + antidepressants) were also evaluated.
3.0 Results
3.1 Subject characteristics
The total number of nursing home residents on the study day was 18,752. The median age of all residents in this study was 75 years. Among the elderly nursing home residents, a majority of the residents receiving a CNS medication were women (70.7%) and were in the age group 85–94 (26.7%) (Table 1).
Table 1.
Characteristics of study subjects
|
Residents, n (%)
| ||||
|---|---|---|---|---|
| Overall | ASDs | Antidepressants | Antipsychotics | |
| Age group | ||||
| <65 | 4704 (37.5%) | 2075 (43.6%) | 3320 (35.2%) | 2700 (49.4%) |
| ≥65 | 7829 (62.5%) | 2687 (56.4%) | 6106 (64.8%) | 2762 (50.6%) |
|
| ||||
| Elderly age group | ||||
|
| ||||
| 65–74 | 1411 (11.3%) | 700 (14.7%) | 1059 (11.2%) | 615 (11.3%) |
| 75–84 | 2446 (19.5%) | 921 (19.3%) | 1934 (20.5%) | 891 (16.3%) |
| 85–94 | 3348 (26.7%) | 928 (19.5%) | 2648 (28.1%) | 1043 (19.1%) |
| 95+ | 624 (5%) | 138 (2.9%) | 465 (4.9%) | 213 (3.9%) |
| Gender | ||||
| Men | 2295 (29.3%) | 826 (30.7%) | 1716 (28.1%) | 841 (30.4%) |
| Women | 5534 (70.7%) | 1861 (69.3%) | 4390 (71.9%) | 1921 (69.6%) |
3.2 Medication Use
Overall, 66.8% of the total population (N=18,752) was prescribed a CNS-active drug. For those 65 years and older, 14.3% of the total population was prescribed an ASD, 14.7% an antipsychotic, and 32.6% an antidepressant medication (Figure 1). Gabapentin prescriptions comprised 7.3%, valproate 3.0%, levetiracetam 1.8%, phenytoin 0.9%, and pregabalin 0.5% (data not shown).
Figure 1. Number and percentage of residents receiving the drugs in each group or combination.
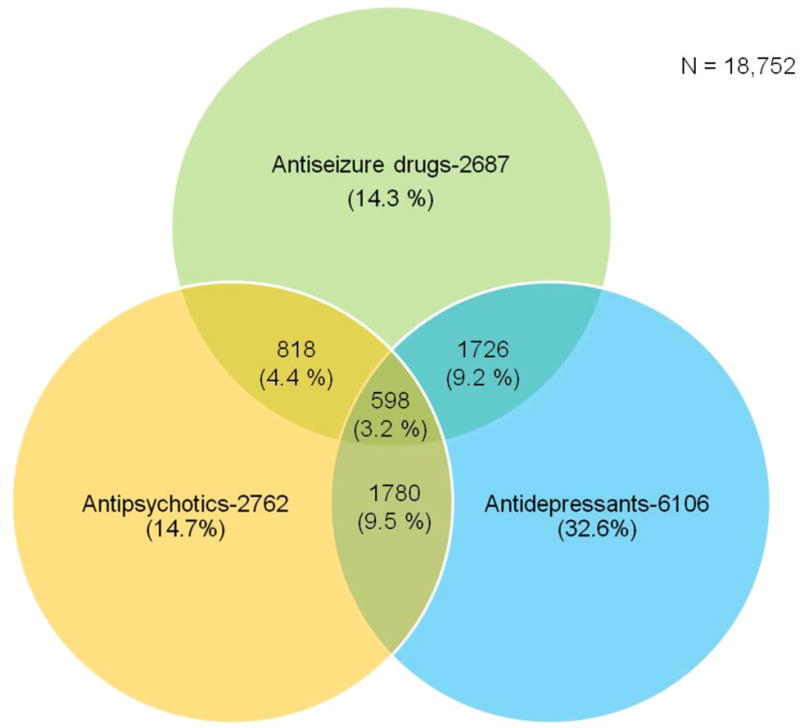
Legend: Percentages were calculated by dividing the number of residents in each group or combination by the total number of residents in the nursing home (denominator; N = 18,752).
Within the ASD group, the five most commonly prescribed ASDs were gabapentin (50.6% of the total ASDs), valproate (20.7%), levetiracetam (12.2%), clonazepam (10.5%), and phenytoin (6.4%) (Table 2). Approximately 60% of the residents in the 95+ age group were prescribed gabapentin, whereas 41% of the residents were prescribed gabapentin in the youngest cohort. In contrast, valproate, levetiracetam, and clonazepam were more frequently prescribed in the youngest compared to the oldest cohort. In the antidepressant medication group, the five most commonly prescribed medications were trazodone (28.4%), citalopram (28.1%), mirtazapine (19.0%), sertraline (16.9%), and paroxetine (7.%) (Table 2). Citalopram and mirtazapine use was higher in the older age groups than the youngest age groups, whereas venlafaxine use was lower in older age groups than the youngest age group. In the antipsychotic medication group, the five most commonly prescribed medications were quetiapine (36.5%), risperidone (19.2%), prochlorperazine (18.6%), haloperidol (14.6%), and olanzapine (13.6%) (Table 2). Prochlorperazine and haloperidol use was higher in the older age groups than in the youngest age group, but olanzapine was lower in the older age groups than the youngest age group.
Table 2.
Number and percentage of elderly residents receiving most commonly prescribed ASDs, antipsychotics, and antidepressants
| Number of residents (% of residents) | |||||
|---|---|---|---|---|---|
|
| |||||
| ASDs | |||||
|
| |||||
| Age > =65 (N=2687) |
Age 65–74 (N=700) |
Age 75–84 (N=921) |
Age 85–94 (N=928) |
Age 95+ (N=138) |
|
|
|
|
|
|
|
|
| Gabapentin | 1360 (50.6 %) | 286 (40.9 %) | 448 (48.6 %) | 543 (58.5 %) | 83 (60.1 %) |
| Valproate | 557 (20.7 %) | 191 (27.3 %) | 184 (20 %) | 163 (17.6 %) | 19 (13.8 %) |
| Levetiracetam | 328 (12.2 %) | 94 (13.4 %) | 133 (14.4 %) | 85 (9.2 %) | 16 (11.6 %) |
| Clonazepam | 283 (10.5 %) | 115 (16.4 %) | 90 (9.8 %) | 70 (7.5 %) | 8 (5.8 %) |
| Phenytoin | 172 (6.4 %) | 47 (6.7 %) | 77 (8.4 %) | 41 (4.4 %) | 7 (5.1 %) |
| Pregabalin | 101 (3.8 %) | 37 (5.3 %) | 28 (3 %) | 33 (3.6 %) | 3 (2.2 %) |
| Carbamazepine | 92 (3.4 %) | 31 (4.4 %) | 34 (3.7 %) | 24 (2.6 %) | 3 (2.2 %) |
| Lamotrigine | 81 (3 %) | 39 (5.6 %) | 29 (3.1 %) | 13 (1.4 %) | 0 (0 %) |
| Topiramate | 48 (1.8 %) | 22 (3.1 %) | 12 (1.3 %) | 11 (1.2 %) | 3 (2.2 %) |
| Primidone | 48 (1.8 %) | 7 (1 %) | 24 (2.6 %) | 15 (1.6 %) | 2 (1.4 %) |
|
| |||||
| Antidepressants | |||||
|
| |||||
| Age > =65 (N=6106) |
Age 65–74 (N=1059) |
Age 75–84 (N=1934) |
Age 85–94 (N=2648) |
Age 95+ (N=465) |
|
|
|
|
|
|
|
|
| Trazodone | 1734 (28.4 %) | 325 (30.7 %) | 554 (28.6 %) | 714 (27 %) | 141 (30.3 %) |
| Citalopram | 1713 (28.1 %) | 240 (22.7 %) | 529 (27.4 %) | 814 (30.7 %) | 130 (28 %) |
| Mirtazapine | 1161 (19 %) | 145 (13.7 %) | 345 (17.8 %) | 551 (20.8 %) | 120 (25.8 %) |
| Sertraline | 1031 (16.9 %) | 179 (16.9 %) | 328 (17 %) | 457 (17.3 %) | 67 (14.4 %) |
| Paroxetine | 440 (7.2 %) | 76 (7.2 %) | 148 (7.7 %) | 188 (7.1 %) | 28 (6 %) |
| Venlafaxine | 438 (7.2 %) | 103 (9.7 %) | 148 (7.7 %) | 167 (6.3 %) | 20 (4.3 %) |
| Duloxetine | 342 (5.6 %) | 103 (9.7 %) | 115 (5.9 %) | 111 (4.2 %) | 13 (2.8 %) |
| Fluoxetine | 299 (4.9 %) | 72 (6.8 %) | 116 (6 %) | 94 (3.5 %) | 17 (3.7 %) |
| Escitalopram | 297 (4.9 %) | 53 (5 %) | 103 (5.3 %) | 125 (4.7 %) | 16 (3.4 %) |
| Bupropion | 257 (4.2 %) | 97 (9.2 %) | 85 (4.4 %) | 68 (2.6 %) | 7 (1.5 %) |
|
| |||||
| Antipsychotics | |||||
|
| |||||
| Age > =65 (N=2762) |
Age 65–74 (N=615) |
Age 75–84 (N=891) |
Age 85–94 (N=1043) |
Age 95+ (N=213) |
|
|
|
|
|
|
|
|
| Quetiapine | 1009 (36.5 %) | 204 (33.2 %) | 357 (40.1 %) | 392 (37.6 %) | 56 (26.3 %) |
| Risperidone | 529 (19.2 %) | 124 (20.2 %) | 165 (18.5 %) | 210 (20.1 %) | 30 (14.1 %) |
| Prochlorperazine | 515 (18.6 %) | 74 (12 %) | 162 (18.2 %) | 211 (20.2 %) | 68 (31.9 %) |
| Haloperidol | 404 (14.6 %) | 62 (10.1 %) | 120 (13.5 %) | 170 (16.3 %) | 52 (24.4 %) |
| Olanzapine | 375 (13.6 %) | 128 (20.8 %) | 124 (13.9 %) | 105 (10.1 %) | 18 (8.5 %) |
| Aripiprazole | 122 (4.4 %) | 65 (10.6 %) | 37 (4.2 %) | 17 (1.6 %) | 3 (1.4 %) |
| Clozapine | 29 (1 %) | 24 (3.9 %) | 4 (0.4 %) | 1 (0.1 %) | 0 (0 %) |
| Lithium carbonate | 29 (1 %) | 13 (2.1 %) | 9 (1 %) | 7 (0.7 %) | 0 (0 %) |
| Ziprasidone | 21 (0.8 %) | 15 (2.4 %) | 2 (0.2 %) | 4 (0.4 %) | 0 (0 %) |
| Chlorpromazine | 17 (0.6 %) | 5 (0.8 %) | 5 (0.6 %) | 6 (0.6 %) | 1 (0.5 %) |
3.3 Distribution based on combinations of ASDs, antidepressants and antipsychotics
In the 18,752 nursing home residents, the percentage prescribed a combination of drugs was 9.2% for ASDs and antidepressants, 4.4% for ASDs and antipsychotics, and 9.5% for antipsychotics and antidepressants (Figure 1). Overall, 3.2% of the residents were prescribed drugs of all three classes.
Among the eight most commonly prescribed ASDs, the use of an antidepressant with an ASD ranged from 50% (with carbamazepine) to 75% (with clonazepam) (Figure 2). The use of antipsychotic medications with an ASD ranged from 16.8% (pregabalin) to 54.2% (valproate). The use of all three medication groups ranged from 12% to 39% for the various ASDs.
Figure 2. Percentage of residents receiving the antipsychotics and antidepressants in combination with ASDs.
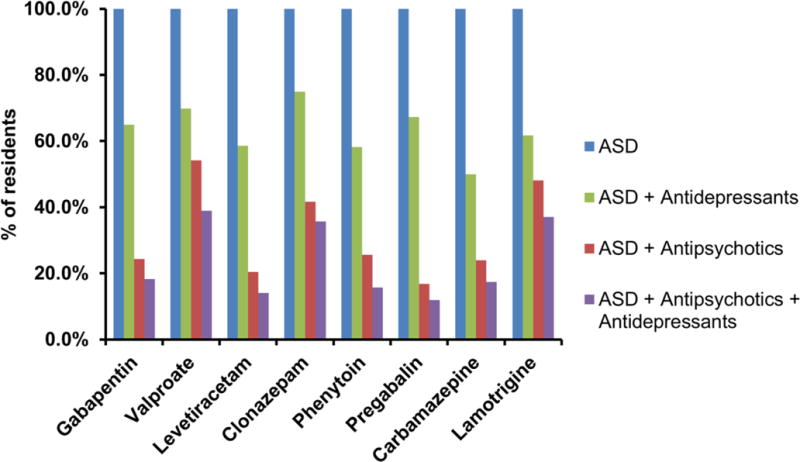
Legend: Percentages were calculated by dividing the number of residents receiving antidepressants or/and antipsychotics in each ASD group to the total number of residents in each ASD group.
3.4 Distribution based on solid and liquid formulations
Among all elderly residents, >90% were prescribed solid formulations for all ASDs (Supplementary Table 1). The percentage of residents that were prescribed liquid formulations of gabapentin was higher in the oldest age group (6% for 95+ group) compared to the youngest age group (3.8% for 65–74 group). For antidepressant, the prescription pattern did not vary across the age groups for trazodone, citalopram, mirtazapine, sertraline, and venlafaxine (99–100%) (data not shown). The percentage of elderly residents receiving antipsychotic medications in solid form (including quetiapine, risperidone, prochlorperazine, olanzapine, and haloperidol) was 100%, 89%, 99%, 100%, and 65%, respectively (data not shown).
3.5 Distribution based on generic and trademarked drugs
Generic drugs were prescribed for almost all the patients receiving ASDs, except for phenytoin (Supplementary Table 2). Generic phenytoin was prescribed for 86% residents, whereas trademarked phenytoin was prescribed for 34% of the residents. The total receiving both generic and brand exceeded 100% for phenytoin, as some residents were prescribed both generic (100 mg) and brand (50 mg) formulations. Most residents were prescribed generic formulations for antidepressant and antipsychotic medications (data not shown).
3.6 Distribution based on route of administration
All residents were prescribed ASDs by the oral route of administration, except for diazepam, which was prescribed on an as-needed basis by the rectal route. All antidepressant medications were prescribed via the oral route of administration except for selegiline (transdermal route). Most residents were prescribed antipsychotic medications by the oral route.
4.0 Discussion
The results of this study show that ASD, antidepressant and antipsychotic medications are frequently used in elderly nursing home residents, and are often used in combination. Compared to earlier data from this geographic area [5], the pattern of prescribing has changed. Phenytoin is no longer the most commonly used ASD, and use of an antidepressant with ASDs has increased considerably. In addition, the concomitant use of both antidepressant and antipsychotic medications with an ASD has increased compared to previous reports in the same geographic area [5]. The increase in frequency is consistent with another report showing that the 2006 rate of antidepressant use increased to 47.5% compared to the 1996 rate of 21.9% [10]. Antipsychotic use also increased significantly in older nursing home residents, from 16.2% in 1996 to 25.5% in 2006 [10].
Overall, drugs categorized as ASDs were prescribed to 14.3% of the total population. Gabapentin, although classified as an ASD, is most commonly used to treat pain, and valproate may be used to modify behavior [4, 11–13]. Levetiracetam, phenytoin, carbamazepine, lamotrigine, topiramate, primidone, oxcarbazepine, zonisamide, felbamate, tiagabine, ethosuximide, and rufinamide were identified as being primarily used to treat epilepsy. Although we did not have data regarding indications, the proportion of ASDs used for epilepsy is very similar to previous studies from the United States, Austria and Germany [6, 14, 15].
The composition of the individual ASDs used in our region has changed. This study was based in a similar geographic region as that reported by Lackner et al. that found phenytoin was used by 6.5% of residents [5]. Previous studies reported that phenytoin was by far the most commonly used ASD in nursing homes in the USA, with more than 6% of the elderly residents receiving it [4, 6]. Phenytoin has many shortcomings including its potential for drug-drug interactions and remarkable intra-patient variability. Selective serotonin reuptake inhibitor (SSRI) antidepressants including fluoxetine, norfluoxetine, sertraline and paroxetine inhibit cytochrome 2C P450 enzymes responsible for phenytoin metabolism [16, 17]. Valproate can inhibit phenytoin metabolism and can compete for albumin binding sites [18, 19]. Risperidone, one of the frequently used atypical antipsychotic drugs, inhibits phenytoin’s clearance probably through the CYP2C9, while phenytoin also inhibits risperidone’s clearance [20]. Carbamazepine and oxcarbazepine induce phenytoin clearance. Use of enzyme-inducing ASDs with antidepressants and antipsychotics may lead to therapeutic complications in the treatment of depression and psychosis in patients with epilepsy [21, 22]. Phenytoin also exhibits highly varying concentrations within elderly nursing home patients [23]. A remarkable decline in phenytoin use, at least in our region, has occurred, as only 0.9% of all residents were prescribed phenytoin in 2013 (6.4% of elderly residents taking ASDs). Levetiracetam is now more widely used than phenytoin in our region. The change in phenytoin prescribing could be due to the known high variability in phenytoin concentrations reported in 2003 and the availability of more water soluble, renally cleared medications that have few drug-drug interactions which make these drugs a less complicated choice for individuals with multiple co-medications.
The overall concomitant use of an antidepressant medication in patients receiving ASDs was 64.2% and was similar for all ASDs and across all ages. The use of an antidepressant medication with an ASD has increased substantially in our region, as Lackner et al. reported combined use of an ASD with an antidepressant as 18.9% compared to the 64.2% in this study [5]. In younger persons with epilepsy, depression can be a significant co-morbidity, in the range of 30 to 35% [24, 25]. Our results suggest that depression may be common in nursing home elderly treated with ASDs and may be much higher than in the overall nursing home population. Clonazepam was the ASD most frequently used with an antidepressant medication; sedation from clonazepam may have been misdiagnosed as depression. An increase in use of antidepressant medications in nursing homes over the past decade has been reported by Hanlon et al; 47.5% of the USA nursing population was prescribed an antidepressant in 2006, and the rate has increased markedly from 21.9% in 1996 [10]. The dramatic increase in use of antidepressants is of concern, as these drugs may have significant CNS side-effects that could lead to long-lasting complications in an elderly population. It is not known if depression was previously underdiagnosed or whether it is being over treated presently. Hanlon et al. suggested that the increased use may be related more to quantity and quality of staffing than to physiologic depression [10].
The use of antipsychotic drugs in residents receiving ASDs was 30.4%. This number is approximately 2.5-fold higher than seen in our previous study of nursing home residents in this geographical area [5]. In 3.2% of our samples, the subjects were receiving all three CNS drugs. The presence of multiple medications that can have an effect on the CNS is of concern, as this group of individuals represents patients at a more vulnerable and fragile time of life, with the possibility of side-effects having a much more devastating outcome (e.g., fractures from falls).
There are several limitations of this study. Indication for why a drug was prescribed was not available. The actual prescribing of these drugs was done by various physicians, and it is not known what criteria were used to make the diagnostic and prescription decisions. With the new classification of epilepsy, a person can be given this diagnosis after a single seizure in the context of a CNS condition known to be associated with recurrent seizures [26]. Thus it is likely that many were treated after a single seizure in the context of a previous stroke, a brain tumor, Alzheimer’s disease or other condition. Information about the prescription of co-medications other than antidepressants and antipsychotics that could possibly interact with ASDs was not available in the current dataset. This study only included data from one day in October. The seasonal and meteorological associations with depressive symptoms in older adults have been reported previously [27]. The levels of depressive symptoms were the highest in winter and the lowest in summer. In addition, participants living in areas with higher levels of rainfall in the preceding and/or current calendar month had greater depressive symptoms while those living in areas with sunnier climates had fewer depressive symptoms. However, to the best of our knowledge, frequency of ASD medication use in different seasons has not been studied/reported. Most of the ASD, antipsychotic, and antidepressant medications are usually for long-term management of treatment. Therefore, we believe the frequency of medications may not change over time within these age groups. In our current study, the information was not available on multiple days covering all seasons to provide definitive conclusions. Future studies should consider evaluating the frequency over an extended period of time (6 months or 1 year) covering all seasons including fall, winter, spring, and summer.
Results from our study raise a number of concerns regarding treatment of elderly nursing home residents. These individuals are frail and suffer from a number of cognitive impairments such as memory loss. Quality of life can also be affected by increasing somnolence. Most importantly, by affecting balance, these medications may contribute to falls and fractures. Unfortunately, very little prospective nursing home research has been done to determine the accuracy of the diagnoses for which these drugs are prescribed or their effectiveness and safety in the vulnerable nursing home population.
Acknowledgments
The authors wish to thank Merwin LTC Pharmacy for providing the nursing home data. This project was funded in part by the National Institutes of Health NIA R01AG026390.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors report no conflicts of interest. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc. 1996;71:576–86. doi: 10.4065/71.6.576. [DOI] [PubMed] [Google Scholar]
- 2.Administration on Aging. A Profile of Older Americans: 2003. US Department of Health and Human Services; Washington, DC: 2005. [Google Scholar]
- 3.Birnbaum AK, Leppik IE, Svensden K, Eberly LE. Prevalence of Epilepsy and Seizure Codes as a Co-morbidity of Neurological Disorders in Nursing Home Residents. Neurology. 2016 In Press. [Google Scholar]
- 4.Schachter SC, Cramer GW, Thompson GD, Chaponis RJ, Mendelson MA, Lawhorne L. An evaluation of antiepileptic drug therapy in nursing facilities. J Am Geriatr Soc. 1998;46:1137–41. doi: 10.1111/j.1532-5415.1998.tb06654.x. [DOI] [PubMed] [Google Scholar]
- 5.Lackner TE, Cloyd JC, Thomas LW, Leppik IE. Antiepileptic drug use in nursing home residents: effect of age, gender, and comedication on patterns of use. Epilepsia. 1998;39:1083–7. doi: 10.1111/j.1528-1157.1998.tb01294.x. [DOI] [PubMed] [Google Scholar]
- 6.Garrard J, Cloyd J, Gross C, Hardie N, Thomas L, Lackner T, Graves N, Leppik I. Factors associated with antiepileptic drug use among elderly nursing home residents. J Gerontol A Biol Sci Med Sci. 2000;55:M384–92. doi: 10.1093/gerona/55.7.m384. [DOI] [PubMed] [Google Scholar]
- 7.Garrard J, Harms S, Hardie N, Eberly LE, Nitz N, Bland P, Gross CR, Leppik IE. Antiepileptic drug use in nursing home admissions. Ann Neurol. 2003;54:75–85. doi: 10.1002/ana.10593. [DOI] [PubMed] [Google Scholar]
- 8.Hauser WA, Annegers JF, Kurland LT. Prevalence of epilepsy in Rochester, Minnesota: 1940–1980. Epilepsia. 1991;32:429–45. doi: 10.1111/j.1528-1157.1991.tb04675.x. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Department of Commerce. We, the American Elderly. Economics and Statistics Administration, Bureau of the Census; Washington, DC: 1993. [Google Scholar]
- 10.Hanlon JT, Handler SM, Castle NG. Antidepressant prescribing in US nursing homes between 1996 and 2006 and its relationship to staffing patterns and use of other psychotropic medications. J Am Med Dir Assoc. 2010;11:320–4. doi: 10.1016/j.jamda.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birnbaum AK, Hardie NA, Conway JM, Bowers SE, Lackner TE, Graves NM, Leppik IE. Valproic acid doses, concentrations, and clearances in elderly nursing home residents. Epilepsy Res. 2004;62:157–62. doi: 10.1016/j.eplepsyres.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Cascade E, Kalali AH, Weisler RH. Varying uses of anticonvulsant medications. Psychiatry (Edgmont) 2008;5:31–3. [PMC free article] [PubMed] [Google Scholar]
- 13.Italiano D, Capuano A, Alibrandi A, Ferrara R, Cannata A, Trifiro G, Sultana J, Ferrajolo C, Tari M, Tari DU, Perrotta M, Pagliaro C, Rafaniello C, Spina E, Arcoraci V. Indications of newer and older anti-epileptic drug use: findings from a southern Italian general practice setting from 2005–2011. Br J Clin Pharmacol. 2015;79:1010–9. doi: 10.1111/bcp.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huber DP, Griener R, Trinka E. Antiepileptic drug use in Austrian nursing home residents. Seizure. 2013;22:24–7. doi: 10.1016/j.seizure.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Huying F, Klimpe S, Werhahn KJ. Antiepileptic drug use in nursing home residents: a cross-sectional, regional study. Seizure. 2006;15:194–7. doi: 10.1016/j.seizure.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Nelson MH, Birnbaum AK, Remmel RP. Inhibition of phenytoin hydroxylation in human liver microsomes by several selective serotonin re-uptake inhibitors. Epilepsy Res. 2001;44:71–82. doi: 10.1016/s0920-1211(00)00203-5. [DOI] [PubMed] [Google Scholar]
- 17.Schmider J, Greenblatt DJ, von Moltke LL, Karsov D, Shader RI. Inhibition of CYP2C9 by selective serotonin reuptake inhibitors in vitro: studies of phenytoin p-hydroxylation. Br J Clin Pharmacol. 1997;44:495–8. doi: 10.1046/j.1365-2125.1997.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patsalos PN, Lascelles PT. In vitro hydroxylation of diphenylhydantoin and its inhibition by other commonly used anticonvulsant drugs. Biochem Pharmacol. 1977;26:1929–33. doi: 10.1016/0006-2952(77)90168-x. [DOI] [PubMed] [Google Scholar]
- 19.Patsalos PN, Lascelles PT. Effect of sodium valproate on plasma protein binding of diphenylhydantoin. J Neurol Neurosurg Psychiatry. 1977;40:570–4. doi: 10.1136/jnnp.40.6.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanderson DR. Drug interaction between risperidone and phenytoin resulting in extrapyramidal symptoms. J Clin Psychiatry. 1996;57:177. [PubMed] [Google Scholar]
- 21.Johannessen SI, Landmark CJ. Antiepileptic drug interactions - principles and clinical implications. Curr Neuropharmacol. 2010;8:254–67. doi: 10.2174/157015910792246254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pugh MJ, Vancott AC, Steinman MA, Mortensen EM, Amuan ME, Wang CP, Knoefel JE, Berlowitz DR. Choice of initial antiepileptic drug for older veterans: possible pharmacokinetic drug interactions with existing medications. J Am Geriatr Soc. 2010;58:465–71. doi: 10.1111/j.1532-5415.2010.02732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birnbaum A, Hardie NA, Leppik IE, Conway JM, Bowers SE, Lackner T, Graves NM. Variability of total phenytoin serum concentrations within elderly nursing home residents. Neurology. 2003;60:555–9. doi: 10.1212/01.wnl.0000052997.43492.e0. [DOI] [PubMed] [Google Scholar]
- 24.Kanner AM, Schachter SC, Barry JJ, Hesdorffer DC, Mula M, Trimble M, Hermann B, Ettinger AE, Dunn D, Caplan R, Ryvlin P, Gilliam F, LaFrance WC., Jr Depression and epilepsy: epidemiologic and neurobiologic perspectives that may explain their high comorbid occurrence. Epilepsy Behav. 2012;24:156–68. doi: 10.1016/j.yebeh.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Kanner AM, Schachter SC, Barry JJ, Hesdorffer DC, Mula M, Trimble M, Hermann B, Ettinger AE, Dunn D, Caplan R, Ryvlin P, Gilliam F, LaFrance WC., Jr Depression and epilepsy, pain and psychogenic non-epileptic seizures: clinical and therapeutic perspectives. Epilepsy Behav. 2012;24:169–81. doi: 10.1016/j.yebeh.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Fisher RS, Leppik I. Debate: When does a seizure imply epilepsy? Epilepsia. 2008;49(Suppl 9):7–12. doi: 10.1111/j.1528-1167.2008.01921.x. [DOI] [PubMed] [Google Scholar]
- 27.O’Hare C, O’Sullivan V, Flood S, Kenny RA. Seasonal and meteorological associations with depressive symptoms in older adults: A geo-epidemiological study. J Affect Disord. 2016;191:172–9. doi: 10.1016/j.jad.2015.11.029. [DOI] [PubMed] [Google Scholar]


