Abstract
Small heat-shock proteins (sHsps), such as αB-crystallin, are one of the major classes of molecular chaperone proteins. In vivo, under conditions of cellular stress, sHsps are the principal defence proteins that prevent large-scale protein aggregation. Progress in determining the structure of sHsps has been significant recently, particularly in relation to the conserved, central and β-sheet structured α-crystallin domain (ACD). However, an understanding of the structure and functional roles of the N- and C-terminal flanking regions has proved elusive mainly because of their unstructured and dynamic nature. In this paper, we propose functional roles for both flanking regions, based around three properties: (i) they act in a localised crowding manner to regulate interactions with target proteins during chaperone action, (ii) they protect the ACD from deleterious amyloid fibril formation and (iii) the flexibility of these regions, particularly at the extreme C-terminus in mammalian sHsps, provides solubility for sHsps under chaperone and non-chaperone conditions. In the eye lens, these properties are highly relevant as the crystallin proteins, in particular the two sHsps αA- and αB-crystallin, are present at very high concentrations.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-017-0789-6) contains supplementary material, which is available to authorized users.
Keywords: AlphaB-crystallin, Small heat-shock proteins, Molecular chaperone, Structure, Function, Unstructured regions
Introduction
The intimate relationship between a protein’s structure and its function is a basic tenet of biology (Bagowski et al. 2010; Worth et al. 2009). Major advances in structural biology techniques over the past 20 or so years have led to the determination of the higher order structures of a wide range of globular proteins. However, amongst the most elusive of proteins whose structures have yet to be fully elucidated are the mammalian small heat-shock proteins (sHsps). Recent progress in this quest has, however, been significant and is summarised in a variety of review articles (Bakthisaran et al. 2015; Basha et al. 2012; Hochberg and Benesch 2014; Treweek et al. 2015). X-ray crystallographic studies have determined the atomic-level structure of the excised α-crystallin domain (ACD) of sHsps which encompasses approximately the central 80 amino acids of the protein. Solid state NMR studies have also provided such information about the ACD, in addition to some detail about the structural arrangement of the N-terminal region (Jehle et al. 2009; Jehle et al. 2011). However, the atomic-level structure of the N- and C-terminal regions and their relationship to the ACD in determining the overall quaternary arrangement have proved refractory to accurate determination. Mass spectrometry, X-ray solution scattering, cryo-electron microscopy and molecular modelling have also provided important structural information that, when combined, has enabled the construction of a variety of models for the oligomeric structure of αB-crystallin (αBc, HspB5), the principal sHsp (Baldwin et al. 2011b; Jehle et al. 2010). However, a detailed understanding of the structural and functional roles of the N- and C-terminal flanking regions of mammalian sHsps represents a significant gap in our knowledge.
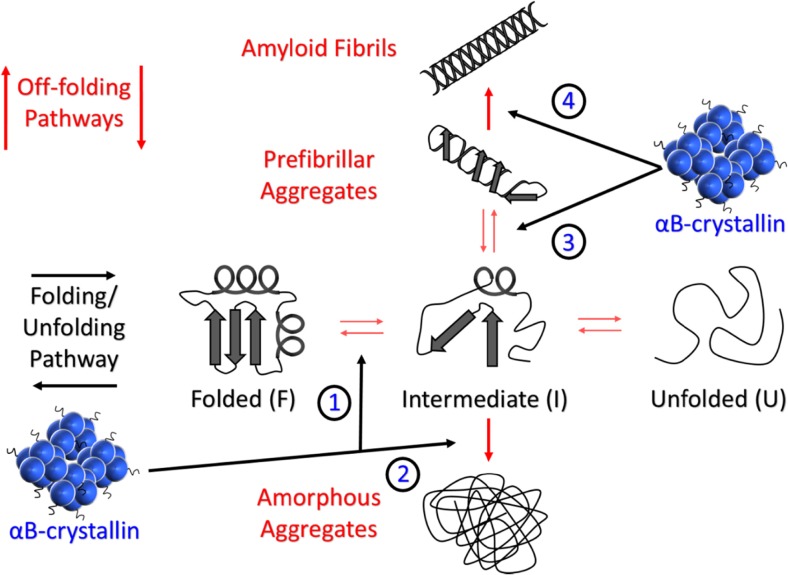
sHsps are ubiquitous and numerous intracellular proteins and are one of the major classes of molecular chaperone proteins. Under conditions of stress (elevated temperature, infection, oxidation, etc.), they selectively interact, in an ATP-independent manner, with target proteins that are destabilised, for example, to form intermediate states that are prone to association and subsequent large-scale aggregation. Accordingly, sHsps exhibit specificity for target proteins that enter off-folding pathways towards either an amorphous or amyloid fibrillar aggregated form (Fig. 1) (Kulig and Ecroyd 2012; Treweek et al. 2015). As a result, sHsp levels are up-regulated markedly under cellular stress conditions, although significant constitutive expression can occur in some cell types presumably to regulate the correct conformation and levels of target proteins. Accordingly, sHsps play an important role in the maintenance of cellular protein homeostasis or proteostasis. Indeed, in a recent study of the proteome of aged nematodes (C. elegans), sHsp levels (and not those of other molecular chaperones) were elevated markedly to compensate for enhanced general protein aggregation that occurs with ageing, highlighting the crucial role of sHsps in maintaining cellular proteostasis (Walther et al. 2015).
Fig. 1.
Schematic of the protein folding/unfolding pathway (horizontal) and the off-folding pathways (vertical). The folding/unfolding pathway depicts both folded (F) and unfolded (U) states whilst the intermediate (I) represents the partially folded state(s) between these two extremes which is characterised by having elements of secondary structure and has the potential to enter the off-folding pathways to form amorphous aggregates and/or amyloid fibrils. The oligomeric sHsp αBc is depicted with small black ‘squiggly’ protrusions representing the solvent-exposed and flexible C-terminal extension. The various junctures at which αBc interacts on the folding/unfolding and off-folding pathways (Cox et al. 2014; Treweek et al. 2015) are indicated with black arrows labelled 1, 2, 3 and 4. Briefly, 1. Interaction with destabilised native-like species, 2. Interaction with intermediate species to prevent or delay amorphous aggregation, 3. Interaction with intermediate species to prevent or delay the generation of prefibrillar aggregates and also to dissociate prefibrillar/oligomeric species back to an intermediate or native state, 4. Binding to amyloid fibrils in order to stabilise them and prevent further elongation and fragmentation which may lead to secondary nucleation
In humans, there are ten sHsps. The most populous and widespread (in terms of tissue distribution) of the sHsps is αBc which is also found at high levels, along with its closely related partner αA-crystallin (αAc, HspB4), in the eye lens. Both sHsps naturally co-associate and function in a chaperone manner to maintain lens transparency via inhibition of crystallin protein aggregation. Most of the recent sHsp structural work has been undertaken on αBc and it will be the focus of much of the discussion in this paper.
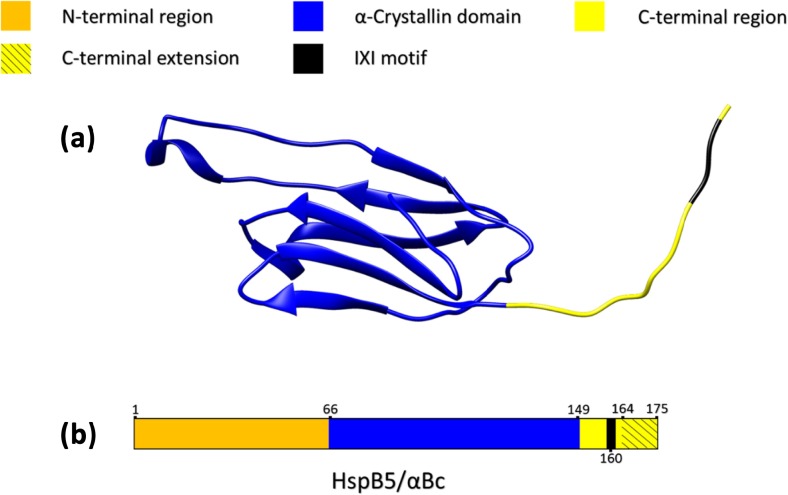
sHsps, in particular the mammalian ones, are difficult to characterise structurally because of their heterogeneous and dynamic nature. Such behaviour is not conducive to their crystallisation and hence structural characterisation by X-ray crystallography. For example, αBc has a monomeric subunit mass of around 20 kDa but exists as an ensemble of oligomers (Aquilina et al. 2003) with a mass distribution of 420 to 980 kDa and an average mass of 650 kDa under physiological conditions (Haley et al. 1998). The conserved central ACD in sHsps is highly β-sheet in character with the β-strands arranged in an immunoglobulin-like fold (Bagneris et al. 2009; Laganowsky et al. 2010). The ACD is flanked by N- and C-terminal regions that are variable in length and lack sequence similarity between sHsps. Apart from short sections of transient helical and β-sheet structure in the N-terminal region (Jehle et al. 2011), the flanking regions adopt little ordered secondary structure and exhibit significant dynamism. Indeed, the last 12 amino acids of αBc (along with similar regions in other mammalian sHsps) have been recognised for over 20 years as a C-terminal extension that is unstructured and has great flexibility, comparable to isolated peptides of the same length (Carver 1999; Carver et al. 1992; Esposito et al. 1998). Figure 2 provides a schematic of the various structural regions in αBc.
Fig. 2.
Schematic diagram of the three structural regions in αBc. The colour scheme for (a) and (b) is indicated at the top of the figure. (a) The crystal structure of a truncated αBc monomer (PDB: 3L1G) incorporating residues 68–162 which is the majority of the structured ACD and part of the unstructured C-terminal region including the conserved IXI sequence (I159-P160-I161). The highly flexible C-terminal extension of 12 amino acids and the N-terminal region were not included in order to facilitate crystallisation (Laganowsky et al. 2010). (b) A linearised αBc sequence displaying the relative lengths of the ACD and the flanking N- and C-terminal regions as well the location of the conserved IXI motif and the flexible C-terminal extension
The polydisperse nature of αBc (and some other mammalian sHsps) is intimately related to the exchange of individual αBc subunits, a process that is highly temperature dependent with subunit exchange rate increasing significantly at higher temperatures (Baldwin et al. 2011c; Bova et al. 1997; Hilton et al. 2013; Sobott et al. 2002). Much insight into the mechanism of subunit exchange has come from recent combined structural investigations of αBc using X-ray crystallography, NMR spectroscopy and mass spectrometry (Baldwin et al. 2011a; Baldwin et al. 2011c; Hochberg and Benesch 2014). The important role of the conserved IXI sequence in the C-terminal region (I159-P160-I161 in αBc) in interacting with an adjacent subunit is now well recognised. Subunit exchange may play an important role in oligomeric sHsp chaperone action by facilitating dissociation from the oligomer and interaction with the target protein.
The role of the various sHsp regions in chaperone action is unclear, more so of late with the observation that the isolated ACD of αBc has significant chaperone ability to prevent the aggregation of target proteins (Hochberg et al. 2014), implying that the chaperone activity (and hence target protein interaction site(s)) is encompassed within this central domain. However, other studies have implicated the N- and C-terminal regions of sHsps in interaction and binding with amorphously aggregating target proteins during chaperone action (Mainz et al. 2015; McDonald et al. 2012; Rajagopal et al. 2015). The obvious question to ask, therefore, is what are the structural and functional roles of the N- and C-terminal flanking regions in sHsps? In this paper, we address this question in a general context in terms of the role of unstructured regions in proteins and specifically in relation to flanking regions in sHsps. The role of these regions has been discussed previously in a review of existing structural data (Sudnitsyna et al. 2012).
Do the flanking regions in sHsps facilitate initial target protein interaction and act as localised crowding agents to regulate interactions with target proteins?
Earlier work by Hall (Hall 2006; Hall and Dobson 2006) examined the effect of conformational changes inherent to an inert biopolymer, through either association/dissociation or undergoing a shape transition from an expanded to a compact form, in regulating macromolecular crowding by altering the excluded volume component of the solution. It was concluded that folding of destabilised proteins was promoted under the conditions that maximised molecular crowding, i.e. when greater excluded volume of the solution occurred. In agreement with this, random polymer chains undergo significant compaction under conditions of macromolecular crowding (Le Coeur et al. 2009).
Conceivably, sHsps could utilise such means to regulate the excluded volume within the crowded environment of the cell, i.e. the highly malleable nature of their unstructured flanking regions would lead to conversion between structural compaction and expansion whilst the extensive subunit exchange would oscillate the proteins between smaller (e.g. dimer) and larger oligomeric species. Subunit exchange in sHsps may simply be a way of facilitating the initial interaction with the target protein by enabling enhanced malleability in the terminal regions, since the dynamic nature of these regions would be no longer relatively constrained within the oligomer. Within the lens fibre cells, where αAc and αBc are present at high concentrations and are by far the predominant species, these properties would be exacerbated. Phosphorylation of large oligomeric sHsps such as Hsp27 (HspB1) and αBc (HspB5) may enable this to occur as well, as has been investigated via the use of phosphomimics of these two sHsps. In these two cases, the phosphomimics have altered oligomeric size and/or mass distribution (Ecroyd et al. 2007; Peschek et al. 2013; Hayes et al. 2009) and, depending on the particular target protein and type of aggregation (amorphous or fibrillar), they exhibit enhanced sHsp chaperone ability (Ecroyd et al. 2007; Jovcevski et al. 2015).
The unfolded flanking regions, particularly when they are associated to form large heterogeneous oligomers as in the mammalian sHsps, would increase molecular crowding in the vicinity of, and when interacting with, intermediately folded (I) target proteins (Fig. 1). In doing so, the sHsps could stabilise these target proteins, stop their unfolding and thereby facilitate their refolding back to the native state via transient interactions. One could conceive of this as a localised molecular crowding phenomenon arising from the close proximity of the two proteins. Hall (2006; Hall and Dobson 2006) has shown that increasing the concentration of a partially folded crowding agent (e.g. a protein) leads to greater structure in the crowding agent, a process that could be applicable to how sHsps function in their initial interaction to stabilise aggregation-prone target proteins. Thus, the unstructured terminal regions of sHsps initially act as akin to a ‘lasso’ to capture the unfolding target protein. The subsequent step of compaction of the sHsp and interaction of the target protein with the structured ACD leads to more intimate association of the two proteins and stabilisation of the intermediately folded target protein. The rationale above provides an explanation for the observation that the ACD is all that is required for the chaperone action of αBc in vitro (Hochberg et al. 2014). Of course, this is an artificial and simple system compared to the crowded nature of the cell where numerous competing interactions are possible with a diversity of cellular components. Finally, interaction and binding of the intermediately folded target protein with the sHsp during chaperone action, and its subsequent refolding, would couple folding to binding (Ganguly and Chen 2011; Shammas et al. 2016). Thereby, sHsps, either as individuals or in partnership with each other and other molecular chaperones (the latter potentially also utilising ATP hydrolysis), would contribute to the maintenance of cellular proteostasis (Jeng et al. 2015).
The importance of the N-terminal region of αBc in capturing amorphously aggregating lysozyme was demonstrated by Mainz et al. (2015). They used a combination of truncation mutants and chaperone assays to show that truncation of the N-terminal region leads to a marked loss of chaperone activity. Furthermore, specific interactions of the N-terminal region in intact αBc were inferred by solid-state NMR through chemical shift changes and alterations in dynamics of resonances in the N-terminal region (Mainz et al. 2015). Similarly, the N-terminal region of Hsp20 (HspB6) has multiple sites of interaction with a target protein as well as a role in regulating chaperone activity (Heirbaut et al. 2014) whilst also being important in the formation of a hetero-oligomer with Hsp27 (Heirbaut et al. 2016). There is evidence from interactome studies that the N-terminal region of plant sHsps is involved in interacting with amorphously aggregating target proteins (Jaya et al. 2009). Another example of the unstructured N-terminal region of sHsps’ involvement in interacting with other proteins comes from the recent work of Sluchanko et al. (2017) who determined the crystal structure of a complex between a phosphorylated form (at Ser16) of the dimeric sHsp, Hsp20 (van de Klundert et al. 1998; Weeks et al. 2014) and the 14-3-3σ dimer, i.e. the two proteins form a 2:2 complex. Phosphoserine 16 in Hsp20 interacts with the binding grove of 14-3-3σ via a long loop containing the N-terminal region of the former protein. The dimeric ACD region of Hsp20, in an immunoglobulin fold conformation, binds in an asymmetric manner to one of the 14-3-3σ monomers.
One key target for sHsps is the intermediate filament (IF) cytoskeleton as evidenced by the range of diseases (cataract, myopathies, neuropathies), caused by mutations in Hsp27, HspB3, αAc, αBc and Hsp22 (HspB8) (Perng and Quinlan 2015), which in all cases cause characteristic histopathological aggregates into which IFs are also concentrated. The mutations span the primary sequence of the sHsps involved, including the N- and C-terminal regions, but there does not seem to be any clustering. Suffice to say that when the C-terminal region is completely removed, as with the cardiomyopathy-causing mutation Q151X in αBc, it is as, if not more, efficient in binding to desmin filaments and also in modulating their assembly. Indeed, data from pin array studies show that IF proteins are bound by multiple sequences throughout αBc (Ghosh et al. 2007). There also appears to be no particular requirement for phosphorylation of sHsps for them to associate with IFs (Nicholl and Quinlan 1994). IF proteins all possess intrinsically disordered domains located on the filament surface (Herrmann and Aebi 2016) and the possibility of synergy (Landsbury et al. 2010) with similarly structured N- and C-terminal regions in sHsps when bound to the filaments has not been explored. Germane to this discussion is that IFs also provide binding sites for other molecular chaperones such as Hsp70 (Perng et al. 1999) as well as the proteasome (Olink-Coux et al. 1994), so the proteostatic machinery is appropriately partitioned on IFs, structures that are integral to the cellular stress response.
The sHsps interact with all elements of the cytoskeleton (Landsbury et al. 2010; Quinlan 2002). Microfilaments, microtubules and IFs are all dependent upon sHsps for their competence (Quinlan 2002), and all are modulated by them. There appear to be multiple binding sequences across the primary sequences of all the sHsps, both in the ACD and in the N- and C-terminal regions (Ghosh et al. 2007). Indeed, deletion of the N-terminal region does not prevent sHsps from binding to actin (Guo and Cooper 2000) or to tubulin and from chaperoning microtubules (Ohto-Fujita et al. 2007). Whilst there is evidence that sequences from the N-terminal region and the ACD of both Hsp27 and αBc are effective inhibitors of actin assembly in vitro (Wieske et al. 2001), the topic is contentious since Hsp27 mutants can stimulate actin polymerisation (Butt et al. 2001) whereas another study using wild-type Hsp27 reported little to no significant change (Graceffa 2011). A common theme emerges from these studies: the assembly and dynamics of all three major cytoskeletal elements in the cell are modulated by sHsps, including Hsp27, αBc, HspB7 and Hsp22. Whilst studies sometimes focus on one specific element of the cytoskeleton (Almeida-Souza et al. 2011; Shimizu et al. 2016), it is obvious that both the competence and the integration of the different elements of the cytoskeleton rely on sHsps.
Do the unstructured flanking (terminal) regions prevent deleterious aggregation of the structured, central α-crystallin domain?
Hall (Hall and Hirota 2009; Hall et al. 2005) and Abeln and Frenkel (Abeln and Frenkel 2008; Abeln and Frenkel 2011) have examined the effect of unstructured flanking polypeptide regions on the aggregation propensity (to form both amyloid fibrillar and amorphous aggregates) of central regions. They conclude that the flanking regions have a marked propensity to prevent the central regions from aggregating; they do so by ‘frustrating the encounter event’ that, of course, is the crucial event in the aggregation process. Furthermore, the presence of the flanking regions on both the N- and C-terminal ends prevents aggregation to an enhanced degree, i.e. the location of the aggregating region in the middle of an unstructured polypeptide chain is most advantageous for the suppression of aggregation.
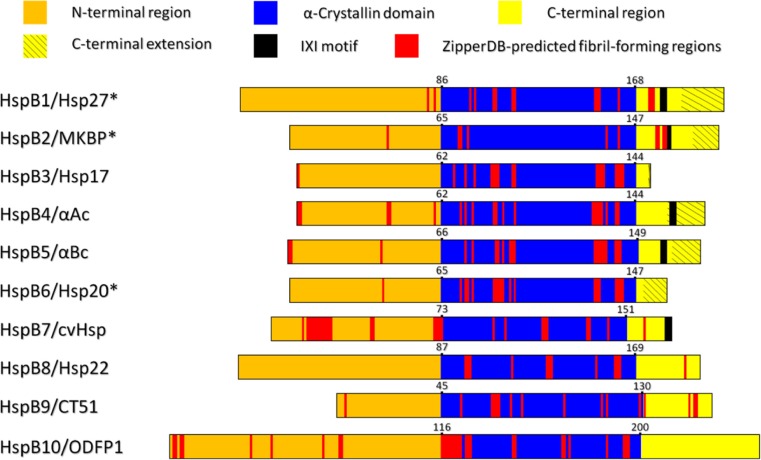
We have undertaken a survey of the regions of the ten human sHsps (HspB1 to HspB10) with a propensity to form amyloid fibrils via the algorithm ZipperDB, which determines the presence of so-called amyloid zipper sequences within the amino acid sequence of a particular protein (Goldschmidt et al. 2010). Figure 3 (along with Fig. S1 and Supplementary Table 1) summarises the results of these analyses for the sHsps. It is readily apparent that all sHsps contain significant regions of fibril-forming propensity that is mainly found in their ACD. In general, in silico analysis with other fibril prediction algorithms (TANGO and Zyggregator) (Fernandez-Escamilla et al. 2004; Tartaglia et al. 2008) gives similar results to the ZipperDB analysis (Fig. 3 and Fig. S1) in implying a significant tendency for the ACD to have more fibril-prone residues than the two other (terminal) regions (Supplementary Table 1).
Fig. 3.
ZipperDB analysis (Goldschmidt et al. 2010) of the amino acid sequences of the ten human sHsps displayed as linearised sequences aligned at the N-terminal end of the ACD. The colour scheme is indicated at the top of the figure. The position of the published IXI sequences (Delbecq et al. 2015) and the C-terminal extensions (Carver 1999) are displayed in the C-terminal region. The red regions are those residues associated with the start of a hexapeptide that has a high propensity to form amyloid fibrils as determined by ZipperDB, i.e. these residues have a Rosetta energy that is less than or equal to the threshold energy of −23 kcal/mol. (*)The C-terminal extensions for HspB1/Hsp27, HspB2/MKBP and HspB6/Hsp20 were identified from 1H NMR spectroscopic studies of sHsps from other mammals, i.e. mouse, rat and rat respectively
Specifically, the ACD of αAc and αBc have large portions of their sequences that are predicted to form amyloid fibrils (18.1 and 20.2% respectively via ZipperDB analysis, Fig. 3, Supplementary Table 1). There is experimental evidence to support this. The isolated peptide encompassing K70 to K88 in αAc (and the corresponding region in αBc, D73 to K92) has marked chaperone ability to prevent amorphous target protein aggregation (Sharma et al. 2000). The peptide has been named ‘mini-chaperone’ by Sharma and co-workers. In addition, F71-K88 αAc forms amyloid fibrils (Raju et al. 2016; Tanaka et al. 2008). However, addition of the last ten amino acids of αAc, i.e. the flexible C-terminal extension, to the C-terminus of the αAc ‘mini-chaperone’ prevents the peptide from forming amyloid fibrils but retains its chaperone ability (Raju et al. 2014). Furthermore, Laganowsky et al. found that the 11-amino acid fragment K90-V100 in αBc (encompassing a loop region between two strands of anti-parallel β-sheet) was highly amyloidogenic based on ZipperDB and experimental analyses (Laganowsky et al. 2012). Indeed, K90-V100, readily formed classic amyloid fibrils, in addition to a β-sheet oligomer whose structure was determined by X-ray crystallography. Furthermore, the oligomer was cytotoxic.
Our work with αAc and αBc has shown that they form amyloid fibrils under slightly destabilising conditions, for example, in the presence of low concentrations of denaturant and elevated temperature (Meehan et al. 2004; Meehan et al. 2007). The E. coli sHsp IbpA forms amyloid fibrils under physiological conditions in vitro, which is prevented by the presence of its co-chaperone IbpB (Ratajczak et al. 2010). IbpB, the other E. coli sHsp, shares 48% sequence identity with IbpA but does not form fibrils under physiological conditions. The ACD of IbpA is slightly more aggregation-prone than the ACD of IbpB by ZipperDB analysis (Supplementary Table 1).
Furthermore, as stated above, the ACD of sHsps adopts an immunoglobulin-like fold, a motif that is prone to amyloid fibril formation, possibly because of its highly β-sheet character which is primed for conversion into the amyloid fold. Thus, immunoglobulin light chains, or its fragments, form amyloid fibrils in amyloid light chain amyloidosis. Likewise, superoxide dismutase 1 and β2-microglobulin both adopt the immunoglobulin fold and are the principal components of the amyloid fibrillar deposits associated with amyotrophic lateral sclerosis and haemodialysis-related amyloidosis (Knowles et al. 2014).
Thus, the ACD of αBc (or at least part(s) of it) is prone to form fibrils which, if were also true for the intact protein, would be highly deleterious to the protein’s functionality in vivo. As αBc (and other sHsps) do not form fibrils under normal physiological conditions, the implication is that the flanking regions have a modulating effect on the amyloidogenicity of the ACD.
The dynamic nature of the flanking regions, particularly the polar, flexible C-terminal extension in mammalian sHsps, acts as a solubilising agent for the protein under chaperone and non-chaperone conditions
The unstructured and dynamic nature of the N- and C-terminal regions is well recognised, particularly so for the C-terminal extension of mammalian sHsps, located at the extreme C-terminal end of the protein that has flexibility comparable to small peptides of comparable length and hence is amenable to observation in solution by NMR spectroscopy (Carver 1999; Carver and Lindner 1998; Treweek et al. 2010). Figures 2b and 3 provide a comparison of the length of the C-terminal extension observed by solution phase NMR spectroscopy for Hsp27 (HspB1) through to Hsp20 (HspB6). It is of note that the remaining four mammalian sHsps (HspB7 to HspB10) have not been studied by NMR spectroscopy to ascertain whether they also possess a flexible C-terminal extension. The structure and function of this region have been well characterised, as summarised in various reviews (Carver 1999; Carver and Lindner 1998). It is suffice to state that removal of the C-terminal extension leads to destabilisation of the protein and reduces its chaperone effectiveness (Lindner et al. 2000) whilst replacement of charged amino acids with uncharged alanine in this region leads to similar effects (Morris et al. 2008; Treweek et al. 2007). It is concluded that the C-terminal extension has an important solubilising role for at least some mammalian sHsps, and is required to offset the inherent exposed hydrophobicity, a factor that is probably of importance for the proteins’ chaperone function. The same role for this extension is utilised under chaperone conditions to solubilise the complex that sHsps form with amorphously aggregating target proteins.
Discussion
In this paper, we have proposed a variety of functional roles for the N- and C-terminal regions of sHsps:
They regulate the interaction and stabilisation of target proteins during chaperone action via localised molecular crowding action.
They effectively shield the central ACD from potential aggregation to form amyloid fibrils.
Their dynamic nature, particularly from the C-terminal extension, acts as solubilising agents for the protein under normal physiological conditions and during chaperone action.
The N- and C-terminal flanking regions in sHsps
The role and importance of structural disorder in molecular chaperone action have been considered by others (Bardwell and Jakob 2012; Tompa and Csermely 2004; Tompa et al. 2015), along with the realisation that many proteins are unstructured in their native state, or have large regions of their polypeptide chain that are disordered (Tompa 2012). Unstructured proteins are classified as intrinsically disordered proteins (Dunker et al. 2008). Mammalian sHsps have many properties of intrinsically disordered proteins because of their mainly unstructured flanking terminal regions.
The flanking regions in mammalian sHsps share very little sequence similarity, are highly variable in length (Fig. 3) and are present in all sHsps (Kappé et al. 2003). The proposed roles of these regions are consistent with the absence of conserved sequence. Thus, all that is required are regions of polypeptide that lack structure and are flexible, malleable and predominantly hydrophilic in character. Many sequences of amino acids can satisfy these requirements.
Our data regarding the unfolding of αAc and αBc in the presence of urea showed that the ACD is more exposed to solution than the N-terminal region (Carver et al. 1993). Likewise, the highly mobile C-terminal extension is very exposed to solution (Treweek et al. 2010). Thus, for the sHsp oligomer, the unstructured C-terminal region most likely undergoes the initial interaction with target proteins, prior to more intimate association with the ACD and/or the N-terminal region, which may be coupled with subunit dissociation.
The mammalian sHsps, Hsp20 and Hsp22 (HspB8) do not form large oligomeric assemblies but exist as smaller species, for example, dimers in the case of Hsp20 (van de Klundert et al. 1998; Weeks et al. 2014; Shemetov et al. 2008). Because of their dissociated nature, it is conceivable that these sHsps largely have their N- and C-terminal regions exposed and, as such, may have some basal level of activity that supports the proteostasis network.
The well-defined oligomeric sHsps, e.g. wheat Hsp16.9 and Methanococcus Hsp16.5, have no flexible C-terminal extension, nor does yeast Hsp26, yet they undergo subunit exchange (Benesch et al. 2010). However, they all have the conserved IXI sequence which facilitates subunit exchange. Hence, they can still potentially act as a ‘lasso’ via their N- or C-terminal regions, as per mammalian sHsps, during chaperone action.
The arguments relating to unstructured regions acting as localised crowding agents could be applied to other molecular chaperones. For example, Hsp70 has large regions of disorder which could function in a similar manner to the terminal regions in sHsps in the initial interaction with an unstructured target protein, prior to the instigation of protein folding along with ATP hydrolysis. For Hsp60 (GroEL), encapsulation of the target protein within the protein cage leads to crowding of the target protein and hence facilitates folding, via an ATP-dependent mechanisms. The concept of ‘molecular shields’ has been proposed to account for the chaperone action of unstructured Late Embryogenesis Abundant (LEA) proteins (Chakrabortee et al. 2012). Their mode of action is via transient interactions that shield the hydrophobic regions of target proteins from association to prevent aggregation. This behaviour is comparable with that of localised molecular crowding proposed for the flanking terminal regions in sHsps. In sHsps, these transient interactions encourage the structurally destabilised target proteins, e.g. potentially amyloid fibril-forming α-synuclein, to return to its natively unfolded (intrinsically disordered) state (Cox et al. 2014; Treweek et al. 2015). From our other studies, it is apparent that the unrelated molecular chaperones, clusterin, caseins and 14-3-3ζ, all exhibit a very similar mechanism of ATP-independent sHsp-like chaperone action (Carver et al. 2003; Holt et al. 2013; Thorn et al. 2015; Williams et al. 2011). In E. coli, curli proteins (e.g. CsgA) form functional amyloid extracellularly. Intracellularly, specific molecular chaperones (e.g. CsgC) prevent inappropriate curli fibril formation via a sHsp-like mechanism (Taylor et al. 2016).
Under stress conditions in vivo, e.g. heat shock, large-scale protein unfolding and potential aggregation occurs. sHsps, such as αBc, are activated (which may involve structural change and/or dissociation from the oligomer to form the dimer species) to interact with and bind to destabilised target proteins to form a high molecular weight complex (Lindner et al. 1998; Stamler et al. 2005). By contrast, under non-heat shock (i.e. constitutive) conditions, transient interaction of destabilised target proteins with non-activated sHsps occurs which does not lead to complex formation (Cox et al. 2014; Kulig and Ecroyd 2012; Treweek et al. 2015). The interaction of αBc with amyloid fibril-forming proteins, e.g. α-synuclein, ataxin-3, apolipoprotein C-II, kappa-casein and β2-microgobulin, is such a situation (Cox et al. 2016; Esposito et al. 2013; Hatters et al. 2001; Rekas et al. 2004; Rekas et al. 2007; Robertson et al. 2010). The variation in sHsp chaperone mechanism depending on conditions and the degree of unfolding of the target protein is consistent with various studies. We have shown that under mild stress conditions, i.e. slightly elevated temperature, target proteins such as malate dehydrogenase and α-lactalbumin interact with the α-crystallin oligomer via complex formation that is consistent with intercalation into the porous surface of the oligomer. The target proteins are readily accessible to interaction with molecular chaperones (e.g. Hsp70) that are capable of refolding target proteins, coupled to ATP hydrolysis (Regini et al. 2010). However, under conditions of significant stress, i.e. high temperature, the target protein (in this case γE-crystallin) is inserted into the central cavity of the α-crystallin oligomer (Clarke et al. 2010). Mchaourab’s work on the chaperone action of sHsps with T4 lysozyme mutants of varying stability also implies sHsp activation during chaperone action that is directly related to the degree of unfolding, and hence binding affinity, of the particular T4 lysozyme mutant (Mchaourab et al. 2002; Shashidharamurthy et al. 2005).
The central α-crystallin domain of sHsps
Goldschmidt et al. (2010) noted that fibril-forming regions in globular, structured proteins are buried and therefore not exposed to solution and any subsequent potential interaction with other similar regions. For unstructured peptides and proteins, the presence of fibril-forming regions in the middle of unstructured peptides and proteins is a general phenomenon (Goldschmidt et al. 2010). Thus, the non-amyloid-β component (NAC), fibril-forming region in α-synuclein, is embedded in the middle of the unstructured protein. Our results (Rekas et al. 2012) showed that when the first 60 amino acids of α-synuclein were absent, i.e. the region immediately N-terminal to the NAC region, fibril formation occurred rapidly. Others have shown that deletion of portions of the C-terminal region leads to enhanced fibril formation of α-synuclein (Hoyer et al. 2004). Consistent with these data, the fibril-forming region of unstructured κ-casein is in the middle of the protein (Ecroyd et al. 2008). Likewise, the crucial fibril-forming residues of amyloid β (Glu11 to Ala21) are in the middle of the peptide (Serpell 2000). Recently, we have shown that a four amino acid tract in the centre of the sequence of SEVI, a peptide which potentiates HIV infection, is crucial in promoting fibril formation (Elias et al. 2014). Finally, the observation that addition of the C-terminal extension to the αAc ‘mini-chaperone’ prevents its fibril formation (Raju et al. 2014) is also consistent with the ability of flexible, unstructured peptide flanking regions to prevent core regions from fibril formation.
Oligomerisation of sHsps
The role of subunit oligomerisation, and the associated subunit exchange, in sHsp chaperone action is an unresolved matter of debate within the literature (Haslbeck et al. 2005). There is evidence that chaperone action is enhanced under conditions of faster subunit exchange, for example, at higher temperature (Carver et al. 2002). However, the cross-linked oligomeric form of α-crystallin is chaperone-active (Augusteyn 2004), as is an immobilised form of the protein (Garvey et al. 2011). One explanation for sHsp oligomerisation is that it may protect against fibril formation, for example, within the ACD, in addition to the protection provided by the unstructured flanking regions. Other protein oligomers associate for such a reason, as we have shown for the unstructured milk casein proteins in which micelle (oligomer) formation by the four unrelated caseins (either with themselves individually, or with other caseins, or all of them to form the casein micelle in milk) prevents amyloid fibril formation by κ- and αs2-casein via mutual chaperone interaction and also (principally within the casein micelle) by the chaperone action of the β- and αs1-caseins (Holt et al. 2013; Holt and Carver 2012; Thorn et al. 2015). Similarly, transthyretin fibril formation requires initial dissociation from a tetrameric species prior to a conformational change within the monomer which leads to an aggregation-prone intermediate (Colon and Kelly 1992). Furthermore, methionine oxidation of apolipoprotein A1 reduces its oligomerisation and leads to enhanced amyloid fibril formation of the protein (Wong et al. 2010).
Relevance to crystallin proteins in the eye lens
The short, flexible C-terminal extensions in mammalian sHsps impart heterogeneity and enhance solubility to the proteins. Via their chaperone action in the eye lens, the α-crystallin subunits also prevent aggregation and precipitation of the β- and γ-crystallins and hence lens opacification. The two α-crystallin subunits, αAc and αBc (in a 3:1 ratio in the human lens), are the predominant lens proteins. The unrelated β-crystallin subunits also have highly flexible and unstructured terminal extensions (at both termini in the basic β-crystallins, but only at the N-terminus in the acidic β-crystallins) and are oligomers (dimers to octamers). The γ-crystallins are structurally related to the β-crystallins and form similar two-domain, Greek key motif, highly β-sheet structures, but are monomers. The major γ-crystallin, γs, has a short, flexible, four amino acid N-terminal extension (Cooper et al. 1994). The other γ-crystallins do not have terminal extensions. In support of the role of unstructured, highly flexible terminal extensions in preventing aggregation of the crystallins, truncation mutants of α- and β-crystallins without terminal extensions (and parts thereof) are prone to aggregation and potential precipitation (Lampi et al. 2002; Treweek et al. unpublished results). In the same vein, deletion of the C-terminal region of αBc (i.e. removal of residues 151 to 175 inclusive) leads to insolubilisation of the protein and the formation of inclusion bodies (Asomugha et al. 2011), although the protein retains some chaperone activity despite losing much of its secondary structure, and has a reduction in its oligomeric status (Hayes et al. 2008).
Goto and co-workers have described the amorphous, glassy state of supersaturated protein solutions and compared it to the amyloid fibril state (Yoshimura et al. 2012). Their conclusions have direct relevance to the arrangement of crystallin proteins in the eye lens. The glassy protein state is present in the lens; the very high concentration of crystallin proteins (up to 400 mg/mL in the centre) is highly stable and maintains solubility (and hence transparency) for tens of years without forming crystals or amyloid fibrils. The amorphous, glassy state of crystallin protein arrays or aggregates that are responsible for lens transparency arise because the proteins are “highly flexible and various intermolecular interactions are possible” (Yoshimura et al. 2012). Specifically, with respect to the lens, the flexibility of the terminal regions in αAc and αBc, the β-crystallins and the N-terminal extension in γs-crystallin, along with extensive subunit exchange of αAc and αBc, ensure that the lens crystallin protein mixture does not crystallise or form amyloid fibrils, occurrences that would be highly deleterious to lens transparency. It is the crystallin mixture that behaves as such, because individual, isolated β- and γ-crystallin subunits readily form well-ordered crystals whose structures have been determined by X-ray crystallography (Lapatto et al. 1991; Moreau and King 2012; Norledge et al. 1996). Thus, from a simple consideration of the highly dynamic nature of the crystallin proteins and supersaturation of a concentrated protein (crystallin) solution, the transparency of the lens can be explained. Transparency occurs despite a very high lens crystallin concentration, a situation that normally favours large-scale aggregation, for example, to form crystals or amyloid fibrils.
Concluding comments
We have proposed that the largely unstructured N- and C-terminal regions of mammalian sHsps have multi-faceted roles: (i) they perform the initial interaction with target proteins during chaperone action, (ii) they protect the structured and sHsp-defining ACD from the possibility of misfolding into potentially non-functional and toxic amyloid fibrils and (iii) because of their dynamic, polar and unstructured nature, they act as solubilising agents for sHsps under chaperone and non-chaperone conditions. Experimentally, (iii) has been shown, in general, to be correct, at least for the C-terminal extension. However, there is plenty of scope and opportunity to undertake experiments to test (i) and (ii) and thereby determine the veracity or not of these two hypotheses, and whether they could be expanded to non-mammalian sHsps.
Electronic supplementary material
ZipperDB analysis (Goldschmidt et al. 2010) of the amino acid sequences of the ten human sHsps. The N-terminal, ACD, and C-terminal regions are segmented by the black vertical dashed lines in that sequential order. The blue, green, yellow and orange lines are hexapeptide residues that are of increasing Rosetta energy, whilst the red lines that cross the threshold of −23 kcal/mol (black horizontal line) are those residues associated with a hexapeptide that has a high propensity of forming amyloid fibrils. (DOCX 88 kb)
Comparison of the predicted fibril-forming or β-aggregation propensity of all ten human sHsps and two E. coli sHsps using three different prediction algorithms, i.e. ZipperDB (Goldschmidt et al. 2010), TANGO (Fernandez-Escamilla et al. 2004) and Zyggregator (Tartaglia et al. 2008). Percentages are given as the number of residues classified as having a ‘high propensity’ to form fibrils within a specific region over all the residues within that specific region of the protein, e.g. the N-terminal, ACD or C-terminal regions. (DOCX 38.5 kb)
Acknowledgements
The Australian National Health and Medical Research Council is thanked for financial support via a project grant to JAC. ABG acknowledges the financial support of an Australian Postgraduate Award. We thank Prof. Yuji Goto, Osaka University, Prof. Roger Truscott, University of Wollongong and Dr. Nicholas Ray and Dr. David Thorn, Australian National University, for the helpful discussions relating to crystallin protein interactions in the eye lens. JAC is indebted to Simon Tognetti whose creative drawings and pottery inspired some of the ideas presented herein.
Abbreviations
- ACD
α-Crystallin domain
- αAc
αA-Crystallin
- αBc
αB-Crystallin
- IF
Intermediate filament
- NAC
Non-amyloid-β component
- sHsps
Small heat-shock proteins
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-017-0789-6) contains supplementary material, which is available to authorized users.
References
- Abeln S, Frenkel D. Disordered flanks prevent peptide aggregation. PLoS Comput Biol. 2008;4(12):e1000241. doi: 10.1371/journal.pcbi.1000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeln S, Frenkel D. Accounting for protein-solvent contacts facilitates design of nonaggregating lattice proteins. Biophys J. 2011;100(3):693–700. doi: 10.1016/j.bpj.2010.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida-Souza L, Asselbergh B, d'Ydewalle C, et al. Small heat-shock protein HSPB1 mutants stabilize microtubules in Charcot-Marie-Tooth neuropathy. J Neurosci. 2011;31(43):15320–15328. doi: 10.1523/JNEUROSCI.3266-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquilina JA, Benesch JL, Bateman OA, Slingsby C, Robinson CV. Polydispersity of a mammalian chaperone: mass spectrometry reveals the population of oligomers in αB-crystallin. Proc Natl Acad Sci U S A. 2003;100(19):10611–10616. doi: 10.1073/pnas.1932958100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asomugha C, Gupta R, Srivastava O. Structural and functional roles of deamidation of N146 and/or truncation of NH2- or COOH-termini in human αB-crystallin. Mol Vis. 2011;17(262):2407–2420. [PMC free article] [PubMed] [Google Scholar]
- Augusteyn R. Dissociation is not required for α-crystallin’s chaperone function. Exp Eye Res. 2004;79(6):781–784. doi: 10.1016/j.exer.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Bagneris C, Bateman OA, Naylor CE, Cronin N, Boelens W, Keep NH, Slingsby C. Crystal structures of α-crystallin domain dimers of αB-crystallin and Hsp20. J Mol Biol. 2009;392(5):1242–1252. doi: 10.1016/j.jmb.2009.07.069. [DOI] [PubMed] [Google Scholar]
- Bagowski CP, Bruins W, te Velthuis AJW. The nature of protein domain evolution: shaping the interaction network. Curr Genomics. 2010;11(5):368–376. doi: 10.2174/138920210791616725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakthisaran R, Tangirala R, Rao CM. Small heat shock proteins: role in cellular functions and pathology. BBA-Proteins Proteom. 2015;1854(4):291–319. doi: 10.1016/j.bbapap.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Baldwin AJ, Hilton GR, Lioe H, Bagnéris C, Benesch JL, Kay LE. Quaternary dynamics of αB-crystallin as a direct consequence of localised tertiary fluctuations in the C-terminus. J Mol Biol. 2011;413(2):310–320. doi: 10.1016/j.jmb.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Baldwin AJ, Lioe H, Hilton GR, Baker LA, Rubinstein JL, Kay LE, Benesch JL. The polydispersity of αB-crystallin is rationalized by an interconverting polyhedral architecture. Structure. 2011;19(12):1855–1863. doi: 10.1016/j.str.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Baldwin AJ, Lioe H, Robinson CV, Kay LE, Benesch JL. αB-crystallin polydispersity is a consequence of unbiased quaternary dynamics. J Mol Biol. 2011;413(2):297–309. doi: 10.1016/j.jmb.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Bardwell JC, Jakob U. Conditional disorder in chaperone action. Trends Biochem Sci. 2012;37(12):517–525. doi: 10.1016/j.tibs.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basha E, O’Neill H, Vierling E. Small heat shock proteins and α-crystallins: dynamic proteins with flexible functions. Trends Biochem Sci. 2012;37(3):106–117. doi: 10.1016/j.tibs.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benesch JL, Aquilina JA, Baldwin AJ, et al. The quaternary organization and dynamics of the molecular chaperone HSP26 are thermally regulated. Chem Biol. 2010;17(9):1008–1017. doi: 10.1016/j.chembiol.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bova MP, Ding L-L, Horwitz J, Fung BK-K. Subunit exchange of αA-crystallin. J Biol Chem. 1997;272(47):29511–29517. doi: 10.1074/jbc.272.47.29511. [DOI] [PubMed] [Google Scholar]
- Butt E, Immler D, Meyer HE, Kotlyarov A, Laass K, Gaestel M. Heat shock protein 27 is a substrate of cGMP-dependent protein kinase in intact human platelets: phosphorylation-induced actin polymerization caused by HSP27 mutants. J Biol Chem. 2001;276(10):7108–7113. doi: 10.1074/jbc.M009234200. [DOI] [PubMed] [Google Scholar]
- Carver JA. Probing the structure and interactions of crystallin proteins by NMR spectroscopy. Prog Retin Eye Res. 1999;18(4):431–462. doi: 10.1016/S1350-9462(98)00027-5. [DOI] [PubMed] [Google Scholar]
- Carver JA, Aquilina JA, Truscott RJ. An investigation into the stability of α-crystallin by NMR spectroscopy; evidence for a two-domain structure. BBA-Protein Struct Mol Enzymol. 1993;1164(1):22–28. doi: 10.1016/0167-4838(93)90107-3. [DOI] [PubMed] [Google Scholar]
- Carver JA, Aquilina JA, Truscott RJ, Ralston GB. Identification by 1H NMR spectroscopy of flexible C-terminal extensions in bovine lens α-crystallin. FEBS Lett. 1992;311(2):143–149. doi: 10.1016/0014-5793(92)81386-Z. [DOI] [PubMed] [Google Scholar]
- Carver JA, Lindner RA. NMR spectroscopy of α-crystallin. Insights into the structure, interactions and chaperone action of small heat-shock proteins. Int J Biol Macromol. 1998;22(3–4):197–209. doi: 10.1016/S0141-8130(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Carver JA, Lindner RA, Lyon C, Canet D, Hernandez H, Dobson CM, Redfield C. The interaction of the molecular chaperone α-crystallin with unfolding α-lactalbumin: a structural and kinetic spectroscopic study. J Mol Biol. 2002;318(3):815–827. doi: 10.1016/S0022-2836(02)00144-4. [DOI] [PubMed] [Google Scholar]
- Carver JA, Rekas A, Thorn DC, Wilson MR. Small heat-shock proteins and clusterin: intra- and extracellular molecular chaperones with a common mechanism of action and function? IUBMB life. 2003;55(12):661–668. doi: 10.1080/15216540310001640498. [DOI] [PubMed] [Google Scholar]
- Chakrabortee S, Tripathi R, Watson M, et al. Intrinsically disordered proteins as molecular shields. Mol BioSyst. 2012;8(1):210–219. doi: 10.1039/C1MB05263B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MJ, Artero JB, Moulin M, et al. Investigation of γE-crystallin target protein binding to bovine lens alpha-crystallin by small-angle neutron scattering. BBA-Gen Subjects. 2010;1800(3):392–397. doi: 10.1016/j.bbagen.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Colon W, Kelly JW. Partial denaturation of transthyretin is sufficient for amyloid fibril formation in vitro. Biochemistry. 1992;31(36):8654–8660. doi: 10.1021/bi00151a036. [DOI] [PubMed] [Google Scholar]
- Cooper PG, Carver JA, Aquilina JA, Ralston GB, Truscott RJ. A 1H NMR spectroscopic comparison of γS- and γB-crystallins. Exp Eye Res. 1994;59(2):211–220. doi: 10.1006/exer.1994.1099. [DOI] [PubMed] [Google Scholar]
- Cox D, Carver JA, Ecroyd H. Preventing α-synuclein aggregation: the role of the small heat-shock molecular chaperone proteins. BBA-Mol Basis Dis. 2014;1842(9):1830–1843. doi: 10.1016/j.bbadis.2014.06.024. [DOI] [PubMed] [Google Scholar]
- Cox D, Selig E, Griffin MD, Carver JA, Ecroyd H. Small heat-shock proteins prevent α-synuclein aggregation via transient interactions and their efficacy is affected by the rate of aggregation. J Biol Chem. 2016;291(43):22618–22629. doi: 10.1074/jbc.M116.739250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbecq SP, Rosenbaum JC, Klevit RE. A mechanism of subunit recruitment in human small heat shock protein oligomers. Biochemistry. 2015;54(28):4276–4284. doi: 10.1021/acs.biochem.5b00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunker AK, Oldfield CJ, Meng J, et al. The unfoldomics decade: an update on intrinsically disordered proteins. BMC Genomics. 2008;9(Suppl 2):S1. doi: 10.1186/1471-2164-9-S2-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecroyd H, Koudelka T, Thorn DC, Williams DM, Devlin G, Hoffmann P, Carver JA. Dissociation from the oligomeric state is the rate-limiting step in fibril formation by κ-casein. J Biol Chem. 2008;283(14):9012–9022. doi: 10.1074/jbc.M709928200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecroyd H, Meehan S, Horwitz J, et al. Mimicking phosphorylation of αB-crystallin affects its chaperone activity. Biochem J. 2007;401(1):129–141. doi: 10.1042/BJ20060981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias AK, Scanlon D, Musgrave IF, Carver JA. SEVI, the semen enhancer of HIV infection along with fragments from its central region, form amyloid fibrils that are toxic to neuronal cells. BBA-Proteins Proteom. 2014;1844(9):1591–1598. doi: 10.1016/j.bbapap.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Esposito G, Garvey M, Alverdi V, et al. Monitoring the interaction between β2-microglobulin and the molecular chaperone αB-crystallin by NMR and mass spectrometry: αB-crystallin dissociates β2-microglobulin oligomers. J Biol Chem. 2013;288(24):17844–17858. doi: 10.1074/jbc.M112.448639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Viglino P, Fogolari F, Gaestel M, Carver JA. Selective NMR experiments on macromolecules: implementation and analysis of QUIET-NOESY. J Magn Reson. 1998;132(2):204–213. doi: 10.1006/jmre.1998.1430. [DOI] [PubMed] [Google Scholar]
- Fernandez-Escamilla A-M, Rousseau F, Schymkowitz J, Serrano L. Prediction of sequence-dependent and mutational effects on the aggregation of peptides and proteins. Nat Biotechnol. 2004;22(10):1302–1306. doi: 10.1038/nbt1012. [DOI] [PubMed] [Google Scholar]
- Ganguly D, Chen J. Topology-based modeling of intrinsically disordered proteins: balancing intrinsic folding and intermolecular interactions. Proteins: Struct, Funct, Bioinf. 2011;79(4):1251–1266. doi: 10.1002/prot.22960. [DOI] [PubMed] [Google Scholar]
- Garvey M, Griesser SS, Griesser HJ, et al. Enhanced molecular chaperone activity of the small heat-shock protein αB-crystallin following covalent immobilization onto a solid-phase support. Biopolymers. 2011;95(6):376–389. doi: 10.1002/bip.21584. [DOI] [PubMed] [Google Scholar]
- Ghosh JG, Houck SA, Clark JI. Interactive sequences in the stress protein and molecular chaperone human αB crystallin recognize and modulate the assembly of filaments. Int J Biochem Cell Biol. 2007;39(10):1804–1815. doi: 10.1016/j.biocel.2007.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt L, Teng PK, Riek R, Eisenberg D. Identifying the amylome, proteins capable of forming amyloid-like fibrils. Proc Natl Acad Sci U S A. 2010;107(8):3487–3492. doi: 10.1073/pnas.0915166107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graceffa P. Hsp27-actin interaction. Biochem Res Int. 2011;2011:901572. doi: 10.1155/2011/901572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Cooper LF. An N-terminal 33-amino-acid-deletion variant of hsp25 retains oligomerization and functional properties. Biochem Bioph Res Co. 2000;270(1):183–189. doi: 10.1006/bbrc.2000.2401. [DOI] [PubMed] [Google Scholar]
- Haley DA, Horwitz J, Stewart PL. The small heat-shock protein, αB-crystallin, has a variable quaternary structure. J Mol Biol. 1998;277(1):27–35. doi: 10.1006/jmbi.1997.1611. [DOI] [PubMed] [Google Scholar]
- Hall D. Protein self-association in the cell: a mechanism for fine tuning the level of macromolecular crowding? Eur Biophys J. 2006;35(3):276–280. doi: 10.1007/s00249-005-0016-8. [DOI] [PubMed] [Google Scholar]
- Hall D, Dobson CM. Expanding to fill the gap: a possible role for inert biopolymers in regulating the extent of the ‘macromolecular crowding’ effect. FEBS Lett. 2006;580(11):2584–2590. doi: 10.1016/j.febslet.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Hall D, Hirota N. Multi-scale modelling of amyloid formation from unfolded proteins using a set of theory derived rate constants. Biophys Chem. 2009;140(1–3):122–128. doi: 10.1016/j.bpc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Hall D, Hirota N, Dobson CM. A toy model for predicting the rate of amyloid formation from unfolded protein. J Mol Biol. 2005;351(1):195–205. doi: 10.1016/j.jmb.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Haslbeck M, Franzmann T, Weinfurtner D, Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol. 2005;12(10):842–846. doi: 10.1038/nsmb993. [DOI] [PubMed] [Google Scholar]
- Hatters DM, Lindner RA, Carver JA, Howlett GJ. The molecular chaperone, α-crystallin, inhibits amyloid formation by apolipoprotein C-II. J Biol Chem. 2001;276(36):33755–33761. doi: 10.1074/jbc.M105285200. [DOI] [PubMed] [Google Scholar]
- Hayes VH, Devlin G, Quinlan RA. Truncation of αB-crystallin by the myopathy-causing Q151X mutation significantly destabilizes the protein leading to aggregate formation in transfected cells. J Biol Chem. 2008;283(16):10500–10512. doi: 10.1074/jbc.M706453200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes D, Napoli V, Mazurkie A, Stafford WF, Graceffa P. Phosphorylation dependence of Hsp27 multimeric size and molecular chaperone function. J Biol Chem. 2009;284(28):18801–18807. doi: 10.1074/jbc.M109.011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heirbaut M, Beelen S, Strelkov SV, Weeks SD. Dissecting the functional role of the N-terminal domain of the human small heat shock protein HSPB6. PLoS One. 2014;9(8):e105892. doi: 10.1371/journal.pone.0105892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heirbaut M, Lermyte F, Martin EM, Beelen S, Verschueren T, Sobott F, Strelkov SV, Weeks SD. The preferential heterodimerization of human small heat shock proteins HSPB1 and HSPB6 is dictated by the N-terminal domain. Arch Biochem Biophys. 2016;610:41–50. doi: 10.1016/j.abb.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Herrmann H, Aebi U. Intermediate filaments: structure and assembly. Cold Spring Harb Perspect Biol. 2016;8(11):a018242. doi: 10.1101/cshperspect.a018242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton GR, Hochberg GK, Laganowsky A, McGinnigle SI, Baldwin AJ, Benesch JL. C-terminal interactions mediate the quaternary dynamics of αB-crystallin. Phil Trans R Soc B. 2013;368(1617):20110405. doi: 10.1098/rstb.2011.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg GK, Benesch JL. Dynamical structure of αB-crystallin. Prog Biophys Mol Biol. 2014;115(1):11–20. doi: 10.1016/j.pbiomolbio.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Hochberg GK, Ecroyd H, Liu C, et al. The structured core domain of αB-crystallin can prevent amyloid fibrillation and associated toxicity. Proc Natl Acad Sci U S A. 2014;111(16):E1562–E1570. doi: 10.1073/pnas.1322673111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt C, Carver JA. Darwinian transformation of a ‘scarcely nutritious fluid’ into milk. J Evolution Biol. 2012;25(7):1253–1263. doi: 10.1111/j.1420-9101.2012.02509.x. [DOI] [PubMed] [Google Scholar]
- Holt C, Carver JA, Ecroyd H, Thorn DC. Invited review: caseins and the casein micelle: their biological functions, structures, and behavior in foods. J Dairy Sci. 2013;96(10):6127–6146. doi: 10.3168/jds.2013-6831. [DOI] [PubMed] [Google Scholar]
- Hoyer W, Cherny D, Subramaniam V, Jovin TM. Impact of the acidic C-terminal region comprising amino acids 109-140 on α-synuclein aggregation in vitro. Biochemistry. 2004;43(51):16233–16242. doi: 10.1021/bi048453u. [DOI] [PubMed] [Google Scholar]
- Jaya N, Garcia V, Vierling E. Substrate binding site flexibility of the small heat shock protein molecular chaperones. Proc Natl Acad Sci U S A. 2009;106(37):15604–15609. doi: 10.1073/pnas.0902177106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehle S, Rajagopal P, Bardiaux B, et al. Solid-state NMR and SAXS studies provide a structural basis for the activation of αB-crystallin oligomers. Nat Struct Mol Biol. 2010;17(9):1037–1042. doi: 10.1038/nsmb.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehle S, van Rossum B, Stout JR, et al. αB-crystallin: a hybrid solid-state/solution-state NMR investigation reveals structural aspects of the heterogeneous oligomer. J Mol Biol. 2009;385(5):1481–1497. doi: 10.1016/j.jmb.2008.10.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehle S, Vollmar BS, Bardiaux B, et al. N-terminal domain of αB-crystallin provides a conformational switch for multimerization and structural heterogeneity. Proc Natl Acad Sci U S A. 2011;108(16):6409–6414. doi: 10.1073/pnas.1014656108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng W, Lee S, Sung N, Lee J, Tsai FT. Molecular chaperones: guardians of the proteome in normal and disease states. F1000Research. 2015;4(F1000 Faculty Rev):1448. doi: 10.12688/f1000research.7214.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovcevski B, Kelly MA, Rote AP, Berg T, Gastall HY, Benesch JL, Aquilina JA, Ecroyd H. Phosphomimics destabilize Hsp27 oligomeric assemblies and enhance chaperone activity. Chem Biol. 2015;22(2):186–195. doi: 10.1016/j.chembiol.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Kappé G, Franck E, Verschuure P, Boelens WC, Leunissen JA, de Jong WW. The human genome encodes 10 α-crystallin–related small heat shock proteins: HspB1–10. Cell Stress Chaperones. 2003;8(1):53–61. doi: 10.1379/1466-1268(2003)8<53:THGECS>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles TP, Vendruscolo M, Dobson CM. The amyloid state and its association with protein misfolding diseases. Nat Rev Mol Cell Biol. 2014;15(6):384–396. doi: 10.1038/nrm3810. [DOI] [PubMed] [Google Scholar]
- Kulig M, Ecroyd H. The small heat-shock protein αB-crystallin uses different mechanisms of chaperone action to prevent the amorphous versus fibrillar aggregation of α-lactalbumin. Biochem J. 2012;448(3):343–352. doi: 10.1042/BJ20121187. [DOI] [PubMed] [Google Scholar]
- Laganowsky A, Benesch JL, Landau M, et al. Crystal structures of truncated alphaA and alphaB crystallins reveal structural mechanisms of polydispersity important for eye lens function. Protein Sci. 2010;19(5):1031–1043. doi: 10.1002/pro.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laganowsky A, Liu C, Sawaya MR, et al. Atomic view of a toxic amyloid small oligomer. Science. 2012;335(6073):1228–1231. doi: 10.1126/science.1213151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampi KJ, Kim YH, Bächinger HP, et al. Decreased heat stability and increased chaperone requirement of modified human βB1-crystallins. Mol Vis. 2002;8:359–366. [PubMed] [Google Scholar]
- Landsbury A, Perng MD, Pohl E, Quinlan RA. Functional symbiosis between the intermediate filament cytoskeleton and small heat shock proteins. In: Arrigo AP, Simon S, editors. Small stress proteins and human diseases. New York: Nova Science; 2010. pp. 55–87. [Google Scholar]
- Lapatto R, Nalini V, Bax B, Driessen H, Lindley P, Blundell T, Slingsby C. High resolution structure of an oligomeric eye lens β-crystallin: loops, arches, linkers and interfaces in βB2 dimer compared to a monomeric γ-crystallin. J Mol Biol. 1991;222(4):1067–1083. doi: 10.1016/0022-2836(91)90594-V. [DOI] [PubMed] [Google Scholar]
- Le Coeur C, Demé B, Longeville S. Compression of random coils due to macromolecular crowding. Phys Rev E. 2009;79(3 Pt 1):031910. doi: 10.1103/PhysRevE.79.031910. [DOI] [PubMed] [Google Scholar]
- Lindner RA, Carver JA, Ehrnsperger M, et al. Mouse Hsp25, a small heat shock protein. Eur J Biochem. 2000;267(7):1923–1932. doi: 10.1046/j.1432-1327.2000.01188.x. [DOI] [PubMed] [Google Scholar]
- Lindner RA, Kapur A, Mariani M, Titmuss SJ, Carver JA. Structural alterations of α-crystallin during its chaperone action. Eur J Biochem. 1998;258(1):170–183. doi: 10.1046/j.1432-1327.1998.2580170.x. [DOI] [PubMed] [Google Scholar]
- Mainz A, Peschek J, Stavropoulou M, et al. The chaperone αB-crystallin uses different interfaces to capture an amorphous and an amyloid client. Nat Struct Mol Biol. 2015;22(11):898–905. doi: 10.1038/nsmb.3108. [DOI] [PubMed] [Google Scholar]
- McDonald ET, Bortolus M, Koteiche HA, Mchaourab HS. Sequence, structure, and dynamic determinants of Hsp27 (HspB1) equilibrium dissociation are encoded by the N-terminal domain. Biochemistry. 2012;51(6):1257–1268. doi: 10.1021/bi2017624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mchaourab HS, Dodson EK, Koteiche HA. Mechanism of chaperone function in small heat shock proteins: two-mode binding of the excited states of T4 lysozyme mutants by αA-crystallin. J Biol Chem. 2002;277(43):40557–40566. doi: 10.1074/jbc.M206250200. [DOI] [PubMed] [Google Scholar]
- Meehan S, Berry Y, Luisi B, Dobson CM, Carver JA, MacPhee CE. Amyloid fibril formation by lens crystallin proteins and its implications for cataract formation. J Biol Chem. 2004;279(5):3413–3419. doi: 10.1074/jbc.M308203200. [DOI] [PubMed] [Google Scholar]
- Meehan S, Knowles TPJ, Baldwin AJ, et al. Characterisation of amyloid fibril formation by small heat-shock chaperone proteins human αA-, αB- and R120G αB-crystallins. J Mol Biol. 2007;372(2):470–484. doi: 10.1016/j.jmb.2007.06.060. [DOI] [PubMed] [Google Scholar]
- Moreau KL, King JA. Protein misfolding and aggregation in cataract disease and prospects for prevention. Trends Mol Med. 2012;18(5):273–282. doi: 10.1016/j.molmed.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AM, Treweek TM, Aquilina JA, Carver JA, Walker MJ. Glutamic acid residues in the C-terminal extension of small heat shock protein 25 are critical for structural and functional integrity. FEBS J. 2008;275(23):5885–5898. doi: 10.1111/j.1742-4658.2008.06719.x. [DOI] [PubMed] [Google Scholar]
- Nicholl I, Quinlan R. Chaperone activity of alpha-crystallins modulates intermediate filament assembly. EMBO J. 1994;13(4):945–953. doi: 10.1002/j.1460-2075.1994.tb06339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norledge B, Mayr E-M, Glockshuber R, Bateman O, Slingsby C, Jaenicke R, Driessen H. The X-ray structures of two mutant crystallin domains shed light on the evolution of multi-domain proteins. Nat Struct Mol Biol. 1996;3(3):267–274. doi: 10.1038/nsb0396-267. [DOI] [PubMed] [Google Scholar]
- Ohto-Fujita E, Fujita Y, Atomi Y. Analysis of the αB-crystallin domain responsible for inhibiting tubulin aggregation. Cell Stress Chaperones. 2007;12(2):163–171. doi: 10.1379/CSC-255.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olink-Coux M, Arcangeletti C, Pinardi F, Minisini R, Huesca M, Chezzi C, Scherrer K. Cytolocation of prosome antigens on intermediate filament subnetworks of cytokeratin, vimentin and desmin type. J Cell Sci. 1994;107(Pt 3):353–366. [PubMed] [Google Scholar]
- Perng MD, Cairns L, van den IJssel P, Prescott A, Hutcheson AM, Quinlan RA (1999) Intermediate filament interactions can be altered by HSP27 and αB-crystallin. J Cell Sci 112(13):2099–2112 [DOI] [PubMed]
- Perng MD, Quinlan RA. The dynamic duo of small heat proteins and IFs maintain cell homeostasis, resist cellular stress and enable evolution in cells and tissues. In: Hightower LE, Tanguay RM, editors. The big book on small heat shock proteins. New York: Springer International Publishing; 2015. pp. 401–434. [Google Scholar]
- Peschek J, Braun N, Rohrberg J, et al. Regulated structural transitions unleash the chaperone activity of αB-crystallin. Proc Natl Acad Sci U S A. 2013;110(40):E3780–E3789. doi: 10.1073/pnas.1308898110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan RA. Cytoskeletal competence requires protein chaperones. In: Arrigo AP, Müller WEG, editors. Small stress proteins. Berlin Heidelberg: Springer; 2002. pp. 219–233. [DOI] [PubMed] [Google Scholar]
- Rajagopal P, Tse E, Borst AJ, Delbecq SP, Shi L, Southworth DR, Klevit RE. A conserved histidine modulates HSPB5 structure to trigger chaperone activity in response to stress-related acidosis. elife. 2015;4:e07304. doi: 10.7554/eLife.07304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju M, Santhoshkumar P, Sharma KK. Alpha-crystallin-derived peptides as therapeutic chaperones. BBA-Gen Subjects. 2016;1860(1 Pt B):246–251. doi: 10.1016/j.bbagen.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju M, Santhoshkumar P, Xie L, Sharma KK. Addition of αA-crystallin sequence 164–173 to a mini-chaperone DFVIFLDVKHFSPEDLT alters the conformation but not the chaperone-like activity. Biochemistry. 2014;53(16):2615–2623. doi: 10.1021/bi4017268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak E, Stróżecka J, Matuszewska M, Ziętkiewicz S, Kuczyńska-Wiśnik D, Laskowska E, Liberek K. IbpA the small heat shock protein from Escherichia coli forms fibrils in the absence of its cochaperone IbpB. FEBS Lett. 2010;584(11):2253–2257. doi: 10.1016/j.febslet.2010.04.060. [DOI] [PubMed] [Google Scholar]
- Regini JW, Ecroyd H, Meehan S, et al. The interaction of unfolding α-lactalbumin and malate dehydrogenase with the molecular chaperone αB-crystallin: a light and X-ray scattering investigation. Mol Vis. 2010;16:2446–2456. [PMC free article] [PubMed] [Google Scholar]
- Rekas A, Adda CG, Aquilina JA, et al. Interaction of the molecular chaperone αB-crystallin with α-synuclein: effects on amyloid fibril formation and chaperone activity. J Mol Biol. 2004;340(5):1167–1183. doi: 10.1016/j.jmb.2004.05.054. [DOI] [PubMed] [Google Scholar]
- Rekas A, Ahn KJ, Kim J, Carver JA. The chaperone activity of α-synuclein: utilizing deletion mutants to map its interaction with target proteins. Proteins: Struct, Funct, Bioinf. 2012;80(5):1316–1325. doi: 10.1002/prot.24028. [DOI] [PubMed] [Google Scholar]
- Rekas A, Jankova L, Thorn DC, Cappai R, Carver JA. Monitoring the prevention of amyloid fibril formation by α-crystallin: temperature dependence and the nature of the aggregating species. FEBS J. 2007;274(24):6290–6304. doi: 10.1111/j.1742-4658.2007.06144.x. [DOI] [PubMed] [Google Scholar]
- Robertson AL, Headey SJ, Saunders HM, Ecroyd H, Scanlon MJ, Carver JA, Bottomley SP. Small heat-shock proteins interact with a flanking domain to suppress polyglutamine aggregation. Proc Natl Acad Sci U S A. 2010;107(23):10424–10429. doi: 10.1073/pnas.0914773107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpell LC. Alzheimer’s amyloid fibrils: structure and assembly. BBA-Mol Basis Dis. 2000;1502(1):16–30. doi: 10.1016/S0925-4439(00)00029-6. [DOI] [PubMed] [Google Scholar]
- Shammas SL, Crabtree MD, Dahal L, Wicky BI, Clarke J. Insights into coupled folding and binding mechanisms from kinetic studies. J Biol Chem. 2016;291(13):6689–6695. doi: 10.1074/jbc.R115.692715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma KK, Kumar RS, Kumar GS, Quinn PT. Synthesis and characterization of a peptide identified as a functional element in αA-crystallin. J Biol Chem. 2000;275(6):3767–3771. doi: 10.1074/jbc.275.6.3767. [DOI] [PubMed] [Google Scholar]
- Shashidharamurthy R, Koteiche HA, Dong J, Mchaourab HS. Mechanism of chaperone function in small heat shock proteins: dissociation of the HSP27 oliogmer is required for recognition and binding of destabilized T4 lysozyme. J Biol Chem. 2005;280(7):5281–5289. doi: 10.1074/jbc.M407236200. [DOI] [PubMed] [Google Scholar]
- Shemetov AA, Seit-Nebi AS, Gusev NB. Structure, properties, and functions of the human small heat-shock protein HSP22 (HspB8, H11, E2IG1): a critical review. J Neurosci Res. 2008;86(2):264–269. doi: 10.1002/jnr.21441. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Tanaka M, Atomi Y. Small heat shock protein αB-crystallin controls shape and adhesion of glioma and myoblast cells in the absence of stress. PLoS One. 2016;11(12):e0168136. doi: 10.1371/journal.pone.0168136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluchanko NN, Beelen S, Kulikova AA, Weeks SD, Antson AA, Gusev NB, Strelkov SV. Structural basis for the interaction of a human small heat shock protein with the 14-3-3 universal signaling regulator. Structure. 2017;25(2):305–316. doi: 10.1016/j.str.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobott F, Benesch JL, Vierling E, Robinson CV. Subunit exchange of multimeric protein complexes. Real-time monitoring of subunit exchange between small heat shock proteins by using electrospray mass spectrometry. J Biol Chem. 2002;277(41):38921–38929. doi: 10.1074/jbc.M206060200. [DOI] [PubMed] [Google Scholar]
- Stamler R, Kappé G, Boelens W, Slingsby C. Wrapping the α-crystallin domain fold in a chaperone assembly. J Mol Biol. 2005;353(1):68–79. doi: 10.1016/j.jmb.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Sudnitsyna MV, Mymrikov EV, Seit-Nebi AB, Gusev NB. The role of intrinsically disordered regions in the structure and functioning of small heat shock proteins. Curr Protein Pept Sci. 2012;13(1):76–85. doi: 10.2174/138920312799277875. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Tanaka R, Tokuhara M, Kunugi S, Lee Y-F, Hamada D. Amyloid fibril formation and chaperone-like activity of peptides from αA-crystallin. Biochemistry. 2008;47(9):2961–2967. doi: 10.1021/bi701823g. [DOI] [PubMed] [Google Scholar]
- Tartaglia GG, Pawar AP, Campioni S, Dobson CM, Chiti F, Vendruscolo M. Prediction of aggregation-prone regions in structured proteins. J Mol Biol. 2008;380(2):425–436. doi: 10.1016/j.jmb.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Taylor JD, Hawthorne WJ, Lo J, et al. Electrostatically-guided inhibition of Curli amyloid nucleation by the CsgC-like family of chaperones. Sci Rep. 2016;6:24656. doi: 10.1038/srep24656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn DC, Ecroyd H, Carver JA, Holt C. Casein structures in the context of unfolded proteins. Int Dairy J. 2015;46:2–11. doi: 10.1016/j.idairyj.2014.07.008. [DOI] [Google Scholar]
- Tompa P. Intrinsically disordered proteins: a 10-year recap. Trends Biochem Sci. 2012;37(12):509–516. doi: 10.1016/j.tibs.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Tompa P, Csermely P. The role of structural disorder in the function of RNA and protein chaperones. FASEB J. 2004;18(11):1169–1175. doi: 10.1096/fj.04-1584rev. [DOI] [PubMed] [Google Scholar]
- Tompa P, Schad E, Tantos A, Kalmar L. Intrinsically disordered proteins: emerging interaction specialists. Curr Opin Struc Biol. 2015;35:49–59. doi: 10.1016/j.sbi.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Treweek TM, Ecroyd H, Williams DM, Meehan S, Carver JA, Walker MJ. Site-directed mutations in the C-terminal extension of human αB-crystallin affect chaperone function and block amyloid fibril formation. PLoS One. 2007;2(10):e1046. doi: 10.1371/journal.pone.0001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treweek TM, Meehan S, Ecroyd H, Carver JA. Small heat-shock proteins: important players in regulating cellular proteostasis. Cell Mol Life Sci. 2015;72(3):429–451. doi: 10.1007/s00018-014-1754-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treweek TM, Rekas A, Walker MJ, Carver JA. A quantitative NMR spectroscopic examination of the flexibility of the C-terminal extensions of the molecular chaperones, αA- and αB-crystallin. Exp Eye Res. 2010;91(5):691–699. doi: 10.1016/j.exer.2010.08.015. [DOI] [PubMed] [Google Scholar]
- van de Klundert FA, Smulders RH, Gijsen ML, Lindner RA, Jaenicke R, Carver JA, de Jong WW. The mammalian small heat-shock protein Hsp20 forms dimers and is a poor chaperone. Eur J Biochem. 1998;258(3):1014–1021. doi: 10.1046/j.1432-1327.1998.2581014.x. [DOI] [PubMed] [Google Scholar]
- Walther DM, Kasturi P, Zheng M, et al. Widespread proteome remodeling and aggregation in aging C. elegans. Cell. 2015;161(4):919–932. doi: 10.1016/j.cell.2015.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks SD, Baranova EV, Heirbaut M, Beelen S, Shkumatov AV, Gusev NB, Strelkov SV (2014) Molecular structure and dynamics of the dimeric human small heat shock protein HSPB6. J Struct Biol 185(3):342–354. doi:10.1016/j.jsb.2013.12.009 [DOI] [PubMed]
- Wieske M, Benndorf R, Behlke J, Dölling R, Grelle G, Bielka H, Lutsch G. Defined sequence segments of the small heat shock proteins HSP25 and αB-crystallin inhibit actin polymerization. Eur J Biochem. 2001;268(7):2083–2090. doi: 10.1046/j.1432-1327.2001.02082.x. [DOI] [PubMed] [Google Scholar]
- Williams DM, Ecroyd H, Goodwin KL, et al. NMR spectroscopy of 14-3-3ζ reveals a flexible C-terminal extension: differentiation of the chaperone and phosphoserine-binding activities of 14-3-3ζ. Biochem J. 2011;437(3):493–503. doi: 10.1042/BJ20102178. [DOI] [PubMed] [Google Scholar]
- Wong YQ, Binger KJ, Howlett GJ, Griffin MD. Methionine oxidation induces amyloid fibril formation by full-length apolipoprotein AI. Proc Natl Acad Sci U S A. 2010;107(5):1977–1982. doi: 10.1073/pnas.0910136107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worth CL, Gong S, Blundell TL. Structural and functional constraints in the evolution of protein families. Nat Rev Mol Cell Biol. 2009;10(10):709–720. doi: 10.1038/nrm2762. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Lin Y, Yagi H, et al. Disthingusihing crystal-like amyloid fibrils and glass-like amorphous aggregates from their kinetics of formation. Proc Natl Acad Sci U S A. 2012;109(36):14446–14451. doi: 10.1073/pnas.1208228109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ZipperDB analysis (Goldschmidt et al. 2010) of the amino acid sequences of the ten human sHsps. The N-terminal, ACD, and C-terminal regions are segmented by the black vertical dashed lines in that sequential order. The blue, green, yellow and orange lines are hexapeptide residues that are of increasing Rosetta energy, whilst the red lines that cross the threshold of −23 kcal/mol (black horizontal line) are those residues associated with a hexapeptide that has a high propensity of forming amyloid fibrils. (DOCX 88 kb)
Comparison of the predicted fibril-forming or β-aggregation propensity of all ten human sHsps and two E. coli sHsps using three different prediction algorithms, i.e. ZipperDB (Goldschmidt et al. 2010), TANGO (Fernandez-Escamilla et al. 2004) and Zyggregator (Tartaglia et al. 2008). Percentages are given as the number of residues classified as having a ‘high propensity’ to form fibrils within a specific region over all the residues within that specific region of the protein, e.g. the N-terminal, ACD or C-terminal regions. (DOCX 38.5 kb)