Abstract
Background
Corneal xenotransplantation is an effective solution for the shortage of human donor corneas, and the porcine cornea may be a suitable candidate for the donor cornea because of its optical similarity with humans. However, it is necessary to administer additional immunosuppressants to overcome antigenic differences. We aimed to investigate the feasibility of porcine corneas with anti-CD40 antibody-mediated co-stimulation blockade in a clinically applicable pig-to-nonhuman primate corneal xenotransplantation model.
Methods
Five Chinese rhesus macaques underwent deep-lamellar corneal transplantation using clinically acceptable sized (7.5 mm diameter) porcine corneal grafts. The anti-CD40 antibody was intravenously administered on a programmed schedule. Graft survival, central corneal thickness, and intraocular pressure were evaluated. Changes in effector and memory T and B cell subsets and anti-αGal and donor-specific antibodies were investigated in the blood, and the changes in complement levels in the aqueous humor and blood were evaluated. Memory cell profiles in the anti-CD40 antibody-treated group were compared with those in from the anti-CD154 antibody-treated group or rejected controls presented in our previous report. The changes in anti-αGal, non-αGal, and donor-specific antibodies after 6 months were compared with baseline values.
Results
Anti-CD40 antibody-mediated co-stimulation blockade resulted in the successful survival of xenocorneal grafts (> 389, > 382, > 236, > 201, and > 61 days), with 80% reaching 6 months of survival. Injection of anti-CD40 antibody considerably reduced the infiltration of inflammatory cells into the grafts and significantly blocked the complement response in the aqueous humor (p = 0.0159, Mann-Whitney U test). Systemic expansion of central or effector memory T cells was abrogated in the anti-CD40 antibody-treated primates compared with those in the rejected controls (p<0.05, Mann-Whitney U test) or those in the anti-CD154 antibody-treated primates (p>0.05, Mann-Whitney U test). The levels of anti-αGal, non-αGal, and donor-specific antibodies at 6 months were not significantly increased compared with baseline levels (p > 0.05, Wilcoxon signed rank test).
Conclusions
An anti-CD40 antibody-mediated blockade appears to be effective immunosuppressive approach for porcine corneal deep lamellar xenotransplantation in primates.
Keywords: anti-CD40 antibody, cornea, deep-lamellar keratoplasty, non-human primates, xeno transplantation
Introduction
The major hurdles of xenotransplantation coming into clinical trials are Galalpha1,3Galbeta1,4GlcNAc-R (αGal) epitope-mediated rejection and porcine endogenous retroviruses (PERVs)-mediated zoonosis[1, 2]. Recent advances in xenotransplantation by development in a-1,3-galactosyltransferase gene knockout (GTKO) pigs and by development of genome-wide inactivation of PERVs using CRISPR-Cas9 takes us a step closer to commercially available xenografts and clinical trials along with the emerging evidence of the lack of transmission of PERVs in human studies[3–5]. Nevertheless, using GTKO pigs as a donor, optimized immune modulatory regimen should be still required as a critical step in xenotransplantation to achieve a successful outcome[6].
Corneal blindness is a worldwide devastating conditions, which can be cured by corneal transplantation[7]. To overcome a shortage of donor corneas in Asian countries, the use of bio-engineered regenerative cornea or xenotransplantation has been actively investigated[8–10]. Recent long-term success of porcine corneal xenografts by anti-CD154 antibody (Ab)-mediated costimulation blockade in non-human primates (NHPs) created the possibility of clinical trials in corneal xenotransplantation. However, preventing the development of thromboembolism in anti-CD154 Ab-mediated costimulation blockade remains a challenge, despite a remarkable effect on transplantation[11–14].
Accordingly, we investigated the effect of anti-CD40 Ab-mediated costimulation blockade on clinical efficacy using a pig-to-NHP deep lamellar-thickness corneal transplantation model with clinically applicable surgical procedures.
Materials and Methods
All procedures used in this study conformed to the ARVO Statement Regarding the Use of Animals in Ophthalmic and Vision Research. The primate study protocol was approved by the Research Ethics Committee at Seoul National University Hospital [IACUC No. 13-0221-C1A0(1)].
Donors and recipients
Porcine corneas were obtained from genetically unmodified, pathogen-free SNU miniature wild type (WT) pigs that had been inbred in the Xenotransplantation Research Center at Seoul National University Hospital. Five Chinese rhesus macaques were used as recipients for deep lamellar-thickness porcine corneal grafts (Table 1).
Table 1. Demographics of donors and recipients and graft survival with anti-CD40 Ab-primates.
Demographics of anti-CD40 Ab-primates are presented. Rejected controls given topical and systemic steroid or steroid with tacrolimus, are included as a reference for use in comparison of memory T cell, antibody or complement responses.
| Recipients | Donors | Immuno-suppressive Regimen | Graft Survival (days) | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Age (months) | Body Weight (kg) | ABO type | Age (months) | ABO type | |||
| Anti-CD40 1 | 140 | 6.32 | B | 28 | A | Topical prednisolone+ Subconjunctival Dexamethasone+ Intramuscular Methylprednisolone+ IVIG+ Intravenous anti-CD40 Ab | >389 |
| Anti-CD40 2 | 74 | 7.12 | A | 28 | A | >382 | |
| Anti-CD40 3 | 56 | 5.02 | AB | 30 | A | >236 | |
| Anti-CD40 4 | 57 | 5.60 | B | 28 | A | >201 | |
| Anti-CD40 5 | 57 | 4.80 | B | 28 | A | >61 | |
| Mean ± SD | 56.7±0.58 | 5.78±0.96 | 28.40±0.89 | MST: Undefined | |||
|
| |||||||
| Control 1* | 48 | 5.48 | A | 37 | A | Topical prednisolone+ Subconjunctival Dexamethasone+ Intramuscular Methylprednisolone | 28 |
| Control 2* | 54 | 4.90 | AB | 35 | A | 29 | |
| Control 3* | 137 | 4.94 | B | 31 | A | 21 | |
|
| |||||||
| Control 4 | 57 | 4.2 | AB | 42 | A | Topical prednisolone+ Subconjunctival Dexamethasone+ Intramuscular Methylprednisolone+ IVIG+Tacrolimus Intramuscular Tacrolimus | 32 |
|
| |||||||
| Mean ± SD | 74.0±42.2 | 4.88±0.45 | 36.25±3.96 | MST : 28.5 | |||
SD: standard deviation, MST: mean survival time
were adopted from previous study[19].
Orthotopic pig-to-rhesus deep lamellar corneal transplantation
The donor corneoscleral buttons had been preserved in Optisol (Chiron Ophthalmics, Irvine, California) for 1~5 days before transplantation. Anterior lamellae in donor cornea were incised 687.5- to 750-μm-thick with a 7.5-mm-diameter Barron vacuum trephine (Katena Products, Denville, NJ) and artificial anterior chamber (Moria, Pennsylvania, USA). Deep-lamellar central portions of corneas were incised from recipients’ eyes using 7.5-mm-diameter Hessburg-Barron vacuum trephines (Katena Products Inc.) and the endothelial layer was separated from the full stromal layer using a big bubble technique as previous reported[15, 16]. After removal of deep lamellar recipient stromal layer, xenografts were placed in each recipient bed and secured with 16~20 interrupted 10-0 nylon sutures (Ethicon, Somerville, NJ). At the end of the surgical procedure, a contact lens (Acuvue® Oasys™, Johnson & Johnson, Jacksonville, FL) was inserted, and eyelids were closed with 6-0 black silk for 1 day.
Postoperative local and systemic immunosuppressive regimen
Postoperatively, all the recipients received the same medications. Levofloxacin 0.5% (Cravit®, Santen Pharmaceutical, Osaka, Japan) and prednisolone acetate 1% (Pred forte®, Allergan, Irvine, CA) were topically administered once a day. Dexamethasone 1.5 mg/0.3 ml (JW Pharmaceutical, Seoul, Republic of Korea) was injected weekly subconjunctivally for up to 6 months and methylprednisolone (Solu-medrol®, Pfizer, New York, NY) was injected intramuscularly at an initial dose of 2 mg/kg/day, tapered over 5 weeks, and discontinued at a final dose of 0.25 mg/kg[17]. Intravenous immunoglobulin G (IVIG) and anti-CD 40 Ab were administered during the 6 months after transplantation. IVIG (1 g/kg) was slowly administered on day 0 and 2 weeks post-transplantation. A mouse-rhesus chimeric monoclonal anti-CD40 (2C10R4, NIH Nonhuman Primate Reagent Resource) previously described[18], was administered intravenously at a dose of 50~30 mg/kg/day on day −1, day 1, postoperative day (POD) 4, POD 7, POD 10, and POD 14, once weekly until week 4, every 2 weeks until week 12, and then every 4 weeks until week 24 (15 times in total, 50 mg/kg/day in first 8 times and 30 mg/kg/day in the remaining 7 times), similar to the time schedules reported previously[19].
Postoperative clinical evaluations
Grafts were evaluated by slit-lamp biomicroscopy, particularly for graft clarity, edema, and neovascularization. Rejection score was graded as described previously on international consensus[20]. Graft rejection in NHPs was defined based on the sum of grades for opacity, edema, and vascularization. Graft scores exceeding 6 was considered rejection. During the follow-up period, loosened, exposed, or new vessel-inducing sutures were selectively removed. Central corneal thickness (CCT) and intraocular pressure (IOP) were measured using an ultrasonic pachymeter (Quantel Medical, Clermont-Ferrand, France) and a Tono-Pen (Medtronic Solan, Jacksonville, FL), respectively. The results are the mean values of 5 measurements. For visualization for living endothelial cells, enucleated eyeballs from the sacrificed recipients were evaluated using a SP-3000 specular microscope (Topcon Medical Laser Systems, Inc., Santa Clara, CA). The changes of endothelial cell density were compared between before and 6 months after transplantation in subjects in which data of endothelial cell density of donor were available before transplantation (n=3).
Immunohistochemistry of xenografts
Corneas from sacrificed recipients were divided into two equal parts and each portion of the cornea was subjected to hematoxylin and eosin (H&E), immunohistochemical and immunofluorescent staining as described previously[19]. Immunohistochemical staining of the grafts was performed using anti-CD68 Abs (Thermo Scientific, Runcorn, United Kingdom; 1:100) and Diaminobenzidine (DAB)-conjugated secondary antibody (Roche, Mannheim, Germany) for visualization. Immunofluorescence staining of the grafts was performed using (1) anti-CD3 Abs (Abcam, Cambridge, USA, 1:200) and goat anti rabbit IgG-FITC (SouthernBiotech, Birmingham, USA, 1:500) as well as anti-CD8 Abs (Abcam, Cambridge, USA; 1:150) and goat anti mouse IgG-Cy3 (Millipore, California, USA, 1:500) for CD3+CD8+ T cells; (2) anti-CD3 Abs (Abcam, Cambridge, USA, 1:200), goat anti rabbit IgG-Cy3 (Millipore, California, USA, 1:200) and anti-CD4-alexa fluor 488 conjugated Abs (Novusbio, Littleton, USA; 1:50) for CD3+CD4+ T cells; and (3) anti-CD3 Abs (Abcam, Cambridge, USA, 1:200) and goat anti rabbit IgG-FITC (SouthernBiotech, Birmingham, USA, 1:500) as well as anti-CD20 Abs (ebioscience, San Diego, USA; 1:100) and goat anti mouse IgG-Cy3 (Millipore, California, USA, 1:500) for CD3−CD20+ B cells. Counter nuclear staining was done using DAPI (4′,6-diamidino-2-phenylindole). To investigate the distribution of αGal epitopes, the Griffonia simplifolia I isolectin B4 (GSIB4; Molecular Probes, Eugene, OR) conjugated with Alexa fluor 488 (I-21411; Molecular Probes) was reacted with the corneal tissue as described previously [21]. To confirm the deposition of IgG Abs in the corneal tissues, fluorescein isothiocyanate (FITC) polyclonal goat anti-monkey IgG (Fc specific) Abs (AP31438FC-N; 1:20, Acris Antibodies Inc., San Diego, CA) were used, per the manufacturer’s protocol. To confirm the deposition of complement C3c in the corneal tissues, polyclonal rabbit anti-human C3c Abs (A0062; 1:100, DAKO) were used as a primary Ab and Alexa Fluor 488 goat anti-rabbit IgG Abs (A11304; 1:500, Life technologies, Grand Island, NY) were used as the secondary Ab. The nuclei of cells were then counterstained with Hoechst33342 and the distribution of IgG Ab and complement C3c was investigated with Venoz AHBT3/Q fluorescent microscopy (Olympus, Tokyo, Japan).
Memory T cell assay
Postoperative changes in memory T-cell sub-populations (CD28+CD95+ central memory and CD28−CD95+ effector memory) were evaluated by flow cytometry analysis of whole blood and draining lymph nodes as previously mentioned[19]. Data were acquired using a FACSCanto flow cytometer (Becton-Dickinson, Mountain View, CA) and data analysis was carried out using FlowJo software (Tree Star, Ashland, OR). The changes of memory T cell expansion of the blood over time in anti-CD40 Ab-treated primates (n=5) were compared to those in anti-CD154 Ab-treated primates (n=4) with porcine corneal grafts which survived successfully as previous described (as a reference) to confirm whether the T cell responses are effectively controlled in tolerable range [17, 19]. In addition, mean percentages and numbers of effector or central memory T cells in draining lymph nodes were compared between anti-CD40 Abs-treated primates (n=4) and rejected controls (n=2, Table 1) that were given topical and systemic steroid after keratoplasty, using a previous study as the reference[19]. The lymph nodes of anti-CD40 treated primates were collected after 6 months and those of the rejected controls in which lymph nodes had been available were collected after 1months on sacrifice.
Anti-αGal and anti-non-αGal antibodies assay
Plasma levels of anti-αGal IgG and IgM Abs were measured by ELISA as described previously[22, 23]. In brief, mean absorbance of anti-αGal Abs was expressed as artificial units (AU)/mL, and was compared with that of a calibrator. As a calibrator, serial dilutions of a selected rhesus monkey plasma were designated as 2,809 AU/mL of anti-αGal IgG and 5,610 AU/mL of anti-αGal IgM. Anti-αGal Ig G Ab or anti-non-αGal Ig G Ab binding responses were assessed by flow cytometry using a GTKO porcine endothelial cell lines (PEC69), and for supplemental purposes, WT porcine endothelial cells (MPN) incubated with 1/10 diluted plasma samples[22]. The level of antibody binding was determined as the net mean fluorescence intensity (nMFI). nMFI values were calculated by subtracting MFI values of the porcine plasma (negative control) from MFI values of the sample. The plasma concentration or MFI were measured every 2 weeks up to 4 months, and thereafter every 1 month until sacrifice. Mean MFI levels and plasma concentration of anti-αGal IgG and IgM Abs or anti-non-αGal Ig G Ab at 6 months were compared with those prior to treatment (n=4).
Donor pig-specific antibodies assay
Plasma concentrations of donor pig-specific IgM/IgG Abs were determined by the flow cross-match technique as described previously[19] using donor peripheral blood mononuclear cells (PBMCs) as targeting cells. Concentrations of donor pig-specific Abs were semi-quantitatively expressed as the MFI. MFIs of donor serum were used as negative controls and net MFI (nMFI) values were calculated by subtracting donor MFI values from respective sample MFI values. The plasma concentration or MFI were measured every 2 weeks up to 4 months, and thereafter every 1 month until sacrifice. Mean MFI levels of donor-specific IgG and IgM Abs at 6 months were compared with those prior to treatment (n=4).
Complement assay in aqueous humor
Concentration of C3a in the aqueous humor was evaluated by commercial ELISA kits (BD OptEIA™ Human C3a ELISA Kit; BD Biosciences, San Diego, CA) according to the manufacturer’s protocols prior to transplantation and 1 months, 3months and 6 months after transplantation.
The changes of complement in anti-CD40 Abs treated primates (n=5) at 1 month were compared to those in controls with rejected grafts (n=4, Table 1) that were given the topical and systemic steroid in previous study [19] or topical and systemic steroid combined with tacrolimus and IVIG after keratoplasty as a reference. The changes of complement in anti-CD40 Abs treated primates at 6 month were also compared with those prior to surgery (n=4).
Statistical analyses
GraphPad Software (GraphPad Prism, Inc., La Jolla, CA, USA) was used for statistical tests. Mean values of cytokines and C3a concentration in the aqueous humour were compared between anti-CD40 Abs-treated primates and rejected controls using the Mann-Whitney U test. Mean percentages or numbers of memory T cells in draining lymph nodes or blood samples were also compared between anti-CD40 Abs-treated primates and rejected controls or between anti-CD40 Abs-treated primates and anti-CD 154 Abs-treated primates using the Mann-Whitney U test. The changes in anti-αGal IgG and IgM Abs or donor-specific antibodies, the changes in complement and corneal endothelial cell density were compared between the pre-operative state and 24 weeks after surgery using the Wilcoxon signed rank test. Data are presented as mean ± standard error (SE) except demographics of donors and recipients. Statistical significance was accepted for p values <0.05.
Results
Clinical outcomes
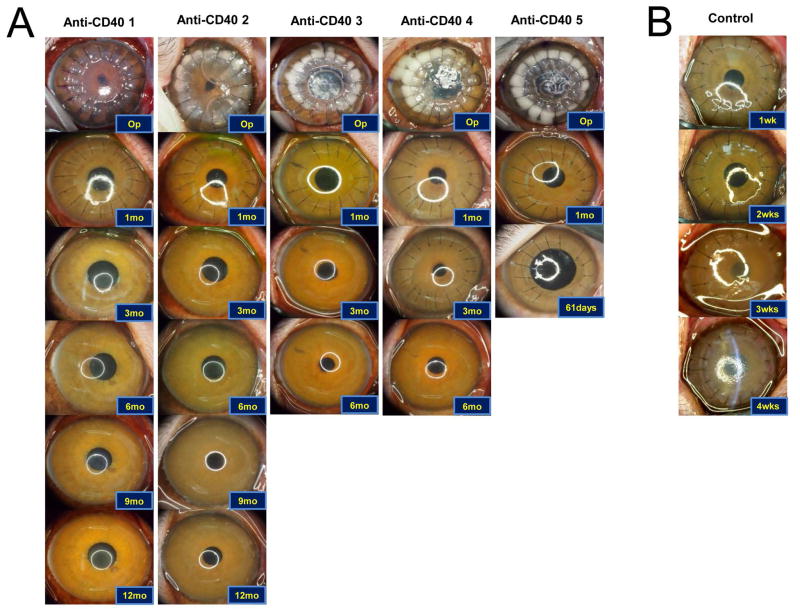
All recipients had transparent xenocorneal grafts longer than 6 months after the transplantation except one that died due to anesthetic accident at 2 months (A clear graft was evident until death). In addition, two recipients (anti-CD40 1 and anti-CD40 2) showed successful survival longer than a year without any signs of rejection (Figure 1). No corneal neovascularization was observed in all primates.
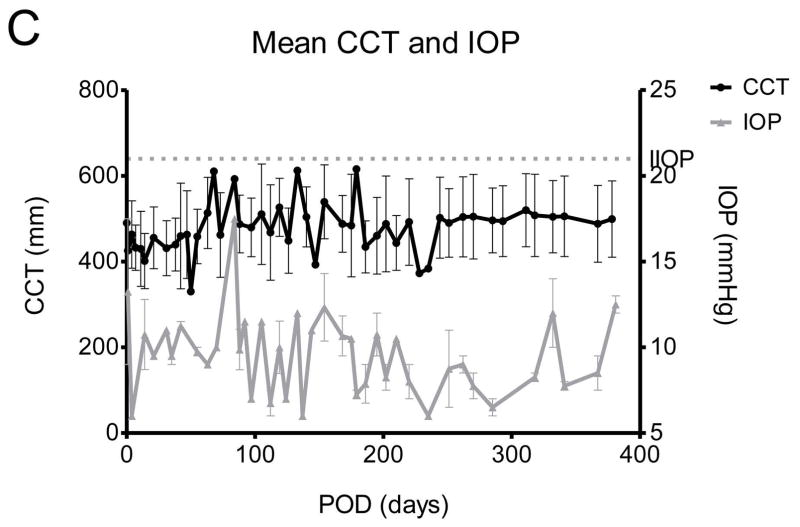
Figure 1. Representative photographs of grafted eyes showing clinical courses, and changes of central corneal thickness and intraocular pressure in deep lamellar porcine keratoplasty in nonhuman primates.
(A) All grafts in the anti-CD40 Abs treated primates survived at least 6 months except one which accidently died due to anesthetic event at POD 61. The primate also showed clear graft until death. Two of the grafted primates showed more than 1year survival. (B) Representative photos of the control showing rejection within 4 weeks. (C) Central corneal thickness was well maintained under 600 μm and increase of intraocular pressure was not observed during the follow-up.
The MST of the anti-CD40 Ab group ((n=5); >389, >382, >236, >201, and >61 days; Table 1) could not to be defined because all xenocorneal grafts of five recipients had survived throughout the whole study. The international guideline for a clinical trial of xenocorneal transplantation demands at least 6 months-survival of 5 in a consecutive 8 NHPs to prove an efficacy as a pre-clinical data [24]. For the welfare and animal-rights of NHPs, we tried to use minimal quantity of NHPs as possible as we could. Unfortunately, a primate had been dead during the anesthetic recovery at 2 months because the neck was accidentally flexed. However, we did not use additional NHPs, because in 5 consecutive NHPs, we did not experience any rejection at all. Although the graft had been still survived after 6 months in remaining NHPs, we had to sacrifice them due to limitation of the funding. The CCT was well maintained under 600 μm throughout the follow-up period and IOPs were not increased (Figure 1C), and the density and shape of endothelial cells were well maintained in all recipients without significant reduction (p>0.05, Wilcoxon signed rank test, Supplementary Figures S1). Anterior segment optical coherence tomographic images of the enucleated eyes showed a well-adapted donor-recipient junction and topography showed tolerable range of the refractive diopter and astigmatism (Supplementary Figure S1).
Neither ocular nor systemic adverse events including side effects of anti-CD40 Ab or CMV infection due to long-term immunosuppressive therapy were observed in any recipients (Supplementary Figure S2 and S3).
Cellular infiltration into the grafted porcine cornea
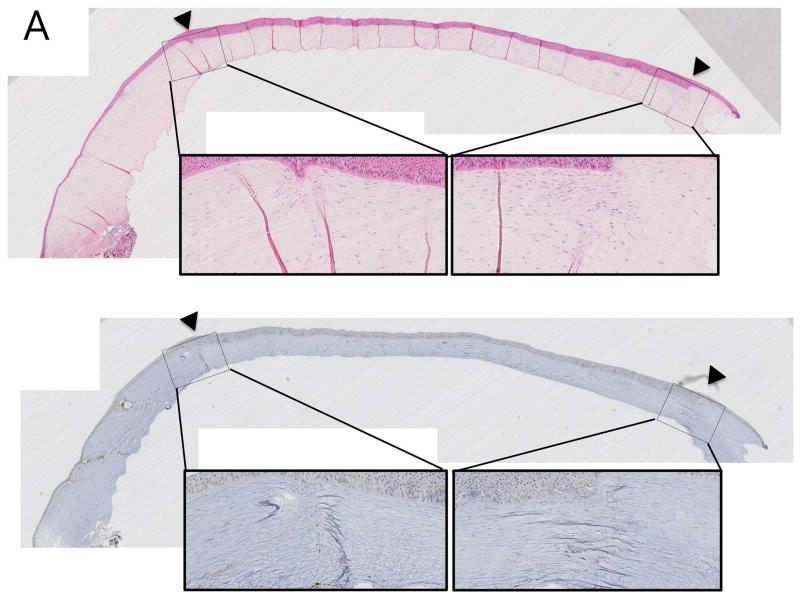
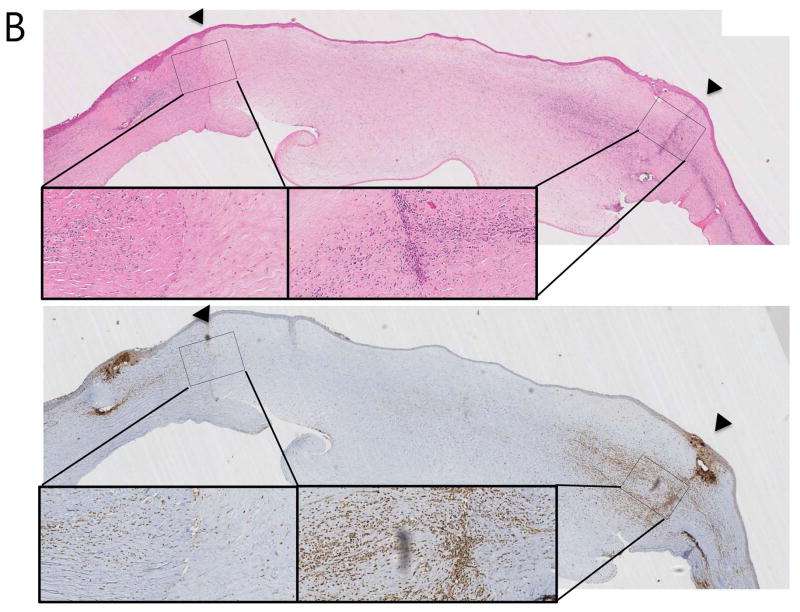
In H&E stains, all recipients with anti-CD40 Abs treatment showed clear grafts with a few inflammatory cells confined in the donor-recipient junction (Figure 2A), while rejected control showed severe edema accompanying by abundant inflammatory cellular infiltration (Figure 2B). In immunohistochemical and immunofluorescent staining, CD3+CD4+ T cells, CD3+CD8+ T cells, CD3−CD20+ B cells and CD68+ macrophages were scarcely found in the surviving grafts (Figure 2A and Figure 3A; upper panel), while infiltration of those cells was abundantly observed in rejected grafts, especially in graft-host junction (Figures 2B and 3B; lower panel).
Figure 2. Representative haematoxylin & eosin (H&E) and immunohistochemical staining (IHC) of porcine corneal grafts in anti-CD40 Ab-treated primate.
(A) Staining of a surviving graft showed less inflammatory cell infiltration without any graft edema (Top; H&E, bottom; IHC using anti-CD 68 Ab; arrowheads indicate donor-recipient junction; x40 and x200). (B) Staining of a rejected graft in a primate without anti-CD40 Ab treatment (control, steroid treated primate) showed severe edema and inflammatory cell infiltration around the donor-recipient junction (Top; H&E, bottom; IHC using anti-CD 68 Ab; arrowheads indicate the donor-recipient junction; magnification x40 and x200).
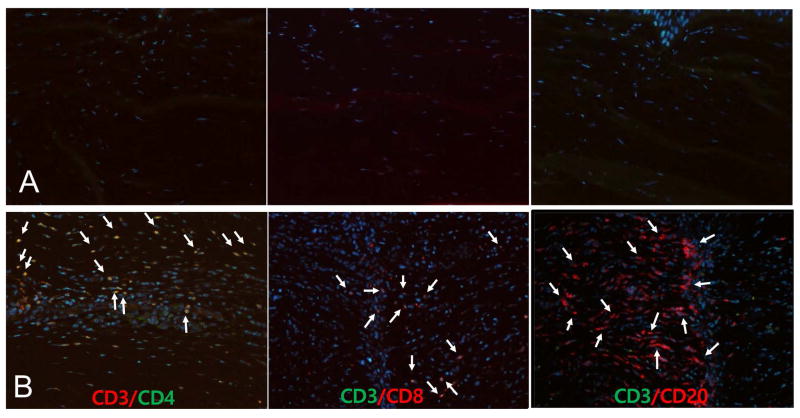
Figure 3. Immunofluorescent staining for inflammatory cells infiltrating into the porcine corneal graft in anti-CD40 Ab-treated primates.
A surviving graft in an anti-CD40 Ab-treated primate (A; upper panel, magnification x200) showed few cells, while a rejected graft (B; lower panel, control; magnification x200) showed significant infiltration of CD3+CD4+ (CD3;red/ CD4;green double staining) and CD3+CD8+ (CD3;green/ CD8;red double staining) T cells as well as CD3−CD20+ (CD3;green/CD20;red) B cells (white arrows).
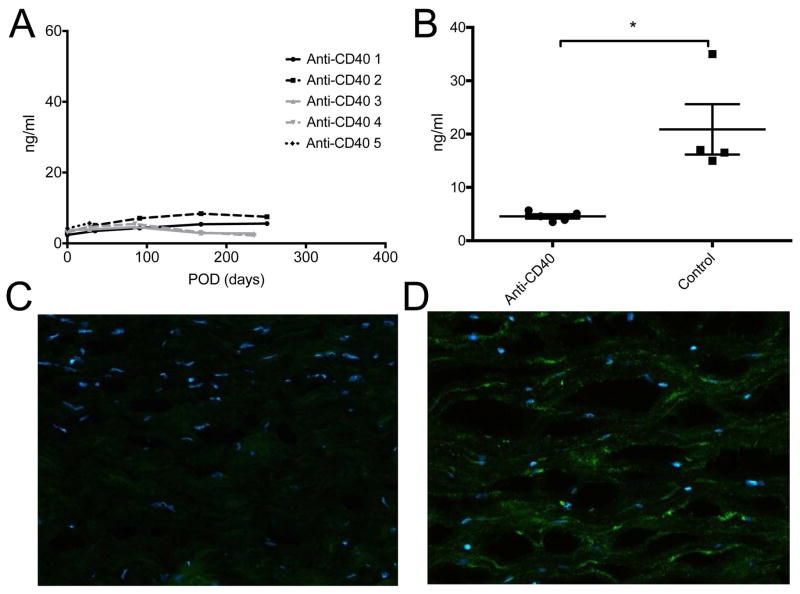
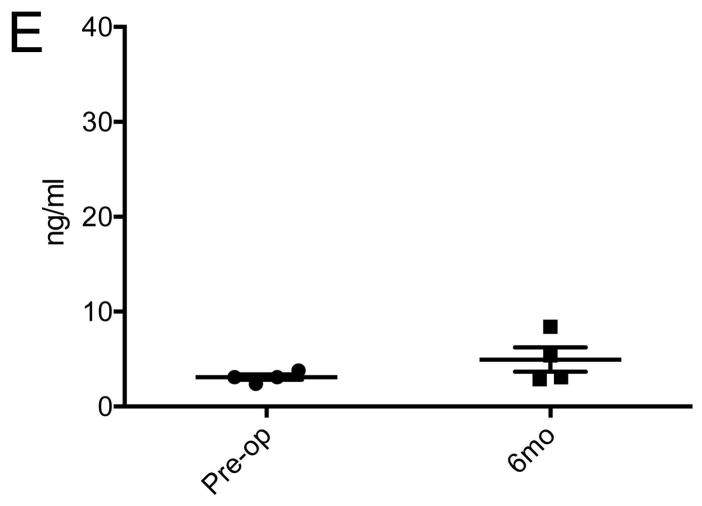
Plasma donor pig-specific antibody assay and IgG deposition in porcine corneal grafts
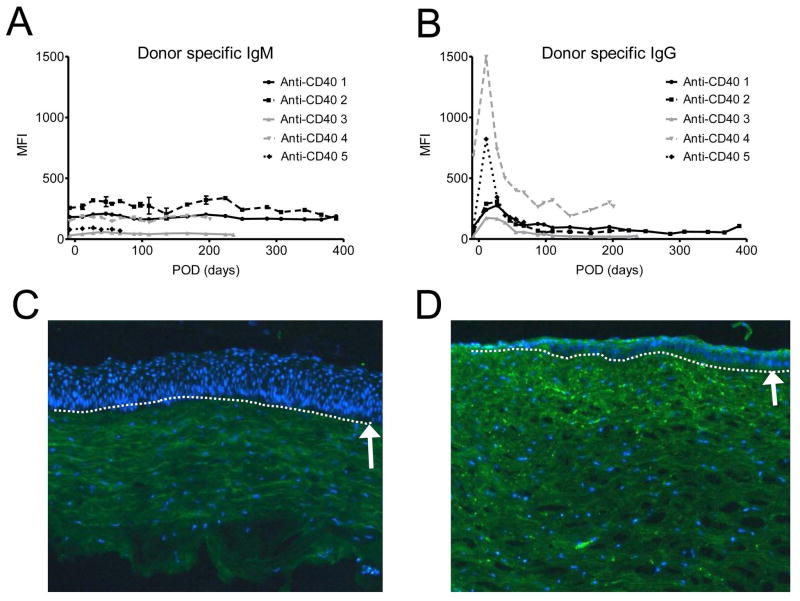
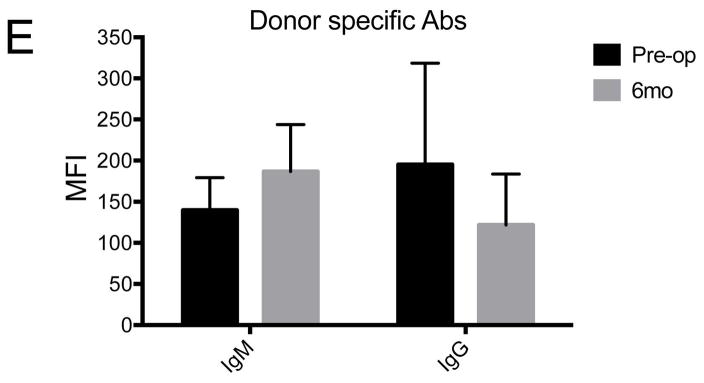
The level of anti-donor specific Ig M and Ig G Ab were not changed throughout the follow-up period (Figures 4A, B and E) and differences were not statistically significant between the baseline and 6 month post-operative time point (p>0.05, Wilcoxon signed rank test). Transient peak of Ig G Abs in early post-operative period may be caused by non-specific bindings of previously existing antibodies in IVIG (supplementary Figure S4 A). Immunofluorescent staining showed IgG deposits were barely observed in the graft with anti-CD40 Ab-treatment (Figure 4C), while deposits were densely found in the rejected graft in the positive control (Figure 4D).
Figure 4. Changes in plasma donor pig-specific antibodies and Ig G deposition in porcine corneal grafts with anti-CD40 Ab-treated primates.
Few changes were observed in donor pig-specific Ig M (A) and Ig G (B) antibodies in anti-CD40 Ab-treated primates during the follow-up. Initial peak of anti-Ig G in early postoperative days was probably caused by intravenous immunoglobulin treatment. Representative figure of anti-CD40 Ab-treated primate showed little Ig G deposition in the stroma (C), while representative figure of the rejected control showed dense IgG deposition mainly in the stroma (D) (X100). Rejected graft showed relatively thin epithelial layers. Dash lines and white arrows indicate epidermal-dermal junction. (E) Compared with basal levels, the MFI level of donor pig-specific Ig M and Ig G antibodies was not significantly high at 6 months (p>0.05, Wilcoxon signed rank test; n=4). “Pre-op” indicates pre-operative baseline data, and “6 mo” indicates data at 6 months post-transplant.
Plasma anti-Gal antibody assay and αGal expression in porcine corneal grafts
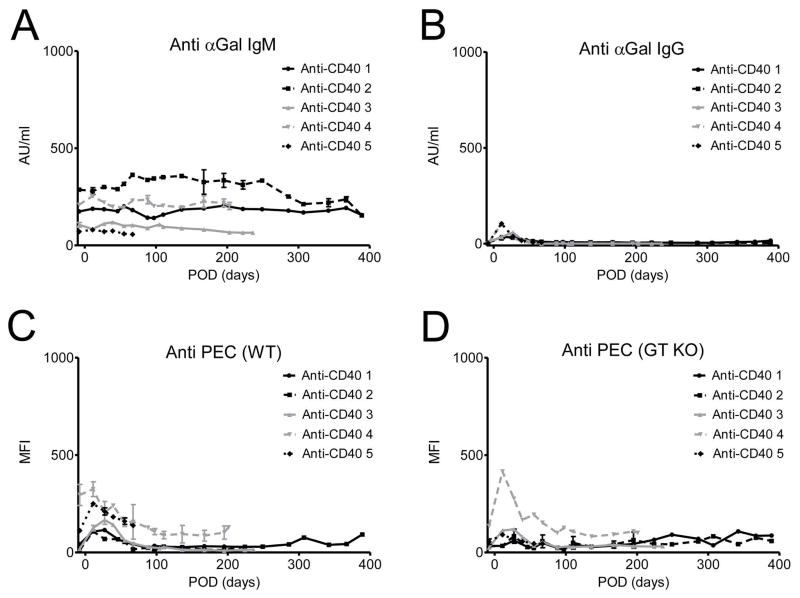
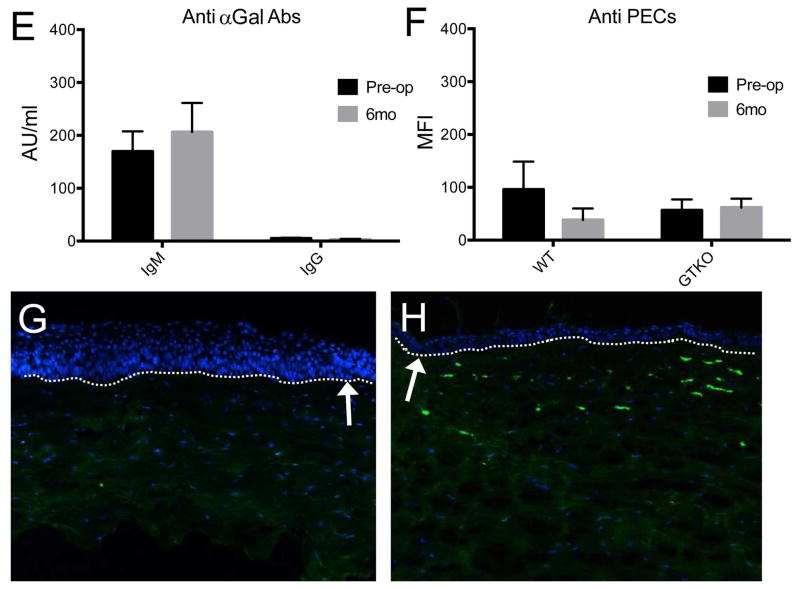
The level of anti-αGal IgM Ab was not increased throughout the study period (Figure 5A, E). The level of anti-αGal IgG Ab showed subtle increase only in early postoperative period due to initial treatment of IVIG, and this peak had no correlation with donor-specific responses (supplementary figure S4). Thereafter, there was no increase of the level of anti αGal IgG Ab through the follow-up, based on baseline verses 6 month values (Figure 5B and E; p>0.05, Wilcoxon signed rank test). There were no significant changes in MFI values in the plasma samples against wild type porcine endothelial cells (PEC; Figure 5C, F; p>0.05, Wilcoxon signed rank test) or against GTKO PEC (Figure 5D) at 6 months compared to baseline (Figure 5F; p>0.05, Wilcoxon signed rank test), suggesting no significant induction of anti-αGal or anti-non-αGal antibodies in the recipients. In immunofluorescent staining, anti-CD40 Ab-treated primates showed few αGal-expressing cells in the graft (Figure 5G), while the rejected control showed dense αGal-expressing cells (Figure 5H).
Figure 5. Changes in plasma anti-αGal antibodies or anti-non αGal antibodies and αGal expression in porcine corneal grafts with anti-CD40 Ab-treated primates.
No significant changes were observed in IgM (A) and IgG (B) anti-αGal antibodies during the follow-up period (E; baseline vs 6 months, p>0.05, Wilcoxon signed rank test; n=4). “Pre-op” indicates pre-operative baseline data, and “6 mo” indicates data at 6 months post-transplant. There were no significant changes in the MFI values of plasma samples against wild-type porcine endothelial cells (PEC; C) or against GTKO PEC (D) during the follow-up period compared to the pre-transplantation level (F; baseline vs 6 months, p>0.05, Wilcoxon signed rank test), suggesting no significant induction of anti-αGal or anti-non-αGal antibodies in the recipients. Representative figure of anti-CD40 Abs treated primate showed few αGal expressions in the stroma (G), while representative figure of the rejected control showed dense αGal expression (H) (X100). Dash lines and white arrows indicate epidermal-dermal junction.
Aqueous complement C3a assay and complement C3c deposition in porcine corneal grafts
There was no clinically relevant increase in C3a concentration in aqueous humor at 6 months compared with baseline level (Figure 6A, E; p>0.05, Wilcoxon signed rank test). At 4 weeks after transplantation, the mean aqueous C3a concentration was significantly lower in anti-CD40 Abs treated primates than that in rejected controls (p=0.0159, Mann-Whitney U test; Figure 6B). The grafts of the anti-CD40 Ab-treated primates did not show a deposition of C3c in the graft (Figure 6C), while rejected graft of the control showed pronounced deposition of C3c in the graft (Figure 6D).
Figure 6. Changes in concentration of aqueous humor C3a and C3c deposition in porcine corneal grafts in anti-CD40 Ab-treated primates.
(A, E) C3a concentration was not increased in the aqueous humor at 6 months compared to baseline levels (p>0.05, Wilcoxon signed rank test). (B) At 4 weeks post-transplantation, C3a concentration in anti-CD40 Ab-treated primates (n=5) was significantly lower compared with that in rejected controls (n=4; *p=0.0159, Mann-Whitney U test). C3c deposits were barely observed in the graft with anti-CD40 Ab-treated primate (C), while extensive C3c deposition were observed in the rejected graft of the control (D; X200).
Memory T cell changes in whole blood and draining lymph nodes
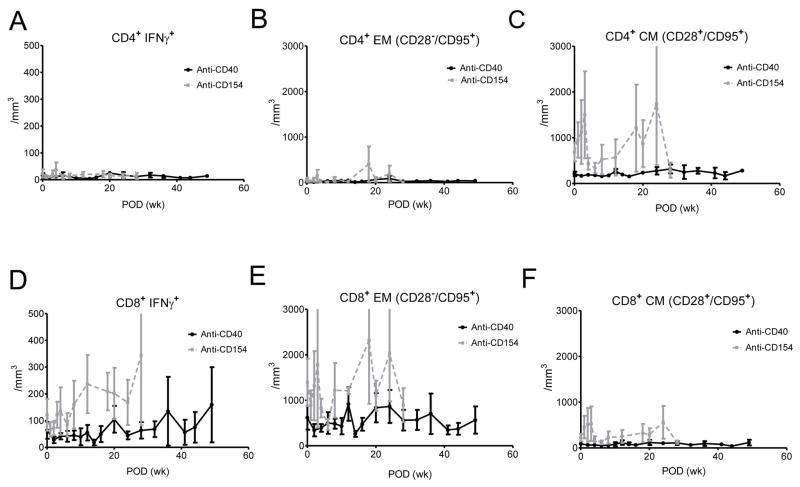
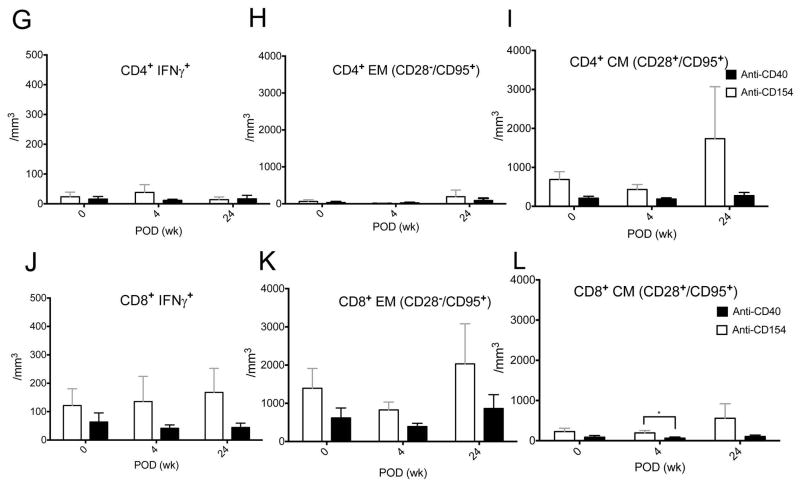
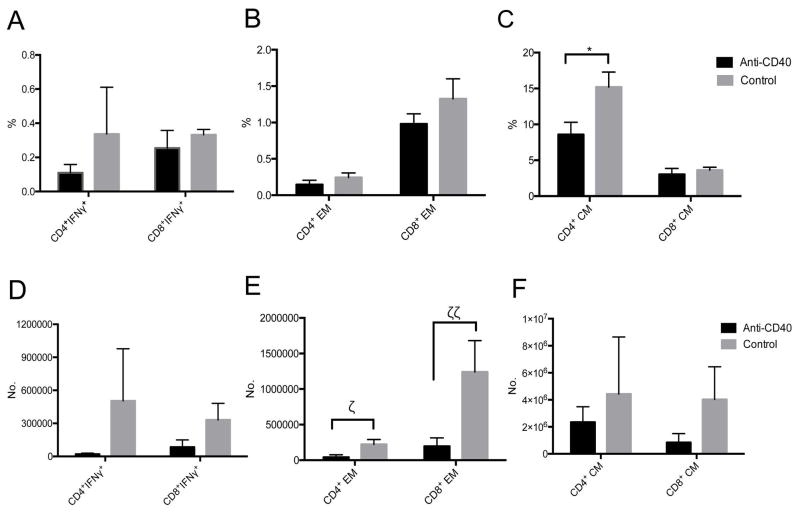
The level of interferon-gamma (IFNγ) secreting CD4+ and CD8+ T cells, effector memory (CD28−CD95+) and central memory (CD28+CD95+) T cells were not higher than those in preoperative states throughout the follow-up (Figure 7A–F). When we compared the IFNγ+ T cells or memory and effector CD4+ and CD8+ T cells between the baseline and 6 months, there was no statistical significant increase over time in anti-CD40 treated primates (Figure 7G–L; p>0.05, Wilcoxon signed rank test). When we compared concentrations of those memory T cells at baseline, 1 month, and 6 months between anti-CD40 treated and anti-CD154 treated primates which showed effective suppression of the clinical rejection, the concentration of CD8+ central memory T cells was significantly lower in anti-CD 40 treated primates at 1 month (Figure 7L; p=0.0317, Mann-Whitney U test). The other memory T cell concentrations tend to be lower without statistical significance, (Figure 7G–K; p>0.05, Mann-Whitney U test). In the draining lymph nodes, mean percentage of central memory (CD28+CD95+) CD4+ T cells was lower in anti-CD40 Ab-treated primates than in rejected controls with a marginal significance (p=0.082, Mann-Whitney U test; Figure 8C). The number of effector memory (CD28−CD95+) CD4+ and CD8+ T cells were lower in anti-CD40 Ab-treated primates than in rejected controls (p=0.055, p=0.032, Mann-Whitney U test; Figure 8E). The changes of IFNγ+ CD4+ and IFNγ+ CD8+ T cells tended to decrease with treatment, but were not statistically significant (Figure 8A, D).
Figure 7. Memory T cell changes in blood of anti-CD40 Ab-treated primates after transplantation.
(A–F) The changes of IFNγ+CD4+ or IFNγ+CD8+ T cells, effector (CD28−CD95+) memory CD4+ and CD8+ T cells, or central (CD28+CD95+) memory CD4+ and CD8+ T cells (black line) were not higher than those of preoperative states throughout the follow-up (baseline vs 6 months; p>0.05, Wilcoxon signed rank test; n=4) (G-K) The level of IFNγ+CD4+ or IFNγ+CD8+ T cells, effector/central memory CD4+ and effector memory CD8+ T cells were not different between anti-CD40 treated and anti-CD154 treated primates at baseline, at 1 month, and at 6 months after transplantation (p>0.05, Mann-Whitney U test). (L) The level of CD8+ central memory T cells was significantly lower in anti-CD 40 treated primates at 1 month after transplantation (p=0.0317, Mann-Whitney U test). CM indicates central memory and EM indicates effector memory.
Figure 8. Comparative analysis of memory T cell changes in draining lymph nodes between anti-CD40 Ab-treated primates and rejected controls.
The mean percentage of central memory(CD28+CD95+) CD4+ T cells was lower in anti-CD40 Ab-treated primates (n=4) than in rejected controls (n=2) with a marginal significance (*p=0.082, Mann-Whitney U test; C). The number of effector memory (CD28−CD95+) CD4+ and CD8+ T cells were significantly lower in anti-CD40 Ab-treated primates than in rejected controls (ζp=0.055, ζζp=0.032, Mann-Whitney U test; E). The changes of IFNγ+ CD4+ T cells was not significant. All data were analyzed after sacrifice (Anti-CD 40 treated primates > 6 months, rejected control > 1month)
Discussion
Our data indicate that anti-CD40 monoclonal antibody (2C10R4) mediated-costimulation blockade effectively suppresses xenograft-related immune reaction and promotes long-term survival of deep-lamellar porcine corneal grafts in rhesus monkeys. Blockade of CD40/CD154 costimulatory pathway has emerged as a promising therapeutic modality in allotransplantation, xenotransplantation as well as autoimmune disease models[14, 25–27]. Although the treatment with anti-CD154 Ab has proven effective in xenotransplantation[28, 29], anti-CD154 Ab has yielded to antagonistic anti-CD40 Ab in the field of transplantation because of the thrombotic complications[11]. Despite a prior demonstration of the comparable xenograft survival outcome following the use of anti-CD154 Ab and anti-CD40 Ab[27], efficacy of anti-CD40 Ab-mediated blockage in various xenotransplantation model has not been clearly established. Compared with the survivals of cellular lamellar keratoplasty using steroids in the other reports (21days-398days)[10], the survival of the cellular lamellar graft with anti-CD40 ab treatment seems to be pretty effective on corneal xenotransplantation. Most of the study achieved 0 to 60% of the 6 months-survival, while our study achieved 80% of the 6 months-survival.
The expression of CD40 or CD154 (CD40L) varies depending on the cells[13, 14, 30]. CD40 is constitutively expressed on B cells, macrophages, dendritic cells (DCs), monocytes, follicular dendritic cells, eosinophils and platelets, as well as on non-hematopoietic cells such as myofibroblasts, fibroblasts, epithelial, and endothelial cells. In addition, CD40 is transiently expressed on activated CD8+ T cells. Meanwhile, CD154 inducibly expresses mainly on activated CD4+ T cells, activated B cells, activated CD8+ and γδ T-cells, and platelets. Under inflammatory conditions, CD154 is also transiently induced on monocytes, natural killer cells, mast cells, and basophils and endothelial cells following activation as well as non-hematopoietic cells including epithelial and vascular endothelial cells, and smooth muscle cells. Therefore, anti-CD40 Ab-mediated blockage appears to have no direct effect on the activated CD4+ T cells unlike the anti-CD154 Ab-mediated blockage, presumably resulting in different efficacy on xeno-transplantation model.
From our data, anti-CD 40 Ab treatment combined with IV IG showed effective down-regulation of donor-specific Ab responses, anti-αGal, as well as anti-non αGal Ab responses, complement changes and the responses of effector memory T cells, resulting in excellent survival of xenografts. We found initial peak of Ig G responses which did not correlate with clinical progress. When we measured in vivo IgG reactivity against non-donor third party PBMCs using plasma from the primates given anti-CD40 Abs and IVIG (n=5), a similar spike was observed in early post-operative period (Supplementary Figure S4 A). The increased Ig G binding against porcine PBMCs was also observed when mixed with plasma of a naïve primate in vitro after addition of IV IG (Supplementary Figure S4 B). Plasma levels of anti-αGal IgG Abs in a naïve primate or a human were also increased in a dose-dependent manner after in vitro application of IVIGs (Supplementary Figure S4 C). In addition, a certain amount of anti-αGal IgG exists in IVIG agents. Taken together, these findings suggest that initial high peak of donor-specific Ig G or anti-αGal level is presumably caused by administration of IVIG.
The level of IFNγ+CD4+/ IFNγ+CD8+ T cells, effector memory and central memory T cells in the PBMCs were not higher than those between baseline and 6 months. It can be assumed that down-regulated dendritic cell activation by blockage of anti-CD40 Abs may contribute to reduced activation of CD4+ T cells. Considering that deep lamellar keratoplasty without endothelial transplantation shows lower antigenicity than the full thickness keratoplasty, direct comparison of the efficacy between the anti-CD154 Ab and the anti-CD40 Ab is not possible in different keratoplasty settings. We compared the changes of T cells between the groups in order to use the data of anti-CD154 treated group as a reference level (negative control) to clarify the range that is tolerable for the T cell changes in successful survival. Compared to previous study with anti-CD154 Ab-treated primates, in which graft survival was excellent[19], no significant changes in effector or central memory T cells were found between anti-CD40 and anti-CD154 Ab treatment, except for changes in CD8+ central memory T cells, which were lower in anti-CD40 abs-treated primates. This indicates that all of the changes in effector and memory T cells in PBMCs appears to be within clinically tolerable ranges, which corresponds well with other clinical data. In addition, the fact that the numbers of effector memory CD4+ and CD8+ T cells were significantly lower in the lymph nodes of anti-CD40 Ab-treated primates than in rejected controls suggests the effective control of the effector memory T cell population by anti-CD40 Abs. We used mouse-rhesus chimeric form of 2C10R4 (Ig G4 isotype) as antagonistic anti-CD40 Abs.
This anti-CD40 Ab-mediated inhibition works well in vitro and as corroborated by our in vivo models[31]. In our study, high dosage of anti-CD40 (50mg/kg) was initially administered for 8 times, and subsequently it was switched to low dosage administration (30mg/kg) for 7 times during 6 months, which worked efficiently. Although, human and rhesus CD40 share 95% homology of amino acid molecules, an in vitro study revealed lower inhibitory action of anti-CD40 Ab (clone of 2C10R4) against proliferation of human PBMCs compared with that against the proliferation of rhesus PBMCs[31]. Therefore, adjustment of the administration may be required in human clinical trials in dosages and frequencies to use anti-CD 40 Abs from the Ig G4 isotype clone, or for human usage, different isotype Ig G should be selected to generate anti-CD40 Abs.
One of the advantages of anti-CD40 from 2C10 appears to induce minimal depletion of B cells, although it effectively inhibited donor-specific and anti-α Gal responses. This property would be important to defense against viral infection under immune suppression after transplantation. In fact, our data (Supplementary Figure S2) revealed no clinically relevant activation of cytomegalovirus (CMV) infection. The antibody titers were subsequently increased which corresponded with the increase of the viral titer, resulting in no clinical activation of CMV. In addition, there were no considerable weight loss, fever, alanine aminotransferase changes, and blood urea nitrogen/creatinine changes in the anti-CD40 treatment (Supplementary Figure S3). All recipients showed weight loss during early postoperative period (average 10.2% loss from the initial weight for 2 weeks; 12.7%, 15.4%, 6.4%, 9.9, and 6.5%), however, they recovered the weight back to normal after 2 weeks of transplantation.
Notably, we found no expression of αGal in surviving graft; by contrast, abundant expression of αGal was found in rejected grafts which corresponds with our previous studies[19, 21]. Although we do not know the exact reason why rejected grafts or grafts in the early post-operative period express αGal abundantly, it can be presumed that damaged or necrotic cells may expose more αGal on the cell surface due to conformational changes that are induced by attack from donor-specific immune cells or surgically induced inflammation. We presume that the reason why surviving grafts do not show any expression of αGals is because donor keratocytes may be slowly replaced in the anterior stromal grafts that have survived at least 6 months by recipient keratocytes from peripheral host tissues.
In conclusion, anti-CD40 Ab-mediated costimulatory blockage works well in deep lamellar keratoplasty in NHPs. The data are a necessary prelude to clinical trials. Currently, we are investigating the efficacy of anti-CD40-mediated blockage in full thickness xenocorneal transplantation using SNU miniature WT pigs in NHPs based on this study.
Supplementary Material
(A) Endothelial cell density (ECD; CD) was well maintained showing 3401/mm2, accompanying by normal range of coefficient of variance (CV; 26) and hexagonality (HEX; 72). (B) Average changes of endothelial cell density in grafted primates after sacrifice with at least 6 months-survival were not significantly different compared to basal levels (mean± standard error; p>0.05, Wilcoxon signed rank test). The primates in which both pre- and post-operative ECD had been available were only included (n=3). (C) Anterior segment optical coherent tomography demonstrated well approximated wound and normal depth of anterior chamber. (D) Topography showed simulated K astigmatism of 7.0 diopter.
The copy numbers of Rhesus CMV (RhCMV, black line) DNA in plasma were determined by quantitative real-time PCR, and the antibody was titrated by immunofluorescence assay. Incidental increase of RhCMV copy number in early postoperative period was observed in anti-CD 40 treated primate 1 (anti-CD40 1, A), followed by subsequent increase of anti-RhCMV antibody responses, resulting in decrease of the copy number during the follow-up. Clinical signs of viremia were never observed. In all the other primates (B–D), anti-RhCMV antibody responses (gray dot line) consistently increased upon increase of the copy number of RhCMV, resulting in no clinically relevant increase of the copy number. It suggests that an intact humoral immune response to CMV reactivation. CMV, cytomegalovirus; FITC, fluorescein isothiocyanate; PCR, polymerase chain reaction.
No clinically significant changes were observed in weight loss, fever, hemoglobin, WBC, alanine aminotransferase changes, and creatinine level.
(A) The in vivo IgG binding responses to 3rd party PBMCs were assessed over time by flow cytometry using plasma of anti-CD40 Ab-treated primates (n=5) given IVIG infusion on day 0 and 2 weeks post-transplantation. Non-specific IgG binding against 3rd party PBMCs was observed during the early post-operative period, possibly due to infusion of IVIG that may already contain IgG Abs against porcine PBMCs. Data are presented as mean ±standard errors from 5 subjects. (B) The in vitro IgG binding responses against porcine PBMCs were assessed by flow cytometry using plasma of a naïve primate when mixed with IVIG agents from different lot numbers (#1, #2). Non-specific IgG binding against porcine PBMCs was also observed in a dose-dependent manner after addition of IVIG into the plasma sample. (C) The levels of anti-αGal IgG Abs were measured by ELISA in IVIG agents from different lot numbers (#1, #2) and in plasma of a naïve primate mixed with these IVIG lots. A detectable amount of basal anti-αGal IgG was found to exist in the IVIG formulations. The addition of IVIG into the naïve monkey plasma elevated the level of anti-αGal IgG in a dose-dependent manner. (PBMCs=peripheral blood mononuclear cells, MP = monkey plasma and HSA = human serum albumin, w/=with)
Acknowledgments
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health & Welfare, Republic of Korea (Project No. HI13C0954). Anti-CD40 antibodies used in this study were provided by the Nonhuman Primate Reagent Resource supported by U.S. National Institutes of Health NIAID contract HHSN 272201300031C and grant OD010976. We thank Dr. Shuji Miyagawa in Osaka University for providing the GTKO porcine endothelial cell line (PEC69).
Abbreviations
- GTKO
a-1,3-galactosyltransferase gene knockout
- Ab
antibody
- CCT
central corneal thickness
- αGal
Galalpha1,3Galbeta1,4GlcNAc-R
- IFN
interferon
- IOP
intraocular pressure
- IVIG
intravenous immunoglobulin
- MFI
mean fluorescence intensity
- MST
median survival time
- NHP
non-human primate
- PBMC
peripheral blood mononuclear cell
- PERVs
porcine endogenous retroviruses
- POD
postoperative day
- WT
wild type
Footnotes
Authors contributions: MKK contributed to study design. HJC contributed to operation. JK, DHK, MKK, HJL, and WRW contributed to acquisition and interpretation of data. HJK contributed to antibody and complement assay. CP contributed to management of nonhuman primate facility and EH contributed to CMV assay. All authors helped to write the manuscript and approved the final version.
References
- 1.MICHEL SG, MADARIAGA ML, VILLANI V, SHANMUGARAJAH K. Current progress in xenotransplantation and organ bioengineering. Int J Surg. 2015;13:239–244. doi: 10.1016/j.ijsu.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 2.PERKEL JM. Xenotransplantation makes a comeback. Nature biotechnology. 2016;34:3–4. doi: 10.1038/nbt0116-3. [DOI] [PubMed] [Google Scholar]
- 3.LAI L, KOLBER-SIMONDS D, PARK KW, CHEONG HT, GREENSTEIN JL, IM GS, et al. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002;295:1089–1092. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- 4.YANG L, GUELL M, NIU D, GEORGE H, LESHA E, GRISHIN D, et al. Genome-wide inactivation of porcine endogenous retroviruses (PERVs) Science. 2015;350:1101–1104. doi: 10.1126/science.aad1191. [DOI] [PubMed] [Google Scholar]
- 5.WYNYARD S, NATHU D, GARKAVENKO O, DENNER J, ELLIOTT R. Microbiological safety of the first clinical pig islet xenotransplantation trial in New Zealand. Xenotransplantation. 2014;21:309–323. doi: 10.1111/xen.12102. [DOI] [PubMed] [Google Scholar]
- 6.COOPER DK, BOTTINO R. Recent advances in understanding xenotransplantation: implications for the clinic. Expert review of clinical immunology. 2015;11:1379–1390. doi: 10.1586/1744666X.2015.1083861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LAMM V, HARA H, MAMMEN A, DHALIWAL D, COOPER DK. Corneal blindness and xenotransplantation. Xenotransplantation. 2014;21:99–114. doi: 10.1111/xen.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.GRIFFITH M, HARKIN DG. Recent advances in the design of artificial corneas. Curr Opin Ophthalmol. 2014;25:240–247. doi: 10.1097/ICU.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 9.HARA H, COOPER DK. Xenotransplantation--the future of corneal transplantation? Cornea. 2011;30:371–378. doi: 10.1097/ICO.0b013e3181f237ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.KIM MK, HARA H. Current status of corneal xenotransplantation. Int J Surg. 2015;23:255–260. doi: 10.1016/j.ijsu.2015.07.685. [DOI] [PubMed] [Google Scholar]
- 11.BUHLER L, ALWAYN IP, APPEL JZ, 3RD, ROBSON SC, COOPER DK. Anti-CD154 monoclonal antibody and thromboembolism. Transplantation. 2001;71:491. doi: 10.1097/00007890-200102150-00028. [DOI] [PubMed] [Google Scholar]
- 12.KAWAI T, ANDREWS D, COLVIN RB, SACHS DH, COSIMI AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6:114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 13.LAW CL, GREWAL IS. Therapeutic interventions targeting CD40L (CD154) and CD40: the opportunities and challenges. Advances in experimental medicine and biology. 2009;647:8–36. doi: 10.1007/978-0-387-89520-8_2. [DOI] [PubMed] [Google Scholar]
- 14.PINELLI DF, FORD ML. Novel insights into anti-CD40/CD154 immunotherapy in transplant tolerance. Immunotherapy. 2015;7:399–410. doi: 10.2217/imt.15.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.FOGLA R. Deep anterior lamellar keratoplasty in the management of keratoconus. Indian journal of ophthalmology. 2013;61:465–468. doi: 10.4103/0301-4738.116061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ARCHILA EA. Deep lamellar keratoplasty dissection of host tissue with intrastromal air injection. Cornea. 1984;3:217–218. [PubMed] [Google Scholar]
- 17.CHOI HJ, KIM MK, LEE HJ, KO JH, JEONG SH, LEE JI, et al. Efficacy of pig-to-rhesus lamellar corneal xenotransplantation. Investigative ophthalmology & visual science. 2011;52:6643–6650. doi: 10.1167/iovs.11-7273. [DOI] [PubMed] [Google Scholar]
- 18.LOWE M, BADELL IR, THOMPSON P, MARTIN B, LEOPARDI F, STROBERT E, et al. A novel monoclonal antibody to CD40 prolongs islet allograft survival. Am J Transplant. 2012;12:2079–2087. doi: 10.1111/j.1600-6143.2012.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CHOI HJ, LEE JJ, KIM DH, KIM MK, LEE HJ, KO AY, et al. Blockade of CD40-CD154 costimulatory pathway promotes long-term survival of full-thickness porcine corneal grafts in nonhuman primates: clinically applicable xenocorneal transplantation. Am J Transplant. 2015;15:628–641. doi: 10.1111/ajt.13057. [DOI] [PubMed] [Google Scholar]
- 20.KIM MK, CHOI HJ, KWON I, PIERSON RN, 3RD, COOPER DK, SOULILLOU JP, et al. The International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of xenocorneal transplantation. Xenotransplantation. 2014;21:420–430. doi: 10.1111/xen.12129. [DOI] [PubMed] [Google Scholar]
- 21.LEE HI, KIM MK, OH JY, KO JH, LEE HJ, WEE WR, et al. Gal alpha(1–3)Gal expression of the cornea in vitro, in vivo and in xenotransplantation. Xenotransplantation. 2007;14:612–618. doi: 10.1111/j.1399-3089.2007.00433.x. [DOI] [PubMed] [Google Scholar]
- 22.KANG HJ, LEE H, PARK EM, KIM JM, SHIN JS, KIM JS, et al. Dissociation between anti-porcine albumin and anti-Gal antibody responses in non-human primate recipients of intraportal porcine islet transplantation. Xenotransplantation. 2015;22:124–134. doi: 10.1111/xen.12152. [DOI] [PubMed] [Google Scholar]
- 23.KANG HJ, LEE H, PARK EM, KIM JM, SHIN JS, KIM JS, et al. Increase in anti-Gal IgM level is associated with early graft failure in intraportal porcine islet xenotransplantation. Annals of laboratory medicine. 2015;35:611–617. doi: 10.3343/alm.2015.35.6.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.KIM MK, CHOI HJ, KWON I, PIERSON RN, 3RD, COOPER DK, SOULILLOU JP, et al. The International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of xenocorneal transplantation. Xenotransplantation. 2014;21:420–430. doi: 10.1111/xen.12129. [DOI] [PubMed] [Google Scholar]
- 25.FORD ML, ADAMS AB, PEARSON TC. Targeting co-stimulatory pathways: transplantation and autoimmunity. Nature reviews Nephrology. 2014;10:14–24. doi: 10.1038/nrneph.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.IWASE H, KOBAYASHI T. Current status of pig kidney xenotransplantation. Int J Surg. 2015;23:229–233. doi: 10.1016/j.ijsu.2015.07.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MOHIUDDIN MM, REICHART B, BYRNE GW, MCGREGOR CG. Current status of pig heart xenotransplantation. Int J Surg. 2015;23:234–239. doi: 10.1016/j.ijsu.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.KUWAKI K, TSENG YL, DOR FJ, SHIMIZU A, HOUSER SL, SANDERSON TM, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 29.KUWAKI K, KNOSALLA C, DOR FJ, GOLLACKNER B, TSENG YL, HOUSER S, et al. Suppression of natural and elicited antibodies in pig-to-baboon heart transplantation using a human anti-human CD154 mAb-based regimen. Am J Transplant. 2004;4:363–372. doi: 10.1111/j.1600-6143.2004.00353.x. [DOI] [PubMed] [Google Scholar]
- 30.ELGUETA R, BENSON MJ, DE VRIES VC, WASIUK A, GUO Y, NOELLE RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunological reviews. 2009;229:152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LEE W, SATYANANDA V, IWASE H, TANAKA T, MIYAGAWA Y, LONG C, et al. In vitro testing of an anti-CD40 monoclonal antibody, clone 2C10, in primates and pigs. Transpl Immunol. 2015;33:185–191. doi: 10.1016/j.trim.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Endothelial cell density (ECD; CD) was well maintained showing 3401/mm2, accompanying by normal range of coefficient of variance (CV; 26) and hexagonality (HEX; 72). (B) Average changes of endothelial cell density in grafted primates after sacrifice with at least 6 months-survival were not significantly different compared to basal levels (mean± standard error; p>0.05, Wilcoxon signed rank test). The primates in which both pre- and post-operative ECD had been available were only included (n=3). (C) Anterior segment optical coherent tomography demonstrated well approximated wound and normal depth of anterior chamber. (D) Topography showed simulated K astigmatism of 7.0 diopter.
The copy numbers of Rhesus CMV (RhCMV, black line) DNA in plasma were determined by quantitative real-time PCR, and the antibody was titrated by immunofluorescence assay. Incidental increase of RhCMV copy number in early postoperative period was observed in anti-CD 40 treated primate 1 (anti-CD40 1, A), followed by subsequent increase of anti-RhCMV antibody responses, resulting in decrease of the copy number during the follow-up. Clinical signs of viremia were never observed. In all the other primates (B–D), anti-RhCMV antibody responses (gray dot line) consistently increased upon increase of the copy number of RhCMV, resulting in no clinically relevant increase of the copy number. It suggests that an intact humoral immune response to CMV reactivation. CMV, cytomegalovirus; FITC, fluorescein isothiocyanate; PCR, polymerase chain reaction.
No clinically significant changes were observed in weight loss, fever, hemoglobin, WBC, alanine aminotransferase changes, and creatinine level.
(A) The in vivo IgG binding responses to 3rd party PBMCs were assessed over time by flow cytometry using plasma of anti-CD40 Ab-treated primates (n=5) given IVIG infusion on day 0 and 2 weeks post-transplantation. Non-specific IgG binding against 3rd party PBMCs was observed during the early post-operative period, possibly due to infusion of IVIG that may already contain IgG Abs against porcine PBMCs. Data are presented as mean ±standard errors from 5 subjects. (B) The in vitro IgG binding responses against porcine PBMCs were assessed by flow cytometry using plasma of a naïve primate when mixed with IVIG agents from different lot numbers (#1, #2). Non-specific IgG binding against porcine PBMCs was also observed in a dose-dependent manner after addition of IVIG into the plasma sample. (C) The levels of anti-αGal IgG Abs were measured by ELISA in IVIG agents from different lot numbers (#1, #2) and in plasma of a naïve primate mixed with these IVIG lots. A detectable amount of basal anti-αGal IgG was found to exist in the IVIG formulations. The addition of IVIG into the naïve monkey plasma elevated the level of anti-αGal IgG in a dose-dependent manner. (PBMCs=peripheral blood mononuclear cells, MP = monkey plasma and HSA = human serum albumin, w/=with)