Abstract
Despite potentially curative surgery pancreatic cancer has a dismal prognosis. Serum cancer antigen 19-9 (CA 19-9) correlates with tumor burden, resectability and survival in patients with pancreatic ductal adenocarcinoma. Identification of novel biomarkers may facilitate early diagnosis of pancreatic cancer and improve survival. Pancreatic juice is a rich source of cancer-specific proteins rendering it a promising tool for identifying biomarkers. Recent proteomic and microRNA expression analyses have identified several biomarkers of potential diagnostic and prognostic value. Tumor markers CA 19-9 and carcinoembryonic antigen (CEA) are widely used in the characterization of premalignant and malignant lesions of the pancreas. Elevated level of CEA in bile is a marker for malignancy and a predictor of hepatic recurrence. The potential value of CA 19-9, CEA and lactate dehydrogenase as prognostic biomarkers in pancreatic juice and bile is unknown. Specimens of pancreatic juice and bile can be readily collected during surgical resection of the tumor. Profiling of pancreatic juice and bile to identify novel prognostic biomarkers may improve selection of patients for adjuvant therapy with a favorable impact on overall survival in patients diagnosed with pancreatic cancer.
Keywords: Prognostic biomarkers, Pancreatic juice, Bile, Pancreatic adenocarcinoma, Surgery
Core tip: Pancreatic juice is a rich source of cancer-specific proteins rendering it a promising tool for identifying novel biomarkers in pancreatic ductal adenocarcinoma. Recent proteomic and microRNA expression analyses have identified several diagnostic and prognostic biomarkers. Elevated carcinoembryonic antigen (CEA) in bile is a marker of malignancy and a predictor of hepatic recurrence. The potential of cancer antigen 19-9, CEA and lactate dehydrogenase as prognostic biomarkers in pancreatic juice and bile is unknown. Specimens of pancreatic juice and bile can be readily collected during pancreatic resection. Profiling of pancreatic juice and bile to identify novel biomarkers may facilitate early diagnosis and improve selection of patients for adjuvant therapy.
INTRODUCTION
Pancreatic cancer is the fourth leading cause of cancer-related mortality in the United States. Despite improvements in adjuvant therapy and identification of novel biomarkers, pancreatic cancer continues to have a dismal prognosis[1]. Pancreatic ductal adenocarcinoma (PDAC) is one of the few cancers for which incidence and mortality rates have changed very little over the past three decades. Surgery is the only potentially curative treatment for prolongation of survival and the use of adjuvant therapy following curative surgery significantly improves 5-year survival[2-4].
Pathologic stage of the tumor is the major determinant of survival after curative resection for PDAC[1]. Serum levels of cancer antigen 19-9 (CA 19-9), carcinoembryonic antigen (CEA) and lactate dehydrogenase (LDH) correlate with the extent of disease and are predictive of survival[5-13]. Serum CA 19-9 correlates with tumor burden, resectability and overall survival. Low preoperative serum CA 19-9, postoperative decline and level < 200 U/mL are independent predictors of survival[5]. Identification of novel diagnostic and prognostic biomarkers in the serum, tissue, bile and pancreatic juice of patients with PDAC may improve early diagnosis and selection of patients for adjuvant therapy.
BIOMARKERS OF PANCREATIC ADENOCARCINOMA
CA 19-9 and CEA in PDAC are well-characterized serum and tissue biomarkers of diagnostic and prognostic value. Recent proteomic and microRNA (miRNA) expression analyses have identified several biomarkers of potential value in the early diagnosis of PDAC and improvement in patient selection for aggressive treatment protocols. Comparative proteomic profiling of tumor and nontumor pancreas samples in patients with PDAC identified a new prognostic biomarker prolargin (PRELP)[14]. Survival analysis demonstrated a significant correlation of protein abundance of PRELP with postoperative survival confirming its value as a candidate prognostic biomarker. Pancreatic juice is a rich source of cancer-specific proteins rendering it a promising tool for identifying novel biomarkers. Additional sources of biomarkers including serum, tumor tissue, pancreatic juice, bile and other body fluids have revealed distinct biomarker patterns in PDAC. These data suggest that analysis of pancreatic juice and bile samples collected at the time of a surgical resection may identify prognostic biomarkers of value in PDAC. Biomarkers may be used to stratify patients based on prognosis and those who will benefit from intensive neoadjuvant protocols or adjuvant hepatic artery infusion therapy.
BIOMARKERS IN PANCREATIC JUICE
Diagnostic biomarkers
During the development of PDAC, malignant ductal cells preferentially shed into the ductal lumen, making pancreatic juice a rich source of cancer-specific proteins. CA 19-9 expression is demonstrated in 90% patients with pancreatic head adenocarcinoma compared to 11%-62% periampullary cancers of duodenal, ampullary or distal bile duct origin[15]. Overexpression of CA 19-9 and CEA in PDAC is shown in Figure 1, Figure 2, Figure 3, Figure 4 and it correlates with a higher histologic grade[15,16]. Elevation of CEA level and presence of K-ras mutation in pancreatic juice is a strong predictor of PDAC[17]. Increased levels of CA 19-9 and CEA in pancreatic juice are predictive of malignant transformation in benign intraductal papillary mucinous neoplasm (IPMN)[18-21]. Immunohistochemical staining of CEA is strongly positive in invasive IPMN and correlates with the grade of cellular atypia[21,22].
Figure 1.
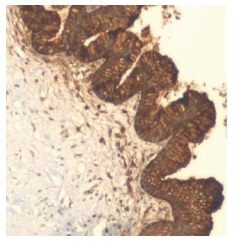
Pancreatic ductal adenocarcinoma with overexpression of carcinoembryonic antigen. The neoplastic cells demonstrate strong cytoplasmic and membranous staining (100 ×). Courtesy, Department of Pathology, Temple University Hospital, Philadelphia, PA, United States.
Figure 2.
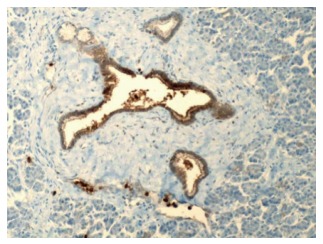
Benign pancreatic ducts and acini with weak staining of the ductal cells for carcinoembryonic antigen (200 ×). Courtesy, Department of Pathology, Temple University Hospital, Philadelphia, PA, United States.
Figure 3.
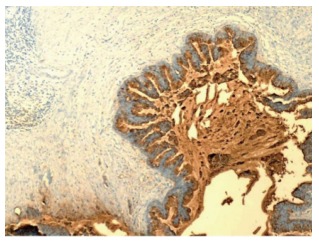
Pancreatic ductal adenocarcinoma with overexpression of cancer antigen 19-9. The neoplastic cells demonstrate strong cytoplasmic staining (100 ×). Courtesy, Department of Pathology, Temple University Hospital, Philadelphia, PA, United States.
Figure 4.
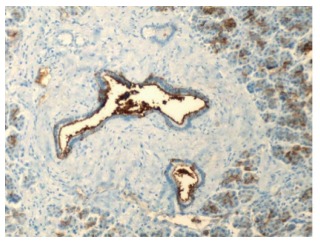
Benign pancreatic ducts and acini with weak staining of the ductal cells for cancer antigen 19-9 (200 ×). Courtesy, Department of Pathology, Temple University Hospital, Philadelphia, PA, United States.
In a comparison of the levels of CA 19-9 in the serum and pancreatic juice of patients with PDAC, the authors reported elevated levels in the pancreatic juice of all patients with normal levels in the sera of several patients[23]. Tumor marker levels are predictive of tumor burden with the level in pancreatic juice correlating with the local tumor and serum level with the systemic burden of disease. This may explain the elevation of tumor markers in pancreatic juice with normal serum levels in patients with malignant IPMN.
Genetic and epigenetic markers such as mutant K-ras, p53 mutations, DNA methylation alterations, mitochondrial DNA mutations and miRNAs in pancreatic juice are under evaluation for their role in distinguishing benign pancreatic pathology or chronic pancreatitis from preinvasive pancreatic neoplasia, IPMN and pancreatic intraepithelial neoplasia (PanIN)[24-33]. Mass spectrometry proteomics of pancreatic juice collected at the time of surgical resection of the tumor suggested distinct proteomic signatures for PDAC[34]. CEA and S100 calcium-binding protein P (S100P) concentrations in duodenal juice were significantly higher in PDAC than the benign conditions and may serve as a useful screening test for the detection of PDAC[35,36]. Immunohistochemical expression of human telomerase reverse transcriptase (hTERT) in preoperative pancreatic juice samples was detectable in 84% PDAC and 88% malignant IPMN and the accuracy of diagnosing PDAC improved when combined with cytology[30,37]. Proteomic analysis of pancreatic juice from patients with PDAC demonstrated three up-regulated proteins, matrix metallo proteinase-9 (MMP-9), oncogene DJ1 (DJ-1) and alpha-1B-glycoprotein precursor (AIBG) indicative of their potential as diagnostic biomarkers in PDAC[38]. Accurate peripheral markers of PDAC are lacking and select miRNAs identified in plasma and bile demonstrated excellent accuracy in distinguishing PDAC from benign conditions[33]. These data highlight the potential value of biomarkers from various biological sources in the early diagnosis of pancreatic cancer.
Prognostic biomarkers
Normal pancreatic juice contains multiple proteins and administration of secretin alters the concentration but not the spectrum of these proteins[39]. The proteome of pancreatic juice in patients with PDAC is markedly altered[39,40]. The proteome of the pancreas after surgical resection contains regenerative and immunomodulatory factors which vary depending on neoadjuvant therapy, history of smoking and vary over time to stimulate restoration of organ function[41]. Profiling of miRNAs in pancreatic juice of patients with PDAC demonstrated higher contents of miR-205 and miR-210 correlating with lymph node metastasis and diminished survival demonstrating their potential value as candidate biomarkers of disease progression and prognosis[42]. Assay of Adnab-9 in pancreaticobiliary secretions and PDAC tumor demonstrated its potential value as a candidate biomarker for diagnosis and prognostication[43]. Elevated level of S100A8 or A9 in pancreatic ductal fluid, a near absence of pancreatic enzymes and high level of mucins (MUC1, 2, 5AC, 5B, 6 and 13) were predictors of poor survival suggesting that pancreatic ductal fluid is a promising tool for identifying prognostic biomarkers[34].
BIOMARKERS IN BILE
Diagnostic and prognostic value
Intraoperative samples of bile from gallbladder in patients with pancreaticobiliary diseases demonstrated significantly higher levels of CA 19-9 in malignancy and correlated with the tumor burden[44]. Biliary CEA > 10 ng/mL in patients undergoing a curative surgery for colorectal cancer is a strong predictor of hepatic recurrence suggesting that it is a marker for occult liver metastases[45,46]. Liver is the site of first recurrence in 50% patients following curative surgery for PDAC[47]. The use of adjuvant liver-directed therapy including hepatic artery infusion chemotherapy (HAI) significantly decreases the incidence of liver metastases with a trend towards improvement in cumulative survival[48,49]. Prediction of the site of early recurrence can impact choice of the optimal modality for adjuvant therapy following curative surgery for PDAC.
Novel diagnostic biliary biomarkers for biliary tract cancer include Mac-2-binding protein (Mac-2BP) identified in bile using tandem mass spectrometry[50]. Alterations in epithelial mucin expression has identified MUC4 in pancreatic juice as a diagnostic and prognostic marker for pancreatic cancer, biliary MUC4 as a diagnostic biomarker and serum MUC5A as a sensitive diagnostic marker correlating negatively with survival in biliary tract cancer[34,51]. Elevated levels of biliary vascular endothelial growth factor (VEGF-1) distinguishes patients with pancreatic cancer from other etiologies of biliary stricture[52]. Potential biomarkers in the pancreatic juice and bile of patients with pancreatic adenocarcinoma are shown in Table 1. These preliminary data demonstrating the diagnostic and prognostic value of biliary markers in cancer require prospective evaluation and validation in large scale multicenter studies.
Table 1.
Potential biomarkers in the pancreatic juice and bile of patients with pancreatic adenocarcinoma
| Body fluid | Biomarker |
| Pancreatic ductal fluid | CA 19-9 |
| CEA | |
| K-ras | |
| p53 mutations | |
| DNA methylation alterations | |
| Mitochondrial DNA mutations | |
| S100 calcium-binding protein P (S100P) | |
| Human telomerase reverse transcriptase | |
| Matrix metalloproteinase-9 | |
| Oncogene DJ1 | |
| Alpha-1B-glycoprotein precursor | |
| MicroRNA- miR-205, miR-210 | |
| Adnab-9 | |
| S100A8 or A9 | |
| Mucins MUC1, 2, 5AC, 5B, 6 and 13 | |
| Bile | CEA |
| Mac-2-binding protein | |
| MUC4 | |
| Vascular endothelial growth factor |
CA 19-9: Cancer antigen 19-9; CEA: Carcinoembryonic antigen.
SELECTION OF BIOMARKERS
Choice of the optimal biomarker
Biomarkers obtained from readily accessible biological materials via non-invasive procedures minimize downstream investigations and costs[53]. Pancreatic juice and/or bile is readily collected during the course of a pancreatic resection for PDAC. In contrast to the recently identified biomarkers requiring further investigation prior to recommendation for clinical use, CA 19-9 and CEA are widely used and validated markers of diagnostic and prognostic value in PDAC and pre-neoplastic lesions of the pancreas[54]. However, the prognostic value of CA 19-9, CEA and LDH levels in the pancreatic juice and bile of patients with PDAC has not been evaluated. Standardized laboratory protocols are available for the assay of CA 19-9 and CEA in body fluids rendering them optimal biomarkers in the evaluation of patients with PDAC.
CONCLUSION
Pancreatic juice is a rich source of cancer-specific proteins rendering it a promising tool for identifying novel prognostic biomarkers in PDAC. Elevated level of CEA in bile is a marker for malignancy and a predictor of hepatic recurrence. CA 19-9, CEA and LDH are widely used in clinical practice as diagnostic markers of pancreatic cancer however, the prognostic value of their levels in pancreatic juice and bile is unknown. Specimens of pancreatic juice and bile can be readily obtained during surgical resection of the tumor and analyzed according to well-established laboratory protocols for assays of CA 19-9, CEA and LDH to evaluate their prognostic value. Profiling of pancreatic juice and bile to identify biomarkers may improve early diagnosis and selection of patients for the optimal adjuvant therapeutic modality.
Footnotes
Conflict-of-interest statement: None.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: December 19, 2016
First decision: March 27, 2017
Article in press: May 13, 2017
P- Reviewer: Kleeff J, Shah OJ, Yamagata M S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
References
- 1.National Cancer Institute. Estimated new cancer cases and deaths for 2013. SEER (Surveillance Epidemiology and End Results) cancer statistics review. Available from: http://www.seer.cancer.gov.
- 2.Corsini MM, Miller RC, Haddock MG, Donohue JH, Farnell MB, Nagorney DM, Jatoi A, McWilliams RR, Kim GP, Bhatia S, et al. Adjuvant radiotherapy and chemotherapy for pancreatic carcinoma: the Mayo Clinic experience (1975-2005) J Clin Oncol. 2008;26:3511–3516. doi: 10.1200/JCO.2007.15.8782. [DOI] [PubMed] [Google Scholar]
- 3.Herman JM, Swartz MJ, Hsu CC, Winter J, Pawlik TM, Sugar E, Robinson R, Laheru DA, Jaffee E, Hruban RH, et al. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol. 2008;26:3503–3510. doi: 10.1200/JCO.2007.15.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 5.Ferrone CR, Finkelstein DM, Thayer SP, Muzikansky A, Fernandez-delCastillo C, Warshaw AL. Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol. 2006;24:2897–2902. doi: 10.1200/JCO.2005.05.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger AC, Garcia M, Hoffman JP, Regine WF, Abrams RA, Safran H, Konski A, Benson AB, MacDonald J, Willett CG. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704. J Clin Oncol. 2008;26:5918–5922. doi: 10.1200/JCO.2008.18.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlieman MG, Ho HS, Bold RJ. Utility of tumor markers in determining resectability of pancreatic cancer. Arch Surg. 2003;138:951–955; discussion 951-955. doi: 10.1001/archsurg.138.9.951. [DOI] [PubMed] [Google Scholar]
- 8.Kinsella TJ, Seo Y, Willis J, Stellato TA, Siegel CT, Harpp D, Willson JK, Gibbons J, Sanabria JR, Hardacre JM, et al. The impact of resection margin status and postoperative CA19-9 levels on survival and patterns of recurrence after postoperative high-dose radiotherapy with 5-FU-based concurrent chemotherapy for resectable pancreatic cancer. Am J Clin Oncol. 2008;31:446–453. doi: 10.1097/COC.0b013e318168f6c4. [DOI] [PubMed] [Google Scholar]
- 9.Ni XG, Bai XF, Mao YL, Shao YF, Wu JX, Shan Y, Wang CF, Wang J, Tian YT, Liu Q, et al. The clinical value of serum CEA, CA19-9, and CA242 in the diagnosis and prognosis of pancreatic cancer. Eur J Surg Oncol. 2005;31:164–169. doi: 10.1016/j.ejso.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Todoroki T, Koike N, Morishita Y, Kawamoto T, Ohkohchi N, Shoda J, Fukuda Y, Takahashi H. Patterns and predictors of failure after curative resections of carcinoma of the ampulla of Vater. Ann Surg Oncol. 2003;10:1176–1183. doi: 10.1245/aso.2003.07.512. [DOI] [PubMed] [Google Scholar]
- 11.Fujioka S, Misawa T, Okamoto T, Gocho T, Futagawa Y, Ishida Y, Yanaga K. Preoperative serum carcinoembryonic antigen and carbohydrate antigen 19-9 levels for the evaluation of curability and resectability in patients with pancreatic adenocarcinoma. J Hepatobiliary Pancreat Surg. 2007;14:539–544. doi: 10.1007/s00534-006-1184-3. [DOI] [PubMed] [Google Scholar]
- 12.Tas F, Aykan F, Alici S, Kaytan E, Aydiner A, Topuz E. Prognostic factors in pancreatic carcinoma: serum LDH levels predict survival in metastatic disease. Am J Clin Oncol. 2001;24:547–550. doi: 10.1097/00000421-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Safi F, Schlosser W, Falkenreck S, Beger HG. Prognostic value of CA 19-9 serum course in pancreatic cancer. Hepatogastroenterology. 1998;45:253–259. [PubMed] [Google Scholar]
- 14.Iuga C, Seicean A, Iancu C, Buiga R, Sappa PK, Völker U, Hammer E. Proteomic identification of potential prognostic biomarkers in resectable pancreatic ductal adenocarcinoma. Proteomics. 2014;14:945–955. doi: 10.1002/pmic.201300402. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi K, Enjoji M, Tsuneyoshi M. Pancreatoduodenal carcinoma: a clinicopathologic study of 304 patients and immunohistochemical observation for CEA and CA19-9. J Surg Oncol. 1991;47:148–154. doi: 10.1002/jso.2930470303. [DOI] [PubMed] [Google Scholar]
- 16.Kamisawa T, Tu Y, Egawa N, Ishiwata J, Tsuruta K, Okamoto A, Hayashi Y, Koike M, Yamaguchi T. Ductal and acinar differentiation in pancreatic endocrine tumors. Dig Dis Sci. 2002;47:2254–2261. doi: 10.1023/a:1020139328215. [DOI] [PubMed] [Google Scholar]
- 17.Futakawa N, Kimura W, Yamagata S, Zhao B, Ilsoo H, Inoue T, Sata N, Kawaguchi Y, Kubota Y, Muto T. Significance of K-ras mutation and CEA level in pancreatic juice in the diagnosis of pancreatic cancer. J Hepatobiliary Pancreat Surg. 2000;7:63–71. doi: 10.1007/s005340050156. [DOI] [PubMed] [Google Scholar]
- 18.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, del Castillo CF, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–1336. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Grobmyer SR, Cance WG, Copeland EM, Vogel SB, Hochwald SN. Is there an indication for initial conservative management of pancreatic cystic lesions? J Surg Oncol. 2009;100:372–374. doi: 10.1002/jso.21260. [DOI] [PubMed] [Google Scholar]
- 20.Maire F, Voitot H, Aubert A, Palazzo L, O’Toole D, Couvelard A, Levy P, Vidaud M, Sauvanet A, Ruszniewski P, et al. Intraductal papillary mucinous neoplasms of the pancreas: performance of pancreatic fluid analysis for positive diagnosis and the prediction of malignancy. Am J Gastroenterol. 2008;103:2871–2877. doi: 10.1111/j.1572-0241.2008.02114.x. [DOI] [PubMed] [Google Scholar]
- 21.Kawai M, Uchiyama K, Tani M, Onishi H, Kinoshita H, Ueno M, Hama T, Yamaue H. Clinicopathological features of malignant intraductal papillary mucinous tumors of the pancreas: the differential diagnosis from benign entities. Arch Surg. 2004;139:188–192. doi: 10.1001/archsurg.139.2.188. [DOI] [PubMed] [Google Scholar]
- 22.Nagai E, Ueki T, Chijiiwa K, Tanaka M, Tsuneyoshi M. Intraductal papillary mucinous neoplasms of the pancreas associated with so-called “mucinous ductal ectasia”. Histochemical and immunohistochemical analysis of 29 cases. Am J Surg Pathol. 1995;19:576–589. doi: 10.1097/00000478-199505000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Schmiegel WH, Kreiker C, Eberl W, Arndt R, Classen M, Greten H, Jessen K, Kalthoff H, Soehendra N, Thiele HG. Monoclonal antibody defines CA 19-9 in pancreatic juices and sera. Gut. 1985;26:456–460. doi: 10.1136/gut.26.5.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maire F, Micard S, Hammel P, Voitot H, Lévy P, Cugnenc PH, Ruszniewski P, Puig PL. Differential diagnosis between chronic pancreatitis and pancreatic cancer: value of the detection of KRAS2 mutations in circulating DNA. Br J Cancer. 2002;87:551–554. doi: 10.1038/sj.bjc.6600475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi C, Fukushima N, Abe T, Bian Y, Hua L, Wendelburg BJ, Yeo CJ, Hruban RH, Goggins MG, Eshleman JR. Sensitive and quantitative detection of KRAS2 gene mutations in pancreatic duct juice differentiates patients with pancreatic cancer from chronic pancreatitis, potential for early detection. Cancer Biol Ther. 2008;7:353–360. doi: 10.4161/cbt.7.3.5362. [DOI] [PubMed] [Google Scholar]
- 26.Rogers CD, Fukushima N, Sato N, Shi C, Prasad N, Hustinx SR, Matsubayashi H, Canto M, Eshleman JR, Hruban RH, et al. Differentiating pancreatic lesions by microarray and QPCR analysis of pancreatic juice RNAs. Cancer Biol Ther. 2006;5:1383–1389. doi: 10.4161/cbt.5.10.3323. [DOI] [PubMed] [Google Scholar]
- 27.Matsubayashi H, Canto M, Sato N, Klein A, Abe T, Yamashita K, Yeo CJ, Kalloo A, Hruban R, Goggins M. DNA methylation alterations in the pancreatic juice of patients with suspected pancreatic disease. Cancer Res. 2006;66:1208–1217. doi: 10.1158/0008-5472.CAN-05-2664. [DOI] [PubMed] [Google Scholar]
- 28.Grønborg M, Bunkenborg J, Kristiansen TZ, Jensen ON, Yeo CJ, Hruban RH, Maitra A, Goggins MG, Pandey A. Comprehensive proteomic analysis of human pancreatic juice. J Proteome Res. 2004;3:1042–1055. doi: 10.1021/pr0499085. [DOI] [PubMed] [Google Scholar]
- 29.Shirai Y, Sogawa K, Yamaguchi T, Sudo K, Nakagawa A, Sakai Y, Ishihara T, Sunaga M, Nezu M, Tomonaga T, et al. Protein profiling in pancreatic juice for detection of intraductal papillary mucinous neoplasm of the pancreas. Hepatogastroenterology. 2008;55:1824–1829. [PubMed] [Google Scholar]
- 30.Nakashima A, Murakami Y, Uemura K, Hayashidani Y, Sudo T, Hashimoto Y, Ohge H, Oda M, Sueda T, Hiyama E. Usefulness of human telomerase reverse transcriptase in pancreatic juice as a biomarker of pancreatic malignancy. Pancreas. 2009;38:527–533. doi: 10.1097/MPA.0b013e3181a16d28. [DOI] [PubMed] [Google Scholar]
- 31.Ohuchida K, Mizumoto K, Ohhashi S, Yamaguchi H, Konomi H, Nagai E, Yamaguchi K, Tsuneyoshi M, Tanaka M. Twist, a novel oncogene, is upregulated in pancreatic cancer: clinical implication of Twist expression in pancreatic juice. Int J Cancer. 2007;120:1634–1640. doi: 10.1002/ijc.22295. [DOI] [PubMed] [Google Scholar]
- 32.Sadakari Y, Ohtsuka T, Ohuchida K, Tsutsumi K, Takahata S, Nakamura M, Mizumoto K, Tanaka M. MicroRNA expression analyses in preoperative pancreatic juice samples of pancreatic ductal adenocarcinoma. JOP. 2010;11:587–592. [PubMed] [Google Scholar]
- 33.Cote GA, Gore AJ, McElyea SD, Heathers LE, Xu H, Sherman S, Korc M. A pilot study to develop a diagnostic test for pancreatic ductal adenocarcinoma based on differential expression of select miRNA in plasma and bile. Am J Gastroenterol. 2014;109:1942–1952. doi: 10.1038/ajg.2014.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen KT, Kim PD, Jones KA, Devarajan K, Patel BB, Hoffman JP, Ehya H, Huang M, Watson JC, Tokar JL, et al. Potential prognostic biomarkers of pancreatic cancer. Pancreas. 2014;43:22–27. doi: 10.1097/MPA.0b013e3182a6867e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mori Y, Ohtsuka T, Kono H, Nagayoshi Y, Ideno N, Aso T, Kozono S, Ohuchida K, Takahata S, Nakamura M, et al. A minimally invasive and simple screening test for detection of pancreatic ductal adenocarcinoma using biomarkers in duodenal juice. Pancreas. 2013;42:187–192. doi: 10.1097/MPA.0b013e3182649979. [DOI] [PubMed] [Google Scholar]
- 36.Ohuchida K, Mizumoto K, Ishikawa N, Fujii K, Konomi H, Nagai E, Yamaguchi K, Tsuneyoshi M, Tanaka M. The role of S100A6 in pancreatic cancer development and its clinical implication as a diagnostic marker and therapeutic target. Clin Cancer Res. 2005;11:7785–7793. doi: 10.1158/1078-0432.CCR-05-0714. [DOI] [PubMed] [Google Scholar]
- 37.Uehara H, Nakaizumi A, Iishi H, Takenaka A, Eguchi H, Ohigashi H, Ishikawa O. In situ telomerase activity in pancreatic juice may discriminate pancreatic cancer from other pancreatic diseases. Pancreas. 2008;36:236–240. doi: 10.1097/MPA.0b013e31815bc1d6. [DOI] [PubMed] [Google Scholar]
- 38.Tian M, Cui YZ, Song GH, Zong MJ, Zhou XY, Chen Y, Han JX. Proteomic analysis identifies MMP-9, DJ-1 and A1BG as overexpressed proteins in pancreatic juice from pancreatic ductal adenocarcinoma patients. BMC Cancer. 2008;8:241. doi: 10.1186/1471-2407-8-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doyle CJ, Yancey K, Pitt HA, Wang M, Bemis K, Yip-Schneider MT, Sherman ST, Lillemoe KD, Goggins MD, Schmidt CM. The proteome of normal pancreatic juice. Pancreas. 2012;41:186–194. doi: 10.1097/MPA.0b013e31822862f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan S, Brentnall TA, Chen R. Proteomics analysis of bodily fluids in pancreatic cancer. Proteomics. 2015;15:2705–2715. doi: 10.1002/pmic.201400476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marchegiani G, Paulo JA, Sahora K, Fernández-Del Castillo C. The proteome of postsurgical pancreatic juice. Pancreas. 2015;44:574–582. doi: 10.1097/MPA.0000000000000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Raimondo M, Guha S, Chen J, Diao L, Dong X, Wallace MB, Killary AM, Frazier ML, Woodward TA, et al. Circulating microRNAs in Pancreatic Juice as Candidate Biomarkers of Pancreatic Cancer. J Cancer. 2014;5:696–705. doi: 10.7150/jca.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tobi M, Kim M, Weinstein DH, Rambus MA, Hatfield J, Adsay NV, Levi E, Evans D, Lawson MJ, Fligiel S. Prospective markers for early diagnosis and prognosis of sporadic pancreatic ductal adenocarcinoma. Dig Dis Sci. 2013;58:744–750. doi: 10.1007/s10620-012-2387-x. [DOI] [PubMed] [Google Scholar]
- 44.Brockmann J, Emparan C, Hernandez CA, Sulkowski U, Dietl KH, Menzel J, Wolters H, Glodny B, Senninger N. Gallbladder bile tumor marker quantification for detection of pancreato-biliary malignancies. Anticancer Res. 2000;20:4941–4947. [PubMed] [Google Scholar]
- 45.Yeatman TJ, Bland KI, Copeland EM, Hollenbeck JI, Souba WW, Vogel SB, Kimura AK. Relationship between colorectal liver metastases and CEA levels in gallbladder bile. Ann Surg. 1989;210:505–512. doi: 10.1097/00000658-198910000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Destri G, Lanteri R, Santangelo M, Torrisi B, Di Cataldo A, Puleo S. Can biliary carcinoembryonic antigen identify colorectal cancer patients with occult hepatic metastases? World J Surg. 2006;30:1494–1499. doi: 10.1007/s00268-005-0698-1. [DOI] [PubMed] [Google Scholar]
- 47.Katz MH, Wang H, Fleming JB, Sun CC, Hwang RF, Wolff RA, Varadhachary G, Abbruzzese JL, Crane CH, Krishnan S, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16:836–847. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayashibe A, Kameyama M, Shinbo M, Makimoto S. Clinical results on intra-arterial adjuvant chemotherapy for prevention of liver metastasis following curative resection of pancreatic cancer. Ann Surg Oncol. 2007;14:190–194. doi: 10.1245/s10434-006-9110-0. [DOI] [PubMed] [Google Scholar]
- 49.Morak MJ, van der Gaast A, Incrocci L, van Dekken H, Hermans JJ, Jeekel J, Hop WC, Kazemier G, van Eijck CH. Adjuvant intra-arterial chemotherapy and radiotherapy versus surgery alone in resectable pancreatic and periampullary cancer: a prospective randomized controlled trial. Ann Surg. 2008;248:1031–1041. doi: 10.1097/SLA.0b013e318190c53e. [DOI] [PubMed] [Google Scholar]
- 50.Koopmann J, Thuluvath PJ, Zahurak ML, Kristiansen TZ, Pandey A, Schulick R, Argani P, Hidalgo M, Iacobelli S, Goggins M, et al. Mac-2-binding protein is a diagnostic marker for biliary tract carcinoma. Cancer. 2004;101:1609–1615. doi: 10.1002/cncr.20469. [DOI] [PubMed] [Google Scholar]
- 51.Matull WR, Andreola F, Loh A, Adiguzel Z, Deheragoda M, Qureshi U, Batra SK, Swallow DM, Pereira SP. MUC4 and MUC5AC are highly specific tumour-associated mucins in biliary tract cancer. Br J Cancer. 2008;98:1675–1681. doi: 10.1038/sj.bjc.6604364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Navaneethan U, Gutierrez NG, Jegadeesan R, Venkatesh PG, Poptic E, Liu X, Sanaka MR, Jang S, Vargo JJ, Parsi MA. Vascular endothelial growth factor levels in bile distinguishes pancreatic cancer from other etiologies of biliary stricture: a pilot study. Dig Dis Sci. 2013;58:2986–2992. doi: 10.1007/s10620-013-2764-0. [DOI] [PubMed] [Google Scholar]
- 53.Verma M, Manne U. Genetic and epigenetic biomarkers in cancer diagnosis and identifying high risk populations. Crit Rev Oncol Hematol. 2006;60:9–18. doi: 10.1016/j.critrevonc.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 54.Duffy MJ, Sturgeon C, Lamerz R, Haglund C, Holubec VL, Klapdor R, Nicolini A, Topolcan O, Heinemann V. Tumor markers in pancreatic cancer: a European Group on Tumor Markers (EGTM) status report. Ann Oncol. 2010;21:441–447. doi: 10.1093/annonc/mdp332. [DOI] [PubMed] [Google Scholar]


