Abstract
Iron Oxide (Fe3O4) nanoparticles were deposited on the surface of low density polyethylene (LDPE) particles by solvothermal method. A magnetic field was introduced to the preparation of Fe3O4/LDPE composites, and the influences of the magnetic field on thermal conductivity and dielectric properties of composites were investigated systematically. The Fe3O4/LDPE composites treated by a vertical direction magnetic field exhibited a high thermal conductivity and a large dielectric constant at low filler loading. The enhancement of thermal conductivity and dielectric constant is attributed to the formation of the conductive chains of Fe3O4 in LDPE matrix under the action of the magnetic field, which can effectively enhance the heat flux and interfacial polarization of the Fe3O4/LDPE composites. Moreover, the relatively low dielectric loss and low conductivity achieved are attributed to the low volume fraction of fillers and excellent compatibility between Fe3O4 and LDPE. Of particular note is the dielectric properties of Fe3O4/LDPE composites induced by the magnetic field also retain good stability across a wide temperature range, and this contributes to the stability and lifespan of polymer capacitors. All the above-mentioned properties along with the simplicity and scalability of the preparation for the polymer nanocomposites make them promising for the electronics industry.
Introduction
Owing to the rapid developments in modern science and technology, the capacitors with remarkable performance were urgently required in the field of electronics and electrical systems. Nowadays, outstanding-performance polymers, which possess a high dielectric constant and flexibility, but low dielectric loss, have attracted great attention owing to their potential application in many cutting-edge industries, including microelectronics, aerospace, and aviation1–3. In general, the polymers possess excellent flexibility and high breakdown strength, but their applications were limited by their low dielectric constant4, 5. The organic-inorganic hybrid strategy, including ceramic/polymer and conductive fillers/polymer composites have been widely adopted6–10. Unfortunately, the ceramic/polymer composites have a limited dielectric constant and often require a large filler loading (>60 vol.%), which causes poor flexibility and uniformity of the materials. The conductive fillers/polymer composites can exhibit very high dielectric constant, but with a relatively high dielectric loss due to the insulator-conductor transition near the percolation threshold, which significantly restricts its practical application.
With the miniaturization of microelectronics and associated increase in power densities, the internal thermal stability of integrated microelectronic devices has become one of the critical factors in achieving advanced performance and extended lifespan11–13. It is crucial for the heat generated from electronics to be dissipated as quickly and effectively as possible, which can maintain the operating temperatures of the electronic equipment at a desired level, because the dielectric strength will decrease with increasing temperature owing to the poor thermal conduction of these dielectric materials. Notably, traditional high dielectric composites would lose their electromechanical stability and displayed a large variation in dielectric constant as well as dielectric loss at a broad temperature range, hindering their reliability and efficiency14.
Previous studies have shown that the polymeric composites, which were integrated with high thermal conductivity fillers, such as metal (Cu)15, oxide(Al2O3)16, aluminum nitride (AlN)17, carbide(SiC)18, and carbon nanotubes19, can endow themselves with superior properties. For example, Cu-filled low-density polyethylene (LDPE) composites were studied by Luyt et al.15 and they found that the thermal conductivity of the composites was 0.35 W m−1 K−1 when the volume fraction of the Cu particles was 7.0 vol.%. Fang et al.18 prepared different dimensional SiC particles to be filled into LDPE composites, and realized that the thermal conductivity of the composites was 0.37 W m−1 K−1 at 10 vol.% SiC content. High thermal conductivity of the composites usually requires a high volume fraction of fillers, and provision of a low dielectric constant, which is not suitable for use for microelectronics. Additionally, most research has just focused on one single side of thermal conductivity or dielectric property of nanocomposites at room temperature. Few in-depth explorations of the cooperative effect of a large thermal conductivity and a high dielectric constant for polymer materials under broader temperature conditions have been investigated until now, and their influential mechanism is still uncertain. Beyond that, how to improve the thermal conductivity and the dielectric performance of composites at low filler loading is one of the key issues.
The external electric and magnetic field could significantly influence the polymer’s molecular arrangement and the conductive particles’ distribution of the polymer composites, in the end the microstructure and macro-properties of the composites are influenced20–22. Hence, in this research, the Fe3O4-LDPE particles were prepared by solvothermal reaction and then the surface was modified by polydopamine (PDA). On this basis, we treated the Fe3O4/LDPE composites under a constant magnetic field for 30 min at 130 °C. Henceforth, the composites of Fe3O4/LDPE treated by the magnetic field can be abbreviated as M-Fe3O4/LDPE, here, “M” means that there has been magnet field treatment applied on the samples. The influence of the external magnetic field on the dielectric properties and thermal conductivity of Fe3O4/LDPE composites were studied intensively. It can be found that the orientation of the Fe3O4 nanoparticles was remote controlled by the external magnetic field because of its ultra-high magnetic response. Compared with the Fe3O4/LDPE composites, the M-Fe3O4/LDPE composites exhibit higher thermal conductivity and dielectric properties at 7.0 vol.% concentration, and the relevant mechanisms are discussed in detail.
Results and Discussion
Morphology of Fe3O4-LDPE particles and LDPE composites
The morphology of the Fe3O4-LDPE particles is directly illustrated by scanning electron microscope (SEM) images, as shown in Fig. 1a. The LDPE particles have a non-spherical shape and with an average diameter of about 90 μm, and the Fe3O4 nanoparticles are uniformly deposited on LDPE particles. Figure 1b shows the X-ray diffraction (XRD) curves of the Fe3O4/LDPE composites with 7.0 vol.% Fe3O4 fillers. The characteristic diffraction peaks of Fe3O4 appear at 2θ = 30.18°, 35.56°, 43.24°, 53.54°, 57.10°, and 62.47°, corresponding to the diffraction peaks from (220), (311), (440), (422), (511), and (440), respectively. The XRD patterns of the composites clearly indicate that the Fe3O4 particles filled the polymer matrix.
Figure 1.
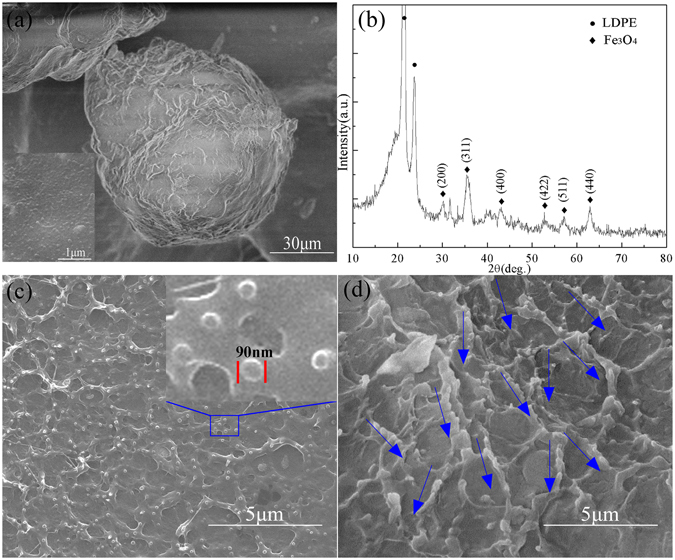
(a) SEM images of the Fe3O4-LDPE particles. The inset shows the partial SEM image of the Fe3O4-LDPE particles. (b) XRD pattern of the Fe3O4/LDPE composites at 7 vol.% concentration. (c) Cross-sectional SEM images of the Fe3O4/LDPE composites at 7 vol.% concentration. The inset shows the partial SEM image of the Fe3O4/LDPE composites. (d) Cross-sectional SEM images of the M-Fe3O4/LDPE composites at 7 vol.% concentration.
The SEM image of the freeze-fractured cross-sections of the LDPE-based composite films containing 7 vol.% Fe3O4 nanoparticles is displayed in Fig. 1c. The Fe3O4 nanoparticles with an the average size of about 90 nm are embedded in the LDPE matrix without agglomeration, and there is no evidence of defects and voids in the Fe3O4/LDPE composites, which is due to the solvothermal reaction process of the Fe3O4-LDPE particles lowering the probability of the agglomeration of the Fe3O4 nanoparticles. The surfactant treatment of PDA greatly improved the compatibility of the Fe3O4 nanoparticles and LDPE matrix23. Figure 1d shows the SEM morphology of the fractured cross-surface of M-Fe3O4/LDPE composite at 7 vol.% concentration. It is clearly found that some Fe3O4 particles come into contact with each other and become short chains along the magnetic direction. That is, the distribution of the Fe3O4 particles in the LDPE matrix is changed and the chains of the Fe3O4 particles are formed under the action of the magnetic field.
Thermal conductivity of Fe3O4/LDPE composites and M-Fe3O4/LDPE composites
In this research, the Fe3O4-LDPE particles were prepared via a solvothermal reaction before preparing the Fe3O4/LDPE composites. To highlight the advantages of this method, the comparison of the thermal conductivity of the Fe3O4/LDPE composites prepared by a simple mixing method and solvothermal reaction is shown in Fig. 2. Compared with the simple mixing method, the Fe3O4/LDPE composites prepared by solvothermal reaction show a higher thermal conductivity at the same filler content. For instance, the maximum thermal conductivity of the Fe3O4/LDPE composites via the solvothermal reaction is 0.384 W m−1 K−1 at 7.0 vol.% concentration, higher than that of Fe3O4/LDPE composites prepared by a simple mixing method (0.350 W m−1 K−1). As shown in the inset of Fig. 2, the voiding occurs due to the agglomeration and the weak compatibility of the Fe3O4 nanoparticles and LDPE matrix prepared by the simple mixing method, which leads to poor thermal conductivity of the Fe3O4/LDPE composites. This also indicated that the solvothermal treatment could effectively improve the dispersion of the Fe3O4 nanoparticles in the LDPE matrix.
Figure 2.
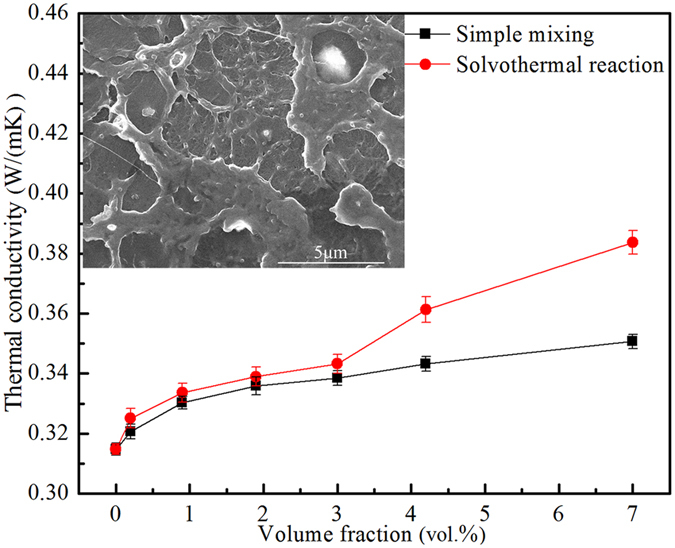
Comparison of thermal conductivity of Fe3O4/LDPE composites prepared by simple mixing method and solvothermal reaction. The inset shows the cross-sectional SEM images of the Fe3O4/LDPE composites at 7 vol.% concentration prepared by simple mixing method.
The curves of thermal conductivity of the composites versus filler content are displayed in Fig. 3. All of the composites show an enhancement in thermal conductivity with increasing Fe3O4 concentration. Moreover, a non-linear increase in thermal conductivity is also observed. The higher thermal conductivity of the composites should result from the higher intrinsic thermal conductivity of the Fe3O4 particles in comparison with the LDPE matrix24, 25. Importantly, the thermal conductivity of the M-Fe3O4/LDPE composites are much higher than that of the Fe3O4/LDPE composites at the same loading. For instance, the thermal conductivity of the M-Fe3O4/LDPE composites is 0.461 W m−1 K−1 at 7.0 vol.% concentration, higher than that of the Fe3O4/LDPE composites (0.384 W m−1 K−1), and this value is superior to that of many previous reports15, 18, 20, 26–28. As shown in Table 1, the thermal conductivity of our composites is larger than that of the high-density polyethylene/fly-ash (HDPE/FA) composites containing 10 vol.% FA (≈0.41 W m−1 K−1)27. It should be pointed out that the filler content of the M-Fe3O4/LDPE composites is smaller than that of other literature materials, with better flexibility.
Figure 3.
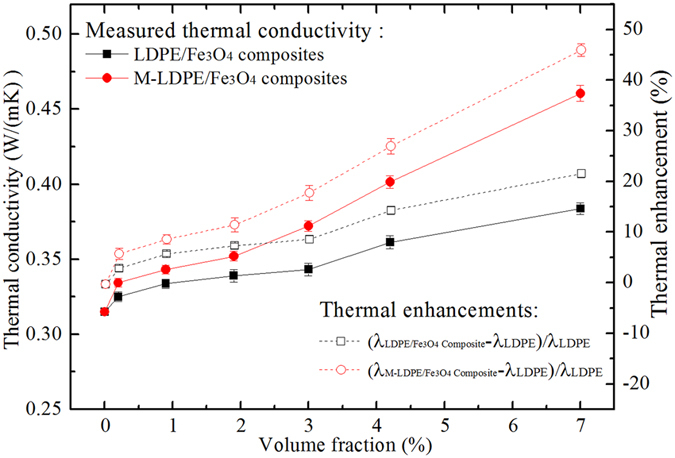
Thermal conductivity of the Fe3O4/LDPE and M-Fe3O4/LDPE composites corresponding to thermal enhancements.
Table 1.
Comparison of the thermal conductivity of our composites and reported literature materials.
Figure 3 also presents the thermal enhancements of the Fe3O4/LDPE and M-Fe3O4/LDPE composites with pure LDPE, respectively. Herein, the thermal enhancement is defined as the percentage of thermal conductivity improvement by Fe3O4 20, 29. It can be found that the thermal enhancement of the Fe3O4/LDPE and M-Fe3O4/LDPE composites increases initially with the filler content, reaching 21.88% and 46.27% at 7.0 vol.% concentration, respectively. The M-Fe3O4/LDPE composites show a better heat conduction effect than the Fe3O4/LDPE composites, owing to the formation of efficient thermal pathways made by the Fe3O4 oriented parallel to the heat flux under the action of the magnetic field (see Fig. 1d).
To further explore the thermal conductivity behavior of the Fe3O4/LDPE and M-Fe3O4/LDPE composites, Maxwell-Eucken’s model has been proposed and the comparisons between the experimental and theoretical values were made30. Maxwell-Eucken assumes that filler particles are homogeneously distributed in the polymer matrix, non-interacting, and roughly spherical. Fewer filler particles would be covered by the polymer and dispersed in the form of isolated islands in the matrix:
| 1 |
where V f is the volume fraction of the filler, and λ c, λ f, and λ p is the thermal conductivity of composites, filler, and matrix, respectively31, 32. In this paper, the value of λ f and λ p used 6.032 W m−1 K−1 and 0.315 W m−1 K−1, respectively.
Figure 4 gives the comparison between the experimental data and the thermal conductivity predicted by the Maxwell-Eucken’s model of the composites. For the Fe3O4/LDPE composites, Fe3O4 nanoparticles are dispersed randomly in the Fe3O4/LDPE composites (shown in Fig. 1c), and the theoretical values match well with the experimental data. However, it is interesting to note that the theoretical values of the M-Fe3O4/LDPE composites are lower than those observed in the experiments. This deviation could be attributed to the easier formation of the thermal conductive net-chain by the interaction of Fe3O4 in the LDPE matrix under the action of the magnetic field.
Figure 4.
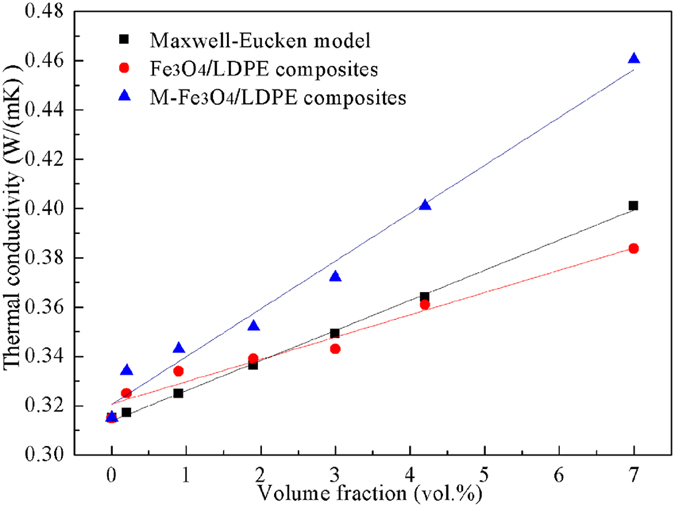
Thermal conductivity of the Fe3O4/LDPE and M-Fe3O4/LDPE composites based on experimental data and Maxwell-Eucken’s model.
Owing to the Maxwell-Eucken model not matching well with the M-Fe3O4/LDPE composites, two heat conduction models (parallel and vertical models) were brought forward to judge the heat flow direction in the polymer composites, based on the formation of the thermal conductive net-chain and mutual interaction of particles33, 34. Considering the crystallinity of the filler and polymer, Agari’s model was created based on a hypothesis of homogeneous dispersion of particles in the polymer:
| 2 |
where C 1 is the constant, which is related to the crystallinity and crystalline dimension of a polymer, and C 2 is the free factor, which indicates the ability of forming a heat conductive net-chain for fillers. C 2 would significant change due to the increase of the particle filling; therefore, Agari’s model should be modified and verified as the following32, 35:
| 3 |
where C p is the formation of the thermal conductive chain free particles, and C f is the reflecting particle formation of the thermal conductivity of difficulty.
Table 2 shows the C p and C f for the above two composites. It can be found that the C p of the M-Fe3O4/LDPE composites did not change significantly compared with the Fe3O4/LDPE composites. However, the C f of the M-Fe3O4/LDPE composites was greater than that of the Fe3O4/LDPE composites. The factor C f related to the ease in forming conductive chains of the filler, that is, the magnetic field shows a strong effect on C f. This also indicates that the external magnetic field could effectively enhance the formation of thermal conductive paths in the LDPE matrix, which is consistent with the results shown in Fig. 1d. In addition, R2 stands for the fitting degree between the theoretical data and the experimental data. The R2 value of the M-Fe3O4/LDPE composites is 0.98522, higher than that of the Fe3O4/LDPE composites (0.96233), showing that the experimental results for the M-Fe3O4/LDPE composites had a better fitting effect than the Fe3O4/LDPE composites. Besides, as shown in Fig. 1d, some changes have taken place in the structure of the LDPE matrix. This phenomenon also indicated that the external magnetic field has some influence on the microstructure of polymer matrix, the similar result has been reported in numerous previous studies36–38. It may be one of the reasons for the thermal conductivity enhancement of M-Fe3O4/LDPE composites.
Table 2.
Values of C p and C f for the Fe3O4/LDPE and M-Fe3O4/LDPE composites.
| Composites | A | B | C p | C f | R2 |
|---|---|---|---|---|---|
| Fe3O4/LDPE | 1.11624 | −0.55891 | 1.02028 | 0.87656 | 0.96233 |
| M-Fe3O4/LDPE | 2.19564 | −0.49094 | 1.02505 | 1.72694 | 0.98522 |
Dielectric properties of Fe3O4/LDPE composites and M-Fe3O4/LDPE composites
The thermal conductivity of the Fe3O4/LDPE composites is enhanced by the formation of conductive chains of the Fe3O4 filler under the action of the magnetic field. Moreover, the distribution of the Fe3O4 nanoparticles also affects the dielectric properties of the corresponding materials. Figure 5 shows the denpendence of volume fraction and dielectric properties of the Fe3O4/LDPE and M-Fe3O4/LDPE composites at 10 Hz and room temperature. It can be found that the dielectric constant of the composites increases with gradually increasing filler content. The maximum dielectric constant of the Fe3O4/LDPE composites is 4.45 when the content of Fe3O4 is 7.0 vol.%. In comparison with pure LDPE (ε = 2.3), the dielectric constant of the composite is nearly 2 times higher. This demonstrates that incorporating conducting fillers into the polymer matrix results in an increase in dielectric constant. Moreover, the dielectric constant of the M-Fe3O4/LDPE is higher than the Fe3O4/LDPE composites at the same concentration. In particular, the dielectric constant of the M-Fe3O4/LDPE composites reaches 51 at 7.0 vol.% concentration, which is 11.6 times higher than that of the Fe3O4/LDPE composites. Fe3O4 nanoparticles are able to move easily and touch together to form clusters in a melting state of the polymer matrix (at 130 °C), and these clusters would array in parallel along the direction of the magnetic field21, 22. The oriented distribution of the Fe3O4 clusters is beneficial for the formation of a conductive network in this direction, which can be regarded as a great amount of parallel-connected micro-capacitors, resulting in a greatly enhanced dielectric constant39, 40. It should be noted that the dielectric properties in our present study is even higher than those of many previous reports21, 23, 41–43. For example, as shown in Table 3, the obtained dielectric constant is higher than that of BZT-BCT/PVDF composites containing 24 vol.% BZT-BCT (ɛ = 37.2 at 10 Hz)41. Moreover, the amount of filler in the M-Fe3O4/LDPE composites is smaller than that in other materials described in the literature, and displayed better flexibility. For embedded capacitor applications, the dielectric loss is an essential parameter. The dielectric loss measured at a certain frequency includes polarization loss and conduction loss44. The dielectric loss tangent for the M-Fe3O4/LDPE composites remain below 0.25 at 10 Hz, and the conductivity of the M-Fe3O4/LDPE composites is also kept at a low value (7 × 10−11 S/cm). These attributed to the improvement of the interfacial adhesion and compatibility between the filler and the matrix by surface treatment of the PDA, no complete conducting path was formed in the composites (shown in Fig. 1d), resulting in a low dielectric loss and low conductivity of the composites within the acceptable ranges45. For example, the achieved dielectric loss and conductivity were found to be significant smaller than that of PEG-Fe3O4/PVDF composites containing 7.5 vol.% PEG-Fe3O4 fillers43.
Figure 5.
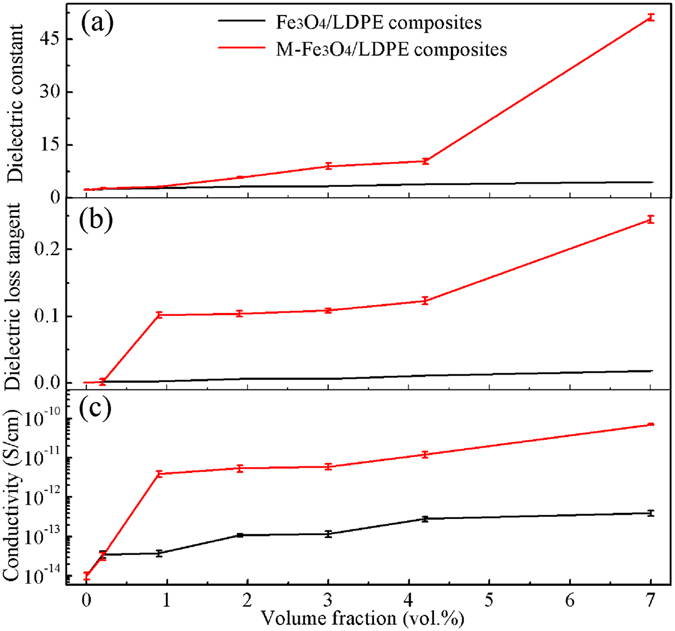
(a) Dielectric constant (b) dielectric loss tangent and (c) conductivity of the Fe3O4/LDPE and M-Fe3O4/LDPE composites at different volume fraction of Fe3O4 filler at 10 Hz.
Table 3.
Comparison of the dielectric properties of our composites and reported literature materials.
| Composites | ε | tan δ | σ (S/cm) | f (vol.%) | Ref. |
|---|---|---|---|---|---|
| Fe3O4/LDPE | 4.45 | 0.0188 | 3.96 × 10−13 | 7 | Our work |
| M-Fe3O4/LDPE | 51 | 0.25 | 7 × 10−11 | 7 | Our work |
| PVDF/Ni | 45 | 0.2 | — | 5 | [21] |
| P(VDF-HFP)/BT-PDA-Ag | ≈44 | 0.213 | — | 20 | [23] |
| BZT-BCT/PVDF | 37.2 | 0.08 | 1.5 × 10−9 | 24 | [41] |
| Fe3O4/SiO2/BECy | 9.5 | 0.09 | — | 20 | [42] |
| PEG-Fe3O4/PVDF | ≈80 | ≈1.25 | ≈7.0 × 10−9 | 7.5 | [43] |
In addition to the movement of particles under the magnetic field, the dielectric relaxation is also likely to affect the dielectric properties. To clarify the mechanism of the dielectric behaviors of the Fe3O4/LDPE and M-Fe3O4/LDPE composites, an electric modulus formalism based analysis of the dielectric relaxation in the composites has been proposed. Complex dielectric modulus formalism M* is demonstrated as follows:46, 47
| 4 |
where M′ is the real and M″ the imaginary part of the electric modulus, respectively. The imaginary part M″ of the electric modulus takes the form of loss curves, allowing us to interpret the relaxation phenomena.
The frequency dependence of M′ and M″ for the Fe3O4/LDPE and M-Fe3O4/LDPE composites is shown in Fig. 6. The change regularity of the Fe3O4/LDPE composites is insignificant with the increasing of Fe3O4 content (Fig. 6a). However, for the M-Fe3O4/LDPE composites, it can be seen that M′ decreases with the increasing of Fe3O4 content (Fig. 6b), and increases with frequency; this behavior is similar to other polymer composites containing conducting fillers48, 49. As shown in Fig. 6c and d, compared with the Fe3O4/LDPE composites, obvious interfacial polarization relaxation peaks occur for the M-Fe3O4/LDPE composites when the Fe3O4 content is higher than 1.9 vol.%. Moreover, the relaxation strength of the composites decreases with the increase of Fe3O4 loading, and the relaxation peak moved toward higher frequency as the Fe3O4 content increased. The inter-particle distance would decrease as the volume fraction of the Fe3O4 increased, and as a result, the probability of Fe3O4 nanoparticles coming into contact increased because of the external magnetic field. As shown in Fig. 1d, some Fe3O4 particles come into contact with each other and become short chains along the direction of the magnetic field, which leads to a higher possibility for the charge carriers to accumulate on the interface between Fe3O4 and LDPE, the polarization and dielectric response are greatly enhanced under the electric field22. Therefore, a high dielectric constant of M-Fe3O4/LDPE composites is achieved at a low volume fraction.
Figure 6.
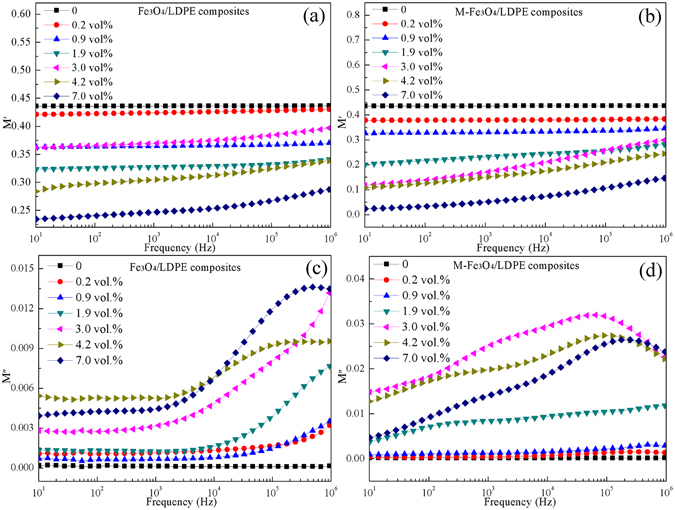
Frequency dependence of the (a,b) real parts and (c,d) imaginary parts of the complex electric modulus for the Fe3O4/LDPE and M-Fe3O4/LDPE composites.
For polymer capacitors, it is essential that the dielectric properties of the composites retain excellent stability across a wide temperature range11, 14. In this research, the temperature dependence of the dielectric constant and dissipation factor of the Fe3O4/LDPE and M-Fe3O4/LDPE composites at 10 Hz are given in Fig. 7. It can be found that a minor variation in dielectric constant of the M-Fe3O4/LDPE composites occurs with the increasing of temperature. The M-Fe3O4/LDPE composites at 7.0 vol.% Fe3O4 concentration display the largest dielectric constant (>51) across the whole temperature ranges. However, for the Fe3O4/LDPE composites, an obvious increasing trend occurred when the temperature was over 100 °C. Concurrently, the dissipation factor of the M-Fe3O4/LDPE composites at 10 Hz also remained at a slightly low value and maintained good stability across the whole temperature range, but an appreciable change has been observed in the Fe3O4/LDPE composites at 7.0 vol.% concentration. It is also evident that, of the dielectric assessed, the M-Fe3O4/LDPE composites offer a stable dielectric constant and dissipation factor at high temperature. This is attributed to the fact that the higher thermal conductivity of the M-Fe3O4/LDPE composites can effectively depress the dielectric loss and conductivity. It also indicates that the magnetic field could largely enhance the dielectric-temperature stability of the Fe3O4/LDPE composites across broad temperature range. There is an undoubtable benefit to the use of Fe3O4/LDPE composites across a wider temperature range in electronics.
Figure 7.
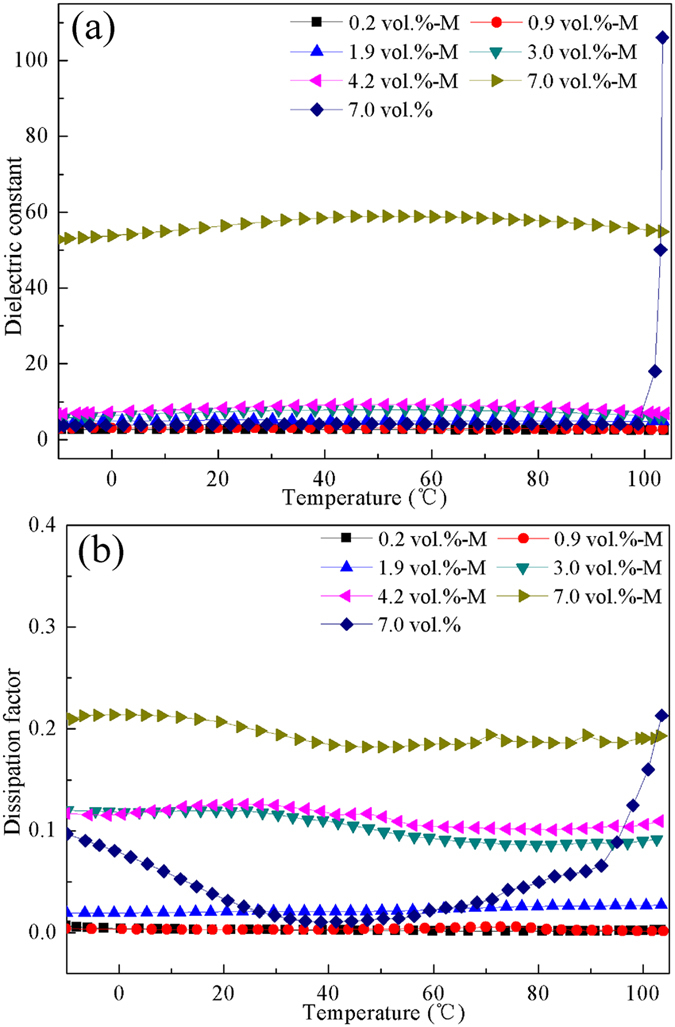
Temperature dependence of (a) dielectric constant and (b) dissipation factor of the Fe3O4/LDPE and M-Fe3O4/LDPE composites at different volume fraction of Fe3O4 filler at 10 Hz.
In our present study, Fe3O4 nanoparticles adopted a directional arrangement along the direction of the magnetic field, and formed a conductive network in the LDPE matrix under the action of a magnetic field, resulting in greatly enhanced thermal conductivity. Moreover, the interfacial polarization of the Fe3O4/LDPE composites is effectively enhanced under magnetic field treatment, and the maximum dielectric constant of the composites reaches 51 at 7.0 vol.% concentration. Additionally, the low volume fraction of the fillers and the excellent compatibility of the Fe3O4 nanoparticles and LDPE matrix, resulted in a relatively low dielectric loss (0.25) and a low conductivity (7 × 10−11 S/cm). Moreover, the dielectric properties of the M-Fe3O4/LDPE composites retain good stability across a wide temperature range (−10 °C~105 °C), resulting from the high thermal conductivity of the Fe3O4/LDPE composites induced by the magnetic field. This good heat dissipation capability prolonged the lifespan of the polymer dielectrics at a higher operating temperature.
Conclusion
In summary, Fe3O4-deposited LDPE hybrid particles were prepared by a solvothermal reaction, and the corresponding Fe3O4/LDPE composites were also prepared. SEM images show that Fe3O4 nanoparticles were embedded in the LDPE matrix without agglomeration and defects. The magnetic field enhanced the probability of forming Fe3O4 conductive chains, which can effectively enhance the heat flux and interfacial polarization of Fe3O4/LDPE composites. The Fe3O4/LDPE composites induced by the magnetic field exhibited higher thermal conductivity and a higher dielectric constant in comparison with the Fe3O4/LDPE composites at the same filler content. Moreover, the relatively low dielectric loss and low conductivity are attributed to the low filler content and the improvement compatibility of the Fe3O4 nanoparticles and LDPE matrix by the surfactant treatment of the PDA. The low filler content leads to good mechanical properties and material processibility. Additionally, the dielectric properties of the M-Fe3O4/LDPE composites also retain good stability across a wide temperature range, due to the high thermal conductivity of the Fe3O4/LDPE composites induced by the magnetic field. This work establishes a facile, yet efficient approach to synthesize polymer materials with high thermal conductivity and high dielectric properties in order to make them suitable candidates for use in the electronics industry.
Materials and Methods
Preparation of Fe3O4 nanoparticles
The typical procedures for the preparation of the super-paramagnetic Fe3O4 nanoparticles were carried out as follows. Firstly, 1.442 g of FeSO4 • 7H2O and 2.472 g of FeCl3 • 6H2O were dissolved in 50 mL distilled water, respectively, then mixed together according to the mole ratio (Fe3+:Fe2+ = 2:1.134) to inhibit the oxidation of Fe2+. The iron solution and NaOH alkaline solution were slowly added to 100 mL distilled water in a 500 mL flask under vigorous stirring at 40 °C for 30 min, the pH of aqueous solution in the whole process was fixed at 12–13. After standing for about 1 h, the precipitate was washed with distilled water and ethanol until the pH reached neutral. The Fe3O4 nanoparticles were obtained and dried under vacuum at 60 °C for 12 h.
Preparation of Fe3O4-LDPE particles
The typical procedures for the preparation of the super-paramagnetic Fe3O4 deposited on the LDPE particles (Fe3O4-LDPE) were carried out as follows. Firstly, the desired amount of LDPE particles was poured into 175 ml ethanol, followed by the addition of Fe3O4 under vigorous stirring at room temperature for 15 min. Then, the obtained mixture was transferred into a 200 ml Teflon-lined stainless steel autoclave. The solvothermal reaction was kept at 120 °C for 4 h, then the precipitate was collected, and washed with distilled water and ethanol until the pH around 7. Finally, the Fe3O4-LDPE particles were obtained and dried under vacuum at 50 °C for 12 h. The volume fractions of Fe3O4 in the Fe3O4-LDPE particles were 0.2 vol.%, 0.9 vol.%,1.9 vol.%, 3.0 vol.%, 4.2 vol.%, and 7.0 vol.%, respectively.
Preparation of Fe3O4/LDPE composites
Before preparing the composites, the 0.6 g Fe3O4-LDPE particles were dispersed in a 200 mL Tris-HCl aqueous solution (10 × 10−3 M, pH = 8.5) followed by sonication for 1 h; then 0.2 g DA-HCl was added and sonicated for another 10 min. The mixture was then stirred vigorously at room temperature for 4 h, and the excess supernatant was removed after sedimentation for about 1 h. The Fe3O4-LDPE particles were obtained and dried under vacuum at 50 °C for 12 h. The Fe3O4/LDPE composites were obtained by a torque rheometer for 30 min at 130 °C and then molded by hot pressing at approximately 130 °C and 15 MPa for 20 min. The M-Fe3O4/LDPE composites were obtained by treating at 130 °C under a vertical direction magnetic field with a magnetic induction density of 1.0 T for 30 min. The preparation process of the Fe3O4/LDPE and M-Fe3O4/LDPE composites is shown in Fig. 8.
Figure 8.
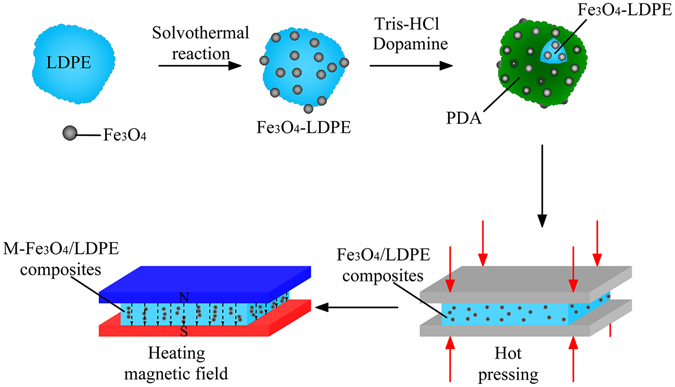
Schematic illustration of the preparation of the Fe3O4/LDPE and M-Fe3O4/LDPE composites.
Characterization
The morphology of the Fe3O4-LDPE particles and the microstructure of the LDPE composites were observed using SEM (Quanta 200 FEI). The phase compositions of the Fe3O4/LDPE composites were analyzed using XRD (Empyrean), using Cu Kα radiation at 40 kV and 40 mA. The thermal diffusivity (α) and specific heat (C) were measured on the disk samples with an LFA447 light flash system (NETZSCH, Selb, Germany) at 25 °C. The thermal conductivity was calculated by λ = αCρ, in which ρ is the density of the Fe3O4/LDPE composite. Prior to performing dielectric measurements, a layer of Al paste (diameter 25 mm) was evaporated on both surfaces to serve as electrodes. Dielectric measurements were employed across a frequency range from 10 to 106 Hz and a temperature range of −10 °C to 105 °C using a broad frequency dielectric spectrometer (Novocontrol Alpha-A).
Acknowledgements
The authors thanks the support of the National Science Foundation of China (61640019), the Open Foundation of State Key Laboratory of Electronic Thin Films and Integrated Devices (KFJJ201601), Science Funds for the Young Innovative Talents of HUST (201102).
Author Contributions
The research was planned by Q.G.C.; Experiments were performed by T.M. and J.F.D.; Q.G.C. and T.M. prepared the manuscript; Y.C. and Y.Z. prepared figures in all paper; C.H.Z. and Q.G.C. analyzed the results; S.C.X., X.W. and Q.Q.L. checked the scientific information and flow of the text to maintain a better readability; All the authors participated in discussing and reviewing of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zheng MS, et al. Enhanced breakdown strength of poly(vinylidene fluoride) utilizing rubber nanoparticles for energy storage application. Appl. Phys. Lett. 2016;109:072902. doi: 10.1063/1.4961252. [DOI] [Google Scholar]
- 2.Rocío PO, Antonio F, Marks TJ. High-k organic, inorganic, and hybrid dielectrics for low-voltage organic field-effect transistors. Chem. Rev. 2010;110:205–239. doi: 10.1021/cr9001275. [DOI] [PubMed] [Google Scholar]
- 3.Chi QG, et al. Effects of Zr doping on the microstructures and dielectric properties of CaCu3Ti4O12 ceramics. J. Alloy Compd. 2013;559:45–48. doi: 10.1016/j.jallcom.2013.01.090. [DOI] [Google Scholar]
- 4.Dang ZM, et al. Fundamentals, processes and applications of high-permittivity polymer–matrix composites. Prog. Mater. Sci. 2012;54:660–723. doi: 10.1016/j.pmatsci.2011.08.001. [DOI] [Google Scholar]
- 5.Zhang CH, et al. Enhanced dielectric properties of poly(vinylidene fluoride) composites filled with nano iron oxide-deposited barium titanate hybrid particles. Sci. Rep. 2016;6:33508. doi: 10.1038/srep33508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang WH, Yu SH, Sun R, Du DX. Nano- and microsize effect of CCTO fillers on the dielectric behavior of CCTO/PVDF composites. Acta Mater. 2011;59:5593–5602. doi: 10.1016/j.actamat.2011.05.034. [DOI] [Google Scholar]
- 7.Chi QG, et al. Enhanced dielectric performance of amorphous calcium copper titanate/polyimide hybrid film. J. Mater. Chem. C. 2014;2:172–177. doi: 10.1039/C3TC31757A. [DOI] [Google Scholar]
- 8.Luo BC, Wang XH, Wang YP, Li LT. Fabrication, characterization, properties and theoretical analysis of ceramic/PVDF composite flexible films with high dielectric constant and low dielectric loss. J. Mater. Chem. A. 2014;2:510–519. doi: 10.1039/C3TA14107A. [DOI] [Google Scholar]
- 9.Dang ZM, Zheng MS, Zha JW. 1D/2D Carbon Nanomaterial-Polymer Dielectric Composites with High Permittivity for Power Energy Storage Applications. Small. 2016;12:1688–1701. doi: 10.1002/smll.201503193. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Dang ZM. Carbon nanotube composites with high dielectric constant at low percolation threshold. Appl. Phys. Lett. 2005;87:042903. doi: 10.1063/1.1996842. [DOI] [Google Scholar]
- 11.Li Q, et al. Flexible high-temperature dielectric materials from polymer nanocomposites. Nature. 2015;523:576–579. doi: 10.1038/nature14647. [DOI] [PubMed] [Google Scholar]
- 12.Zha JW, et al. Tuning of thermal and dielectric properties for epoxy composites filled with electrospun alumina fibers and graphene nanoplatelets through hybridization. J. Mater. Chem. C. 2015;3:7195–7202. doi: 10.1039/C5TC01552A. [DOI] [Google Scholar]
- 13.Schelling PK, Li S, Goodson KE. Managing heat for electronics. Mater. Today. 2005;8:30–35. doi: 10.1016/S1369-7021(05)70935-4. [DOI] [Google Scholar]
- 14.Zhou WY, et al. Effect of the particle size of Al2O3 on the properties of filled heat-conductive silicone rubber. J. Appl. Polym. Sci. 2007;104:1312–1318. doi: 10.1002/app.25789. [DOI] [Google Scholar]
- 15.Luyt AS, Moldefi JA, Krump H. Thermal, mechanical and electrical properties of copper powder filled low-density and linear low-density polyethylene composites. Polym. Degrad. Stabil. 2006;91:1629–1636. doi: 10.1016/j.polymdegradstab.2005.09.014. [DOI] [Google Scholar]
- 16.Yang Y, He JL, Wu GN, Hu J. “Thermal Stabilization Effect” of Al2O3 nano-dopants improves the high-temperature dielectric performance of polyimide. Sci. Rep. 2015;5:16986. doi: 10.1038/srep16986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Min C, Yu D, Cao J, Wang G, Feng L. A graphite nanoplatelet/epoxy composite with high dielectric constant and high thermal conductivity. Carbon. 2013;55:116–125. doi: 10.1016/j.carbon.2012.12.017. [DOI] [Google Scholar]
- 18.Ren F, Ren PG, Di YY, Chen DM, Liu GG. Thermal, Mechanical and Electrical Properties of Linear Low-Density Polyethylene Composites Filled with Different Dimensional SiC Particles. Polym. Plast. Technol. 2011;50:791–796. doi: 10.1080/03602559.2011.551967. [DOI] [Google Scholar]
- 19.Zeng JL, et al. Effects of MWNTs on phase change enthalpy and thermal conductivity of a solid-liquid organic PCM. J. Therm. Anal. Calorim. 2009;95:507–512. doi: 10.1007/s10973-008-9275-9. [DOI] [Google Scholar]
- 20.Yuan C, et al. Thermal Conductivity of Polymer-Based Composites with Magnetic Aligned Hexagonal Boron Nitride Platelets. ACS Appl. Mater. Interfaces. 2015;7:13000–13006. doi: 10.1021/acsami.5b03007. [DOI] [PubMed] [Google Scholar]
- 21.Li WP, Yu LJ, Zhu YJ, Hua DY. External Magnetic Field Induced Percolation in Polyvinylidene Fluoride and Nickel Composites. J. Phys. Chem. C. 2010;114:14004–14007. doi: 10.1021/jp103086y. [DOI] [Google Scholar]
- 22.Chi QG, et al. Nano iron oxide-deposited calcium copper titanate/polyimide hybrid films induced by an external magnetic field: toward a high dielectric constant and suppressed loss. J. Mater. Chem. C. 2016;4:8179–8188. doi: 10.1039/C6TC01655C. [DOI] [Google Scholar]
- 23.Yang K, Huang XY, He JL, Jiang PK. Strawberry-like Core-Shell Ag@Polydopamine@BaTiO3 Hybrid Nanoparticles for High-k Polymer Nanocomposites with High Energy Density and Low Dielectric Loss. Adv. Mater. Interfaces. 2015;2:1500361. doi: 10.1002/admi.201500361. [DOI] [Google Scholar]
- 24.Li Y, et al. Large Dielectric Constant and High Thermal Conductivity in Poly(vinylidene fluoride)/Barium Titanate/Silicon Carbide Three-Phase Nanocomposites. ACS Appl. Mater. Interfaces. 2011;3:4396–4403. doi: 10.1021/am2010459. [DOI] [PubMed] [Google Scholar]
- 25.Jin WQ, Yuan L, Liang GZ, Gu J. Multifunctional cyclotriphosphazene/hexagonal boron nitride hybrids and their flame retarding bismaleimide resins with high thermal conductivity and thermal stability. ACS Appl. Mater. Interfaces. 2014;6:14931–14944. doi: 10.1021/am502364k. [DOI] [PubMed] [Google Scholar]
- 26.Lee GW, Park M, Kim JY, Lee JI, Yoon HG. Enhanced thermal conductivity of polymer composites filled with hybrid filler. Compos. Part A-Appl. S. 2006;37:727–734. doi: 10.1016/j.compositesa.2005.07.006. [DOI] [Google Scholar]
- 27.Baglari S, Kole M, Dey TK. Effective thermal conductivity and coefficient of linear thermal expansion of high-density polyethylene-flyash composites. Indian J. Phys. 2011;85:559–573. doi: 10.1007/s12648-011-0059-x. [DOI] [Google Scholar]
- 28.Xie SH, Zhu BK, Li JB, Wei XZ, Xu ZK. Preparation and properties of polyimide/aluminum nitride composites. Polym Test. 2004;23:797–801. doi: 10.1016/j.polymertesting.2004.03.005. [DOI] [Google Scholar]
- 29.Lin ZY, et al. Magnetic alignment of hexagonal boron nitride platelets in polymer matrix: toward high performance anisotropic polymer composites for electronic encapsulation. ACS Appl. Mater. Interfaces. 2013;5:7633–7640. doi: 10.1021/am401939z. [DOI] [PubMed] [Google Scholar]
- 30.Su NC, Smith ZP, Freeman BD, Singh JJ. Size-Dependent Permeability Deviations from Maxwell’s Model in Hybrid Cross-Linked Poly(ethylene glycol)/Silica Nanoparticle Membranes. Chem. Mater. 2015;27:2421–2429. doi: 10.1021/cm504463c. [DOI] [Google Scholar]
- 31.Shen MX, Cui YX, He J. Thermal conductivity model of filled polymer composites. International Journal of Minerals Metallurgy & Materials. Int. J. Min. Met. Mater. 2011;18:623–631. doi: 10.1007/s12613-011-0487-9. [DOI] [Google Scholar]
- 32.Agari Y, Uno T. Estimation on Thermal Conductivities of Filled Polymers. J. Appl. Polym. Sci. 2003;32:5705–5712. doi: 10.1002/app.1986.070320702. [DOI] [Google Scholar]
- 33.Agari Y, Tanaka M, Nagai S, Uno T. Thermal conductivity of a polymer composite filled with mixtures of particles. J. Appl. Polym. Sci. 1987;34:1429–1437. doi: 10.1002/app.1987.070340408. [DOI] [Google Scholar]
- 34.Zhou YC, et al. The use of polyimide-modified aluminum nitride fillers in AlN@PI/epoxy composites with enhanced thermal conductivity for electronic encapsulation. Sci. Rep. 2014;4:4779. doi: 10.1038/srep04779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agari Y, Ueda A, Tanaka M, Nagai S. Thermal Conductivity of a Polymer Filled with Particles in the Wide Range From Low to Super-high Volume Content. J. Appl. Polym. Sci. 1990;5:929–941. doi: 10.1002/app.1990.070400526. [DOI] [Google Scholar]
- 36.Florian E, Thomas TA. Controlling the Orientantion of Semicrystalline Polymers by Crystallization in Magnetic Fields. Macromolecules. 2003;36:8685–8694. doi: 10.1021/ma034760g. [DOI] [Google Scholar]
- 37.Tsunehisa K. Study on the Effect of Magnetic Fields on Polymeric Materials and Its Application. Polym. J. 2003;35:823–843. doi: 10.1295/polymj.35.823. [DOI] [Google Scholar]
- 38.Vilens’kyi VO, Demchenko VL. Influence of the intensity of constant magnetic field on the structure and properties of composites based on epoxy polymers and Fe (III) or Al(III) oxides. Mater. Sci. 2009;45:409–416. doi: 10.1007/s11003-009-9199-y. [DOI] [Google Scholar]
- 39.Wang G. Enhanced dielectric properties of three-phase-percolative composites based on thermoplastic-ceramic matrix (BaTiO3 + PVDF) and ZnO radial nanostructures. ACS Appl. Mater. Interfaces. 2010;2:1290–1293. doi: 10.1021/am100296u. [DOI] [PubMed] [Google Scholar]
- 40.Xie LY, Huang XY, Huang YH, Yang K, Jiang PK. Core-shell structured hyperbranched aromatic polyamide/BaTiO3 hybrid filler for poly (vinylidene fluoride-trifluoroethylene-chlorofluoroethylene) nanocomposites with the dielectric constant comparable to that of percolative composites. ACS Appl. Mater. Interfaces. 2013;5:1747–1756. doi: 10.1021/am302959n. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y, et al. High performance of polyimide/CaCu3Ti4O12@Ag hybrid films with enhanced dielectric permittivity and low dielectric loss. J. Mater. Chem. A. 2015;3:4916–4921. doi: 10.1039/C4TA05673F. [DOI] [Google Scholar]
- 42.Ramani R, et al. A Free Volume Study on the Origin of Dielectric Constant in a Fluorine-Containing Polyimide Blend: Poly(vinylidene fluoride-co-hexafluoro propylene)/Poly(ether imide) J. Phys. Chem. B. 2014;118:12282–12296. doi: 10.1021/jp506039y. [DOI] [PubMed] [Google Scholar]
- 43.Chi QG, et al. Effect of particle size on the dielectric properties of 0.5Ba(Zr0.2Ti0.8)O3–0.5(Ba0.7Ca0.8)TiO3/polyvinylidene fluoride hybrid films. Ceram. Int. 2015;41:15446–15121. [Google Scholar]
- 44.Sun WX, et al. Multifunctional Properties of Cyanate Ester Composites with SiO2 Coated Fe3O4 Fillers. ACS Appl. Mater. Interfaces. 2013;5:1636–1642. doi: 10.1021/am302520e. [DOI] [PubMed] [Google Scholar]
- 45.Zhu JJ, et al. An ultrahigh dielectric constant composite based on polyvinylidene fluoride and polyethylene glycol modified ferroferric oxide. J. Phys. D: Appl. Phys. 2015;48:355301. doi: 10.1088/0022-3727/48/35/355301. [DOI] [Google Scholar]
- 46.Havriliak S, Negami SA. Complex Plane Representation of Dielectric and Mechanical Relaxation Processes in Some Polymers. Polymer. 1967;8:161–210. doi: 10.1016/0032-3861(67)90021-3. [DOI] [Google Scholar]
- 47.Yang WH, et al. Electrical modulus analysis on the Ni/CCTO/PVDF system near the percolation threshold. J. Phys. D:Appl. Phys. 2011;44:475305–475312. doi: 10.1088/0022-3727/44/47/475305. [DOI] [Google Scholar]
- 48.Laurati M, et al. Dynamics of Water Absorbed in Polyamides. Macromolecules. 2012;45:1676–1687. doi: 10.1021/ma202368x. [DOI] [Google Scholar]
- 49.Huang XY, et al. Temperature-dependent electrical property transition of graphene oxide paper. Nanotechnology. 2012;23:1–8. doi: 10.1155/2012/214783. [DOI] [PubMed] [Google Scholar]


