Abstract
OBJECTIVES
Spinal cord stimulation (SCS) has emerged as an appropriate modality of treatment for intractable chronic pain. The present study examines variations in SCS trial-to-permanent conversion rates based on provider types performing the procedure.
MATERIALS AND METHODS
We designed a large, retrospective analysis using the Truven MarketScan database analyzing adult SCS patients with provider information available, with or without IPG implantation from the years 2007 to 2012. Patients were categorized based on provider type performing the implantation including anesthesiologists, neurosurgeons, orthopedic surgeons, and physical medicine and rehabilitation (PM&R). Univariate and multivariate models identified factors associated with successful conversion.
RESULTS
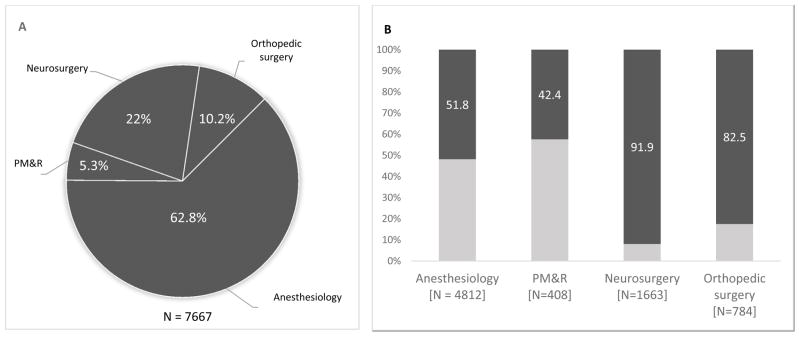
A total of 7,667 unique instances of SCS implants were identified across five providers. Overall, 4,842 (63.2%) of those receiving trials underwent permanent SCS system implantation. Anesthesiology performed the majority of implants (62.8%), followed by neurosurgery (22.0%), orthopedic surgery (10.2%), and PM&R (5.3%). Compared to anesthesiologists, both neurosurgeons (OR 10.99, 95% CI [9.11, 13.25]; p < 0.001) and orthopedic surgeons (OR 4.64, 95% CI [3.81, 5.65]; p < 0.001) had significantly higher conversion rates, while PM&R (OR 0.71, 95% CI [0.58, 0.87]; p = 0.001) had significantly lower. Percutaneous implants comprised 5473 (71.4%) of all implants. Neurosurgeons and orthopedic surgeons performed a significantly greater number of paddle implants among the different providers (p < 0.0001). Explant rates were similar across all cohorts analyzed (average 11.6%; p = 0.546).
CONCLUSIONS
In this nationwide analysis, our results suggest that over a recent five year period, conversion rates are highest when SCS trials are performed by neurosurgeons and orthopedic surgeons. The study has important implications for establishing uniform guidelines for training, patient selection and education of physicians across multiple disciplines.
Keywords: Spinal cord stimulator (SCS), trial conversion, provider type, chronic pain, costs, outcomes
INTRODUCTION
Chronic pain affects approximately 100 million individuals in the United States, with a significant economic impact resulting in $635 billion in health care costs annually1. Traditional treatment with pharmacological therapies has been shown to inadequately treat neuropathic pain with nearly 20% of chronic pain patients reporting poor pain relief with opioid control2, 3. In the context of these poor outcomes and high cost burden, spinal cord stimulation (SCS) has emerged an appropriate modality of treatment. SCS has been shown to reduce pain intensity, and improve quality of life and overall burden of pain management4, 5.
Previously a last-line treatment, SCS is now considered an effective treatment for a variety of neuropathic pain conditions. SCS is particularly effective in treating neuropathic pain derived from failed back surgery syndrome (FBSS), complex regional pain syndrome (CRPS), peripheral neuropathy, phantom limb pain, angina, and ischemic limb pain6–8. The procedure involves a short trial of 3–7 days, typically performed in an outpatient setting, in which a temporary stimulator lead is implanted to determine whether adequate pain relief is achieved. If significant (>50%) pain relief is seen during the trial, a complete (permanent) system is implanted9, and successful trial-to-permanent conversion is achieved.
As a growing body of evidence demonstrates that SCS is both more cost-effective and creates better outcomes than conventional opioid therapies, it has become more commonly used. This rise in use has been accompanied by an increasing diversity of providers performing the procedure, which now includes anesthesiologists, pain management or pain medicine specialists (hereafter anesthesiologists), neurosurgeons, orthopedic surgeons and physical medicine and rehabilitation (PM&R) specialists. With different surgical and medical disciplines participating in patient selection, implantation, and follow-up, it has also become increasingly clear that SCS outcomes may be influenced by physician expertise in such areas. Furthermore, with a nationwide trial-to-permanent conversion rate recently reported for SCS at 41.4%, there is a need to evaluate and address factors that have the potential to improve outcomes10.
Although there is ample evidence that SCS is best for managing certain types of neuropathic pains, little work has been done to understand whether provider type impacts the success of SCS procedures and influences patient outcomes or healthcare resource utilization. The availability of large secondary databases that track outcome measures and overall healthcare spending across government, community, and academic institutions provides a unique lens through which to observe how different provider types may impact these important metrics for successful management of chronic pain. In this large, retrospective study, we used patient data from the Truven MarketScan database to examine variations in SCS trial-to-permanent conversion rates from 2007 to 2012 based on the specialty performing the procedure. We hypothesize that specialties traditionally trained in surgical implantation of SCS systems will have a higher trial-to-permanent conversion rates and will better utilize healthcare resources.
METHODS
Data source
Truven Marketscan was utilized to design a retrospective review and establish a patient cohort for analysis. Marketscan is a national database that includes information from Commercial Claims and Encounters, Medicare Supplemental and Coordination of Benefits, and Medicaid databases. Patients have an encrypted ID that serves a unique identifier as well as allows for de-identification and a linkage variable across different files. The database is constructed from paid claims from 100 data sources, and includes United States inpatient admissions, inpatient services, outpatient services, and enrollment tables between 2007 and 2012, which is de-identified and collected from a third party, requiring no patient contact or consent.
Outcome measures
Trial-to-permanent conversion rates comprised the main outcome measure. A successful trial was defined by percutaneous lead implant trial followed by permanent implantation. Failed trial was defined by percutaneous lead implant not accompanied by an internal pulse generator (IPG) implant. Separate cohorts for chronic pain patients were obtained based on successful vs failed trial with respect to each provider type performing the implant.
Patient selection and cohorts
Unique instances of SCS trials were identified from the Marketscan national database. These individuals underwent implantation of a percutaneous SCS lead (CPT-4: 63650) or a paddle SCS lead (CPT: 63655) without a simultaneous implantation of a percutaneous pulse generator (CPT-4: 63685) (Supplementary Table 1). Inclusion criteria included age at the first SCS lead implantation ≥18 years old and available medication data in the database. The provider inclusion criteria included involvement in a minimum of three SCS trial implants and the provider type, where the latter was represented by at least 100 unique instances to represent providers that typically perform these procedures. Provider types included in our analysis were anesthesiology, PM&R, neurosurgery and orthopedic surgery. Providers that self-identified as pain management or medicine specialists were grouped together with anesthesiology. Providers without enrollment and baseline data were excluded in our study. Any instances that were coded ambiguously were also excluded from analyses including IPG placement one week prior to SCS. This yielded 7,667 unique instances of patients undergoing trial implantation (Supplementary Figure 1). All unique instances of patients were also categorized with ICD-9 pain diagnoses into seven commonly used groupings including back pain, chronic pain syndrome, chronic regional pain syndrome, degenerative spine disease, neuritis/radiculitis, limb pain and post-laminectomy syndrome (Table S1).
Statistical analysis
Descriptive statistics were reported and separated by provider type performing procedure. Univariate logistic regression model was used to test the significance of provider type. Continuous variables such as trial-to-permanent conversion rates, explant rates, pain diagnosis, gender, employment status, age, geographic region were summarized using means, standard deviations, medians, and quartiles. Variables of healthcare resource utilization were also included to understand the effect of provider type on cost outcomes. A multivariate logistic regression model was used to quantify the likelihood of permanent conversion using categorical variables. These covariates included age at first SCS lead implant, gender, year of procedure, insurance source, employment status, provider type, and Charlson score. Kruskal Wallis test was used for the group difference for the continuous variable, and Chi-square test was used for the group difference for categorical variables. Further, variables of healthcare resource utilization were included to understand the effect of provider type on cost outcomes. Adjusted odds ratios (ORs) with 95% confidence intervals (CIs) are reported for trial-to-permanent conversion rates. All analyses were conducted with SAS version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Patient cohort
7,667 unique instances of patients that underwent a percutaneous SCS trial between 2007 and 2012 were identified. There were four total provider types that were included in the study. The patient cohort was grouped based on these provider types, which included anesthesiologists (62.8% of all procedures), neurosurgery (22.0%), orthopedic surgery (10.2%), and PM&R (5.3%). Overall, 60.0% of patients were female, 21.4% were actively employed and 73.6% were commercially insured. Average Charlson score did not significantly differ among the providers, with the average score being 0 among 50.1% of patients. Majority of the patients (67.6%) were located in the Southern region. Description statistics are provided in Table 1.
Table 1.
Baseline characteristics by provider type
| Pain medicine/anesthesiology (N=4812) | PM&R (N=408) | Neurosurgery (N=1663) | Orthopedic Surgery (N=784) | Total (N=7667) | p value | |
|---|---|---|---|---|---|---|
| Successful Trial, N (%) | <0.0001 | |||||
| 0 | 2319 (48.2%) | 235 (57.6%) | 134 (8.1%) | 137 (17.5%) | 2825 (36.8%) | |
| 1 | 2493 (51.8%) | 173 (42.4%) | 1529 (91.9%) | 647 (82.5%) | 4842 (63.2%) | |
| SCS Type, N (%) | <0.0001 | |||||
| Both | 213 (4.4%) | 24 (5.9%) | 1222 (73.5%) | 444 (56.6%) | 1903 (24.8%) | |
| Paddle | 11 (0.2%) | 2 (0.5%) | 222 (13.3%) | 56 (7.1%) | 291 (3.8%) | |
| Percutaneous | 4588 (95.3%) | 382 (93.6%) | 219 (13.2%) | 284 (36.2%) | 5473 (71.4%) | |
| Charlson Index, N (%) | 0.7724 | |||||
| Missing | 69 (1.4%) | 4 (1.0%) | 15 (0.9%) | 12 (1.5%) | 100 (1.3%) | |
| 0 | 2414 (50.2%) | 201 (49.3%) | 832 (50.0%) | 393 (50.1%) | 3840 (50.1%) | |
| 1 | 1092 (22.7%) | 88 (21.6%) | 361 (21.7%) | 181 (23.1%) | 1722 (22.5%) | |
| 2 | 598 (12.4%) | 61 (15.0%) | 212 (12.7%) | 98 (12.5%) | 969 (12.6%) | |
| >=3 | 639 (13.3%) | 54 (13.2%) | 243 (14.6%) | 100 (12.8%) | 1036 (13.5%) | |
| Employment Status, N (%) | <0.0001 | |||||
| Active Full Time | 994 (20.7%) | 112 (27.5%) | 334 (20.1%) | 204 (26.0%) | 1644 (21.4%) | |
| Retired | 557 (11.5%) | 68 (16.6%) | 180 (10.7%) | 102 (13%) | 907 (11.9%) | |
| Other | 3261 (67.8%) | 228 (56%) | 1149 (69%) | 478 (61%) | 5116 (66.8%) | |
| Gender of Patient, N (%) | 0.2346 | |||||
| Male | 1916 (39.8%) | 156 (38.2%) | 697 (41.9%) | 299 (38.1%) | 3068 (40.0%) | |
| Female | 2896 (60.2%) | 252 (61.8%) | 966 (58.1%) | 485 (61.9%) | 4599 (60.0%) | |
| Age at SCS, N (%) | 0.0271 | |||||
| Mean (SD) | 54.0 (13.3) | 53.2 (13.5) | 53.1 (12.3) | 52.7 (11.3) | 53.6 (12.9) | |
| Median | 54.0 | 53.0 | 53.0 | 53.0 | 53.0 | |
| Region, N (%) | <0.0001 | |||||
| Northeast Region | 172 (3.6%) | 18 (4.4%) | 80 (4.8%) | 2 (0.3%) | 272 (3.5%) | |
| North Central Region | 684 (14.2%) | 32 (7.8%) | 132 (7.9%) | 41 (5.2%) | 889 (11.6%) | |
| South Region | 3049 (63.4%) | 307 (75.2%) | 1183 (71.1%) | 641 (81.8%) | 5180 (67.6%) | |
| West Region | 423 (8.8%) | 25 (6.1%) | 112 (6.7%) | 71 (9.1%) | 631 (8.2%) | |
| Unknown Region | 13 (0.3%) | 0 (0.0%) | 1 (0.1%) | 0 (0.0%) | 14 (0.2%) | |
| M | 471 (9.8%) | 26 (6.4%) | 155 (9.3%) | 29 (3.7%) | 681 (8.9%) | |
| Source, N (%) | <0.0001 | |||||
| CCAE | 3416 (71.0%) | 299 (73.3%) | 1273 (76.5%) | 658 (83.9%) | 5646 (73.6%) | |
| MAID | 471 (9.8%) | 26 (6.4%) | 155 (9.3%) | 29 (3.7%) | 681 (8.9%) | |
| MDCR | 925 (19.2%) | 83 (20.3%) | 235 (14.1%) | 97 (12.4%) | 1340 (17.5%) | |
| Year of SCS, N (%) | <0.0001 | |||||
| 2007 | 733 (15.2%) | 57 (14.0%) | 201 (12.1%) | 109 (13.9%) | 1100 (14.3%) | |
| 2008 | 891 (18.5%) | 59 (14.5%) | 315 (18.9%) | 135 (17.2%) | 1400 (18.3%) | |
| 2009 | 1316 (27.3%) | 76 (18.6%) | 412 (24.8%) | 188 (24.0%) | 1992 (26.0%) | |
| 2010 | 538 (11.2%) | 53 (13.0%) | 239 (14.4%) | 119 (15.2%) | 949 (12.4%) | |
| 2011 | 698 (14.5%) | 82 (20.1%) | 286 (17.2%) | 124 (15.8%) | 1190 (15.5%) | |
| 2012 | 636 (13.2%) | 81 (19.9%) | 210 (12.6%) | 109 (13.9%) | 1036 (13.5%) | |
| Explant | 0.5465 | |||||
| 0 | 2192 (87.9%) | 152 (87.9%) | 1366 (89.3%) | 568 (87.8%) | 4278 (88.4%) | |
| 1 | 301 (12.1%) | 21 (12.1%) | 163 (10.7%) | 79 (12.2%) | 564 (11.6%) | |
| Explant days after SCS lead | 0.6838 | |||||
| N | 301 | 21 | 163 | 79 | 564 | |
| Mean (SD) | 395.7 (392.0) | 446.4 (531.6) | 360.3 (377.3) | 341.1 (313.8) | 379.7 (383.6) | |
| Median | 252.0 | 336.0 | 220.0 | 257.0 | 243.0 |
SD, standard deviation
SCS, spinal cord stimulation
Predictors of conversion rates
Overall, 4,842 patients (63.2%) underwent a permanent SCS implantation after the initial trial. 2825 patients (36.8%) failed an SCS trial. Demographic factors associated with increased trial-to-permanent conversion rates included female gender (OR 1.11, 95% CI [1.00, 1.23]; p = 0.050). Compared to commercial insurance type, no significant difference was found in conversion rates with Medicaid (OR 0.74, 95% CI [0.53, 1.03], p = 0.075) while no difference was found with patients on Medicare (OR 0.99, 95% CI [0.82, 1.18], p = 0.878). Year of SCS implantation was not a significant predictor of trial-to-permanent conversion rates (OR 0.98, 95% CI [0.95, 1.01], p = 0.219). Finally, age of patient and Charlson score index did not play a role in predicting trial-to-permanent conversion rates.
Provider-based outcomes
Provider type was a significant predictor of trial-to-permanent conversion rates (Table 1). Overall, higher conversion rates were found among neurosurgeons (91.9% of all patients had successful permanent implantation) and orthopedic surgeons (82.5%) compared to anesthesiology (51.8%), a group that commanded the largest portion of the patient cohort. In a univariate analysis, significantly lower conversion rates were found in PM&R (OR 0.71, 95% CI [0.58, 0.87]; p = 0.001). Significantly higher conversion rates were present with neurosurgeons (OR 10.99, 95% CI [9.11, 13.25]; p < 0.001) and orthopedic surgeons (OR 4.64, 95% CI [3.81, 4.65]; p < 0.001).
An overwhelming majority of anesthesiologists and PM&R implanted percutaneous leads only (95.3% and 93.6% of all implants) compared to neurosurgeons and orthopedic surgeons (13.2% and 36.2% of all implants). Neurosurgeons (13.3%) and orthopedic surgeons (7.1%) implanted significantly more paddle implants compared to anesthesiologists (0.2%) and PM&R (0.5%). Overall, paddle trials comprised 3.8% of all implants.
While explant rates were slightly higher among the anesthesiologists (12.1% of implanted individuals) and lower among the neurosurgeons (10.7%), overall, there were no significant differences among the providers (median explant rate 11.6%; p = 0.546). Similarly, average time to explant was not significantly different among the providers (median time to explant 243.0 days; p = 0.681).
The predominant diagnoses for each provider type are listed in Table 2. Overall, the median number of comorbid pain diagnoses for each group was four. A prior history of back pain was most commonly present in the orthopedic surgery patient cohort (75.6%), followed by PM&R (73.5%), neurosurgery (72.4%), and anesthesiology (65.2%; overall median 68.3%, p < 0.0001). A prior history of CRPS was most commonly found among the anesthesiology patient cohort (9.3%), followed by PM&R (8.3%), neurosurgery (7.3%), and orthopedic surgery (5.2%; overall median 8.4%, p = 0.0004). Diagnosis of degenerative spine disease was most common among the orthopedic surgery patient cohort (74.5%) and least common among the anesthesiology cohort (65.2%; overall median 67.1%, p < 0.0001). A diagnosis of neuritis/radiculitis was most commonly present among PM&R (77.9%) and least common among the anesthesiology cohort (72.5%; overall median 73.4%, p = 0.0194). The provider composition of limb pain diagnosis included neurosurgery (26.9%), PM&R (27.0%), orthopedic surgery (25.0%), and anesthesiology (23.5%; overall median 24.6%, p = 0.0227). A diagnosis of post-laminectomy syndrome did not significantly differ among the groups.
Table 2.
Pain diagnosis by provider type
| Pain Medicine/anesthesiology (N=4812) | PM&R (N=408) | Neurosurgery (N=1663) | Orthopedic Surgery (N=784) | Total (N=7667) | p value | |
|---|---|---|---|---|---|---|
| Back Pain | <0.0001 | |||||
| 0 | 1676 (34.8%) | 108 (26.5%) | 459 (27.6%) | 191 (24.4%) | 2434 (31.7%) | |
| 1 | 3136 (65.2%) | 300 (73.5%) | 1204 (72.4%) | 593 (75.6%) | 5233 (68.3%) | |
| Chronic Pain Syndrome | . | |||||
| 0 | 4812 (100.0%) | 408 (100.0%) | 1663 (100.0%) | 784 (100.0%) | 7667 (100.0%) | |
| CRPS Hx | 0.0004 | |||||
| 0 | 4363 (90.7%) | 374 (91.7%) | 1542 (92.7%) | 743 (94.8%) | 7022 (91.6%) | |
| 1 | 449 (9.3%) | 34 (8.3%) | 121 (7.3%) | 41 (5.2%) | 645 (8.4%) | |
| Degenerative Spine Disease | <0.0001 | |||||
| 0 | 1674 (34.8%) | 113 (27.7%) | 536 (32.2%) | 200 (25.5%) | 2523 (32.9%) | |
| 1 | 3138 (65.2%) | 295 (72.3%) | 1127 (67.8%) | 584 (74.5%) | 5144 (67.1%) | |
| Neuritis / Radiculitis | 0.0194 | |||||
| 0 | 1323 (27.5%) | 90 (22.1%) | 409 (24.6%) | 214 (27.3%) | 2036 (26.6%) | |
| 1 | 3489 (72.5%) | 318 (77.9%) | 1254 (75.4%) | 570 (72.7%) | 5631 (73.4%) | |
| Limb Pain | 0.0227 | |||||
| 0 | 3683 (76.5%) | 298 (73.0%) | 1215 (73.1%) | 588 (75.0%) | 5784 (75.4%) | |
| 1 | 1129 (23.5%) | 110 (27.0%) | 448 (26.9%) | 196 (25.0%) | 1883 (24.6%) | |
| Post-laminectomy Syndrome | 0.4005 | |||||
| 0 | 2752 (57.2%) | 219 (53.7%) | 961 (57.8%) | 436 (55.6%) | 4368 (57.0%) | |
| 1 | 2060 (42.8%) | 189 (46.3%) | 702 (42.2%) | 348 (44.4%) | 3299 (43.0%) | |
| Multiple chronic pain diagnoses | <0.0001 | |||||
| Mean (SD) | 3.5 (1.2) | 3.7 (1.2) | 3.6 (1.2) | 3.7 (1.1) | 3.6 (1.2) | |
| Median | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
CRPS, chronic regional pain syndrome
Healthcare resource utilization
Trends in healthcare resource utilization are listed in Table 3. There were no significant differences in the total cost of treatment across the provider types (overall median cost $8343.6; p = 0.361) or the total number of inpatient admissions (p = 0.0776). Total cost attributed to pain encounters was higher amongst the orthopedic surgeons (median $4353.4) and lowest for anesthesiology (median $3436.3) and neurosurgery (median $3759.5; p = 0.0011). Time to permanent IPG implantation after SCS lead was shortest among the anesthesiologists (median 32.0 days) and longest among the neurosurgeons (median 35.0 days; p = 0.0006).
Table 3.
Healthcare resource utilization by provider type
| Pain Medicine/Anesthesiology (N=4812) | PM&R (N=408) | Neurosurgery (N=1663) | Orthopedic Surgery (N=784) | Total (N=7667) | p-value | |
|---|---|---|---|---|---|---|
| Total Cost | 0.3611 | |||||
| Mean (SD) | 12746.1 (14719.0) | 12373.5 (13406.6) | 12579.3 (13872.5) | 13235.0 (14333.7) | 12740.1 (14430.9) | |
| Median | 8215.1 | 8016.1 | 8362.1 | 9116.4 | 8343.6 | |
| Pain encounters | 0.0037 | |||||
| Mean (SD) | 43.1 (44.5) | 45.7 (43.5) | 43.1 (45.8) | 46.4 (43.8) | 43.6 (44.6) | |
| Median | 31.0 | 35.0 | 30.0 | 34.5 | 31.0 | |
| Total cost of pain encounters | 0.0011 | |||||
| Mean (SD) | 6022.9 (7969.6) | 6196.5 (8109.3) | 6065.6 (8273.1) | 7074.0 (8549.4) | 6148.9 (8109.0) | |
| Median | 3436.3 | 3759.5 | 3420.9 | 4353.4 | 3500.6 | |
| Inpatient admissions | 0.0776 | |||||
| Mean (SD) | 0.4 (1.2) | 0.5 (1.3) | 0.5 (1.1) | 0.5 (1.3) | 0.5 (1.2) | |
| Median | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| IPG implantation post-SCS lead (days) | 0.0006 | |||||
| N | 2485 | 172 | 1528 | 647 | 4832 | |
| Mean (SD) | 49.3 (89.7) | 53.4 (50.5) | 52.1 (85.4) | 54.8 (86.1) | 51.1 (86.8) | |
| Median | 32.0 | 39.0 | 35.0 | 35.0 | 34.0 | 0 |
Total cost, pain encounters, total cost of pain encounters, and inpatient admission data were obtained 1 year prior to match DT
SD, standard devisation; SCS, spinal cord stimulation; IPG, internal pulse generator
DISCUSSION
Chronic pain is a major public health care concern with the yearly cost of treatment at approximately $635 billion in the United States alone1. In this study, we utilized the MarketScan database to design a large, retrospective study trial and identify 7,667 patients that underwent a percutaneous or paddle SCS trial. Data was classified across four provider types including anesthesiology, PM&R, neurosurgery and orthopedic surgery. We subsequently analyzed trial-to-permanent conversion rates associated with different provider types, as well as independent factors associated with a successful conversion. Variables of healthcare resource utilization included total cost of treatment and cost of pain prescriptions. Our hypothesis was largely supported, showing increased conversion rates among specialties that traditionally perform surgical implantation of SCS systems. However, there were no significant differences in healthcare resource utilization across the providers.
A primary finding in our study relates to trial-to-permanent conversion rates across provider types that performed SCS procedures (Figure 1). Anesthesiologists performed the majority of the SCS implantations in our patient cohort. Both neurosurgery and orthopedic surgery had significantly higher conversion rates compared to other specialties, and accounted for 32.2% of all implants (Table 1). PM&R was associated with significantly lower conversion rates. Furthermore, due to increased trial-to-permanent conversion rates among some provider groups, we evaluated whether this also translated into higher explant rates. We found no significant differences, and explant rates and average days to explantation remained similar across all providers. Overall, age was distributed equally across the provider types. While female gender was an independent predictor of a successful conversion in our study (Table 4), gender distribution in itself was not significantly different across the providers. Finally, while there were no differences in total cost of treatment or inpatient admissions across the major providers, differences were found for the total cost of pain encounters prior to SCS implantation, which was lowest among the anesthesiologists, and highest among the orthopedic surgeons.
Figure 1.
Patient cohort and conversion rates across provider types. Neurosurgeons and orthopedic surgeons display higher rates of trial-to-permanent spinal cord stimulator conversion.
Patient cohort and conversion rates across provider types. A. Total patient cohort is represented across four provider types analyzed in study. Pie chart insets represent percentage of patient cohort undergoing SCS procedure by specific provider type. B. Trial-to-conversion rates of SCS procedure are shown across the provider types. Bar graph insets represent successful percentage conversion to permanent implantation. PM&R, physical medicine and rehabilitation.
Table 4.
Multivariate logistic regression for successful conversion
| OR (95% CI) | p-value | |
|---|---|---|
| Age | ||
| 1.00 (0.99, 1.00) | 0.817 | |
| Gender | ||
| Female | 1.11 (1.00, 1.23) | 0.050 |
| Male | reference | . |
| Year of SCS | ||
| 0.98 (0.95, 1.01) | 0.219 | |
| Insurance source | ||
| MDCR | 0.99 (0.82, 1.18) | 0.878 |
| MAID | 0.74 (0.53, 1.03) | 0.075 |
| CCAE | reference | . |
| Employment status | ||
| Other | 1.02 (0.90, 1.16) | 0.772 |
| Retiree/Medicare Eligible/Disabled | 0.83 (0.68, 1.00) | 0.048 |
| FT/PT | reference | . |
| Region | ||
| West region | 1.05 (0.76, 1.46) | 0.749 |
| South region | 0.83 (0.62, 1.09) | 0.183 |
| North Central region | 1.07 (0.79, 1.46) | 0.670 |
| Northeast region | reference | . |
| Provider type | ||
| Orthopedic Surgery | 4.64 (3.81, 5.65) | <.001 |
| Neurosurgery | 10.99 (9.11, 13.25) | <.001 |
| PM&R | 0.71 (0.58, 0.87) | 0.001 |
| Pain medicine/anesthesiology | reference | . |
| Charlson Score | ||
| >=3 | 10.92 (0.77, 1.10) | 0.380 |
| 2 | 1.09 (0.92, 1.28) | 0.321 |
| 1 | 1.04 (0.92, 1.18) | 0.528 |
| 0 | reference | . |
MDCR, Medicare; MAID, Medicaid; CCAE, commercially available insurance
OR, odds ratio; CI, confidence interval
Higher trial-to-permanent conversion rates among the neurosurgeons and orthopedic surgeons may be attributed to different factors. One of these may be availability of post-residency training programs targeting surgical experience with techniques involved in SCS. However, as discussed in previous efforts to standardize guidelines11, surgical experience alone may not account for the increased success. While patient selection criteria across the different providers could not be measured in this study, such criteria might further contribute to higher conversion rates among the surgical specialties in our study, such that these patients may have already failed conventional medical management. We also considered the impact of differences in the predominant pain diagnoses among the providers (Table 2). The median number of pain diagnoses across all providers was four. Significant albeit small differences were found among the majority of the pain diagnoses. Most notably, a prior history of back pain and degenerative spine disease was most common among the orthopedic surgeons and least common among the anesthesiologists. CRPS was most common among the anesthesiologists and least common among the orthopedic surgeons. While certain pain diagnoses such as CRPS carry a lower trial-to-permanent conversion rate and may contribute to the trend observed in our dataset, these alone do not explain the vast differences in trial-to-permanent conversion rates that exist among the provider types. Finally, the total number of procedures performed might serve as an indicator of physician experience. Previously, a minimum case load has been suggested as one aspect of core competency in providers performing SCS implantation 11. Recent work from our group has analyzed how procedure volume predicts conversion rates, as well as healthcare resource utilization 12. Overall, high volume providers were found to be independent predictors of successful trial-to-permanent conversion rates. Thus, it is possible that provider types with higher trial-to-permanent conversion rates are part of practices that see a higher volume of patients.
An important aspect of these findings is consideration of which specialties perform the IPG. Because the surgical specialties often perform both the trial and the IPG permanent implants, one could suggest that there are differing inertias to continue towards permanent based on the trial period13, 14. Patients of surgeons do not need a referral for IPG, whereas the patients of non-surgeons may sometimes need a referral if the trial physician does not perform the permanent implantation. Consequently, patient autonomy—where decision to proceed is made by the patient’s subjective interpretation of >50% pain improvement—is diluted. However, PM&R specialists and anesthesiologists may have greater inertia to make the referral to a surgeon for IPG placement, whereas surgeon’s operative schedules might incentivize subsequent placement. Further, a significantly higher number of paddle implants are performed by neurosurgeons and orthopedic surgeons. Such inertia differences might inflate the conversion rates in surgeons—where it is much harder for a patient to subjectively admit the trial “failed” when their surgery for IPG is already scheduled for the next week. However, we also evaluated the possible consequence of this using explant rates, and did not find significant differences among the provider types. This is similar to evidence in the literature showing poor correlation of explant rates between different providers15. Furthermore, average number of days to IPG after trial SCS lead was 34.0 days and similar across all providers, with anesthesiology having shorter (32.0 days) and neurosurgeons with longer (35.0 days) lengths. Nonetheless, while success has been seen in permanent implantation despite poor response in the trial16, we suggest that SCS training reinforce the importance of patient rating of pain improvement in guiding the scheduling of permanent device placement. Maintaining the integrity of the trial period will importantly remove this as a variable in the comparison of the specialists who perform implants. Ultimately, higher conversion rates among the surgical specialties should be considered multifactorial.
Previously, the North American Neuromodulation Society provided guidelines towards adequate training and competency among providers performing SCS in an effort to standardize knowledge across specialties with marked differences in training11. As SCS procedures have expanded to include different specialties over the last few decades, so have the challenges in implantation and management of SCS systems that require dedicated multidisciplinary training in an academic setting. Competency in SCS systems extends beyond expertise in surgical techniques, and includes training in epidural access techniques, programming and management of SCS systems, as well as core knowledge in mechanisms of action, appropriate screening, patient selection, and post-operative follow-up with recognition of complications. Indeed, an appropriate SCS practice may include a physician or group of physicians that have been trained to provide care across these facets11. Such services would also include an adequate case load and appropriate medical personnel familiar with SCS systems from more than one manufacturer. Existing guidelines by NANS have proposed three tracks to train physicians performing SCS that complete preliminary training across neurosurgery, pain medicine, orthopedic spine surgery, anesthesiology and interventional pain medicine. Besides desirable competency in core knowledge and management of SCS systems, active participation in at least 25 follow-up visits implanted with SCS in the capacity of a primary operator and evaluator must be demonstrated.
As more SCS procedures are performed annually in the US17, 18, our study provides the first concrete evidence of variations in success rates with provider type performing SCS trials and implantation, and underscores the importance of standardizing education and training through uniform guidelines. In exploring prognostic factors associated with optimal SCS outcomes, previous studies demonstrated the importance of appropriate patient selection, pain pattern, and physician expertise19, 20. Accumulating evidence in the last 20 years has also shown a significant correlation between procedural volume and surgical outcomes21–23. In the past 15 years, a number of randomized control trials and meta-analyses have demonstrated SCS as a therapeutically beneficial and cost-effective approach to chronic pain management in carefully selected patients24–26. Over this same period, the number of SCS procedures by anesthesiologists, PM&R and orthopedic surgeons have grown to service and provide options to major portions of patients undergoing implantation. Yet successful trial-to-permanent conversion rates for SCS remain below 50% nationwide10 and the predictors of SCS trial outcome remain poorly understood beyond pain etiology27, 28. Going forward, a concerted effort is needed to establish and follow uniform guidelines for standardization of training across the different medical and surgical specialties involved in patient selection, SCS trial and permanent implantation, and follow-up, will prove useful in improving the overall quality of care being offered.
This is the first study to show variations in trial-to-permanent conversion rates in different provider specialties that perform SCS trial or permanent implantation, a treatment modality that is being increasingly utilized in treating chronic pain. There are, however, several limitations to this study. First, this is a retrospective cohort analysis that uses unique instances of patients from a Truven MarketScan database. To address this, we performed univariate and multivariate analysis with regression covariates, and adjustment of appropriate patient and cost-related factors. Second, baseline clinical states and other patient-related factors including pain intensity and quality of life could not be analyzed in the patient cohort. Third, as the database does not distinguish between the prior training of pain medicine specialists, this group was categorized into a larger group with anesthesiology due to common background training. While both groups had strikingly similar conversion rates, other differences between the two groups may not be ruled out. Fourth, the database may not take into account the type of procedures or pain encounters that may contribute to varied healthcare resource utilization costs across the different providers. Furthermore, the database does not account for physician experience, patient selection or customization of spinal cord stimulation characteristics.
Despite the aforementioned limitations, the comprehensive and inclusive nature of the Truven MarketScan database provides us a useful trend in trial-to-permanent conversion rates for SCS implantations across various provider types. Follow up studies may utilize separate metrics of healthcare resource utilization to delineate the impact of the results of this study. As SCS trial and permanent implantation becomes more commonly performed, an important component would be addressing the current variations in training and education of providers performing SCS procedures.
CONCLUSION
In this large, nationwide analysis of 7,667 patients, we demonstrated significant variations among providers in trial-to-permanent rates for SCS implantations. Our study is the first in literature that provides evidence of significant variation in SCS outcomes among providers. This has implications for development of appropriate training programs for improved competency across provider types that are performing large numbers of SCS procedures. As the field of neuromodulation continues to expand and neurostimulation procedures are performed by an expanding number of medical and surgical specialties, standardization and guidelines for expected training requirements will ensure quality and optimal outcomes for patients undergoing treatment of chronic pain.
Supplementary Material
7,796 unique instances recorded. *Includes all provider disciplines over 2007–2012. **Includes 1,053 unique providers #Providers included had >100 implants. Analysis included five providers performing majority of SCS implantations (anesthesiology, pain medicine, neurosurgery, orthopedic surgery, and physical medicine and rehabilitation.
Codes utilized for procedures and pain diagnoses
Acknowledgments
The study received IRB approval from the Institutional Review Board. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001117. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Sources of Financial Support
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001117.
Siyun Yang and Jichun Xie had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The study received IRB approval from the Institutional Review Board. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001117. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Authorship Statement
Mr. Hussaini helped lead the study, and had major inputs into data analysis and manuscript preparation. Mr. Hussaini, Ms. Kelly, Ms. Han, Mr. Elsamadicy and Mr. Premji were involved in the literature review, interpretation of the data, and manuscript preparation. Ms. Yang and Dr. Xie helped lead statistical support, and had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Ms. Parente and Dr. Pagadala were involved with intellectual input into data inpretation and editorial support. Dr. Lad provided critical input into study design and final manuscript preparation.
Conflicts of Interest
Dr. Lad has received fees for serving as a speaker and consultant for Medtronic Inc., Boston Scientific, and St. Jude Medical. He serves as the Director of the Duke Neuro-outcomes Center which has received research funding from NIH KM1 CA 156687, Medtronic Inc. and St. Jude Medical. The remaining authors report no conflicts of interest or financial disclosures.
References
- 1.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012 Aug;13(8):715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan MD, Von Korff M, Banta-Green C, Merrill JO, Saunders K. Problems and concerns of patients receiving chronic opioid therapy for chronic non-cancer pain. Pain. 2010 May;149(2):345–353. doi: 10.1016/j.pain.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003 Nov 13;349(20):1943–1953. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- 4.Kumar K, Taylor RS, Jacques L, et al. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery. 2008 Oct;63(4):762–770. doi: 10.1227/01.NEU.0000325731.46702.D9. discussion 770. [DOI] [PubMed] [Google Scholar]
- 5.Kumar K, Rizvi S. Cost-effectiveness of spinal cord stimulation therapy in management of chronic pain. Pain Med. 2013 Nov;14(11):1631–1649. doi: 10.1111/pme.12146. [DOI] [PubMed] [Google Scholar]
- 6.Jeon YH. Spinal cord stimulation in pain management: a review. Korean J Pain. 2012 Jul;25(3):143–150. doi: 10.3344/kjp.2012.25.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barolat G. Spinal cord stimulation for chronic pain management. Arch Med Res. 2000 May-Jun;31(3):258–262. doi: 10.1016/s0188-4409(00)00075-8. [DOI] [PubMed] [Google Scholar]
- 8.Wolter T. Spinal cord stimulation for neuropathic pain: current perspectives. J Pain Res. 2014;7:651–663. doi: 10.2147/JPR.S37589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pain SA, Pain SA, Raff M, Melvill R, Coetzee G, Smuts J. Spinal cord stimulation for the management of pain: Recommendations for best clinical practice. S Afr Med J. 2013 Jun;103(6 Pt 2):423–430. doi: 10.7196/samj.6323. [DOI] [PubMed] [Google Scholar]
- 10.Huang KT, Martin J, Marky A, et al. A national survey of spinal cord stimulation trial-to-permanent conversion rates. Neuromodulation. 2015 Feb;18(2):133–139. doi: 10.1111/ner.12199. discussion 139–140. [DOI] [PubMed] [Google Scholar]
- 11.Henderson JM, Levy RM, Bedder MD, et al. NANS Training Requirements for Spinal Cord Stimulation Devices: Selection, Implantation, and Follow-up. Neuromodulation. 2009 Jul;12(3):171–174. doi: 10.1111/j.1525-1403.2009.00211.x. [DOI] [PubMed] [Google Scholar]
- 12.Murphy KR, Han JL, Hussaini SM, et al. The Volume-Outcome Effect: Impact on Trial-to-Permanent Conversion Rates in Spinal Cord Stimulation. Neuromodulation. 2016 Oct 3; doi: 10.1111/ner.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coiera E. Why system inertia makes health reform so difficult. Bmj. 2011;342:d3693. doi: 10.1136/bmj.d3693. [DOI] [PubMed] [Google Scholar]
- 14.HABAL MB, KARLAN MS, LEAKE D. Direction or inertia: the future for regulation of surgical implant devices. Journal of clinical engineering. 1978;3(2):186–188. doi: 10.1097/00004669-197804000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Hayek SM, Veizi E, Hanes M. Treatment-Limiting Complications of Percutaneous Spinal Cord Stimulator Implants: A Review of Eight Years of Experience From an Academic Center Database. Neuromodulation. 2015 Oct;18(7):603–608. doi: 10.1111/ner.12312. discussion 608–609. [DOI] [PubMed] [Google Scholar]
- 16.Oakley JC, Krames ES, Stamatos J, Foster AM. Successful long-term outcomes of spinal cord stimulation despite limited pain relief during temporary trialing. Neuromodulation. 2008 Jan;11(1):66–73. doi: 10.1111/j.1525-1403.2007.00145.x. [DOI] [PubMed] [Google Scholar]
- 17.Gharibo C, Laux G, Forzani BR, Sellars C, Kim E, Zou S. State of the Field Survey: Spinal Cord Stimulator Use by Academic Pain Medicine Practices. Pain Medicine. 2014;15(2):188–195. doi: 10.1111/pme.12264. [DOI] [PubMed] [Google Scholar]
- 18.LETTER T. Estimates of annual spinal cord stimulator implant rises in the United States. 2009 doi: 10.1111/j.1525-1403.2009.00264.x. [DOI] [PubMed] [Google Scholar]
- 19.Taylor RS, Van Buyten JP, Buchser E. Spinal cord stimulation for chronic back and leg pain and failed back surgery syndrome: a systematic review and analysis of prognostic factors. Spine (Phila Pa 1976) 2005 Jan 1;30(1):152–160. doi: 10.1097/01.brs.0000149199.68381.fe. [DOI] [PubMed] [Google Scholar]
- 20.Taylor RS, Desai MJ, Rigoard P, Taylor RJ. Predictors of pain relief following spinal cord stimulation in chronic back and leg pain and failed back surgery syndrome: a systematic review and meta-regression analysis. Pain Pract. 2014 Jul;14(6):489–505. doi: 10.1111/papr.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khuri SF, Daley J, Henderson W, et al. Relation of surgical volume to outcome in eight common operations: results from the VA National Surgical Quality Improvement Program. Ann Surg. 1999 Sep;230(3):414–429. doi: 10.1097/00000658-199909000-00014. discussion 429–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boudourakis LD, Wang TS, Roman SA, Desai R, Sosa JA. Evolution of the surgeon-volume, patient-outcome relationship. Ann Surg. 2009 Jul;250(1):159–165. doi: 10.1097/SLA.0b013e3181a77cb3. [DOI] [PubMed] [Google Scholar]
- 23.Livingston EH, Cao J. Procedure volume as a predictor of surgical outcomes. JAMA. 2010 Jul 7;304(1):95–97. doi: 10.1001/jama.2010.905. [DOI] [PubMed] [Google Scholar]
- 24.North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56(1):98–106. doi: 10.1227/01.neu.0000144839.65524.e0. discussion 106–107. [DOI] [PubMed] [Google Scholar]
- 25.North RB, Kidd D, Shipley J, Taylor RS. Spinal cord stimulation versus reoperation for failed back surgery syndrome: a cost effectiveness and cost utility analysis based on a randomized, controlled trial. Neurosurgery. 2007 Aug;61(2):361–368. doi: 10.1227/01.NEU.0000255522.42579.EA. discussion 368–369. [DOI] [PubMed] [Google Scholar]
- 26.Taylor RS, Ryan J, O’Donnell R, Eldabe S, Kumar K, North RB. The cost-effectiveness of spinal cord stimulation in the treatment of failed back surgery syndrome. Clin J Pain. 2010 Jul-Aug;26(6):463–469. doi: 10.1097/AJP.0b013e3181daccec. [DOI] [PubMed] [Google Scholar]
- 27.Williams KA, Gonzalez-Fernandez M, Hamzehzadeh S, et al. A multi-center analysis evaluating factors associated with spinal cord stimulation outcome in chronic pain patients. Pain Med. 2011 Aug;12(8):1142–1153. doi: 10.1111/j.1526-4637.2011.01184.x. [DOI] [PubMed] [Google Scholar]
- 28.Kumar K, Toth C, Nath RK, Laing P. Epidural spinal cord stimulation for treatment of chronic pain--some predictors of success. A 15-year experience. Surg Neurol. 1998 Aug;50(2):110–120. doi: 10.1016/s0090-3019(98)00012-3. discussion 120–111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
7,796 unique instances recorded. *Includes all provider disciplines over 2007–2012. **Includes 1,053 unique providers #Providers included had >100 implants. Analysis included five providers performing majority of SCS implantations (anesthesiology, pain medicine, neurosurgery, orthopedic surgery, and physical medicine and rehabilitation.
Codes utilized for procedures and pain diagnoses