Abstract
Objective
To test the hypothesis that age-related demineralization of otoconia will result in an age-related increase in blood levels of otoconia matrix protein, otolin-1.
Study design
Cross-sectional observational clinical trial.
Setting
Clinical research center.
Patients
Seventy-nine men and women ranging in age from 22 to 95 years old.
Interventions
Diagnostic.
Main outcome measures
Blood levels of otolin-1 in relation to age.
Results
Levels of otolin-1 of subjects divided into four age groups (1: 20–30 [n=20], 2: 50–65 [n=20], 3: 66–80 [n=20], 4: 81–95 [n=19] years old) demonstrated an increasing trend with age The difference between otolin levels of groups 2 and 3, as well as, (P=0.04) and 2 and 4 (P=0.031) were statistically significant, but there was no significant difference between the two oldest groups.
Conclusions
Otolin-1 blood levels are significantly higher in patients older than 65 years of age. This is consistent with previous scanning electron microscopy findings of age-related otoconia degeneration and increased prevalence of benign paroxysmal positional vertigo (BPPV) with age. Normative data provided here can serve as important reference values against which levels from BPPV patients can be compared to further evaluate otolin-1 as a circulatory biomarker for otoconia degeneration.
Introduction
Biomarkers in circulation are powerful indicators of normal and pathological biological processes, as well as, response to pharmacological treatments1. Until recently no specific biomarker for inner ear diseases was available. The inner ear has been found to possess a number of unique proteins that mediate its specialized functions. We have demonstrated that one of these proteins, otolin-1, has increased levels in circulation in patients with benign paroxysmal positional vertigo (BPPV), thus providing proof of concept for use of inner ear proteins as biomarkers2,3. Otolin-1 is a secreted glycoprotein whose mRNA expression is restricted to the inner ear, specifically the support cells of the vestibular maculae, semicircular canal cristae, organ of Corti and marginal cells of the stria vascularis4. Its functions include interaction with other specialized inner ear proteins, such as otoconin 90, to form and maintain otoconia4–6. Before otologic serological biomarkers such as otolin-1, can be adopted into clinical practice, the characteristics of otolin-1 in circulation need to be defined. Here we take the initial step toward this objective. To guide our work, we have maintained our focus on BPPV. BPPV increases in prevalence with age7. This characteristic of the disease appears to be related to age-related demineralization of otoconia6,8. Towards the goal of evaluating the potential of otolin-1 as a serological biomarker, here we test the hypothesis that with demineralization of otoconia, otolin-1 levels in blood will increase with age.
Methods
Subjects
This study was approved by the Institutional Review Board (15-006-3). Subjects were recruited as a part of an ongoing study conducted by the UConn Center of Aging on examining the impact of aging on the response to flu vaccination. With a recruitment goal of 20 subjects, participants were enrolled into the following age groups: young (20–30 years old), middle aged (50–65 years old), old (66–80 years old) and the oldest old (81–95 years old). We successfully met the recruitment goal for all groups except the oldest old which had 19 subjects. Inclusion criteria were male and female gender. Exclusion criterion for the youngest three age groups was major health conditions with the exception common chronic conditions such as hypertension, hypothyroidism, hypercholesterolemia, and reflux. For the oldest old, the only exclusion criterion was the inability to consent. Major health conditions did not serve as an exclusion criterion in order to increase recruitment. In this group, frailty was quantified using the Rockwood Frailty Index9. No vulnerable populations (trainees and prisoners) were recruited.
Data Collection
Non-fasting blood samples (‘green tops’) were collected from all subjects. Collected specimens were spun at 1,300G for 10 minutes. Plasma was then removed and frozen at −80° C until time of assay. Otolin-1 was measured in the plasma using the Human-OTOL1 enzyme-linked immunosorbent assay (ELISA) kit (MyBiosource.com, San Diego, Ca) as described in the manufacturer’s instruction manual. A 1:5 dilution was prepared, and each plasma sample was assayed in triplicate. The optical density in the wells of the ELISA microplate was measured at 450 nm using a BioTek ELx808 plate reader, and data were compiled using the KCJunior software package (BioTek Instruments, Winooski, Vermont).
Statistics
SPSS v 22 was used for statistical analyses. Normality of distributions was assessed with the Shapiro-Wilk test. Non-parametric tests were carried out when distributions departed from normal. Correlation analysis was performed using Spearman’s rho (rs). Group comparisons were performed using Mann Whitney U tests of independent samples. Level of significance was set at p < 0.05, two tailed. According to convention, undetectable values were defined as being one-half the minimal detectable concentration for the assay.
Results
Seventy-nine subjects, 57 female and 22 male, participated in this study. In the oldest group the Frailty Index scores ranged from 1 (very fit) to 7 (severely frail), with mode of the distribution being 3 (multiple medical problems, but managing well).
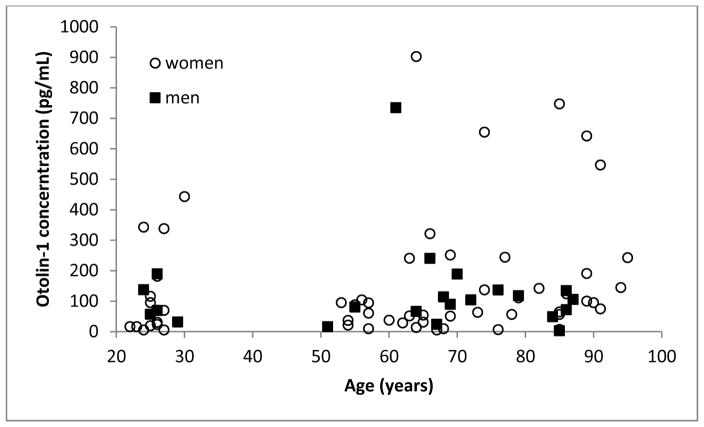
Figure 1 shows otolin-1 levels as a function of age and gender. In the 20–30-year-old group, most subjects had levels below 200 pg/mL, but there were a few outliers with otolin-1 levels between 300–500 pg/mL. Similarly, in the older groups, two clusters were present, with the majority being below 350 pg/mL and a minority between 500–900 pg/mL. Among the subset of subjects with high values, female subjects outnumbered the males by 8 to 1.
Figure 1.
Otolin-1 concentration of male (squares) and female (circles) subjects as a function of age.
In the older group, using a conservative index for outliers (g=2.2)10,11, all values greater than 354 pg/mL were considered statistical outliers. These subjects likely represent a distinct entity and are deserving of focused attention. In considering the trends in the majority pool, to avoid an outlier bias in the statistical testing, these outliers were not included in the subsequent analyses. The Shapiro-Wilk tests of normality were performed on the four separate age groups after removal of the outliers. The distributions of the young- and middle-aged-groups were both statistically significantly different from normal distributions (p = 0.023 and p = 0.01, respectively). Therefore, nonparametric testing will be utilized to compare the four age groups.
Table 1 shows the results of correlation of otolin-1 levels and age. The male subgroups are underpowered and will not be discussed in detail, although their results are reported for completeness. Among, females, there were moderate to strong correlations noted between age and otolin-1 levels, however, they were not statistically significant. A weak, positive, but statistically significant, correlation was found between age and otolin-1 level (rs = 0.3, p = 0.01) when all subjects were combined. Analysis by age groups showed a moderate, positive correlation between age and otolin-1 level (rs = 0.57, p = 0.02) in the oldest old. Using a linear regression model for the oldest old, otolin-1 level could be predicted from age by the following formula: otolin-1 level = −744.6 + age × 10 (i.e., for every year increase in age above 81, otolin-1 level increased by 10 pg/mL).
Table 1.
Spearman rho (rs) correlation coefficient of otolin-1 concentrations with as a function of gender
| Age (years) | All (n) | Male (n) | Female (n) |
|---|---|---|---|
| 20–30 | 0.17 (17) | −0.41 (5) | 0.26 (12) |
| 50–65 | −0.06 (18) | 0.5 (3) | −0.19 (15) |
| 66–80 | 0.012 (19) | 0.07 (8) | 0.46 (11) |
| 81–95 | 0.57*(16) | 0.72 (5) | 0.51 (11) |
| All | 0.3**(70) | 0.03 (21) | 0.37 (49) |
p = 0.02;
p = 0.012
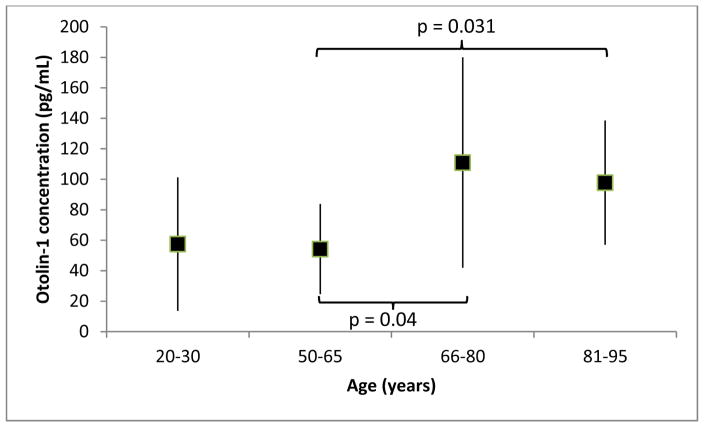
Figure 2 shows the median otolin-1 levels with interquartile ranges for the four age groups. The median of the two youngest groups were below 60 pg/mL while those of the two older groups were near or greater than 100 pg/mL. Mann Whitney U tests of independent samples demonstrated statistically significant differences between the middle aged and the old (p = 0.04), as well as, the middle aged and the oldest old (p = 0.031) groups. Besides age, the difference between the two oldest groups is that the oldest old included vulnerable and frail subjects. The absence of a difference in otolin-1 levels in these two groups implies that frailty had no significant impact. This is further supported by the absence of a correlation between frailty index and otolin-1 level.
Figure 2.
Otolin-1 medians for the four age groups after removal of outliers. The number of subjects in each group was: young = 17, middle aged = 19, old = 19 and oldest old = 16. Error bars represent interquartile range.
Discussion
Although current tools for investigating the inner ear are very helpful, there remain significant constraints on our ability to gain a full understanding of inner ear function and pathophysiology. Biomarkers represent an under explored avenue which could aid a better understanding of inner ear pathophysiology. Highly specialized inner ear functions are made possible by unique proteins which upon disruption of function or cellular injury may be released into circulation where they could provide useful information. Specifically, we have proposed otolin-12 and prestin12 as a serological biomarkers and provided proof of concept in BPPV2 and noise-induced hearing loss13, respectively. Paving the path to practical application of inner ear biomarkers, however, requires additional validation. Toward that goal, in this study, we hypothesized that with the known age-related demineralization of otoconia, otolin-1 levels in plasma should increase with age. Our results support this hypothesis, as subjects above age 65 have higher blood levels of otolin-1 than those less than 65. The design of the current study, a cross-sectional observational human trial, is a limitation in that it only permits the association between otolin-1 levels in circulation and demineralization of otoconia by inference. Nevertheless, given current state of knowledge, this is a very strong inference. Certainly, future animal studies could be useful in further strengthening our conclusions. Animal models which have demonstrated changes in the morphometry and distributional pattern of otoconia include those in aged rats 14 and in an ovariectomized rat model of osteoporosis15.
Comparison to BPPV subjects - Dizziness was not a criterion for participation in the study. We invited participants without major health conditions to participate in this study for the age groups below 80 years of age. We placed no restriction on older participants so as not to limit recruitment. Therefore, whether any subjects in this study had BPPV is unknown.
It is, however, instructive to compare the present results to those of subjects with known BPPV. In female, post-menopausal BPPV subjects, otolin-1 levels averaged around 600 pg/mL (range: 324.7–1002.3 pg/mL), while those of age-matched control subjects with osteoporosis averaged around 450 pg/mL (range: 86.3–607 pg/mL)2. The otolin-1 levels for both BPPV and osteoporosis groups were much higher than those reported in the present study. This further highlights the potential of otolin-1 as a biomarker for otolithic degeneration and BPPV and suggests that upper normal range could be 300–350 pg/mL.
In our sample, we had subjects in both the young and older age groups that were outliers. The outliers in the older groups, had otolin-1 levels comparable to those of BPPV subjects in our previous study2. It is tempting to speculate that the outliers have either undiagnosed BPPV or severe otolithic degeneration which places them at high risk of developing BPPV. Five out of 59 subjects older than 50 in the present study had very high otolin-1 levels, making up 8.5% of our sample. This is comparable to the 9% rate of undiagnosed BPPV in the geriatric population 16. However, we emphasize that an elevated otolin-1 level is not necessarily equivalent to a diagnosis of BPPV, keeping in mind the intraoperative observation that presence of particulate matter in the membranous portion of the posterior semicircular canal does not necessarily coincide with a diagnosis of BPPV17. We have also observed otolin-1 level over 600 pg/mL in post-menopausal, osteoporotic women who did not have BPPV2.
Alternative interpretation
All of our subjects were healthy, with the exception of the oldest old. Since age-related changes were first noted in the old group, age-related decline in clearance mechanisms (e.g., renal), are unlikely to account for our observation of an age-related increase in blood otolin-1. Above we suggested an increase in circulating otolin-1 with age was consistent with age-related degeneration of otoconia. An alternative, but related, interpretation is that presence of otolin-1 in the blood is consistent with the notion that there is turnover in otoconia 18–20. In line with this interpretation, persistent elevation of blood otolin-1 level could be the product of increased turnover with degeneration out pacing deposition/repair in the older subjects. This interpretation would be analogous to disrupted bone turnover in favor of bone resorption in osteoporosis, which has been shown to be strongly associated with BPPV 18–24.
Sex differences
Women are two times more likely to suffer from BPPV25. Based on this epidemiological knowledge we expected to find significant differences in otolin-1 levels between male and female subjects in our sample. However, the number of male participants in this study was much smaller than females and therefore, comparisons between male and females were underpowered. Larger samples of both male and females will be needed to elucidate gender-related trends.
How can an otolithic degeneration biomarker be useful - Since otolithic degeneration is a hallmark of BPPV, a biomarker for otolithic degeneration can be used to promote better understanding of BPPV. Majority of BPPV cases have no known precipitating etiology (75% are idiopathic). Even when there is a precipitating cause (e.g., URI or head trauma), it is not clear why some suffer from BPPV, but most don’t.
This work represents the early phase of biomarker evaluation. Biomarker evaluation consists of analytical validation of the assay, quantification and assessment of associations between the biomarker and disease states and contextual analysis of utilization.1 Additional studies to determine if our methods are biomarker-specific and reliable enough or alternative methodologies are needed to utilize otolin-1 and other possible biomarkers for otologic diseases.
Aging is a risk factor for a majority of chronic diseases and pathologies which were previously thought to be disparate, but are now understood to be connected26. Understanding how aging enables chronic disease and developing novel multi-disease preventive and therapeutic approaches, i.e., geroscience, is an emerging priority26,27. Recent recognition of strong associations between BPPV, osteopenia/osteoporosis and vitamin D deficiency21,23,24,28–31 are changing the way we think of BPPV: a BPPV episode may be manifestation of a progressive, chronic disorder involving otolithic degeneration. A thorough understanding of such a progressive condition would be inherently limited, if we focus on characteristics of episodic manifestations (i.e., during BPPV attack) without acknowledgement of coexisting morbidities. By extension, clinical management of BPPV is suboptimal if management is directed at relieving individual BPPV episodes without attending to chronic predisposing factors. Easy to measure biomarkers in circulation that inform on the status of otoconia could provide useful insights into a structure which would otherwise be difficult to evaluate.
Otolin-1 can also be convenient tools for assessing effectiveness of novel pharmaceutical treatment strategies. For example, as our understanding of the associations of BPPV and osteopenia/osteoporosis and vitamin D deficiency translates into therapeutic strategies, otolin-1 level in circulation could be one measure to assess their effectiveness. Also, given the important role of structural proteins such as otolin-1, novel pharmaceuticals that stabilize the structure of otoconia could be explored and monitored to prevent BPPV attacks.
Finally, a biomarker in circulation could be a convenient tool to aid diagnosis of challenging presentations suspected of being BPPV. These include cases of subjective BPPV, multicanal or bilateral disease and when it is difficult to perform diagnostic positional maneuvers such as in kyphotic, frail elderly.
Acknowledgments
Sources of Support: The UCONN Center on Aging, The Connecticut Institute on Clinical and Translational Science(Parham), NIH R01 AG048023 (Kuchel) and P01 AG021600 (Haynes)
References
- 1.Micheel CM, Ball JR. Evaluation of Biomarkers and Surrogate Endpoints in Chronic Disease. Washington, D.C: National Academies Press; 2010. [PubMed] [Google Scholar]
- 2.Parham K, Sacks D, Bixby C, Fall P. Inner ear protein as a biomarker in circulation? Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2014;151(6):1038–1040. doi: 10.1177/0194599814551127. [DOI] [PubMed] [Google Scholar]
- 3.Sacks D, Parham K. Preliminary Report on the Investigation of the Association Between BPPV and Osteoporosis Using Biomarkers. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2015;36(9):1532–1536. doi: 10.1097/MAO.0000000000000853. [DOI] [PubMed] [Google Scholar]
- 4.Deans MR, Peterson JM, Wong GW. Mammalian Otolin: a multimeric glycoprotein specific to the inner ear that interacts with otoconial matrix protein Otoconin-90 and Cerebellin-1. PLoS One. 2010;5(9):e12765. doi: 10.1371/journal.pone.0012765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang H, Zhao X, Xu Y, Wang L, He Q, Lundberg YW. Matrix recruitment and calcium sequestration for spatial specific otoconia development. PLoS One. 2011;6(5):e20498. doi: 10.1371/journal.pone.0020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrade LR, Lins U, Farina M, Kachar B, Thalmann R. Immunogold TEM of otoconin 90 and otolin - relevance to mineralization of otoconia, and pathogenesis of benign positional vertigo. Hear Res. 2012;292(1–2):14–25. doi: 10.1016/j.heares.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parham K, Kuchel GA. A Geriatric Perspective on Benign Paroxysmal Positional Vertigo. J Am Geriatr Soc. 2016;64(2):378–385. doi: 10.1111/jgs.13926. [DOI] [PubMed] [Google Scholar]
- 8.Walther LE, Wenzel A, Buder J, Bloching MB, Kniep R, Blodow A. Detection of human utricular otoconia degeneration in vital specimen and implications for benign paroxysmal positional vertigo. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies. 2013 doi: 10.1007/s00405-013-2784-6. [DOI] [PubMed] [Google Scholar]
- 9.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 2005;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoaglin DC, Iglewicz B, Tukey JW. Performance of some resistant rues for outlier labeling. Journal of the American Statistical Association. 1986;81:991–999. [Google Scholar]
- 11.Hoaglin DC, Iglewicz B. Fine tuning some resitant rules for outlier labeling. Journal of Am Stat Assoc. 1987;82:1147–1149. [Google Scholar]
- 12.Parham K. Prestin as a biochemical marker for early detection of acquired sensorineural hearing loss. Medical hypotheses. 2015;85(2):130–133. doi: 10.1016/j.mehy.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Parham K, Dyhrfjeld-Johnsen J. Outer Hair Cell Molecular Protein, Prestin, as a Serum Biomarker for Hearing Loss: Proof of Concept. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2016;37(9):1217–1222. doi: 10.1097/MAO.0000000000001164. [DOI] [PubMed] [Google Scholar]
- 14.Jang YS, Hwang CH, Shin JY, Bae WY, Kim LS. Age-related changes on the morphology of the otoconia. The Laryngoscope. 2006;116(6):996–1001. doi: 10.1097/01.mlg.0000217238.84401.03. [DOI] [PubMed] [Google Scholar]
- 15.Vibert D, Sans A, Kompis M, et al. Ultrastructural changes in otoconia of osteoporotic rats. Audiology & neuro-otology. 2008;13(5):293–301. doi: 10.1159/000124277. [DOI] [PubMed] [Google Scholar]
- 16.Oghalai JS, Manolidis S, Barth JL, Stewart MG, Jenkins HA. Unrecognized benign paroxysmal positional vertigo in elderly patients. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2000;122(5):630–634. doi: 10.1016/S0194-5998(00)70187-2. [DOI] [PubMed] [Google Scholar]
- 17.Kveton JF, Kashgarian M. Particulate matter within the membranous labyrinth: pathologic or normal? Am J Otol. 1994;15(2):173–176. [PubMed] [Google Scholar]
- 18.Preston RE, Johnsson LG, Hill JH, Schacht J. Incorporation of radioactive calcium into otolithic membranes and middle ear ossicles of the gerbil. Acta oto-laryngologica. 1975;80(3–4):269–275. doi: 10.3109/00016487509121327. [DOI] [PubMed] [Google Scholar]
- 19.Ross MD. Calcium ion uptake and exchange in otoconia. Advances in oto-rhino-laryngology. 1979;25:26–33. doi: 10.1159/000402913. [DOI] [PubMed] [Google Scholar]
- 20.Kawamata S, Igarashi Y. Growth and turnover of rat otoconia as revealed by labeling with tetracycline. Anat Rec. 1995;242(2):259–266. doi: 10.1002/ar.1092420216. [DOI] [PubMed] [Google Scholar]
- 21.Vibert D, Kompis M, Hausler R. Benign paroxysmal positional vertigo in older women may be related to osteoporosis and osteopenia. The Annals of otology, rhinology, and laryngology. 2003;112(10):885–889. doi: 10.1177/000348940311201010. [DOI] [PubMed] [Google Scholar]
- 22.Jeong SH, Choi SH, Kim JY, Koo JW, Kim HJ, Kim JS. Osteopenia and osteoporosis in idiopathic benign positional vertigo. Neurology. 2009;72(12):1069–1076. doi: 10.1212/01.wnl.0000345016.33983.e0. [DOI] [PubMed] [Google Scholar]
- 23.Jang YS, Kang MK. Relationship between bone mineral density and clinical features in women with idiopathic benign paroxysmal positional vertigo. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2009;30(1):95–100. doi: 10.1097/MAO.0b013e31818f5777. [DOI] [PubMed] [Google Scholar]
- 24.Parham K, Leonard G, Feinn RS, Lafreniere D, Kenny AM. Prospective clinical investigation of the relationship between idiopathic benign paroxysmal positional vertigo and bone turnover: a pilot study. The Laryngoscope. 2013;123(11):2834–2839. doi: 10.1002/lary.24162. [DOI] [PubMed] [Google Scholar]
- 25.von Brevern M, Radtke A, Lezius F, et al. Epidemiology of benign paroxysmal positional vertigo: a population based study. Journal of neurology, neurosurgery, and psychiatry. 2007;78(7):710–715. doi: 10.1136/jnnp.2006.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burch JB, Augustine AD, Frieden LA, et al. Advances in geroscience: impact on healthspan and chronic disease. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S1–3. doi: 10.1093/gerona/glu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamanaka T, Shirota S, Sawai Y, Murai T, Fujita N, Hosoi H. Osteoporosis as a risk factor for the recurrence of benign paroxysmal positional vertigo. The Laryngoscope. 2013;123(11):2813–2816. doi: 10.1002/lary.24099. [DOI] [PubMed] [Google Scholar]
- 29.Jeong SH, Kim JS, Shin JW, et al. Decreased serum vitamin D in idiopathic benign paroxysmal positional vertigo. Journal of neurology. 2013;260(3):832–838. doi: 10.1007/s00415-012-6712-2. [DOI] [PubMed] [Google Scholar]
- 30.Talaat HS, Abuhadied G, Talaat AS, Abdelaal MS. Low bone mineral density and vitamin D deficiency in patients with benign positional paroxysmal vertigo. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies. 2015;272(9):2249–2253. doi: 10.1007/s00405-014-3175-3. [DOI] [PubMed] [Google Scholar]
- 31.Sheikhzadeh M, Lotfi Y, Mousavi A, Heidari B, Monadi M, Bakhshi E. Influence of supplemental vitamin D on intensity of benign paroxysmal positional vertigo: A longitudinal clinical study. Caspian J Intern Med. 2016;7(2):93–98. [PMC free article] [PubMed] [Google Scholar]