Abstract
Objective
To critically review and evaluate the proposed mechanisms and documented results of the therapeutics currently in active clinical drug trials for the treatment of sensorineural hearing loss.
Data Sources
U.S. National Institutes of Health (NIH) Clinical Trials registry, MEDLINE/PubMed.
Study Selection & Data Extraction
A review of the NIH Clinical Trials registry identified candidate hearing loss therapies, and supporting publications were acquired from MEDLINE/PubMed. Proof-of-concept, therapeutic mechanisms, and clinical outcomes were critically appraised.
Data Synthesis
22 active clinical drug trials registered in the United States were identified, and six potentially therapeutic molecules were reviewed. Of the six molecules reviewed, four comprised mechanisms pertaining to mitigating oxidative stress pathways that presumably lead to inner ear cell death. One remaining therapy sought to manipulate the cell death cascade, and the last remaining therapy was a novel cell replacement therapy approach to introduce a transcription factor that promotes hair cell regeneration.
Conclusion
A common theme in recent clinical trials registered in the United States appears to be the targeting of cell death pathways and influence of oxidant stressors on cochlear sensory neuroepithelium. In addition, a virus-delivered cell replacement therapy would be the first of its kind should it prove safe and efficacious. Significant challenges for bringing these bench-to-bedside therapies to market remain. It is never assured that results in non-human animal models translate to effective therapies in the setting of human biology. Moreover, as additional processes are described in association with hearing loss, such as an immune response and loss of synaptic contacts, additional pathways for targeting become available.
Keywords: Hearing loss, molecular therapy, gene therapy
INTRODUCTION
According to the most recent estimates of the World Health Organization, 360 million people, or approximately 5.3% of the world’s population, live with disabling hearing loss.1 In children, hearing loss has repeatedly been demonstrated to affect their academic, behavioral and cognitive development as well decreased overall quality of life.2–7 Deleterious effects of hearing loss in adults also generate morbidity as hearing loss has been linked to poor overall physical functioning and social interaction, as well decreased overall quality of life.8–11 Medical therapies for hearing loss have remained elusive despite the number of persons living with disabling hearing loss world-wide and the multi-dimensional burden of hearing loss.
The most common form of hearing loss world-wide is sensorineural hearing loss (SNHL). 12,13 The development of drugs to treat or prevent SNHL has proven challenging. Many investigators have sought to characterize the biochemical, molecular, and intra-cellular mechanisms in both normal-state hearing function and in pathological processes that impair hearing function. Over the past decade, academic institutions and pharmaceutical firms around the world have begun to commit significant resources to development of therapies for treatment of SNHL. These combined efforts have resulted in a relatively recent burst of clinical trial activity and increased hope that these emerging therapies can be brought to market. The objective of this review is to critically review and evaluate the proposed mechanisms and data of the therapeutics currently in active randomized clinical drug trials for the treatment of sensorineural hearing loss. In addition, we briefly discuss new drugs proposed to be on the short track for clinical trials.
METHODS
This review comprised periodicals and previously published data and therefore IRB approval was not required.
Clinical Trial Registry Review and Therapy Identification
Interventional clinical trials that investigated sensorineural hearing loss in phase 1 or later were included. Clinical trials that evaluated steroids, natural supplements (e.g. Gingko Biloba), treatments for conditions other than specifically for sensorineural hearing loss (e.g. Meniere’s Disease, autoimmune inner ear disease, cerumen impaction, congenital cytomegalovirus infection), or trials that were withdrawn/terminated were excluded.
RESULTS
We identified 22 active clinical drug trials registered in the United States (Table 1) as of the writing of this manuscript. Of the 22 active clinical trials, we reviewed six potentially therapeutic molecules (Table 2; Figure 1). Sufficient basic science and available clinical data were obtained for each molecule to inform a balanced discussion on the foundation and rationale of the therapy. Of the six molecules reviewed, four addressed mechanisms pertaining to mitigating oxidative stress pathways that presumably lead to auditory cell death. One remaining therapy sought to manipulate the cell death cascade, and the last remaining therapy was a novel cell replacement therapy approach to introduce a transcription factor that induces hair cell regeneration.
Table 1.
Curated list of active clinical drug trials for the prevention or treatment of sensorineural hearing loss. Source: Clinicaltrials.gov, as of July 9, 2016. DB – Double Blind, OL – Open Label. NIDCD - National Institute on Deafness and Other Communication Disorders.
| Trial Drug | First Listed Sponsor | Recruitment | Trial Phase | Funding | Masking | Start Date | End Date |
|---|---|---|---|---|---|---|---|
| PF-04958242 | Pfizer | Completed | Phase 1 | Industry | DB | Dec. 2011 | Feb. 2013 |
| SPI-1005 | Sound Pharmaceuticals Inc. | Completed | Phase 1 | Industry | DB | May 2006 | Aug. 2006 |
| HPN-07 | Otologic Pharmaceutics, Inc. | Completed | Phase 1 | Industry | DB | Oct. 2014 | Jan. 2015 |
| N-Acetylcysteine | Children’s Hospital Los Angeles | Recruiting | Phase 1 | Other | OL | Mar. 2016 | Feb. 2018 |
| Anakinra | Northwell Health | Completed | Phase 1 & 2 | Other | OL | Oct. 2013 | Jul. 2015 |
| Ancrod | Nordmark Arzneimittel GmbH & Co. KG | Recruiting | Phase 1 & 2 | Industry & Other | DB | May 2013 | Aug. 2016 |
| Zonisamide | Washington University School of Medicine | Not yet | Phase 1 & 2 | Other | OL | Jun. 2017 | Dec.2019 |
| Anakinra | NIDCD | Completed | Phase 1 & 2 | Other& NIH | OL | Jun. 2011 | Sept. 2014 |
| CGF166 | Novartis Pharmaceuticals | Recruiting | Phase 1 & 2 | Industry | OL | Oct. 2014 | Aug. 2017 |
| AUT00063 | Autifony Therapeutics Limited | Active, not recruiting | Phase 2 | Industry | DB | Jan. 2015 | Apr. 2016 |
| AM-111 | Auris Medical, Inc. | Completed | Phase 2 | Industry | DB | Dec.2008 | Jul. 2012 |
| N-acetylcysteine | National Taiwan University Hospital | Completed | Phase 2 | Other | DB | Nov. 2007 | Jan. 2008 |
| SPI-1005 | Sound Pharmaceuticals, Inc. | Not yet | Phase 2 | Industry & U.S. Federal | DB | Aug. 2016 | Jan. 2018 |
| SPI-1005 | Sound Pharmaceuticals, Inc. | Not yet | Phase 2 | Industry | DB | Sept. 2016 | Aug. 2017 |
| EPI-743 | Edison Pharmaceuticals Inc. | Completed | Phase 2 | Industry | DB | Oct. 2014 | Nov. 2015 |
| SPI-1005 | Sound Pharmaceuticals Inc | Completed | Phase 2 | Industry & Other | DB | Sept. 2011 | Dec. 2013 |
| Vestipitant | GlaxoSmithKline | Completed | Phase 2 | Industry | DB | Dec. 2006 | Aug. 2009 |
| N-acetylcysteine | TC Erciyes University | Completed | Phase 2 & 3 | Other | OL | Jun. 2010 | Nov. 2011 |
| Alpha-lipoic acid | VA Office of Research and Development | Completed | Phase 2 & 3 | U.S. Federal & Other | DB | Oct. 2007 | Mar. 2011 |
| Sodium thiosulfate | Children’s Oncology Group | Active, not recruiting | Phase 3 | Other & NIH | OL | Jun. 2008 | Sept. 2012 |
| AM-111 | Auris Medical, Inc. | Recruiting | Phase 3 | Industry | DB | Jun. 2016 | Jun. 2018 |
| D-methionine | Southern Illinois University | Recruiting | Phase 3 | U.S. Federal | DB | Sept. 2013 | Dec. 2016 |
Table 2.
Currently investigated therapies and mechanisms for sensorineural hearing loss in humans.
| Trial Agent | Application | Mechanism | Therapeutic Molecule | Administration Method | Clinical Data Available |
|---|---|---|---|---|---|
| AM-111 | Sensorineural Hearing Loss, Multiple Applications | Cell Death Cascade Manipulation | JNK Stress Kinase Inhibitor | Trans-Tympanic Gel | No |
| CGF166 | Sensorineural Hearing Loss, Multiple Applications | Hair Cell Regeneration | Atoh1 Transcription Factor | Intra-Labyrinthine Infusion | No |
| D-methionine | Noise-Induced Hearing Loss, Oxidant Induced | Oxidant Stress Mitigation | D-methionine | Oral | No |
| N-acetylcysteine | Sensorineural Hearing Loss, Oxidant Induced | Oxidant Stress Mitigation | N-acetylcysteine | Oral, Trans-Tympanic | Yes |
| Sodium thiosulfate | Sensorineural Hearing Loss, Chemotherapy-Induced | Oxidant Stress Mitigation | Sodium thiosulfate | Systemic | Yes |
| SPI-1005 | Sensorineural Hearing Loss, Oxidant Induced | Oxidant Stress Mitigation | Ebselen | Oral | Yes |
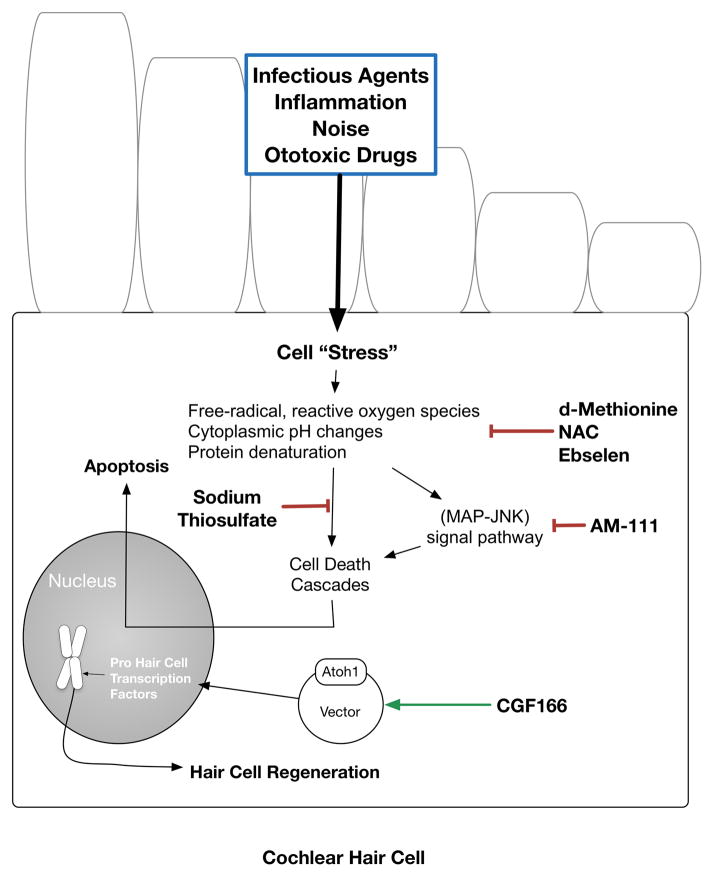
Figure 1.
Schematic of reviewed candidate molecules for the treatment of sensorineural hearing loss.
Several studies were not included as specific details for therapies registered in the ClinicalTrials.gov registry were not available on either sponsor-controlled web sites or media, or through directed searches in MEDLINE. The omitted therapies included anakinra (interleukin-1 (IL-1) receptor antagonist), ancrod (Malayan Pit Viper venom), AUT00063 (voltage gated potassium ion channel modulator), EPI-743 (AKA. Vatiquinone), HPN-07 (anti-oxidant 2,4-disulfonyl α-phenyl tertiary butyl nitrone), PF-04958242 (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid potentiator), vestipitant (NK1 receptor selective antagonist), and zonisamide (anti-convulsant).
DISCUSSION
Efforts to develop drugs to treat SNHL have resulted in over twenty active randomized clinical drug trials for the prevention or treatment of hearing loss registered in the United States in the past decade (Table 1). The number of late phase trials is promising and the prospect of the near-availability of a drug to address hearing loss carries the hope that sensorineural hearing loss will no longer be a permanent disability. An understanding of the therapies and mechanisms is needed to accurately counsel patients on the potential role of these hearing loss therapies should they make it to market. Considering the need for this understanding, we sought to critically evaluate the proposed mechanisms and data related to the therapeutics currently in active randomized clinical drug trials. We identified 22 active randomized clinical drug trials, and reviewed 6 therapeutic molecules.
Cochlear Hair Cell Regeneration Therapy – The ‘Atoh1’ Gene
Sensorineural hearing loss is most commonly caused by the degeneration of hair cells from either internal or external pathologic factors including infectious or inflammatory processes, ototoxic drugs, noise over-stimulation as well as the normal process of aging. Hair cells are essential to hearing and balance function, and are the mechanotransducers that convert mechanical energy produced by sound or head movement into electrical potentials that are subsequently relayed to the brain. While many genes contribute to the normal differentiation and development of the cochlear sensory epithelium, the ‘Atoh1’ gene, also known as ‘Math1,’ was first discovered as a vital regulator of cochlear and vestibular hair cell generation in mice.14 It has been shown that the natural expression of Atoh1 occurs in a population of the primordial sensory cells of the cochlea, and plays an essential role in the differentiation of these cells into hair cells.15 If in mouse models expression of Atoh1 is blocked, the sensory epithelium of the cochlea is completely disrupted and hair cell development is stymied.16 Conversely, if Atoh1 expression is induced in sensory epithelia of embryonic or neonatal mouse or rat cochleae hair cell formation occurs.16–18
Other animal models used for testing Atoh-1 have produced interesting results. Using a guinea pig model, 4–5 week old normal hearing animals were inoculated with the Atoh1-containing virus vector through direct delivery to the cochlea.19 The investigators reported the formation of occasional, immature hair cell-like cells in non-sensory regions of the cochlea four days after inoculation. Because these were normal hearing animals, it was difficult to determine if new hair cell formation occurred within the boundaries of the organ of Corti. Therefore, with this encouraging result, subsequent studies tested the ability of Atoh1 to create hair cells in deafened mammalian models. One such study evaluated the effects of Atoh1 overexpression on hair cell regeneration in the deafened cochlea of young guinea pigs.20 In this study, guinea pigs were deafened with systemic injection of ototoxic drugs and confirmed deaf by auditory brainstem response (ABR). The Atoh1 gene was placed in an adenovirus vector, and infused into the scala media of the deafened guinea pig cochleae 4 days after the ototoxic injury. Scanning electron microscopy 8 weeks after infusion demonstrated a variable degree of inner and outer hair cell regeneration in the organ of Corti along with a dramatic improvement in hearing. The new hair cells, were noted to be positioned on the basement membrane, suggestive of trans-differentiation of supporting cells into hair cells. The authors suggested, based on these findings, that expression of the Atoh1 gene alone is sufficient to restore hearing through hair cell regeneration.20 However, when the study was repeated with a delay of 7 days to the viral treatment after deafening, no hair cell regeneration occurred.21 The authors examined the cochleae and noted that within six days of treatment differentiated supporting cells were absent. Of note, the deafening treatment was also different from the treatment used in the first study: in lieu of using kanamycin and ethacrynic acid, neomycin, which can also induce changes in supporting cells, was used. The authors concluded that differentiated supporting cells are necessary for the successful regeneration of hair cells using the Atoh1 gene delivery approach. However, with such a narrow therapeutic window, a question remains to the appropriate indication for the use of this treatment in humans. It has been shown that people with a sudden sensorineural hearing loss have a variable loss of supporting cells and their cochleae consist of areas with flat epithelium.22,23 In addition, to date, no other studies have been published replicating these findings of cochlear hair cell regeneration and hearing restoration following Atoh1 gene delivery to adult inner ears, thereby raising concerns regarding the feasibility of this approach.
Novartis Pharmaceuticals is currently evaluating ‘CGF166’ – an adenovirus vector encoding the human Atonal transcription factor ‘Hath1’ in safety and efficacy Phase 1 and Phase 2 clinical trials24 (Table 1). Little background information has been made available through the clinical trial registry or commercial websites. Inclusion criteria include adults with a long standing, stable (greater than a year with minimal change), non-fluctuating hearing loss that is at least 50 dB HL at 125 and 250 Hz and greater than 70 dB HL in higher frequencies. The adenovirus vector with Hath1 is to be administered as an infusion via a stapedotomy into the vestibule. While peer-reviewed published data to directly support feasibility of hearing restoration of long standing hearing loss using Atoh1 gene is not available, the study was approved by the United States Food and Drug Administration (FDA), and represents the first gene transfer clinical trial for hearing restoration.
In parallel to the development of targeted therapies for hearing loss, methods of drug delivery to the inner ear have been investigated. Various methods for delivery have been explored including osmotic mini-pump infusion, direct microinjection, and dissolvable drug-infused packing.25 The most effective approach will depend upon the properties of the molecule or agent to be delivered. Directed surgical feasibility studies of inner ear adenovirus delivery have also demonstrated that both a round window and cochleostomy approach are feasible, but a round window approach may result in less hearing loss than a cochleostomy approach in mice.26 The efficacy and safety of the adenovirus delivery vector to the inner ear in humans remains unknown. No current human study data are available to characterize the risks of direct intra-labyrinthine application of an adenovirus vector and/or spread to the CSF or other routes. Direct intra-labyrinthine inoculation many not be the most practical means for drug delivery in humans given the need for an invasive approach. It is possible that direct inoculation of the labyrinth may confer efficacy and tolerability benefit versus systemic administration as used with traditional oral and parenteral methods, but this remains to be explored.
Manipulating the Cell Death Cascade – JNK Stress Kinase Inhibition
When a cell experiences significant stress or injury, intrinsic cell death cascades may be activated that result in death of the injured cell. Upon activation of cell death pathways, biochemical changes can occur including the generation of free-radical and reactive oxygen species, acidic cytoplasmic pH changes, and protein denaturation.27,28 With respect to hearing, it has been demonstrated that significant stressors can cause cell death, as well as structural damage, resulting in sensorineural hearing loss.29 If the sensory neuroepithelium were made more resilient to cell death by changing the balance between the signaling pathways for death (e.g., caspase 9, MAPK, cJNK) and survival (e.g., Bcl-2), it is plausible that sensorineural hearing loss could be prevented.
The mitogen-activated protein kinase/c-Jun N-terminal kinase (MAP-JNK) signaling pathway has been shown to be activated following damaging levels of aminoglycoside and noise trauma stressors.30–34 Specifically, it is hypothesized that cellular stress such as aminoglycoside exposure in the cochlea results in the formation of reactive oxygen species and free radicals that cause the JNK kinase to activate c-Jun.35 The activated c-Jun transcription factor binds other transcriptional complexes which ultimately results in the complete loss of cochlear hair cells.36 Regardless of the molecular pathway under consideration, it is believed that a therapy designed to intervene prior or immediately following the cochlear neuroepthielial injury may prevent sensorineural hearing loss.29
Animal studies using synthetically created JNK inhibitory molecules have demonstrated protective effects in the face of established cochlear neuroepthielial stressors. Mouse in vitro and adult guinea pig in vivo models exposed to noise trauma, ototoxic agents, and trauma-induced hearing loss (such as with electrode implantation stressors), when treated with direct co-application of synthetic JNK inhibitor peptides, demonstrated near-complete prevention of hair cell death.37,38 However, most studies did not perform dose-response curves which are critical to development of a full understanding of the therapeutic potential of a drug. One such study, for example, found that CEP-11004, an indirect inhibitor of JNK signaling, can inhibit hair cell death in utricles exposed to moderate but not high doses of neomycin.39 Prevention of cell death in cochlear neuroepithelia is the basis of treatment with AM-111 – the Auris Medical AG JNK inhibitor peptide currently in clinical trials.40 AM-111 purportedly prevents cellular death from different stressors leading to sensorineural hearing loss by preventing JNK-mediated apoptosis.41 Specifically, AM-111 binds to JNK and prevents activation by the c-jun and c-fos transcription factors. In animal studies, AM-111 has prevented cochlear hair cell death in response to noise trauma, ischemic cochlear damage, acute labyrinthitis and aminoglycoside ototoxcity.37,42–44 AM-111 has also been shown to protect against hearing loss in cases of semicircular canal transection in a guinea pig otitis media model.45 AM-111 is administered topically in a biodegradable gel scaffold that is applied at the round-window membrane. Reported benefits of this delivery method include potential for intraoperative application and ease of trans-tympanic injection.42
AM-111 Phase 2 trial efficacy data from a prospective double-blind randomized placebo-controlled study was made available by Auris in 2015.46,47 In patients with severe-to-profound sudden sensorineural hearing loss, patients treated with a single trans-tympanic dose of AM-111 realized significant improvements in absolute and relative pure tone average and speech discrimination scores compared to the placebo group. The patients were treated within a mean 29 hours of acute onset sensorineural hearing loss. No treatment benefits were observed for patients with mild-moderate sensorineural hearing loss. The investigators reported only mild procedure-related adverse effects including otalgia, incision site complications, and otitis media in less than 5% of their patient population.
Oxidative Stress Mitigation – D-Methionine, N-Acetylcysteine, Glutathione Peroxidase Mimicry, & Sodium Thiosulfate
As discussed previously, reactive oxygen species and free radicals are generated in cochlear hair cells from exposure to stressors of different etiologies. The release of these molecules can result in cellular damage, and in some cases cell death. Molecules with antioxidant properties prevent the formation of or disarm potentially destructive free radicals and reactive oxygen species that facilitate cellular death.48 After a measurable noise exposure, reactive oxygen species and free radicals increase in hair cells.49,50 In current clinical trials for sensorineural hearing loss, several studies are investigating possible benefit of various antioxidant molecules including D-methionine, N-acetylcysteine, glutathione peroxidase mimicry, and sodium thiosulfate.
D-methionine
The exact antioxidant mechanism of D-methionine is not known, however it is purported to be a scavenger of free radicals and supporter of other antioxidant enzymatic processes that serve to decrease oxidant stressors within a cell.51,52 Multiple in vitro and in vivo animal studies with D-methionine have demonstrated otoprotective properties when rat, mice, and guinea pig models were subjected to ototoxic agents such as cisplatin chemotherapies, aminoglycoside antibiotics, and noise-induced trauma.53–58 In a recent investigation of the optimal timing of administration of D-methionine after noise exposure, chinchillas who were administered the agent between three and seven hours post-exposure had decreased ABR threshold shifts three weeks after exposure.59 The fact that D-methionine is available in a stable oral formulation, coupled with evidence of otoprotective effects with post-exposure administration, has spurred interest in human studies for noise induced hearing loss,59 especially in military settings.60 However, again, investigation did not include a drug dose-response curve or utilization of a range of loudness of noise exposures. Can results from otoprotection of chinchillae exposed for six hours to 105 dB SPL octave band noise (centered at 4 kHz) be generalized to a variety of noise exposures in human?
Military environments are fraught with potential expected and unexpected noise trauma. New onset hearing loss has been positively associated with combat deployment, exposure to improvised explosive devices (IED), and head trauma.61,62 Economic models are currently in development to assess the burden of hearing loss in the U.S. military, but the burden is thought to be significant.63 The objective of a recent Department of National Defense trial was to augment hearing protection devices, such as ear plugs, for noise exposure that may exceed the protective capability of these devices.60 The trial will determine the efficacy of D-methionine in preventing either noise-induced hearing loss or tinnitus after eleven days of weapons training for drill sergeant instructor trainees.60
N-acetylcysteine
The mechanism of action of N-acetylcysteine (NAC) is thought to be one of free radical scavenger, as well as augmentation of glutathione enzymatic activity, similar to D-methionine described above.64–66 NAC has been extensively studied in medicine and is FDA-approved for use as a mucolytic for pulmonary disorders and in the treatment of acute hepatotoxicity after acetaminophen overdose. Given the established link between oxidant-induced cellular injury in the cochlea and sensorineural hearing loss, considerable interest in NAC as a possible therapy for the prevention or treatment of sensorineural hearing loss has emerged in numerous settings and applications.
Significant evidence of the protective effects of NAC across multiple animal model and simulated pathological circumstances has amassed. NAC has been demonstrated to protect against hair cell loss and/or temporal threshold shifts in numerous noise-induced hearing loss models in chinchillas and rats, as well as blast-noise exposed rats, and rabbits.67–77 In other pathologic conditions, such as meningitis-induced hearing loss, NAC appeared to reduce long-term hearing loss measured at 14 days in a rat model, in age-related hearing loss in γ-glutamyl transferase 1 deficient mice, and in a murine industrial-solvent induced cochlear injury model.78–80 In cochlear implant explant rat and guinea pig cochlea, the administration of NAC partially preserved inner hair cells compared to controls in response to simulated surgical trauma.81,82
Previously completed clinical evaluations of NAC have shown promise. An Iranian study of textile workers exposed to continuous noise greater than 85 decibels for at least 8 hours daily were randomly allocated to either receiving placebo, ginseng, or two doses of NAC.83 Both NAC and ginseng reduced temporary threshold shifts at 14 days, with the larger response seen in the NAC treated cohort. A similar prospective, randomized, double-blinded, placebo-controlled clinical trial of military-based individuals receiving NAC to prevent hearing loss after weapons training showed a small reduction in threshold shifts in the participants’ trigger-hand ear, but there was no significant reduction in the overall rate of threshold shifts.84 No significant adverse effects were reported. Another retrospective study evaluated the use of oral NAC in conjunction with intratympanic dexamethasone injections for sudden sensorineural hearing loss, and found that the addition of NAC conferred increased benefit in hearing recovery.85 Multiple active clinical trials are investigating NAC in the treatment of sensorineural hearing loss. Three active trials are seeking to characterize the protective effects of N-acetylcysteine on either cisplatin-, aminoglycoside-, or noise-induced ototoxicity. A pediatric trial is investigating cisplatin-induced ototoxicity in children being treated for different malignancies and is open for recruitment as of the writing of this manuscript.86 In Turkey, NAC is being investigated as an otoprotective adjunct in patients who have developed peritonitis while receiving continuous ambulatory peritoneal dialysis.87 Peritonitis is a common complication of this form of dialysis and the microbiology of these infections has called for aminoglycosides and vancomycin as the antibiotic of choice, despite their known ototoxicity.87 The last is a closed trial in Taiwan that studied 53 steel industry workers exposed to noise. In a double cross-over placebo design, the steel workers were provided with 1200 mg/day of NAC with complete pre- and post-treatment audiometry performed.88 The authors reported that NAC administration significantly reduced temporary threshold shifts by 2.45 dB compared to 2.75 after placebo when exposed to daily ambient noise exposure of 88.4 to 89.4 dB. However, the clinical significance of this difference is questioned.
Glutathione Peroxidase Mimicry
Chemotherapeutic drugs, particularly the platinum-based agents, have been shown to be directly ototoxic to the cochlea through the generation of a free radicals.89–91 Specifically, cisplatin causes depletion of glutathione and other antioxidant enzymes in the cochlea resulting in outer hair cell loss and damage to the organ of Corti.92 Early studies sought to counter the deleterious effects of cisplatin by introducing various molecules with anti-oxidant properties, and it was shown that these properties can prevent hair cell loss in the cochlea.92 Around the time of this work, ischemia and stroke researchers introduced ebselen [2-phenyl-1, 2-benzisoselenazol-3 (2H)-one], a neuroprotective molecule that mimics the activity of endogenous glutathione peroxidase and phospholipids hydroperoxide glutathione peroxidase.93 Providing glutathione to glutathione-deficient guinea pigs has also been shown to limit noise-induced hearing loss.94 With the characterization of the hearing protective effects of glutathione, and the anti-oxidant effects of ebselen, investigators interested in stress-induced oxidizing damage of the cochlea demonstrated ebselen as protective of auditory cells when subjected to cisplatin in vitro.95 Moreover, endogenous glutathione peroxidase was demonstrated as active and present in the organ of Corti, spiral ganglia, stria vascularis, and spiral ligament, suggesting that ebselen may have direct activity at multiple cochlear sites where oxidant-stress related injury may occur.96
Soon after ebselen was shown to be protective against cisplatin-mediated ototoxicity in vitro, data from animal studies followed that were increasingly encouraging, demonstrating ebselen as a potential therapy for preventing oxidative sensorineural hearing loss for stress mechanisms including and beyond that caused by cisplatin. In noise-exposed rats, ebselen has been demonstrated to protect against the loss of outer hair cells and reduce acute stria vascularis edema purportedly through direct free radical scavenging activity, and by promoting endogenous glutathione peroxidase expression.96 Studies utilizing auditory brainstem responses in rats after noise-exposures demonstrated that ebselen protected against both temporary and permanent threshold shifts.97
Clinical data in the past five years has kindled the prospect for ebselen-mediated sensorineural hearing loss protection in human subjects. Preliminary data of a randomized double-blind, placebo-controlled Phase 2 clinical trial in adults with normal or slight hearing loss who were subjected to four hours of noise exposure through a MP3 player has been made available in an abstract.98 Oral ebselen administration resulted in a significant reduction in temporary threshold shift incidence, severity and duration when compared to the placebo group. The full details of this study were not available to review at the writing of this manuscript. Currently, ebselen is under evaluation for safety and efficacy in 4 clinical trials sponsored by Sound Pharmaceuticals as the trial drug ‘SPI-1005’ in settings of noise exposure and cisplatin-mediated ototoxicity.99–102
Sodium Thiosulfate
As discussed above, platinum-based chemotherapeutics have well-established ototoxicity. Sodium thiosulfate is a sulfur containing molecule that has the ability to bind and inactivate platinum-based chemotherapeutics such as cisplatin.103
Specifically, sodium thiosulfate has been shown to bind and inactivate carboplatin, allowing renal excretion and ultimately less systemic toxicity, without a decrease the anti-tumor activity of carboplatin, if infused in a delayed fashion.104,105 Sodium thiosulfate has also been shown to have direct otoprotective effects against carboplatin in the guinea pig cochlea. There may be a dose-dependent relationship as continuous, direct infusion to the middle ear space resulted in improved hearing preservation, as compared with single daily doses, as measurable by auditory brainstem responses.106,107 However, the time course of protective effect of sodium thiosulfate is limited to co-administration with cisplatin, and protection is lost if administered even a few hours after cisplatin.108 Beyond direct binding and inactivation of platinum-based chemotherapeutics, sodium thiosulfate may also have intrinsic anti-oxidant properties that may serve to protect hearing, but this has yet to be extensively explored.109
Clinical data for the use of sodium thiosulfate to prevent chemotherapy-induced ototoxicity has produced promising results. A cohort study of adult patients receiving parenteral cisplatin for advance carcinoma of the head and neck analyzed the effect of co-administered systemic sodium thiosulfate, and reported a decrease in measured hearing loss except for higher frequencies.110 A retrospective study with a similar patient population also demonstrated that co-infusion of parenteral sodium thiosulfate results in less severe cisplatin ototoxicity than when intravenous cisplatin is provided exclusively.111 Specifically, patients who did not receive sodium thiosulfate had detectable hearing loss at both high and ultra-high frequencies, whereas patients who received sodium thiosulfate had hearing loss only at ultra-high frequencies. Last, a randomized control trial of patients receiving cisplatin-based chemoradiation analyzed the effect of providing co-administered sodium thiosulfate and found that it conferred a significant protective effect at frequencies in the range of speech perception, and fewer ears qualified for hearing aids.112 As of the writing of this paper, one active phase III clinical trial is evaluating the otoprotective effect of intravenous sodium thiosulfate in children receiving cisplatin for a variety of malignancy types.113
Presbycusis & Mitochondrial Dysfunction – The Holy Grail of Hearing Loss?
Presbycusis, or ‘age-related hearing loss,’ has received much attention as the prevalence of presbycusis exceeds 40% in adults over 65 years-old in the United States.114 Despite this substantial unmet need, an effective therapy has yet to be developed. Akin to the causes of other forms of sensorineural hearing loss, the discovery of causal mechanisms of presbycusis has remained elusive. Theories to explain the pathophysiology of presbycusis have evolved over time, but contemporary impressions report that a diffuse pattern of cochlear degeneration, including a combination of strial hearing loss with both primary and secondary loss of hair cells, may be more credible than aberrancies in cochlear conductive processes.115 The extent to which presbycusis shares similar pathologic molecular mechanisms with other forms of sensorineural hearing loss reviewed in this paper is notable. In a novel presbycusis mouse model, an inbred mouse strain which suffers from early multi-system aging (senescence-accelerated mouse prone 8; SAMP8), oxidative stress and markers of chronic inflammation were all demonstrated using lipid peroxidation product measurements as well as oxidative mitochondrial and nuclear DNA damage biomarker assays.116 Interestingly, ultrastructural analysis of mitochondria from the organ of Corti in these animals demonstrated unrecognizable mitochondria cristae and evidence of mitochondrial wall damage. Moreover, mitochondrial complex I and II, as well as cytochrome c oxidase enzymatic activity showed perturbations that signaled significant mitochondrial dysfunction. As a caveat, one should consider that this model of age related hearing loss may be secondary to a process that is unique to the SAMP8 strain, or a result of a combination of mutations that is different from those which underlie age related hearing loss in humans. Other animal models of hearing loss have been used extensively to study presbycusis, specifically the Fischer 344 rat as outlined in a recent review.117 However, the review synthesizes evidence that the mechanism of degenerative hearing loss in the Fischer 344 rat may not be related to cochlear hair cell loss. Despite the disappearance of otoacoustic emissions and elevated ABR thresholds, cochlear hair cells can still be found on histologic analysis.118 This suggests a significant limitation for the use of the Fischer 344 rat for the study of presbycusis in humans. The generation of an animal model that closely mimics the pathophysiology of human presbycusis will be needed for the development and testing of targeted therapies.
Evidence in the last 20 years suggests that mitochondrial DNA defects and resulting dysfunction play a key role in inherited hearing loss, and possibly presbycusis.119–123 Mitochondrial-mediated cell death has been postulated to be a causal factor for presbycusis.120 This cell death pathway is governed in part by the Bcl-2 family of genes and gene products.124 Silencing of one of the Bcl-2 genes implicated in mitochondrial-mediated cell death, the pro-apoptotic gene Bak, has been shown to prevent presbycusis in a mouse model.121 However, a recent review reports that there may be other mechanisms behind mitochondrial-mediated presbycusis including calcium-regulation aberrancies, and specific mitochondrial DNA deletions.125 What remains to be confirmed is if mitochondrial dysfunction in presbycusis occurs through mechanisms either shared or distinct to that of the well-described oxidant-antioxidant pathways. If there are separate mitochondrial mechanisms driving presbycusis, the investigation of pathways leading to mitochondrial dysfunction in presbycusis may yield novel therapy targets.
Conclusion & Future Directions
We are entering an exciting era in the development of directed therapies for sensorineural hearing loss. A common theme in recent clinical trials registered in the United States appears to be the targeting of cell death pathways and influence of oxidant stressors on cochlear sensory neuroepithelium. In addition, a virus-delivered cell replacement therapy using the ATOH1 gene, or other genes which would likely follow, would be revolutionary, if proven safe and efficacious. Finally, there is an increase in our knowledge and understanding of the pathophysiology of hearing loss, leading to the identification of additional pathways for targeting.126–128 For example, of particular interest will be molecular pathways identified through cell type-specific transcriptomic analyses of the signaling cascades induced after noise exposure. Significant challenges for bringing these bench-to-bedside therapies to market still remain. It is never assured that in vitro and in vivo results in non-human animal models may translate to effective therapies in the setting of human biology. Characterization of dose-response curves in animals not only for the proposed treatments, but also for the known offending stressors will be important to validate and calibrate therapy. Moreover, as additional processes that impact etiology of hearing loss - such as an immune response and neuronal retraction - are discovered, additional pathways for targeting become available.
Acknowledgments
The authors wish to thank Edwin W Rubel, Ph.D. for his helpful comments on a prior draft of this manuscript. Funding (R.H.) this work was supported by NIDCD/NIH R01DC013817 and R01DC03544 and DOD CDMRP MR130240.
Footnotes
- Each of the authors signed below have contributed to, read and approved this manuscript.
- None of the authors has any conflict of interest, financial or otherwise.
- This manuscript is original and it, or any part of it, has not been previously published; nor is it under consideration for publication elsewhere.
- In consideration of the journal reviewing and editing my submission, “Emerging Therapies for Sensorineural Hearing” the authors undersigned transfers, assigns and otherwise conveys all copyright ownership if such work is published.
References
- 1.World Health Organization. Estimates. Prevention of blindness and deafness. http://www.who.int/pbd/deafness/estimates/en/. Published 2012.
- 2.Wake M, Hughes EK, Poulakis Z, Collins C, Rickards FW. Outcomes of Children with Mild-Profound Congenital Hearing Loss at 7 to 8 Years: A Population Study. Ear Hear. 2004;25(1):1–8. doi: 10.1097/01.AUD.0000111262.12219.2F. [DOI] [PubMed] [Google Scholar]
- 3.Wake M, Hughes EK, Collins CM, Poulakis Z. Parent-Reported Health-Related Quality of Life in Children With Congenital Hearing Loss: A Population Study. Ambul Pediatr. 2004;4(5):411–417. doi: 10.1367/A03-191R.1. [DOI] [PubMed] [Google Scholar]
- 4.Bess FH, Dodd-Murphy J, Parker Ra. Children with minimal sensorineural hearing loss: prevalence, educational performance, and functional status. Ear Hear. 1998;19(5):339–354. doi: 10.1097/00003446-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Borton SA, Mauze E, Lieu JEC. Quality of life in children with unilateral hearing loss: a pilot study. Am J Audiol. 2010;19(1):61–72. doi: 10.1044/1059-0889(2010/07-0043). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Culbertson JL, Gilbert LE. Children with unilateral sensorineural hearing loss: cognitive, academic, and social development. Ear Hear. 1986;7(1):38–42. doi: 10.1097/00003446-198602000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Nasralla HR, Bento RF, Valéria M, Gof S, Magalhaes AT. Important Factors in the Cognitive Development of Children with Hearing Impairment: Case Studies of Candidates for Cochlear Implants. Int Arch Otorhinolaryngol. 2014;18(4):357–361. doi: 10.1055/s-0034-1382095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlsson P-I, Hjaldahl J, Magnuson A, et al. Severe to profound hearing impairment: quality of life, psychosocial consequences and audiological rehabilitation. Disabil Rehabil. 2015;37(20):1849–1856. doi: 10.3109/09638288.2014.982833. [DOI] [PubMed] [Google Scholar]
- 9.Liljas AEM, Wannamethee SG, Papacosta O, et al. Social and lifestyle characteristics and burden of ill-health associated with self-reported hearing and vision impairments in older men in the British community: a cross-sectional study. Lancet. 2014;384(2):S45. doi: 10.1016/S0140-6736(14)62171-1. [DOI] [PubMed] [Google Scholar]
- 10.Dalton DS, Cruickshanks KJ, Klein BEK, Klein R, Wiley TL, Nondahl DM. The impact of hearing loss on quality of life in older adults. Gerontologist. 2003;43(5):661–668. doi: 10.1093/geront/43.5.661. [DOI] [PubMed] [Google Scholar]
- 11.Mulrow CD, Aguilar C, Endicott JE, et al. Quality-of-life changes and hearing impairment A Randomized trial Quality-of-life changes and hearing impairment. A randomized trial. Ann Intern Med. 1990;113(June 1988):188–194. doi: 10.7326/0003-4819-113-3-188. [DOI] [PubMed] [Google Scholar]
- 12.Cruickshanks KJ, Tweed TS, Wiley TL, et al. The 5-year incidence and progression of hearing loss: the epidemiology of hearing loss study. Arch Otolaryngol Head Neck Surg. 2003;129(10):1041–1046. doi: 10.1001/archotol.129.10.1041. [DOI] [PubMed] [Google Scholar]
- 13.Cruickshanks KJ, Wiley TL, Tweed TS, et al. Prevalence of Hearing Loss in Older Adults in Beaver Dam, Wisconsin. Am J Audiol. 1998;148(9):879–886. doi: 10.1093/oxfordjournals.aje.a009713. [DOI] [PubMed] [Google Scholar]
- 14.Bermingham NA. Math1: An Essential Gene for the Generation of Inner Ear Hair Cells. Science (80- ) 1999;284(5421):1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- 15.Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129(10):2495–2505. doi: 10.1007/s00267-008-9138-y. [DOI] [PubMed] [Google Scholar]
- 16.Woods C, Montcouquiol M, Kelley MW. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci. 2004;7(12):1310–1318. doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- 17.Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3(6):580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- 18.Shou J, Zheng JL, Gao WQ. Robust generation of new hair cells in the mature mammalian inner ear by adenoviral expression of Hath1. Mol Cell Neurosci. 2003;23(2):169–179. doi: 10.1016/S1044-7431(03)00066-6. [DOI] [PubMed] [Google Scholar]
- 19.Kawamoto K, Ishimoto S-I, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23(11):4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. 23/11/4395 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izumikawa M, Minoda R, Kawamoto K, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11(3):271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- 21.Izumikawa M, Batts SA, Miyazawa T, Swiderski DL, Raphael Y. Response of the flat cochlear epithelium to forced expression of Atoh1. Hear Res. 2008;240(1–2):52–56. doi: 10.1016/j.heares.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merchant SN, Adams JC, Nadol JB. Pathology and pathophysiology of idiopathic sudden sensorineural hearing loss. Otol Neurotol. 2005;26(2):151–160. doi: 10.1097/00129492-200503000-00004. http://www.ncbi.nlm.nih.gov/pubmed/5793397. [DOI] [PubMed] [Google Scholar]
- 23.Hinojosa R, Marion M. Histopathology of profound sensorineural deafness. Ann N Y Acad Sci. 1983;405:459–484. doi: 10.1111/j.1749-6632.1983.tb31662.x. [DOI] [PubMed] [Google Scholar]
- 24.U.S. National Institutes of Health. Safety, Tolerability and Efficacy for CGF166 in Patients With Bilateral Severe-to-profound Hearing Loss. [Accessed July 5, 2016];ClinicalTrials.gov. http://clinicaltrials.gov/show/NCT02132130. Published 2016.
- 25.Lalwani AK, Jero J, Mhatre AN. Current issues in cochlear gene transfer. Audiol Neuro-Otology. 2002;7(3):146–151. doi: 10.1159/000058300. [DOI] [PubMed] [Google Scholar]
- 26.Chien WW, McDougald DS, Roy S, Fitzgerald TS, Cunningham LL. Cochlear gene transfer mediated by adeno-associated virus: Comparison of two surgical approaches. Laryngoscope. 2015;125(11):2557–2564. doi: 10.1002/lary.25317. [DOI] [PubMed] [Google Scholar]
- 27.Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. 2012;92(2):689–737. doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]
- 28.Coffey ET. Nuclear and cytosolic JNK signalling in neurons. Nat Rev Neurosci. 2014;15(5):285–299. doi: 10.1038/nrn3729. [DOI] [PubMed] [Google Scholar]
- 29.Wong ACY, Ryan AF. Mechanisms of sensorineural cell damage, death and survival in the cochlea. Front Aging Neurosci. 2015;7(APR):1–15. doi: 10.3389/fnagi.2015.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kyriakis JM, Banerjee P, Nikolakaki E, et al. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369(6476):156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Ruel J, Ladrech S, Bonny C, van de Water TR, Puel J-L. Inhibition of the c-Jun N-terminal kinase-mediated mitochondrial cell death pathway restores auditory function in sound-exposed animals. Mol Pharmacol. 2007;71(3):654–666. doi: 10.1124/mol.106.028936.such. [DOI] [PubMed] [Google Scholar]
- 32.Dinh CT, Goncalves S, Bas E, Van De Water TR, Zine A. Molecular regulation of auditory hair cell death and approaches to protect sensory receptor cells and/or stimulate repair following acoustic trauma. Front Cell Neurosci. 2015;9(March):96. doi: 10.3389/fncel.2015.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meltser I, Tahera Y, Canlon B. Differential activation of mitogen-activated protein kinases and brain-derived neurotrophic factor after temporary or permanent damage to a sensory system. Neuroscience. 2010;165(4):1439–1446. doi: 10.1016/j.neuroscience.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 34.Alagramam KN, Stepanyan R, Jamesdaniel S, Chen DH-C, Davis RR. Noise exposure immediately activates cochlear mitogen-activated protein kinase signaling. Noise Health. 1974;16(73):400–409. doi: 10.4103/1463-1741.144418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabuchi K, Nishimura B, Nakamagoe M, Hayashi K, Nakayama M, Hara A. Ototoxicity: mechanisms of cochlear impairment and its prevention. Curr Med Chem. 2011;18(31):4866–4871. doi: 10.2174/092986711797535254. BSP/CMC/E-Pub/2011/360 [pii] [DOI] [PubMed] [Google Scholar]
- 36.Scarpidis U, Madnani D, Shoemaker C, et al. Arrest of apoptosis in auditory neurons: implications for sensorineural preservation in cochlear implantation. Otol Neurotol. 2003;24(3):409–417. doi: 10.1097/00129492-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Van De Water TR, Bonny C, de Ribaupierre F, Puel JL, Zine A. A peptide inhibitor of c-Jun N-terminal kinase protects against both aminoglycoside and acoustic trauma-induced auditory hair cell death and hearing loss. J Neurosci. 2003;23(24):8596–8607. doi: 10.1523/JNEUROSCI.23-24-08596.2003. 23/24/8596 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eshraghi AA, He J, Mou CH, et al. D-JNKI-1 treatment prevents the progression of hearing loss in a model of cochlear implantation trauma. Otol Neurotol. 2006;27(4):504–511. doi: 10.1097/01.mao.0000217354.88710.13. [DOI] [PubMed] [Google Scholar]
- 39.Sugahara K, Rubel EW, Cunningham LL. JNK signaling in neomycin-induced vestibular hair cell death. Hear Res. 2006;221(1–2):128–135. doi: 10.1016/j.heares.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.U.S. National Institutes of Health. AM-111 for the treatment of acute inner ear hearing loss. ClinicalTrials.gov. https://clinicaltrials.gov/show/NCT02561091. Published 2016.
- 41.Auris Medical. [Accessed May 7, 2016];AM-111 for the treatment of acute inner ear hearing loss. http://www.aurismedical.com/product-candidates/am-111. Published 2015.
- 42.Coleman JKM, Littlesunday C, Jackson R, Meyer T. AM-111 protects against permanent hearing loss from impulse noise trauma. Hear Res. 2007;226(1–2):70–78. doi: 10.1016/j.heares.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 43.Barkdull GC, Hondarrague Y, Meyer T, Harris JP, Keithley EM. AM-111 reduces hearing loss in a guinea pig model of acute labyrinthitis. Laryngoscope. 2007;117(12):2174–2182. doi: 10.1016/S1041-892X(09)79440-5. [DOI] [PubMed] [Google Scholar]
- 44.Omotehara Y, Hakuba N, Hato N, Okada M, Gyo K. Protection Against Ischemic Cochlear Damage by Intratympanic Administration of AM-111. Otol Neurotol. 2011;32(9):1422–1427. doi: 10.1097/MAO.0b013e3182355658. [DOI] [PubMed] [Google Scholar]
- 45.Grindal TC, Sampson EM, Antonelli PJ. AM-111 prevents hearing loss from semicircular canal injury in otitis media. Laryngoscope. 2010;120(1):178–182. doi: 10.1002/lary.20759. [DOI] [PubMed] [Google Scholar]
- 46.Auris Medical. [Accessed May 7, 2016];AM-111 Acute Hearing Loss Therapy Fact Sheet. http://www.aurismedical.com/images/auris/downloads/auris_factsheet_AM111.pdf. Published 2015.
- 47.Suckfuell M, Canis M, Strieth S, Scherer H, Haisch A. Intratympanic treatment of acute acoustic trauma with a cell-permeable JNK ligand: a prospective randomized phase I/II study. Acta Otolaryngol. 2007;127(9):938–942. doi: 10.1080/00016480601110212. [DOI] [PubMed] [Google Scholar]
- 48.Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging. 2007;2(2):219–236. http://dx.doi.org/ [PMC free article] [PubMed] [Google Scholar]
- 49.Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006;27(1):1–19. doi: 10.1097/01.aud.0000191942.36672.f3. [DOI] [PubMed] [Google Scholar]
- 50.Yang C-H, Schrepfer T, Schacht J. Age-related hearing impairment and the triad of acquired hearing loss. Front Cell Neurosci. 2015;9(July):276. doi: 10.3389/fncel.2015.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campbell KCM, Meech RP, Rybak LP, Hughes LF. The effect of D-methionine on cochlear oxidative state with and without cisplatin administration: mechanisms of otoprotection. J Am Acad Audiol. 2003;14(3):144–156. [PubMed] [Google Scholar]
- 52.Vogt W. Oxidation of methionyl residues in proteins: Tools, targets, and reversal. Free Radic Biol Med. 1995;18(1):93–105. doi: 10.1016/0891-5849(94)00158-G. [DOI] [PubMed] [Google Scholar]
- 53.Campbell KCM, Meech RP, Klemens JJ, et al. Prevention of noise- and drug-induced hearing loss with d-methionine. Hear Res. 2007;226(1–2):92–103. doi: 10.1016/j.heares.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 54.Campbell KCM, Meech RP, Rybak LP, Hughes LF. D-Methionine protects against cisplatin damage to the stria vascularis. Hear Res. 1999;138(1–2):13–28. doi: 10.1016/S0378-5955(99)00142-2. [DOI] [PubMed] [Google Scholar]
- 55.Campbell KCM, Rybak LP, Meech RP, Hughes L. D-Methionine provides excellent protection from cisplatin ototoxicity in the rat. Hear Res. 1996;102(1–2):90–98. doi: 10.1016/S0378-5955(96)00152-9. [DOI] [PubMed] [Google Scholar]
- 56.Kopke RD, Coleman JKM, Liu J, Campbell KCM, Riffenburgh RH. Enhancing Intrinsic Cochlear Stress Defenses to Reduce Noise-Induced Hearing Loss. Laryngoscope. 2002;112(9):1515–1532. doi: 10.1097/00005537-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 57.Sha SH, Schacht J. Antioxidants attenuate gentamicin-induced free radical formation in vitro and ototoxicity in vivo: D-methionine is a potential protectant. Hear Res. 2000;142(1–2):34–40. doi: 10.1016/S0378-5955(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 58.Samson J, Wiktorek-Smagur A, Politanski P, et al. Noise-induced time-dependent changes in oxidative stress in the mouse cochlea and attenuation by d-methionine. Neuroscience. 2008;152(1):146–150. doi: 10.1016/j.neuroscience.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 59.Campbell K, Claussen A, Meech R, Verhulst S, Fox D, Hughes L. D-methionine (d-met) significantly rescues noise-induced hearing loss: Timing studies. Hear Res. 2011;282(1–2):138–144. doi: 10.1016/j.heares.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 60.U.S. National Institutes of Health. Phase 3 Clinical Trial: D-methionine to Reduce Noise-Induced Hearing Loss (NIHL) [Accessed May 7, 2016];ClinicalTrials.gov. https://clinicaltrials.gov/show/NCT01345474. Published 2015.
- 61.Wells TS, Seelig AD, Ryan MA, et al. Hearing loss associated with US military combat deployment. Noise Heal. 2015;17(74):34–42. doi: 10.4103/1463-1741.149574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Orsello CA, Moore JE, Reese C. Sensorineural hearing loss incidence among U.S. military aviators between 1997 and 2011. Aviat Sp Environ Med. 2013;84(9):975–979. doi: 10.3357/ASEM.3660.2013. [DOI] [PubMed] [Google Scholar]
- 63.Alamgir H, Tucker DL, Kim S-Y, et al. Economic Burden of Hearing Loss for the U.S. Military: A Proposed Framework for Estimation. Mil Med. 2016;181(April):301–306. doi: 10.7205/MILMED-D-14-00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Vries N, De Flora S. N-Acetyl-cysteine. J Cell Biochem. 1993;277(1993):270–277. doi: 10.1002/jcb.240531040. [DOI] [PubMed] [Google Scholar]
- 65.Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine:its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Rad Biol Med. 1989;6(6):593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 66.Zafarullah M, Li WQ, Sylvester J, Ahmad M. Review Molecular mechanisms of N -acetylcysteine actions Cellular and Molecular Life Sciences Review Molecular mechanisms of N -acetylcysteine actions. Cell Mol Life Sci Rev. 2003;60:6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kopke R, Bielefeld E, Liu J, et al. Prevention of impulse noise-induced hearing loss with antioxidants. Acta Otolaryngol. 2005;125(3):235–243. doi: 10.1080/00016480410023038. [DOI] [PubMed] [Google Scholar]
- 68.Ewert DL, Lu J, Li W, Du X, Floyd R, Kopke R. Antioxidant treatment reduces blast-induced cochlear damage and hearing loss. Hear Res. 2012;285(1–2):29–39. doi: 10.1016/j.heares.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 69.Lorito G, Giordano P, Petruccelli J, Martini A, Hatzopoulos S. Different strategies in treating noiseinduced hearing loss with N-acetylcysteine. Med Sci Monit. 2008;14(8):BR159–R164. 865761 [pii] [PubMed] [Google Scholar]
- 70.Duan M, Qiu J, Laurell G, Olofsson Å, Counter SA, Borg E. Dose and time-dependent protection of the antioxidant N-L-acetylcysteine against impulse noise trauma. Hear Res. 2004;192(1–2):1–9. doi: 10.1016/j.heares.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 71.Motalebi Kashani M, Saberi H, Hannani M. Prevention of Acoustic Trauma-Induced Hearing Loss by N-acetylcysteine Administration in Rabbits. Arch trauma Res. 2013;1(4):145–150. doi: 10.5812/atr.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu HP, Hsu CJ, Cheng TJ, Guo YL. N-acetylcysteine attenuates noise-induced permanent hearing loss in diabetic rats. Hear Res. 2010;267(1–2):71–77. doi: 10.1016/j.heares.2010.03.082. [DOI] [PubMed] [Google Scholar]
- 73.Lorito G, Giordano P, Prosser S, Martini A, Hatzopoulos S. Noise-induced hearing loss: a study on the pharmacological protection in the Sprague Dawley rat with N-acetyl-cysteine. Acta Otorhinolaryngol Ital. 2006;26(3):133–139. [PMC free article] [PubMed] [Google Scholar]
- 74.Coleman JKM, Kopke RD, Liu J, et al. Pharmacological rescue of noise induced hearing loss using N-acetylcysteine and acetyl-l-carnitine. Hear Res. 2007;226(1–2):104–113. doi: 10.1016/j.heares.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 75.Fetoni AR, Ralli M, Sergi B, Parrilla C, Troiani D, Paludetti G. Protective effects of N-acetylcysteine on noise-induced hearing loss in guinea pigs. Acta Otorhinolaryngol Ital. 2009;29(2):70–75. http://www.ncbi.nlm.nih.gov/pubmed/20111615%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2808688&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- 76.Hamernik RP, Qiu W, Davis B. The effectiveness of N-acetyl-l-cysteine (l-NAC) in the prevention of severe noise-induced hearing loss. Hear Res. 2008;239(1–2):99–106. doi: 10.1016/j.heares.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 77.Choi C-H, Du X, Floyd RA, Kopke RD. Therapeutic effects of orally administrated antioxidant drugs on acute noise-induced hearing loss. Free Radic Res. 2014;48(3):264–272. doi: 10.3109/10715762.2013.861599. [DOI] [PubMed] [Google Scholar]
- 78.Klein M, Koedel U, Pfister HW, Kastenbauer S. Meningitis-associated hearing loss: Protection by adjunctive antioxidant therapy. Ann Neurol. 2003;54(4):451–458. doi: 10.1002/ana.10684. [DOI] [PubMed] [Google Scholar]
- 79.Ding Dalian, Jiang Haiyan, Chen Guang-Di, Longo-Guess Chantal, Muthaiah Vijaya Prakash Krishnan, Tian Cong, Sheppard Adam, Salvi Richard, KRJ N-acetyl-cysteine prevents age-related hearing loss and the progressive loss of inner hair cells in γ-glutamyl transferase 1 deficient mice. Aging (Albany NY) 2016;8(4):730–750. doi: 10.18632/aging.100927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang WP, Hu BH, Chen GD, Bielefeld EC, Henderson D. Protective effect of N-acetyl-L-cysteine (L-NAC) against styrene-induced cochlear injuries. Acta Otolaryngol. 2009;129(10):1036–1043. doi: 10.1080/00016480802566261. 906243629 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eshraghi AA, Roell J, Shaikh N, et al. A novel combination of drug therapy to protect residual hearing post cochlear implant surgery. Acta Otolaryngol. 2016;6489(February):1–5. doi: 10.3109/00016489.2015.1134809. [DOI] [PubMed] [Google Scholar]
- 82.Eastwood H, Pinder D, James D, et al. Permanent and transient effects of locally delivered n-acetyl cysteine in a guinea pig model of cochlear implantation. Hear Res. 2010;259(1–2):24–30. doi: 10.1016/j.heares.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 83.Doosti A, Lotfi Y, Moossavi A, Bakhshi E, Talasaz AH, Hoorzad A. Comparison of the effects of N-acetyl-cysteine and ginseng in prevention of noise induced hearing loss in male textile workers. Noise Health. 2014;16(71):223–227. doi: 10.4103/1463-1741.137057. [DOI] [PubMed] [Google Scholar]
- 84.Kopke R, Slade MD, Jackson R, et al. Efficacy and safety of N-acetylcysteine in prevention of noise induced hearing loss: A randomized clinical trial. Hear Res. 2015;323:40–50. doi: 10.1016/j.heares.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 85.Angeli SI, Abi-Hachem RN, Vivero RJ, Telischi FT, Machado JJ. L-N-Acetylcysteine treatment is associated with improved hearing outcome in sudden idiopathic sensorineural hearing loss. Acta Otolaryngol. 2012;132(4):369–376. doi: 10.3109/00016489.2011.647359. [DOI] [PubMed] [Google Scholar]
- 86.U.S. National Institutes of Health. NAC to Prevent Cisplatin-induced Hearing Loss. [Accessed May 7, 2016];ClinicalTrials.gov. https://clinicaltrials.gov/show/NCT02094625. Published 2016.
- 87.U.S. National Institutes of Health. Protective Effect of N-acetylcysteine Against From Ototoxicity This. [Accessed May 7, 2016];ClinicalTrials.gov. https://clinicaltrials.gov/show/NCT01271088. Published 2011.
- 88.Lin C, Wu J, Shih T, et al. N-Acetyl-cysteine against noise-induced temporary threshold shift in male workers. Hear Res. 2010;269(1–2):42–47. doi: 10.1016/j.heares.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 89.Rybak LP. Cis-platinum associated hearing loss. J Laryngol Otol. 1981;95(7):745–747. doi: 10.1017/s0022215100091374. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=emed1a&AN=1981219027%5Cnhttp://sfx.leidenuniv.nl:9003/sfx_local?sid=OVID:Embase&issn=0022-2151&isbn=&volume=95&issue=7&spage=745&date=1981&pid=%3Cauthor%3ERybak+L.P.%3C*uthor%3E. [DOI] [PubMed] [Google Scholar]
- 90.Fausti SA, Henry JA, Schaffer HI, Olson DJ, Frey RH, Bagby GC. High-frequency monitoring for early detection of cisplatin ototoxicity. Arch Otolaryngol Head Neck Surg. 1993;119(6):661–666. doi: 10.1001/archotol.1993.01880180081015. [DOI] [PubMed] [Google Scholar]
- 91.Clerici WJ, Hensley K, DiMartino DL, Butterfield DA. Direct detection of ototoxicant-induced reactive oxygen species generation in cochlear explants. Hear Res. 1996;98(1–2):116–124. doi: 10.1016/0378-5955(96)00075-5. [DOI] [PubMed] [Google Scholar]
- 92.Rybak LP, Whitworth C, Somani S. Application of antioxidants and other agents to prevent cisplatin ototoxicity. Laryngoscope. 1999;109(11):1740–1744. doi: 10.1097/00005537-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 93.Lapchak PA, Zivin JA. Ebselen, a seleno-organic antioxidant, is neuroprotective after embolic strokes in rabbits: Synergism with low-dose tissue plasminogen activator. Stroke. 2003;34(8):2013–2018. doi: 10.1161/01.STR.0000081223.74129.04. [DOI] [PubMed] [Google Scholar]
- 94.Ohinata Y, Yamasoba T, Schacht J, Miller JM. Glutathione limits noise-induced hearing loss. Hear Res. 2000;146(1–2):28–34. doi: 10.1016/s0378-5955(00)00096-4. [DOI] [PubMed] [Google Scholar]
- 95.Kim SJ, Park C, Han AL, et al. Ebselen attenuates cisplatin-induced ROS generation through Nrf2 activation in auditory cells. Hear Res. 2009;251(1–2):70–82. doi: 10.1016/j.heares.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 96.Kil J, Pierce C, Tran H, Gu R, Lynch ED. Ebselen treatment reduces noise induced hearing loss via the mimicry and induction of glutathione peroxidase. Hear Res. 2007;226(1–2):44–51. doi: 10.1016/j.heares.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 97.Lynch ED, Gu R, Pierce C, Kil J. Ebselen-mediated protection from single and repeated noise exposure in rat. Laryngoscope. 2004;114(2):333–337. doi: 10.1097/00005537-200402000-00029. [DOI] [PubMed] [Google Scholar]
- 98.Kil J. Efficacy of SPI-1005 for Prevention of Noise-Induced Hearing Loss: Phase 2 Clinical Trial Results. Otolaryngol Head Neck Surg. 2011;33(December 2012):7–16. doi: 10.1177/0194599814541627a172. [DOI] [Google Scholar]
- 99.U.S. National Institutes of Health. SPI-1005 for Prevention and Treatment of Chemotherapy Induced Hearing Loss. [Accessed May 7, 2016];ClinicalTrials.gov. https://clinicaltrials.gov/show/NCT01451853. Published 2016.
- 100.U.S. National Institutes of Health. Otoprotection With SPI-1005 for Prevention of Temporary Auditory. ClinicalTrials.gov. https://clinicaltrials.gov/show/NCT01444846. Published 2014.
- 101.U.S. National Institutes of Health. Study to Evaluate the Safety and Pharmacokinetics of SPI-1005. [Accessed May 7, 2016];ClinicalTrials.Gov. http://clinicaltrials.gov/show/NCT01452607. Published 2016.
- 102.U.S. National Institutes of Health. A Phase 2b Study of SPI-1005 to Reduce the Incidence, Severity, and Duration of Acute Noise Induced Hearing Loss (NIHL) [Accessed May 7, 2016];ClinicalTrials.gov. https://clinicaltrials.gov/show/NCT02779192. Published 2016.
- 103.Gandara DR, Wiebe VJ, Perez EA, Makuch RW, DeGregorio MW. Cisplatin rescue therapy: Experience with sodium thiosulfate, WR2721, and diethyldithiocarbamate. Crit Rev Oncol Hematol. 1990;10(4):353–365. doi: 10.1016/1040-8428(90)90010-P. [DOI] [PubMed] [Google Scholar]
- 104.Elferink F, Van Der Vijgh WJF, Klein I, Pinedo HM. Interaction of cisplatin and carboplatin with sodium thiosulfate: Reaction rates and protein binding. Clin Chem. 1986;32(4):641–645. [PubMed] [Google Scholar]
- 105.Muldoon LL, Pagel MA, Kroll RA, et al. Delayed administration of sodium thiosulfate in animal models reduces platinum ototoxicity without reduction of antitumor activity. Clin Cancer Res. 2000;6(1):309–315. [PubMed] [Google Scholar]
- 106.Neuwelt EA, Brummett RE, Remsen LG, et al. In vitro and animal studies of sodium thiosulfate as a potential chemoprotectant against carboplatin-induced ototoxicity. Cancer Res. 1996;56(4):706–709. [PubMed] [Google Scholar]
- 107.Stocks RMS, Gould HJ, Bush AJ, Dudney BW, Pousson M, Thompson JW. Ototoxic protection of sodium thiosulfate: Daily vs constant infusion. Otolaryngol - Head Neck Surg. 2004;131(1):115–119. doi: 10.1016/j.otohns.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 108.Saito T, Zhang ZJ, Manabe Y, Ohtsubo T, Saito H. The effect of sodium thiosulfate on ototoxicity and pharmacokinetics after cisplatin treatment in guinea pigs. Eur Arch Oto-rhino-laryngology. 1997;254(6):281–286. doi: 10.1007/BF02905989. http://www.ncbi.nlm.nih.gov/pubmed/9248736. [DOI] [PubMed] [Google Scholar]
- 109.Hochman J, Blakley BW, Wellman M, Blakley L. Prevention of aminoglycoside-induced sensorineural hearing loss. J Otolaryngol. 2006;35(3):153–156. http://www.ncbi.nlm.nih.gov/pubmed/16929990. [PubMed] [Google Scholar]
- 110.Madasu R, Ruckenstein MJ, Leake F, Steere E, Robbins KT. Ototoxic effects of supradose cisplatin with sodium thiosulfate neutralization in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 1997;123(9):978–981. doi: 10.1001/archotol.1997.01900090094014. [DOI] [PubMed] [Google Scholar]
- 111.Ishikawa E, Sugimoto H, Hatano M, et al. Protective effects of sodium thiosulfate for cisplatin-mediated ototoxicity in patients with head and neck cancer. Acta Otolaryngol. 2015;135(9):919–924. doi: 10.3109/00016489.2015.1035797. [DOI] [PubMed] [Google Scholar]
- 112.Zuur CL, Simis YJ, Lansdaal PE, et al. Ototoxicity in a randomized phase III trial of intra-arterial compared with intravenous cisplatin chemoradiation in patients with locally advanced head and neck cancer. J Clin Oncol. 2007;25(24):3759–3765. doi: 10.1200/JCO.2006.08.9540. [DOI] [PubMed] [Google Scholar]
- 113.U.S. National Institutes of Health. Sodium Thiosulfate in Preventing Hearing Loss in Young Patients Receiving Cisplatin for Newly Diagnosed. [Accessed May 7, 2016];ClinicalTrials.gov. https://clinicaltrials.gov/show/NCT00716976. Published 2016.
- 114.Ries PW. Prevalence and characteristics of persons with hearing trouble: United States, 1990–91. Vital Health Stat 10. 1994;(188):1–75. [PubMed] [Google Scholar]
- 115.Nelson EG, Hinojosa R. Presbycusis: a human temporal bone study of individuals with downward sloping audiometric patterns of hearing loss and review of the literature. Laryngoscope. 2006;116(9 Pt 3 Suppl 112):1–12. doi: 10.1097/01.mlg.0000236089.44566.62. [DOI] [PubMed] [Google Scholar]
- 116.Menardo J, Tang Y, Ladrech S, et al. Oxidative Stress, Inflammation, and Autophagic Stress as the Key Mechanisms of Premature Age-Related Hearing Loss in SAMP8 Mouse Cochlea. Antioxid Redox Signal. 2012;16(3):263–274. doi: 10.1089/ars.2011.4037. [DOI] [PubMed] [Google Scholar]
- 117.Syka J. The Fischer 344 rat as a model of presbycusis. Hear Res. 2010;264(1–2):70–78. doi: 10.1016/j.heares.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 118.Bielefeld EC, Coling D, Di Chen G, et al. Age-related hearing loss in the Fischer 344/NHsd rat substrain. Hear Res. 2008;241(1–2):26–33. doi: 10.1016/j.heares.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Seidman MD, Ahmad N, Bai U. Molecular mechanisms of age-related hearing loss. Ageing Res Rev. 2002;1(3):331–343. doi: 10.1016/s1568-1637(02)00004-1. [DOI] [PubMed] [Google Scholar]
- 120.Someya S, Prolla TA. Mitochondrial oxidative damage and apoptosis in age-related hearing loss. Mech Ageing Dev. 2010;131(7–8):480–486. doi: 10.1016/j.mad.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Someya S, Xu J, Kondo K, et al. Age-related hearing loss in C57BL/6J mice is mediated by Bak-dependent mitochondrial apoptosis. Proc Natl Acad Sci U S A. 2009;106(46):19432–19437. doi: 10.1073/pnas.0908786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yamasoba T, Someya S, Yamada C, Weindruch R, Prolla TA, Tanokura M. Role of mitochondrial dysfunction and mitochondrial DNA mutations in age-related hearing loss. Hear Res. 2007;226(1–2):185–193. doi: 10.1016/j.heares.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 123.Ohlemiller KK. Mechanisms and genes in human strial presbycusis from animal models. Brain Res. 2009;1277:70–83. doi: 10.1016/j.brainres.2009.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Han C, Someya S. Mouse models of age-related mitochondrial neurosensory hearing loss. Mol Cell Neurosci. 2013;55:95–100. doi: 10.1016/j.mcn.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bottger EC, Schacht J. The mitochondrion: A perpetrator of acquired hearing loss. Hear Res. 2013;303:12–19. doi: 10.1016/j.heares.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Clifford ÃRE, Hoffer M, Rogers ÃR. The Genomic Basis of Noise-induced Hearing Loss: A Literature Review Organized by Cellular Pathways. Otol Neurotol. 2016;37(8):309–316. doi: 10.1097/MAO.0000000000001073. [DOI] [PubMed] [Google Scholar]
- 127.Kujawa SG, Liberman MC. Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hear Res. 2015;330:191–199. doi: 10.1016/j.heares.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sly ÃDJ, Campbell ÃL, Uschakov ÃA, Saief ÃST, Lam ÃM, Leary ÃSJO. Applying Neurotrophins to the Round Window Rescues Auditory Function and Reduces Inner Hair Cell Synaptopathy After Noise-induced Hearing Loss. Otol Neurotol. 2016;37(7):1223–1230. doi: 10.1097/MAO.0000000000001191. [DOI] [PubMed] [Google Scholar]