Abstract
Rho GTPases are critical signal transducers of multiple pathways. They have been proposed to be useful anti-neoplastic targets for over two decades, especially in Ras-driven cancers. Until recently, however, few in vivo studies had been carried out to test this premise. Several recent mouse model studies have verified that Rac1, RhoA, and some of their effector proteins such as PAK and ROCK, are likely anti-cancer targets for treating K-Ras-driven tumors. Other seemingly contradictory studies have suggested that at least in certain instances inhibition of individual Rho GTPases may paradoxically result in pro-neoplastic effects. Significantly, both RhoA GTPase gain- and loss-of-function mutations have been discovered in primary leukemia/lymphoma and gastric cancer by human cancer genome sequencing efforts, suggesting both pro- and anti-neoplastic roles. In this review we summarize and integrate these unexpected findings and discuss the mechanistic implications in the design and application of Rho GTPase targeting strategies in future cancer therapies.
Keywords: Rho GTPases, tumorigenesis, tumor suppression, anti-neoplasia target, rational drug design
Introduction
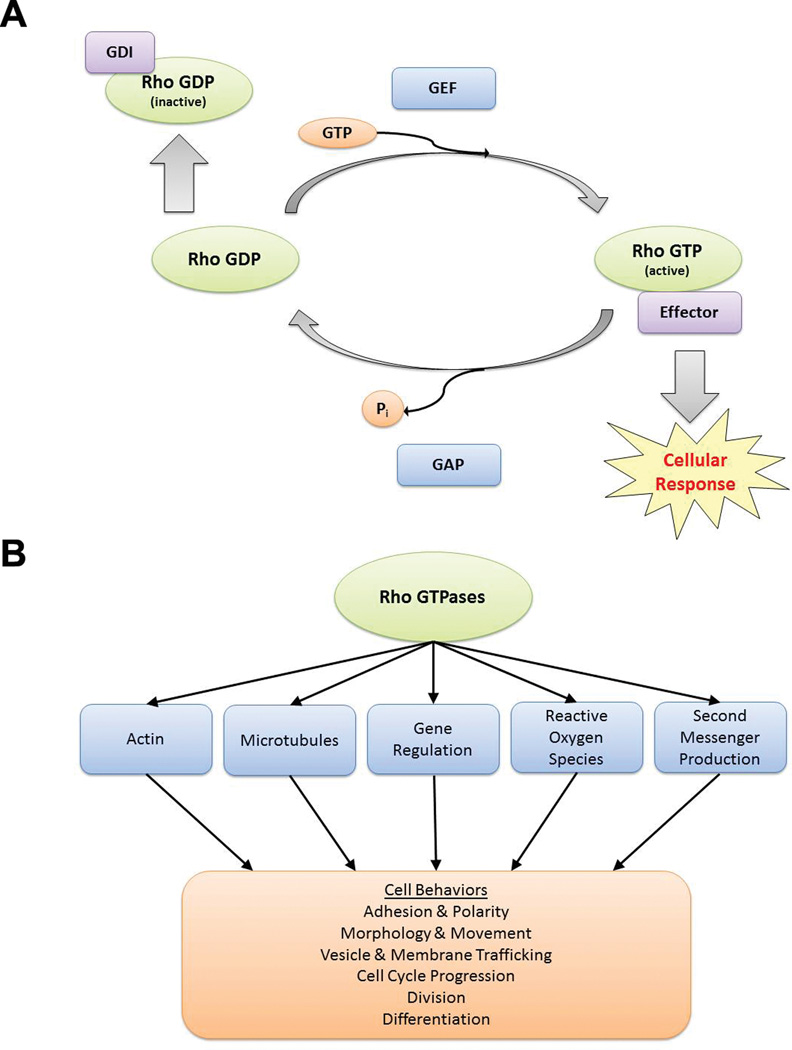
Rho GTPases are a family of signaling proteins that belong to the Ras GTPase superfamily.1 Mammalian Rho GTPases include 22 members,2 of which the most-studied are RhoA, Rac1, and Cdc42. These proteins relay intracellular signals by acting as tightly regulated molecular switches (Figure 1a).3 The GTPase-activating proteins (GAPs) facilitate the slow intrinsic GTP-hydrolysis reaction to turn off signaling, while the guanine nucleotide exchange factors (GEFs) catalyze the GTP-loading reaction to turn on Rho GTPase signaling. An additional level of regulation is provided by Rho GDP dissociation inhibitors (GDIs) which bind to the GDP-bound Rho GTPases, inhibiting GDP dissociation and sequestering them away from the active intracellular membrane sites. RhoGDIs can also prevent degradation of prenylated Rho GTPases when in the inactive state.4 This dynamic cycle of GTP-loading/GTP-hydrolysis is essential for proper Rho GTPase signaling function. Alterations of this process may result in changes in Rho GTPase regulated cell functions including cell morphogenesis, adhesion, migration, cytokinesis, polarity, proliferation, and survival (Figure 1b).5
Figure 1.
The GTP-binding and GTP-hydrolysis cycle and signaling functions of Rho GTPases in cells. (a) The biochemical model shows the signal regulation of Rho GTPases by GEFs, GAPs, and GDIs cycling in the GTP-bound active, and GDP-bound inactive, states. (b) Major cellular processes directly affected by Rho GTPases and the cell behaviors subsequently affected by those processes are depicted.
RhoA, Rac1, and Cdc42 are thought to be positively involved in cancer cell growth and potential anti-cancer targets in tumor initiation and metastasis.6, 7 Evidence that they are pro-neoplastic emerged over 20 years ago with studies showing that constitutively active RhoA and Rac1 mutants possess weak transforming activity.8–10 Dominant negative forms of Rho GTPases could block K-Ras or other oncogene-driven transformation of fibroblasts, suggesting an interconnection between Rho and Ras signaling pathways. Additional evidence for Rho and Ras crosstalk includes that RhoA or Rac1 could transform fibroblasts synergistically with oncogenic Raf8 and a shared connection between RhoA and Ras with serum response factors (SRFs)11 that bind to serum response elements and induce the expression of genes important for cell cycle progression, growth, division, and differentiation. A number of Ras effector pathways had been found to lead to Rho GTPase activation.12, 13 For example, Ras binds to and activates T-lymphoma invasion and metastasis-inducing protein 1 (TIAM1), a GEF for Rac1 that may activate Rac1 to contribute to Ras transformation.14 PI3Ks, one of the main effector pathways of Ras, can also activate Rac1 via the PIP3-dependent Rac Exchanger (P-Rex) family of Rac GEFs.15
Although tremendous efforts have been dedicated to further implicating individual Rho GTPases in subsequent studies,16–20 limited information from primary human cancers or mouse cancer model studies was available until recently. Several key findings came to light in the past several years yielding more complicated implications related to the role of individual Rho GTPases in several cancer types. In parallel, small molecule targeting of Rho GTPases has progressed in preclinical studies, making available lead chemical probes that have translational values. It is thus timely to review recent findings of Rho GTPase signaling in cancer biology, and refresh the implications in rational targeting of Rho pathways in neoplasia.
Rho GTPases as pro-oncogenic signal transducers
Rho GTPases and signaling molecules that activate their activity – such as GEFs – have traditionally been considered as oncogenic, and therefore potential anti-neoplastic targets.21–23 Indeed, in the past two decades, numerous studies have demonstrated the roles of multiple Rho GTPases in tumorigenesis. Much of the earlier work used loss- or gain-of-function mutant Rho GTPase overexpression in cancer cell or fibroblast cell lines in vitro, and the results supported the pro-proliferation and pro-metastasis functions of RhoA, Rac1, and Cdc42. In agreement with this, overexpression or hyper-activation of many Rho GTPases have been implicated in various human cancers and have been extensively reviewed elsewhere.24, 25 Recent cancer genetic studies by whole-genome sequencing have identified a growing list of recurrent mutations in Rho GTPases (reviewed in Ref.25, 26), which were previously thought to be rare. Further, an intimate involvement of Rho GTPases in modulating multiple cell types in the tumor microenvironment, which have active roles in angiogenesis, chemotaxis, and inflammatory responses, also strongly implicates their pro-neoplastic roles.7, 27
Adding to the understanding of Rho GTPase signaling pathways and functions characterized by biochemical and cell biological approaches, in vivo cell-type specific and cancer-type specific functions of mammalian Rho GTPases have been delineated recently by murine conditional-knockout genetic models,28–30 including in Ras-driven cancer models (Table 1). These mouse genetic models and human cancer genetic findings of “hot-spot” mutations of Rho GTPases further invigorate the interests.
Table 1.
Function of Rho GTPases in in vivo cancer models.
| Rho GTPases |
Pro- or anti- oncogenic |
Model | Remarks | References (year) |
|---|---|---|---|---|
| RhoC | Pro-oncogenic | RhoC−/−; pyV-MT mouse model of mammary tumor |
Loss of RhoC did not affect tumor development but decreases tumor cell motility and metastatic cell survival leading to a drastic inhibition of metastasis |
Hakem et al.47 (2005) |
| Rac1 | Pro-oncogenic | Rac1flox/flox and LSL-K-RasG12D mouse, lung infections with adenovirus expressing Cre (Ad-Cre) |
Rac1 function is required for tumorigenesis in this oncogenic K-Ras-induced lung cancer model |
Kissil et al.50 (2007) |
| Rac1 | Pro-oncogenic | Rac1flox/flox; K5-Cre mouse, treated with DMBA/TPA to induce skin tumors |
Rac1 is crucial for skin tumor formation and mice with keratinocyte-restricted deletion of Rac1 are resistant to skin tumor formation. |
Wang et al.52 (2010) |
| Rac1 | Pro-oncogenic | LSL-K-RasG12D; K14-Cre:ER; Rac1WT/− mouse model of epidermal papilloma |
Active Rac1 level was high in this model and genetic removal of one Rac1 allele significantly impaired K- Ras induced oral papilloma growth |
Samuel et al.51 (2011) |
| Rac1/Rac2 | Pro-oncogenic | Transplant of Low density bone marrow from Rac1flox/flox; Mx1-Cre or Rac2−/−; Mx1- Cre mouse, transfected with retrovirus expressing MLL-AF9 to induce leukemia. |
Rac2, but not Rac1, is critical to the initiation of acute myeloid leukemia in this model; however, loss of either Rac1 or Rac2 is sufficient to impair survival and growth of the transformed MLL-AF9 leukemia |
Mizukawa et al.54 (2011) |
| RhoA/Rac1 | Pro-oncogenic | Oncogenic Ras driven eye hyperplasia /tumorigenesis in Drosophila (ey-GAL4; UAS-Ras85DV12) |
Rho1, Rac1 and RhoGEF2 were identified to enhance oncogenic Ras driven tumorigenesis in a genome-wide screen |
Brumby et al.31 (2011) |
| RhoA | Pro-oncogenic | K-RasLA2-G12D mouse model of non-small cell lung cancer (NSCLC) |
Combined inhibition of the proteasome and ROCK robustly suppresses K-Ras mutant tumor growth |
Kumar et al.32 (2012) |
| Rac1b | Pro-oncogenic | LSL-K-RasG12D; Rosa26-LSL-Rac1b mouse model of lung cancer, lung infection with adenovirus expressing Cre (Ad-Cre) |
Expression of Rac1b synergized with oncogenic K- Ras resulting in increased cellular proliferation and accelerated tumor growth. |
Zhou et al.64 (2013) |
| Rac1 | Pro-oncogenic | Rac1flox/flox; K-Ras(LSL-G12D)/+; Ptf1acre/+ mouse model of pancreatic ductal adenocarcinoma (PDA) with pancreas- specific deletion of Rac1 |
Pancreas-specific deletion of Rac1 prevented the development of pancreatic tumors. |
Wu et al.53 (2014) |
| Cdc42 | Pro-oncogenic | APCmin/+; Cdc42flox/flox; Vil-Cre or Catnb(ex3)/+; Cdc42flox/flox; Vil-Cre mouse model of colorectal cancer with intestinal epithelial cell specific Cdc42 deletion |
Reduction of Cdc42 alleviates the tumorigenicity of mutant intestinal cells carrying single APC or β- catenin mutations |
Sakamori et al.58 (2014) |
| RhoA | Anti-oncogenic | TO(K-RasG12V/RhoA), TO(K- RasG12V/RhoAT19N), or TO(K- RasG12V/RhoAG14V) zebra fish model of hepatocellular carcinoma |
Liver enlargement and hepatocyte proliferation induced by tet-on-inducible, liver-specific expression K-RasG12V was augmented by dominant- negative RhoAT19N, but reduced by constitutive- active RhoAG14V. |
Chew et al.94 (2014) |
| RhoA | Anti-oncogenic | APCmin/+; RhoAT19N/−; Vil-Cretg/− mouse model of colorectal cancer with expression of dominant-negative RhoAT19N |
RhoA inactivation contributes to colorectal cancer progression/metastasis, largely through the activation of Wnt/β-catenin signaling |
Rodrigues et al.97 (2014) |
| RhoB | Anti-oncogenic | RhoB−/− mouse with cutaneous squamous cell carcinomas (SCC) induced with UVB |
RhoB deletion lowered the incidence of SCC precursor tumors following chronic exposure to UVB. |
Meyer et al.112 (2014) |
| Rac1 | Pro-oncogenic | K14-ΔNLef1; K14-Rac1Q61L mouse model of sebaceous adenoma with epidermis- specific active Rac1 |
Active Rac1 did not change the incidence or frequency of tumors, but could suppress tumor cell differentiation and enable malignant progression of sebaceous tumors. |
Frances et al.56 (2015) |
| RhoA/RhoC | Anti-oncogenic | LSL-K-RasG12D with RhoAflox/flox or RhoC−/− or both, mouse models of lung carcinoma induced by lung infection of adenovirus expressing Cre (Ad-Cre) |
Deletion of RhoA or RhoC alone did not suppress K- RasG12D induced lung adenoma initiation; rather, deletion of RhoA along accelerated lung adenoma formation. |
Zandvakili et al.98 (2015) |
RhoA
A previous Drosophila study by a forward genetic screen had identified genes that cooperate with oncogenic Ras to drive eye hyperplasia/tumorigenesis.31 Interestingly, many positive hits in the screen belonged to the Rho GTPase pathway including Rho1, Rac1 and RhoGEF2, which all enhanced Ras-driven tumorigenesis in a JNK-dependent manner. The positive influence of RhoA in cancer was strengthened by two recent reports exploring therapeutic targets in K-Ras-driven murine models of lung cancer. The first report found that K-Ras-mutant cancer cells, but not K-Ras wild-type cancer cells, were vulnerable to perturbations in a GATA2 regulated set of signaling pathways including Rho signaling, IL-1/NF-κB signaling, and Nrf1/proteasome function.32 Using a mutant K-Ras non-small cell lung cancer (NSCLC) mouse model, the authors further showed that the adenomas could be induced to regress by combination treatment with proteasome and Rho signaling inhibitors. In the second paper, study of a lung adenocarcinoma model in mutant K-Ras; Cdkn2a-null mice found that RhoA signaling was upregulated in adenocarcinomas and downstream FAK (focal adhesion kinase) was a key for malignant phenotypes in both lung adenocarcinoma cell lines as well as in a mouse model.33 Additionally, studies of RhoA contribution to gastric cancer implicate RhoA activity as a permissive signal for G1-S-transition of the cell cycle progression through INK4 family members.34
Recent human cancer genetic studies utilizing whole genome sequencing have found recurrent RhoA mutations in gastric cancer (GC),35–38 peripheral T cell lymphoma (PTCL),39–42 adult T-cell leukemia/lymphoma (ATLL),43 Burkitt Lymphoma (BL),44 and head and neck cancer.45 Some of them appear to be gain-of-function while a significant portion are loss-of-function, and their functional significance has yet to be causally defined. Consistent with a pro-cancer progression role, duplication of exons in ROCK, a RhoA effector, presumably resulting in a gain-of-function, has also been described in lung adenocarcinoma.46
RhoC
RhoC has long been associated with cancer cell invasion and metastasis.18 The first mouse model to address the role of RhoC in metastasis in vivo was that of Hakem and colleagues where they produced a constitutively RhoC-null mouse that, surprisingly, showed no abnormal phenotype at its basal state.47 To assess the effect of RhoC on metastasis, the authors used the MMTV-PyVT transgenic mouse [Mouse Mammary Tumor Virus (MMTV) driven Polyoma Virus middle T antigen (PyVT)], which developed mammary tumors that metastasized to the lung with high penetrance. In this genetic background, RhoC loss led to dramatically fewer metastases to the lung, and the resulting mammary tumor cells showed reduced invasion activity in vitro. This evidence correlates with clinical observations of elevated RhoC levels associated with metastatic grade in human breast and gastric cancers.48, 49
Rac1 and Cdc42
Both Rac1 and Cdc42 were shown to be pro-transformation in early fibroblast studies. Rac1 conditional knockout mouse studies have shown that Rac1 is necessary for K-Ras-driven lung adenoma formation,50 epidermal papilloma initiation and growth,51 and DMBA/TPA-induced skin tumor formation.52 In a K-Ras-driven pancreatic ductal adenocarcinoma (PDA) mouse model, pancreas-specific disruption of Rac1 or p110α, but not p110β, prevented the development of pancreatic tumors, and the loss of transformation was independent of AKT regulation.53 In a retroviral expression model of MLL-AF9 induced leukemogenesis, Rac2, but not Rac1, is critical to the initiation of acute myeloid leukemia. However, loss of either Rac1 or Rac2 is sufficient to impair survival and growth of the transformed MLL-AF9 leukemia.54 Induced deletion of Rac1 in endothelial cells suggested that Rac1 is required for embryonic vascular integrity and angiogenesis, representing potential anti-angiogenetic therapeutic targets for cancer.55 In a mouse model for benign, more differentiated sebaceous skin tumors, epidermis-specific Rac1 activity did not alter tumor incidence and frequency, but suppressed tumor cell differentiation leading to malignant progression of sebaceous tumors.56 Likewise, conditional deletion of Cdc42 in Ras-transformed fibroblast cells drastically alters cell morphology and inhibits proliferation, cell-cycle progression and tumorigenicity.57 In a mouse colorectal cancer model, Cdc42 ablation suppressed the malignant progression of early-stage intestinal epithelial cancer cells carrying single APC or beta-catenin mutations.58
Rac1 and Cdc42 protein levels have been shown to be upregulated in multiple human cancers. Rac1b, the splice variant of Rac1 that contains a 19 amino acid insert adjacent to the switch II domain resulting in increased signaling and ROS generation, has emerged as a variant that is upregulated in several cancers such as lung, breast, colon and thyroid cancers.48, 59–62 Also, Rac1b may cooperate with B-Raf oncogenic mutant V600E in promoting tumorigenesis.61, 63 In a mouse model of K-Ras induced lung adenocarcinoma, expression of Rac1b synergized with oncogenic K-Ras and caused increased proliferation and accelerated tumor growth.64
Recent cancer genome sequencing efforts have revealed functionally relevant Rac1 gain-of-function mutations. Two studies utilizing whole genome sequencing of over 120 melanoma samples found a frequent and previously undescribed Rac1 mutation: 5% of melanomas65 and 9.2% of sun-exposed melanomas66 contain a recurrent Rac1P29S mutation that increases its GTP-bound active state and binding to effector proteins with enhanced signaling. A similar mutation, P29L, was found in Rac2.65 Additional activating Rac1 mutations A159V and Q61R were later found in head and neck cancer and prostate cancer, respectively.67
GEFs and Effectors
Historically, the positive Rho GTPase regulators, GEFs, and Rho effectors are considered pro-oncogenic and pro-growth, whereas negative regulators such as Rho GAPs are considered tumor suppressing. For instance, the Rac GEF TIAM1 is also a potential effector for H- and K-Ras, and both its upregulation and deletion have been implicated in tumor initiation and metastasis, respectively (reviewed in Ref.22). Another Rac GEF, PREX2, is found to be mutated in human melanoma,68 pancreatic cancer,69 and colorectal cancer.70 Certain PREX2 mutations, when expressed in murine melanoma models, increased Rac1 activity, PI3K-AKT pathway signaling and tumorigenesis, along with shortened survival life of the mice.71 Vav1, a normally hematopoietic cell-specific RhoGEF, has been identified to be mutated in ATLL72 and lung adenocarcinoma,73 and found to be involved in novel fusions in PTCLs.74 An analysis of the genome data set by Kakiuchi et al.35 using CHASM75 identified a possible driver mutation in ArfGEF1 in gastric cancer.76 Mutations in DOCK2 and DOCK3, which belong to a different family of Rho GEFs from the Dbl-like molecules, have also been identified recently in colorectal cancer.70 Interestingly, certain functions of RhoGEFs in tumors could be independent of their nucleotide exchange activity. For example, P-Rex2a can act as a component of the PI3K pathway by interacting with PTEN to suppress its lipid phosphatase activity in tumor cells.77 Rho GEF H1, a RhoA GEF, is a critical part of a positive feedback loop in the Ras pathway via direct interaction with scaffolding protein Kinase suppressor of Ras 1 (KSR-1).78
With regards to effector proteins, abrogation of ROCK signaling has been shown to have a mortality benefit in murine models of leukemia, hepatocellular carcinoma, as well as breast and lung carcinomas.79–81 Deletion of both ROCK isoforms, Rock1 and Rock2, but not individually, blocked tumor formation in mouse models of non-small cell lung cancer and melanoma, suggesting indispensable yet redundant roles for ROCK1/2 in cell cycle progression and tumorigenesis.82 ROCK inhibitors may have high potential for treating cancer and other physiological conditions, and they are being investigated in clinical trials for human diseases including solid tumors.83 While multiple ROCK inhibitors target the ATP binding pocket, classic inhibitors such as fasudil and Y27632 are generally not ROCK isoform selective and also interfere with other AGC kinases such as protein kinase N (PKN) kinases (also known as PRKs), another effector of Rho, with slightly lower potency.84 Considerable efforts are being devoted to developing new ROCK inhibitors with higher potency and selectivity.81 Studies of other downstream proteins such as RhoA effector formins have yielded positive results with regards to inhibiting cancer cell motility and progression.85 Both Rac1 and Cdc42 directly bind to and activate p110β, a subunit of PI3K, via the Rho Binding Domain of p110β.86, 87 The Rac1/Cdc42 effector PAK family kinases (p21-activated kinase), known to phosphorylate important cell signaling proteins such as Bcl-2, MEK, and Raf1 and is a part of the MAPK, JNK and NF-κB pathways, have been found to be upregulated in human cancers.88 Deletion of PAK1 led to a dramatically reduced tumorigenesis and tumor progression in a K-Ras-induced skin cancer model,89 and inhibition of PAK1 attenuated tumor growth and metastasis in a model of pancreatic adenocarcinoma.90 These crucial effector pathways of Rho GTPases appear consistently involved in pro-oncogenic signaling.
Rho GTPases with potential tumor suppressing roles
While most Rho GTPases, especially RhoA, Rac1, and Cdc42, along with their signaling components, have been considered pro-neoplastic (exceptions include RhoB,91 RhoE,92 and RhoH93), recent mouse model and human genomic data has emerged to suggest that RhoA, Rac1, and Cdc42 can also act in a tumor suppressing role under defined conditions. This new information (Table 1; Figure 2) raises the question whether individual Rho GTPases are pro- or anti-neoplastic in a given tumor, and sheds new light into therapeutic targeting strategies of Rho pathways.
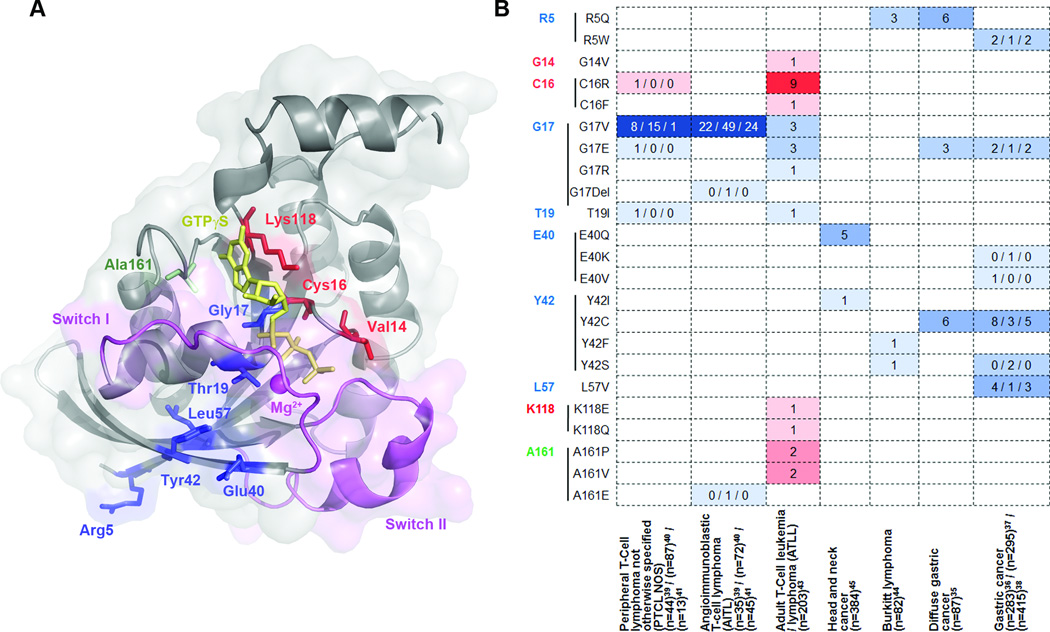
Figure 2.
Recurrent RhoA mutations found in human cancers. (a) Most commonly mutated amino acid positions identified in recent reports are mapped to the 3D structure of activated RhoA161 (PDB#: 1A2B). GTPγS is shown as yellow sticks and the magnesium ion is shown as magenta sphere. Sites for gain-of-function mutations G14 (shown as V14, mutated in the original structure), C16, and K118 are shown as red sticks; sites for loss-of-function mutations R5, G17, T19, E40, Y42, and L57 are shown as blue sticks; while A161 is shown as green sticks as both gain-of-function mutations (A161P and A161V) and lost-of-function mutations (A161E) have been identified. (b) The occurrence of the hot-spot mutations are listed across tumor types. Hotspots are ordered by amino acid position and colored in the same scheme as in (a). Gain-of-function and loss-of-function mutations are classified based on preliminary biochemical studies and need further characterization. In Palomero et al.39, RhoA mutations other than G17V were identified in a single case each and the authors didn’t specify the PTCL subtype. They are included in PTCL-NOS here for simplicity.
RhoA
A recent study of K-Ras-induced hepatic adenoma formation in Zebra fish found that constitutively active RhoA reduced adenoma size and increased animal survival, while dominant negative RhoA resulted in larger adenomas and decreased survival.94 These observations appear opposite of results from in vitro studies of K-Ras transformed fibroblasts by dominant RhoA mutant expression. The authors found that increased neoplasia resulting from dominant negative RhoA was in part due to increased AKT and S6 signaling and upregulation of cyclin D1. This finding is in line with two in vitro studies which found RhoA negatively regulated AKT phosphorylation and decreased cyclin D1 levels in endothelial cells and K-Ras-driven adrenocortical cancer cell lines.95, 96 Another recent study of a murine colon cancer model induced by mutant APC found that simultaneous expression of dominant negative RhoA resulted in larger and more frequent adenomas and decreased survival.97 Perhaps more intriguing, conditional gene deletion of either RhoA or RhoC alone did not suppress K-RasG12D induced lung adenoma initiation. Rather, deletion of RhoA alone exacerbated lung adenoma formation, whereas dual deletion of both RhoA and RhoC significantly reduced K-RasG12D induced adenoma formation.98 In this context, deletion of RhoA seems to induce a compensatory mechanism that exacerbates adenoma formation, which is at least partly mediated by RhoC.
The strongest evidence that RhoA may have a tumor suppressor role has come to light in human cancer genomic studies (Figure 2) (commented in Ref.99–103). A compelling finding is the recent whole exome sequencing of T cell lymphoma in several studies which found that 50.3% – 70.8% of angioimmunoblastic T cell lymphoma (AITL) and 7.7% – 18% of PTCL, not otherwise specified (PTCL-NOS), share a recurrent RhoAG17V mutation. RhoAG17V causes a loss of nucleotide binding, enhanced GEF interaction, and may act as a dominant negative.39–42 Another study of Burkitt lymphoma in children found recurrent RhoA mutations such as RhoAR5Q which appear to be loss-of-function and predominately disrupt RhoA interactions with GEFs.44 Further characterization of RhoAR5Q mutation confirmed its impaired activity using biochemical and cell functional assays.104 Most recently, a study of adult T-cell leukemia and lymphomas (ATLL) found that ~15% of ATLLs have several recurrent RhoA mutations in the GTP binding pocket, some of which were previously undescribed.43 Interestingly, some of the recurrent mutations are gain-of-function mutations, while others are loss-of-function or even dominant-negative mutations. These genetic data indicate that both gain- and loss-of-function RhoA mutations may be pro-oncogenic depending on the cell of origin of the ATLL, such that gain-of-function RhoA mutations are pro-oncogenic in Tregs, whereas loss-of-function mutations are pro-oncogenic in T memory cells.
Inactivating RhoA mutations have also been found in solid tumors. A large scale human cancer genetic study of paired normal and tumor tissues across multiple cancer types identified recurrent RhoA mutations at E40 and Y42 in seven tumors (six head and neck, one breast) that are likely to disrupt the interaction of RhoA with effectors.45 Similar mutations thought to abrogate or modulate RhoA effector interactions have been recently described in gastric cancer.35–38 RhoA mutation prevalence was estimated at 14.3% – 25.3% in diffuse-type gastric cancer and 3.9% – 5.4% in the whole cohort. RhoA mutations were noted in hotspot sites including Y42, G17, L57, and R5 (Figure 2). SiRNA-knocking down of RhoA in gastric cell lines containing mutant RhoA in Y42 or G17, but not wild-type RhoA, significantly impairs proliferation.35 Further rescue experiments in cells suggested that Y42C and G17E are gain-of-function mutations that may provide a strong growth advantage.35 However, biochemical analysis showed that Y42C and L57V are reduced in the active form, suggesting they work in a loss-of-function manner.36 Indeed, RhoAY42C has been evaluated in earlier biochemical assays and shown attenuated activation of PKN, but not mDia2 and ROCK1.105 Most recently, by applying an unsupervised method, ParsSNP, to the gastric cancer genome data set from Kakiuchi et al.,35 Kumar et al. confirmed that RhoAY42C may be a driver mutation.76 Recent studies of Gα13-RhoA signaling axis with a tumor suppressor role in Burkitt's lymphoma and diffuse large B-cell lymphoma have begun to causally associate the loss of RhoA signaling with tumorigenesis.92 It will be important to better define the mechanism of loss-of-function RhoA mutants, to contrast with gain-of-function mutants.
RhoB and other Rho family members
In contrast to findings suggestive of increased RhoA and/or RhoC expression or activity in many cancers, it has been long known that RhoB is deleted in multiple cancers, including lung cancer.106–108 Although RhoA, B, and C can all regulate actin stress fibers, cytoskeleton organization, and vesicle transportation redundantly, RhoB differs from RhoA/C in cellular localization and has distinct functions. RhoB is primarily localized to endosomes and regulates cytokine trafficking and cell survival,109 and has anti-proliferative and pro-apoptotic effects in cancer cells.91 RhoB is unique in that it can be modified by either a farnesyl or a geranylgeranyl moity, and its prenylation state seems to affect RhoB actions. Whereas farnesylated RhoB can be either pro- or anti-growth in different settings, geranylgeranylated RhoB displays consistent anti-growth activity.110 RhoB appears to act more than an inhibitory isoform that opposes the effects of RhoA and RhoC signaling.110 In agreement with its tumor suppressor role, RhoB deletion was shown to accelerate chemically induced skin tumors in mice.111 In a recent study, RhoB deletion lowered the risk of UVB-induced skin carcinogenesis, but tumors that did form were preferentially undifferentiated and highly proliferative, suggesting RhoB may promote skin cancer initiation but limits the tumor aggressiveness.112
There is evidence that Cdc42, which is typically considered an oncoprotein, can also present a tumor suppressing function as exemplified by the finding that mice with Cdc42-deficiency in blood developed a lethal myeloproliferative disorder.113 Likewise, mouse with Cdc42 ablated in hepatocytes and bile duct cells developed hepatomegaly soon after birth, and signs of transformation and hepatocellular carcinoma was observed later.114 Intestinal deletion of Cdc42 resulted in a hyperplasia of intestinal epithelial cells and drastically increased intestine length and thickness.115 Recently, Rac1 and Cdc42 activities were found to be decreased in human pheochromocytomas, possibly resulting from reduced expression level of two RhoGEFs, ARHGEF1 and FARP1.116
GAPs and Other Rho GTPase regulators
Since Rho GAPs act to decrease Rho-GTP species, they are generally considered tumor suppressors by virtual of their capability to downregulate Rho/Rac/Cdc42 activities.117 For instance, DLC-1 gene, the product of which is a Rac GAP, so named because it is often deleted in liver cancer, is either deleted or methylated in a wide variety of cancers.118, 119 However, another GAP that regulates RhoA activity, p190GAP, has been found to have upregulated in expression in inflammatory breast cells, and may have a pro-growth role.120 P190B heterozygous mouse showed reduced tumor penetrance and remarkably delayed tumor onset in MMTV-Neu breast cancer model.121 Similarly, expression of several RhoGAP genes was increased in basal-like breast cancer (BLBC), and knockdown of two of them, ArhGAP11A and RacGAP1, resulted in significant defects in the proliferations of BLBC cells.122 In the same study using CHASM75 to analyze the data set from Kakiuchi et al.,35 a potential driver mutation in one RhoGAP, ArhGAP28, was identified.76 While the role of individual RhoGAP in specific cancer awaits further characterization, their apparent pro- and anti-cancer cell proliferative functions may be associated with the paradoxical role of their respective substrates, Rho GTPases. Alternatively, RhoGAPs may be associated with the cycling regulation of Rho GTPases. Overexpression of RhoGAPs may allow proper cycling of Rho GTPase substrates under conditions of exacerbated Rho-dependent signaling in cancer cells.
Changes of RhoGDIs expression levels have also been associated with cancer.123 The changes vary by cancer types (reviewed in Ref.124). For example, RhoGDI1 expression is upregulated in colorectal and ovarian cancers,125–127 but downregulated in brain cancers.128 Increased expression of RhoGDI2 has been found in pancreatic cancers,129, 130 while the opposite occurs in bladder cancers.131 In breast cancer, conflicting results have been found for RhoGDI1 expression,48, 132 whereas GDI2 seemed to have a biphasic expression pattern.133 Genetic studies of specific RhoGDI in murine cancer models have been lacking and the full degree of complexity of RhoGDI function and regulation in cancer remains to be appreciated.
Balancing the pro- and anti-neoplastic roles of Rho GTPases in developing targeted therapy
An outstanding question is how to reconcile the pro- and anti-neoplastic effects attributable to individual Rho GTPases. While the majority of in vitro studies examining the role of RhoA, Cdc42 and Rac1 support their pro-neoplastic function, it is within the mouse in vivo and human genetic findings where the opposite effects arise. How can we reconcile such seemingly paradoxical findings? This may be partly explained by the difference between in vitro and in vivo experimental systems. First, in vitro studies may be biased due to extensive culture resulting in clonal variability of cell lines. The cell lines are well adapted towards rapid growth with reliance on key signaling pathways including Rho GTPases, and could be hypersensitive to perturbations of key signal transduction pathways. In contrast, malignant cells in an in vivo environment may be more resilient to cell signaling perturbations and possess a malleable signaling network more plastic for adaptive compensations. Second, Rho GTPases may also affect the tumor microenvironment in vivo, which is missing in vitro, to either favor or antagonize tumor growth in a cell-type specific manner. Third, manipulating the level of one Rho GTPase in cell lines may affect the level and activity of other endogenous Rho GTPases, thereby, some of the conclusions from in vitro studies need to be cautioned. It is increasingly clear that our current understanding of Rho signaling in tumorigenicity is still incomplete.
Analogy to other targeted approaches in compensatory response
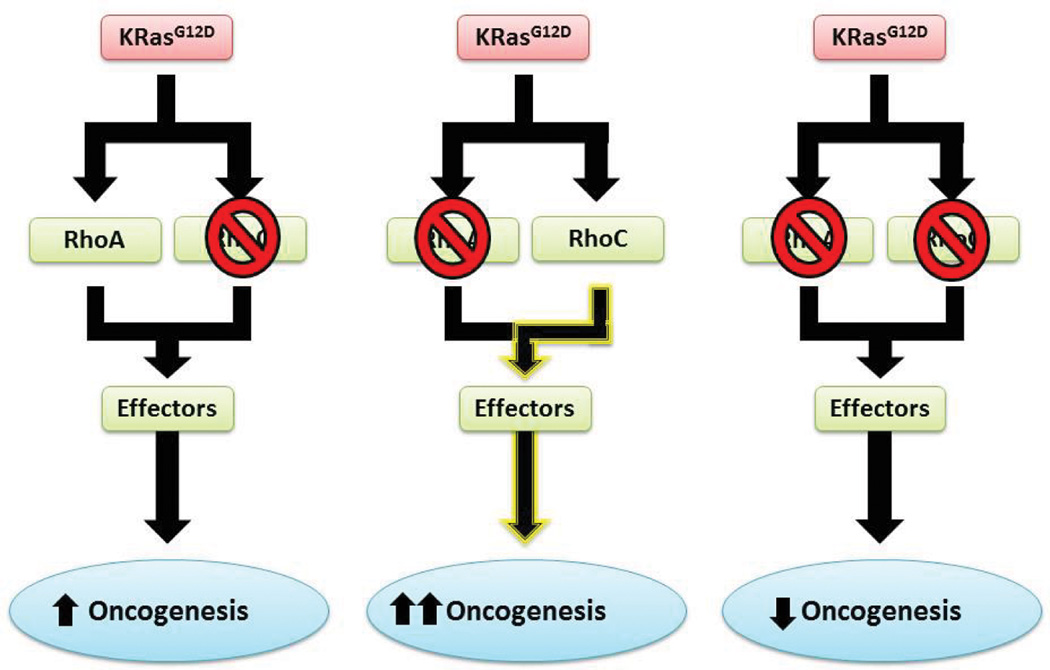
In a recent study, RhoA was found to be pro-neoplastic in the absence of RhoC, but anti-neoplastic in the presence of RhoC in murine lung adenoma model.98 This seemingly paradoxical observation shares an analogy with other targeted therapy situations. A prominent example is the adverse effects of B-Raf inhibitors.134 Despite the well appreciated pro-neoplastic role of BRAF-activating mutations in metastatic melanoma, clinical trials of the B-Raf inhibitors, vemurafenib and dabrafenib, yielded unexpected results that B-Raf targeting can mediate the related C-Raf activation leading to a relapse of more malignant tumors.135, 136 This compensatory interplay of B-Raf and C-Raf parallels that of Rho GTPase crosstalk, including the roles of RhoA and RhoC, despite that they may be mediated by distinct mechanisms (Figure 3). Such a compensatory response is not surprising in the context of cancer cell signaling network. In a recently report, inhibition of MEK in K-Ras-mutant lung and pancreatic cancer cells provokes a signaling rebound via FGFR1. Combinational inhibitors of MEK and FGFR1 enhance tumor death in vitro and in vivo.137 The compensatory response may reflect another way that Rho family members can contribute to the development of resistance to targeted therapies: the level or activity of one Rho protein can affect the level and/or activity of others.
Figure 3.
A scheme of the interplay between RhoA and RhoC signaling in K-Ras-driven cancer. The model summarizes the possible effects of RhoA inhibition on K-Ras-driven tumor formation. RhoA loss can paradoxically result in increased oncogenesis through a compensatory elevated RhoC activity, endowing RhoA to behave in a tumor suppressing role. In the absence of RhoC, RhoA is required for oncogenesis, displaying pro-oncogenic signal as an antineoplastic drug target.
Rational targeting of Rho GTPase signaling
Due to their globular structure, small GTPases such as Rho and Ras have been deemed “undruggable” by traditional drug design approaches. However, there have been advances in the field over the past decade with pre-clinical and clinical progress of several targeting strategies of Rho GTPase pathways. One strategy is targeting the interaction of Rho GTPase with its GEFs, which has been achieved at the preclinical level in the three prototypical Rho GTPases, i.e. Rac1, RhoA, and Cdc42. For example, NSC23766 is an inhibitor which disrupts the interaction of Rac1 with GEFs such as TIAM1, and CASIN is a Cdc42 inhibitor which disrupts the Rho:GEF interaction.138, 139 In fact, Rac1 inhibitors are especially desirable in the treatment of many melanomas, as common Rac1P29S mutation in melanoma has been shown to confer resistance to B-Raf inhibitors for the treatment of B-Raf driven metastatic melanomas.140 The chemical probe Rhosin (also termed G04) was developed to bind between the two important “switch” regions of RhoA and inhibit its interaction with GEFs such as leukemia-associated RhoGEF (LARG). Not only does this probe decrease phosphorylation of myosin and have antineoplastic effects in vitro, but importantly it inhibits signaling of RhoC as well.141, 142 The alternate approach of targeting RhoGEFs rather than Rho GTPase itself has also been useful conceptually for inhibiting Rho activities. For example, the chemical probe Y16 binds to LARG and inhibits Rho GEF activity.141, 143
The other major target for disrupting GTPase activity is the activity of downstream effectors. With regard to Rho GTPases, a successful example is the ROCK inhibitors fasudil and Y-27632.144, 145 Both inhibitors bind the ATP-binding pocket of ROCK and inhibit serine-threonine kinase activity. Fasudil is the only clinically used Rho GTPase pathway inhibitor and is used to treat pulmonary hypertension and cerebral hypertension in Japan, along with a sister compound, ripasudil, being used to treat glaucoma in Japan. Other GTPase effector inhibitors include several developed against the Cdc42 and Rac effector, PAK.146 Another notable lead inhibitor, Phox-I, inhibits the Rac effector p67phox of the NOX2 enzyme complex which produces Rac-mediated superoxide.147 Extensive reviews can be found elsewhere describing the development of Rho GTPase inhibitors and strategies to inhibit GTPase signaling including targeting GDIs or post-transcriptional modifications.24, 148, 149
Despite the important roles of Rho GTPases in cancer and considerable efforts to target Rho-dependent pathways, no inhibitor of Rho GTPase signaling has yet been used clinically to treat cancer, and the number of clinical trials is still limited. Besides the technical difficulty to target small GTPases per se, one major challenge is the lack of knowledge about the actual roles of Rho GTPase-dependent pathways at the organismal level, which is reflected by the recent human cancer genetic studies. It will be important to carry out further analysis of the signaling network changes of the gain- and loss-function mutation bearing tumors, particularly the downstream pathways that may better explain possible compensatory effects and selection pressure of the cancer cells under the driver mutations and defined microenvironment. Such comprehensive characterization of Rho GTPase signaling activities will guide the rationale in pharmacological targeting of Rho GTPases for anti-cancer therapy development.
In addition to modulate cancer cell proliferation, survival and migration, Rho GTPase pathways play a role in the resistance of anti-cancer radiotherapy and chemotherapy. For example, the RhoA/ROCK pathway was implicated in cancer cell stemness and radioresistance.150 Overexpression of RhoGDI1 increased resistance of cancer cells to the induction of apoptosis by chemotherapeutic agents etoposide and doxorubicin,151 and RhoGDI2 was identified as a key player in resistance to a cyclin-dependent kinases inhibitor.152 Thus, combined inhibition of Rho signaling with other anti-cancer therapy may be useful to achieve greater efficacy while reducing potential resistance. To this end, ROCK inhibitor fasudil and MEK inhibitor trametinib cooperatively induced apoptosis in N-Ras mutant melanoma.153 Using a synthetic lethal drug screen to identify innovative drug combinations to treat K-Ras mutant cancers, Wang et al. recently showed that dual inhibition of Rho signaling components polo-like kinase 1 (PLK1) and ROCK leads to synergistic effects to induce apoptosis and cell-cycle arrest in vitro and causing potent tumor regressions in vivo.154
Conclusions and Perspectives
Rho GTPase signaling is important in Ras pathways and other cancer-driven mechanisms. Multiple Rho GTPases haven been found to be potential anti-neoplastic targets in a wide variety of cancers including colon cancer, breast cancer, and leukemia.7, 155, 156 Fasudil, a ROCK inhibitor, has been used to treat pulmonary hypertension and is now being researched for the treatment of refractory angina.157–159 Recent studies show that Rho GTPases, RhoA and Cdc42 in particular, may also behave as “tumor suppressors” in certain cancer and defined circumstances. Such a complex, and sometimes paradoxical, interpretation of Rho GTPase functions also applies to their regulators and effectors, and redundant functions between Rho family homologs and interplays of feedback signaling loops may be involved. Dynamic selection pressure upon loss or gain of a Rho GTPase function is likely a contributing factor in the specific tumor context, as evidenced in T cell leukemia and lymphoma.43 Loss- and gain-of-function mutations of RhoA may endow the tumor cells selective advantage in early vs. late stages of the cancer progression, respectively. Further stringent demonstrations of their causal role and unveiling the underlying mechanism in driving tumorigenesis or tumor suppression in specific cancer types are warranted.
Considering recent findings, development of novel approaches inhibiting individual Rho GTPase activities need a more careful consideration. The timing and balance of downstream signals and possible compensatory feedback mechanisms after effective inhibition of one Rho GTPase must be assessed in order to ensure a beneficial tumor suppressing outcome. For example, the possible redundant and compensatory signaling from RhoC should be considered upon specific targeting of RhoA in tumors from the onset. These considerations suggest that the use of relatively more “promiscuous” drugs that inhibit multiple Rho GTPases such as RhoA/RhoC or further downstream signaling, such as ROCK or PAK, with acceptable toxicity, may provide better efficacies and also be beneficial for reducing potential resistance to the therapy against a single Rho GTPase target. In congruence with this idea, agents such as phytochemical Rocaglamide that inhibits the activities of Rho, Cdc42, and Rac160 may represent a new class of anticancer drugs. Although inhibitors of Rho GTPase pathways have not been clinically used for cancer treatment, rational targeting of Rho GTPase signaling nevertheless carries significant potentials in anti-cancer drug discovery, especially in future combinatory therapies.
Acknowledgments
We would like to acknowledge support from the University of Cincinnati Medical Scientist Training Program and the Molecular and Developmental Biology Graduate Program at Cincinnati Children’s Hospital Medical Center. The work in the Y. Z. lab is partly supported by NIH grants R01 CA193350, R01 DK104814 and R01 HL134617.
Footnotes
Conflict of Interest
The authors declare no conflict of interests.
References
- 1.Hall A. Rho family GTPases. Biochem Soc Trans. 2012;40:1378–1382. doi: 10.1042/BST20120103. [DOI] [PubMed] [Google Scholar]
- 2.Rojas AM, Fuentes G, Rausell A, Valencia A. The Ras protein superfamily: evolutionary tree and role of conserved amino acids. J Cell Biol. 2012;196:189–201. doi: 10.1083/jcb.201103008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev. 2013;93:269–309. doi: 10.1152/physrev.00003.2012. [DOI] [PubMed] [Google Scholar]
- 4.Boulter E, Garcia-Mata R. RhoGDI: A rheostat for the Rho switch. Small GTPases. 2010;1:65–68. doi: 10.4161/sgtp.1.1.12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 6.Orgaz JL, Herraiz C, Sanz-Moreno V. Rho GTPases modulate malignant transformation of tumor cells. Small GTPases. 2014;5:e29019. doi: 10.4161/sgtp.29019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H, Peyrollier K, Kilic G, Brakebusch C. Rho GTPases and cancer. Biofactors. 2014;40:226–235. doi: 10.1002/biof.1155. [DOI] [PubMed] [Google Scholar]
- 8.Khosravi-Far R, Solski PA, Clark GJ, Kinch MS, Der CJ. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol Cell Biol. 1995;15:6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiu RG, Chen J, Kirn D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature. 1995;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- 10.Qiu RG, Chen J, McCormick F, Symons M. A role for Rho in Ras transformation. Proc Natl Acad Sci U S A. 1995;92:11781–11785. doi: 10.1073/pnas.92.25.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–342. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- 12.Bar-Sagi D, Hall A. Ras and Rho GTPases: a family reunion. Cell. 2000;103:227–238. doi: 10.1016/s0092-8674(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 13.Mitin N, Rossman KL, Der CJ. Signaling interplay in Ras superfamily function. Curr Biol. 2005;15:R563–R574. doi: 10.1016/j.cub.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Lambert JM, Lambert QT, Reuther GW, Malliri A, Siderovski DP, Sondek J, et al. Tiam1 mediates Ras activation of Rac by a PI(3)K-independent mechanism. Nature cell biology. 2002;4:621–625. doi: 10.1038/ncb833. [DOI] [PubMed] [Google Scholar]
- 15.Welch HC. Regulation and function of P-Rex family Rac-GEFs. Small GTPases. 2015;6:49–70. doi: 10.4161/21541248.2014.973770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ridley AJ. Rho proteins and cancer. Breast Cancer Res Treat. 2004;84:13–19. doi: 10.1023/B:BREA.0000018423.47497.c6. [DOI] [PubMed] [Google Scholar]
- 17.Vega FM, Ridley AJ. Rho GTPases in cancer cell biology. FEBS Lett. 2008;582:2093–2101. doi: 10.1016/j.febslet.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 18.Karlsson R, Pedersen ED, Wang Z, Brakebusch C. Rho GTPase function in tumorigenesis. Biochim Biophys Acta. 2009;1796:91–98. doi: 10.1016/j.bbcan.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Parri M, Chiarugi P. Rac and Rho GTPases in cancer cell motility control. Cell Commun Signal. 2010;8:23. doi: 10.1186/1478-811X-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridley AJ. RhoA, RhoB and RhoC have different roles in cancer cell migration. J Microsc. 2013;251:242–249. doi: 10.1111/jmi.12025. [DOI] [PubMed] [Google Scholar]
- 21.Barrio-Real L, Kazanietz MG. Rho GEFs and cancer: linking gene expression and metastatic dissemination. Sci Signal. 2012;5:pe43. doi: 10.1126/scisignal.2003543. [DOI] [PubMed] [Google Scholar]
- 22.Cook DR, Rossman KL, Der CJ. Rho guanine nucleotide exchange factors: regulators of Rho GTPase activity in development and disease. Oncogene. 2014;33:4021–4035. doi: 10.1038/onc.2013.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vigil D, Cherfils J, Rossman KL, Der CJ. Ras superfamily GEFs and GAPs: validated and tractable targets for cancer therapy? Nat Rev Cancer. 2010;10:842–857. doi: 10.1038/nrc2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Y, Zheng Y. Approaches of targeting Rho GTPases in cancer drug discovery. Expert Opin Drug Discov. 2015;10:991–1010. doi: 10.1517/17460441.2015.1058775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porter AP, Papaioannou A, Malliri A. Deregulation of Rho GTPases in cancer. Small GTPases. 2016:1–16. doi: 10.1080/21541248.2016.1173767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alan JK, Lundquist EA. Mutationally activated Rho GTPases in cancer. Small GTPases. 2013;4:159–163. doi: 10.4161/sgtp.26530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pajic M, Herrmann D, Vennin C, Conway JR, Chin VT, Johnsson AK, et al. The dynamics of Rho GTPase signaling and implications for targeting cancer and the tumor microenvironment. Small GTPases. 2015;6:123–133. doi: 10.4161/21541248.2014.973749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays. 2007;29:356–370. doi: 10.1002/bies.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nature reviews Molecular cell biology. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 30.Zhou X, Zheng Y. Cell type-specific signaling function of RhoA GTPase: lessons from mouse gene targeting. J Biol Chem. 2013;288:36179–36188. doi: 10.1074/jbc.R113.515486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brumby AM, Goulding KR, Schlosser T, Loi S, Galea R, Khoo P, et al. Identification of novel Ras-cooperating oncogenes in Drosophila melanogaster: a RhoGEF/Rho-family/JNK pathway is a central driver of tumorigenesis. Genetics. 2011;188:105–125. doi: 10.1534/genetics.111.127910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar MS, Hancock DC, Molina-Arcas M, Steckel M, East P, Diefenbacher M, et al. The GATA2 transcriptional network is requisite for RAS oncogene-driven non-small cell lung cancer. Cell. 2012;149:642–655. doi: 10.1016/j.cell.2012.02.059. [DOI] [PubMed] [Google Scholar]
- 33.Konstantinidou G, Ramadori G, Torti F, Kangasniemi K, Ramirez RE, Cai YR, et al. RHOA-FAK Is a Required Signaling Axis for the Maintenance of KRAS-Driven Lung Adenocarcinomas. Cancer Discov. 2013;3:444–457. doi: 10.1158/2159-8290.CD-12-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang SY, Tang QL, Xu F, Xue Y, Zhen ZP, Deng Y, et al. RhoA Regulates G(1)-S Progression of Gastric Cancer Cells by Modulation of Multiple INK4 Family Tumor Suppressors. Mol Cancer Res. 2009;7:570–580. doi: 10.1158/1541-7786.MCR-08-0248. [DOI] [PubMed] [Google Scholar]
- 35.Kakiuchi M, Nishizawa T, Ueda H, Gotoh K, Tanaka A, Hayashi A, et al. Recurrent gain-of-function mutations of RHOA in diffuse-type gastric carcinoma. Nat Genet. 2014;46:583–587. doi: 10.1038/ng.2984. [DOI] [PubMed] [Google Scholar]
- 36.Wang K, Yuen ST, Xu J, Lee SP, Yan HH, Shi ST, et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet. 2014;46:573–582. doi: 10.1038/ng.2983. [DOI] [PubMed] [Google Scholar]
- 37.Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rocken C, Behrens HM, Boger C, Kruger S. Clinicopathological characteristics of RHOA mutations in a Central European gastric cancer cohort. J Clin Pathol. 2016;69:70–75. doi: 10.1136/jclinpath-2015-202980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palomero T, Couronne L, Khiabanian H, Kim MY, Ambesi-Impiombato A, Perez-Garcia A, et al. Recurrent mutations in epigenetic regulators, RHOA and FYN kinase in peripheral T cell lymphomas. Nat Genet. 2014;46:166–170. doi: 10.1038/ng.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakata-Yanagimoto M, Enami T, Yoshida K, Shiraishi Y, Ishii R, Miyake Y, et al. Somatic RHOA mutation in angioimmunoblastic T cell lymphoma. Nat Genet. 2014;46:171–175. doi: 10.1038/ng.2872. [DOI] [PubMed] [Google Scholar]
- 41.Yoo HY, Sung MK, Lee SH, Kim S, Lee H, Park S, et al. A recurrent inactivating mutation in RHOA GTPase in angioimmunoblastic T cell lymphoma. Nat Genet. 2014;46:371–375. doi: 10.1038/ng.2916. [DOI] [PubMed] [Google Scholar]
- 42.Manso R, Sanchez-Beato M, Monsalvo S, Gomez S, Cereceda L, Llamas P, et al. The RHOA G17V gene mutation occurs frequently in peripheral T-cell lymphoma and is associated with a characteristic molecular signature. Blood. 2014;123:2893–2894. doi: 10.1182/blood-2014-02-555946. [DOI] [PubMed] [Google Scholar]
- 43.Nagata Y, Kontani K, Enami T, Kataoka K, Ishii R, Totoki Y, et al. Variegated RHOA mutations in adult T-cell leukemia/lymphoma. Blood. 2016;127:596–604. doi: 10.1182/blood-2015-06-644948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rohde M, Richter J, Schlesner M, Betts MJ, Claviez A, Bonn BR, et al. Recurrent RHOA mutations in pediatric Burkitt lymphoma treated according to the NHL-BFM protocols. Genes Chromosomes Cancer. 2014;53:911–916. doi: 10.1002/gcc.22202. [DOI] [PubMed] [Google Scholar]
- 45.Lawrence MS, Stojanov P, Mermel CH, Robinson JT, Garraway LA, Golub TR, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505:495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imielinski M, Hernandez B, Lawrence M, Hodis E, Kryukov G, Stojanov P, et al. Uncovering signals of somatic selection through whole exome and whole genome sequencing of lung adenocarcinoma. Cancer Research. 2012;72 [Google Scholar]
- 47.Hakem A, Sanchez-Sweatman O, You-Ten A, Duncan G, Wakeham A, Khokha R, et al. RhoC is dispensable for embryogenesis and tumor initiation but essential for metastasis. Genes Dev. 2005;19:1974–1979. doi: 10.1101/gad.1310805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fritz G, Brachetti C, Bahlmann F, Schmidt M, Kaina B. Rho GTPases in human breast tumours: expression and mutation analyses and correlation with clinical parameters. British journal of cancer. 2002;87:635–644. doi: 10.1038/sj.bjc.6600510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu N, Zhang G, Bi F, Pan Y, Xue Y, Shi Y, et al. RhoC is essential for the metastasis of gastric cancer. J Mol Med (Berl) 2007;85:1149–1156. doi: 10.1007/s00109-007-0217-y. [DOI] [PubMed] [Google Scholar]
- 50.Kissil JL, Walmsley MJ, Hanlon L, Haigis KM, Bender Kim CF, Sweet-Cordero A, et al. Requirement for Rac1 in a K-ras induced lung cancer in the mouse. Cancer Res. 2007;67:8089–8094. doi: 10.1158/0008-5472.CAN-07-2300. [DOI] [PubMed] [Google Scholar]
- 51.Samuel MS, Lourenco FC, Olson MF. K-Ras mediated murine epidermal tumorigenesis is dependent upon and associated with elevated Rac1 activity. PLoS One. 2011;6:e17143. doi: 10.1371/journal.pone.0017143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z, Pedersen E, Basse A, Lefever T, Peyrollier K, Kapoor S, et al. Rac1 is crucial for Ras-dependent skin tumor formation by controlling Pak1-Mek-Erk hyperactivation and hyperproliferation in vivo. Oncogene. 2010;29:3362–3373. doi: 10.1038/onc.2010.95. [DOI] [PubMed] [Google Scholar]
- 53.Wu CY, Carpenter ES, Takeuchi KK, Halbrook CJ, Peverley LV, Bien H, et al. PI3K regulation of RAC1 is required for KRAS-induced pancreatic tumorigenesis in mice. Gastroenterology. 2014;147:1405 e1407–1416 e1407. doi: 10.1053/j.gastro.2014.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mizukawa B, Wei J, Shrestha M, Wunderlich M, Chou FS, Griesinger A, et al. Inhibition of Rac GTPase signaling and downstream prosurvival Bcl-2 proteins as combination targeted therapy in MLL-AF9 leukemia. Blood. 2011;118:5235–5245. doi: 10.1182/blood-2011-04-351817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nohata N, Uchida Y, Stratman AN, Adams RH, Zheng Y, Weinstein BM, et al. Temporal-specific roles of Rac1 during vascular development and retinal angiogenesis. Dev Biol. 2016;411:183–194. doi: 10.1016/j.ydbio.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 56.Frances D, Sharma N, Pofahl R, Maneck M, Behrendt K, Reuter K, et al. A role for Rac1 activity in malignant progression of sebaceous skin tumors. Oncogene. 2015;34:5505–5512. doi: 10.1038/onc.2014.471. [DOI] [PubMed] [Google Scholar]
- 57.Stengel KR, Zheng Y. Essential role of Cdc42 in Ras-induced transformation revealed by gene targeting. PLoS One. 2012;7:e37317. doi: 10.1371/journal.pone.0037317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sakamori R, Yu S, Zhang X, Hoffman A, Sun J, Das S, et al. CDC42 inhibition suppresses progression of incipient intestinal tumors. Cancer Res. 2014;74:5480–5492. doi: 10.1158/0008-5472.CAN-14-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jordan P, Brazao R, Boavida MG, Gespach C, Chastre E. Cloning of a novel human Rac1b splice variant with increased expression in colorectal tumors. Oncogene. 1999;18:6835–6839. doi: 10.1038/sj.onc.1203233. [DOI] [PubMed] [Google Scholar]
- 60.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Silva AL, Carmo F, Bugalho MJ. RAC1b overexpression in papillary thyroid carcinoma: a role to unravel. Eur J Endocrinol. 2013;168:795–804. doi: 10.1530/EJE-12-0960. [DOI] [PubMed] [Google Scholar]
- 62.Singh A, Karnoub AE, Palmby TR, Lengyel E, Sondek J, Der CJ. Rac1b, a tumor associated, constitutively active Rac1 splice variant, promotes cellular transformation. Oncogene. 2004;23:9369–9380. doi: 10.1038/sj.onc.1208182. [DOI] [PubMed] [Google Scholar]
- 63.Matos P, Oliveira C, Velho S, Goncalves V, da Costa LT, Moyer MP, et al. B-Raf(V600E) cooperates with alternative spliced Rac1b to sustain colorectal cancer cell survival. Gastroenterology. 2008;135:899–906. doi: 10.1053/j.gastro.2008.05.052. [DOI] [PubMed] [Google Scholar]
- 64.Zhou C, Licciulli S, Avila JL, Cho M, Troutman S, Jiang P, et al. The Rac1 splice form Rac1b promotes K-ras-induced lung tumorigenesis. Oncogene. 2013;32:903–909. doi: 10.1038/onc.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krauthammer M, Kong Y, Ha BH, Evans P, Bacchiocchi A, McCusker JP, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet. 2012;44:1006–1014. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chang MT, Asthana S, Gao SP, Lee BH, Chapman JS, Kandoth C, et al. Identifying recurrent mutations in cancer reveals widespread lineage diversity and mutational specificity. Nat Biotechnol. 2016;34:155–163. doi: 10.1038/nbt.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berger MF, Hodis E, Heffernan TP, Deribe YL, Lawrence MS, Protopopov A, et al. Melanoma genome sequencing reveals frequent PREX2 mutations. Nature. 2012;485:502–506. doi: 10.1038/nature11071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robles AI, Traverso G, Zhang M, Roberts NJ, Khan MA, Joseph C, et al. Whole-Exome Sequencing Analyses of Inflammatory Bowel Disease-Associated Colorectal Cancers. Gastroenterology. 2016;150:931–943. doi: 10.1053/j.gastro.2015.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lissanu Deribe Y, Shi Y, Rai K, Nezi L, Amin SB, Wu CC, et al. Truncating PREX2 mutations activate its GEF activity and alter gene expression regulation in NRAS-mutant melanoma. Proc Natl Acad Sci U S A. 2016;113:E1296–E1305. doi: 10.1073/pnas.1513801113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kataoka K, Nagata Y, Kitanaka A, Shiraishi Y, Shimamura T, Yasunaga J, et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet. 2015;47:1304–1315. doi: 10.1038/ng.3415. [DOI] [PubMed] [Google Scholar]
- 73.Campbell JD, Alexandrov A, Kim J, Wala J, Berger AH, Pedamallu CS, et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat Genet. 2016;48:607–616. doi: 10.1038/ng.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boddicker RL, Razidlo GL, Dasari S, Zeng Y, Hu G, Knudson RA, et al. Integrated mate-pair and RNA sequencing identifies novel, targetable gene fusions in peripheral T-cell lymphoma. Blood. 2016;128:1234–1245. doi: 10.1182/blood-2016-03-707141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carter H, Chen S, Isik L, Tyekucheva S, Velculescu VE, Kinzler KW, et al. Cancer-specific high-throughput annotation of somatic mutations: computational prediction of driver missense mutations. Cancer Res. 2009;69:6660–6667. doi: 10.1158/0008-5472.CAN-09-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kumar RD, Swamidass SJ, Bose R. Unsupervised detection of cancer driver mutations with parsimony-guided learning. Nat Genet. 2016;48:1288–1294. doi: 10.1038/ng.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fine B, Hodakoski C, Koujak S, Su T, Saal LH, Maurer M, et al. Activation of the PI3K pathway in cancer through inhibition of PTEN by exchange factor P-REX2a. Science. 2009;325:1261–1265. doi: 10.1126/science.1173569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cullis J, Meiri D, Sandi MJ, Radulovich N, Kent OA, Medrano M, et al. The RhoGEF GEF-H1 is required for oncogenic RAS signaling via KSR-1. Cancer Cell. 2014;25:181–195. doi: 10.1016/j.ccr.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 79.Morgan-Fisher M, Wewer UM, Yoneda A. Regulation of ROCK activity in cancer. J Histochem Cytochem. 2013;61:185–198. doi: 10.1369/0022155412470834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rath N, Olson MF. Rho-associated kinases in tumorigenesis: re-considering ROCK inhibition for cancer therapy. EMBO Rep. 2012;13:900–908. doi: 10.1038/embor.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wei L, Surma M, Shi S, Lambert-Cheatham N, Shi J. Novel Insights into the Roles of Rho Kinase in Cancer. Arch Immunol Ther Exp (Warsz) 2016;64:259–278. doi: 10.1007/s00005-015-0382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kumper S, Mardakheh FK, McCarthy A, Yeo M, Stamp GW, Paul A, et al. Rho-associated kinase (ROCK) function is essential for cell cycle progression, senescence and tumorigenesis. Elife. 2016;5 doi: 10.7554/eLife.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feng Y, LoGrasso PV, Defert O, Li R. Rho Kinase (ROCK) Inhibitors and Their Therapeutic Potential. J Med Chem. 2016;59:2269–2300. doi: 10.1021/acs.jmedchem.5b00683. [DOI] [PubMed] [Google Scholar]
- 84.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kitzing TM, Wang Y, Pertz O, Copeland JW, Grosse R. Formin-like 2 drives amoeboid invasive cell motility downstream of RhoC. Oncogene. 2010;29:2441–2448. doi: 10.1038/onc.2009.515. [DOI] [PubMed] [Google Scholar]
- 86.Fritsch R, de Krijger I, Fritsch K, George R, Reason B, Kumar MS, et al. RAS and RHO families of GTPases directly regulate distinct phosphoinositide 3-kinase isoforms. Cell. 2013;153:1050–1063. doi: 10.1016/j.cell.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang HW, Shin MG, Lee S, Kim JR, Park WS, Cho KH, et al. Cooperative activation of PI3K by Ras and Rho family small GTPases. Mol Cell. 2012;47:281–290. doi: 10.1016/j.molcel.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rev Cancer. 2006;6:459–471. doi: 10.1038/nrc1892. [DOI] [PubMed] [Google Scholar]
- 89.Chow HY, Jubb AM, Koch JN, Jaffer ZM, Stepanova D, Campbell DA, et al. p21-Activated kinase 1 is required for efficient tumor formation and progression in a Ras-mediated skin cancer model. Cancer Res. 2012;72:5966–5975. doi: 10.1158/0008-5472.CAN-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou W, Jubb AM, Lyle K, Xiao Q, Ong CC, Desai R, et al. PAK1 mediates pancreatic cancer cell migration and resistance to MET inhibition. J Pathol. 2014;234:502–513. doi: 10.1002/path.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Prendergast GC. Actin' up: RhoB in cancer and apoptosis. Nat Rev Cancer. 2001;1:162–168. doi: 10.1038/35101096. [DOI] [PubMed] [Google Scholar]
- 92.Riou P, Villalonga P, Ridley AJ. Rnd proteins: multifunctional regulators of the cytoskeleton and cell cycle progression. Bioessays. 2010;32:986–992. doi: 10.1002/bies.201000060. [DOI] [PubMed] [Google Scholar]
- 93.Aspenstrom P, Ruusala A, Pacholsky D. Taking Rho GTPases to the next level: the cellular functions of atypical Rho GTPases. Exp Cell Res. 2007;313:3673–3679. doi: 10.1016/j.yexcr.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 94.Chew TW, Liu XJ, Liu L, Spitsbergen JM, Gong Z, Low BC. Crosstalk of Ras and Rho: activation of RhoA abates Kras-induced liver tumorigenesis in transgenic zebrafish models. Oncogene. 2014;33:2717–2727. doi: 10.1038/onc.2013.240. [DOI] [PubMed] [Google Scholar]
- 95.Forti FL, Armelin HA. Vasopressin triggers senescence in K-ras transformed cells via RhoA-dependent downregulation of cyclin D1. Endocr Relat Cancer. 2007;14:1117–1125. doi: 10.1677/ERC-07-0154. [DOI] [PubMed] [Google Scholar]
- 96.Ming XF, Viswambharan H, Barandier C, Ruffieux J, Kaibuchi K, Rusconi S, et al. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol. 2002;22:8467–8477. doi: 10.1128/MCB.22.24.8467-8477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rodrigues P, Macaya I, Bazzocco S, Mazzolini R, Andretta E, Dopeso H, et al. RHOA inactivation enhances Wnt signalling and promotes colorectal cancer. Nat Commun. 2014;5:5458. doi: 10.1038/ncomms6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zandvakili I, Davis AK, Hu G, Zheng Y. Loss of RhoA Exacerbates, Rather Than Dampens, Oncogenic K-Ras Induced Lung Adenoma Formation in Mice. PLoS One. 2015;10:e0127923. doi: 10.1371/journal.pone.0127923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou J, Hayakawa Y, Wang TC, Bass AJ. RhoA mutations identified in diffuse gastric cancer. Cancer Cell. 2014;26:9–11. doi: 10.1016/j.ccr.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chiba S, Enami T, Ogawa S, Sakata-Yanagimoto M. G17V RHOA: Genetic evidence of GTP-unbound RHOA playing a role in tumorigenesis in T cells. Small GTPases. 2015;6:100–103. doi: 10.4161/21541248.2014.988088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gilbert-Ross M, Marcus AI, Zhou W. RhoA, a novel tumor suppressor or oncogene as a therapeutic target? Genes Dis. 2015;2:2–3. doi: 10.1016/j.gendis.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ishikawa S. Opposite RHOA functions within the ATLL category. Blood. 2016;127:524–525. doi: 10.1182/blood-2015-12-683458. [DOI] [PubMed] [Google Scholar]
- 103.Maeda M, Ushijima T. RHOA mutation may be associated with diffuse-type gastric cancer progression, but is it gain or loss? Gastric Cancer. 2016;19:326–328. doi: 10.1007/s10120-015-0525-9. [DOI] [PubMed] [Google Scholar]
- 104.O'Hayre M, Inoue A, Kufareva I, Wang Z, Mikelis CM, Drummond RA, et al. Inactivating mutations in GNA13 and RHOA in Burkitt's lymphoma and diffuse large B-cell lymphoma: a tumor suppressor function for the Galpha13/RhoA axis in B cells. Oncogene. 2016;35:3771–3780. doi: 10.1038/onc.2015.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sahai E, Alberts AS, Treisman R. RhoA effector mutants reveal distinct effector pathways for cytoskeletal reorganization, SRF activation and transformation. EMBO J. 1998;17:1350–1361. doi: 10.1093/emboj/17.5.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bousquet E, Mazieres J, Privat M, Rizzati V, Casanova A, Ledoux A, et al. Loss of RhoB expression promotes migration and invasion of human bronchial cells via activation of AKT1. Cancer Res. 2009;69:6092–6099. doi: 10.1158/0008-5472.CAN-08-4147. [DOI] [PubMed] [Google Scholar]
- 107.Mazieres J, Antonia T, Daste G, Muro-Cacho C, Berchery D, Tillement V, et al. Loss of RhoB expression in human lung cancer progression. Clin Cancer Res. 2004;10:2742–2750. doi: 10.1158/1078-0432.ccr-03-0149. [DOI] [PubMed] [Google Scholar]
- 108.Sato N, Fukui T, Taniguchi T, Yokoyama T, Kondo M, Nagasaka T, et al. RhoB is frequently downregulated in non-small-cell lung cancer and resides in the 2p24 homozygous deletion region of a lung cancer cell line. International journal of cancer Journal international du cancer. 2007;120:543–551. doi: 10.1002/ijc.22328. [DOI] [PubMed] [Google Scholar]
- 109.Wheeler AP, Ridley AJ. Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp Cell Res. 2004;301:43–49. doi: 10.1016/j.yexcr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 110.Huang M, Prendergast GC. RhoB in cancer suppression. Histol Histopathol. 2006;21:213–218. doi: 10.14670/HH-21.213. [DOI] [PubMed] [Google Scholar]
- 111.Liu AX, Rane N, Liu JP, Prendergast GC. RhoB is dispensable for mouse development, but it modifies susceptibility to tumor formation as well as cell adhesion and growth factor signaling in transformed cells. Mol Cell Biol. 2001;21:6906–6912. doi: 10.1128/MCB.21.20.6906-6912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Meyer N, Peyret-Lacombe A, Canguilhem B, Medale-Giamarchi C, Mamouni K, Cristini A, et al. RhoB promotes cancer initiation by protecting keratinocytes from UVB-induced apoptosis but limits tumor aggressiveness. J Invest Dermatol. 2014;134:203–212. doi: 10.1038/jid.2013.278. [DOI] [PubMed] [Google Scholar]
- 113.Yang L, Wang L, Kalfa TA, Cancelas JA, Shang X, Pushkaran S, et al. Cdc42 critically regulates the balance between myelopoiesis and erythropoiesis. Blood. 2007;110:3853–3861. doi: 10.1182/blood-2007-03-079582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.van Hengel J, D'Hooge P, Hooghe B, Wu X, Libbrecht L, De Vos R, et al. Continuous cell injury promotes hepatic tumorigenesis in cdc42-deficient mouse liver. Gastroenterology. 2008;134:781–792. doi: 10.1053/j.gastro.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 115.Melendez J, Liu M, Sampson L, Akunuru S, Han X, Vallance J, et al. Cdc42 coordinates proliferation, polarity, migration, and differentiation of small intestinal epithelial cells in mice. Gastroenterology. 2013;145:808–819. doi: 10.1053/j.gastro.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Croise P, Houy S, Gand M, Lanoix J, Calco V, Toth P, et al. Cdc42 and Rac1 activity is reduced in human pheochromocytoma and correlates with FARP1 and ARHGEF1 expression. Endocr Relat Cancer. 2016;23:281–293. doi: 10.1530/ERC-15-0502. [DOI] [PubMed] [Google Scholar]
- 117.Grogg M, Zheng Y. Rho GTPase-activating proteins in cancer. In: Golen KV, editor. The Rho GTPases in cancer, 2010 edn. Springer Publisher; 2010. pp. 93–110. [Google Scholar]
- 118.Braun AC, Olayioye MA. Rho regulation: DLC proteins in space and time. Cell Signal. 2015;27:1643–1651. doi: 10.1016/j.cellsig.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 119.Wang D, Qian X, Rajaram M, Durkin ME, Lowy DR. DLC1 is the principal biologically-relevant down-regulated DLC family member in several cancers. Oncotarget. 2016 doi: 10.18632/oncotarget.9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lin M, van Golen KL. Rho-regulatory proteins in breast cancer cell motility and invasion. Breast Cancer Res Treat. 2004;84:49–60. doi: 10.1023/B:BREA.0000018424.43445.f3. [DOI] [PubMed] [Google Scholar]
- 121.Heckman-Stoddard BM, Vargo-Gogola T, McHenry PR, Jiang V, Herrick MP, Hilsenbeck SG, et al. Haploinsufficiency for p190B RhoGAP inhibits MMTV-Neu tumor progression. Breast Cancer Res. 2009;11:R61. doi: 10.1186/bcr2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lawson CD, Fan C, Mitin N, Baker NM, George SD, Graham DM, et al. Rho GTPase Transcriptome Analysis Reveals Oncogenic Roles for Rho GTPase-Activating Proteins in Basal-like Breast Cancers. Cancer Res. 2016;76:3826–3837. doi: 10.1158/0008-5472.CAN-15-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Harding MA, Theodorescu D. RhoGDI signaling provides targets for cancer therapy. Eur J Cancer. 2010;46:1252–1259. doi: 10.1016/j.ejca.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Garcia-Mata R, Boulter E, Burridge K. The 'invisible hand': regulation of RHO GTPases by RHOGDIs. Nature reviews Molecular cell biology. 2011;12:493–504. doi: 10.1038/nrm3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jones MB, Krutzsch H, Shu H, Zhao Y, Liotta LA, Kohn EC, et al. Proteomic analysis and identification of new biomarkers and therapeutic targets for invasive ovarian cancer. Proteomics. 2002;2:76–84. [PubMed] [Google Scholar]
- 126.Zhao L, Wang H, Li J, Liu Y, Ding Y. Overexpression of Rho GDP-dissociation inhibitor alpha is associated with tumor progression and poor prognosis of colorectal cancer. J Proteome Res. 2008;7:3994–4003. doi: 10.1021/pr800271b. [DOI] [PubMed] [Google Scholar]
- 127.Zhao L, Wang H, Sun X, Ding Y. Comparative proteomic analysis identifies proteins associated with the development and progression of colorectal carcinoma. FEBS J. 2010;277:4195–4204. doi: 10.1111/j.1742-4658.2010.07808.x. [DOI] [PubMed] [Google Scholar]
- 128.Forget MA, Desrosiers RR, Del M, Moumdjian R, Shedid D, Berthelet F, et al. The expression of rho proteins decreases with human brain tumor progression: potential tumor markers. Clin Exp Metastasis. 2002;19:9–15. doi: 10.1023/a:1013884426692. [DOI] [PubMed] [Google Scholar]
- 129.Abiatari I, DeOliveira T, Kerkadze V, Schwager C, Esposito I, Giese NA, et al. Consensus transcriptome signature of perineural invasion in pancreatic carcinoma. Mol Cancer Ther. 2009;8:1494–1504. doi: 10.1158/1535-7163.MCT-08-0755. [DOI] [PubMed] [Google Scholar]
- 130.Koide N, Yamada T, Shibata R, Mori T, Fukuma M, Yamazaki K, et al. Establishment of perineural invasion models and analysis of gene expression revealed an invariant chain (CD74) as a possible molecule involved in perineural invasion in pancreatic cancer. Clin Cancer Res. 2006;12:2419–2426. doi: 10.1158/1078-0432.CCR-05-1852. [DOI] [PubMed] [Google Scholar]
- 131.Seraj MJ, Harding MA, Gildea JJ, Welch DR, Theodorescu D. The relationship of BRMS1 and RhoGDI2 gene expression to metastatic potential in lineage related human bladder cancer cell lines. Clin Exp Metastasis. 2000;18:519–525. doi: 10.1023/a:1011819621859. [DOI] [PubMed] [Google Scholar]
- 132.Jiang WG, Watkins G, Lane J, Cunnick GH, Douglas-Jones A, Mokbel K, et al. Prognostic value of rho GTPases and rho guanine nucleotide dissociation inhibitors in human breast cancers. Clin Cancer Res. 2003;9:6432–6440. [PubMed] [Google Scholar]
- 133.Hu LD, Zou HF, Zhan SX, Cao KM. Biphasic expression of RhoGDI2 in the progression of breast cancer and its negative relation with lymph node metastasis. Oncol Rep. 2007;17:1383–1389. [PubMed] [Google Scholar]
- 134.Holderfield M, Nagel TE, Stuart DD. Mechanism and consequences of RAF kinase activation by small-molecule inhibitors. British journal of cancer. 2014;111:640–645. doi: 10.1038/bjc.2014.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Oberholzer PA, Kee D, Dziunycz P, Sucker A, Kamsukom N, Jones R, et al. RAS mutations are associated with the development of cutaneous squamous cell tumors in patients treated with RAF inhibitors. J Clin Oncol. 2012;30:316–321. doi: 10.1200/JCO.2011.36.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Su F, Viros A, Milagre C, Trunzer K, Bollag G, Spleiss O, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366:207–215. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Manchado E, Weissmueller S, Morris JP, Chen CC, Wullenkord R, Lujambio A, et al. A combinatorial strategy for treating KRAS-mutant lung cancer. Nature. 2016;534:647–651. doi: 10.1038/nature18600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Florian MC, Dorr K, Niebel A, Daria D, Schrezenmeier H, Rojewski M, et al. Cdc42 Activity Regulates Hematopoietic Stem Cell Aging and Rejuvenation. Cell Stem Cell. 2012;10:520–530. doi: 10.1016/j.stem.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci U S A. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Watson IR, Li L, Cabeceiras PK, Mahdavi M, Gutschner T, Genovese G, et al. The RAC1 P29S hotspot mutation in melanoma confers resistance to pharmacological inhibition of RAF. Cancer Res. 2014;74:4845–4852. doi: 10.1158/0008-5472.CAN-14-1232-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shang X, Marchioni F, Evelyn CR, Sipes N, Zhou X, Seibel W, et al. Small-molecule inhibitors targeting G-protein-coupled Rho guanine nucleotide exchange factors. Proc Natl Acad Sci U S A. 2013;110:3155–3160. doi: 10.1073/pnas.1212324110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shang X, Marchioni F, Sipes N, Evelyn CR, Jerabek-Willemsen M, Duhr S, et al. Rational design of small molecule inhibitors targeting RhoA subfamily Rho GTPases. Chemistry & biology. 2012;19:699–710. doi: 10.1016/j.chembiol.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Evelyn CR, Ferng T, Rojas RJ, Larsen MJ, Sondek J, Neubig RR. High-Throughput Screening for Small-Molecule Inhibitors of LARG-Stimulated RhoA Nucleotide Binding via a Novel Fluorescence Polarization Assay. J Biomol Screen. 2009;14:161–172. doi: 10.1177/1087057108328761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nagumo H, Sasaki Y, Ono Y, Okamoto H, Seto M, Takuwa Y. Rho kinase inhibitor HA-1077 prevents Rho-mediated myosin phosphatase inhibition in smooth muscle cells. Am J Physiol Cell Physiol. 2000;278:C57–C65. doi: 10.1152/ajpcell.2000.278.1.C57. [DOI] [PubMed] [Google Scholar]
- 145.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 146.He H, Huynh N. p21-activated kinase family: promising new drug targets. Research and Reports in Biochemistry. 2015;5:119–128. [Google Scholar]
- 147.Bosco EE, Kumar S, Marchioni F, Biesiada J, Kordos M, Szczur K, et al. Rational design of small molecule inhibitors targeting the Rac GTPase-p67(phox) signaling axis in inflammation. Chemistry & biology. 2012;19:228–242. doi: 10.1016/j.chembiol.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Smithers CC, Overduin M. Structural Mechanisms and Drug Discovery Prospects of Rho GTPases. Cells. 2016;5 doi: 10.3390/cells5020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Biro M, Munoz MA, Weninger W. Targeting Rho-GTPases in immune cell migration and inflammation. Br J Pharmacol. 2014;171:5491–5506. doi: 10.1111/bph.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pranatharthi A, Ross C, Srivastava S. Cancer Stem Cells and Radioresistance: Rho/ROCK Pathway Plea Attention. Stem Cells Int. 2016;2016:5785786. doi: 10.1155/2016/5785786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang B, Zhang Y, Dagher MC, Shacter E. Rho GDP dissociation inhibitor protects cancer cells against drug-induced apoptosis. Cancer Res. 2005;65:6054–6062. doi: 10.1158/0008-5472.CAN-05-0175. [DOI] [PubMed] [Google Scholar]
- 152.Skalnikova H, Martinkova J, Hrabakova R, Halada P, Dziechciarkova M, Hajduch M, et al. Cancer drug-resistance and a look at specific proteins: Rho GDP-dissociation inhibitor 2, Y-box binding protein 1, and HSP70/90 organizing protein in proteomics clinical application. J Proteome Res. 2011;10:404–415. doi: 10.1021/pr100468w. [DOI] [PubMed] [Google Scholar]
- 153.Vogel CJ, Smit MA, Maddalo G, Possik PA, Sparidans RW, van der Burg SH, et al. Cooperative induction of apoptosis in NRAS mutant melanoma by inhibition of MEK and ROCK. Pigment Cell Melanoma Res. 2015;28:307–317. doi: 10.1111/pcmr.12364. [DOI] [PubMed] [Google Scholar]
- 154.Wang J, Hu K, Guo J, Cheng F, Lv J, Jiang W, et al. Suppression of KRas-mutant cancer through the combined inhibition of KRAS with PLK1 and ROCK. Nat Commun. 2016;7:11363. doi: 10.1038/ncomms11363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.McHenry PR, Vargo-Gogola T. Pleiotropic functions of Rho GTPase signaling: a Trojan horse or Achilles' heel for breast cancer treatment? Curr Drug Targets. 2010;11:1043–1058. doi: 10.2174/138945010792006852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Walker K, Olson MF. Targeting Ras and Rho GTPases as opportunities for cancer therapeutics. Curr Opin Genet Dev. 2005;15:62–68. doi: 10.1016/j.gde.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 157.Fukumoto Y, Mohri M, Inokuchi K, Ito A, Hirakawa Y, Masumoto A, et al. Anti-ischemic effects of fasudil, a specific Rho-kinase inhibitor, in patients with stable effort angina. J Cardiovasc Pharmacol. 2007;49:117–121. doi: 10.1097/FJC.0b013e31802ef532. [DOI] [PubMed] [Google Scholar]
- 158.Shimokawa H, Hiramori K, Iinuma H, Hosoda S, Kishida H, Osada H, et al. Anti-anginal effect of fasudil, a Rho-kinase inhibitor, in patients with stable effort angina: a multicenter study. J Cardiovasc Pharmacol. 2002;40:751–761. doi: 10.1097/00005344-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 159.Vicari RM, Chaitman B, Keefe D, Smith WB, Chrysant SG, Tonkon MJ, et al. Efficacy and safety of fasudil in patients with stable angina: a double-blind, placebo-controlled, phase 2 trial. J Am Coll Cardiol. 2005;46:1803–1811. doi: 10.1016/j.jacc.2005.07.047. [DOI] [PubMed] [Google Scholar]
- 160.Becker MS, Muller PM, Bajorat J, Schroeder A, Giaisi M, Amin E, et al. The anticancer phytochemical rocaglamide inhibits Rho GTPase activity and cancer cell migration. Oncotarget. 2016 doi: 10.18632/oncotarget.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ihara K, Muraguchi S, Kato M, Shimizu T, Shirakawa M, Kuroda S, et al. Crystal structure of human RhoA in a dominantly active form complexed with a GTP analogue. J Biol Chem. 1998;273:9656–9666. doi: 10.1074/jbc.273.16.9656. [DOI] [PubMed] [Google Scholar]