Abstract
Purpose
The ocular dimensional changes in myopia reflect increased scleral remodeling, and in high myopia, loss of scleral integrity leads to biomechanical weakening and continued scleral creep. As integrins, a type of cell surface receptors, have been linked to scleral remodeling, they represent potential targets for myopia therapies. As a first step, this study aimed to characterize the integrin subunits at the messenger RNA level in the sclera of the guinea pig, a more recently added but increasingly used animal model for myopia research.
Methods
Primers for α and β integrin subunits were designed using NCBI/UCSC Genome Browser and Primer3 software tools. Total RNA was extracted from normal scleral tissue and isolated cultured scleral fibroblasts, as well as liver and lung, as reference tissues, all from guinea pig. cDNA was produced by reverse transcription, PCR was used to amplify products of predetermined sizes, and products were sequenced using standard methods.
Results
Guinea pig scleral tissue expressed all known integrin alpha subunits except αD and αE. The latter integrin subunits were also not expressed by cultured guinea pig scleral fibroblasts; however, their expression was confirmed in guinea pig liver. In addition, isolated cultured fibroblasts did not express integrin subunits αL, αM, and αX. This difference between results for cultured cells and intact sclera presumably reflects the presence in the latter of additional cell types. Both guinea pig scleral tissue and isolated scleral fibroblasts expressed all known integrin beta subunits. All results were verified through sequencing.
Conclusion
The possible contributions of integrins to scleral remodeling make them plausible targets for myopia prevention. Data from this study will help guide future ex vivo and in vitro studies directed at understanding the relationship between scleral integrins and ocular growth regulation in the guinea pig model for myopia.
Keywords: Myopia, sclera, integrin, guinea pig
Introduction
Myopia describes the condition in which light rays from distant objects are focused in front of the retina. Increased ocular axial length in myopia increases the risk of potentially blinding retinal pathology such as retinal detachment and maculopathies.1 Elongation of the globe, at least in mammalian eyes, has been linked to remodeling of the sclera, which is largely made of up collagen. For example, in tree shrews, collagen accounts for 90% of the scleral dry weight.2 Scleral remodeling is accelerated in myopia leading to altered tissue integrity, increased scleral creep, and continued ocular elongation.3 Thus, a plausible route for addressing the problem of excessive ocular elongation in myopia would be to inhibit scleral remodeling, thereby preserving scleral integrity and preventing further ocular elongation. To date, with the exception of topical atropine and some novel optical therapies,4 the management of myopia has been largely symptomatic, directed toward reducing the blur symptoms resulting from the mismatch between the refracting power of the eye and its length, rather than controlling myopia progression.
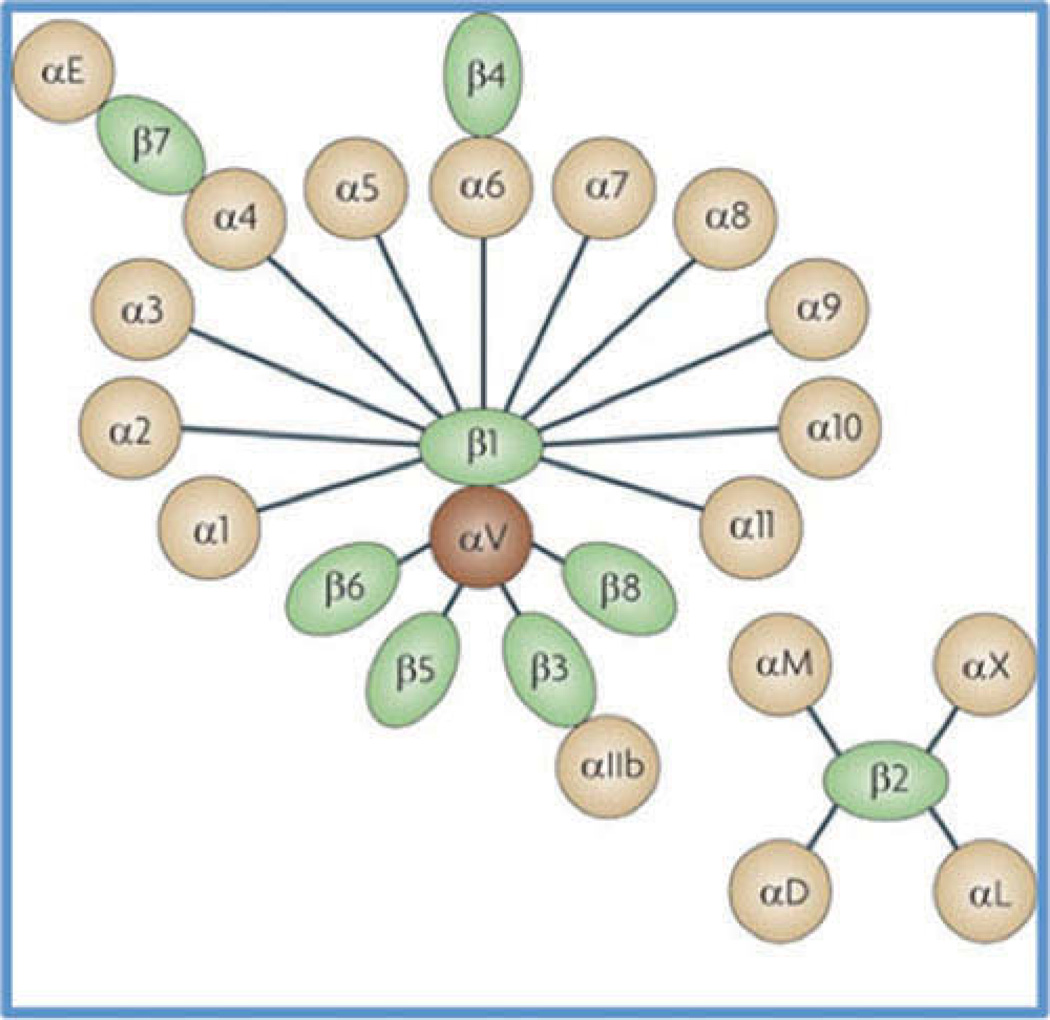
Collagen-binding integrins have been linked to scleral remodeling and represent plausible therapeutic targets for myopia.5–7 Integrins are transmembrane linkers that are responsible for binding cells to the extracellular matrix (ECM). Structurally, integrins exist as heterodimers made up of alpha and beta subunits. Mammals are known to have 18 different alpha subunits and eight different beta subunits. A total of 24 unique integrins have been described (in the form of dimers), representing combinations of these various alpha and beta subunits, and are shown in Figure 1.8
Figure 1.
Integrin heterodimers. Schematic diagram showing the different known mammalian integrin alpha and beta subunits and the unique integrins that can arise from the various combinations of these subunits.41
Ligands within the ECM bind to integrins, activating their cytoplasmic tail and thereby binding to several intracellular anchor proteins. The intracellular connections made by integrins are often to actin filaments and occasionally to intermediate filaments. The anchor proteins in turn can bind to other anchor proteins or actin filaments in the cell cortex. Activation of multiple integrin receptors together also allows for the formation of focal adhesions between the cell and the ECM. In addition to their connections to the ECM, integrins are capable of passing information from the ECM to the cell and vice versa.9 Thus, integrins are important in providing cells with the capability of responding rapidly to environmental changes; they are known to activate intracellular pathways, interact with growth factors, and have been implicated in mechanotransduction as well as cell attachment.10 Signaling is made possible by assembly of complexes at the cytoplasmic side of the plasma membrane. A unique characteristic of integrins is that once activated, changes are generally kept localized in the cytoplasm close to the cell-matrix contact area, whereas activation of conventional receptors often induces global cell responses.9 In the eye, integrins appear to have many important roles, including but not limited to development, wound healing, inflammation, and thrombosis of the eye.11
Animal models have proved valuable tools for investigating treatment options for myopia.12 In this context, the guinea pig represents a more recently introduced mammalian model, joining the well-studied tree shrew model for myopia. Importantly, guinea pigs have a well-developed visual system and reliably develop myopia in response to imposed hyperopic defocus (via negative lenses) and form deprivation.13 Nonetheless, relatively little is known about the guinea pig sclera. The objective of the study described here was to establish the integrin subunit expression profile of the guinea pig sclera, specifically, to identify the integrin subunits expressed at the level of messenger RNA, in both intact scleral tissue and isolated cultured scleral fibroblasts. The expression of integrin subunits at the protein level was not investigated. Nonetheless, gene expression profiles for these two sources (and how they differ between each other) may inform the design and interpretation of future ex vivo and in vitro studies into anti-myopia therapies specifically targeting the sclera.
Methods
Animals and Tissue Extraction
All procedures were conducted according to the ARVO Statement for the use of animals in ophthalmic and vision research and were approved by Institutional Animal Care and Use Committee. Young normal 6-week-old guinea pigs (Elm Hill Labs, MA, USA) randomly allocated (unknown gender and no experimentally induced refractive error) were chosen for experiments. Animals were maintained on a 12-hour light/dark cycle, and food and water were provided ad libitum. Animals were anesthetized with ketamine (30 mg/ml) and xylazine (3 mg/ml) and euthanized with an intracardial injection of sodium pentobarbital. Eyes were enucleated, excessive orbital fat was removed, and eyes were cut into anterior and posterior segments. The posterior segment was flat-mounted; the vitreous, retina, and choroid were separated; and the sclera was isolated (for either RNA extraction or cell culture). To ensure that there was no contamination with neural tissue, the sclera was thoroughly cleaned with phosphate buffered saline, and the optic nerve head was removed with a surgical trephine. Lung and liver tissues were also collected to act as positive controls for the ex vivo study.
Cell Culture
Primary cultures of scleral fibroblasts were obtained from whole sclera explants. The explants were grown in polystyrene culture dishes in Dulbecco’s Modified Eagle’s Medium (DMEM):F12 (1:1), supplemented with 10% fetal bovine serum (FBS), and 1% penicillin and streptomycin (Invitrogen). Fibroblast cultures were maintained and incubated at 37°C with 95% air and 7% carbon dioxide until confluent (2 to 3 weeks). Once the dishes reached 75% confluence, cells were passaged and subcultures were established. Fibroblasts between P1 and P4 were used for experiments.
RNA Extraction and Reverse Transcription
RNA samples were collected from several tissue sources: guinea pig sclera (ex vivo sample), liver and lung, as well as cultured scleral fibroblasts (in vitro sample). Harvested tissue samples were stored in Qiagen RNAlater prior to RNA extraction (Qiagen, Redwood City, CA, USA). Prior to RNA extraction, tissue samples were homogenized using a Bead Ruptor Omni 24 (OMNI International Inc., Kennesaw, GA, USA). Total RNA was extracted from all tissue samples using Qiagen RNeasy Mini Kits (Qiagen). The quality and concentration of RNA was then quantified using a Thermo Scientific NanoDrop 2000c (Thermo Scientific, Denver, CO, USA). Conversion of RNA to cDNA was accomplished using Invitrogen SuperScript III First- Strand Synthesis kits for RT-PCR (Life Technologies, Carlsbad, CA, USA).
Primer Design
A total of 26 pairs of primers were designed to target known mammalian integrin subunits (Tables 1 and 2). Primer design was based on the information obtained from predictions made by computational analyses on NCBI entries from a guinea pig genome sequencing project,14 Primer3 program, and UCSC Genome Browser Bioinformatics. The primers were designed to span across multiple introns to ensure that genomic DNA contamination did not confound results. Product resulting from genomic DNA would be much larger in size than the desired products.
Table 1.
Primers for integrin alpha subunit family and corresponding amplicon sizes.
| Oligonucleotide Primer Sequence α-Integrin Subunits | |||
|---|---|---|---|
| Subunit | Forward | Reverse | Size (bp) |
| α1 | AGATTCTGCAGGCATTCCAT | ATTGGTTCCAGGCTCATTTG | 208 |
| α2 | TAGTGAAAGTGAGGAAGCAAACA | AGTGCACGACAGAAGGAACA | 150 |
| α2b | TGACCGGCACACAGCTCTAC | ATCGATGTCTGTGGCACCTC | 245 |
| α3 | TCCCTCAACATGGACAACAA | CTTCCACAGCAAGAGGATGA | 156 |
| α4 | GAATGGATTGCCCTCTGTGT | TACTTGGATGCTGCCTGTGA | 187 |
| α5 | TATTCTGTGGCTGTGGGTGA | CGGCATAGCCAAAGTAGGAG | 169 |
| α6 | CTCGTGCGAGCTTACATTGA | CAAGCATCAGTATCCCAGCA | 169 |
| α7 | TGTGGACAACAGGGATAGGAG | CGCGGTCAAAGCTGTAGAGT | 175 |
| α8 | CCTGTGCTCCTTTATATCACTGG | TCCTGCTTGGCAGTAACCTT | 162 |
| α9 | TCTAAACATCTCCATCTCCAACC | ACGCTGCACTTGAGGAAGTC | 153 |
| α10 | GGGAGCTGAGGTCTCTATTGG | AGAAGAGCAAGCAGGAGCAG | 249 |
| α11 | TCCTGGCCTCCAGTACTTTG | TGCAGTCCTTGTGGAAGATG | 185 |
| αD | GGCAGTCACAGTCGATCAAA | GTTGTACGATGGGCTTCACC | 500 |
| αE | CCTCCAGACTTCCAGAAAGC | CTTGGCGTGAAGATGTTGTC | 245 |
| αL | AGAACACGCACAGTCAGCAG | ATGTTGAAGGATGCCAGGTC | 169 |
| αM | CAATGTGACGCTCTTCTCCA | CTCAGGGACTGCCCAAAGTA | 244 |
| αV | TACCGGCTGGATTACAGGAC | CCTTGGTTCTGAGCCTTCAC | 224 |
| αX | GAGCACCTACTGCAGCATCA | AAGTCCCTCTGACCCAGGTT | 300 |
Table 2.
Primers for integrin beta subunit family and corresponding amplicon sizes.
| Oligonucleotide Primer Sequence β-Integrin Subunits | |||
|---|---|---|---|
| Subunit | Forward | Reverse | Size (bp) |
| β1 | CCCGAGGACATCACTCAGAT | TCAGCTCTGTCCCGAGACTT | 187 |
| β2 | GCTGGTGCACAAACTGACTG | GCCTTCTTGATGAGGTCCAC | 159 |
| β3 | CAGTGGGAAGTCCATCCTGT | TGGCTCGTTCTTCCTCAAAC | 188 |
| β4 | AGGATGTGGACGAGTTCAGG | AGGTGGCACTTCTCGTCATT | 238 |
| β5 | GGAAGATCTACGGGCCTTTC | CCGGCATGTACTGATGTCTG | 164 |
| β6 | GTGAGTGATGCCGATTCTCA | TCTTGGGTTACAGCAAAGATCA | 194 |
| β7 | GCTGAGTGAAGACTCCAGCA | GGCTCTTGGAGGCAACTCTT | 243 |
| β8 | ATGCCTTCACCCTCACAATC | ACCTTCAGCAACCCAATCAA | 174 |
Polymerase Chain Reaction (PCR)
The presence of various integrin subunits was first assessed using polymerase chain reaction (PCR). Specificity and sensitivity was increased by amplification using touchdown PCR parameters as suggested by Korbie et al.15 PCR was carried out using hot-start polymerase (Qiagen) and a thermal cycler (Bio-Rad, Hercules, CA, USA). PCR products were then subjected to electrophoresis and imaged using FluorChem Multiimage III (Protein Simple, San Jose, CA, USA). Qiagen QlAquick PCR purification kits (Qiagen) were then used to purify and prepare the PCR products for sequencing.
Sequencing
Purified PCR products were sequenced by University of California Berkeley’s DNA Sequencing Facility, using the Sanger sequencing method. Data were subsequently analyzed using an Applied Bioscience Sequence Scanner. Finally, predicted integrin sequences, as determined from computational analyses on NCBI entries from the guinea pig genome-sequencing project, were compared with sequencing results for the PCR products, using the CLUSTAL 2.1 program.
Results
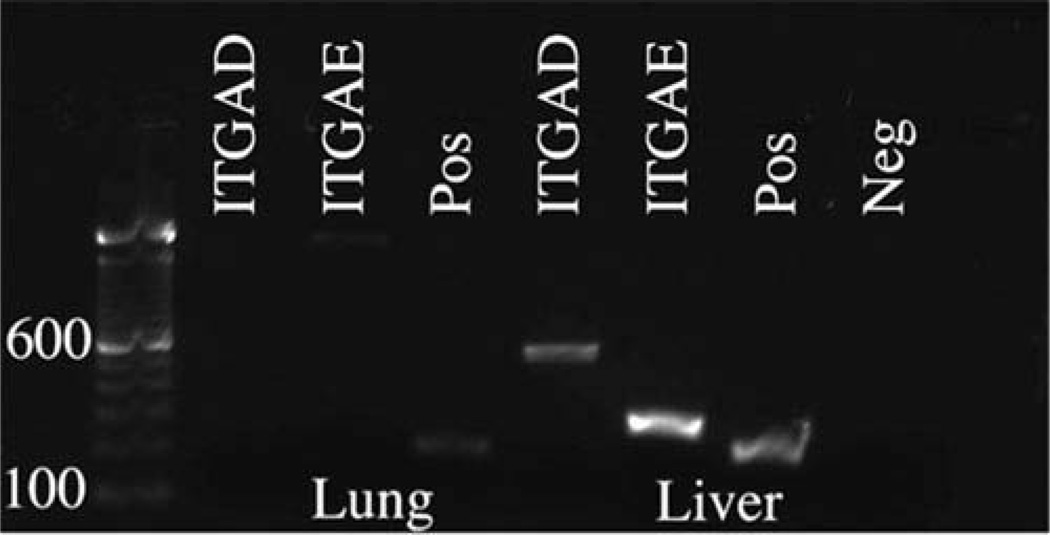
PCR analysis of intact guinea pig scleral (ex vivo) samples revealed 24 of the 26 possible integrin subunits to be expressed at the mRNA level, producing 22 possible integrins when combined. Results for isolated cultured scleral fibroblasts (in vitro samples) differed in that only 21 of the 26 possible subunits were detected, allowing for only 19 potential integrin dimer combinations at the mRNA level. More specifically, ex vivo samples failed to show integrin αD and αE subunits, while in vitro samples lacked αD, αE, αL, αM, and αX subunits at the mRNA level. Both ex vivo and in vitro samples expressed the full spectrum of known integrin β subunits at the mRNA level (Figure 2). Results from guinea pig liver and lung tissue samples validated our primer designs for the missing integrin subunits, integrin αD and αE, which were both detected at the mRNA level in the liver sample (Figure 3). In other words, our primer designs for integrin subunits αD and αE were adequate for detecting the presence of these targets at the mRNA level.
Figure 2.
Integrin subunits expressed in intact guinea pig scleral tissue and cultured guinea pig scleral fibroblasts. Amplification products for guinea pig scleral tissue showed all known integrin alpha subunits to be present except αD and αE (top left) and all known beta subunits (top right). Amplification products for cultured guinea pig scleral fibroblasts showed all known integrin alpha subunits to be present except integrin αD, αE, αL, αM, and αX (bottom left), and all beta subunits (bottom right). Molecular markers (100 bp) are included for product size comparison. Arrows represent integrin subunits that were not expressed.
Figure 3.
Identification of integrin subunits expressed in guinea pig liver and lung. Amplification products for guinea pig liver and lung; integrin β2 was used as a positive control. Integrin αD and αE were detected in the liver sample and the lung sample exhibited a non-specific band for integrin αE as verified with sequencing. Molecular markers (100 bp) are included for product size comparison. These integrin alpha subunits were not detected in either intact guinea pig sclera or isolated cultured scleral fibroblasts.
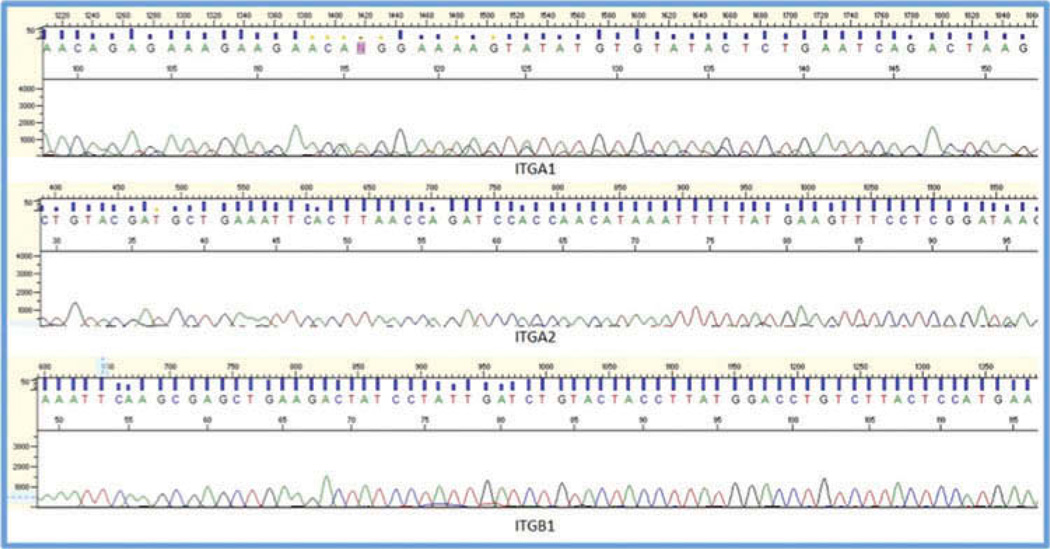
Qualitative analysis of PCR products derived from both intact scleral tissue and isolated scleral fibroblasts confirmed that all products were within expected size ranges. Comparisons of PCR products with predicted integrin sequences yielded over 95% identity, validating the specificity of our findings. As further confirmation of the validity of our results, all PCR products were sequenced, with the chromatograms thus obtained being analyzed for both reliability and specificity (Figures 4 and 5). These analyses confirmed that the amplicons detected in the scleral samples were neither products of non-specific binding of primers nor the products of genomic DNA contamination.
Figure 4.
Comparison of sequencing results and predicted guinea pig sequences. Results from the sequencing experiments were compared with predicted guinea pig sequences, determined from computational analyses performed on NCBI entries from the guinea pig genome sequencing project using CLUSTAL 2.1. There is over 95% identity between the two sets of sequences, validating the specificity of our findings.
Figure 5.
Representative sequence chromatogram results showing the nucleotide sequences for the purified PCR products, for integrin subunits α1, α2, and α1.
Discussion
The current study was motivated by earlier studies in the tree shrew, reporting expression and changes in scleral integrins linked to myopia progression. In the context of myopia research, scleral integrin subunits have been well characterized in the tree shrew model,6 which represents one of the early mammalian models of myopia to be described.16 A related study described decreased expression levels of collagen-binding integrins α1 and β1 during the progression of myopia.5 Also of relevance are the results of another study involving the guinea pig pointing to the potential for integrins as therapeutic targets for slowing of axial elongation of the globe during myopia. In brief, basic fibroblast growth factor (bFGF) was found to not only inhibit form deprivation myopia (FDM) in the guinea pig model but to increase the expression level of type 1 collagen, α2 integrin, and β1 integrin.7 However, the latter study did not attempt to fully characterize the integrin subunit profile of the guinea pig sclera, yet it would seem an important first step for any study attempting to target scleral integrins for myopia control. Instead, attention was directed at the α2 and β1 integrins, which are two major receptors for type 1 collagen.17 While it is clear from these results that integrins play an important role in the pathophysiology of experimentally induced myopia in the guinea pig model, the findings of the current study provide the framework needed to further characterize the involvement of scleral integrins in the excessive ocular elongation underlying myopia in this model.
Interactions between ligands present in the scleral ECM and scleral integrins appear to play many important physiological roles in the sclera. Table 3 lists known scleral ligands along with possible integrin receptors that may be present in guinea pig sclera, based on its integrin subunit profile as reported here. Two of these ligands, fibronectin and laminin, are thought to be important in directing developmental events in the sclera,18 with overexpression of scleral fibronectin also being linked to nanophthalmos.19 The maintenance of scleral collagen matrix integrity and thus its biomechanical stability have been linked to interactions between integrins (mostly α1β1 and α2β1) and other ligands, including collagen.20 Finally, osteopontin and tenascin appear to be involved in regulating matricellular proteins during scleral remodeling.21 These few examples serve to highlight the varied and potentially important roles of integrins in eye elongation. Thus, characterizing the integrin subunits present in the guinea pig sclera may not only benefit research aimed at understanding potential mechanisms underlying the development of myopia but may also lead to potential novel therapies for myopia and other eye size abnormalities. In the context of myopia control therapies, information about the integrin subunits present on sclera fibroblasts may allow for customization of synthetic scleral explants such as the biomimetic hyaluronic acid-based hydrogels currently being explored.22,23 Specifically, knowledge of the integrin profiles of scleral fibroblasts can be applied to optimize the biocompatibility of these hydrogels.
Table 3.
Example of interactions between ligands present in the scleral ECM and scleral integrins.
| Ligand | Integrin | Evidence for extracellular ligands and integrin interaction |
Evidence for the expression of ligand in sclera |
|---|---|---|---|
| Fibronectin | α2β1, α3β1, α4β1, α4β7, α5β1, αVβ1, αVβ3, αVβ6, αVβ8, αMβ2, αXβ2, αLβ2 |
(8, 10) | (36) |
| Laminin | α1β1, α2β1, α6β1, α6β4, α7β1, αVβ8 | (8, 10) | (37, 38) |
| Osteopontin | α8β1, αVβ3 | (8, 10) | (21) |
| Tenascin | α9β1 | (8, 10) | (39) |
| Collagen | α1β1, α2β1, α10β1, α11β1 | (8, 10) | (40) |
Our study revealed significant differences in the integrin mRNA expression profiles of intact guinea pig sclera and those of isolated, cultured guinea pig scleral fibroblasts, the former expressing 24 of the 26 known mammalian integrin subunits while the later expressed only 21 of the 26 integrin subunits. One explanation for the discrepancy between the results for intact scleral tissue and cultured scleral fibroblasts could be the presence in intact scleral tissue of additional cells, with different integrin expression profiles. Specifically, cells other than fibroblasts such as histiocytes, blast cells, granulocytes, lymphocytes, and plasma cells have all been previously noted within the normal scleral stroma although in low numbers.24 Integrins are important for the migration of leukocytes from the vasculature, interaction with target and antigen-presenting cells, as well as binding to iC3b and fibrinogen,25 and corresponding to our findings, integrin subunits αL, αM, and αX have been reported to be expressed in combination with β2 by leukocytes. Cultured scleral fibroblasts represent a more controlled condition, where contributions to expression profiles from other cell types are unlikely. Thus, it is perhaps not surprising that αL, αM, and αX were not expressed by our cultures. Nonetheless, it is not possible to rule out as a contributing factor, differences in the immediate environments of cells in the 2D environment of cell cultures compared to the 3D environment of the intact tissue, with impacts on intercellular and extracellular interactions, potentially leading to changes in cell signaling, cell adhesion, integrin ligation, and mechanotranduction.26 It is possible that the native ECM also contains growth factors, which will be absent in the case of cells grown under standard 2D cell culture conditions, removing also any potential for interaction between ECM and growth factors. Other considerations that may differentially affect integrin expression in intact scleral tissue compared to cultured scleral fibroblasts include the type and intensity of stresses experienced by scleral fibroblasts. For example, prior to harvesting, cells in the intact sclera (ex vivo samples) would have been subject to mechanical forces resulting from intraocular pressure (IOP), which undergoes frequent, physiological fluctuations related to the vascular pulse, blinking, eye movements, and diurnal rhythms. These forces are known to influence scleral cell mitosis and collagen synthesis,27 and it is plausible that they also influence the expression of integrins, given that stress-induced changes in integrin expression have already been reported in other cell systems.28–30
Although there were differences in expression between intact guinea pig sclera and cultured scleral fibroblasts, PCR analysis did not detect integrin αD and αE in either at the mRNA level. These findings were validated when the same primers as used in the latter analyses revealed expression of both integrin subunits in intact guinea pig liver, implying that the primer designs were not the origin of these negative results, i.e., the results for the liver samples rule out the possibility of primer failure. It is possible that guinea pig sclera fibroblasts do not express either αD or αE integrin subunits, or that these integrin subunits are expressed at very low levels. Understanding the physiological roles of these integrin subunits elsewhere in the body may provide some insight into why these two integrin subunits are not strongly expressed in the sclera. Interestingly, both integrin αD and αE subunits have been linked to inflammation. Specifically, the αD subunit integrin has been shown to be associated with integrin β2, with the resulting Integrin αDβ2 heterodimer apparently involved in regulating the ability of eosinophils and macrophages to adhere to sites of inflammation, through interaction with vascular cell adhesion molecule 1 (VCAM-1).31,32 Integrin αE is thought to serve as a secondary ligand, with a possible role in the selective recruitment of a certain subset of T cells into the intestinal mucosa, and expression of integrin αEβ7 has also been linked to the retention of cells within epithelial sites.33,34 Note that the sclera is composed mainly of nonvascular fibroblasts and is itself relatively avascular, with inflammatory response cells being generally only temporary residents, coming from the nearby highly vascular choroid.35 Thus, the lack of cellular diversity within the sclera, combined with its avascular nature, offers a plausible explanation for the lack of expression in the sclera of integrin αD and αE subunits.
In summary, most but not all of known integrin subunits were found to be expressed in the guinea pig sclera at the mRNA level. Although this study answers the question of which integrin subunits are present in the guinea pig sclera at the level of messenger RNA, it does not answer the questions of which of these genes are translated into proteins and which functional heterodimers are present. Future studies will be directed toward addressing these questions and also differences between the scleras of myopic and normal eyes, thereby allowing for selective targeting of integrins in subsequent studies aimed at identifying plausible therapeutic targets for myopia control. The ultimate goal is to develop an effective therapy for myopia, by preventing or reversing the adverse changes in scleral integrity and biomechanical strength, so the risk of pathological complications can be minimized.
Acknowledgments
Supported by funds from T35EY007139 to KW, K08EY022670 to RM, and R01EY012392 to CFW.
Footnotes
Color versions of one or more of the figures in the article can be found online at www.tandfonline.com/icey.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Jonas JB, Xu L. Histological changes of high axial myopia. Eye (Lond) 2014 Feb;28(2):113–117. doi: 10.1038/eye.2013.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norton T, Miller E. Collagen and protein levels in sclera during normal development, induced myopia, and recovery in tree shrews. Invest Ophthalmol Vis Sci. 1995;36(S760) [Google Scholar]
- 3.McBrien NA, Gentle A. Role of the sclera in the development and pathological complications of myopia. Prog Retin Eye Res. 2003 May;22(3):307–338. doi: 10.1016/s1350-9462(02)00063-0. [DOI] [PubMed] [Google Scholar]
- 4.Walline JJ, Lindsley K, Vedula SS, Cotter SA, Mutti DO, Twelker JD. Interventions to slow progression of myopia in children. Cochrane Database Syst Rev. 2011;(12):CD004916. doi: 10.1002/14651858.CD004916.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McBrien NA, Metlapally R, Jobling AI, Gentle A. Expression of collagen-binding integrin receptors in the mammalian sclera and their regulation during the development of myopia. Invest Ophthalmol Vis Sci. 2006 Nov;47(11):4674–4682. doi: 10.1167/iovs.05-1150. [DOI] [PubMed] [Google Scholar]
- 6.Metlapally R, Jobling AI, Gentle A, McBrien NA. Characterization of the integrin receptor subunit profile in the mammalian sclera. Mol Vis. 2006;12:725–734. [PubMed] [Google Scholar]
- 7.Tian XD, Cheng YX, Liu GB, Guo SF, Fan CL, Zhan LH, et al. Expressions of type I collagen, alpha2 integrin and beta1 integrin in sclera of guinea pig with defocus myopia and inhibitory effects of bFGF on the formation of myopia. Int J Ophthalmol. 2013;6(1):54–58. doi: 10.3980/j.issn.2222-3959.2013.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010 Jan;339(1):269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alberts BJA, Lewis J, et al. Molecular Biology of the Cell. 4th. New York: Garland Sciences; 2002. [Google Scholar]
- 10.Haas TA, Plow EF. Integrin-ligand interactions: a year in review. Curr Opin Cell Biol. 1994 Oct;6(5):656–662. doi: 10.1016/0955-0674(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 11.Elner SG, Elner VM. The integrin superfamily and the eye. Invest Ophthalmol Vis Sci. 1996 Apr;37(5):696–7701. [PubMed] [Google Scholar]
- 12.Schaeffel F, Feldkaemper M. Animal models in myopia research. Clin Exp Optometry. 2015 Nov;98(6):507–517. doi: 10.1111/cxo.12312. [DOI] [PubMed] [Google Scholar]
- 13.Jiang L, Schaeffel F, Zhou X, Zhang S, Jin X, Pan M, et al. Spontaneous axial myopia and emmetropization in a strain of wild-type guinea pig (Cavia porcellus) Invest Ophthalmol Vis Sci. 2009 Mar;50(3):1013–1019. doi: 10.1167/iovs.08-2463. [DOI] [PubMed] [Google Scholar]
- 14.Weyrich A, Schullermann T, Heeger F, Jeschek M, Mazzoni CJ, Chen W, et al. Whole genome sequencing and methylome analysis of the wild guinea pig. BMC Genomics. 2014 Nov 28;15(1):1036. doi: 10.1186/1471-2164-15-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korbie DJ, Mattick JS. Touchdown PCR for increased specificity and sensitivity in PCR amplification. Nat Protoc. 2008;3(9):1452–1456. doi: 10.1038/nprot.2008.133. [DOI] [PubMed] [Google Scholar]
- 16.Sherman SM, Norton TT, Casagrande VA. Myopia in the lid-sutured tree shrew (Tupaia glis) Brain Res. 1977 Mar 18;124(1):154–157. doi: 10.1016/0006-8993(77)90872-1. [DOI] [PubMed] [Google Scholar]
- 17.Taubenberger A, Cisneros DA, Friedrichs J, Puech PH, Muller DJ, Franz CM. Revealing early steps of alpha2beta1 integrin-mediated adhesion to collagen type I by using single-cell force spectroscopy. Mol Biol Cell. 2007 May;18(5):1634–1644. doi: 10.1091/mbc.E06-09-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maza MSdl, Tauber J, Foster CS. The Sclera. 2nd. New York: Springer; 2012. p. xiii.p. 320. [Google Scholar]
- 19.Yue BY, Kurosawa A, Duvall J, Goldberg MF, Tso MO, Sugar J. Nanophthalmic sclera. Fibronectin studies. Ophthalmology. 1988 Jan;95(1):56–60. doi: 10.1016/s0161-6420(88)33215-x. [DOI] [PubMed] [Google Scholar]
- 20.Hu S, Cui D, Yang X, Hu J, Wan W, Zeng J. The crucial role of collagen-binding integrins in maintaining the mechanical properties of human scleral fibroblasts-seeded collagen matrix. Mol Vis. 2011;17:1334–1342. [PMC free article] [PubMed] [Google Scholar]
- 21.Gao H, Frost MR, Siegwart JT, Jr, Norton TT. Patterns of mRNA and protein expression during minus-lens compensation and recovery in tree shrew sclera. Mol Vis. 2011;17:903–919. [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia M, Jha A, Healy K, Wildsoet C. Myopia control in guinea pigs with a biomimetic hyaluronic acid-based hydrogel. Invest Ophthalmol Vis Sci. 2013 Jun 16;54(6):4702. 2013. [Google Scholar]
- 23.Garcia MB. Myopia control in guinea pigs [Doctoral] University of California at Berkeley; 2014. [Google Scholar]
- 24.Watson PG, Young RD. Scleral structure, organisation and disease. A review. Exp Eye Res. 2004 Mar;78(3):609–623. doi: 10.1016/s0014-4835(03)00212-4. [DOI] [PubMed] [Google Scholar]
- 25.Wong DA, Davis EM, LeBeau M, Springer TA. Cloning and chromosomal localization of a novel gene-encoding a human beta 2-integrin alpha subunit. Gene. 1996 Jun 1;171(2):291–294. doi: 10.1016/0378-1119(95)00869-1. [DOI] [PubMed] [Google Scholar]
- 26.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006 Mar;7(3):211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 27.Ganesan O’Brien P. Scleral Bioreactor: Design and Use for Evaluation of Myopia Therapies [Doctoral] University of Calfiornia at Berkeley; 2011. [Google Scholar]
- 28.Danias J, Gerometta R, Ge Y, Ren L, Panagis L, Mittag TW, et al. Gene expression changes in steroid-induced IOP elevation in bovine trabecular meshwork. Invest Ophthalmol Vis Sci. 2011 Nov;52(12):8636–8645. doi: 10.1167/iovs.11-7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soghomonians A, Barakat AI, Thirkill TL, Blankenship TN, Douglas GC. Effect of shear stress on migration and integrin expression in macaque trophoblast cells. Biochim Biophys Acta. 2002 May 8;1589(3):233–246. doi: 10.1016/s0167-4889(02)00179-9. [DOI] [PubMed] [Google Scholar]
- 30.Sun X, Liu X, Zhang Y, Kuang X, Lv B, Ge J. A simple and effective pressure culture system modified from a transwell cell culture system. Biol Res. 2013;46(1):47–52. doi: 10.4067/S0716-97602013000100007. [DOI] [PubMed] [Google Scholar]
- 31.Van der Vieren M, Crowe DT, Hoekstra D, Vazeux R, Hoffman PA, Grayson MH, et al. The leukocyte integrin alpha D beta 2 binds VCAM-1: evidence for a binding interface between I domain and VCAM-1. J Immunol. 1999 Aug 15;163(4):1984–1990. [PubMed] [Google Scholar]
- 32.Yakubenko VP, Belevych N, Mishchuk D, Schurin A, Lam SC, Ugarova TP. The role of integrin alpha D beta2 (CD11d/CD18) in monocyte/macrophage migration. Exp Cell Res. 2008 Aug 15;314(14):2569–2578. doi: 10.1016/j.yexcr.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehmann J, Huehn J, de la Rosa M, Maszyna F, Kretschmer U, Krenn V, et al. Expression of the integrin alpha Ebeta 7 identifies unique subsets of CD25+ as well as CD25− regulatory T cells. Proc Natl Acad Sci USA. 2002 Oct 1;99(20):13031–13036. doi: 10.1073/pnas.192162899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strauch UG, Mueller RC, Li XY, Cernadas M, Higgins JM, Binion DG, et al. Integrin alpha E(CD103)beta 7 mediates adhesion to intestinal microvascular endothelial cell lines via an E-cadherin-independent interaction. J Immunol. 2001 Mar 1;166(5):3506–3514. doi: 10.4049/jimmunol.166.5.3506. [DOI] [PubMed] [Google Scholar]
- 35.Bron AJ, Tripathi RC, Tripathi BJ, Wolff E. Wolff’s Anatomy of the Eye and Orbit. 8th. London: New York: Chapman & Hall Medical; 1997. p. ix.p. 736. [Google Scholar]
- 36.Chapman SA, Ayad S, O’Donoghue E, Bonshek RE. Glycoproteins of trabecular meshwork, cornea and sclera. Eye (Lond) 1998;12(Pt 3a):440–448. doi: 10.1038/eye.1998.102. [DOI] [PubMed] [Google Scholar]
- 37.Dietlein TS, Jacobi PC, Paulsson M, Smyth N, Krieglstein GK. Laminin heterogeneity around Schlemm’s canal in normal humans and glaucoma patients. Ophthalmic Res. 1998;30(6):380–387. doi: 10.1159/000055499. [DOI] [PubMed] [Google Scholar]
- 38.Marshall GE. Human scleral elastic system: an immunoelectron microscopic study. Br J Ophthalmol. 1995 Jan;79(1):57–64. doi: 10.1136/bjo.79.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tervo K, Latvala T, Suomalainen VP, Tervo T, Immonen I. Cellular fibronectin and tenascin in experimental perforating scleral wounds with incarceration of the vitreous. Graefes Arch Clin Exp Ophthalmol. 1995 Mar;233(3):168–172. doi: 10.1007/BF00166610. [DOI] [PubMed] [Google Scholar]
- 40.Gentle A, Liu Y, Martin JE, Conti GL, McBrien NA. Collagen gene expression and the altered accumulation of scleral collagen during the development of high myopia. J Biol Chem. 2003 May 9;278(19):16587–16594. doi: 10.1074/jbc.M300970200. [DOI] [PubMed] [Google Scholar]
- 41.Cox D, Brennan M, Moran N. Integrins as therapeutic targets: lessons and opportunities. Nat Rev Drug Discov. 2010 Oct;9(10):804–820. doi: 10.1038/nrd3266. [DOI] [PubMed] [Google Scholar]