Abstract
Children who are deaf and receive bilateral cochlear implants (BiCIs) perform better on spatial hearing tasks using bilateral rather than unilateral inputs; however, they underperform relative to normal-hearing (NH) peers. This gap in performance is multi-factorial, including the inability of speech processors to reliably deliver binaural cues. Although much is known regarding binaural sensitivity of adults with BiCIs, less is known about how the development of binaural sensitivity in children with BiCIs compared to NH children. Sixteen children (ages 9–17 years) were tested using synchronized research processors. Interaural time differences and interaural level differences (ITDs and ILDs, respectively) were presented to pairs of pitch-matched electrodes. Stimuli were 300-ms, 100-pulses-per-second, constant-amplitude pulse trains. In the first and second experiments, discrimination of interaural cues (either ITDs or ILDs) was measured using a two-interval left/right task. In the third experiment, subjects reported the perceived intracranial position of ITDs and ILDs in a lateralization task. All children demonstrated sensitivity to ILDs, possibly due to monaural level cues. Children who were born deaf had weak or absent sensitivity to ITDs; in contrast, ITD sensitivity was noted in children with previous exposure to acoustic hearing. Therefore, factors such as auditory deprivation, in particular, lack of early exposure to consistent timing differences between the ears, may delay the maturation of binaural circuits and cause insensitivity to binaural differences.
I. INTRODUCTION
Humans rely on binaural cues to localize sounds and to segregate speech from interfering sounds in the environment. Normal-hearing (NH) individuals benefit from having access to robust acoustic cues that are used to localize sounds in the environment. Sounds reach the ears with varying interaural timing differences (ITDs) and interaural level differences (ILDs) based on location (Bronkhorst and Plomp, 1988; Carlile et al., 2005; Middlebrooks and Green, 1991). ILDs result from differences in sound pressure level between the two ears. ITDs result from disparities in the timing of the acoustic waveform at the two ears. For low-frequency stimuli of a few hundred Hz, ITDs that result from disparities in the temporal fine structure of the acoustic waveform at the two ears are particularly salient. JNDs (just-noticeable differences, the smallest changes in the stimulus magnitude that can be reliably discriminated) for tones can be as small as a few tens of μs. JNDs in NH adults decrease up to 750 Hz and then increase with frequency until about 1400 Hz, where the usability of ITD completely disappears (e.g., Brughera et al., 2013). ITDs can also be detected when a stimulus with a high-frequency carrier has a relatively slow amplitude modulation (AM) of the temporal envelope. Envelope ITD JNDs are lowest at modulation rates of 100–200 Hz, and sensitivity decreases rapidly as the modulation rate is increased further (McFadden and Pasanen, 1978; Bernstein, 2001). These findings suggest that when high-frequency channels in the binaural system receive information similar to what is typically provided to low-frequency channels, ITD sensitivity can be similar in low- and high-frequency regions (Colburn and Esquissaud, 1976). The magnitude of physical ILDs increase with increasing frequencies for sounds presented in the free field (Fedderson, 1957) and ILD JNDs in NH adults are roughly independent of frequency when presented over headphones (Yost and Dye, 1988).
Children spend much of their time attending to sound sources in complex acoustic environments, like a classroom, where there is one target talker with various distracting auditory signals, a situation known as the “cocktail party” environment (Cherry, 1953). In general, NH listeners show very good sensitivity to binaural cues and are able to make use of these cues to complete spatial hearing tasks effectively. For example, children with NH have shown spatial release from masking (i.e., better speech understanding when the target and competing speech are spatially separated compared to when they are co-located) as early as 3–4 years of age (Garadat and Litovsky, 2007). Although spatial release from masking seems to develop at such a young age, this auditory skill might continue to develop and improve throughout adolescence. In particular, when the spatial configuration of the competing speech minimizes availability of monaural head shadow and necessitates the use of binaural cues (binaural release from masking or binaural squelch), NH children show more variability than adults (Misurelli and Litovsky, 2012).
Given the importance of binaural cues for functioning in complex listening environments, patients who are deaf and eligible for cochlear implants (CIs) are often implanted with bilateral CIs (BiCIs), in order to provide them with some of the spatial cues that are known to assist NH subjects with spatial hearing (for reviews see Litovsky, 2015; Litovsky and Gordon, 2016). While adults with BiCIs have been studied extensively with regard to their binaural sensitivity, much less is known in children with BiCIs, and how their performance compares to binaural sensitivity in NH children. Studies with NH children show that sound localization ability is fairly well-developed by 4–5 years of age; in fact, some children show adult-like free-field localization performance, with root-mean-square (RMS) errors as low as 8° (Grieco-Calub and Litovsky, 2010; Litovsky and Godar, 2010; Zheng et al., 2015). In addition, ITD JNDs in the envelope of high-frequency sounds are similar between NH children age 8–10 years and adults (Ehlers et al., 2016). Young children who use BiCIs perform significantly worse on spatial hearing tasks conducted in the free-field than their NH peers (Grieco-Calub and Litovsky, 2010; Litovsky, 2015; Zheng et al., 2015), even after several years of experience with BiCIs (Zheng et al., 2015). That is not to say that children with BiCIs do not receive benefits from access to sound in both ears; there is ample evidence to suggest that they perform better while using two CIs than while listening through a single CI alone (Grieco-Calub and Litovsky, 2010; Litovsky et al., 2006).
The reason for poorer performance in individuals with BiCIs compared with NH peers has been a topic of considerable interest in recent years (Gordon et al., 2014; Kan and Litovsky, 2015; Kan et al., 2013; Litovsky and Gordon, 2016). One known limitation is the inability of today's CIs to deliver binaural cues with fidelity (Baumgaertel et al., 2017; van Hoesel, 2004; Kan and Litovsky, 2015; Wilson and Dorman, 2008), a problem which arises from multiple potential causes. First, is the lack of synchronization of the CI sound processors, which limits the ability of the two processors to present binaural cues to the electrode arrays in a coordinated and consistent manner between ears. Second, CI sound processors discard temporal fine structure, which is an important cue for ITD processing of frequencies below 1400 Hz (Brughera et al., 2013). Third, spread of excitation in the cochlea typically results in large effective bandwidths (Nelson et al., 2008), stimulation of multiple auditory channels, which minimizes specificity of stimulation at specific locations and can distort the ITD cues for multi-electrode stimulation (Kan and Litovsky, 2015). Fourth, current sound processing algorithms use high-rate, amplitude-modulated stimulation with the rates of stimulation often ≥900 pulses per second (pps) (Loizou, 2006). This high rate of stimulation is thought to provide better speech understanding in many users, but is not ideal for ITD sensitivity, which is known to be better at lower rates (van Hoesel et al., 2009). On the contrary, speech understanding is poorer at low rates, suggesting there is a trade-off between binaural sensitivity and speech understanding (Churchill et al., 2014). Fifth, frequency-to-electrode allocation occurs without taking into account the actual place of stimulation. This can be problematic as the electrode arrays may be inserted at different depths between ears. Therefore, electrodes in the two ears that have the same numbers will be matched for frequency regardless of specific anatomical locations. This approach has the potential to cause inputs between the two ears to stimulate cochlear regions that are not matched for frequency, thereby resulting in a reduced convergence of binaural information due to frequency offset, which causes reduced binaural sensitivity (Kan and Litovsky, 2015; Kan et al., 2013). Finally, auditory deprivation results in loss of neural function, both peripherally and centrally, which may lead to degraded abilities to use binaural inputs (Shepherd and McCreery, 2006). Research has shown that profound hearing loss early in life can cause a lack of tonotopic organization of the primary auditory cortex (Kral et al., 2009). Therefore, it may be that other parts of the auditory pathway are affected as well, including pathways responsible for conveying binaural cues.
In the event that CI speech processors were able to better deliver binaural cues to BiCI users, it would be imperative to understand the development of sensitivity to ITDs and ILDs in BiCI users and to compare their development to their NH peers (for further review see Dietz, 2016). Studies on adults with BiCIs have been conducted for nearly two decades using research processors that carefully control which electrodes are stimulated, and that deliver synchronized binaural stimulation to selected pairs of electrodes in the right and left cochlear arrays. Research has shown that there is great variability across patients in sensitivity to ITDs but sensitivity to static ILDs is generally very good (Kan et al., 2013; Laback et al., 2015; Litovsky et al., 2010). The reason that ILD sensitivity may occur is that none of those studies used level roving to minimize access to monaural level cues. However, it seems that BiCI listeners were likely performing a binaural comparison because studies that include interaural place-of-stimulation mismatch (i.e., Kan et al., 2013; Kan et al., 2015b) show elevated ILD JNDs as the electrodes became less matched in place. In addition, if one presents dynamic ILDs through envelopes that are interaurally decorrelated, performance drops to chance with sufficient place mismatch likely because there are no monaural level cues in such a task (Goupell and Litovsky, 2015).
One of the primary factors that may account for the variability in ITD sensitivity is the age at onset of deafness. Adults with early onset of deafness generally have poor ITD sensitivity whereas adults with auditory experience prior to onset of deafness typically show relatively better ITD sensitivity, even if they experience many years of auditory deprivation between the time of onset of deafness and time of implantation (Laback et al., 2015; Litovsky et al., 2010; Litovsky et al., 2012). A fundamental difference between research on adults with BiCIs and pediatric research is that most testing on adults with BiCIs uses high-performing listeners with a late onset of deafness, while most children with BiCIs will have an early onset of deafness. Many children who are fitted with BiCIs are congenitally deaf, and have never been exposed to normal binaural acoustic cues. Here, we were able to test two groups of children: children who were exposed to normal acoustic hearing prior to becoming deaf and children who were not. Thus, one of the goals of this work was to compare binaural sensitivity in both groups of children with adult BiCI users.
To date, only a small number of studies with children who use BiCIs have been conducted using synchronized research processors to present binaural cues with fidelity to a single pair of electrodes. There is evidence to suggest that children with BiCIs show reliable sensitivity to ILDs (again, with the caveat that monaural cues are available), but are less reliable in detecting the presence of ITDs (Gordon et al., 2014; Salloum et al., 2010). Those studies did not mirror the procedures that have been used to date in studies with adult bilateral CI users (Lawson et al., 1998; van Hoesel and Tyler, 2003; van Hoesel et al., 2009), i.e., procedures that are based on the classic binaural hearing literature. Traditionally, left-right discrimination is used to probe sensitivity to changes in ITDs and ILDs, measuring perceived changes in intracranial sound image location. In contrast to the classic task, Salloum et al. (2010) used a four-alternative forced-choice task where children reported if sounds were perceived to be on the left, right, center or towards both ears. Some limitations of this method are that JNDs cannot be calculated and it is unclear what cue was used to perform the task. Nor did that procedure effectively evaluate perceived intracranial lateralization of sources on a continuous scale, which limits the ability to interpret the data as a change in intracranial location (Litovsky et al., 2010; Kan et al., 2013). Gordon et al. (2014) measured children's detection of any change in the stimuli, rather than the discrimination of a binaural cue that relied on perceiving the change in an intracranial location. Therefore, another goal of the present study was to use the same psychophysical procedures as in the binaural literature. This enables the results of this study to be compared more directly to the results of adult BiCI users (e.g., Litovsky et al., 2010; Kan et al., 2013) and more broadly to NH listeners. In this set of experiments, we are able to establish what gaps there are, if any, between children who are fitted with BiCIs at a young age, and adults with pre- or post-lingual onset of deafness. Having the same procedures in children and adults is paramount to being able to ultimately draw conclusions about the binaural hearing sensitivity in BiCI users across the age span. Another important distinction between the current study and prior studies on binaural hearing in children with BiCIs is the combination of the intracranial lateralization task and the discrimination task. By pairing these data, one can better understand if the discrimination performance truly relied on changes in intracranial location and if a binaural comparison actually occurred.
The present study evaluated binaural sensitivity in children with BiCIs in three experiments using low-rate pulsatile stimulation delivered to the electrode arrays through synchronized research processors. In experiments I and II, children performed a left-right discrimination task that measured ITD and ILD JNDs. In experiment III, the perceived intracranial location of sound sources was measured using a lateralization task, which offers a more direct estimate regarding the contribution of binaural cues to spatial mapping abilities. Overall, the purpose of this work was to understand whether children who are deaf and use unsynchronized bilateral processors in everyday situations can use ITD and/or ILD cues on acute psychophysical tasks when binaural cues are controlled with a research processor.
II. GENERAL METHODS
A. Subjects
Sixteen children who were profoundly hearing impaired and used BiCIs participated in three experiments. The subjects' mean age was 11.9 years (±2.2). Table I shows the profiles for each subject. All children wore Cochlear Ltd. devices that were either from the CI24 or CI512 family of CIs. These internal devices have an electrode array of 22 intra-cochlear stimulation electrodes and two extra-cochlear ground electrodes. The electrodes are numbered from the basal end to the apical end, or 1 to 22, respectively.
TABLE I.
Subject information.
| Subjects | Sex | Age at first test (years) | Early Acoustic Hearing Experience (months) | Age at 1st implant (months) | Ear 1st CI | Inter-implantation Delay (years, months) | BiCI Exp. (years, months) |
|---|---|---|---|---|---|---|---|
| CIDX | M | 10 | None | 29 | Right | 1,2 | 8,2 |
| CIAY | M | 12 | 42 | 62 | Right | 0, 10 | 6,9 |
| CIEB | F | 11 | ID at 19, progressive loss | 43 | Right | 0,5 | 7,3 |
| CIAQ | M | 17 | ID at 14 | 48 | Right | 4,3 | 9,4 |
| CIAP | F | 14 | ID at 16, progressive loss | 42 | Right | 1,8 | 9,7 |
| CIBO | F | 14 | ID at 25, fluctuating loss | 34 | Right | 1,1 | 10,4 |
| CIAG | M | 12 | ID at birth, progressive loss | 21 | Right | 1,5 | 9,3 |
| CIAW | M | 12 | None | 15 | Right | 4,3 | 6,5 |
| CIFF | M | 10 | None | 13 | Right | 5 | 4,7 |
| CIEC | M | 9 | ID at birth, progressive loss | 28 | Right | 0,5 | 7,2 |
| CIDJ | F | 10 | None | 19 | Right | 3,5 | 5,1 |
| CIEV | F | 11 | Birth | 32 | Right | 3,1 | 2,0 |
| CIEU | F | 13 | ID at 6, progressive loss | 51 | Right | 6,2 | 3,9 |
| CIBK | M | 15 | ID at 17 | 26 | Right | 5 | 8,1 |
| CIDQ | F | 12 | None | 46 | Right | 3,6 | 7,11 |
| CIEH | M | 9 | None | 13 | Simultaneous | 0 | 8,0 |
Subjects traveled to Madison, WI with family members and testing was conducted at the Waisman Center on the University of Wisconsin-Madison campus. Travel costs were covered and a stipend was provided. To complete the battery of tests, subjects were typically in Madison, WI for 2 to 3 days. All experiments conducted followed regulations created by the National Institute of Health and were also approved by the University of Wisconsin's Human Subjects Institutional Review Board.
B. Equipment
The experiments were controlled by a laptop computer using matlab software (The Mathworks, Natick, MA). Nucleus Implant Communicator (NIC2; Cochlear Ltd., Sydney, Australia) software and bilaterally synchronized L34 research processors were used to deliver all stimuli to each subject's internal devices. Subjects provided all responses by interfacing with a touch screen that was connected to the laptop.
C. Stimuli
Bilaterally synchronized, electric pulse trains were used. The pulses were biphasic, with a 25-μs phase duration and an 8-μs interphase gap, and the grounding configuration was monopolar. The pulses were delivered in trains at a rate of 100 pps. The individual pulses had a constant amplitude. The pulse train was 300 ms in duration. The rate of stimulation was lower than typical clinical CI stimulation in order to maximize ITD sensitivity, which is best at low stimulation rates (van Hoesel et al., 2009). This stimulation rate is also consistent with previous research completed with adults (Kan et al., 2013; Litovsky et al., 2010).
D. Implant mapping
Subjects' clinical sound processor programs (i.e., MAPs) were provided by their audiologists through direct request from the children's parents. These MAPs were used as a starting point for setting stimulation levels during the experiments. Three stimulation levels were carefully determined using the test stimuli for all even-numbered electrodes in both ears: Threshold (T, the lowest level of audibility), Comfortable (C, a level that was comfortable enough for a patient to tolerate listening to for an extended period of time), and Maximum Comfortable (M, the highest amount of current that a subject could accept briefly without the stimulation being uncomfortably loud).
Once these loudness levels were determined, C-levels were loudness-balanced within each ear, and then across ears. For within-ear balancing, subjects were presented with five-electrode sets at a time and asked to judge the relative loudness, in particular focusing on any electrodes that were judged to be “soft” or “loud” relative to the others in the series. Adjustments were manually made, until all C-levels within each group of five were perceived as having “equal loudness.” The procedure was iterated within each ear, until all 11 electrodes were tested, and a final sweep across all 11 electrodes was made in order to ensure balanced loudness. This approach is consistent with methods used in previous studies with adults (Kan et al., 2013; Litovsky et al., 2010).
E. Selection of electrode pairs
Numerous studies to date have assumed that in order to maximize binaural sensitivity, stimulation should be provided to a pair of electrodes across the two ears that are perceived to be matched by pitch, so as to stimulate populations of neurons that are tuned to the same frequency (van Hoesel, 2004; Kan et al., 2013; Litovsky et al., 2010). In fact, there is evidence to suggest that mismatched pairs of electrodes result in reduced ITD sensitivity (Kan et al., 2013; Poon et al., 2009). Thus, here too, two tasks were employed to achieve matched electrode pairs, which assumes that similar pitches will interaurally align place-of-stimulation. The first pitch-matching task had subjects assign a value to the perceived pitch, also known as pitch magnitude estimation (Kan et al., 2013; Litovsky et al., 2010). Subjects were instructed to provide a value that represents the perceived pitch of the stimulus on an arbitrary scale ranging from 1 (low pitch) to 100 (high pitch). The stimuli were presented at C level, in random order to the 22 electrodes (11 in each ear) and were repeated ten times at each electrode. Prior to initiation of the task, subjects were familiarized with assigning a value to the perceived pitch of a stimulus, via verbal discussion and practice as well as encouragement to use the full scale from 1 to 100 when subjectively judging the pitch.
The second pitch-matching task involved a direct comparison of perceived pitch between select pairs of electrodes in the two ears. On the basis of the rankings in the pitch magnitude estimation task, electrodes in the right and left ear that had similar rankings were selected. Of those two electrodes, the one in the left ear was held constant, and compared to five electrodes in the right ear, including the matched electrode, as well as two electrodes more basal and two electrodes more apical. The direct pitch comparison task was conducted using a two-interval, five-alternative forced-choice task. The subject was asked to directly compare the pitch of the electrode in the left ear, with each of the electrodes in the right ear and to determine if the pitch in the right relative to the left was: “much higher,” “higher,” “the same,” “lower,” or “much lower.” Twenty repetitions were completed per electrode pair. Pitch-matched electrode pairs chosen for testing with binaural stimuli are shown for each subject in Table II. This method has been used in adults with BiCIs in the past to select binaural pairs of electrodes (e.g., Litovsky et al., 2010).
TABLE II.
Chosen electrode pairs.
| Subject | Experiment I and III (L/R) | Experiment II Base, Mid, Apex (L/R) |
|---|---|---|
| CIAW | 14/16 | DNT |
| CIEB | 12/12 | DNT |
| CIDX | 12/12 | DNT |
| CIEV | 14/14 | DNT |
| CIFF | 14/14 | DNT |
| CIEC | 12/14 | DNT |
| CIAG | 12/10 | DNT |
| CIEU | 14/14 | 4/4, 12/12, 18/18 |
| CIAY | 12/12 | DNT, 12/12, 20/18 |
| CIDJ | 12/12 | 6/6, 12/12, 20/18 |
| CIAP | DNT | 4/4, 12/10, 20/16 |
| CIBK | DNT | 4/4, 12/12, 20/18 |
| CIBO | DNT | 4/4, 12/12, 20/18 |
| CIDQ | DNT | 4/4, 12/12, 20/20 |
| CIEH | DNT | 4/6, 12/14, 20/20 |
| CIAQ | DNT | 4/4, 12/13, 20/19 |
F. Interaural loudness balancing
A task aimed at interaural loudness balancing was performed in order to ensure the C levels of the pitch-matched pairs were perceived to be of equal loudness. In this task the stimulation level in the left electrode was held constant, while the level in the right varied. Subjects were able to control the stimulation level of the right electrode themselves, increasing or decreasing the amplitude until the stimulation in the right and left electrodes was judged to be of equal loudness. Single-electrode stimulation was used for the loudness balancing with sequential sets of electrodes being used, causing overlap in the electrodes across sets. This allowed for more stable loudness balancing within each ear. The entire mapping procedure including finding T and C levels, selection of electrode pairs, and loudness balancing took approximately two-three hours depending on subject attentiveness and consistency of responses. If subjects returned for multiple visits, the entire mapping procedure and selection of electrode pairs was repeated. At each visit, the electrode pair chosen was deemed the best pitch matched pair, even if it was not consistent with previous electrode pairs tested.
III. EXPERIMENT I AND II: DISCRIMINATION
A. Methods
In experiments I and II, ITD and ILD sensitivity were measured for each subject on electrode pairs that had been matched in pitch and balanced in loudness. In experiment I, discrimination for both ITD and ILD was measured at an electrode pair that was in the middle of the electrode arrays. In experiment II, ITD and ILD sensitivity was measured on three electrode pairs located at the basal, middle, and apical regions of the electrode arrays. An exception to this is subject CIAY, who was not tested with a basal electrode pair due to time constraints. Three subjects completed both experiments I and II. Some subjects in experiment II were not tested on both ITDs and ILDs due to time constraints, hence the slightly different N sizes.
Testing was conducted in blocks of trials where either a non-zero ITD or ILD was imposed on the pulsatile stimulation. The inter-stimulus interval was 300 ms. In experiment II, within each block of ITD or ILD testing the electrode pairs tested were randomized.
Subjects participated in a left-right discrimination task using a two-interval, two-alternative forced-choice task. In the first interval, the cue favored one ear, and in the second interval the cue favored the opposite ear (left-right followed by right-left, or vice versa). Subjects responded by indicating the direction of the sound in the second interval when compared to the first. Subjects were given feedback after each trial. When testing non-zero ITDs, ILD values were set to 0, and vice versa when testing non-zero ILDs. Typical ITD values were ±100, ±200, ±400, and ±800 μs. Typical ILD values were ±2, ±5, ±10, ±20 current units (CUs). For Cochlear-brand devices, they have a logarithmic conversion from dB to uA. For example, for the Nucleus Freedom there is a 1.82% increase in current per current level step. However, these values varied for some subjects depending on their sensitivity to these cues. Subjects were tested on 40 trials (20 right-left and 20 left-right) per cue per condition. All subjects were tested with ITD cues; due to time constraints, subjects CIDX and CIDQ were not tested on ILDs. Percent correct data were fit to a psychometric function and JNDs were calculated at the point on the psychometric function intersecting with 70.7% correct (Wichmann and Hill, 2001).
B. Analysis
One of the questions that arises regarding binaural sensitivity in children with BiCIs is whether they perform similarly to or significantly worse than their peers with NH. Data from the current study were compared to NH children tested on the same task (Ehlers et al., 2016). It is worth noting that there is currently no accepted way to directly compare electric CUs to acoustic decibels (dB). However, for the purposes of comparison, expressing ILD JNDs as a percentage of dynamic range (DR) between NH children and children with BiCIs can be informative. This approach shares some characteristics with a previous study that compared ILD lateralization ranges between NH and BiCI adults (Stakhovskaya and Goupell, 2017). For the current study, the DR for NH subjects was estimated to be 65 dB sound pressure level (SPL) based on the loudness growth function in NH adults for a 4-kHz tone (Allen et al., 1990), which was the carrier frequency for the pulse train presented to NH children in Ehlers et al. (2016). Research has shown that children and adults show similar loudness growth functions (Serpanos and Gravel, 2000). For subjects with BiCIs, the DR was estimated as
where T, C, and M are the threshold, comfortable, and maximum comfortable levels, respectively; the subscripts R and L denote the right and left ears, respectively. As there are two DRs that may differ between the ears, the smaller of the two was chosen in order to be conservative. ILD JNDs were then calculated as a percentage of DR for both subjects with NH (Ehlers et al., 2016) and subjects with BiCIs. For example, if a NH subject had an ILD JND of 5 dB, this would utilize 8.3% of their DR. If a subject with BiCIs had an across ear DR of 50 and an ILD JND of 5 CUs, this would utilize 10% of their DR.
C. Results
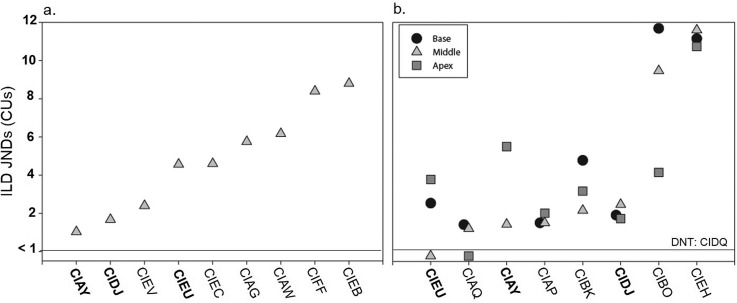
Figures 1 and 2 show ILDs and ITD JNDs, respectively. Individual JNDs from experiment I are shown in Figs. 1(a) and 2(a), while JNDs from experiment II are shown in Figs. 1(b) and 2(b). Subjects whose code is in bold font completed both experiments. Results show that all subjects had measureable ILD JNDs at all tested electrode pairs; however, performance varied across children in a number of ways. First, one subject (CIEH) had extremely large ILD JNDs at all electrode pairs tested [Fig. 1(b)]. Second, four subjects had ILD JNDs that did not vary much across electrode pair location [Fig. 1(b): CIAQ, CIAP, CIDJ, CIEH]. Finally, the remaining subjects showed larger variability in ILD JND with electrode pair location; and the best (lowest) ILD JND was measured at different electrode locations for different individuals. Similar variability in binaural JNDs across electrode pair locations has been found in adults (Kan et al., 2015a; Laback et al., 2015) and the implications are discussed in more detail below.
FIG. 1.
Individual ILD JNDs for subjects tested on a single middle electrode pair (n = 9) in experiment I (a) and for subjects tested on three electrode pairs located at the base, middle, and apex of the electrode array (n = 8) in experiment II (b). Subject codes in bold were tested in both experiments (n = 3).
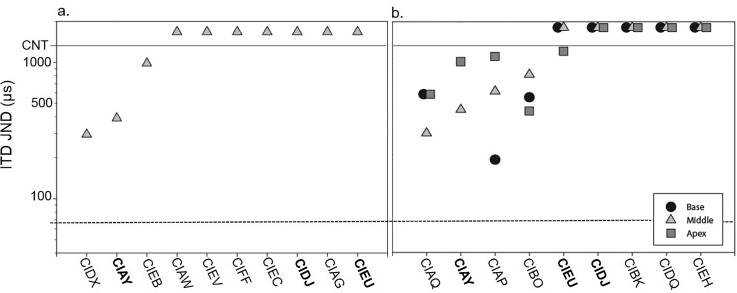
FIG. 2.
Individual ITD JNDs for subjects tested on a single middle electrode pair (n = 10) in Experiment I (a) and for subjects tested on three electrode pairs located at the base, middle, and apex of the electrode array (n = 9) in experiment II (b). Subject codes in bold were tested in both experiments (n = 3). JND values represent the ITD difference from 0 μs at which thresholds were reached. For example, a JND of 200 μs would mean that there was a total difference of 400 μs between the two ears. The dotted line represents the average NH ITD JND (Ehlers et al., 2016).
A different pattern of results emerged for ITD discrimination (Fig. 2). Of the subjects tested in experiment I (only the middle of the electrode array was tested), only 3/10 (CIDX, CIAY, and CIEB) had measureable ITD JNDs [Fig. 2(a)]. In experiment II when subjects were tested at three locations along the electrode arrays, 5/10 (CIAQ, CIAY, CIAP, CIBO, and CIEU) showed measureable ITD JNDs on at least one electrode pair. For the purpose of this study, only JNDs <1600 μs were considered measureable, a value over twice as large as the largest ITD produced by an adult human head (approximately 700 μs; Fedderson et al., 1957). This cutoff is consistent with previous studies conducted using similar paradigms (Kan et al., 2013; Litovsky et al., 2010). In addition, it can be seen in Fig. 2(b) that 4/9 subjects (CIAQ, CIAY, CIAP, CIBO) demonstrated ITD sensitivity at more than one electrode location, and one subject demonstrated sensitivity to ITDs at only the apical electrode pair (CIEU).
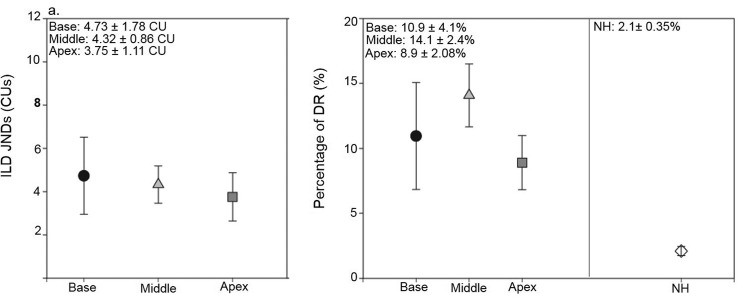
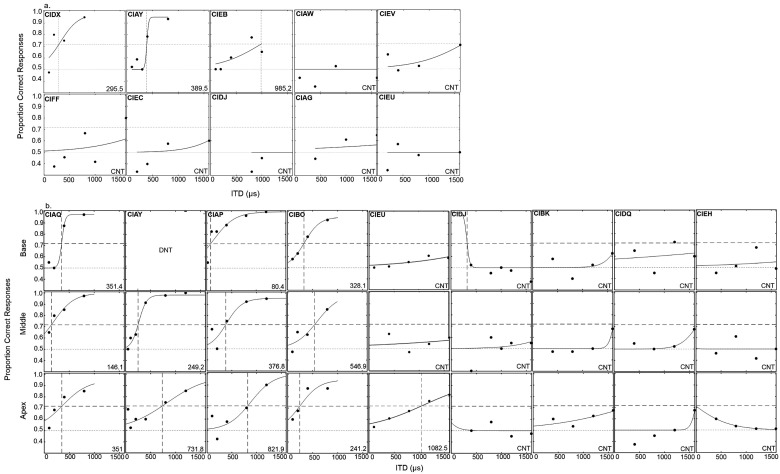
In Fig. 3(a), average data for the BiCI subjects are shown for ILDs. In addition, for middle electrode pairs, data from experiments I and II were both included in the average ILD JNDs. Data were analyzed using two one-way analysis of variance (ANOVA) with unequal N size and with factor location (base, middle, and apex) for ILDs. There was no significant main effect of location for the ILD data [F(2, 31) = 0.164, p = 0.849]. Not all subjects had measurable ITD sensitivity, making it difficult to compare averages across location. Therefore, results are discussed further for individual data and the psychometric functions of ITD sensitivity are shown in Fig. 4 for experiment I (a) and experiment II (b). If the subject had sensitivity, the JND was chosen as the point on the psychometric function intersecting with 70.7% correct. However, it can be seen that some subjects (i.e., CIFF, CIEC, CIEV, etc.) demonstrated performance above 50% when given larger ITDs. Therefore, although ITD JNDs could not be measured in this population using the 70.7% criterion; some degree of ITD sensitivity may be present in these subjects.
FIG. 3.
Average (±1 standard error) ILD JNDs (a) and ILD JNDs expressed as a percentage of DR (b) are shown for the three locations across the array. JND values represent the ILD difference from 0 CU at which thresholds were reached. For example, a JND of 2 CU would mean that there was a total difference of 4 CU between the two ears. In addition, comparisons are made with data that were previously published in NH children (Ehlers et al., 2016).
FIG. 4.
Proportion correct for each ITD tested for (a) experiment I and (b) experiment II. Proportion correct data were fit to a psychometric function and JNDs were calculated at the point on the psychometric function intersecting with 70.7% correct.
Ehlers et al. (2016) recently measured binaural sensitivity in children with NH using a Gaussian envelope tone (GET) vocoder, which has a high-frequency carrier and ITDs conveyed in the temporal envelopes of the bandlimited pulse train. The GET stimuli has a 4-kHz carrier frequency thus rendering temporal fine structure ITD cue unusable, but a salient envelope ITD cue. In addition, a GET pulse train (Goupell et al., 2013; Kan et al., 2013) can be used to approximate the spread of current that occurs with monopolar stimulation in CIs (Boëx et al., 2003). Therefore, the GET pulse trains approximately match the temporal and spectral characteristics of monopolar electrical stimulation.
Group comparisons were made for ILD findings and it is important to note how we compared and converted ILDs in current units (from the CI research processor) to ILDs in dB SPL. The ILD JND was considered in the context of each subject's DR; Fig. 3(b) shows ILD JNDs as a percentage of DR, for children with BiCIs tested here, and also DR data calculated from the NH data (Ehlers et al., 2016). A between group one-way ANOVA with unequal N was conducted on ILD JND DR for the two groups of children; for the BiCI group each subject's best JND was used. The ANOVA confirmed significantly higher ILD JNDs in percent DR for the children with BiCIs when compared to the NH children [F(1,23) = 12.720, p = 0.002]. Group comparisons for ITD JNDs are difficult to make as not all children with BiCIs had measurable sensitivity. It can be seen based on Fig. 4, that even the best performers in the BiCI group demonstrate ITD JNDs that are much higher than where the NH average JND occurs.
IV. EXPERIMENT III: LATERALIZATION
A. Methods
Nine of the subjects that participated in experiment I also participated in experiment III. All nine subjects performed the ITD measurements. Because of time constraints, one subject (CIAG) was not tested with ILDs. Testing was conducted separately for ITD and ILD cues. The values used for ITDs were: 0, ±100, ±200, ±400, ±800, and ±1600 μs. The values used for ILDs were: 0, ±2, ±4, ±10, and, ±20 CUs. An ILD of 0 was measured in order to provide a data point where a centered image might be expected, which is consistent with previous lateralization experiments conducted with BiCI subjects (Litovsky et al., 2010). There were 20 trials per condition, and cue values were randomized within blocks of either ILD or ITD. Similar to the discrimination task in experiments I and II, positive cue values indicate that the cue favored the right ear and negative values indicate that the cue favored the left ear. Subjects sat facing a computer monitor that displayed a cartoon of a head; a red shaded area between the left and right ears provided a visual scale designed to enable them to indicate the perceived intracranial position of sound sources. After each stimulus presentation, subjects were asked to indicate the perceived intracranial position of the sound using a computer mouse to move a pointer positioned in the horizontal red shaded area of the head. Responses were coded using an arbitrary scale from −10 to +10 (−10 = at the left ear, 0 = center, + 10 = at the right ear). Prior to testing, subjects were familiarized with the task for approximately 10–15 min.
B. Analysis
A linear effects model predicted slope values for the lateralization results in experiment III. The psychometric functions relating perceived intra-cranial position to ILD or ITD value were modeled using the R software (R Development Core Team, 2014) with a non-linear least squares (NLS) curve fitting procedure using the Levenberg-Marquardt algorithm available in the “minipack.lm” package. Further details of this analysis can be found in Ehlers et al. (2016).
C. Results
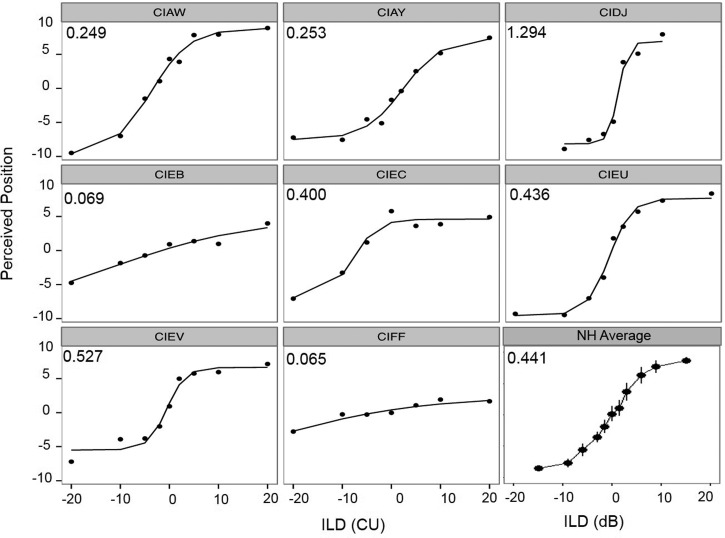
Individual ILD data from experiment III are plotted in Fig. 5, showing each subject's average responses as a function of ILD, with the model's predicted slope located in the upper left-hand corner.
FIG. 5.
Individual data from the lateralization task are shown for ILD data. In each panel, data from a single subject indicate the average perceived intracranial position as a function of ILD. Slope values are inserted in the top left corner of each panel. In the bottom right panel, the overall average intracranial positions for NH children as a function of ILD are replotted from Ehlers et al. (2016). The average ILD slope value for the NH children is inserted in the top left corner of the panel.
ILD data could be modeled using the NLS curve fitting for all subjects. In the bottom right panel of the figure, average data from NH children are shown (Ehlers et al., 2016). They have been re-plotted and analyzed using the same NLS curve-fitting model. A between groups one-way ANOVA for unequal N sizes revealed no significant differences in slope between the NH and BiCI groups [F(1,17) = 0.135, p = 0.717]. Two subjects' data (CIEB and CIFF) had very shallow slopes, implying that even the largest ILD (±20 CUs) was not enough to alter the perceived location of the auditory image completely to the right or completely to the left. However, other subjects showed reasonable changes in perceived intracranial location for changes in ILD values. For example, subject CIDJ did not require testing at ILDs larger than 10 CUs in order to demonstrate perceived lateralization towards each ear. This subject also had a small overall across ear DR of 12 CUs (as calculated in experiment II); therefore, an ILD of 10 CUs would represent a large percentage (83%) of the DR.
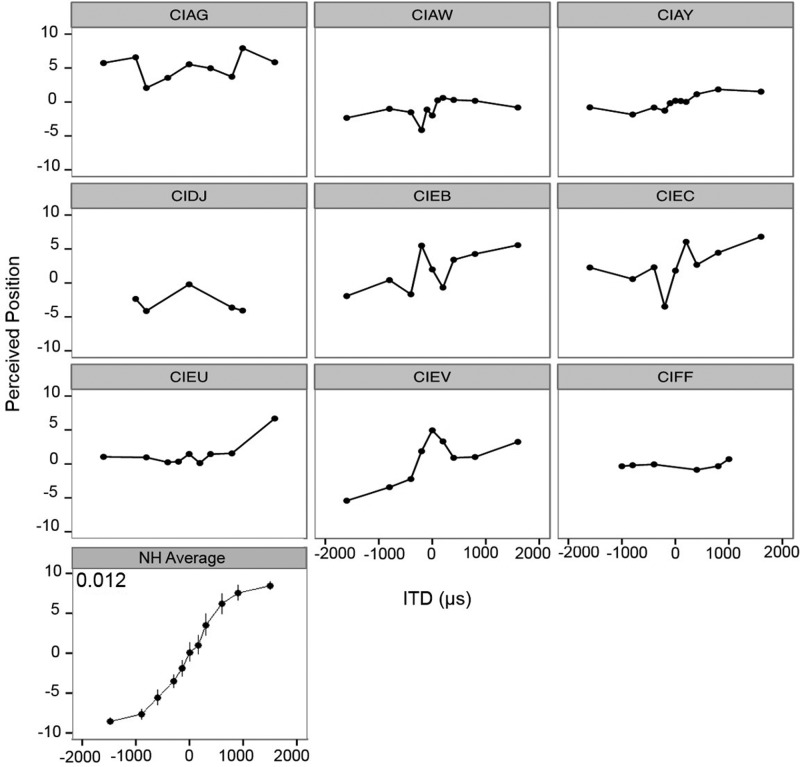
Individual ITD data from experiment II are plotted in Fig. 6 showing each subject's average responses as a function of ITD. Because of the fact that subjects' performance was highly variable, the model was not able to predict performance in a meaningful way. Therefore, there are no slope values for the ITD data. Once again, the bottom panel represents the NH average intracranial location as a function of ITD (Ehlers et al., 2016). The general trends of the functions can be compared across subject groups. Results suggest that NH children could provide a more cue-dependent, finely-grained response distribution. In other words, NH children distinguished a more gradual shift in perceived position as the ITDs varied. In contrast, children with BiCIs were unable to use the ITD cues reliably to indicate intracranial position. For example, subject CIAG had a very strong right-sided bias, never reporting sound on the left. Another example is subject CIFF, who reported that nearly all given ITDs were perceived in the center of the head.
FIG. 6.
Individual data from the lateralization task are shown for ITD data. In each panel, data from a single subject indicate the average perceived intracranial position as a function of ITD. Slope values are not listed as the model could not be run for the ITD condition. In the bottom left panel, the overall average intracranial positions for NH children as a function of ITD are replotted from Ehlers et al. (2016). The average ITD slope value for the NH children is inserted in the top left corner of the panel.
V. GENERAL DISCUSSION
The experiments reported here were motived by the fact that children with BiCIs perform notably poorer than NH peers on spatial hearing tasks. There are a variety of reasons as to why this might occur, including lack of fine structure temporal information in current clinical processing, the lack of temporal synchronization between the two CI devices, potential neural degradation due to lack of early acoustic hearing, surgical procedural issues that cause differing depths of electrode array insertion between the two ears, etc. (for further review see Kan and Litovsky, 2015). In the present set of experiments, the abilities of children with BiCIs to utilize individual binaural cues on discrimination and lateralization tasks were investigated. Results were compared to those of NH children studied in Ehlers et al. (2016) using the same tasks and stimuli intended to mimic aspects of CI processing.
The left-right discrimination and lateralization tasks have been used frequently (e.g., Ehlers et al., 2016; Goupell et al., 2013; Kan et al., 2013; Kan et al., 2015b; van Hoesel, 2004). However, this is the first study to test children with BiCIs on discrimination and perceived lateralization. On the discrimination task, all children with BiCIs were able to make use of ILD cues. As described in the results, in order to compare ILD sensitivity in BiCI users and NH children, the DR was taken into account based on the loudness growth functions for a 4-kHz tone (Allen et al., 1990), which was the high-frequency carrier presented to NH children (Ehlers et al., 2016). ILD JNDs for children with BiCIs consumed a significantly larger portion of DR than did ILD JNDs in the NH children, suggesting that they may not be as sensitive to small changes in ILD as their NH peers. However, some caution is warranted when interpreting this finding, given the lack of direct comparison for ILDs across acoustic and electric hearing (Stakhovskaya and Goupell, 2017). Regardless of the extent to which ILD sensitivity is similar in NH and BiCI users, the fact that all children with BiCIs do show sensitivity to ILD cues suggests that salience of ILD information by the auditory system is resilient to auditory deprivation. The reason this may occur is that the subjects used monaural loudness cues rather than true ILDs. In the current study, static ILDs were given to subjects. Research has shown that if dynamic ILDs are presented through interaurally decorrelated envelopes, subject performance can decrease as there are no monaural cues to rely on (Goupell and Litovsky, 2015).
For ITDs, not all children showed sensitivity to the ITD cue using the 70.7% criterion. However, some children demonstrated the ability to detect differences in ITDs at performance levels of 60%–70% (just under criterion to establish a JND), which suggests that there may be some amount of ITD sensitivity in this population even if it could not be tested. However, when comparing children with BiCIs and children with NH, the small number of children with BiCIs who had sensitivity to ITDs showed higher JNDs than the NH children ages 8–10 years who were tested using stimuli that mimic CI processing. This difference occurs even when comparing the best BiCI performers to all NH listeners. Therefore, even the best performers in the BiCI group still do not perform as well as their NH peers. The fact that NH children show sensitivity to ITDs in the envelopes of high-frequency carriers suggests that the NH binaural system is well developed by age 8–10 years as measured with the discrimination task (Ehlers et al., 2016). The finding with children who use BiCIs in the present study suggests that poor sensitivity to ITDs may be related to other factors such as neural degradation, which are discussed below in greater detail.
Previous research in NH children showed a correlation between ITD and ILD JNDs when provided with the GET stimuli (Ehlers et al., 2016). Because of limited sample size a similar statistical analysis was not run but comparisons of subject performance across cues can be discussed in further detail. For example, CIAQ, CIAY, and CIAP, who demonstrated the best performance with ILD cues, also demonstrate the best sensitivity to ITD cues. Similarly, subject CIEH had the poorest sensitivity to ILDs and did not show sensitivity to ITDs, while subject CIEV had one of the poorest JNDs for ILD performance as well as the poorest measureable JND for ITD performance. It appears binaural sensitivity to these cues may be correlated but further research on a larger sample size should be completed.
An additional factor to consider in regards to ITD sensitivity in this population is the fact that children with BiCIs were tested on pitch-matched electrode pairs, as evidence shows pitch-matched pairs are near optimal for binaural sensitivity (van Hoesel, 2004; Kan et al., 2013; Litovsky et al., 2010; Litovsky et al., 2012). However, some research shows that pitch-matched pairs do not always yield the best ITD JND (Hu and Dietz, 2015; Poon et al., 2009; Kan et al., 2015); therefore, it may be that other electrode pairs might yield better ITD sensitivity than the ones tested. In addition, the perception of pitch may be malleable and change overtime (Reiss et al., 2008). Hence, the pitch-matching tasks may not provide a fully accurate measure of electrode place mapping. However, given the large spatial bandwidth of monopolar stimulation (∼4.6 mm) and that large interaural mismatches are necessary to show degradation of binaural performance (>3 mm, ∼4 electrodes for a Cochlear-brand array; Poon et al., 2009; Kan et al., 2015; Goupell and Litovsky, 2015), it is likely that the electrode pairs used in this study provided near the best ITD sensitivity.
Experiment III continued the investigation of sensitivity to binaural cues through the perceptual mapping of auditory stimuli to intracranial positions indicated on a continuous scale. All children were able to perceive an intracranial position associated with ILD cues. When comparing the slopes of the ILD data for the children with BiCIs to the children with NH, there were no significant differences. One advantage of the lateralization task, even though no level roving was applied, the data can better be interpreted that the children with BiCIs were truly perceiving a change in intracranial location, which more strongly suggests than the discrimination data that that salience of ILD information by the auditory system is resilient to auditory deprivation. In contrast to ILDs, children with BiCIs were not able to map perceptual position of ITDs in any meaningful or predictable manner. Performance across the discrimination (I and II) and lateralization (III) experiments appears to be related. For example, subjects CIEB and CIFF showed the poorest ILD sensitivity in experiment 1, and did not appear to use the ILD cues in a continuous manner (perceiving all the cues as coming roughly from the center) in the lateralization task. Similarly, CIAW, CIAY, and CIEV had relatively low ILD JNDs in experiment 1 and steeper lateralization slopes in experiment III. However, Subject CIDJ demonstrated a low ILD JND in experiment I but did not use the full range of ILDs provided, rather he appeared to discriminate left vs right without using the cues in a continuous manner. In addition, subject CIAY demonstrated good sensitivity to ITDs, but showed very little change in perceived lateralization when given ITD cues. It may be that performance on these tasks is linked but that access to binaural cues in a discrimination paradigm does not ensure the ability to use the cue on a lateralization task. In addition, there was a large amount of variability among subjects on both tasks. Previous research with NH children demonstrated large amounts of variability on the lateralization task when compared with NH adults (Ehlers et al., 2016). This is also consistent with previous research with adult BiCI subjects tested on a similar task with 100 pps (Baumgaertel et al., 2017). Therefore, lateralization, particularly with ILDs, may be a skill that could improve in children with BiCIs as their auditory systems continue to develop.
In contrast to the static ITD and ILD studies, other studies have focused on children's ability to use interaural envelope decorrelation to better detect tones in noise (van Deun et al., 2009; Todd et al., 2016). Interaural decorrelation occurs under conditions in which detection thresholds are measured for diotic vs dichotic stimuli. Binaural masking level differences (BMLDs) are thought to relate to better understanding of speech in noise (Todd et al., 2016). For BiCI users who are presented only temporal envelopes, the BMLD paradigm measures the ability to detect changes in interaural envelope correlation or fluctuating ILDs, and may not be highly revealing about the use of ITDs. In fact, children with poor static ITD sensitivity can demonstrate positive BMLDs (Todd et al., 2016); thus, ITD sensitivity per se may not be required for BMLDs. However, with regard to spatial hearing, there continues to be an open question regarding the development of binaural processing through the electric binaural hearing pathways in CI users.
Previous reports on children with BiCIs have examined lateralization of binaural stimuli with a discrete, not continuous, response format (Gordon et al., 2014; Salloum et al., 2010). Salloum et al. (2010) asked children to describe stimuli as coming from the left side, right side, middle of the head, or from both right and left simultaneously. Performance was poor for stimuli with non-zero ITDs and good for stimuli with non-zero ILDs. Gordon et al. (2014) reported that sensitivity to ITDs occurs in children with BiCIs after 4 years of BiCI use. However, there is a notable difference in the task used in that study and the present study. Gordon et al. (2014) showed the percentage of times that subjects reported the sound as coming from either the left or the right. In contrast, in the present study, both the ITD discrimination and lateralization tasks required that subjects be able to hear the sounds as lateralized. In the ITD discrimination task, they had to note the direction of the sound movement (right to left or left to right). In the lateralization task, they had to perceptually map binaural cues to a continuous range of intracranial positions. These two tasks are more consistent with a well-established set of binaural literature (e.g., Baumgaertel et al., 2017; Kan and Litovsky, 2015; Labeck et al., 2015) and may be more challenging than the task used by Gordon et al. (2014), which was more akin to a same/different task. This same/different task required that any change in the stimuli be detected; however, a detected change in stimuli does not necessarily requiring use of a spatial cue.
Finally, the population tested in this set of experiments was dissimilar to the groups of children tested by Gordon et al. (2013a), who tested many children who were simultaneously implanted. In the present study, most of the children were sequentially implanted, with the mean inter-implantation time of 35.1 ± 30.0 months. Gordon et al (2014) found that performance was better in subjects who had an inter-implantation delay of less than 18 months (Gordon et al., 2013a; Gordon et al., 2014), and have argued that a large inter-implantation delay is related to unilateral reorganization of the auditory system, thus affecting the ability to process binaural cues (see Gordon et al., 2013a,b). This factor may have played a role in the current study, although direct comparison with prior work is not possible because the impact of inter-implant delay on ITD sensitivity per se has not been previously measured.
The lack of ITD sensitivity in 10/16 children studied here is consistent with what is known about binaural sensitivity in pre-lingually deafened adult BiCI users, tested on the same task as the one used in the present study (Litovsky et al., 2010; Laback et al., 2015). Adults who had NH for a long period of time tend to show good sensitivity to ITDs; however, adults that were pre-lingually deafened and experienced little to no early acoustic hearing did not show any sensitivity to ITDs (Litovsky et al., 2010). The subjects in the current study had variable early acoustic hearing experience (see Table I). Prior to the onset of deafness, 5/6 subjects with ITD sensitivity had a progressive or fluctuating loss, and one subject (CIAY) had 42 months of normal acoustic hearing prior to the onset of deafness. The ITD JNDs for these five children are comparable to the group with a childhood onset of deafness in the Litovsky et al. (2010) study with the range of ITD JNDs for the adult group (276–2106 μs) overlapping with the current subject's range of JNDs (156–1082 μs). The subject with ITD sensitivity but no early acoustic hearing (CIDX) had relatively short inter-implantation delay of 14 months, which is within the window suggested by Gordon et al. (2013a) for more proper development of the binaural pathways.
With regard to the effect of auditory deprivation on the use of binaural cues, it is unclear that deprivation obliterates binaural processing altogether. Two studies in children with BiCIs have measured BMLDs, which require the detection of tone in noise in conditions with vs without interaural difference cues, i.e., the detection cue responsible for BMLDs is the interaural envelope decorrelation. BMLDs are found regardless of whether the children have ITD sensitivity or not (van Deun et al., 2009; Todd et al., 2016). It thus appears that the ability to detect interaural decorrelation, similar to the ability to detect a sound with an ITD (Gordon et al., 2014), reveal aspects of binaural sensitivity not evident from ITD discrimination and lateralization measures. Another issue for consideration is the underlying neural substrates involved in the complex tasks used here. As has been demonstrated in many studies over the years, the auditory system undergoes continued maturation into the teenage years (Litovsky, 2015). Because of many factors, including auditory deprivation early in life and the need to learn to use auditory input through electrical stimulation provided by the CI, it is possible that binaural hearing abilities are undergoing developmental changes that are more protracted in children with BiCIs than in NH children. Future research investigating these issues in greater detail may be more revealing regarding the developmental trajectory of binaural sensitivity in this specific population.
Aside from auditory perception, we consider the potential influence of cognitive abilities such as working memory and attention. In particular, the lateralization task used here may be tapping non-auditory abilities that require more mature executive function. This task differs from the discrimination task as there is not a perceptual reference for making a judgment. While some familiarization was provided, it could be that best performance is only achieved after hours of testing. To date, work on training of auditory cues has focused on improved performance measured with discrimination tasks, similar to that used in experiment I (Wright and Zhang, 2009), but little is known about the effect of training on auditory spatial mapping in humans. As mentioned above, in the ferret model, training appears to facilitate learning new spatial maps after unilateral auditory deprivation (Kacelnik et al., 2006), although the hearing loss was conductive in nature which may be entirely different from the electrical stimulation scenario in the present experiment.
A final note with regard to the impact of early acoustic exposure on the development of binaural hearing. In humans, very little is known about the impact of auditory deprivation on binaural hearing. In adult BiCI users, ILD sensitivity appears to recover function following deprivation more easily than ITD sensitivity (Kan and Litovsky, 2015; Litovsky et al., 2010). It may be that early access to acoustic cues is required for the development of good ITD sensitivity (Laback et al., 2015). Some lessons may be gleaned from studies in non-human mammalian species. Deafness-induced plasticity is thought to result in changes in the response properties of auditory neurons at all levels of the auditory pathway (for review, see Shepherd and Hardie, 2001). Studies in either congenitally deaf or early-deafened animal models can lend great insight into the nature of the auditory system, but the way that hearing loss is imposed may not be a perfect model for how children acquire deafness. Nonetheless, deafness causes changes that extend from auditory nerve to the cortex. Pertinent to binaural hearing, several types of changes occur. These changes have a high likelihood of contributing to degraded sensitivity to ITD cues such as those seen here, but the impact on ILD sensitivity is less clear. In the periphery, auditory nerve fibers have diminished myelin sheath, and a reduction of dendritic connections and synaptic vesicles (Ryugo et al., 1998). Further, at the level of the cochlear nucleus there is decline in the number and size of neurons, and reduced neuronal activity, which together is likely to compromise temporal processing (Zhou et al., 1995).
Chung et al. (2015) recently modeled degraded ITD sensitivity in BiCIs, and suggest that ITD sensitivity might require strong synaptic connections and fast membrane responses at the level of the brainstem. Consistent with their model, anatomical and physiological changes have been observed in all three primary nuclei involved in binaural hearing (for further review, Dietz, 2016). These are the medial superior olive (MSO), lateral superior olive (LSO), and the medial nucleus of the geniculate body (MNTB). The MSO which is known for processing low-frequency ITD information, receives bilateral excitatory input from the cochlear nucleus, and precisely timed inhibition from the MNTB that influences ITD sensitivity (Brand et al., 2002). ITD sensitivity is likely to be modulated by the biophysical properties and the way that ion channels are spatially distributed along the cell soma (Kapfer et al., 2002; Matthews et al., 2010). In deafened animals, the MSO undergoes atrophy of dendrites, and disturbance in the cellular properties that provide the inhibitory input and help to shape gradients of ITD sensitive neurons along the MSO (Tirko and Ryugo, 2012). Finally, in deaf animals, loss of a cochlear traveling wave delay might lead to abnormal relative latencies of MSO inputs (Colburn et al., 2009), potentially degrading ITD tuning. The LSO, which is known for processing high-frequency ILD information and ITD information in the envelopes of high-frequency sounds, receives ipsilateral excitatory and contralateral inhibitory inputs from cochlear nucleus, the latter being via the MNTB. While the LSO shows some decrease in cell size following deafness (e.g., Moore, 1992), the central synapse in the MNTB known as the calyx of Held appears to undergo normal maturation in animals that are deafened (e.g., Oleskevich and Walmsley, 2002). Thus, it is possible that the neural structures that mediate ILD sensitivity are more resilient to auditory deprivation. These findings might help shed light on the fact that in the present study ITD sensitivity seems to be disrupted in children who are pre-lingually deaf much more substantially than ILD sensitivity.
In summary, the motivation behind this study was to understand possible reasons why children with BiCIs might perform more poorly than children with NH on spatial hearing tasks (van Deun et al., 2009; Grieco-Calub and Litovsky, 2010; Zheng et al., 2015). A known limitation contributing to poor ITD sensitivity is the lack of temporal fine structure present in everyday clinical CI processing. However, previous research with NH children demonstrates that when providing ITD cues through the envelope of stimuli, performance on a discrimination task remained excellent, even adult-like (Ehlers et al., 2016). Essentially, children with BiCIs using pulse-based ITDs perform poorer than children with NH. This is consistent with data found in adults with BiCIs on similar tasks (Baumgaertel et al., 2017), suggesting that deficits in children with BiCIs may not be solely related to current CI processing; rather, other factors might be responsible for this gap in performance. For example, lack of exposure to binaural cues during development, and inexperience with ITDs in particular could have compromised the children's ability to utilize the cues on the tasks used in this study. The impact of these factors on spatial hearing is not fully understood, even in adults with BiCIs, and therefore further research is necessary to identify the reasons for performance gaps observed to date. Ultimately, such work has the potential to identify possible ways in which performance can be improved, perhaps by implementing better engineering of bilateral devices and through protection of the auditory system from the effects of auditory deprivation.
Two tasks were conducted in children with BiCIs: discrimination and lateralization. The following results were found:
-
(1)
On the discrimination task, all children with BiCIs had measurable sensitivity to ILDs. However, only approximately 50% of children had measureable sensitivity to ITDs, and those that did had significantly higher JNDs than NH children tested on stimuli where ITDs were conveyed in the envelope of a modulated tone.
-
(2)
On the lateralization task, children with BiCIs had measureable slopes and showed sensitivity to ILDs. For ITDs, children with BiCIs did not have results that could be modeled in any predictable manner. This is different than children with NH who showed sensitivity to both ITDs and ILDs when tested on the same task.
-
(3)
This work serves as an important benchmark for future studies regarding binaural sensitivity in this population. Further research is necessary to investigate other factors that may be barriers to binaural sensitivity, specifically with ITDs, such as neural degradation, interaural frequency mismatch, or non-auditory factors like working memory and attention.
ACKNOWLEDGMENTS
The authors are grateful to the families and children who participated in this study. This work was supported by NIH-NIDCD Grant No. 5R01DC00835 (R.Y.L.), R01DC014948 (M.J.G.), and in part by a core grant to the Waisman Center from the National Institute of Health–National Institute of other Communication Disorders (Grant No. P30 HD03352).
References
- 1. Allen, J. B. , Hall, J. L. , and Jeng, P. S. (1990). “ Loudness growth in 1/2-octave bands (LGOB)–a procedure for the assessment of loudness,” J. Acoust. Soc. Am. 88, 745–753. 10.1121/1.399778 [DOI] [PubMed] [Google Scholar]
- 2. Baumgaertel, R. M. , Hu, H. , Kollmeier, B. , and Dietz, M. (2017). “ Extent of lateralization at large interaural time differences in simulated electric hearing and bilateral cochlear implant users,” J. Acoust. Soc. Am. 141(4), 2338–2352. 10.1121/1.4979114 [DOI] [PubMed] [Google Scholar]
- 3. Bernstein, L. R. (2001). “ Auditory processing of interaural timing information: New insights,” J. Neurosci. Res. 66(6), 1035–1046. 10.1002/jnr.10103 [DOI] [PubMed] [Google Scholar]
- 4. Boëx, C. , de Balthasar, C. , Kós, M. I. , and Pelizzone, M. (2003). “ Electrical field interactions in different cochlear implant systems,” J. Acoust. Soc. Am. 114, 2049–2057. 10.1121/1.1610451 [DOI] [PubMed] [Google Scholar]
- 5. Brand, A. , Behrend, O. , Marquardt, T. , McApline, D. , and Grothe, B. (2002). “ Precise inhibition is essential for microsecond interaural time difference coding,” Nature 417, 543–547. 10.1038/417543a [DOI] [PubMed] [Google Scholar]
- 6. Bronkhorst, A. W. , and Plomp, R. (1988). “ The effect of head-induced interaural time and level differences on speech intelligibility in noise,” J. Acoust. Soc. Am. 83, 1508–1516. 10.1121/1.395906 [DOI] [PubMed] [Google Scholar]
- 7. Brughera, A. , Dunai, L. , and Hartmann, W. M. (2013). “ Human interaural time difference thresholds for sine tones: The high-frequency limit,” J. Acoust. Soc. Am. 133(5), 2839–2855. 10.1121/1.4795778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carlile, S. , Martin, R. , and McAnally, K. (2005). “ Spectral information in sound localization,” Int. Rev. Neurobiol. 70, 399–434. 10.1016/S0074-7742(05)70012-X [DOI] [PubMed] [Google Scholar]
- 9. Cherry, E. C. (1953). “ Some experiments on the recognition of speech, with one and with two ears,” J. Acoust. Soc. Am. 25, 975–979. 10.1121/1.1907229 [DOI] [Google Scholar]
- 10. Chung, Y. , Delgutte, B. , and Colburn, H. S. (2015). “ Modeling binaural responses in auditory brainstem to electric stimulation of the auditory nerve,” J. Assoc. Res. Otolaryngol. 16, 135–158. 10.1007/s10162-014-0492-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Churchill, T. J. , Kan, A. H. , Goupell, M. J. , Ihlefeld, A. , and Litovsky, R. Y. (2014). “ Speech perception in noise with a harmonic complex excited vocoder,” J. Assoc. Res. Otolaryngol. 15(2), 265–278. 10.1007/s10162-013-0435-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Colburn, H. S. , Chung, Y. , Zhou, Y. , and Brughera, A. (2009). “ Models of brainstem responses to bilateral electrical stimulation,” J. Assoc. Res. Otolaryngol. 10(1), 91–110. 10.1007/s10162-008-0141-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Colburn, H. S. , and Equissaud, P. (1976). “ An auditory nerve model for interaural time discriminaton of high-frequency complex stimuli,” J. Acoust. Soc. Am. 59(1), S23. 10.1121/1.2002503 [DOI] [Google Scholar]
- 14. Dietz, M. (2016). “ Models of the electricallly stimulated binaural system: A review,” Network: Comput. Neural Syst. 27(2–3), 186–211. [DOI] [PubMed] [Google Scholar]
- 15. Ehlers, E. , Kan, A. , Winn, M. , Stoelb, C. , and Litovsky, R. (2016). “ Binaural hearing in children using Gaussian envelope and transposed tones,” J. Acoust. Soc. Am. 139(4), 1724–1733. 10.1121/1.4945588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feddersen, W. E. , Sandel, T. T. , Teas, D. C. , and Jeffress, L. A. (1957). “ Localization of high-frequency tones,” J. Acoust. Soc. Am. 29, 988–991. 10.1121/1.1909356 [DOI] [Google Scholar]
- 17. Garadat, S. N. , and Litovsky, R. Y. (2007). “ Speech intelligibility in free field: Spatial unmasking in preschool children,” J. Acoust. Soc. Am. 121, 1047–1055. 10.1121/1.2409863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gordon, K. A. , Deighton, M. R. , Abbasalipour, P. , and Papsin, B. C. (2014). “ Perception of binaural cues develops in children who are deaf through bilateral cochlear implantation,” PLoS One 9, e114841. 10.1371/journal.pone.0114841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gordon, K. A. , Jiwani, S. , and Papsin, B. C. (2013a). “ Benefits and detriments of unilateral cochlear implant use on bilateral auditory development in children who are deaf,” Front. Psychol. 4, 719. 10.3389/fpsyg.2013.00719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gordon, K. A. , Wong, D. D. E. , and Papsin, B. C. (2013b). “ Bilateral input protects the cortex from unilaterally-driven reorganization in children who are deaf,” Brain 136, 1609–1625. 10.1093/brain/awt052 [DOI] [PubMed] [Google Scholar]
- 21. Goupell, M. J. , and Litovsky, R. Y. (2015). “ Sensitivity to interaural envelope correlation changes in bilateral cochlear-implant users,” J. Acoust. Soc. Am. 137(1), 335–349. 10.1121/1.4904491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goupell, M. J. , Stoelb, C. , Kan, A. , and Litovsky, R. Y. (2013). “ Effect of mismatched place-of-stimulation on the salience of binaural cues in conditions that simulate bilateral cochlear-implant listening,” J. Acoust. Soc. Am. 133, 2272–2287. 10.1121/1.4792936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grieco-Calub, T. M. , and Litovsky, R. Y. (2010). “ Sound localization skills in children who use bilateral cochlear implants and in children with normal acoustic hearing,” Ear Hear. 31, 645–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu, H. , and Dietz, M. (2015). “ Comparison of interaural electrode pairing methods for bilateral cochlear implants,” Trends Hear. 19, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kacelnik, O. , Nodal, F. R. , Parsons, C. H. , and King, A. J. (2006). “ Training-induced plasticity of auditory localization in adult mammals,” PLoS Biol. 4, e71. 10.1371/journal.pbio.0040071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kan, A. , Jones, H. G. , and Litovsky, R. Y. (2015a). “ Effect of multi-electrode configuration on sensitivity to interaural timing differences in bilateral cochlear-implant users,” J. Acoust. Soc. Am. 138, 3826–3833. 10.1121/1.4937754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kan, A. , and Litovsky, R. Y. (2015). “ Binaural hearing with electrical stimulation,” Hear. Res. 322, 127–137. 10.1016/j.heares.2014.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kan, A. , Litovsky, R. Y. , and Goupell, M. J. (2015b). “ Effects of interaural pitch matching and auditory image centering on binaural sensitivity in cochlear implant users,” Ear Hear. 36, e62–68. 10.1097/AUD.0000000000000135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kan, A. , Stoelb, C. , Litovsky, R. Y. , and Goupell, M. J. (2013). “ Effect of mismatched place-of-stimulation on binaural fusion and lateralization in bilateral cochlear-implant users,” J. Acoust. Soc. Am. 134, 2923–2936. 10.1121/1.4820889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kapfer, C. , Seidl, A. H. , Schweizer, H. , and Grothe, B. (2002). “ Experience dependent refinement of inhibitory inputs to auditory coincidence-detector neurons,” Nat Neurosci. 5(3), 247–253. 10.1038/nn810 [DOI] [PubMed] [Google Scholar]
- 31. Kral, A. , Tillein, J. , Hubka, P. , Schiemann, D. , Heid, S. , Hartmann, R. , and Engel, A. K. (2009). “ Spatiotemporal patterns of cortical activity with bilateral cochlear implants in congenital deafness,” J. Neurosci. 29, 811–827. 10.1523/JNEUROSCI.2424-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Laback, B. , Egger, K. , and Majdak, P. (2015). “ Perception and coding of interaural time differences with bilateral cochlear implants,” Hear. Res. 322, 138–150. 10.1016/j.heares.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 33. Lawson, D. T. , Wilson, B. S. , Zerbi, M. , van den Honert, C. , Finley, C. C. , Farmer, J. C., Jr. , McElveen, J. R., Jr. , and Roush, P. A. (1998). “ Bilateral cochlear implants controlled by a single speech processor,” Am. J. Otol. 19, 758–761. [PubMed] [Google Scholar]
- 34. Litovsky, R. (2015). “ Development of the auditory system,” Handb. Clin. Neurol. 129, 55–72. 10.1016/B978-0-444-62630-1.00003-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Litovsky, R. Y. , and Godar, S. (2010). “ Difference in precedence effect between children and adults signifies development of sound localization abilities in complex listening tasks,” J. Acoust. Soc. Am. 128, 1979–1991. 10.1121/1.3478849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Litovsky, R. Y. , and Gordon, K. (2016). “ Bilateral cochlear implants in children: Effects of auditory experience and deprivation on auditory perception,” Hear. Res. 338, 76–87. 10.1016/j.heares.2016.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Litovsky, R. Y. , Goupell, M. J. , Godar, S. , Grieco-Calub, T. , Jones, G. L. , Garadat, S. N. , Agrawal, S. , Kan, A. , Todd, A. , Hess, C. , and Misurelli, S. (2012). “ Studies on bilateral cochlear implants at the University of Wisconsin's Binaural Hearing and Speech Laboratory,” J. Am. Acad. Audiol. 23, 476–494. 10.3766/jaaa.23.6.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Litovsky, R. Y. , Johnstone, P. M. , Godar, S. , Agrawal, S. , Parkinson, A. , Peters, R. , and Lake, J. (2006). “ Bilateral cochlear implants in children: Localization acuity measured with minimum audible angle,” Ear Hear. 27, 43–59. 10.1097/01.aud.0000194515.28023.4b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Litovsky, R. Y. , Jones, G. L. , Agrawal, S. , and van Hoesel, R. (2010). “ Effect of age at onset of deafness on binaural sensitivity in electric hearing in humans,” J. Acoust. Soc. Am. 127, 400–414. 10.1121/1.3257546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Loizou, P. C. (2006). “ Speech processing in vocoder-centric cochlear implants,” Prog. Oto. Rhino Laryngol. 64, 109–143. 10.1159/000094648 [DOI] [PubMed] [Google Scholar]
- 41. Matthews, P. J. , Jercog, P. E. , Rinzel, J. , Scott, L. L. , and Golding, N. L. (2010). “ Control of submillisecond synaptic timing in binaural coincidence detectors by K(v)1 channels,” Nat. Neurosci. 13, 601–609. 10.1038/nn.2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McFadden, D. , and Pasanen, E. G. (1978). “ Binaural detection at high frequencies with time delayed waveforms,” J. Acoust. Soc. Am. 63, 1120–1131. 10.1121/1.381820 [DOI] [PubMed] [Google Scholar]
- 43. Middlebrooks, J. C. , and Green, D. M. (1991). “ Sound localization by human subjects,” Annu. Rev. Psychol. 42, 135–159. 10.1146/annurev.ps.42.020191.001031 [DOI] [PubMed] [Google Scholar]
- 44. Misurelli, S. M. , and Litovsky, R. Y. (2012). “ Spatial release from masking in children with normal hearing and with bilateral cochlear implants: Effect of interferer asymmetry,” J. Acoust. Soc. Am. 132(1), 380–391. 10.1121/1.4725760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moore, D. R. (1992). “ Trophic influences of excitatory and inhibitory synapses on neurons in the auditory brainstem,” Neuroreport 3(3), 269–272. 10.1097/00001756-199203000-00014 [DOI] [PubMed] [Google Scholar]
- 46. Nelson, D. A. , Donaldson, G. S. , and Kreft, H. (2008). “ Forward-masked spatial tuning curves in cochlear implant users,” J. Acoust. Soc. Am. 123, 1522–1543. 10.1121/1.2836786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oleskevich, S. , and Walmsley, B. (2002). “ Synaptic transmission in the auditory brainstem of normal and congenitally deaf mice,” J. Physiol. 540, 447–455. 10.1113/jphysiol.2001.013821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Poon, B. B. , Eddington, D. K. , Noel, V. , and Colburn, H. S. (2009). “ Sensitivity to interaural time difference with bilateral cochlear implants: Development over time and effect of interaural electrode spacing,” J. Acoust. Soc. Am. 126, 806–815. 10.1121/1.3158821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.R Development Core Team (2014). “ R: A language and environment for statistical computing,” R Foundation for Statistical Computing, Vienna, Austria [Computer software: version 3.1.0].
- 50. Reiss, L. A. , Gantz, B. J. , and Turner, C. W. (2008). “ Cochlear implant speech processor frequency allocations may influence pitch perception,” Otol Neurotol. 29, 160–167. 10.1097/mao.0b013e31815aedf4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ryugo, D. K. , Rosenbaum, B. T. , Kim, P. J. , Niparko, J. K. , and Saada, A. A. (1998). “ Single unit recordings in the auditory nerve of congenitally deaf white cats: Morphological correlated in the cochlea and cochlear nucleus,” J. Comp. Neurol. 397, 532–548. [DOI] [PubMed] [Google Scholar]
- 52. Salloum, C. A. M. , Valero, J. , Wong, D. D. E. , Papsin, B. C. , van Hoesel, R. , and Gordon, K. A. (2010). “ Lateralization of interimplant timing and level differences in children who use bilateral cochlear implants,” Ear Hear. 31, 441–456. 10.1097/AUD.0b013e3181d4f228 [DOI] [PubMed] [Google Scholar]
- 53. Serpanos, Y. C. , and Gravel, J. S. (2000). “ Assessing growth of loudness in children by cross-modality matching,” J. Am. Acad. Audiol. 11, 190–202. [PubMed] [Google Scholar]
- 54. Shepherd, R. K. , and Hardie, N. K. (2001). “ Deafness-induced changes in the auditory pathway: Implications for cochlear implants,” Audiol Neurotol. 6, 305–318. 10.1159/000046843 [DOI] [PubMed] [Google Scholar]
- 55. Shepherd, R. K. , and McCreery, D. B. (2006). “ Basis of electrical stimulation of the cochlea and the cochlear nucleus,” Adv. Otorhinolaryngol. 64, 186–205. 10.1159/000094652 [DOI] [PubMed] [Google Scholar]
- 56. Stakhovskaya, O. A. , and Goupell, M. J. (2017). “ Lateralization of interaural level differences with multiple electrode stimulation in bilateral cochlear-implant subjects,” Ear Hear. 38(1), e22–e38. 10.1097/AUD.0000000000000360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tirko, N. N. , and Ryugo, D. K. (2012). “ Synaptic plasticity in the medial superior olive of hearing, deaf, and cochlear implanted cats,” J. Comp. Neurol. 20(10), 2202–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Todd, A. E. , Goupell, M. J. , and Litovsky, R. Y. (2016). “ Binaural release from masking with single- and multi-electrode stimulation in children with cochlear implants,” J. Acoust. Soc. Am. 140(1), 59–73. 10.1121/1.4954717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. van Deun, L. , Van Wieringen, A. , Francart, T. , Scherf, F. , Dhooge, I. J. , Deggouj, N. , Desloovere, C. , Van de Heyning, P. H. , Offeciers, F. E. , De Raeve, L. , and Wouters, J. (2009). “ Bilateral cochlear implants in children: Binaural unmasking,” Audiol. Neurotol. 14, 240–247. 10.1159/000190402 [DOI] [PubMed] [Google Scholar]
- 60. van Hoesel, R. J. M. (2004). “ Exploring the benefits of bilateral cochlear implants,” Audiol. Neuro-Otology 9, 234–246. 10.1159/000078393 [DOI] [PubMed] [Google Scholar]
- 61. van Hoesel, R. J. M. , Jones, G. L. , and Litovsky, R. Y. (2009). “ Interaural time-delay sensitivity in bilateral cochlear implant users: Effects of pulse rate, modulation rate, and place of stimulation,” J. Assoc. Res. Otolaryngol. 10, 557–567. 10.1007/s10162-009-0175-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. van Hoesel, R. J. M. , and Tyler, R. S. (2003). “ Speech perception, localization, and lateralization with bilateral cochlear implants,” J. Acoust. Soc. Am. 113, 1617–1630. 10.1121/1.1539520 [DOI] [PubMed] [Google Scholar]
- 63. Wichmann, F. A. , and Hill, N. J. (2001). “ The psychometric function: I. Fitting, sampling, and goodness of fit,” Percept. Psychophys. 63, 1293–1313. 10.3758/BF03194544 [DOI] [PubMed] [Google Scholar]
- 64. Wilson, B. S. , and Dorman, M. F. (2008). “ Cochlear implants: A remarkable past and a brilliant future,” Hear. Res. 242, 3–21. 10.1016/j.heares.2008.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wright, B. A. , and Zhang, Y. (2009). “ A review of the generalization of auditory learning,” Philos. Trans. R. Soc. Lond. B. Biol. Sci. 364, 301–311. 10.1098/rstb.2008.0262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yost, W. A. , and Dye, R. H. (1988). “ Discrimination of interaural differences of level as a function of frequency,” J. Acoust. Soc. Am. 83(5), 1846–1851. 10.1121/1.396520 [DOI] [PubMed] [Google Scholar]
- 67. Zheng, Y. , Godar, S. P. , and Litovsky, R. Y. (2015). “ Development of sound localization strategies in children with bilateral cochlear implants,” PLoS One 10(8), e0135790. 10.1371/journal.pone.0135790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhou, R. , Abbas, P. J. , and Assouline, J. G. (1995). “ Electrically evoked auditory brainstem response in peripherally myelin-deficient mice,” Hear Res. 88, 98–106. 10.1016/0378-5955(95)00105-D [DOI] [PubMed] [Google Scholar]