Abstract
AIM
To evaluate the potential effectiveness of robot-assisted gastrectomy (RAG) in comparison to open gastrectomy (OG) for gastric cancer patients.
METHODS
A comprehensive systematic literature search using PubMed, EMBASE, and the Cochrane Library was carried out to identify studies comparing RAG and OG in gastric cancer. Participants of any age and sex were considered for inclusion in comparative studies of the two techniques independently from type of gastrectomy. A meta-analysis of short-term perioperative outcomes was performed to evaluate whether RAG is equivalent to OG. The primary outcome measures were set for estimated blood loss, operative time, conversion rate, morbidity, and hospital stay. Secondary among postoperative complications, wound infection, bleeding and anastomotic leakage were also analysed.
RESULTS
A total of 6 articles, 5 retrospective and 1 randomized controlled study, involving 6123 patients overall, with 689 (11.3%) cases submitted to RAG and 5434 (88.7%) to OG, satisfied the eligibility criteria and were included in the meta-analysis. RAG was associated with longer operation time than OG (weighted mean difference 72.20 min; P < 0.001), but with reduction in blood loss and shorter hospital stay (weighted mean difference -166.83 mL and -1.97 d respectively; P < 0.001). No differences were found with respect to overall postoperative complications (P = 0.65), wound infection (P = 0.35), bleeding (P = 0.65), and anastomotic leakage (P = 0.06). The postoperative mortality rates were similar between the two groups. With respect to oncological outcomes, no statistical differences among the number of harvested lymph nodes were found (weighted mean difference -1.12; P = 0.10).
CONCLUSION
RAG seems to be a technically valid alternative to OG for performing radical gastrectomy in gastric cancer resulting in safe complications.
Keywords: Robot-assisted gastrectomy, Gastric resection, Open gastrectomy, Gastric cancer
Core tip: We took into consideration how safe and efficient robot-assisted gastrectomy (RAG) is compared to open gastrectomy (OG) for gastric cancer via systematic review and meta-analysis. The available studies to date and the analysis of pooled data extracted from these showed that RAG is safe and feasible, making it possible to obtain lower blood loss related to surgery and a more rapid patient recovery. At the same time similar lymph node dissection between the two techniques were revealed. We can reasonably expect that the innovative robotic technique could represent a valid alternative with potential benefit to equal oncological adequacy with respect to OG.
INTRODUCTION
Since its introduction by Kitano et al[1] in 1994, laparoscopy has been increasingly used for the treatment of gastric cancer. During this period of time, a number of works have shown laparoscopic gastrectomy (LG) to be a feasible option in treating gastric cancer and level III studies provided the evidence that laparoscopic assisted distal gastrectomy (LADG) leads to better results in the short-term than conventionally performed open gastrectomy (OG) for early gastric cancer[2-5]. Due to the high incidence of gastric cancer and the extremely high levels of expertise achieved by Asian surgeons, LG is now a routine procedure for early gastric cancer in eastern states[6]. On the other hand, laparoscopic surgery did not meet the same widespread requirements for the management of advanced gastric cancer, mainly due to the technical difficulties posed by the D2 lymphadenectomy and the intestinal reconstruction after total gastrectomy. Concerns regarding oncological adequacy, in particular for potential inadequate lymphadenectomy and long-term outcomes[6,7], make LG for advanced gastric cancer still questionable. Therefore, a significant proportion of patients with advanced stage disease are still treated with OG, especially in Western countries. In an effort to overcome the technical disadvantages of laparoscopic technique, robotic surgery has been introduced and it has gradually spread worldwide. Robotic systems have three-dimensional (3D) high-resolution imaging, tremor filter, and internal articulated endoscopic wrist (EndoWrist™ System), which lead to significant improvements in visibility and manipulation with respect to conventional laparoscopy. With this advanced equipment, robot-assisted gastrectomy (RAG) has been advocated to give a global advantage over the traditional laparoscopic approach, particularly in performing the D2 lymphadenectomy and facilitating complex reconstruction after gastrectomy[8-10]. A variety of reports have demonstrated the safety and feasibility of this technique[6]. However, so far most of the reports derives from not large, retrospective, non-randomized studies.
In order to achieve a confirmed acceptance, an innovative technique with minimum invasiveness absolutely has to show that it is not disadvantageous to oncologic result. As LG still has not reached a comprehensive validation for the treatment of all (advanced) gastric cancer, the introduction of robotic surgery can represent a fair cue of advancement potentially able to make the laparoscopic technique more oncologically adequate, and so to increase its use as alternative procedure to the conventional open approach. Yet, to date a mere handful of trials have shown high quality comparative analysis of RAG vs OG in the treatment of gastric cancer, and most of these studies have been limited to small sample size and a single institution design. To overcome these limitations, we performed a systematic review and meta-analysis which can increase the statistical power of short-term results available so far on these two techniques. Thus, relevant trials comparing the safety and efficacy of RAG vs OG in treating gastric cancer were analyzed, to verify if at present there is actual evidence of an advantage to the introduction of this new minimally invasive technique with respect to the validated open procedure. Positive results could represent the preliminary impulse to potentiate the robotic tool which, by overcoming some intrinsic limits of the conventional laparoscopic method, might increase the use and acceptance of the minimally invasive procedure for gastric cancer in the future.
MATERIALS AND METHODS
Literature search
A comprehensive systematic literature search was conducted using PubMed, EMBASE, and the Cochrane Library to identify relevant articles comparing RAG vs OG for the treatment of gastric cancer published up to December 2016. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement guidelines were adopted for performing and reporting meta-analysis data[11]. No restriction was set for type and date of publication, and for age and sex of participants. Article language was limited to English. The following terms were used for the search strategy: “Robot” or “robotic” or “robot-assisted” or “robotic-assisted” and “open” in combination with “gastrectomy” or “gastric resection” or “gastric cancer” or “stomach cancer” or “gastric carcinoma”. Either free-text and medical subject heading (MeSH) searches were used for keywords. The search was further broadened by extensive cross-checking of all reference in the retrieved articles fulfilling the inclusion criteria in order to identify eventual additional non indexed literature. Discrepancies in the search were resolved by consensus discussion among the entire author group. All relevant texts, tables and figures were reviewed for data extraction.
Study selection
Two authors (FF and LDF) independently screened the primary data from the studies identified in the electronic search. The initial assessed data included authors, titles and abstract. Then, the following inclusion criteria were set for inclusion the studies in the meta-analysis: (1) trials comparing robotic and OG for gastric adenocarcinoma, independently from the type of gastrectomy (distal gastrectomy, proximal gastrectomy and total gastrectomy) and tumour stage (early or advanced gastric cancer); and (2) Studies reporting at least one of the perioperative outcome measures among the following: Operative blood loss, operative time, numbers of harvested lymph nodes, postoperative complication rate, postoperative mortality, and hospital stay (interval from operation to discharge).
The following exclusion criteria were set: (1) duplicate studies; (2) non-comparative studies; (3) if publications are reviews, conference abstracts, letters, comments or case reports; (4) non-relevant topic papers or when all the reported appropriate outcomes were not included; (5) studies where it was not possible to extract or calculate data of interest from the published results; and (6) if more than one study was reported by the same institute, the most recent work or that containing more complete data was selected.
Primary relevant data from the original included studies were independently extracted and summarized by the same two authors. In addition, in terms of postoperative complications, anastomotic leakage, bleeding, as well as wound infection were also analyzed when reported. Any disagreement was resolved by consensus among the author group.
Quality assessment
The modified Newcastle-Ottawa Scale was used to assess the methodological quality of retrospective non randomized studies[12]. The scale consists of eight multiple-choice questions assessed essentially on three major categories: Patient selection, comparability (of cases and controls in case-control studies, of cohorts in cohort studies), and the assessment of the outcome (in case-control studies) or exposure (in cohort studies)[12]. The number of possible answers per question ranges from 2 to 5. High-quality responses earn a star, totaling up to nine stars (the comparability question earns up to two stars).
The quality of randomized clinical trials was assessed using Jadad’s scoring system[13]. The Jadad’s scale, widely validated for reporting randomized controlled trials quality, assess a score (ranging 0 to 6) on the base of three major elements: randomization (0-2 points), blinding (0-2 points) and patients withdrawal (0-1 point).
Risk of bias
Assessment for potential publication bias was analysed through drawing of funnel plots which were inspected for asymmetry for all outcome measures and evaluated by the Begg’s[14] and Egger’s tests[15].
Statistical analysis
The statistical analysis was performed using Review Manager software version 5.3 (RevMan 5.3, Cochrane Collaboration, Oxford, United Kingdom). Weighted mean differences (WMD) and odds ratios (OR) were used as a summary measure of efficacy for continuous and dichotomous variables respectively. A 95%CI was reported.
Statistical heterogeneity among the studies was evaluated using the χ2 test and according to the Higgins’ I2 statistic[16]. I2 values of 0-25%, 25%-50% and > 50% were considered as indicative of homogeneity, moderate heterogeneity and high heterogeneity, respectively[17]. To estimate the pooled WMD or OR, the inverse variance method with fixed-effects model was applied when no or moderate heterogeneity was detected among studies (I2 < 50%) according to Mantel-Haenszel method[18], whereas the random-effect model was used for analysis when I2 was greater than 50% (DerSimonian and Laird method)[19]. WMD was pooled by using the inverse variance model. The Z test was used to determine the pooled WMD or OR. Sensitivity analyses and funnel plots were assumed to investigate potential publication bias.
Funnel plot asymmetry, which reflects the presence of publication bias in the studies, was assessed using Begg’s and Egger’s tests. Begg and Mazumdar’s rank correlation tests the rank correlation (Kendall’s tau) between the standardized effect size and the variances (or standard errors) of these effects[14]; the Egger’s linear regression method[20] quantify the bias captured by the funnel plot. P value < 0.05 were considered to indicate statistical significance.
RESULTS
Study selection
The literature search yielded a total of 384 articles (Figure 1). After elimination of duplicates (n = 165), the remaining 219 titles and abstracts were reviewed. Based on the methodological inclusion and exclusion criteria, 196 studies were excluded: 115 did not compare techniques, 21 were non English studies, 60 were review articles, letters, case reports or comment. The full text of the remaining 23 articles were reviewed; of these, 1 was excluded because it was a redundant and lower level series, 11 contained non relevant topics, 5 because it was impossible to retrieve or calculate data of interest. Finally, a total of 6 articles[21-26] (South Korea 2, China 2, Italy 1, Romania 1) were considered eligible for inclusion in the meta-analysis (Figure 1).
Figure 1.
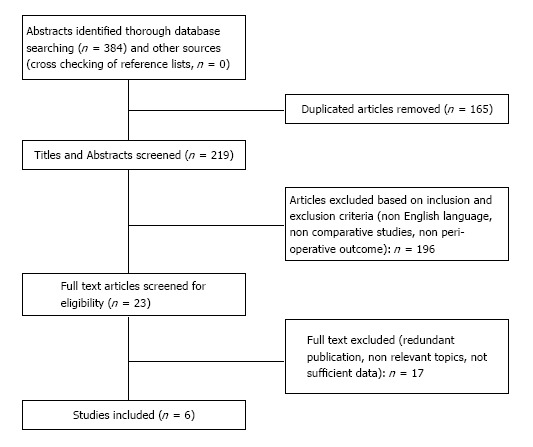
Flow chart of study selection.
Only one of these studies was a randomized controlled trial[26], while the others were retrospective non-randomized trials. The same two authors extracted the number and characteristics of patients of both the RAG and OG groups, which globally included 6.123 patients.
Huang et al[23] did not provide in their original papers data regarding means and standard deviations of perioperative outcome, which instead were expressed as medians and ranges. This additional initially unpublished information was retrieved from a previous meta-analysis[27], in which the data was obtained by contacting the authors.
The baseline characteristic, quality assessment and main perioperative data of the included studies were listed in Tables 1 and 2.
Table 1.
Baseline characteristics of include studies and quality assessment
| Ref. | Year | Country | Type of study | Total patients (n) | Group | n | Sex (M/F) | P value | Age (mean ± SD) | P value | BMI (mean ± SD) | P value | Quality assessment |
| Kim et al[21] | 2010 | South Korea | Retrospective clinical trial | 28 | RAG | 16 | 10/6 | NS | 53.8 ± 15.6 56.0 ± 12.4 | NS | 21.3 ± 3.4 25.2 ± 1.9 | > 0.05 | 6 stars1 |
| OG | 12 | 9/3 | |||||||||||
| Caruso et al[22] | 2011 | Italy | Retrospective clinical trial | 149 | RAG | 29 | 18/11 | NS | 64.8 ± 12.4 65.1 ± 11 | NS | 27 ± 3 28 ± 4 | NS | 6 stars1 |
| OG | 120 | 65/55 | |||||||||||
| Huang et al[23] | 2012 | China | Retrospective clinical trial | 625 | RAG | 39 | 19/20 | < 0.05 | 65.1 ± 15.9 67.9 ± 30.1 | NS | 24.2 ± 3.7 23.7 ± 3.6 | NS | 5 stars1 |
| OG | 586 | 406/180 | |||||||||||
| Kim et al[24] | 2012 | South Korea | Retrospective clinical trial | 4978 | RAG | 436 | 265/171 | NS | 54.2 ± 12.5 57.7 ± 11.8 | < 0.05 | 23.6 ± 3.1 23.8 ± 8.0 | NS | 5 stars1 |
| OG | 4542 | 3008/1534 | |||||||||||
| Procopiuc et al[25] | 2015 | Romania | Retrospective clinical trial | 47 | RAG | 18 | 13/5 | NS | 59.1 ± 13.7 60.1 ± 12.4 | NS | 26.0 ± 3.24 24.8 ± 4.58 | NS | 6 Stars1 |
| OG | 29 | 21/8 | |||||||||||
| Wang et al[26] | 2016 | China | Randomized clinical trial | 296 | RAG | 151 | 109/42 | NS | 57.5 ± 12.7 55.9 ± 13.1 | NS | 22.1 ± 2.9 21.3 ± 2.5 | NS | 3 points2 |
| OG | 145 | 89/56 |
According to the NOS (Newcastle-Ottawa Scale) classification;
According to Jadad’s scale for reporting randomized controlled trials. RAG: Robot-assisted gastrectomy; OG: Open gastrectomy; NS: Not statistically significant.
Table 2.
Main perioperative data of the included studies
| Ref. | Open conversion (%) | Group | Operation time (min ± SD)1 | P value | Blood loss (mL ± SD)1 | P value | Harvested nodes (n ± SD)1 | P value | Morbidity (%) | P value | Mortality (%) | P value | Hospital stay (d ± SD)1 | P value |
| Kim et al[21] | 0 | RAG OG | 259.2 ± 38.9 126.7 ± 24.1 | < 0.05 | 30.3 ± 15.1 78.8 ± 74.1 | < 0.05 | 41.1 ± 10.9 43.3 ± 10.4 | NS | 0 20 | NS | 0 0 | NS | 5.1 ± 0.3 6.7 ± 1.4 | < 0.05 |
| Caruso et al[22] | 0 | RAG OG | 290 ± 67 222 ± 94 | < 0.05 | 197.6 ± 202.1 386.1 ± 95.5 | < 0.05 | 28.0 ± 11.2 31.7 ± 15.6 | NS | 10.32 10.02 | NS | 0 3.3 | NS | 9.6 ± 2.8 13.4 ± 8.5 | < 0.05 |
| Huang et al[23] | NR | RAG OG | 415.9 ± 101.2 331.8 ± 92.9 | < 0.05 | 93.9 ± 89 192 ± 193 | < 0.05 | 32 ± 13.7 34 ± 14.8 | NS | 15.4 14.7 | NS | 1.4 2.6 | NS | 11.3 ± 14.4 16.5 ± 13.6 | < 0.05 |
| Kim et al[24] | NR | RAG OG | 226 ± 54 158 ± 52 | < 0.05 | 85 ± 160 192 ± 193 | < 0.05 | 40.2 ± 15.5 40.5 ± 16.6 | NS | 10.1 10.7 | NS | 0.5 0.5 | NS | 7.5 10.2 | < 0.05 |
| Procopiuc et al[25] | 0 | RAG OG | 320.8 ± 85.1 243.3 ± 57.9 | < 0.05 | 208.2 ± 139.8 564.6 ± 468.4 | < 0.05 | 22.0 ± 8.9 25.2 ± 9.0 | NS | 11.12 20.72 | NS | 0 0 | NS | 8.1 ± 2.0 11.4 ± 2.9 | < 0.05 |
| Wang et al[26] | 1.93 | RAG OG | 242.7 ± 43.8 192.4 ± 31.5 | < 0.05 | 94.2 ± 51.5 152.8 ± 94.2 | < 0.05 | 29.1 ± 6.7 30.1 ± 7.2 | NS | 9.3 10.3 | NS | 0 0 | NS | 5.7 ± 2.3 6.4 ± 2.5 | < 0.05 |
Mean value;
Major complications rate base on Clavien-Dindo classification ≥ 3, such as anastomotic and duodenal lekeage;
Rate of patients excluded from the study analysis. RAG: Robot-assisted gastrectomy; OG: Open gastrectomy; NS: Not statistically significant difference.
Operative time
All included studies[21-26] reported a significantly longer operation time of the RAG group than OG (Table 2). The meta-analysis of pooled data (Figure 2A) confirmed the result showing a significantly lower operative time in the group of OG compared to RAG group (WMD: 72.20 min, 95%CI: 48.82 to 105.13 min, P < 0.001). There was significant heterogeneity (I2 = 85%) (Figure 2A).
Figure 2.
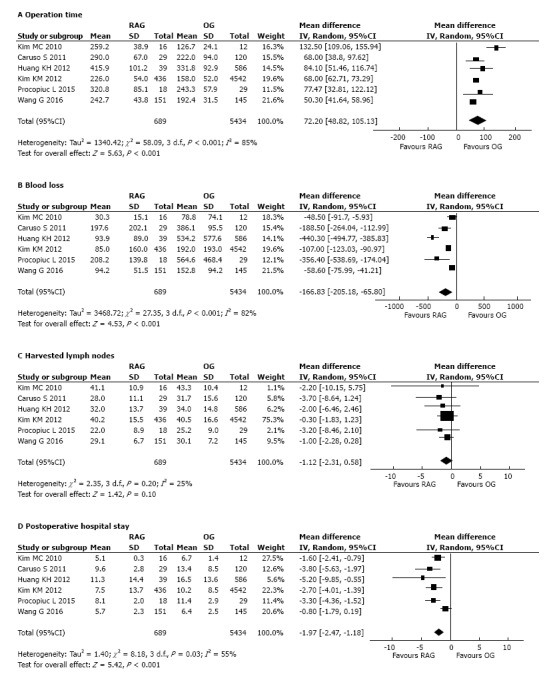
Forest plot showing the meta-analysis of pooled data on robot-assisted gastrectomy vs open gastrectomy. A: Operation time; B: Estimated blood loss; C: Harvested lymph nodes; D: Postoperative hospital stay. RAG: Robot-assisted gastrectomy; OG: Open gastrectomy.
Estimated blood loss
All the included studies reported the mean intra-operative related to surgery estimated blood loss. A concordant result of statistical significantly lower blood loss volume in the RAG group than in the OG group (Table 2) was reported. The pool meta-analyzed data confirmed that blood loss was notably less in the RAG group as opposed to OG (WMD: -166.83 mL, 95%CI: -205.18 to -65.80 mL, P < 0.001) with a significant heterogeneity between studies (I2 = 82%) (Figure 2B).
Harvested lymph nodes
The mean number of harvested lymph nodes was reported in all studies (Table 2). The pooled data from the included studies showed that the two groups did not differ significantly in the number of harvested lymph nodes (WMD = -1.12; 95%CI: -2.31 to 0.58; P = 0.10), with low heterogeneity between studies (I2 = 25%) (Figure 2C).
Postoperative hospital stay: All the 6 included studies reported the length of hospital stay, showing in agreement a statistically significant reduction in favour of the RAG group compared to OG (Table 2). The meta-analysis of combined data confirmed the result, showing shorter postoperative hospital stay in the RAG group compared to OG (Figure 2D). The robotic approach reduced the postoperative stay by a mean of 1.97 d (WMD = -1.97; 95%CI: -2.47 to -1.18 d; P < 0.001). Although there was a significant heterogeneity among the studies (I2 = 55%) (Figure 2D).
Postoperative complications: Short-term postoperative complications were recorded in all analyzed studies. The meta-analysis did not significantly differ in the overall postoperative complication rate of the two groups (OR = 0.95, 95%CI: 0.60-1.34, P = 0.65) with low heterogeneity (I2=12%) (Figure 3A).
Figure 3.
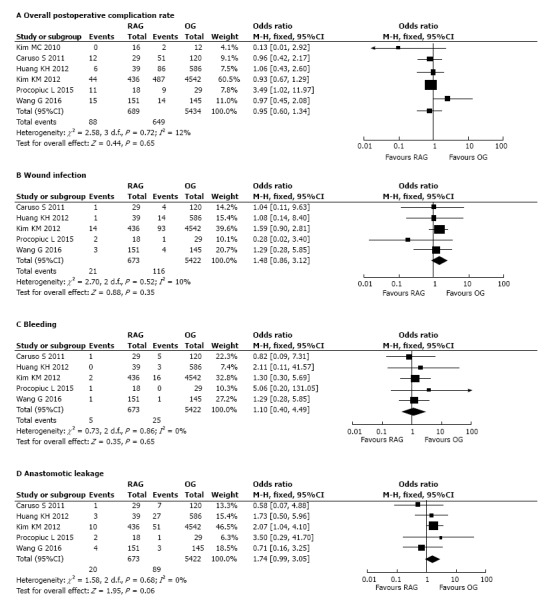
Forest plot showing the meta-analysis of postoperative complication between robot-assisted gastrectomy and open gastrectomy. A: Overall postoperative complications; B: Wound infection; C: Bleeding; D: Anastomotic leakage. RAG: Robot-assisted gastrectomy; OG: Open gastrectomy.
Five out of 6 studies[22-26] reported the incidence by group of the following subtype of early postoperative complications: wound infection, bleeding and anastomotic leakage. The meta-analysis of pooled data regarding these complications showed no difference between the two groups (respectively: Wound infection, OR = 1.48, 95%CI: 0.86-3.12, P = 0.35, I2 = 10%; bleeding, OR = 1.10, 95%CI: 0.40-4.49, P = 0.65, I2 = 0%; anastomotic leakage OR = 1.74, 95%CI: 0.99-3.05, P = 0.06, I2 = 0%) (Figure 3B-D).
Three studies out of 6[22-24] reported postoperative mortality rate value ranging from 0.5% to 3.3%, without statistically significant differences between the robotic and open procedures, while the rest of the studies[21,25,26] did not detect any case of mortality related to both surgical techniques (Table 2). A meta-analysis of pooled data was therefore considered unnecessary, as 50% of studies did not report any event of mortality in both groups and the data are insufficient to calculate an objective OR, thus the combined data reflected the evident equality of mortality rates among RAG and OG groups.
Publication bias
A standard-error based funnel plot using fix effect size between RAG and OG was constructed for morbidity (Figure 4). The overall postoperative complication rate of all the studies lay within the limits of 95%CIs with just a slight asymmetry, indicating no serious publication biases. No evidence of publication bias was revealed among the studies from statistical tests for any primary outcomes (Begg’s test all P > 0.10; Egger’s test all P > 0.10).
Figure 4.
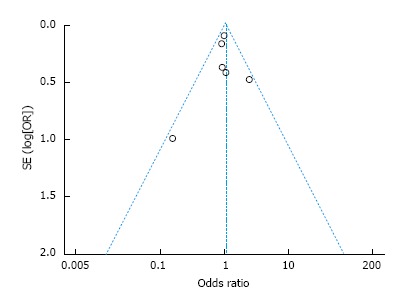
Funnel plot for results from each study comparing overall morbidity between robot-assisted gastrectomy and open gastrectomy. OR: Odds ratio; SE: Standard error.
Sensitivity analysis: Sensitivity analysis was performed by excluding the study with the lowest quality score and the smallest sample size[21]. All variables were conducted for sensitivity analysis. The results were not affected by sensitivity analysis as shown in Table 3.
Table 3.
Sensitivity analysis of outcomes
| Outcomes | No. of studies |
Patients |
WMD/OR | Analysis model | 95%CI | P value |
Heterogeneity |
||
| RAG | OG | I2 (%) | P value | ||||||
| Operative time (min) | 5[22-26] | 673 | 5422 | 60.12 | Random | 41.31, 98.06 | < 0.00001 | 80 | 0.41 |
| Estimated blood loss (mL) | 5[22-26] | 673 | 5422 | -193.78 | Random | -215.77, -72.13 | < 0.0001 | 72 | 0.007 |
| Harvested lymph nodes | 5[22-26] | 673 | 5422 | -1.05 | Random | -2.01, 0.39 | 0.35 | 0 | 0.12 |
| Overall postoperative complication | 5[22-26] | 673 | 5422 | 0.92 | Fixed | 0.61, 1.36 | 0.6 | 12 | 0.72 |
| Postoperative hospital stay | 5[22-26] | 673 | 5422 | -2.57 | 135.8 ± 133.9 | -2.68, -1.56 | < 0.001 | 0 | 0.54 |
RAG: Robot-assisted gastrectomy; OG: Open gastrectomy; WMD: Weighted mean difference; OR: Odds ratio.
DISCUSSION
Procedures which offer minimum invasiveness would present a perfectly acceptable alternative to open surgery, with better short-term results, if it were possible to respect oncologic criteria to the same degree as the open approach, and if there were no compromising effect on long-term survival. Even though long-term survival is one of the major oncologically prominent issue, lymph node metastasis has long been seen as the element which most significantly predicts recurrence and therefore survival in patients suffering from gastric cancer[28]. Thus, the amount of harvested lymph nodes is an accurate reflection of whether gastric resection for adenocarcinoma is an adequate option, and can be used as indicator of oncological adequacy when no long follow-up times are available.
Total and distal gastrectomy with D2 lymphadenectomy node is the recommended surgical procedure for most resectable gastric cancer patients[29]. LG with lymph node dissection has developed as a minimally invasive surgery for gastric cancer over the last two decades and it has been utilized principally for early gastric cancer. Some randomized studies and meta-analysis showed that LG with limited lymph node dissection for patients with early-stage gastric lesion provided oncologic results which were not inferior compared to OG, with however improved short-term outcomes[2-5].
In contrast, a handful of trials, which all contained not large cohort of patients, outline the safety of laparoscopic assisted distal and total gastrectomy with D2 lymph node dissection in advanced-stage of gastric cancer. Several meta-analysis regarding this issue have been recently published. However, the outcomes were contradictory, especially regarding postoperative complications and the amount of harvested lymph nodes[30-32].
Thus, although LADG has been widely developed for early gastric cancer, the global effectiveness in therapeutic terms of LG still has not been extensively looked into with regards to the treatment of advanced-stage of gastric cancer. Although a totally LG with extended D2 lymphadenectomy has been demonstrated to be feasible by several authors[33-36], owing to the intrinsic difficulty of execution, oncologic concerns still exist regarding the possibility of performing a D2 lymphadenectomy radically and suitably. Indeed, the meta-analysis of the randomized controlled trials (RCTs) demonstrates that whenever results on LADG is gathered from advanced gastric cancers together with the early stage the same extent of lymph node dissection as in traditional surgery could not be guaranteed[37,38].
Although laparoscopic sub-D2 lymphadenectomy may be seen as suitable for nearly all early gastric cancer in which lymph node metastases rarely occur (2%-20% of cases)[37], and so far is routine in Asia[39], the same cannot be said about advanced gastric cancer and so LG cannot be advised as a standard approach for all patients with gastric cancer.
With the development of technology, the introduction of a robotic tool as a useful adjunctive method to assist laparoscopy has gradually increased the use of minimally invasive procedures in several fields of surgery. For the treatment of gastric cancer, RAG has been widely demonstrated to be feasible and safe in many studies[8,40-49]. Robotic surgery is progressively becoming an attractive option for surgeons, in particular because it may overcome some intrinsic limitations of conventional laparoscopy, in particular for the D2 lymphadenectomy, expanding the application of minimally invasive procedures. In fact, this technique has certain indisputable advantages, such as high definition 3D imaging, improved dexterity enabled by the endowristed movements, tremors filtration, motion scaling, stereoscopic visualization, which are particular useful when precise dissection is needed, such as during the lymphadenectomy along major abdominal vessels (gastric, gastroepiploic, common hepatic, and celiac artery lymph nodes). Thus, as long as drawbacks of the LG technique exist, the introduction of new innovative technologies, such as robotic gastrectomy, are desirable. In fact, the median number of retrieved nodes, reported by many authors through the use of robotic system for D2 lymphadenectomy, is not dissimilar to that of traditional open technique, and in several instances even superior to laparoscopy[27,40,41,50-57].
However, significant limitations exist in the interpretation of data available so far regarding the comparison of RAG with respect to OG, as a result of the shortage of randomized trials, the restricted amount of observational and comparative studies of high quality, the small sample sizes so far, and the shortened length of follow-up. Therefore, there has been difficulty in drawing final conclusions regarding the superiority of one approach over another.
A meta-analysis is a suitable way to widen the source of evidence. Evaluating pooled data among the most relevant studies is a quantitative method that may increase the statistical power of otherwise poorly consistent results and may resolve some controversy of evidence.
Robotic surgery is a technical innovation which improves the effectiveness of laparoscopic technique, which is used through the same laparoscopic way as a non independent adjunctive tool. Thus, we strictly limit the research by focusing exclusively on RAG with the intent to evaluate the real merit of the addition of robotic assistance to laparoscopy over the traditional OG for gastric cancer, performing a comprehensive systematic review and meta-analysis. Such a way of conducting the trial will provide a more objective appraisal of the effectiveness of RAG in gastric cancer patients, in order to confirm the single-institute promising results in favour of this innovative technique to date reported. This could represent the preliminary cue in support of the increasingly widespread view which considers robotics to be a completion of laparoscopy, making it possible to fill the existing performance gap with respect to OG.
Six studies, of which 5 retrospective clinical trials and 1 RCT, involving 6123 patients with 689 (11.3%) cases of RAG and 5434 (88.7%) of OG, were considered eligible for inclusion in this meta-analysis.
The results show globally that RAG provided short-term results which can be compared to OG, with outcomes which can be considered as satisfactory with regards to perioperative results and oncological effectiveness.
The operation time was significantly longer with RAG than OG (P < 0.001). The greater length of robotic surgery is principally due to the additional time for set-up and docking of the robotic system[58]. Nevertheless, it should be noted that the time of operation notably diminished as surgical experience increased and the robotic procedure was standardized[8,47,59].
An advantageous lower blood loss and shorter hospital stay were revealed in favor of RAG, that can be principally due to globally lesser surgical damage than OG. The robotic system enables a meticulous and precise dissection in a magnified vision, which minimizes the risk of bleeding. Moreover, the technical advancement of the robotic device, which is provided by a high definition 3D stereoscopic vision, enabling a better detection of vascular structures and allowing to easier inspect the bleeding occurring intra abdominally with tremor filtration and stable haemostatic strain provided with the robotic instrument.
No statistical difference was observed between RAG and OG in terms of postoperative complication rate (P = 0.65), and also specifically referring to subcategories of complications, such as wound infection, bleeding, anastomotic leakage. In particular regarding the most feared adverse event after gastric cancer, the rate of anastomotic leakage is comparable to that reported by previous studies[60,61], ranging from 1% to 10%, and the rate among pooled data was 2.97% (20/673) for RAG and 1.64% (89/5422) for OG (P = 0.06).
Analysis of the pooled data revealed that the number of harvested lymph nodes was similar between RAG and OG. The feeling is that the technically advantageous properties of robotic surgery can easily and safely execute an effective, and oncologically adequate lymphadenectomy[62,63]. In particular, the meticulous dissection, together with the high 3D definition image and dexterity provided by the robotic system, seems to make the lymph node dissection safely feasible in difficult lymphatic stations around major vessels or in difficult area[6], with less blood loss[6,38].
The main limitation of this meta-analysis is that it does not resolve certain heterogeneity of the included studies, such as in terms of baseline characteristics of patients, type of gastrectomy, stage of disease, details of surgery, difference in reporting perioperative outcomes. For example, in the study of Kim et al[21] the body mass index (BMI) of the RAG group was significantly lower than that of the open (P = 0.0004). Huang et al[23] included patients in the robotic group which were associated with female predominance and were reconstructed mainly by Roux-en-Y anastomosis. In the study of Kim et al[24], the patients of RAG group were significantly younger than OG. Kim et al[24] and Huang et al[23] reported in their series a significantly higher proportion (P < 0.001) of advanced gastric cancers in the OG gastrectomy group with respect to the RAG group, that would suggest a corresponding higher number of lymph nodes retrieved in advanced stages than in early stages. Effectively, that reflects a trend of a higher amount of lymph nodes dissected with the open procedure than with the robotic technique, both in the single institute reports and in the pooling data meta-analysis, however this difference did not reach a statistical significance. Globally, this result suggests that RAG, even if applied in a greater proportion of early gastric cancer than OG, guarantees an adequate removal of lymph nodes, similar to that of OG in a larger amount of advanced gastric cancer. Since it was difficult to match baseline characters in all selected studies, the meta-analytic method planned the use of a random effected model to evaluate these parameters. However, high heterogeneity still existed in terms of operation time, blood loss and postoperative hospital stay, which the meta-analysis cannot completely resolve.
However, the meta-analytic method can represent a valid preliminary analysis of the global framework of these data, eventually susceptible to a sub-set analysis of more homogeneous groups. Two previous meta-analysis[27,57], comparing RAG with conventional laparoscopy and OG, conducted a subgroup analysis matched for some of these parameters, such as the extent of lymphadenectomy, type of gastrectomy (total or subtotal), and blood loss. However, the final results were substantially equal to the pooled data here presented in our meta-analysis. Moreover, although sensitivity analysis using matched data should reduce some of these potential bias, it cannot eliminate all of them and essentially it was impossible to match patient characteristics in all studies. For example, robotic procedures included the initial learning period, which may have resulted in an unequal surgical quality comparison. Moreover, most of the studies had small sample sizes with fewer than 50 RAG procedures and one single high-volume centre (Kim et al[24]) contributed more than half of the total number of RAG; this uneven distribution in the number of patients contributed to heterogeneity.
An advantage of our meta-analysis with respect to previous ones is that it included, even if only one, RCT and presently it is the most up to date work with the largest sample size comparing RAG and OG.
In conclusion, RAG seems to offer a viable option to OG in treating gastric cancer patients. It allows the reduction of the estimated blood loss and the length of postoperative stay with respect to OG with, at the same time, a comparable oncologically adequate lymphadenectomy. The longer operative time did not seem to affect the patient’s recovery, with equal postoperative complications rate, risk of bleeding, wound infection and anastomotic leakage compared to open procedure.
Moreover, by overcoming some of the intrinsic limits of conventional laparoscopy, robotic gastrectomy probably represents the most promising technological innovation able to fill the gap still existing between laparoscopy and traditional open approach, particularly in the performance of D2 lymphadenectomy.
That could make LG when assisted with the robotic tool more oncologically adequate and then more widespread, so as to maintain and expand the well-known advantages of a minimally invasive surgery with respect to the open procedure.
Future research should be directed towards comparing RAG to OG, to delineating significantly quantifiable advantages between the two techniques, also in terms of cost analysis, especially in well-designed prospective randomized controlled trials. Finally, as a result of a lacking adequate follow-up and a small amount of high quality studies, it is too soon to formulate certain conclusive opinions.
COMMENTS
Background
Robot-assisted gastrectomy (RAG) is an innovative technique which improves the effectiveness of traditional laparoscopy, making it possible to overcome some of its typical limits. Several reports have demonstrated that this new procedure is technically feasible and safe, but no consensus is available in literature yet about the potential benefit of this technique with respect to the traditional open procedure.
Research frontiers
Minimally invasive surgery has progressively improved and spread, because it offers a number of patient benefits compared to open surgery. Future research will be directed towards innovative techniques which could further minimize the surgical invasiveness for patients, so as to improve postoperative outcomes. From this point of view, RAG appears to be a promising advancement of minimally invasive surgery, and will probably continue to be increasingly used in the treatment of gastric cancer.
Innovations and breakthroughs
Here the authors presented the meta-analysis of pooled data originating from the systematic review of relevant studies which compared short-term outcomes between RAG and open gastrectomy. Presently, this is the most up to date and largest clinical work comparing the effectiveness of these two techniques, and the only one that included a randomized controlled trial.
Applications
The present work elucidates the current scientific evidence concerning the hypothesized beneficial application of RAG in gastric cancer patients.
Peer-review
This paper is a meta-analysis of 6 reports comparing the outcomes of robot-assisted laparoscopic gastrectomy for early gastric cancer with open gastrectomy, with favourable results for the former group. The information is important and needs to be made known. It is well written.
Footnotes
Conflict-of-interest statement: All authors disclose any potential or actual personal, political or financial conflict of interest in the material, information or techniques described in the paper.
Data sharing statement: No additional data are available.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: February 8, 2017
First decision: March 8, 2017
Article in press: May 5, 2017
P- Reviewer: Czupryna A, Tovey FI, Wang M S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
References
- 1.Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146–148. [PubMed] [Google Scholar]
- 2.Yakoub D, Athanasiou T, Tekkis P, Hanna GB. Laparoscopic assisted distal gastrectomy for early gastric cancer: is it an alternative to the open approach? Surg Oncol. 2009;18:322–333. doi: 10.1016/j.suronc.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Chen XZ, Hu JK, Yang K, Wang L, Lu QC. Short-term evaluation of laparoscopy-assisted distal gastrectomy for predictive early gastric cancer: a meta-analysis of randomized controlled trials. Surg Laparosc Endosc Percutan Tech. 2009;19:277–284. doi: 10.1097/SLE.0b013e3181b080d3. [DOI] [PubMed] [Google Scholar]
- 4.Zeng YK, Yang ZL, Peng JS, Lin HS, Cai L. Laparoscopy-assisted versus open distal gastrectomy for early gastric cancer: evidence from randomized and nonrandomized clinical trials. Ann Surg. 2012;256:39–52. doi: 10.1097/SLA.0b013e3182583e2e. [DOI] [PubMed] [Google Scholar]
- 5.Deng Y, Zhang Y, Guo TK. Laparoscopy-assisted versus open distal gastrectomy for early gastric cancer: A meta-analysis based on seven randomized controlled trials. Surg Oncol. 2015;24:71–77. doi: 10.1016/j.suronc.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Caruso S, Patriti A, Roviello F, De Franco L, Franceschini F, Coratti A, Ceccarelli G. Laparoscopic and robot-assisted gastrectomy for gastric cancer: Current considerations. World J Gastroenterol. 2016;22:5694–5717. doi: 10.3748/wjg.v22.i25.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham D, Chua YJ. East meets west in the treatment of gastric cancer. N Engl J Med. 2007;357:1863–1865. doi: 10.1056/NEJMe078182. [DOI] [PubMed] [Google Scholar]
- 8.Song J, Oh SJ, Kang WH, Hyung WJ, Choi SH, Noh SH. Robot-assisted gastrectomy with lymph node dissection for gastric cancer: lessons learned from an initial 100 consecutive procedures. Ann Surg. 2009;249:927–932. doi: 10.1097/01.sla.0000351688.64999.73. [DOI] [PubMed] [Google Scholar]
- 9.Alimoglu O, Atak I, Eren T. Robot-assisted laparoscopic (RAL) surgery for gastric cancer. Int J Med Robot. 2014;10:257–262. doi: 10.1002/rcs.1566. [DOI] [PubMed] [Google Scholar]
- 10.Kim HI, Park MS, Song KJ, Woo Y, Hyung WJ. Rapid and safe learning of robotic gastrectomy for gastric cancer: multidimensional analysis in a comparison with laparoscopic gastrectomy. Eur J Surg Oncol. 2014;40:1346–1354. doi: 10.1016/j.ejso.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells GA, Shea B, O’Connell D. Ottawa, ON: Ottawa Hospital Research Institute;; 2011. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [webpage on the Internet] Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 13.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 14.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 15.Sterne JAC, Egger M, Davey Smith G. London: bMJ Publishing Group; 2001. Investigating dealing with publication other biases. In: Egger M, Davey Smith G, Altman DG, editors. Systematic Reviews in Health Care: Meta-Analysis in Context; pp. 189–208. [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 19.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim MC, Heo GU, Jung GJ. Robotic gastrectomy for gastric cancer: surgical techniques and clinical merits. Surg Endosc. 2010;24:610–615. doi: 10.1007/s00464-009-0618-9. [DOI] [PubMed] [Google Scholar]
- 22.Caruso S, Patriti A, Marrelli D, Ceccarelli G, Ceribelli C, Roviello F, Casciola L. Open vs robot-assisted laparoscopic gastric resection with D2 lymph node dissection for adenocarcinoma: a case-control study. Int J Med Robot. 2011;7:452–458. doi: 10.1002/rcs.416. [DOI] [PubMed] [Google Scholar]
- 23.Huang KH, Lan YT, Fang WL, Chen JH, Lo SS, Hsieh MC, Li AF, Chiou SH, Wu CW. Initial experience of robotic gastrectomy and comparison with open and laparoscopic gastrectomy for gastric cancer. J Gastrointest Surg. 2012;16:1303–1310. doi: 10.1007/s11605-012-1874-x. [DOI] [PubMed] [Google Scholar]
- 24.Kim KM, An JY, Kim HI, Cheong JH, Hyung WJ, Noh SH. Major early complications following open, laparoscopic and robotic gastrectomy. Br J Surg. 2012;99:1681–1687. doi: 10.1002/bjs.8924. [DOI] [PubMed] [Google Scholar]
- 25.Procopiuc L, Tudor S, Manuc M, Diculescu M, Vasilescu C. Open vs robotic radical gastrectomy for locally advanced gastric cancer. Int J Med Robot. 2016;12:502–508. doi: 10.1002/rcs.1674. [DOI] [PubMed] [Google Scholar]
- 26.Wang G, Jiang Z, Zhao J, Liu J, Zhang S, Zhao K, Feng X, Li J. Assessing the safety and efficacy of full robotic gastrectomy with intracorporeal robot-sewn anastomosis for gastric cancer: A randomized clinical trial. J Surg Oncol. 2016;113:397–404. doi: 10.1002/jso.24146. [DOI] [PubMed] [Google Scholar]
- 27.Hyun MH, Lee CH, Kim HJ, Tong Y, Park SS. Systematic review and meta-analysis of robotic surgery compared with conventional laparoscopic and open resections for gastric carcinoma. Br J Surg. 2013;100:1566–1578. doi: 10.1002/bjs.9242. [DOI] [PubMed] [Google Scholar]
- 28.Isozaki H, Tanaka N, Okajima K. General and specific prognostic factors of early gastric carcinoma treated with curative surgery. Hepatogastroenterology. 1999;46:1800–1808. [PubMed] [Google Scholar]
- 29.Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–449. doi: 10.1016/S1470-2045(10)70070-X. [DOI] [PubMed] [Google Scholar]
- 30.Viñuela EF, Gonen M, Brennan MF, Coit DG, Strong VE. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann Surg. 2012;255:446–456. doi: 10.1097/SLA.0b013e31824682f4. [DOI] [PubMed] [Google Scholar]
- 31.Ding J, Liao GQ, Liu HL, Liu S, Tang J. Meta-analysis of laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for gastric cancer. J Surg Oncol. 2012;105:297–303. doi: 10.1002/jso.22098. [DOI] [PubMed] [Google Scholar]
- 32.Martínez-Ramos D, Miralles-Tena JM, Cuesta MA, Escrig-Sos J, Van der Peet D, Hoashi JS, Salvador-Sanchís JL. Laparoscopy versus open surgery for advanced and resectable gastric cancer: a meta-analysis. Rev Esp Enferm Dig. 2011;103:133–141. doi: 10.4321/s1130-01082011000300005. [DOI] [PubMed] [Google Scholar]
- 33.Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, Ponzano C. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232–237. doi: 10.1097/01.sla.0000151892.35922.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uyama I, Sugioka A, Fujita J, Komori Y, Matsui H, Hasumi A. Laparoscopic total gastrectomy with distal pancreatosplenectomy and D2 lymphadenectomy for advanced gastric cancer. Gastric Cancer. 1999;2:230–234. doi: 10.1007/s101200050069. [DOI] [PubMed] [Google Scholar]
- 35.Pugliese R, Maggioni D, Sansonna F, Costanzi A, Ferrari GC, Di Lernia S, Magistro C, De Martini P, Pugliese F. Subtotal gastrectomy with D2 dissection by minimally invasive surgery for distal adenocarcinoma of the stomach: results and 5-year survival. Surg Endosc. 2010;24:2594–2602. doi: 10.1007/s00464-010-1014-1. [DOI] [PubMed] [Google Scholar]
- 36.Tanimura S, Higashino M, Fukunaga Y, Takemura M, Tanaka Y, Fujiwara Y, Osugi H. Laparoscopic gastrectomy for gastric cancer: experience with more than 600 cases. Surg Endosc. 2008;22:1161–1164. doi: 10.1007/s00464-008-9786-2. [DOI] [PubMed] [Google Scholar]
- 37.Hosono S, Arimoto Y, Ohtani H, Kanamiya Y. Meta-analysis of short-term outcomes after laparoscopy-assisted distal gastrectomy. World J Gastroenterol. 2006;12:7676–7683. doi: 10.3748/wjg.v12.i47.7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Memon MA, Khan S, Yunus RM, Barr R, Memon B. Meta-analysis of laparoscopic and open distal gastrectomy for gastric carcinoma. Surg Endosc. 2008;22:1781–1789. doi: 10.1007/s00464-008-9925-9. [DOI] [PubMed] [Google Scholar]
- 39.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 40.Patriti A, Ceccarelli G, Bellochi R, Bartoli A, Spaziani A, Di Zitti L, Casciola L. Robot-assisted laparoscopic total and partial gastric resection with D2 lymph node dissection for adenocarcinoma. Surg Endosc. 2008;22:2753–2760. doi: 10.1007/s00464-008-0129-0. [DOI] [PubMed] [Google Scholar]
- 41.Anderson C, Ellenhorn J, Hellan M, Pigazzi A. Pilot series of robot-assisted laparoscopic subtotal gastrectomy with extended lymphadenectomy for gastric cancer. Surg Endosc. 2007;21:1662–1666. doi: 10.1007/s00464-007-9266-0. [DOI] [PubMed] [Google Scholar]
- 42.Lee HH, Hur H, Jung H, Jeon HM, Park CH, Song KY. Robot-assisted distal gastrectomy for gastric cancer: initial experience. Am J Surg. 2011;201:841–845. doi: 10.1016/j.amjsurg.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 43.D’Annibale A, Pende V, Pernazza G, Monsellato I, Mazzocchi P, Lucandri G, Morpurgo E, Contardo T, Sovernigo G. Full robotic gastrectomy with extended (D2) lymphadenectomy for gastric cancer: surgical technique and preliminary results. J Surg Res. 2011;166:e113–e120. doi: 10.1016/j.jss.2010.11.881. [DOI] [PubMed] [Google Scholar]
- 44.Jiang ZW, Zhao K, Wang G, Bao Y, Xie LF, Liu FT, Pan HF, Zhang XL, Ruan H, Li N, et al. [Application of surgical robotic system in patients with gastric cancer: a report of 120 cases] Zhonghua Weichang Waike Zazhi. 2012;15:801–803. [PubMed] [Google Scholar]
- 45.Isogaki J, Haruta S, Man-I M, Suda K, Kawamura Y, Yoshimura F, Kawabata T, Inaba K, Ishikawa K, Ishida Y, et al. Robot-assisted surgery for gastric cancer: experience at our institute. Pathobiology. 2011;78:328–333. doi: 10.1159/000330172. [DOI] [PubMed] [Google Scholar]
- 46.Liu XX, Jiang ZW, Chen P, Zhao Y, Pan HF, Li JS. Full robot-assisted gastrectomy with intracorporeal robot-sewn anastomosis produces satisfying outcomes. World J Gastroenterol. 2013;19:6427–6437. doi: 10.3748/wjg.v19.i38.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park JY, Kim YW, Ryu KW, Eom BW, Yoon HM, Reim D. Emerging Role of Robot-assisted Gastrectomy: Analysis of Consecutive 200 Cases. J Gastric Cancer. 2013;13:255–262. doi: 10.5230/jgc.2013.13.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coratti A, Fernandes E, Lombardi A, Di Marino M, Annecchiarico M, Felicioni L, Giulianotti PC. Robot-assisted surgery for gastric carcinoma: Five years follow-up and beyond: A single western center experience and long-term oncological outcomes. Eur J Surg Oncol. 2015;41:1106–1113. doi: 10.1016/j.ejso.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 49.Tokunaga M, Makuuchi R, Miki Y, Tanizawa Y, Bando E, Kawamura T, Terashima M. Late phase II study of robot-assisted gastrectomy with nodal dissection for clinical stage I gastric cancer. Surg Endosc. 2016;30:3362–3367. doi: 10.1007/s00464-015-4613-z. [DOI] [PubMed] [Google Scholar]
- 50.Marano A, Choi YY, Hyung WJ, Kim YM, Kim J, Noh SH. Robotic versus Laparoscopic versus Open Gastrectomy: A Meta-Analysis. J Gastric Cancer. 2013;13:136–148. doi: 10.5230/jgc.2013.13.3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liao G, Chen J, Ren C, Li R, Du S, Xie G, Deng H, Yang K, Yuan Y. Robotic versus open gastrectomy for gastric cancer: a meta-analysis. PLoS One. 2013;8:e81946. doi: 10.1371/journal.pone.0081946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiong B, Ma L, Zhang C. Robotic versus laparoscopic gastrectomy for gastric cancer: a meta-analysis of short outcomes. Surg Oncol. 2012;21:274–280. doi: 10.1016/j.suronc.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 53.Xiong J, Nunes QM, Tan C, Ke N, Chen Y, Hu W, Liu X, Mai G. Comparison of short-term clinical outcomes between robotic and laparoscopic gastrectomy for gastric cancer: a meta-analysis of 2495 patients. J Laparoendosc Adv Surg Tech A. 2013;23:965–976. doi: 10.1089/lap.2013.0279. [DOI] [PubMed] [Google Scholar]
- 54.Shen WS, Xi HQ, Chen L, Wei B. A meta-analysis of robotic versus laparoscopic gastrectomy for gastric cancer. Surg Endosc. 2014;28:2795–2802. doi: 10.1007/s00464-014-3547-1. [DOI] [PubMed] [Google Scholar]
- 55.Liao GX, Xie GZ, Li R, Zhao ZH, Sun QQ, Du SS, Ren C, Li GX, Deng HJ, Yuan YW. Meta-analysis of outcomes compared between robotic and laparoscopic gastrectomy for gastric cancer. Asian Pac J Cancer Prev. 2013;14:4871–4875. doi: 10.7314/apjcp.2013.14.8.4871. [DOI] [PubMed] [Google Scholar]
- 56.Chuan L, Yan S, Pei-Wu Y. Meta-analysis of the short-term outcomes of robotic-assisted compared to laparoscopic gastrectomy. Minim Invasive Ther Allied Technol. 2015;24:127–134. doi: 10.3109/13645706.2014.985685. [DOI] [PubMed] [Google Scholar]
- 57.Zong L, Seto Y, Aikou S, Takahashi T. Efficacy evaluation of subtotal and total gastrectomies in robotic surgery for gastric cancer compared with that in open and laparoscopic resections: a meta-analysis. PLoS One. 2014;9:e103312. doi: 10.1371/journal.pone.0103312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giulianotti PC, Coratti A, Angelini M, Sbrana F, Cecconi S, Balestracci T, Caravaglios G. Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 2003;138:777–784. doi: 10.1001/archsurg.138.7.777. [DOI] [PubMed] [Google Scholar]
- 59.Park JY, Jo MJ, Nam BH, Kim Y, Eom BW, Yoon HM, Ryu KW, Kim YW, Lee JH. Surgical stress after robot-assisted distal gastrectomy and its economic implications. Br J Surg. 2012;99:1554–1561. doi: 10.1002/bjs.8887. [DOI] [PubMed] [Google Scholar]
- 60.Ichikawa D, Kurioka H, Yamaguchi T, Koike H, Okamoto K, Otsuji E, Shirono K, Shioaki Y, Ikeda E, Mutoh F, et al. Postoperative complications following gastrectomy for gastric cancer during the last decade. Hepatogastroenterology. 2004;51:613–617. [PubMed] [Google Scholar]
- 61.Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453–462. doi: 10.1056/NEJMoa0707035. [DOI] [PubMed] [Google Scholar]
- 62.Pugliese R, Maggioni D, Sansonna F, Ferrari GC, Forgione A, Costanzi A, Magistro C, Pauna J, Di Lernia S, Citterio D, et al. Outcomes and survival after laparoscopic gastrectomy for adenocarcinoma. Analysis on 65 patients operated on by conventional or robot-assisted minimal access procedures. Eur J Surg Oncol. 2009;35:281–288. doi: 10.1016/j.ejso.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 63.Jacob BP, Gagner M. Robotics and general surgery. Surg Clin North Am. 2003;83:1405–1419. doi: 10.1016/S0039-6109(03)00159-2. [DOI] [PubMed] [Google Scholar]


