Abstract
Small heat shock proteins (sHSPs) are gatekeepers of cellular homeostasis across species, preserving proteome integrity under stressful conditions. Nonetheless, recent evidence suggests that sHSPs are more than molecular chaperones with merely auxiliary role. In contrast, sHSPs have emerged as central lifespan determinants, and their malfunction has been associated with the manifestation of neurological disorders, cardiovascular disease and cancer malignancies. In this review, we focus on the role of sHSPs in ageing and age-associated diseases and highlight the most prominent paradigms, where impairment of sHSP function has been implicated in human pathology.
Keywords: Small heat shock proteins, Proteome, Ageing, Neurodegeneration, Cardiovascular disease, Immunity, Cancer
Introduction
Proteins, due to their inherent, unique, biochemical properties and structural diversity, have been selected as the cellular executive macromolecules. The protein reservoir functions amid an environment which is constantly challenged by extrinsic stress factors (such as heat or oxidative stress insults) and intrinsic, potentially harmful agents. For instance, reactive oxygen species (ROS), which are natural by-products of mitochondrial oxidative phosphorylation, can carbonylate polypeptides, damaging them in an irreversible manner (Suzuki et al. 2010). To fulfil the pivotal task of preserving the proteome integrity, evolution has invented elaborate mechanisms to remove polypeptides which have accumulated damage. These include the ubiquitin-proteasome system (UPS) and autophagy which constantly survey cells to eliminate damaged proteins. The activity of the UPS system and autophagy is believed to decline with age and this is accompanied by concomitant trend for aggregation (Labbadia and Morimoto 2014). Considering the importance of the proteome for the cellular functions, it is not surprising that loss of protein homeostasis (proteostasis) is considered as a key hallmark of the ageing process across species (López-Otín et al. 2013).
In parallel to UPS and autophagy, a repertoire of chaperone machines, which are denoted according to their molecular weight (HSP60, HSP70, HSP90, HSP100), functions to re-fold polypeptides which have acquired an erroneous conformation and prevent the aggregation of misfolded intermediates (Saibil 2013). The genome also encodes for small heat shock proteins (sHSPs), with a molecular mass ranging from 12 to 42 kDa (Haslbeck and Vierling 2015). The members of this protein subfamily are characterized by the presence of a conserved alpha-crystallin domain that is flanked by two amino-acid stretches of lower conservation in the amino and carboxy termini (Franck et al. 2004; Kriehuber et al. 2010). sHSPs form complexes with abnormal or disordered protein intermediates through both low and high affinity interactions to facilitate their refolding to native conformation or degradation. As such, they are often referred as ‛holdases’ or ‛stabilizers’ in the literature (Treweek et al. 2015). The numerous biological functions of each sHSP may lead to a variety of cell biological consequences with implications on diverse maladies. Here, we review the current knowledge regarding the interplay between sHSP function and ageing, and we particularly emphasize cases where sHSP function or dysfunction is linked with human disease (Table 1).
Table 1.
sHSPs influence lifespan and human pathology. Summary of prominent examples where sHSP function has been associated with lifespan and manifestation of age-associated disease
| sHSPs | Organism | Ageing | Mutation | Pathology | References |
|---|---|---|---|---|---|
| HSP-16.48, HSP-43, HSP-17 and SIP-1 | C. elegans | Elevated, required for normal lifespan | – | – | (Hsu et al. 2003; Walther et al. 2015) |
| HSP22 | D. melanogaster | O/E prolongs lifespan | – | – | (Morrow et al. 2016) |
| Α-Crystallin(HSPB4-HSPB5 complex) | M. musculus, H. sapiens | – | R120G | Presbyopia, Cataracts | (Andley et al. 2011; Heys et al. 2007) |
| HSP22/HspB8 | M. musculus, H. sapiens | – | K141E K141N | Charcot–Marie-Tooth | (Irobi et al. 2004) |
| HSP27/HSPB1 | M. musculus, H. sapiens | – | R127W S135F R136W T151I P182L P182S |
Charcot–Marie-tooth | (Evgrafov et al. 2004) |
| HSP22/HspB8 | M. musculus, H. sapiens | – | K141N | Cardiomyopathy | (Sanbe et al. 2013) |
| HSP20/HSPB6 | M. musculus, H. sapiens | – | S16A S16D |
Cardiac disease | (Martin et al. 2014a; Qian et al. 2009) |
| αB-crystallin/HSPB5 | M. musculus, H. sapiens | – | R120G | Myopathy and heart failure | (Rajasekaran et al. 2007; Vicart et al. 1998) |
| HSPB7 | H. sapiens | – | SNP (rs1739843) | Dilated cardiomyopathy | (Stark et al. 2010) |
| HSP27/HSPB1 | M. musculus, H. sapiens | – | CVD and inflammation | (Batulan et al. 2016; Seibert et al. 2013) | |
| HSP27/HSPB1 | M. musculus, H. sapiens | – | Cancer development | (Acunzo et al. 2012; Arrigo and Gibert 2014) | |
| αA-crystallin/HSPB4, αB-crystallin/HSPB5 HSP22/HSPB8 | M. musculus, H. sapiens | – | Cancer development | (Acunzo et al. 2012; Arrigo and Gibert 2014) |
sHSPs as ageing modulators: lessons from invertebrate models
Studies in the nematode Caenorhabditis elegans have introduced the intriguing notion that loss of proteostasis is an event which occurs early in adulthood, far before the manifestation of age-related phenotypes (Ben-Zvi et al. 2009; David et al. 2010). The ageing process is characterized by an extensive alteration in the organism’s proteome profile (Walther et al. 2015). The abundance of several sHSP homologues (such as HSP-16.48, HSP-43, HSP-17 and SIP-1) increases in aged nematodes. Moreover, elevated HSP-16.2 and HSP-16.11 expression can be utilized as an efficient predictor of shorter lifespan in an electrotaxis-based assay for assessing lifespan (Manière et al. 2014). Genetic inhibition of various molecular chaperones significantly shortens the organism’s lifespan (Hsu et al. 2003). Intriguingly, daf-2 mutants for the Insulin/IGF receptor, which live up to three times more than wild type animals, display enormous protein aggregation during ageing. sHSPs are integral components of these aggregates, which may sequester aggregation-sensitive or potentially harmful proteins (Walther et al. 2015). DAF-16, the worm homologue of the FOXO transcription factor and master regulator of ageing downstream of the Insulin/IGF receptor, cooperates with HSF-1 to regulate the expression of several target genes, among which several small heat shock protein genes. Direct regulation of sHSP expression by FOXO has been also observed in Drosophila melanogaster in response to oxidative stress (Donovan and Marr 2016). Our research has also elucidated a protective sHSP function in neuron survival upon a heat stroke. In that context HSP-16.1, which localizes to the medial Golgi, fortifies cells against heat stroke-induced neurodegeneration and mediates the beneficial effect of preconditioning in neuron survival (Kourtis et al. 2012). Overall, the activity of sHSPs appears indispensable for longevity and survival under acute stress conditions.
Hsp22 provides a remarkable paradigm of a sHSP family member which lies at the crossroad of mitochondrial metabolism, stress resistance and longevity in Drosophila melanogaster (Morrow and Tanguay 2015). Hsp22 localizes in mitochondria and its expression, which is markedly induced as the flies age, can be used as a reliable ageing biomarker (Tower et al. 2014; Yang and Tower 2009). Ubiquitous Hsp22 overexpression is sufficient to prolong lifespan and protect animals against oxidative and heat stress insults, while its heterologous expression in human fibroblasts can delay the onset of senescence in human primary fibroblasts (Morrow et al. 2004; Wadhwa et al. 2010). A recent study also highlights extensive changes in the mitoproteome of Hsp22 overexpressing flies, with several electron transport chain (ETC) and Krebs cycle components markedly affected (Morrow et al. 2016). Interestingly, Hsp22 was shown to associate with ETC complex I components and its overexpression upregulates Hsp70 levels in mitochondria. This implicates Hsp22 in the amplification of a mitoUPR, a salvation response for mitochondrial homeostasis.
sHSPs in neurological disorders
α-Crystallin, a complex of αA-crystallin/HSPB4 and αB-crystallin/HSPB5 sHSPs ensure proper light refraction in eye lenses, preventing the detrimental aggregation of itself and other crystallins (β- and γ-). Along ageing, a progressive decline in α-crystallin concentration, which can lead to a concomitant increase in lens stiffness and presbyopia, has been documented (Heys et al. 2007). Furthermore, several mutations in the α-crystallin domain have been associated with cataract. Prominently, knock-in mice for the R120G missense mutation, which affects its oligomeric state and renders HSPB5 unstable (Treweek et al. 2005), exhibits lens abnormalities, reminiscent of those observed in patients who carry this mutation (Andley et al. 2011). Interestingly, these mice also display elevated levels of p62 close to the nuclear membrane of lens’ epithelial cells, indicating that impaired clearance of insoluble aggregates by autophagy may occur (Wignes et al. 2013). Whether defective autophagy may arise from excessive protein aggregation in the HSPB5R120G overexpressing background, or it rather drives the latter, remains an open question. Intriguingly, a recent report reported the development of ‛pharmacological chaperones’, chemical compounds, which can potentially bind to α-crystallins and reverse their deleterious aggregation (Makley et al. 2015). Their administration can ameliorate protein aggregation defects in both mouse and human tissues.
Some of the most severe neurological disorders, such as Alzheimer’s, Parkinson’s, Huntington and Creutzfeldt Jacobs diseases, are characterized by aberrant formation of insoluble protein inclusions. sHSPs, due to their inherent ability to prevent protein aggregation, have been proposed as potential therapeutic candidates (Carra et al. 2013; Muchowski and Wacker 2005). HSPB5 and HSPB1 interact with mature α-synuclein fibrils and inhibit their elongation (Cox et al. 2016; Waudby et al. 2010). A similar protective effect of αB-crystallin was demonstrated in the case of Aβ42 amyloid fibrils, which are the hallmarks of Alzheimer’s disease (Shammas et al. 2011). Similarly, upregulation of HSPB8 by administration of colchicine and doxorubicin facilitates the clearance of protein aggregates from neurons. In this case, HSPB8 in cooperation with BAG-3 nucleotide exchange factor facilitates the removal of protein aggregates, probably through autophagy activation (Crippa et al. 2016).
Apart from protein aggregation diseases, sHSPs seem to exert beneficial effects in paradigms where neurons are challenged by harmful insults. For instance, overexpression of αB-crystallin in cultured rat hippocampal neurons protects against heat shock through enhanced dendritic branching (Bartelt-Kirbach et al. 2016). HSPB3, an sHSP which is highly expressed in motor neurons confers protection against lesion-induced neurodegeneration (La Padula et al. 2016). Collectively, sHSPs act protectively in the context of neurodegenerative disease to ameliorate excessive protein aggregation and promote neuron survival.
sHSPs in motor neuropathies
Charcot-Marie-Tooth (CMT) belongs to the family of hereditary motor neuropathies (HMN) and is the most widespread inherited neuromuscular disorder affecting approximately 1:2500 individuals. It is a peripheral nervous system disease, since patients with CMT exhibit atrophy in the muscles of the arms and legs, sensory abnormalities, foot deformities and walking difficulties. The disorder has a late-onset, with its symptoms being manifested during adulthood or midlife. Almost a decade ago, two hallmark studies linked specific missense mutations in HSP22/HSPB8 and HSP27/HSPB1 with the manifestation of CMT and HMN (Evgrafov et al. 2004; Irobi et al. 2004). These two chaperones have been reported to physically interact (Sun et al. 2004). Both HSPB8 mutations alter a single amino-acid residue (K141) located in its conserved a-crystallin domain. Intriguingly, mutations in K141 of HSPB8 do not disturb its interaction with HSPB1, but rather enhance it, leading to the formation of cytoplasmic aggregates (Fontaine et al. 2006; Irobi et al. 2004). As for HSP27, numerous mutations predominantly in the a-crystallin domain (R127W, S135F, R136W, T151I) but also in the carboxy-terminal domain (P182L, P182S) have been identified (Evgrafov et al. 2004; Kijima et al. 2005). Overexpression of mutated HSPB1 variants is sufficient to compromise cell viability, while the S135F mutation has been shown to perturb neurofilament assembly in vitro (Evgrafov et al. 2004). The list of variations associated with CMT and HMNs has greatly expanded and nowadays includes mutations which are propagated in both autosomal dominant and recessive manner (Houlden et al. 2008; Ikeda et al. 2009; Kim et al. 2015; Nakhro et al. 2013; Stancanelli et al. 2015).
The recent generation of mouse models has provided novel insights concerning the relation between HSP mutations and CMT. Overexpression of either mutant HSPB1S135F or HSPB1P182L isoforms in the neuronal system leads to pronounced motor defects and reduced muscle strength, as monitored through behavioural tests (d’Ydewalle et al. 2011; Lee et al. 2015). While there is support for aberrant myelination in the latter study using the HSPB1S135F model, a decrease in the levels of a-tubulin acetylation seems to be a common denominator of the observed pathology. Tubulin hypoacetylation is associated with impaired transport of mitochondria across axons and in neurites in HSPB1S135F overexpressing animals, and this defect can be reversed upon administration of histone deacetylase inhibitors (d’Ydewalle et al. 2011). In addition, overexpression of the HSPB1R136W mutant isoform results in severe axonopathy, defective neurofilament cytoskeleton and accumulation of mitochondria along axons (Srivastava et al. 2012). Moreover, the hyperactive HSPB1 mutants (R127W, S135F and R136W) have been reported to display enhanced affinity to tubulin and microtubule stabilization (Almeida-Souza et al. 2011). Thus, the recapitulation of the CMT condition in mice supports the requirement for intact HSPB1 function for preserving cytoskeleton integrity in neurons. Nonetheless, since HSPB1 can have non-conventional and often unanticipated roles in cell physiology, for instance the regulation of abundance and activity of the master regulator GATA-1 during erythroid differentiation (de Thonel et al. 2010), other potential HSPB1 modes of action in neurons should not be a priori excluded.
sHSPs in cardiovascular disease
Cardiovascular disease (CVD) is the leading cause of death throughout the developed world. In recent years, huge advancements in the understanding of CVD led to innovative therapeutic interventions and sensational improvement in age-adjusted cardiovascular mortality rates. Undoubtedly, thorough understanding of the underlying mechanisms and identification of novel targets will aid in curtailing CVD prevalence. Emerging evidence implicates sHSPs in the pathogenesis of CVD, and several studies uncovered mutations and single nucleotide polymorphisms in sHSP genes associated with cardiomyopathies (Martin et al. 2014a; Martin et al. 2014b; Raizman et al. 2013; Rajasekaran et al. 2007; Seibert et al. 2013; Stark et al. 2010; Vicart et al. 1998). For instance, even a single point mutation of HSPB8 can cause cardiac disease (Sanbe et al. 2013). Among all sHSP family members, HSPB1 and HSPB6 have traditionally been the main focus of interest of cardiovascular research. A compilation of recent scientific knowledge highlights the cardioprotective properties of these molecules which are predominantly expressed in the cardiovascular system (Edwards et al. 2011; Fan et al. 2005a; Martin et al. 2014a; Weintraub and Rubinstein 2013). The anti-platelet aggregation properties of HSPB6 attracted further attention in the field of cardiovascular research (Kozawa et al. 2002). Under physiological conditions, platelets prevent haemorrhage of the injured vasculature. During atherosclerotic plaque rupture, however, platelet-mediated thrombus may lead to acute coronary syndrome (ACS). HSPB6 specifically binds to platelets, attenuating their aggregation through mechanisms that are incompletely understood. Nevertheless, inducing HSPB6 expression levels in rupture-prone atherosclerotic microenvironments may provide a novel therapeutic approach in ACS. The potential role of HSPB1 in CVD originated from studies of human vascular tissue. HSPB1 secretion decreases in atherosclerotic plaques, compared to healthy tissues, and soluble HSPB1 plasma levels also decline in atherosclerotic patients compared to healthy subjects (Martin-Ventura et al. 2004), whereas overexpression of HSBP1 in atherosclerotic mouse models ameliorates foam cell formation and reduces atherosclerotic plaque area (Rayner et al. 2008). These findings suggest a protective role for sHSPs in atherogenesis, however, the precise mechanisms of action remain obscure, largely due to the diverse nature of each sHSP family member.
Posttranslational modifications of sHSPs and implications in CVD
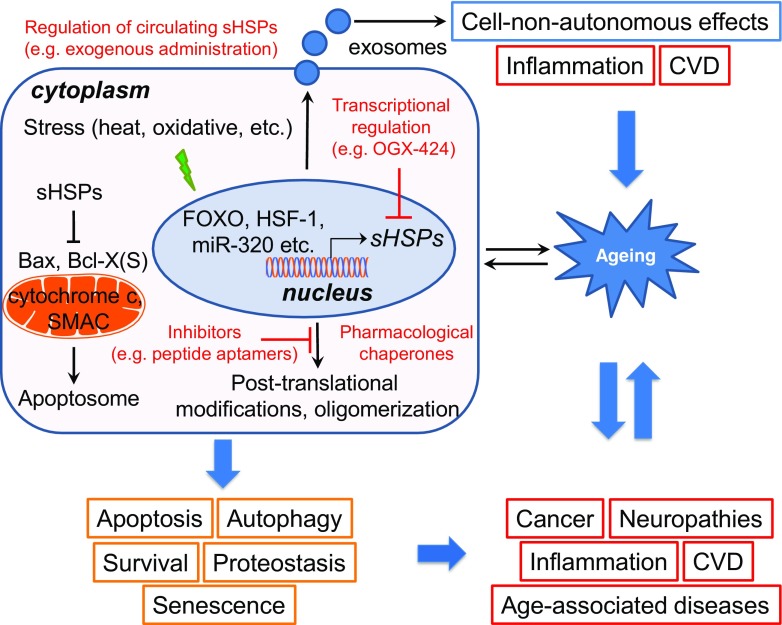
Given the diversity of homo- and hetero-oligomerization as well as their potential for post-translational modification, the functional diversification of sHSPs is further expanded and so is their involvement in CVD progression. sHSPs undergo a variety of post-translational modifications, with phosphorylation being the most prominent (Mymrikov et al. 2011). HSBP1 was reported to be methylglyoxal modified in endothelial cells exposed to conditions of excessive glucose (Schalkwijk et al. 2006). This methylglyoxal modification modulates the oligomeric population of HSPB1, increases its chaperone activity, reduces its interaction with cytochrome c and modulates its anti-apoptotic activity (Oya-Ito et al. 2006; Padival et al. 2003; Sakamoto et al. 2002; van Heijst et al. 2006). This can potentially contribute to endothelial dysfunction associated to metabolic syndrome and various cardiovascular diseases, such as atherosclerosis. Direct association of methylglyoxal modifications of sHSPs and ageing remains unascertained. An in vitro study suggests that methylglyoxal modification of α-crystallin can enhance its chaperone function and protect the lens against environmental and metabolic stress by preventing nascent polypeptide accumulation associated with ageing (Nagaraj et al. 2003; Nagaraj et al. 2012b). Similarly, HSPB4 acetylation in human lens alters the chaperone’s function, a process that is essential for impeding the age-associated accumulation of insoluble lens proteins (Nagaraj et al. 2012a). Interestingly, HSPB1 contains an S-thiolation site that is absent in HSPB5 (αB crystallin). During ischemia, reperfusion and oxidative damage, which contributes to the injury sustained by the heart during ischemia and reperfusion, rat cardiac HSPB1 is modified (Eaton et al. 2002a, b). It is proposed that S-thiolation increases the affinity of dissociated HSPB1 multimers for specific target proteins; however, evidence on the functional significance of this posttranslational modification remains scarce. Although anticipated, the in vivo role of sHSP phosphorylation is largely ambiguous, and research towards this direction is imperative. Accumulating evidence suggests that it may function as a switch, to enable access to their target clients (Bakthisaran et al. 2015). In vitro experiments suggest that phosphorylation influences the oligomeric state of sHSPs and interaction with client proteins and consequently their chaperone-like activity. During precarious and stressful conditions, such as heat shock, serine phosphorylation of several HSPB members such as HSPB1, HSPB4, HSPB5, HSPB6 and HSPB8 may occur. As a consequence, their function is redirected, and a variety of cellular processes are modulated accordingly. For instance, although both HSPB1 and HSPB6 regulate vascular smooth muscle tone, HSPB1 phosphorylation is associated with vasorelaxation, whereas HSPB6 phosphorylation leads to smooth muscle contraction (Dreiza et al. 2010; McLemore et al. 2005), further perplexing the already complicated mode of actions. Furthermore, the phosphorylation status seems to modulate the pro-apoptotic and anti-apoptotic effects of sHSPs; however, further studies are required to elucidate to what extent these modifications are directly related to downstream apoptotic cascades. Further evidence also suggests that it is involved in the regulation of autophagy. Along these lines, it is noteworthy to mention that cell death and autophagy are tightly interrelated with ageing (Pollack and Leeuwenburgh 2001; Rubinsztein et al. 2011). sHSPs may constitute an interchange between autophagy, apoptosis and ageing. Inhibition of HSPB6 phosphorylation at Ser16 results in hearts with larger infracts, via increased apoptosis and necrosis and reduced autophagy (Qian et al. 2009). In support of these findings, it was demonstrated that miR-320, an miRNA that is upregulated in ischemic hearts, negatively regulates HSPB1 expression. Likewise, HMGB1 induces HSPB1 to attenuate cardiomyocyte apoptosis associated with doxorubicin-induced cardiomyopathy and regulate autophagy and mitochondrial autophagy (mitophagy) (Narumi et al. 2015; Tang et al. 2011). However, involvement of protein phosphorylation in this process remains unknown. Thus, sHSPs phosphorylation status and regulation at the transcriptional and post-transcriptional level may constitute an important modality not only for cardioprotection, but for ageing as well (Fig. 1).
Fig. 1.
sHSPs at the crossroad of ageing and age-related diseases. sHSPs are strongly upregulated during ageing and under stressful conditions to preserve proteostasis. sHSP functional diversification is further expanded, due to their ability to homo- and hetero-oligomerize, interact with several client proteins and be post-translationally modified. Intriguingly, circulating sHSPs have been identified, indicating putative cell-non-autonomous effects. Several vital cellular processes such as apoptosis, autophagy, senescence (orange boxes), which have been associated with human disorders (red boxes), are influenced by sHSP function or dysfunction. Hence, sHSPs constitute promising targets for the confrontation of age-associated diseases. Fine-tuning of sHSP activity could prove beneficial in various pathologies. In red, the current approaches undertaken for the development of future targeted pharmaceutical interventions are highlighted
Extracellular sHSPs in CVD and inflammation
In recent years, intensive research activity has focused in delineating the role of extracellular sHSPs in the immune system and the modulation of atherosclerosis. Albeit sHSPs lack the N-terminal signal peptide required for extracellular secretion; they have been detected extracellularly. The mechanism involved in sHSPs secretion remains elusive, and several theories have been proposed. Accumulating evidence suggests an exosome-mediated secretion; however, alternative pathways are not yet excluded (Batulan et al. 2016; van Noort et al. 2012). The mode of action of secreted sHSPs is beginning to be addressed, albeit their presence on exosomes, and surmises a possible cell-non-autonomous activity. Exosomes have been detected in various body fluids, including serum, saliva and breast milk and have been associated to cellular senescence and ageing (Xu and Tahara 2013). Hence, they may act not only in an autocrine fashion, but also in a paracrine and endocrine fashion. It is believed that tissue communication may affect longevity in an endocrine fashion, by coupling physiological pathways which regulate ageing. It is suggested that manipulation of cell-non-autonomous signalling pathways may increase health- and life-span by regulating organismal survival during reduced metabolism and under stress conditions. Direct association of secreted sHSPs with cell-non-autonomous effects which impinge on ageing remains elusive. HSPB6 has been characterized as a cardiokine promoting myocardial angiogenesis through activation of VEGFR. Physical interactions of circulating HSPB6 with VEGFR increase capillary density providing a novel protective mechanism against cardiac injury, given that coronary angiogenesis is vital for heart restoration. However, neovessel formation may destabilize atherosclerotic plaques leading to plaque rupture and ACS (Bentzon et al. 2014). Thus, the protective role of HSPB6 in cardiovascular disease needs to be carefully considered and re-evaluated. Lower circulating HSPB1 serum levels were documented in atherosclerotic coronary arteries and were inversely associated with increasing plaque progression and age. Thus, serum release of HSPB1 seems to have an atheroprotective and potentially an anti-ageing role (Batulan et al. 2016; Kardys et al. 2008; Lepedda et al. 2009; Martin-Ventura et al. 2004; Miller et al. 2005; Rayner et al. 2008). In a mouse model of atherosclerosis, exogenous administration of HSPB1 reduced plaque progression, corroborating its atheroprotective properties and further suggesting a potential therapeutic benefit (Seibert et al. 2013). Intriguingly, HSPB1 was first identified as an oestrogen-responsive protein and was subsequently characterized as a sHSP (Ciocca et al. 1983). The association with circulating oestrogen levels further suggests sex-related effects of HSPB1. Indeed, the atheroprotective effects of HSPB1 were more pronounced in female mouse models (Rayner et al. 2008). Since, atherosclerosis is primarily a chronic inflammatory disease, the link between secreted sHSPs and the immune system was soon appraised. Overall, these effects were, at least partially, attributed to the inflammatory effects of extracellular HSPB1. It has been shown to reduce macrophage infiltration and free serum cholesterol and attenuate foam cell formation. This occurs via downregulation of scavenger receptor-A expression via NF-κB signalling and interaction with toll-like receptors (TLRs) in both cardiovascular and immune cells inter allia (Batulan et al. 2016; Jin et al. 2014; Raizman et al. 2013). Secreted sHSPs are now considered potential therapeutic agents to prevent or ameliorate inflammatory processes (van Noort et al. 2012). Interestingly, the association between ageing and immune response mechanisms begins to be appreciated (Nikoletopoulou et al. 2014). During ageing, the immune responses gradually deteriorate, increasing susceptibility to infections and causing low-grade inflammation. This age-associated proinflammatory phenotype, referred to as ‛inflammageing’, is attributed to the imbalance between anti- and pro-inflammatory mechanisms. Deeper understanding of the mechanisms and factors involved in immunosenescence and inflammageing may aid healthy ageing through more efficient pharmacological strategies, where circulating sHSPs could serve as a bona fide therapeutic target.
sHSPs in cancer
Although, sHSPs are often overexpressed in cancer cells and are associated with increased tumorigenicity, their precise mode of action remains enigmatic (Acunzo et al. 2012; Arrigo and Gibert 2014). Evading apoptosis is one of the major cancer traits, and since sHSPs have an extremely intricate role in the regulation of apoptosis, these proteins attracted scientific interest to confront carcinogenesis. sHSPs possess mainly anti-apoptotic properties; however, pro-apoptotic features have also been reported (Acunzo et al. 2012; Arrigo and Gibert 2014; Bakthisaran et al. 2015; Guo et al. 2015; Ju et al. 2015; Mymrikov et al. 2011; Zeng et al. 2013). Interestingly, sHSPs interfere with both extrinsic (dependent on death ligands and receptors) and intrinsic (mitochondrial-mediated) apoptotic pathways. For instance, HSPB1 and HSPB5 negatively modulate TNF-α and Fas-induced cell apoptosis; whereas, HSPB2 has been reported to prevent TRAIL and TNF-α-induced apoptosis (Mehlen et al. 1996; Oshita et al. 2010; Pinz et al. 2008; Qi et al. 2014). Cytosolic HSPB1 interacts with the mitochondrial outer membrane and directly binds to released cytochrome c to inhibit apoptosome formation (Bruey et al. 2000). Interestingly, HSPB1 expression protects from mitochondrial injury-mediated apoptosis and promotes cell survival by a phosphatidylinositol 3-kinase (PI3-K)-dependent mechanism (Havasi et al. 2008). Subsequent Akt activation inhibits Bax activation, oligomerization and translocation to mitochondria, preventing cytochrome c release. Apparently, it appears that HSPB1 interferes with apoptosis on multiple levels. Apart from preventing cytochrome c release from injured mitochondria, it simultaneously exerts its protective effects upon mitochondrial membrane injury by impeding cytochrome c activity upon release. Furthermore, HSPB1 controls the release of second mitochondria-derived activator of caspases (Smac), which promotes apoptosis via caspase activation, and also plays important role in the p53 pathway, underlining its crucial role in apoptosis and in the regulation of cellular senescence (Bakthisaran et al. 2015; Chauhan et al. 2003; O’Callaghan-Sunol et al. 2007; Xu et al. 2013). Similarly, alpha-crystallins (HSPB4 and HSPB5) and HSPB6 prevent apoptotic cell death through interaction with pro-apoptotic proteins of the Bcl-2 family, such as Bax and Bcl-X(S), intercepting their translocation from cytosol into mitochondria (Fan et al. 2005b; Mao et al. 2004). Additionally, alpha-crystallins preserve mitochondrial integrity and inhibit apoptosis by interacting with cytochrome c and caspase subtypes and by repressing caspase-3 activation (Bakthisaran et al. 2015; Mao et al. 2004; McGreal et al. 2012). Detailed information on the interplay between sHSPs and the extrinsic and intrinsic apoptotic pathways can be found in excellent recent articles (Acunzo et al. 2012; Arrigo and Gibert 2014; Bakthisaran et al. 2015; Mymrikov et al. 2011).
Intriguingly, sHSPs also play essential role in cancer cell migration, invasion and metastasis, most probably due to their biochemical cytoskeletal related functions (Mounier and Arrigo 2002), and in cancer-related angiogenesis, expanding their pleiotropic effects on cancer development. Metastasis is the leading cause of death for patients suffering from malignancies. The importance of angiogenesis and lymphangiogenesis in cancer development is fundamental, since tumour growth and metastasis primarily depend on the formation of new vessels (Hanahan and Weinberg 2011). HSPB1 increases cancer cell motility, and its overexpression and phosphorylation are associated with increased metastatic and invasive potential of cells both in vivo and in vitro in various cancer types (Gibert et al. 2012; Voll et al. 2014; Yang et al. 2010). Moreover, alpha-crystallins play an important role in cell motility and invasion. Whereas HSPB4 seems to restrain cell migration and to negatively regulate pancreatic carcinogenesis, HSPB5 induces epidermal growth factor (EGF)-independent growth and increases cell migration and invasion (Deng et al. 2010; Moyano et al. 2006). HSPB6 has been recently shown to regulate transforming growth factor-α (TGF-α)-induced migration and invasion of hepatocellular carcinoma cells (Matsushima-Nishiwaki et al. 2016). Interestingly, a phospho-HSPB6 mimetic reduces their invasive potential, illustrating the importance of sHSPs’ post-translational modifications. Moreover, the fact that sHSPs contribute to the metastatic potential and maintenance of cancer stem cells through regulation of epithelial to mesenchymal transition (EMT) is of particular interest. EMT has been shown to play a fundamental role in metastasis. Intriguingly, HSPB1 mediates EMT, metastasis and circulating tumour cells in prostate cancer and breast cancer via modulation of STAT3/Twist signalling and nuclear factor-kappaB (NF-κB) respectively (Shiota et al. 2013; Wei et al. 2011). Endothelial to mesenchymal transition (EndMT) is another parameter known to be important for metastatic extravasation in brain endothelial cells and to promote invasiveness of infected endothelial cells, thus contributing to cancer progression (Gasperini et al. 2012; Krizbai et al. 2015). Endothelial cell function is vital for the maintenance of the vasculature and for neovascularization under pathophysiological conditions. HSPB1 is expressed in tumour vessels and is a key regulator of EndMT in cancer (Choi et al. 2016). Although contradictory to the effects on EMT, the abovementioned effects suggest a key role of HSBP1 in maintaining the vasculature during cancer, by impeding EndMT. The effects of HSPB1 during tumour angiogenesis are also contradictory. Although HSPB1 administration leads to VEGF secretion and angiogenesis, overexpression of HSPB1 leads to aggressive tumour growth in vivo and its downregulation leads to reduced endothelial cell proliferation and decreased secretion of VEGF, suggesting an involvement of HSPB1 in tumour angiogenesis. EC-secreted HSPB1 can also prevent tumour angiogenesis via direct binding with VEGF (Lee et al. 2012; Straume et al. 2012; Thuringer et al. 2013). Other sHSPs promote tumour angiogenesis, mainly through increasing vascular survival. Tumours which develop in HSPB5 mutant mice are significantly less vascularized than wild-type tumours and display increased areas of apoptosis/necrosis (Dimberg et al. 2008).
Overall, sHSPs are mainly considered as oncoproteins due to their anti-apoptotic mode of action; however, more studies are required to unveil their exact role during carcinogenesis. The cytoprotective, anti-apoptotic, pro-angiogenic and pro-metastatic properties of sHSPs, position them as promising targets for anticancer treatment, and a thorough understanding of sHSPs mode of action will allow the development of specific and targeted pharmaceutical interventions. HSPB1 inhibition by OGX-427 is currently in multicentre phase II clinical trials for treatment of metastatic cancer. OGX-427 is a second-generation antisense technology inhibitor that enables targeted downregulation of gene expression at the transcriptional level. It has been shown to potently reduce HSPB1 levels and in synergy with other cancer treatments to inhibit tumour growth, further validating these proteins as potential therapeutic targets for cancer treatment (Baylot et al. 2011; Gleave and Monia 2005; Lamoureux et al. 2014; Lelj-Garolla et al. 2015; Shiota et al. 2013).
Concluding remarks and outlook
Ageing affects many cellular processes and is considered one of the major risk factors for the development and progression of cardiovascular disease, neurodegeneration and cancer. The average lifespan of humans and concomitantly the percentage of elderly people have immensely increased over the past decades. Cardiovascular disease (CVD) will remain the leading cause of mortality within that group and together with cancer and neuropathies will burden societies with high socioeconomic costs. Till recently, the fields of ageing biology and CVD have remained largely unrelated, although it was known that ageing has a remarkable impact on the heart and arterial system, evidenced as atherosclerosis, hypertension, myocardial infarction and stroke incidents (Lakatta and Levy 2003a, b; North and Sinclair 2012). A plethora of longevity genes initially identified in model organisms, including the nematode Caenorhabditis elegans and the fly Drosophila melanogaster, are currently appraised for their role in CVD (Nair and Ren 2012; North and Sinclair 2012). The link between ageing and cancer has been well documented. Given that lifespan has rapidly increased over the past decades, it is not surprising that worldwide prevalence of cancer will continue to rise and become the prevailing cause of mortality. Understanding fundamental mechanisms that orchestrate the ageing process can lead to significant advancements into both preventative and therapeutic interventions of CVD, neurodegeneration and cancer. Fine-tuning of sHSPs could have beneficial outcomes in various pathologies and in lifespan itself. In CVD and neurodegenerative disorders, tissue-specific upregulation of sHSPs could prove beneficial; whereas, confining or completely abrogating several sHSPs could facilitate cancer mitigation. The use of functional inhibitors would be another, perhaps more plausible approach. sHSP function highly depends on posttranslational modifications as well as their ability to form homo- and hetero-oligomeric complexes and interact with various client proteins. In fact, the diverse biochemical functional activities of HSPBs might explain the divergent outcomes. Thus, a deeper understanding of the underlying mechanisms and the precise mode of action of each sHSP is imperative for the design of effective strategies that could be beneficial for increasing life- and health-span.
Acknowledgements
We apologize to those colleagues whose work could not be referenced directly owing to space limitations. Work in the authors’ laboratory is funded by grants from the European Research Council (ERC), the European Commission Framework Programmes, and the Greek Ministry of Education. E.K. is supported by Stiftung für Herz- und Kreislaufkrankheiten.
Footnotes
Nikolaos Charmpilas and Emmanouil Kyriakakis contributed equally.
References
- Acunzo J, Katsogiannou M, Rocchi P. Small heat shock proteins HSP27 (HspB1), alphaB-crystallin (HspB5) and HSP22 (HspB8) as regulators of cell death. Int J Biochem Cell Biol. 2012;44:1622–1631. doi: 10.1016/j.biocel.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Almeida-Souza L, Asselbergh B, d’Ydewalle C, Moonens K, Goethals S, de Winter V, Azmi A, Irobi J, Timmermans J-P, Gevaert K, et al. Small heat-shock protein HSPB1 mutants stabilize microtubules in Charcot–Marie-tooth neuropathy. J Neurosci. 2011;31:15320–15328. doi: 10.1523/JNEUROSCI.3266-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andley UP, Hamilton PD, Ravi N, Weihl CC. A knock-in mouse model for the R120G mutation of αB-crystallin recapitulates human hereditary myopathy and cataracts. PLoS One. 2011;6:e17671. doi: 10.1371/journal.pone.0017671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo AP, Gibert B. HspB1, HspB5 and HspB4 in human cancers: potent oncogenic role of some of their client proteins. Cancers (Basel) 2014;6:333–365. doi: 10.3390/cancers6010333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakthisaran R, Tangirala R, Rao Ch M. Small heat shock proteins: role in cellular functions and pathology. Biochim Biophys Acta. 2015;1854:291–319. doi: 10.1016/j.bbapap.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Bartelt-Kirbach B, Moron M, Glomb M, Beck C-M, Weller M-P, Golenhofen N. HspB5/αB-crystallin increases dendritic complexity and protects the dendritic arbor during heat shock in cultured rat hippocampal neurons. Cell Mol Life Sci. 2016;73:3761–3775. doi: 10.1007/s00018-016-2219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batulan Z, Pulakazhi Venu VK, Li Y, Koumbadinga G, Alvarez-Olmedo DG, Shi C, O’Brien ER. Extracellular release and signaling by heat shock protein 27: role in modifying vascular inflammation. Front Immunol. 2016;7:285. doi: 10.3389/fimmu.2016.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylot V, Andrieu C, Katsogiannou M, Taieb D, Garcia S, Giusiano S, Acunzo J, Iovanna J, Gleave M, Garrido C, et al. OGX-427 inhibits tumor progression and enhances gemcitabine chemotherapy in pancreatic cancer. Cell Death Dis. 2011;2:e221. doi: 10.1038/cddis.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zvi A, Miller EA, and Morimoto RI (2009) Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proceedings of the National Academy of Sciences [DOI] [PMC free article] [PubMed]
- Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–1866. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000;2:645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- Carra S, Rusmini P, Crippa V, Giorgetti E, Boncoraglio A, Cristofani R, Naujock M, Meister M, Minoia M, Kampinga HH, et al. Different anti-aggregation and pro-degradative functions of the members of the mammalian sHSP family in neurological disorders. Philosophical Transactions of the Royal Society B: Biological Sciences. 2013;368:20110409. doi: 10.1098/rstb.2011.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan D, Li G, Hideshima T, Podar K, Mitsiades C, Mitsiades N, Catley L, Tai YT, Hayashi T, Shringarpure R, et al. Hsp27 inhibits release of mitochondrial protein Smac in multiple myeloma cells and confers dexamethasone resistance. Blood. 2003;102:3379–3386. doi: 10.1182/blood-2003-05-1417. [DOI] [PubMed] [Google Scholar]
- Choi SH, Nam JK, Kim BY, Jang J, Jin YB, Lee HJ, Park S, Ji YH, Cho J, Lee YJ. HSPB1 inhibits the endothelial-to-mesenchymal transition to suppress pulmonary fibrosis and lung tumorigenesis. Cancer Res. 2016;76:1019–1030. doi: 10.1158/0008-5472.CAN-15-0952. [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Adams DJ, Edwards DP, Bjercke RJ, McGuire WL. Distribution of an estrogen-induced protein with a molecular weight of 24,000 in normal and malignant human tissues and cells. Cancer Res. 1983;43:1204–1210. [PubMed] [Google Scholar]
- Cox D, Selig E, Griffin MDW, Carver JA, and Ecroyd H (2016) Small Heat Shock Proteins Prevent Alpha-Synuclein Aggregation via Transient Interactions and their Efficacy is Affected by the Rate of Aggregation. Journal of Biological Chemistry [DOI] [PMC free article] [PubMed]
- Crippa V, D’Agostino VG, Cristofani R, Rusmini P, Cicardi ME, Messi E, Loffredo R, Pancher M, Piccolella M, Galbiati M, et al. Transcriptional induction of the heat shock protein B8 mediates the clearance of misfolded proteins responsible for motor neuron diseases. Scientific Reports. 2016;6:22827. doi: 10.1038/srep22827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Ydewalle C, Krishnan J, Chiheb DM, Van Damme P, Irobi J, Kozikowski AP, Berghe PV, Timmerman V, Robberecht W, Van Den Bosch L. HDAC6 inhibitors reverse axonal loss in a mouse model of mutant HSPB1-induced Charcot–Marie-tooth disease. Nat Med. 2011;17:968–974. doi: 10.1038/nm.2396. [DOI] [PubMed] [Google Scholar]
- David DC, Ollikainen N, Trinidad JC, Cary MP, Burlingame AL, Kenyon C. Widespread protein aggregation as an inherent part of aging in C. elegans. PLoS Biol. 2010;8:e1000450. doi: 10.1371/journal.pbio.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Thonel A, Vandekerckhove J, Lanneau D, Selvakumar S, Courtois G, Hazoume A, Brunet M, Maurel S, Hammann A, Ribeil JA, et al. HSP27 controls GATA-1 protein level during erythroid cell differentiation. Blood. 2010;116:85. doi: 10.1182/blood-2009-09-241778. [DOI] [PubMed] [Google Scholar]
- Deng M, Chen PC, Xie S, Zhao J, Gong L, Liu J, Zhang L, Sun S, Ma H, Batra SK, et al. The small heat shock protein alphaA-crystallin is expressed in pancreas and acts as a negative regulator of carcinogenesis. Biochim Biophys Acta. 2010;1802:621–631. doi: 10.1016/j.bbadis.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Dimberg A, Rylova S, Dieterich LC, Olsson AK, Schiller P, Wikner C, Bohman S, Botling J, Lukinius A, Wawrousek EF, et al. alphaB-crystallin promotes tumor angiogenesis by increasing vascular survival during tube morphogenesis. Blood. 2008;111:2015–2023. doi: 10.1182/blood-2007-04-087841. [DOI] [PubMed] [Google Scholar]
- Donovan MR, and Marr MT (2016) dFOXO Activates Large and Small Heat Shock Protein Genes in Response to Oxidative Stress to Maintain Proteostasis in Drosophila. Journal of Biological Chemistry [DOI] [PMC free article] [PubMed]
- Dreiza CM, Komalavilas P, Furnish EJ, Flynn CR, Sheller MR, Smoke CC, Lopes LB, Brophy CM. The small heat shock protein, HSPB6, in muscle function and disease. Cell Stress Chaperones. 2010;15:1–11. doi: 10.1007/s12192-009-0127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton P, Byers HL, Leeds N, Ward MA, Shattock MJ. Detection, quantitation, purification, and identification of cardiac proteins S-thiolated during ischemia and reperfusion. J Biol Chem. 2002;277:9806–9811. doi: 10.1074/jbc.M111454200. [DOI] [PubMed] [Google Scholar]
- Eaton P, Fuller W, Shattock MJ. S-thiolation of HSP27 regulates its multimeric aggregate size independently of phosphorylation. J Biol Chem. 2002;277:21189–21196. doi: 10.1074/jbc.M200591200. [DOI] [PubMed] [Google Scholar]
- Edwards HV, Cameron RT, Baillie GS. The emerging role of HSP20 as a multifunctional protective agent. Cell Signal. 2011;23:1447–1454. doi: 10.1016/j.cellsig.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Evgrafov OV, Mersiyanova I, Irobi J, Van Den Bosch L, Dierick I, Leung CL, Schagina O, Verpoorten N, Van Impe K, Fedotov V, et al. Mutant small heat-shock protein 27 causes axonal Charcot-Marie-tooth disease and distal hereditary motor neuropathy. Nat Genet. 2004;36:602–606. doi: 10.1038/ng1354. [DOI] [PubMed] [Google Scholar]
- Fan GC, Chu G, Kranias EG. Hsp20 and its cardioprotection. Trends Cardiovasc Med. 2005;15:138–141. doi: 10.1016/j.tcm.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Fan GC, Ren X, Qian J, Yuan Q, Nicolaou P, Wang Y, Jones WK, Chu G, Kranias EG. Novel cardioprotective role of a small heat-shock protein, Hsp20, against ischemia/reperfusion injury. Circulation. 2005;111:1792–1799. doi: 10.1161/01.CIR.0000160851.41872.C6. [DOI] [PubMed] [Google Scholar]
- Fontaine J-M, Sun X, Hoppe AD, Simon S, Vicart P, Welsh MJ, Benndorf R. Abnormal small heat shock protein interactions involving neuropathy-associated HSP22 (HSPB8) mutants. FASEB J. 2006;20:2168–2170. doi: 10.1096/fj.06-5911fje. [DOI] [PubMed] [Google Scholar]
- Franck E, Madsen O, van Rheede T, Ricard G, Huynen MA, de Jong WW. Evolutionary diversity of vertebrate small heat shock proteins. J Mol Evol. 2004;59:792–805. doi: 10.1007/s00239-004-0013-z. [DOI] [PubMed] [Google Scholar]
- Gasperini P, Espigol-Frigole G, McCormick PJ, Salvucci O, Maric D, Uldrick TS, Polizzotto MN, Yarchoan R, Tosato G. Kaposi sarcoma herpesvirus promotes endothelial-to-mesenchymal transition through notch-dependent signaling. Cancer Res. 2012;72:1157–1169. doi: 10.1158/0008-5472.CAN-11-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibert B, Eckel B, Gonin V, Goldschneider D, Fombonne J, Deux B, Mehlen P, Arrigo AP, Clezardin P, Diaz-Latoud C. Targeting heat shock protein 27 (HspB1) interferes with bone metastasis and tumour formation in vivo. Br J Cancer. 2012;107:63–70. doi: 10.1038/bjc.2012.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleave ME, Monia BP. Antisense therapy for cancer. Nat Rev Cancer. 2005;5:468–479. doi: 10.1038/nrc1631. [DOI] [PubMed] [Google Scholar]
- Guo Y, Ziesch A, Hocke S, Kampmann E, Ochs S, De Toni EN, Goke B, Gallmeier E. Overexpression of heat shock protein 27 (HSP27) increases gemcitabine sensitivity in pancreatic cancer cells through S-phase arrest and apoptosis. J Cell Mol Med. 2015;19:340–350. doi: 10.1111/jcmm.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Haslbeck M, Vierling E. A first line of stress defense: small heat shock proteins and their function in protein homeostasis. J Mol Biol. 2015;427:1537–1548. doi: 10.1016/j.jmb.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havasi A, Li Z, Wang Z, Martin JL, Botla V, Ruchalski K, Schwartz JH, Borkan SC. Hsp27 inhibits Bax activation and apoptosis via a phosphatidylinositol 3-kinase-dependent mechanism. J Biol Chem. 2008;283:12305–12313. doi: 10.1074/jbc.M801291200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heys KR, Friedrich MG, Truscott RJW. Presbyopia and heat: changes associated with aging of the human lens suggest a functional role for the small heat shock protein, α-crystallin, in maintaining lens flexibility. Aging Cell. 2007;6:807–815. doi: 10.1111/j.1474-9726.2007.00342.x. [DOI] [PubMed] [Google Scholar]
- Houlden H, Laura M, Wavrant-De Vrièze F, Blake J, Wood N, Reilly M. Mutations in the HSP27 (HSPB1) gene cause dominant, recessive, and sporadic distal HMN/CMT type 2. Neurology. 2008;71:1660–1668. doi: 10.1212/01.wnl.0000319696.14225.67. [DOI] [PubMed] [Google Scholar]
- Hsu A-L, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Abe A, Ishida C, Takahashi K, Hayasaka K, Yamada M. A clinical phenotype of distal hereditary motor neuronopathy type II with a novel HSPB1 mutation. J Neurol Sci. 2009;277:9–12. doi: 10.1016/j.jns.2008.09.031. [DOI] [PubMed] [Google Scholar]
- Irobi J, Impe KV, Seeman P, Jordanova A, Dierick I, Verpoorten N, Michalik A, Vriendt ED, Jacobs A, Gerwen VV, et al. Hot-spot residue in small heat-shock protein 22 causes distal motor neuropathy. Nat Genet. 2004;36:597–601. doi: 10.1038/ng1328. [DOI] [PubMed] [Google Scholar]
- Jin C, Cleveland JC, Ao L, Li J, Zeng Q, Fullerton DA, Meng X. Human myocardium releases heat shock protein 27 (HSP27) after global ischemia: the proinflammatory effect of extracellular HSP27 through toll-like receptor (TLR)-2 and TLR4. Mol Med. 2014;20:280–289. doi: 10.2119/molmed.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YT, Kwag SJ, Park HJ, Jung EJ, Jeong CY, Jeong SH, Lee YJ, Choi SK, Kang KR, Hah YS, et al. Decreased expression of heat shock protein 20 in colorectal cancer and its implication in tumorigenesis. J Cell Biochem. 2015;116:277–286. doi: 10.1002/jcb.24966. [DOI] [PubMed] [Google Scholar]
- Kardys I, Rifai N, Meilhac O, Michel JB, Martin-Ventura JL, Buring JE, Libby P, Ridker PM. Plasma concentration of heat shock protein 27 and risk of cardiovascular disease: a prospective, nested case-control study. Clin Chem. 2008;54:139–146. doi: 10.1373/clinchem.2007.094961. [DOI] [PubMed] [Google Scholar]
- Kijima K, Numakura C, Goto T, Takahashi T, Otagiri T, Umetsu K, Hayasaka K. Small heat shock protein 27 mutation in a Japanese patient with distal hereditary motor neuropathy. J Hum Genet. 2005;50:473–476. doi: 10.1007/s10038-005-0280-6. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Lee J, Hong YB, Kim YJ, Lee JH, Nam SH, Choi B-O, Chung KW. Ser135Phe mutation in HSPB1 (HSP27) from Charcot–Marie–tooth disease type 2F families. Genes & Genomics. 2015;37:295–303. doi: 10.1007/s13258-014-0259-9. [DOI] [Google Scholar]
- Kourtis N, Nikoletopoulou V, Tavernarakis N. Small heat-shock proteins protect from heat-stroke-associated neurodegeneration. Nature. 2012;490:213–218. doi: 10.1038/nature11417. [DOI] [PubMed] [Google Scholar]
- Kozawa O, Matsuno H, Niwa M, Hatakeyama D, Oiso Y, Kato K, Uematsu T. HSP20, low-molecular-weight heat shock-related protein, acts extracellularly as a regulator of platelet functions: a novel defense mechanism. Life Sci. 2002;72:113–124. doi: 10.1016/S0024-3205(02)02144-6. [DOI] [PubMed] [Google Scholar]
- Kriehuber T, Rattei T, Weinmaier T, Bepperling A, Haslbeck M, Buchner J. Independent evolution of the core domain and its flanking sequences in small heat shock proteins. FASEB J. 2010;24:3633–3642. doi: 10.1096/fj.10-156992. [DOI] [PubMed] [Google Scholar]
- Krizbai IA, Gasparics A, Nagyoszi P, Fazakas C, Molnar J, Wilhelm I, Bencs R, Rosivall L, Sebe A. Endothelial-mesenchymal transition of brain endothelial cells: possible role during metastatic extravasation. PLoS One. 2015;10:e0123845. doi: 10.1371/journal.pone.0119655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Padula V, Staszewski O, Nestel S, Busch H, Boerries M, Roussa E, Prinz M, Krieglstein K. HSPB3 protein is expressed in motoneurons and induces their survival after lesion-induced degeneration. Exp Neurol. 2016;286:40–49. doi: 10.1016/j.expneurol.2016.08.014. [DOI] [PubMed] [Google Scholar]
- Labbadia J, Morimoto RI. Proteostasis and longevity: when does aging really begin? F1000Prime Rep. 2014;6:1–7. doi: 10.12703/P6-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a "set up" for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.CIR.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.CIR.0000048893.62841.F7. [DOI] [PubMed] [Google Scholar]
- Lamoureux F, Thomas C, Yin MJ, Fazli L, Zoubeidi A, Gleave ME. Suppression of heat shock protein 27 using OGX-427 induces endoplasmic reticulum stress and potentiates heat shock protein 90 inhibitors to delay castrate-resistant prostate cancer. Eur Urol. 2014;66:145–155. doi: 10.1016/j.eururo.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Jung S-C, Joo J, Choi Y-R, Moon HW, Kwak G, Yeo HK, Lee J-S, Ahn H-J, Jung N, et al. Overexpression of mutant HSP27 causes axonal neuropathy in mice. J Biomed Sci. 2015;22:43. doi: 10.1186/s12929-015-0154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Lee HJ, Choi SH, Jin YB, An HJ, Kang JH, Yoon SS, Lee YS. Soluble HSPB1 regulates VEGF-mediated angiogenesis through their direct interaction. Angiogenesis. 2012;15:229–242. doi: 10.1007/s10456-012-9255-3. [DOI] [PubMed] [Google Scholar]
- Lelj-Garolla B, Kumano M, Beraldi E, Nappi L, Rocchi P, Ionescu DN, Fazli L, Zoubeidi A, Gleave ME. Hsp27 inhibition with OGX-427 sensitizes non-small cell lung cancer cells to Erlotinib and chemotherapy. Mol Cancer Ther. 2015;14:1107–1116. doi: 10.1158/1535-7163.MCT-14-0866. [DOI] [PubMed] [Google Scholar]
- Lepedda AJ, Cigliano A, Cherchi GM, Spirito R, Maggioni M, Carta F, Turrini F, Edelstein C, Scanu AM, Formato M. A proteomic approach to differentiate histologically classified stable and unstable plaques from human carotid arteries. Atherosclerosis. 2009;203:112–118. doi: 10.1016/j.atherosclerosis.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makley LN, McMenimen KA, DeVree BT, Goldman JW, McGlasson BN, Rajagopal P, Dunyak BM, McQuade TJ, Thompson AD, Sunahara R, et al. Pharmacological chaperone for α-crystallin partially restores transparency in cataract models. Science. 2015;350:674–677. doi: 10.1126/science.aac9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manière X, Krisko A, Pellay FX, Di Meglio JM, Hersen P, Matic I. High transcript levels of heat-shock genes are associated with shorter lifespan of Caenorhabditis elegans. Exp Gerontol. 2014;60:12–17. doi: 10.1016/j.exger.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Mao YW, Liu JP, Xiang H, Li DW. Human alphaA- and alphaB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 2004;11:512–526. doi: 10.1038/sj.cdd.4401384. [DOI] [PubMed] [Google Scholar]
- Martin-Ventura JL, Duran MC, Blanco-Colio LM, Meilhac O, Leclercq A, Michel JB, Jensen ON, Hernandez-Merida S, Tunon J, Vivanco F, et al. Identification by a differential proteomic approach of heat shock protein 27 as a potential marker of atherosclerosis. Circulation. 2004;110:2216–2219. doi: 10.1161/01.CIR.0000136814.87170.B1. [DOI] [PubMed] [Google Scholar]
- Martin TP, Currie S, Baillie GS. The cardioprotective role of small heat-shock protein 20. Biochem Soc Trans. 2014;42:270–273. doi: 10.1042/BST20130272. [DOI] [PubMed] [Google Scholar]
- Martin TP, Hortigon-Vinagre MP, Findlay JE, Elliott C, Currie S, Baillie GS. Targeted disruption of the heat shock protein 20-phosphodiesterase 4D (PDE4D) interaction protects against pathological cardiac remodelling in a mouse model of hypertrophy. FEBS Open Bio. 2014;4:923–927. doi: 10.1016/j.fob.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima-Nishiwaki R, Toyoda H, Nagasawa T, Yasuda E, Chiba N, Okuda S, Maeda A, Kaneoka Y, Kumada T, Kozawa O. Phosphorylated heat shock protein 20 (HSPB6) regulates transforming growth factor-alpha-induced migration and invasion of hepatocellular carcinoma cells. PLoS One. 2016;11:e0151907. doi: 10.1371/journal.pone.0151907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGreal RS, Kantorow WL, Chauss DC, Wei J, Brennan LA, Kantorow M. alphaB-crystallin/sHSP protects cytochrome c and mitochondrial function against oxidative stress in lens and retinal cells. Biochim Biophys Acta. 2012;1820:921–930. doi: 10.1016/j.bbagen.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLemore EC, Tessier DJ, Thresher J, Komalavilas P, Brophy CM. Role of the small heat shock proteins in regulating vascular smooth muscle tone. J Am Coll Surg. 2005;201:30–36. doi: 10.1016/j.jamcollsurg.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Kretz-Remy C, Preville X, Arrigo AP. Human hsp27, drosophila hsp27 and human alphaB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNFalpha-induced cell death. EMBO J. 1996;15:2695–2706. [PMC free article] [PubMed] [Google Scholar]
- Miller H, Poon S, Hibbert B, Rayner K, Chen YX, O’Brien ER. Modulation of estrogen signaling by the novel interaction of heat shock protein 27, a biomarker for atherosclerosis, and estrogen receptor beta: mechanistic insight into the vascular effects of estrogens. Arterioscler Thromb Vasc Biol. 2005;25:e10–e14. doi: 10.1161/01.ATV.0000156536.89752.8e. [DOI] [PubMed] [Google Scholar]
- Morrow G, Kim H-J, Pellerito O, Bourrelle-Langlois M, Le Pécheur M, Groebe K, Tanguay RM. Changes in drosophila mitochondrial proteins following chaperone-mediated lifespan extension confirm a role of Hsp22 in mitochondrial UPR and reveal a mitochondrial localization for cathepsin D. Mech Ageing Dev. 2016;155:36–47. doi: 10.1016/j.mad.2016.02.011. [DOI] [PubMed] [Google Scholar]
- Morrow G, Samson M, Michaud S, and Tanguay RM (2004) Overexpression of the small mitochondrial Hsp22 extends Drosophila life span and increases resistance to oxidative stress. The FASEB Journal [DOI] [PubMed]
- Morrow G, and Tanguay RM (2015) Drosophila melanogaster Hsp22: a mitochondrial small heat shock protein influencing the aging process. Frontiers in Genetics 6. [DOI] [PMC free article] [PubMed]
- Mounier N, Arrigo AP. Actin cytoskeleton and small heat shock proteins: how do they interact? Cell Stress Chaperones. 2002;7:167–176. doi: 10.1379/1466-1268(2002)007<0167:ACASHS>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyano JV, Evans JR, Chen F, Lu M, Werner ME, Yehiely F, Diaz LK, Turbin D, Karaca G, Wiley E, et al. AlphaB-crystallin is a novel oncoprotein that predicts poor clinical outcome in breast cancer. J Clin Invest. 2006;116:261–270. doi: 10.1172/JCI25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci. 2005;6:11–22. doi: 10.1038/nrn1587. [DOI] [PubMed] [Google Scholar]
- Mymrikov EV, Seit-Nebi AS, Gusev NB. Large potentials of small heat shock proteins. Physiol Rev. 2011;91:1123–1159. doi: 10.1152/physrev.00023.2010. [DOI] [PubMed] [Google Scholar]
- Nagaraj RH, Nahomi RB, Shanthakumar S, Linetsky M, Padmanabha S, Pasupuleti N, Wang B, Santhoshkumar P, Panda AK, Biswas A. Acetylation of alphaA-crystallin in the human lens: effects on structure and chaperone function. Biochim Biophys Acta. 2012;1822:120–129. doi: 10.1016/j.bbadis.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj RH, Oya-Ito T, Padayatti PS, Kumar R, Mehta S, West K, Levison B, Sun J, Crabb JW, Padival AK. Enhancement of chaperone function of alpha-crystallin by methylglyoxal modification. Biochemistry. 2003;42:10746–10755. doi: 10.1021/bi034541n. [DOI] [PubMed] [Google Scholar]
- Nagaraj RH, Panda AK, Shanthakumar S, Santhoshkumar P, Pasupuleti N, Wang B, Biswas A. Hydroimidazolone modification of the conserved Arg12 in small heat shock proteins: studies on the structure and chaperone function using mutant mimics. PLoS One. 2012;7:e30257. doi: 10.1371/journal.pone.0030257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S, Ren J. Autophagy and cardiovascular aging: lesson learned from rapamycin. Cell Cycle. 2012;11:2092–2099. doi: 10.4161/cc.20317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhro K, Park J-M, Kim YJ, Yoon BR, Yoo JH, Koo H, Choi B-O, Chung KW. A novel Lys141Thr mutation in small heat shock protein 22 (HSPB8) gene in Charcot–Marie–tooth disease type 2 L. Neuromuscul Disord. 2013;23:656–663. doi: 10.1016/j.nmd.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Narumi T, Shishido T, Otaki Y, Kadowaki S, Honda Y, Funayama A, Honda S, Hasegawa H, Kinoshita D, Yokoyama M, et al. High-mobility group box 1-mediated heat shock protein beta 1 expression attenuates mitochondrial dysfunction and apoptosis. J Mol Cell Cardiol. 2015;82:1–12. doi: 10.1016/j.yjmcc.2015.02.018. [DOI] [PubMed] [Google Scholar]
- Nikoletopoulou V, Kyriakakis E, Tavernarakis N. Cellular and molecular longevity pathways: the old and the new. Trends Endocrinol Metab. 2014;25:212–223. doi: 10.1016/j.tem.2013.12.003. [DOI] [PubMed] [Google Scholar]
- North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. 2012;110:1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan-Sunol C, Gabai VL, Sherman MY. Hsp27 modulates p53 signaling and suppresses cellular senescence. Cancer Res. 2007;67:11779–11788. doi: 10.1158/0008-5472.CAN-07-2441. [DOI] [PubMed] [Google Scholar]
- Oshita SE, Chen F, Kwan T, Yehiely F, Cryns VL. The small heat shock protein HspB2 is a novel anti-apoptotic protein that inhibits apical caspase activation in the extrinsic apoptotic pathway. Breast Cancer Res Treat. 2010;124:307–315. doi: 10.1007/s10549-010-0735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oya-Ito T, Liu BF, Nagaraj RH. Effect of methylglyoxal modification and phosphorylation on the chaperone and anti-apoptotic properties of heat shock protein 27. J Cell Biochem. 2006;99:279–291. doi: 10.1002/jcb.20781. [DOI] [PubMed] [Google Scholar]
- Padival AK, Crabb JW, Nagaraj RH. Methylglyoxal modifies heat shock protein 27 in glomerular mesangial cells. FEBS Lett. 2003;551:113–118. doi: 10.1016/S0014-5793(03)00874-3. [DOI] [PubMed] [Google Scholar]
- Pinz I, Robbins J, Rajasekaran NS, Benjamin IJ, Ingwall JS. Unmasking different mechanical and energetic roles for the small heat shock proteins CryAB and HSPB2 using genetically modified mouse hearts. FASEB J. 2008;22:84–92. doi: 10.1096/fj.07-8130com. [DOI] [PubMed] [Google Scholar]
- Pollack M, Leeuwenburgh C. Apoptosis and aging: role of the mitochondria. J Gerontol A Biol Sci Med Sci. 2001;56:B475–B482. doi: 10.1093/gerona/56.11.B475. [DOI] [PubMed] [Google Scholar]
- Qi Z, Shen L, Zhou H, Jiang Y, Lan L, Luo L, Yin Z. Phosphorylation of heat shock protein 27 antagonizes TNF-alpha induced HeLa cell apoptosis via regulating TAK1 ubiquitination and activation of p38 and ERK signaling. Cell Signal. 2014;26:1616–1625. doi: 10.1016/j.cellsig.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Qian J, Ren X, Wang X, Zhang P, Jones WK, Molkentin JD, Fan GC, Kranias EG. Blockade of Hsp20 phosphorylation exacerbates cardiac ischemia/reperfusion injury by suppressed autophagy and increased cell death. Circ Res. 2009;105:1223–1231. doi: 10.1161/CIRCRESAHA.109.200378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizman JE, Chen YX, Seibert T, Hibbert B, Cuerrier CM, Salari S, Zhao X, Hu T, Shi C, Ma X, et al. Heat shock protein-27 attenuates foam cell formation and atherogenesis by down-regulating scavenger receptor-A expression via NF-kappaB signaling. Biochim Biophys Acta. 2013;1831:1721–1728. doi: 10.1016/j.bbalip.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Rajasekaran NS, Connell P, Christians ES, Yan LJ, Taylor RP, Orosz A, Zhang XQ, Stevenson TJ, Peshock RM, Leopold JA, et al. Human alpha B-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell. 2007;130:427–439. doi: 10.1016/j.cell.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner K, Chen YX, McNulty M, Simard T, Zhao X, Wells DJ, de Belleroche J, O’Brien ER. Extracellular release of the atheroprotective heat shock protein 27 is mediated by estrogen and competitively inhibits acLDL binding to scavenger receptor-A. Circ Res. 2008;103:133–141. doi: 10.1161/CIRCRESAHA.108.172155. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- Saibil H. Chaperone machines for protein folding, unfolding and disaggregation. Nat Rev Mol Cell Biol. 2013;14:630–642. doi: 10.1038/nrm3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H, Mashima T, Yamamoto K, Tsuruo T. Modulation of heat-shock protein 27 (Hsp27) anti-apoptotic activity by methylglyoxal modification. J Biol Chem. 2002;277:45770–45775. doi: 10.1074/jbc.M207485200. [DOI] [PubMed] [Google Scholar]
- Sanbe A, Marunouchi T, Abe T, Tezuka Y, Okada M, Aoki S, Tsumura H, Yamauchi J, Tanonaka K, Nishigori H, et al. Phenotype of cardiomyopathy in cardiac-specific heat shock protein B8 K141 N transgenic mouse. J Biol Chem. 2013;288:8910–8921. doi: 10.1074/jbc.M112.368324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalkwijk CG, van Bezu J, van der Schors RC, Uchida K, Stehouwer CD, van Hinsbergh VW. Heat-shock protein 27 is a major methylglyoxal-modified protein in endothelial cells. FEBS Lett. 2006;580:1565–1570. doi: 10.1016/j.febslet.2006.01.086. [DOI] [PubMed] [Google Scholar]
- Seibert TA, Hibbert B, Chen YX, Rayner K, Simard T, Hu T, Cuerrier CM, Zhao X, de Belleroche J, Chow BJ, et al. Serum heat shock protein 27 levels represent a potential therapeutic target for atherosclerosis: observations from a human cohort and treatment of female mice. J Am Coll Cardiol. 2013;62:1446–1454. doi: 10.1016/j.jacc.2013.05.041. [DOI] [PubMed] [Google Scholar]
- Shammas SL, Waudby CA, Wang S, Buell AK, Knowles TPJ, Ecroyd H, Welland ME, Carver JA, Dobson CM, Meehan S. Binding of the molecular chaperone αB-crystallin to Aβ amyloid fibrils inhibits fibril elongation. Biophys J. 2011;101:1681–1689. doi: 10.1016/j.bpj.2011.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota M, Bishop JL, Nip KM, Zardan A, Takeuchi A, Cordonnier T, Beraldi E, Bazov J, Fazli L, Chi K, et al. Hsp27 regulates epithelial mesenchymal transition, metastasis, and circulating tumor cells in prostate cancer. Cancer Res. 2013;73:3109–3119. doi: 10.1158/0008-5472.CAN-12-3979. [DOI] [PubMed] [Google Scholar]
- Srivastava AK, Renusch SR, Naiman NE, Gu S, Sneh A, Arnold WD, Sahenk Z, Kolb SJ. Mutant HSPB1 overexpression in neurons is sufficient to cause age-related motor neuronopathy in mice. Neurobiol Dis. 2012;47:163–173. doi: 10.1016/j.nbd.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancanelli C, Fabrizi GM, Ferrarini M, Cavallaro T, Taioli F, Di Leo R, Russo M, Gentile L, Toscano A, Vita G, et al. Charcot–Marie–tooth 2F: phenotypic presentation of the Arg136Leu HSP27 mutation in a multigenerational family. Neurol Sci. 2015;36:1003–1006. doi: 10.1007/s10072-014-2050-8. [DOI] [PubMed] [Google Scholar]
- Stark K, Esslinger UB, Reinhard W, Petrov G, Winkler T, Komajda M, Isnard R, Charron P, Villard E, Cambien F, et al. Genetic association study identifies HSPB7 as a risk gene for idiopathic dilated cardiomyopathy. PLoS Genet. 2010;6:e1001167. doi: 10.1371/journal.pgen.1001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straume O, Shimamura T, Lampa MJ, Carretero J, Oyan AM, Jia D, Borgman CL, Soucheray M, Downing SR, Short SM, et al. Suppression of heat shock protein 27 induces long-term dormancy in human breast cancer. Proc Natl Acad Sci U S A. 2012;109:8699–8704. doi: 10.1073/pnas.1017909109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Fontaine J-M, Rest JS, Shelden EA, Welsh MJ, Benndorf R. Interaction of human HSP22 (HSPB8) with other small heat shock proteins. J Biol Chem. 2004;279:2394–2402. doi: 10.1074/jbc.M311324200. [DOI] [PubMed] [Google Scholar]
- Suzuki YJ, Carini M, Butterfield DA. Protein Carbonylation. Antioxid Redox Signal. 2010;12:323–325. doi: 10.1089/ars.2009.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Kang R, Livesey KM, Kroemer G, Billiar TR, Van Houten B, Zeh HJ, 3rd, Lotze MT. High-mobility group box 1 is essential for mitochondrial quality control. Cell Metab. 2011;13:701–711. doi: 10.1016/j.cmet.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuringer D, Jego G, Wettstein G, Terrier O, Cronier L, Yousfi N, Hebrard S, Bouchot A, Hazoume A, Joly AL, et al. Extracellular HSP27 mediates angiogenesis through toll-like receptor 3. FASEB J. 2013;27:4169–4183. doi: 10.1096/fj.12-226977. [DOI] [PubMed] [Google Scholar]
- Tower J, Landis G, Gao R, Luan A, Lee J, Sun Y. Variegated expression of Hsp22 transgenic reporters indicates cell-specific patterns of aging in drosophila Oenocytes. J Gerontol Ser A Biol Med Sci. 2014;69A:253–259. doi: 10.1093/gerona/glt078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treweek TM, Meehan S, Ecroyd H, Carver JA. Small heat-shock proteins: important players in regulating cellular proteostasis. Cell Mol Life Sci. 2015;72:429–451. doi: 10.1007/s00018-014-1754-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treweek TM, Rekas A, Lindner RA, Walker MJ, Aquilina JA, Robinson CV, Horwitz J, Der Perng M, Quinlan RA, Carver JA. R120G αB-crystallin promotes the unfolding of reduced α-lactalbumin and is inherently unstable. FEBS J. 2005;272:711–724. doi: 10.1111/j.1742-4658.2004.04507.x. [DOI] [PubMed] [Google Scholar]
- van Heijst JW, Niessen HW, Musters RJ, van Hinsbergh VW, Hoekman K, Schalkwijk CG. Argpyrimidine-modified heat shock protein 27 in human non-small cell lung cancer: a possible mechanism for evasion of apoptosis. Cancer Lett. 2006;241:309–319. doi: 10.1016/j.canlet.2005.10.042. [DOI] [PubMed] [Google Scholar]
- van Noort JM, Bsibsi M, Nacken P, Gerritsen WH, Amor S. The link between small heat shock proteins and the immune system. Int J Biochem Cell Biol. 2012;44:1670–1679. doi: 10.1016/j.biocel.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Vicart P, Caron A, Guicheney P, Li Z, Prevost MC, Faure A, Chateau D, Chapon F, Tome F, Dupret JM, et al. A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet. 1998;20:92–95. doi: 10.1038/1765. [DOI] [PubMed] [Google Scholar]
- Voll EA, Ogden IM, Pavese JM, Huang X, Xu L, Jovanovic BD, Bergan RC. Heat shock protein 27 regulates human prostate cancer cell motility and metastatic progression. Oncotarget. 2014;5:2648–2663. doi: 10.18632/oncotarget.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa R, Ryu J, Gao R, Choi I-K, Morrow G, Kaur K, Kim I, Kaul SC, Yun C-O, Tanguay RM. Proproliferative functions of drosophila small mitochondrial heat shock protein 22 in human cells. J Biol Chem. 2010;285:3833–3839. doi: 10.1074/jbc.M109.080424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther DM, Kasturi P, Zheng M, Pinkert S, Vecchi G, Ciryam P, Morimoto RI, Dobson CM, Vendruscolo M, Mann M, et al. Widespread proteome remodeling and aggregation in Aging C. elegans. Cell. 2015;161:919–932. doi: 10.1016/j.cell.2015.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waudby CA, Knowles TPJ, Devlin GL, Skepper JN, Ecroyd H, Carver JA, Welland ME, Christodoulou J, Dobson CM, Meehan S. The interaction of αB-crystallin with mature α-synuclein amyloid fibrils inhibits their elongation. Biophys J. 2010;98:843–851. doi: 10.1016/j.bpj.2009.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Liu TT, Wang HH, Hong HM, Yu AL, Feng HP, Chang WW. Hsp27 participates in the maintenance of breast cancer stem cells through regulation of epithelial–mesenchymal transition and nuclear factor-kappaB. Breast Cancer Res. 2011;13:R101. doi: 10.1186/bcr3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub NL, Rubinstein J. Cooling the fire of atherosclerosis with heat shock protein 27. J Am Coll Cardiol. 2013;62:1455–1456. doi: 10.1016/j.jacc.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wignes JA, Goldman JW, Weihl CC, Bartley MG, Andley UP. p62 expression and autophagy in αB-crystallin R120G mutant knock-in mouse model of hereditary cataract. Exp Eye Res. 2013;115:263–273. doi: 10.1016/j.exer.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Tahara H. The role of exosomes and microRNAs in senescence and aging. Adv Drug Deliv Rev. 2013;65:368–375. doi: 10.1016/j.addr.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Xu Y, Diao Y, Qi S, Pan X, Wang Q, Xin Y, Cao X, Ruan J, Zhao Z, Luo L, et al. Phosphorylated Hsp27 activates ATM-dependent p53 signaling and mediates the resistance of MCF-7 cells to doxorubicin-induced apoptosis. Cell Signal. 2013;25:1176–1185. doi: 10.1016/j.cellsig.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Yang F, Yin Y, Wang F, Wang Y, Zhang L, Tang Y, Sun S. miR-17-5p promotes migration of human hepatocellular carcinoma cells through the p38 mitogen-activated protein kinase-heat shock protein 27 pathway. Hepatology. 2010;51:1614–1623. doi: 10.1002/hep.23566. [DOI] [PubMed] [Google Scholar]
- Yang J, Tower J. Expression of hsp22 and hsp70 transgenes is partially predictive of drosophila survival under normal and stress conditions. J Gerontol Ser A Biol Med Sci. 2009;64A:828–838. doi: 10.1093/gerona/glp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Tan J, Lu W, Lu T, Hu Z. The potential role of small heat shock proteins in mitochondria. Cell Signal. 2013;25:2312–2319. doi: 10.1016/j.cellsig.2013.07.027. [DOI] [PubMed] [Google Scholar]