Abstract
Diet and inflammation have been suggested to be important risk factors for oral and pharyngeal cancer. We examined the association between dietary inflammatory index (DII) and oral and pharyngeal cancer in a large case-control study conducted between 1992 and 2009 in Italy. This study included 946 cases with incident, histologically confirmed oral and pharyngeal cancer, and 2492 controls hospitalized for acute non-neoplastic diseases. The DII was computed based on dietary intake assessed by a valid 78-item food frequency questionnaire and was adjusted for non-alcohol energy intake using the residual approach (E-DII). Logistic regression models were used to estimate odds ratios (ORs), and 95% confidence intervals (CIs), adjusted for age, sex, non-alcohol energy intake, study center, year of interview, education, body mass index, tobacco smoking, and alcohol drinking. Subjects with higher DII scores (i.e., with a more pro-inflammatory diet) had a higher risk of oral and pharyngeal cancer, the OR being 1.80 (95% CI 1.36–2.38) for the highest versus the lowest DII quartile and 1.17 (95 % CI 1.10–1.25) for a one-unit increase (8 % of the DII range). When stratified by selected covariates, a stronger association was observed among women (ORquartile4 v.1 3.30, 95% CI 1.95–5.57). We also observed stronger association for oral cancer subsite and a strong combined effect of higher DII and tobacco smoking or alcohol consumption on oral and pharyngeal cancer. These results indicate that the pro-inflammatory potential of the diet, as shown by higher DII scores, is associated with higher odds of oral and pharyngeal cancer.
Introduction
Oral and pharyngeal cancer is the eight most common neoplasm and the eleventh leading cause of cancer mortality in Europe, with higher incidence in men as compared with women 1, 2. Although tobacco 3 and alcohol 4 are the major recognized risk factors for oral and pharyngeal cancer, there is a role of diet and inflammation in the development of this cancer 5, 6. Inflammation is the body’s response to any kind of tissue injury or insult in the presence of inflammatory stimulants such as cytokines 7, 8. Chronic inflammation – which is characterized by the continuous presence of inflammatory cytokines in circulation and in the tissues – is known to play a key role in the development of various epithelial cancers, including oral and pharyngeal neoplasms 6, 9. Aspirin has been inversely related to the risk of oral and pharyngeal cancer 10, 11.
There is growing evidence that specific dietary components influence both inflammation 12–14 and oral and pharyngeal cancer 15–18. Research on the role of diet in inflammation suggests that diet represents a complicated set of exposures that often interact, and whose cumulative effect modifies both inflammatory responses and health outcomes. A literature-derived, population-based dietary inflammatory index (DII) was developed to assess the inflammatory potential of an individual’s diet 14. It has been validated with various inflammatory markers, including C-reactive protein 19, 20, interleukin-6 21, and homocysteine 20. The DII has been associated with colorectal 22, pancreatic 23 and hepatocellular 24 cancers. DII has also been shown to be strongly associated with esophageal squamous cell25 laryngeal26 and nasopharyngeal cancers27 in Italy.
Using a case-control study conducted in Italy, this is the first study to examine the association between the DII and oral and pharyngeal cancer. Our working hypothesis is that increasing inflammatory potential of diet is positively associated with oral and pharyngeal cancer.
Methods
A multicentric case-control study of oral and pharyngeal cancer was conducted from 1992 to 2009 in the greater Milan area in northern Italy, the provinces of Pordenone in northeastern Italy, and Rome and Latina in central Italy 5, 28. Cases were 946 patients (756 men and 190 women; median age 58 years, range 22–79 years) admitted to major hospitals in the study areas, with incident, diagnosed within 1 year from the interview, histologically confirmed cancer of the oral cavity and of the pharynx. Controls were 2,492 subjects (1497 men and 995 women; median age 58 years, range 19–82 years) admitted to the same hospitals as cases for a wide spectrum of acute, non-neoplastic conditions, unrelated to known risk factors for oral and pharyngeal cancer, and not associated with long-term dietary modifications. Aspirin and other anti-inflammatory medications use was not an exclusion criterion 10, 11. To compensate for the rarity of oral and pharyngeal cancer in women, a control-to-case ratio of ~5 was chosen for women, as opposed to ~2 for men. Of the controls, 24% were admitted for traumas, 27% for other orthopedic disorders, 22% for surgical conditions, 9% for eye diseases, and 19% for miscellaneous various other illnesses. Less than 5% of both cases and controls contacted refused to participate. The same structured questionnaire and coding manual were used in each centre, and all interviewers were centrally trained and routinely supervised. It was not possible to blind the interviewers to the case-control status. The study was approved by the referent ethics committee and have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
The questionnaire included information on socio-demographic characteristics, such as education and occupation, lifetime smoking and alcohol-drinking habits, physical activity, anthropometric measures at various ages, a problem oriented personal medical history, and family history of cancer. A reproducible 29, 30 and valid 31 food frequency questionnaire (FFQ) was used to assess the patients’ usual diet in the two years preceding cancer diagnosis (for cases) or hospital admission (for controls). The FFQ included the average weekly consumption of 78 food items or food groups and of five alcoholic beverages. All subjects (cases and controls) in the study have a complete FFQ. Intakes lower than once a week, but at least once per month were coded as 0.5 per week.
FFQ-derived dietary data were used to calculate DII scores for each study subject. A complete description of the DII is available elsewhere 14. Briefly, to calculate DII for the subjects in this study, the dietary data were first linked to a world database that provided a robust estimate of a mean and standard deviation for each food parameter included in the DII. These parameters then became the multipliers to express an individual’s exposure relative to the “standard global mean” as a z-score. This was achieved by subtracting the “standard global mean” from the amount reported and dividing this value by the standard deviation. To minimize the effect of “right skewing,” this value was then converted to a centered percentile score. The centered percentile score of each food parameter for each subject was then multiplied by the respective food parameter effect score, in order to obtain a food parameter-specific DII score. All the food parameter-specific DII scores were then summed up to create the overall DII score for each study subject. DII was adjusted for (non-alcohol) energy using the residual approach 32 to get energy adjusted DII (E-DII).
The E-DII was analyzed both by quartiles of exposure, determined on the basis of its distribution among controls, and as a continuous variable (i.e., for a one-unit increment in the DII corresponding to approximately 8% of its global range). Odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) were estimated using logistic regression models, including terms for age (quinquennia), sex, study center, year of interview, education (<7, 7–11, ≥12 years, categorically), body mass index (BMI, <20, 20–25, 25–30, ≥30 kg/m2, categorically), tobacco smoking (never smoker, ex-smoker, current smoker of <15, 15–24, and ≥25 cigarettes/day, categorically), and alcohol drinking (<14, 14–<28, 28–<56, ≥56 drinks/week, categorically), and non-alcohol energy intake (quintiles among controls). Linear tests for trend were performed using the median value within each quartile as an ordinal variable. We also analysed the association between the E-DII and oral and pharyngeal cancer by anatomic subsites. To investigate whether the effect of the E-DII was homogeneous across strata of selected covariates, we conducted analyses stratified by age, sex, education, alcohol, tobacco smoking. To test for homogeneity between strata, the difference between the −2 log(likelihood) of the models with and without the interaction terms was compared with the χ2 distribution with the same number of degrees of freedom as the number of interaction terms.
We have then evaluated interactions between alcohol or smoking and E-DII as a deviation from additivity and as multiplicative joint effects. In order to estimate the departure from additivity of effects we used the relative excess risk due to interaction (RERI) and the synergy index (S) 33. CI for the indices were obtained using the delta method 34. In order to evaluate the significance of the multiplicative interaction we used the likelihood ratio tests of significance comparing models including versus excluding the interaction term. Statistical analyses were performed using SAS 9.3 (SAS Institute, Inc.).
Results
Table 1 gives the distribution of 946 cases and 2492 controls according to center, age, sex, and other selected covariates. Age distribution was similar in cases and controls. Cases were less educated and reported a higher tobacco and alcohol consumption than controls.
Table 1.
Distribution of 946 patients with oral and pharyngeal cancer and 2492 control patients according to age, sex, education, and other selected variables. Italy, 1992–2009.
| Characteristics | Cases | Controls | P-valuea |
|---|---|---|---|
| N. (%) | N. (%) | ||
| Age (years) | 0.07 | ||
| <50 | 190 (20.1) | 583 (23.4) | |
| 50–59 | 313 (33.1) | 734 (29.5) | |
| 60–69 | 329 (34. 8) | 837 (33.6) | |
| ≥70 | 114 (12.1) | 338 (13.6) | |
| Sex | <0.0001 | ||
| Men | 756 (79.9) | 1497 (60.1) | |
| Women | 190 (20.1) | 995 (39.9) | |
| Education (years)b | <0.0001 | ||
| <7 | 553 (58.8) | 1272 (51.3) | |
| 7–11 | 260 (27.6) | 726 (29.3) | |
| ≥12 | 128 (13.6) | 483 (19.5) | |
| Smoking b | <0.0001 | ||
| Non-smoker | 137 (14.5) | 1077 (43.3) | |
| Ex-smoker | 255 (27.1) | 761 (30.6) | |
| Current smoker | |||
| <15 cigarettes/day | 125 (13.3) | 280 (11.3) | |
| 15–24 cigarettes/day | 275 (29.2) | 283 (11.4) | |
| ≥25 cigarettes/day | 150 (15.9) | 86 (3.5) | |
| Alcohol intake (drinks/week)b | <0.0001 | ||
| <14 | 188 (19.9) | 1209 (48.5) | |
| 14–<28 | 176 (18.6) | 653 (26.2) | |
| 28–<56 | 201 (21.3) | 448 (18.0) | |
| ≥56 | 381 (40.3) | 182 (7.3) |
p value for Chi-square test
The sum does not add up to the total because of some missing values
The mean E-DII value was 0.45 (SD=1.46) among cases and −0.17 (SD=1.43) among controls, indicating a more pro-inflammatory diet for cases. Control characteristics across quartiles of E-DII are provided in Table 2. Participants in the highest quartiles were more often males, current smokers and consumed high amount of alcohol compared to subjects in the lowest quartile.
Table 2.
Participants’ characteristics across quartiles of energy-adjusted dietary inflammatory index (E-DII) among 2492 controls. Italy, 1992–2009.
| Characteristics | E-DII quartiles | ||||
|---|---|---|---|---|---|
| −5.29, −1.19 | −1.18, −0.31 | −0.30, 0.73 | 0.74, 7.66 | P-valuea | |
| N (%) | N (%) | N (%) | N (%) | ||
| Age (years) | 0.12 | ||||
| <50 | 133 (21.4) | 137 (22.0) | 144 (23.1) | 169 (27.1) | |
| 50–59 | 183 (29.4) | 203 (32.6) | 174 (27.9) | 174 (27.9) | |
| 60–69 | 230 (36.9) | 205 (32.9) | 211 (33.9) | 191 (30.7) | |
| >69 | 77 (12.4) | 78 (12.5) | 94 (15.1) | 89 (14.3) | |
| Sex | <0.001 | ||||
| Men | 303 (48.6) | 395 (63.4) | 402 (64.5) | 397 (63.7) | |
| Women | 320 (51.4) | 228 (36.6) | 221 (35.5) | 226 (36.3) | |
| Education (years)b | 0.52 | ||||
| <7 | 306 (49.5) | 312 (50.2) | 331 (53.4) | 323 (52.0) | |
| 7–11 | 186 (30.1) | 190 (30.6) | 182 (29.4) | 168 (27.1) | |
| ≥12 | 126 (20.4) | 120 (19.3) | 107 (17.3) | 130 (20.9) | |
| Smoking b | <0.0001 | ||||
| Non-smoker | 320 (51.5) | 256 (41.1) | 257 (41.4) | 244 (39.2) | |
| Ex-smoker | 183 (29.5) | 198 (31.8) | 193 (31.1) | 187 (30.1) | |
| Current smoker | |||||
| <15 cigarettes/day | 60 (9.7) | 68 (10.9) | 83 (13.4) | 69 (11.1) | |
| 15–24 cigarettes/day | 49 (7.9) | 80 (12.8) | 64 (10.3) | 90 (14.5) | |
| ≥25 cigarettes/day | 9 (1.5) | 21 (3.4) | 24 (3.9) | 32 (5.1) | |
| Alcohol intake (drinks/week)b | <0.0001 | ||||
| <14 | 323 (51.9) | 276 (44.3) | 291 (46.7) | 319 (51.2) | |
| 14–<28 | 187 (30.0) | 184 (29.5) | 156 (25.0) | 126 (20.2) | |
| 28–<56 | 87 (14.0) | 118 (18.9) | 122 (19.6) | 121 (19.4) | |
| ≥56 | 26 (4.2) | 45 (7.2) | 54 (8.7) | 57 (9.2) | |
p value for Chi-square test
The sum does not add up to the total because of some missing values
Table 3 shows the distribution of 10 food groups across E-DII quartiles among controls. Servings of fruit, vegetables, fish, coffee, and poultry decreased significantly across quartiles, whereas servings of pork, sugar, cheese, and desserts increased significantly.
Table 3.
Distribution of servings/week of food groups across quartiles of energy-adjusted dietary inflammatory index (E-DII) among 2492 controls (mean ± standard deviation). Italy, 1992–2009.
| Servings/week | E-DII quartiles | ||||
|---|---|---|---|---|---|
| −5.29, −1.19 | −1.18, −0.31 | −0.30, 0.73 | 0.74, 7.66 | p valuea | |
| Fruit | 32.32±16.70 | 24.15±12.59 | 17.63±9.93 | 11.69±10.34 | <0.0001 |
| Vegetables | 17.49±7.30 | 15.13±7.03 | 12.55±5.88 | 8.24±4.83 | <0.0001 |
| Fish | 2.02±1.31 | 1.85±1.09 | 1.68±1.06 | 1.47±1.09 | <0.0001 |
| Red meat | 3.92±2.27 | 4.76±4.23 | 4.27±2.47 | 3.98±3.89 | 0.61 |
| Coffee | 19.41±12.63 | 19.19±11.35 | 18.50±12.03 | 17.00±11.90 | 0.0002 |
| Poultry | 1.91±1.30 | 1.90±1.28 | 1.76±1.37 | 1.58±1.38 | <0.0001 |
| Pork | 2.56±2.20 | 2.76±2.07 | 2.85±2.48 | 2.78±2.67 | 0.07 |
| Sugar | 26.85±23.77 | 31.89±24.62 | 34.52±28.70 | 31.28±27.36 | 0.0007 |
| Cheese | 3.54±1.92 | 4.31±2.29 | 4.68±3.47 | 4.43±4.05 | <0.0001 |
| Desserts | 4.66±4.22 | 6.15±5.35 | 6.71±7.33 | 8.09±11.97 | <0.0001 |
p values were obtained from ANOVA
ORs and 95% CIs of oral and pharyngeal cancer according to quartiles of E-DII and continuous E-DII are shown in Table 4. In the full model, significant positive associations were observed between E-DII and oral and pharyngeal cancer risk when used as both a categorical variable (ORquartile4 v.1 1.80, 95% CI 1.36–2.38; p for trend < 0.0001) and as a continuous variable (ORcontinuous 1.17, 95 % CI 1.10–1.25). When analysed by cancer subsite, stronger association was observed with oral cancer (ORQuartile4vs1= 2.08, 95% CI 1.47, 2.93), although significant trends were observed for all cancer subtypes (hypopharynx and oropharynx).
Table 4.
Odds ratios (OR) of oral and pharyngeal cancer and corresponding confidence intervals (CI) for energy-adjusted dietary inflammatory index (E-DII), among 946 cases and 2492 controls. Italy, 1992–2009.
| E-DII quartiles | P-value for trend | E-DII continuousa | ||||
|---|---|---|---|---|---|---|
| −5.29, −1.19 | −1.18, −0.31 | −0.30, 0.73 | 0.74, 7.66 | |||
| No. of controls | 623 | 623 | 623 | 623 | 2492 | |
| Overall | ||||||
| No. of cases | 122 | 160 | 279 | 385 | 946 | |
| Model 1, OR (95% CI)b | 1c | 1.11 (0.84, 1.45) | 1.81 (1.41, 2.33) | 2.54 (1.96, 2.33) | <0.0001 | 1.28 (1.21, 1.36) |
| Model 2, OR (95% CI)d | 1c | 0.95 (0.71, 1.28) | 1.52 (1.15, 2.01) | 1.80 (1.36, 2.38) | <0.0001 | 1.17 (1.10, 1.25) |
| Cancer subtypee | ||||||
| Oral | ||||||
| No. of cases | 66 | 85 | 147 | 208 | 506 | |
| Model 2, OR (95% CI)d | 1 b | 1.06 (0.73, 1.53) | 1.78 (1.26, 2.52) | 2.08 (1.47, 2.93) | <0.0001 | 1.20 (1.11, 1.30) |
| Hypopharynx | ||||||
| No. of cases | 23 | 22 | 45 | 70 | 160 | |
| Model 2, OR (95% CI)d | 1 b | 0.60 (0.31, 1.14) | 1.09 (0.61, 1.95) | 1.64 (0.93, 2.89) | 0.005 | 1.15 (1.01, 1.32) |
| Oropharynx | ||||||
| No. of cases | 31 | 50 | 81 | 96 | 258 | |
| Model 2, OR (95% CI)d | 1 b | 1.06 (0.63, 1.80) | 1.51 (0.92, 2.48) | 1.60 (0.97, 2.63) | 0.03 | 1.10 (0.98, 1.23) |
One unit increase equals to approximately 8 % of E-DII global range.
Adjusted for age, sex, center, year of interview, and non-alcohol energy intake.
Reference category.
Adjusted as in Model 1 and additionally adjusted for education, tobacco smoking, and alcohol drinking.
The sum of the cancer subsites does not add up to the total of cases because of 22 cases with ‘unspecified’ cancer subsite.
Table 5 shows full-adjusted ORs of oral and pharyngeal cancer in strata of selected covariates. Stronger associations were observed among subjects ≥60years (ORQuartile4vs1= 2.12, 95% CI 1.42, 3.16), women (ORQuartile4vs1= 3.30, 95% CI 1.95, 5.57), less educated subjects (ORQuartile4vs1= 2.07, 95% CI 1.37, 3.13), and among never smokers (ORQuartile4vs1= 3.06, 95% CI 1.69, 5.51). However, significant heterogeneity was observed only for sex (p value=0.03). A sensitivity analyses conducted after additionally adjusting for family history of cancer, did not materially change the results (data not shown).
Table 5.
Odds ratios (OR) of oral and pharyngeal cancer and corresponding 95% confidence intervals (CI) according to quartiles of energy-adjusted dietary inflammatory index (E-DII), among 946 cases and 2492 controls, in strata of selected covariates. Italy, 1992–2009.
| Cases/Controls | E-DII quartiles, OR (95% CI)a | p value for trend | p value for homogeneity | ||||
|---|---|---|---|---|---|---|---|
| −5.29, −1.19 | −1.18, −0.31 | −0.30, 0.73 | 0.74, 7.66 | ||||
| Age (years) | 0.52 | ||||||
| <60 | 503/1317 | 1 b | 0.84 (0.54, 1.8) | 1.41 (0.94, 2.11) | 1.45 (0.96, 2.17) | 0.02 | |
| ≥60 | 443/1175 | 1 b | 1.07 (0.70, 1.62) | 1.53 (1.03, 2.27) | 2.12 (1.42, 3.16) | <0.0001 | |
| Sex | 0.03 | ||||||
| Male | 756/1497 | 1 b | 0.72 (0.50, 1.04) | 1.40 (1.00, 1.96) | 1.38 (0.98, 1.96) | 0.002 | |
| Female | 190/995 | 1 b | 1.69 (0.98, 2.91) | 1.66 (0.95, 2.89) | 3.30 (1.95, 5.57) | <0.0001 | |
| Education (years)c | 0.38 | ||||||
| <7 | 553/1272 | 1 b | 1.31 (0.86, 2.00) | 1.67 (1.11, 2.52) | 2.07 (1.37, 3.13) | 0.0003 | |
| ≥7 | 388/1209 | 1 b | 0.64 (0.41, 1.00) | 1.41 (0.95, 2.10) | 1.62 (1.09, 2.41) | 0.0002 | |
| Alcohol (drinks/week) | 0.76 | ||||||
| <28 | 364/1862 | 1 b | 1.00 (0.68, 1.45) | 1.49 (1.05, 2.12) | 1.85 (1.30, 2.63) | <0.0001 | |
| ≥28 | 582/630 | 1 b | 1.07 (0.67, 1.73) | 1.84 (1.18, 2.88) | 2.28 (1.46, 3.58) | 0.0003 | |
| Tobacco smokingc | 0.37 | ||||||
| Non-smoker | 137/1077 | 1 b | 1.84 (1.02, 3.33) | 1.88 (1.04, 3.41) | 3.06 (1.69, 5.51) | 0.0003 | |
| Ex-smoker | 255/761 | 1 b | 0.90 (0.53, 1.52) | 1.72 (1.05, 2.80) | 1.47 (0.88, 2.44) | 0.04 | |
| Current smoker | 550/649 | 1 b | 0.70 (0.43, 1.15) | 1.35 (0.86, 2.14) | 1.72 (1.09, 2.72) | <0.0001 | |
Adjusted for age, sex, centre, year of interview, education, body mass index, tobacco smoking, alcohol consumption, and non-alcohol energy intake.
Reference category.
The sum does not add up to the total because of some missing values
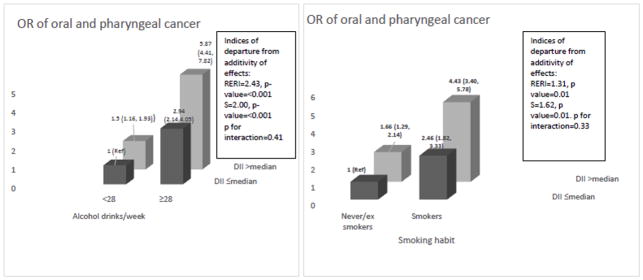
Figure 1 shows the joint effects of E-DII and alcohol drinking or tobacco smoking on oral and pharyngeal cancer risk, controlling for centre and potential confounders. Compared to the lowest risk category, that is never to moderate drinkers (<28 drinks/week) with the lower E-DII category (≤median), the OR for oral and pharyngeal cancer was 5.87 (95% CI 4.41,7.82) for heavy drinkers with the higher E-DII category (> median). RERI was 2.43 (p-value<0.001), S was 2.00 (p-value<0.001), and p-value for multiplicative interaction was 0.41. With reference to smoking, compared to never/ex smokers with the lower E-DII category, the OR for oral and pharyngeal cancer was 4.43 (95% CI 3.40,5.78) for current smokers with the higher E-DII category. RERI was 1.31 (p-value=0.01), S was 1.62 (p-value=0.01), and p-value for multiplicative interaction was 0.33, suggestive of positive additive interaction of a pro-inflammatory diet with smoking and alcohol consumption in the association with oral and pharyngeal cancer.
Fig. 1.
Odds ratios (OR)a and 95 % confidence intervals for oral and pharyngeal cancer according to the combination of of dietary inflammatory index (E-DII) and alcohol and smoking. Italy, 1992–2009.
Footnote:
Abbreviation: RERI: Relative excess risk due to interaction; S: Synergy index.
aEstimated from multiple logistic regression models, including terms for age, sex, centre, education, body mass index, non-alcohol energy intake, and tobacco smoking and alcohol consumption when appropriate.
Discussion
In this large case-control study, a more pro-inflammatory diet, as reflected in higher DII scores, was associated with an increased risk of oral and pharyngeal cancer with a stronger association observed among women. As was observed among controls (Table 2), compared to men women tend to be much more well represented in the lowest E-DII quartile. Having a high concentration of lower (i.e., referent) values among female controls would tend to amplify risk in the higher quartiles of E-DII among women, as was observed in these analyses.
This is the first report on this association. There has been a growing interest in conducting research on dietary scores and patterns and cancer, rather than any single nutrient 35. These results indicate a diet rich in anti-inflammatory food items like fruit, vegetables, fish, coffee, and poultry and low in pork, sugar, cheese, and desserts can help in reducing the risk of oral and pharyngeal cancer.
Previous results obtained in this case-control study showed protective effects of flavonones, flavonols and total flavonoids 36 and high fruit and vegetable intake 5, all of which have anti-inflammatory properties or components, and increased risks with foods rich in pro-inflammatory components like animal protein, animal fat, saturated fatty acids and cholesterol 5. Adherence to healthy dietary pattern like Mediterranean diet was also protective in this case-control study 37. A diet with a high Mediterranean diet score (MDS), as defined by Trichopoulou et al. 2003 38, would also have a low DII (or E-DII) score as we have observed previously in other Mediterranean populations 39–41. In this study, we observed an inverse Spearman correlation of −0.43 (p-value <0.0001). We also carried out sensitivity analyses adjusting for MDS in the analyses and there was a marginal drop off in the OR for continuous DII, i.e., 1.14 (95 % CI 1.06–1.22). The inverse correlation that was observed may reflect the importance of dietary features common to both indices: higher intakes of whole grains, fish, vegetables, fruit and plant-based components which contribute a range of nutrients with potential to impact cancer risk through different mechanisms. Differences between these indices and outcomes may reflect how other items such as potatoes and eggs were considered differently by the different indices.
In formulating the DII, an approach uniquely different to that of other indices was taken by focusing on the functional effects of foods and nutrients. As such, it relies on reviewing and scoring of the peer-reviewed literature on the subject of diet and inflammation. Also, it standardizes individuals’ dietary intakes of pro- and anti-inflammatory food constituents to world referent values that results in values that are not dependent on units of consumption and can be used for comparison across studies 42. The DII is based on the idea that many chronic inflammation-related diseases such as cancer operate in a pro-inflammatory environment. The most anti-inflammatory diets are likely to include foods such as fruit and vegetables, fish, and olive oil that feature prominently in a Mediterranean diet. A Mediterranean diet consists of components such as whole grains, which are rich in anti-oxidants, so a likely mechanism through which the Mediterranean diet would exert its protective effect could involve oxidative process. Antioxidants manifest themselves in an inhibition of the synthesis and activity of growth factors that promote development of cancer cells, starting from activation of carcinogens, through regulating the cell cycle, to the process of angiogenesis and carcinogenesis 43. These effects are more likely in a strongly pro-inflammatory substrate. Other possible mechanisms for this positive association of the DII with oral and pharyngeal cancer might be through the excess production of proto-oncogenic cytokines like IL-6, IL-8, platelet-derived growth factor, and vascular endothelial growth factor in the tumor microenvironment, which are then responsible for carcinogenic activities such as anti-apoptosis, tumor angiogenesis and metastasis 44. Hypoxia is a common state in cancers and inflamed tissues can cause DNA damage and induce tumorigenic factors. Finally, tissue vasculature is a vital part of its microenvironment, supplying oxygen, nutrients, and growth factors to rapidly dividing cells and providing a mechanism for metastatic spread 6.
Previous studies have been conducted to examine various other dietary components, patterns and indices in relation to oral and pharyngeal cancer 45–51. In the pooled analyses within the International Head and Neck Cancer Epidemiology Consortium folate 47, vitamin C 45, coffee and tea intake 48 were found to be protective against oral and pharyngeal cancer. Moreover, a meta-analyses based on 15 case-control and one cohort studies revealed a protective role of fruit and vegetables consumption 49. In the Netherlands Cohort Study, an inverse association was observed between intake of nutrients like vitamin C and food items like fruits and vegetables; and the incidence of head and neck cancers including oral cancers 50, 51.
Among the strengths of the study are its large sample size and the availability of major confounding factors, including tobacco smoking and alcohol drinking 52. Dietary recall of cases can be influenced by recent diagnosis of cancer. However, the information collected in our study refers to the habitual diet before diagnosis or hospital admission. Reproducibility was tested in a separate sample a few months after the first interview 29 as well as few years after original data collection in another sub-sample of controls 53. Both studies gave satisfactory reproducibility, with correlation coefficients between 0.5 and 0.7 for most frequently consumed foods. The dietary habits of hospital controls may differ from those of the general population, but only patients admitted to the hospital for acute conditions, not related to major changes in diet and other lifestyle factors were included. Although – as in most case-control studies53, 54 – interviewers could not be blinded to the case or control status, recall bias in the intake of several dietary components from the FFQ is unlikely, as awareness of a link between diet and oral and pharyngeal cancer risk in Italy was not widely known at the time of this study. Moreover, the same interview setting and catchment areas for cases and controls, and the almost complete participation, provide some reassurance against any strong selection bias.
In conclusion, subjects – especially women – who consumed a more pro-inflammatory diet were at increased risk of oral and pharyngeal cancer compared to those who consumed a more anti-inflammatory diet in this Italian population. This is the first study to examine the association between inflammatory potential of diet and oral and pharyngeal cancer and the results suggest that encouraging intake of more anti-inflammatory dietary factors, such as plant-based foods rich in fiber and phytochemicals, and reducing intake of pro-inflammatory factors, such as fried foods or processed foods rich in saturated fat or animal protein, is a promising strategy for reducing the risk of oral and pharyngeal cancer. Hence, targeting an improvement in the DII of the diet may be added to the list of general health recommendations for the reduction in risk of oral and pharyngeal cancer. These results should be replicated in other studies in order to increase confidence in making this recommendation.
Novelty & Impact Statements.
In this large case-control study, we report a positive association between inflammatory potential of diet and of oral and pharyngeal cancer in Italy. This is the first study to examine this association. Results show that a diet rich in pro-inflammatory components may be a risk factor for oral and pharyngeal carcinogenesis. Public health steps should be taken to promote an anti-inflammatory diet; i.e. one rich in green, leafy vegetables and fruits.
Acknowledgments
Funding: This study was supported by the Italian Foundation for Cancer Research (FIRC). Drs. Shivappa and Hébert were supported by grant number R44DK103377 from the United States National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Valentina Rosato was supported by a fellowship from the Italian Foundation for Cancer Research (FIRC #18107).
Footnotes
Disclosure: Dr. James R. Hébert owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the dietary inflammatory index (DII) from the University of South Carolina in order to develop computer and smart phone applications for patient counseling and dietary intervention in clinical settings. Dr. Nitin Shivappa is an employee of CHI. The subject matter of this paper will not have any direct bearing on that work, nor has that activity exerted any influence on this project.
References
- 1.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. European journal of cancer. 2010;46:765–81. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Bosetti C, Bertuccio P, Malvezzi M, Levi F, Chatenoud L, Negri E, La Vecchia C. Cancer mortality in Europe, 2005–2009, and an overview of trends since 1980. Annals of Oncology. 2013;24:2657–71. doi: 10.1093/annonc/mdt301. [DOI] [PubMed] [Google Scholar]
- 3.Thun MJ, Henley SJ, Gansler T. Inflammation and cancer: an epidemiological perspective. Novartis Found Symp. 2004;256:6–21. discussion 2–8, 49–52, 266–9. [PubMed] [Google Scholar]
- 4.Alcohol consumption and ethyl carbamate. IARC Monogr Eval Carcinog Risks Hum. 2010;96:3–1383. [PMC free article] [PubMed] [Google Scholar]
- 5.Bravi F, Bosetti C, Filomeno M, Levi F, Garavello W, Galimberti S, Negri E, La Vecchia C. Foods, nutrients and the risk of oral and pharyngeal cancer. British journal of cancer. 2013;109:2904–10. doi: 10.1038/bjc.2013.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonomi M, Patsias A, Posner M, Sikora A. The role of inflammation in head and neck cancer. Advances in experimental medicine and biology. 2014;816:107–27. doi: 10.1007/978-3-0348-0837-8_5. [DOI] [PubMed] [Google Scholar]
- 7.Keibel A, Singh V, Sharma MC. Inflammation, microenvironment, and the immune system in cancer progression. Curr Pharm Des. 2009;15:1949–55. doi: 10.2174/138161209788453167. [DOI] [PubMed] [Google Scholar]
- 8.Pan MH, Lai CS, Dushenkov S, Ho CT. Modulation of inflammatory genes by natural dietary bioactive compounds. J Agric Food Chem. 2009;57:4467–77. doi: 10.1021/jf900612n. [DOI] [PubMed] [Google Scholar]
- 9.Tezal M, Scannapieco FA, Wactawski-Wende J, Hyland A, Marshall JR, Rigual NR, Stoler DL. Local inflammation and human papillomavirus status of head and neck cancers. Archives of otolaryngology--head & neck surgery. 2012;138:669–75. doi: 10.1001/archoto.2012.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosetti C, Talamini R, Franceschi S, Negri E, Garavello W, La Vecchia C. Aspirin use and cancers of the upper aerodigestive tract. British journal of cancer. 2003;88:672–4. doi: 10.1038/sj.bjc.6600820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosetti C, Rosato V, Gallus S, Cuzick J, La Vecchia C. Aspirin and cancer risk: a quantitative review to 2011. Annals of oncology : official journal of the European Society for Medical Oncology. 2012;23:1403–15. doi: 10.1093/annonc/mds113. [DOI] [PubMed] [Google Scholar]
- 12.de Mello VD, Schwab U, Kolehmainen M, Koenig W, Siloaho M, Poutanen K, Mykkanen H, Uusitupa M. A diet high in fatty fish, bilberries and wholegrain products improves markers of endothelial function and inflammation in individuals with impaired glucose metabolism in a randomised controlled trial: the Sysdimet study. Diabetologia. 2011;54:2755–67. doi: 10.1007/s00125-011-2285-3. [DOI] [PubMed] [Google Scholar]
- 13.Khoo J, Piantadosi C, Duncan R, Worthley SG, Jenkins A, Noakes M, Worthley MI, Lange K, Wittert GA. Comparing effects of a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. J Sex Med. 2011;8:2868–75. doi: 10.1111/j.1743-6109.2011.02417.x. [DOI] [PubMed] [Google Scholar]
- 14.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–96. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leoncini E, Nedovic D, Panic N, Pastorino R, Edefonti V, Boccia S. Carotenoid Intake from Natural Sources and Head and Neck Cancer: A Systematic Review and Meta-analysis of Epidemiological Studies. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24:1003–11. doi: 10.1158/1055-9965.EPI-15-0053. [DOI] [PubMed] [Google Scholar]
- 16.Lucenteforte E, Garavello W, Bosetti C, La Vecchia C. Dietary factors and oral and pharyngeal cancer risk. Oral oncology. 2009;45:461–7. doi: 10.1016/j.oraloncology.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Ide R, Fujino Y, Hoshiyama Y, Mizoue T, Kubo T, Pham TM, Shirane K, Tokui N, Sakata K, Tamakoshi A, Yoshimura T, Group JS. A prospective study of green tea consumption and oral cancer incidence in Japan. Annals of epidemiology. 2007;17:821–6. doi: 10.1016/j.annepidem.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 18.De Stefani E, Boffetta P, Ronco AL, Correa P, Oreggia F, Deneo-Pellegrini H, Mendilaharsu M, Leiva J. Dietary patterns and risk of cancer of the oral cavity and pharynx in Uruguay. Nutrition and cancer. 2005;51:132–9. doi: 10.1207/s15327914nc5102_2. [DOI] [PubMed] [Google Scholar]
- 19.Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Tabung F, Hebert JR. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS) Public Health Nutrition. 2013;10:1–9. doi: 10.1017/S1368980013002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wirth MD, Burch J, Shivappa N, Violanti JM, Burchfiel CM, Fekedulegn D, Andrew ME, Hartley TA, Miller DB, Mnatsakanova A, Charles LE, Steck SE, et al. Association of a dietary inflammatory index with inflammatory indices and metabolic syndrome among police officers. J Occup Environ Med. 2014;56:986–9. doi: 10.1097/JOM.0000000000000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shivappa N, Hebert JR, Rietzschel ER, De Buyzere ML, Langlois M, Debruyne E, Marcos A, Huybrechts I. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. The British journal of nutrition. 2015;113:665–71. doi: 10.1017/S000711451400395X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tabung FK, Steck SE, Zhang J, Ma Y, Liese AD, Agalliu I, Hingle M, Hou L, Hurley TG, Jiao L, Martin LW, Millen AE, et al. Construct validation of the dietary inflammatory index among postmenopausal women. Annals of epidemiology. 2015;25:398–405. doi: 10.1016/j.annepidem.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shivappa N, Bosetti C, Zucchetto A, Serraino D, La Vecchia C, Hebert JR. Dietary inflammatory index and risk of pancreatic cancer in an Italian case-control study. The British journal of nutrition. 2014:1–7. doi: 10.1017/S0007114514003626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shivappa N, Hebert JR, Polesel J, Zucchetto A, Crispo A, Montella M, Franceschi S, Rossi M, La Vecchia C, Serraino D. Inflammatory potential of diet and risk for hepatocellular cancer in a case-control study from Italy. The British journal of nutrition. 2016;115:324–31. doi: 10.1017/S0007114515004419. [DOI] [PubMed] [Google Scholar]
- 25.Shivappa N, Zucchetto A, Serraino D, Rossi M, La Vecchia C, Hebert JR. Dietary inflammatory index and risk of esophageal squamous cell cancer in a case-control study from Italy. Cancer causes & control : CCC. 2015;26:1439–47. doi: 10.1007/s10552-015-0636-y. [DOI] [PubMed] [Google Scholar]
- 26.Shivappa N, Hebert JR, Rosato V, Serraino D, La Vecchia C. Inflammatory potential of diet and risk of laryngeal cancer in a case-control study from Italy. Cancer causes & control : CCC. 2016;27:1027–34. doi: 10.1007/s10552-016-0781-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shivappa N, Hebert JR, Zucchetto A, Montella M, Libra M, Garavello W, Rossi M, La Vecchia C, Serraino D. Increased Risk of Nasopharyngeal Carcinoma with Increasing Levels of Diet-Associated Inflammation in an Italian Case-Control Study. Nutrition and cancer. 2016;68:1123–30. doi: 10.1080/01635581.2016.1216137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franceschi S, Favero A, Conti E, Talamini R, Volpe R, Negri E, Barzan L, La Vecchia C. Food groups, oils and butter, and cancer of the oral cavity and pharynx. British journal of cancer. 1999;80:614–20. doi: 10.1038/sj.bjc.6690400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franceschi S, Negri E, Salvini S, Decarli A, Ferraroni M, Filiberti R, Giacosa A, Talamini R, Nanni O, Panarello G, et al. Reproducibility of an Italian food frequency questionnaire for cancer studies: results for specific food items. European journal of cancer. 1993;29A:2298–305. doi: 10.1016/0959-8049(93)90225-5. [DOI] [PubMed] [Google Scholar]
- 30.Franceschi S, Barbone F, Negri E, Decarli A, Ferraroni M, Filiberti R, Giacosa A, Gnagnarella P, Nanni O, Salvini S, et al. Reproducibility of an Italian food frequency questionnaire for cancer studies. Results for specific nutrients. Annals of epidemiology. 1995;5:69–75. doi: 10.1016/1047-2797(95)92893-d. [DOI] [PubMed] [Google Scholar]
- 31.Decarli A, Franceschi S, Ferraroni M, Gnagnarella P, Parpinel MT, La Vecchia C, Negri E, Salvini S, Falcini F, Giacosa A. Validation of a food-frequency questionnaire to assess dietary intakes in cancer studies in Italy. Results for specific nutrients. Annals of epidemiology. 1996;6:110–8. doi: 10.1016/1047-2797(95)00129-8. [DOI] [PubMed] [Google Scholar]
- 32.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. The American journal of clinical nutrition. 1997;65:1220S–8S. doi: 10.1093/ajcn/65.4.1220S. discussion 9S–31S. [DOI] [PubMed] [Google Scholar]
- 33.Rothman K. Measuring interactions. Oxford: Oxford University Press; 2002. Epidemiology: an introduction; pp. 168–80. [Google Scholar]
- 34.Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3:452–6. doi: 10.1097/00001648-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Current opinion in lipidology. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Rossi M, Garavello W, Talamini R, Negri E, Bosetti C, Dal Maso L, Lagiou P, Tavani A, Polesel J, Barzan L, Ramazzotti V, Franceschi S, et al. Flavonoids and the risk of oral and pharyngeal cancer: a case-control study from Italy. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16:1621–5. doi: 10.1158/1055-9965.EPI-07-0168. [DOI] [PubMed] [Google Scholar]
- 37.Filomeno M, Bosetti C, Garavello W, Levi F, Galeone C, Negri E, La Vecchia C. The role of a Mediterranean diet on the risk of oral and pharyngeal cancer. British journal of cancer. 2014;111:981–6. doi: 10.1038/bjc.2014.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. The New England journal of medicine. 2003;348:2599–608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 39.Pimenta AM, Toledo E, Rodriguez-Diez MC, Gea A, Lopez-Iracheta R, Shivappa N, Hebert JR, Martinez-Gonzalez MA. Dietary indexes, food patterns and incidence of metabolic syndrome in a Mediterranean cohort: The SUN project. Clinical nutrition. 2015;34:508–14. doi: 10.1016/j.clnu.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maisonneuve P, Shivappa N, Hebert JR, Bellomi M, Rampinelli C, Bertolotti R, Spaggiari L, Palli D, Veronesi G, Gnagnarella P. Dietary inflammatory index and risk of lung cancer and other respiratory conditions among heavy smokers in the COSMOS screening study. Eur J Nutr. 2016;55:1069–79. doi: 10.1007/s00394-015-0920-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia-Calzon S, Zalba G, Ruiz-Canela M, Shivappa N, Hebert JR, Martinez JA, Fito M, Gomez-Gracia E, Martinez-Gonzalez MA, Marti A. Dietary inflammatory index and telomere length in subjects with a high cardiovascular disease risk from the PREDIMED-NAVARRA study: cross-sectional and longitudinal analyses over 5 y. Am J Clin Nutr. 2015;102:897–904. doi: 10.3945/ajcn.115.116863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hebert JR, Wirth M, Davis L, Davis B, Harmon BE, Hurley TG, Drayton R, Angela Murphy E, Shivappa N, Wilcox S, Adams SA, Brandt HM, et al. C-reactive protein levels in African Americans: a diet and lifestyle randomized community trial. American journal of preventive medicine. 2013;45:430–40. doi: 10.1016/j.amepre.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Potentas E, Witkowska AM, Zujko ME. Mediterranean diet for breast cancer prevention and treatment in postmenopausal women. Przeglad menopauzalny = Menopause review. 2015;14:247–53. doi: 10.5114/pm.2015.56381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pries R, Wollenberg B. Cytokines in head and neck cancer. Cytokine & growth factor reviews. 2006;17:141–6. doi: 10.1016/j.cytogfr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Edefonti V, Hashibe M, Parpinel M, Turati F, Serraino D, Matsuo K, Olshan AF, Zevallos JP, Winn DM, Moysich K, Zhang ZF, Morgenstern H, et al. Natural vitamin C intake and the risk of head and neck cancer: A pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. International journal of cancer Journal international du cancer. 2014 doi: 10.1002/ijc.29388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sapkota A, Hsu CC, Zaridze D, Shangina O, Szeszenia-Dabrowska N, Mates D, Fabianova E, Rudnai P, Janout V, Holcatova I, Brennan P, Boffetta P, et al. Dietary risk factors for squamous cell carcinoma of the upper aerodigestive tract in central and eastern Europe. Cancer causes & control : CCC. 2008;19:1161–70. doi: 10.1007/s10552-008-9183-0. [DOI] [PubMed] [Google Scholar]
- 47.Galeone C, Edefonti V, Parpinel M, Leoncini E, Matsuo K, Talamini R, Olshan AF, Zevallos JP, Winn DM, Jayaprakash V, Moysich K, Zhang ZF, et al. Folate intake and the risk of oral cavity and pharyngeal cancer: A pooled analysis within the International Head and Neck Cancer Epidemiology Consortium. International Journal of Cancer. 2015;136:904–14. doi: 10.1002/ijc.29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galeone C, Tavani A, Pelucchi C, Turati F, Winn DM, Levi F, Yu GP, Morgenstern H, Kelsey K, Dal Maso L, Purdue MP, McClean M, et al. Coffee and tea intake and risk of head and neck cancer: pooled analysis in the international head and neck cancer epidemiology consortium. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19:1723–36. doi: 10.1158/1055-9965.EPI-10-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pavia M, Pileggi C, Nobile CGA, Angelillo IF. Association between fruit and vegetable consumption and oral cancer: a meta-analysis of observational studies. The American journal of clinical nutrition. 2006;83:1126–34. doi: 10.1093/ajcn/83.5.1126. [DOI] [PubMed] [Google Scholar]
- 50.de Munter L, Maasland DH, van den Brandt PA, Kremer B, Schouten LJ. Vitamin and carotenoid intake and risk of head-neck cancer subtypes in the Netherlands Cohort Study. Am J Clin Nutr. 2015;102:420–32. doi: 10.3945/ajcn.114.106096. [DOI] [PubMed] [Google Scholar]
- 51.Maasland DH, van den Brandt PA, Kremer B, Goldbohm RA, Schouten LJ. Consumption of vegetables and fruits and risk of subtypes of head-neck cancer in the Netherlands Cohort Study. International journal of cancer Journal international du cancer. 2015;136:E396–409. doi: 10.1002/ijc.29219. [DOI] [PubMed] [Google Scholar]
- 52.Negri E, La Vecchia C, Franceschi S, Tavani A. Attributable risk for oral cancer in northern Italy. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1993;2:189–93. [PubMed] [Google Scholar]
- 53.D’Avanzo B, La Vecchia C, Katsouyanni K, Negri E, Trichopoulos D. An assessment, and reproducibility of food frequency data provided by hospital controls. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation. 1997;6:288–93. doi: 10.1097/00008469-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 54.MacMahon B, Trichopoulos D. Epidemiology. Principles and Methods. 2. Boston: Little, Brown and Co; 1996. [Google Scholar]