Abstract
Background
Severe asthma is largely unexplored in the Chinese population. Patients with asthma underwent systematic evaluation, by investigating the characteristics of uncontrolled asthma and of asthma treated with three different controller therapies.
Methods
This multi-centre, real-world study was conducted from March 2014 to September 2015. Adults with stable asthma underwent assessment of medication use, asthma control, quality of life, psychological symptoms, work productivity and activity impairment, bronchodilator response and sputum induction.
Results
Participants (n=379) had a mean (SD) age of 47.4 (14.0) years, and 57.0% were female. There were 14.8% (n=56) of patients receiving treatment with Step 4/5 as severe asthma, but only 13 (3.4%) met ERS/ATS severe refractory asthma criteria. The patients with severe asthma usually used triple controller therapy: ICS/LABA, additional leukotriene modifier or theophylline, and reported better asthma control. Two fifths of patients (n=147) had uncontrolled asthma, with worse symptoms, psychological symptoms (both P<0.001), health-related work productivity and activity impairment, increased eosinophilic inflammation in sputum [1.68% (0.0, 17.1%) vs. 0.2% (0.0, 1.3%), P<0.0001] and more exacerbations (P<0.05). Multiple regression analysis indicated that triple controller therapy significantly reduced the risk of uncontrolled asthma [OR =0.32, 95% CI =(0.14, 0.75)].
Conclusions
Although there is a relatively low proportion of severe refractory asthma based on ERS/ATS criteria, two of five patients with asthma in China are uncontrolled, displaying more psychological symptoms and reduced work productivity. Substantial gain in asthma control is obtained by triple controller therapy and this may be a promising therapeutic option for persistent asthma.
Keywords: Severe asthma, uncontrolled asthma, controller therapy
Introduction
Asthma has an increasing prevalence in China (1,2). Severe asthma and poorly controlled asthma remain important global issues (2). Population-based studies found that control of asthma was not achieved in the majority of Chinese patients with asthma (2). Severe asthma is an important subset of asthma (about 5–10%). It is difficult-to-treat and accounts for a large proportion of resource expenditure (3). Severe asthma is largely unexplored in the Chinese population.
Controller medications, such as inhaled corticosteroids (ICS) and long-acting beta2-agonists (LABA) are recommended for asthma, with escalating doses of ICS and LABA added for poor asthma control. Second line controllers including leukotriene receptor antagonists (LTRA), theophylline, and long-acting muscarinic agents (LAMA) can be effective (4,5), and some guidelines provide triple controller therapy as an option (6,7). This is an important issue since triple combination inhalers are currently in development. While use of triple controller therapy is common in chronic obstructive pulmonary disease (COPD), there is little data on this approach in asthma. This real-life study systematically evaluates severe asthma in China, by investigating the characteristics of uncontrolled asthma and of asthma treated with triple controller therapies.
Methods
Study oversight and design
This study was as a part with Chinese population from the Severe Asthma Web-based Database (SAWD) via secure web site to facilitate data collection, which was manipulated by the Australasian Severe Asthma Network (ASAN). ASAN provided training to site staff regarding the study protocol, data collection requirements and sputum induction, performed quality control with selected source data verification and performed the analysis.
Data was collected from three sites in Sichuan and Jilin provinces, China from March 2014 to September 2015. Adult patients (≥18 years of age) with stable asthma, and confirmed by variable airflow obstruction, were recruited from the outpatient clinics of West China Hospital, Sichuan University, the People’s Hospital of Jilin Province and No. 2 Affiliate Hospital, Jilin University. The subjects were excluded if they were pregnant, had cognitive impairment, current solid organ malignancy, or an inability to attend study visits. This study was conducted according to the International Conference on Harmonisation Good Clinical Practice Guidelines and was approved by the Hunter New England and University of Newcastle human research ethics committees and local Institutional Review Boards from China. All participants gave informed written consent.
Data collection and assessments
Baseline data were collected during a period of stable asthma and included demographics, medications, asthma history, the 6-item Asthma Control Questionnaire (ACQ-6) (8) and Asthma Quality of Life Questionnaire (AQLQ) (9). Spirometry was performed according to American Thoracic Society (ATS)/European Respiratory Society (ERS) standards. Predicted FEV1 and FVC were calculated using data from the Chinese population (10). Bronchodilator reversibility (BDR) was defined as a ≥12% or 200 mL improvement in FEV1 at 15 minutes following inhaled salbutamol 200 mcg.
Sputum was induced using nebulised 4.5% saline as described (11), with salbutamol 400 mcg (GSK, Avda de Extremadura, Spain) pre-treatment. If the baseline pre or post FEV1 was ≤40% of predicted, sputum induction was completed with 0.9% saline after it was deemed safe by the supervising physician. Selected sputum was dispersed using dithiothreitol, a total cell count performed, and cytospins prepared for differential cell count. Inflammatory phenotypes were classified as: eosinophilic (eosinophils ≥3%), neutrophilic (neutrophils >61%), paucigranulocytic (eosinophils <3% and neutrophils ≤61%) and mixed granulocytic (eosinophils ≥3% and neutrophils >61%).
Anxiety and depression symptoms were assessed using the Hospital Anxiety and Depression Scale (HADS) (12). The effect of health problems on work productivity was assessed using the Work productivity and impairment: general health (WPAI:GH) questionnaire (13). The WPAI: GH questionnaire, as a validated and reliable tool, captures the work time lost due to absenteeism and presenteeism, with a recall period of 1 week. For presenteeism, the patients were asked about the extent to which patients’ health problems affected their productivity while they were working. WPAI:GH outcomes are expressed as impairment percentages, with higher numbers indicating greater impairment and less productivity (worse outcomes).
Atopy was assessed by skin prick testing with allergen extracts for house dust mites (Dermatophagoides pteronyssinus, Dermatophagoides farinae), mold (Alternaria tenuis, Aspergillus), dog hair, cat hair, pollen (ragweed, birch, London plane) and cockroach, together with positive (histamine) and negative (saline) controls.
Definitions of severe asthma
This study used the ERS/ATS guideline (3) definition of severe refractory asthma, which required treatment with high dose ICS plus a second controller to prevent it from becoming uncontrolled or which remained uncontrolled despite this therapy. Furthermore, for the treatment based severe asthma classification, we had an alternative definition for severe asthma as patients receiving treatment with Step 4/5 (moderate or high dose ICS/LABA ± add-on), or remaining uncontrolled despite this treatment (1). In addition, the triple controller therapy was defined as at least moderate dose ICS/LABA plus LTRA and/or theophylline or LAMA.
Statistical analysis
Comparisons were conducted using Chi-squared or Fisher’s exact test for categorical data and Student’s t-test or Wilcoxon rank sum test for continuous data as appropriate. Predictors of response were determined using single and multiple logistic regression adjusted for age, gender, smoking and site. All analyses were conducted using STATA 13 (College Station, Texas, USA). A P value ≤0.05 was considered significant.
Results
Characterisation of the participants
A total of 379 patients with asthma from Northeast and West China were included (Table 1). Participants were 57.0% female, had a mean (standard deviation, SD) age of 47.4 (14.0) years, a body mass index (BMI) of 23.4 (4), and 37% were current or ex-smokers. Most (61.4%) were atopic, with a mean (SD) asthma duration of 4.3 (7.0) years, and moderate impairment of lung function with mean (SD) 71.5% (23.3) of FEV1% predicted. About one third had at least one exacerbation requiring oral corticosteroid (OCS) in the previous year. Asthma was objectively confirmed in all patients, by BDR in 201/233 (86.3%), airways hyperresponsiveness in 149/212 (70.3%), and peak flow variability in 5/11. According to sputum inflammatory counts, 26.7% of participants displayed an eosinophilic phenotype, 11.4% were neutrophilic, 1.3% were mixed granulocytic and 60.6% were paucigranulocytic.
Table 1. Patient characteristics.
| Variables | Data |
|---|---|
| N | 379 |
| Agea | 47.4 (14.0) |
| Male/female (N=379) | 163/216 |
| Smokingc (N=344) | |
| Never | 217 (63.1) |
| Current | 57 (16.6) |
| Ex | 70 (20.4) |
| Pack years (ex/current smokers)b | 19.50 (7.50, 31.50) |
| BMIb | 23.44 (21.12, 25.87) |
| Atopyc | 78/127 (61.4) |
| Pre β2 agonist spirometrya (N=375) | |
| FEV1% predicted | 71.48 (23.30) |
| FVC % predicted | 88.35 (20.22) |
| FEV1/FVC | 66.45 (14.38) |
| Post β2 agonist spirometrya (N=340) | |
| FEV1% predicted | 78.60 (23.47) |
| FVC % predicted | 93.92 (19.66) |
| FEV1/FVC | 68.89 (14.25) |
| Sputum inflammatory phenotypec (N=236) | |
| Neutrophilic | 27 (11.4) |
| Eosinophilic | 63 (26.7) |
| Paucigranulocytic | 143 (60.6) |
| Mixed | 3 (1.3) |
BMI, body mass index; Data given as amean (SD), bmedian (Q1, Q2), cNo. (%).
Uncontrolled asthma
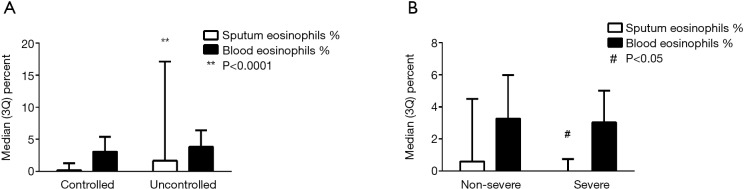
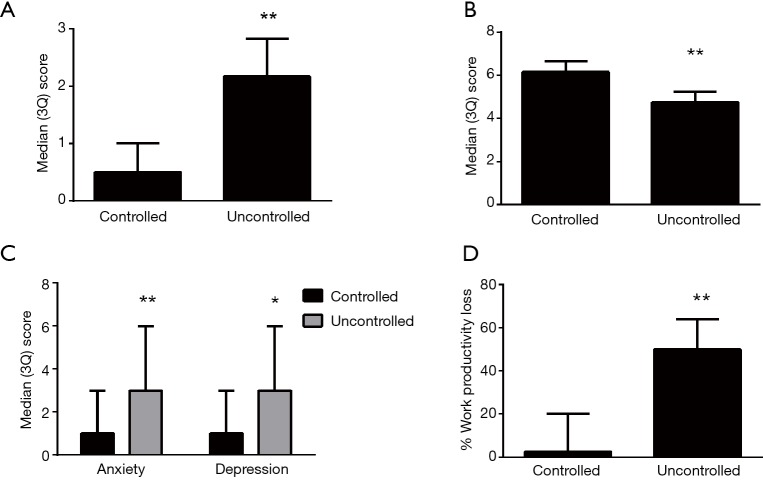
Based on ACQ cut-points, 39.4% (n=147) of patients had uncontrolled asthma (Table 2). In comparison with controlled asthma, the uncontrolled asthma group had worse eosinophilic inflammation in sputum [1.68% (0.0, 17.1%) vs. 0.2% (0.0, 1.3%), P<0.0001, Figure 1] (Table 3), and reduced lung function (FEV1% pre 64.8±23.9 vs. 75.7±22.0, P<0.0001; FEV1/FVC 65.0±13.6 vs. 71.6±14.1, P<0.0001) (Table 2). A greater proportion of patients with uncontrolled asthma reported ≥1 severe exacerbations in the past year that required OCS treatment (41.8% vs. 31.6%, P=0.044), more exacerbations requiring parenteral CS (24.0% vs. 15.8%, P=0.05) and unscheduled doctor visits (34.3% vs. 21.8%, P=0.008). The patients with uncontrolled asthma had worse AQLQ [4.75 (4.06, 5.25) vs. 6.16 (5.59, 6.56), P<0.0001, Figure 2] than patients with controlled asthma. A greater proportion of patients with uncontrolled asthma had psychological symptoms (15.2% vs. 4.1%, P<0.0001 for HADS-A score ≥8; 17.8% vs. 6.3%, P<0.0001 for HADS-D score ≥8) and their symptoms were worse [3 (0, 6) vs. 1 (0, 3), P<0.0001 for HADS-A and P=0.0002 for HADS-D, Figure 2].
Table 2. Characteristics of patients with controlled and uncontrolled asthma.
| Variables | Controlled asthma (ACQ score <1.5) | Uncontrolled asthma (ACQ score ≥1.5) | P value |
|---|---|---|---|
| N | 226 | 147 | |
| Agea | 46.8 (14.02) | 48.0 (13.66) | 0.425 |
| Male/Female | 93/133 | 66/81 | 0.475 |
| Smokingc: never | 135/201 (67.2) | 82/141 (58.2) | 0.085 |
| Pack years (ex/current smokers)b | 19.5 (8.0, 41.0) | 19.5 (7.5, 30.0) | 0.321 |
| Atopyc | 58/95 (61.1) | 19/30 (63.3) | 0.823 |
| Pre-β2 agonist spirometrya (N) | 223 | 147 | |
| FEV1% predicted | 75.69 (22.04) | 64.83 (23.90) | <0.0001 |
| FVC % predicted | 90.14 (18.30) | 85.53 (22.84) | 0.045 |
| FEV1/FVC | 69.38 (13.97) | 61.65 (13.76) | <0.0001 |
| Post β2 agonist spirometrya (N) | 194 | 142 | |
| FEV1% predicted | 81.76 (22.13) | 74.35 (24.87) | 0.004 |
| FVC % predicted | 94.42 (17.67) | 93.49 (22.33) | 0.668 |
| FEV1/FVC | 71.64 (14.14) | 64.98 (13.55) | <0.0001 |
| Asthma control | |||
| ACQ6 score | 0.5 (0.17, 1.0) | 2.17 (1.83, 2.83) | <0.0001 |
| Severe refractory asthma (ATS/ERS criteria)c | 9/226 (4.0) | 3/147 (2.0) | 0.378 |
| Severe asthma requiring Step 4/5c | 47/226 (20.8) | 9/147 (6.1) | <0.0001 |
| Exacerbation history | |||
| ≥1 severe exacerbation in past year requiring OCS | 71/225 (31.6) | 61/146 (41.8) | 0.044 |
| Hospital admission | 64/226 (28.3) | 48/147 (32.7) | 0.395 |
| ICU admission | 1/226 (0.4) | 1/147 (0.7) | 1.0 |
| ER visit | 28/224 (12.5) | 19/145 (13.1) | 0.865 |
| Unscheduled doctor visit | 49/225 (21.8) | 50/146 (34.3) | 0.008 |
| Respiratory medications | |||
| OCS usec | 2/226 (0.9) | 4/147 (2.7) | 0.217 |
| ICS/LABA usec | 101/226 (44.7) | 54/146 (37.0) | 0.141 |
| ICS dose (BDPmcg/day)b | 400 (200, 1,000) | 400 (400, 1,000) | 0.609 |
| Long Acting anticholinergicc | 3/226 (1.3) | 5/147 (3.4) | 0.272 |
| Leukotriene modifierc | 56/226 (24.8) | 14/146 (9.6) | <0.0001 |
| Theophyllinec | 43/226 (19.0) | 6/147 (4.0) | <0.0001 |
ACQ, Asthma Control Questionnaire; ATS, American Thoracic Society; BDP, beclomethasone dipropionate; ER, emergency room; ERS, European Respiratory Society; ICS, Inhaled corticosteroid; ICU, intensive care unit; LABA, long-acting beta2-agonist; OCS, oral corticosteroid. Data given as amean (SD), bmedian (Q1, Q2), cNo. (%). Italic face indicates significant.
Figure 1.
Blood and sputum eosinophils in (A) controlled and uncontrolled asthma and (B) non-severe and severe asthma. #, P<0.05, **, P<0.0001.
Table 3. Blood and sputum biomarkers in controlled and uncontrolled asthma.
| Variables | Controlled asthma | Uncontrolled asthma | P value |
|---|---|---|---|
| Sputum cell count | |||
| Sputumb (N) | 146 | 90 | |
| Eosinophils (%) | 0.2 (0, 1.3) | 1.68 (0, 17.1) | <0.0001 |
| Neutrophils (%) | 8.75 (0.6, 39.25) | 3.85 (0.2, 32.25) | 0.215 |
| Macrophage (%) | 82.38 (55.0, 94.0) | 71.63 (34.2, 91.0) | 0.050 |
| Lymphocyte (%) | 0.95 (0.25, 2.300 | 0.88 (0.25, 1.900 | 0.619 |
| Sputum inflammatory phenotypec | <0.0001 | ||
| Eosinophilic | 25 (17.1%) | 38 (42.2%) | |
| Neutrophilic | 18 (12.3%) | 9 (10.0%) | |
| Paucigranulocytic | 102 (69.9%) | 41 (45.6%) | |
| Mixed | 1 (0.7%) | 2 (2.2%) | |
| Full blood countb (N) | 210 | 135 | |
| White cell count, ×109/L | 6.51 (5.30, 8.03) | 7.30 (6.0, 8.80) | 0.001 |
| Eosinophils, ×109/L | 0.20 (0.10, 0.34) | 0.25 (0.10, 0.50) | 0.052 |
| Monocytes, ×109/L | 0.39 (0.30, 0.50) | 0.47 (0.33, 0.60) | 0.0005 |
| Basophils, ×109/L | 0.03 (0.01, 0.04) | 0.02 (0, 0.07) | 0.507 |
| Lymphocytes, ×109/L | 1.85 (1.49, 2.26) | 2.19 (1.70, 2.70) | <0.0001 |
| Neutrophils, ×109/L | 3.79 (3.05, 5.02) | 4.11 (3.20, 5.30) | 0.101 |
| Platelets, ×109/L | 194.50 (155.0, 236.0) | 222.0 (177.0, 262.0) | 0.0003 |
| Red cell count, ×109/L | 4.68 (4.40, 5.06) | 4.83 (4.47, 5.20) | 0.056 |
| Serum biomarkers (N) | 135 | 43 | |
| Serum IgEb, IU/mL | 102.71 (36.26, 213.44) | 187.0 (43.52, 441.15) | 0.057 |
Data given as bmedian (Q1, Q2), cNo. (%), Italic face indicates significant.
Figure 2.
Asthma control score (A), quality of life (B), psychological symptoms (C) and work productivity (D) in controlled and uncontrolled asthma. *P<0.001; **P<0.0001.
While ICS plus LABA use in uncontrolled asthma was similar to controlled asthma, a greater proportion of the patients with controlled asthma received additional treatment, i.e., triple controller therapy, with a LTRA (24.8% vs. 9.6%, P<0.0001) or theophylline (19.0% vs. 4.0%, P<0.0001). Using triple controller therapy (ICS/LABA and one of LTRA, LAMA or theophylline) was associated with a significantly reduced risk of uncontrolled asthma [OR =0.32, 95% CI =(0.14, 0.75), P<0.0001] adjusted for age, gender, smoking and site.
Health-related work productivity impairment
The percentage of patients at work was similar in uncontrolled and controlled asthma (48.4% vs. 50.4%, P=0.796) (Table 4). Of those working, 86% (18) of patients with uncontrolled asthma reported productivity loss due to health problems in comparison to 50% (23) of those with controlled asthma (P=0.007). More patients with uncontrolled asthma reported presenteeism (85.2% vs. 47.5%, P=0.001), and they had higher presenteeism scores [30 (10, 50), vs. 0 (0, 20), P<0.0001] compared to patients with controlled asthma. High scores for % absenteeism occurred in more patients with uncontrolled asthma (Table 4). Patients with uncontrolled asthma had greater overall health-problem related work productivity impairment [50.0 (20.0, 64.0) vs. 2.5 (0, 20), P<0.0001] and greater impairment of regular daily activities [30.0 (20.0, 60.0) vs. 10.0 (0.0, 30.0), P<0.0001] than those with controlled asthma.
Table 4. Work productivity outcomes in uncontrolled asthma and severe asthma.
| WPAI:GH questionnaire | Patients grouped by ACQ score | Patients grouped by GINA step 4/5 or not | |||||
|---|---|---|---|---|---|---|---|
| Controlled asthma | Uncontrolled asthma | P value | Non-severe asthma | Severe asthma | P value | ||
| Participants currently workingc | 70/139 (50.4%) | 30/62 (48.4%) | 0.796 | 80/164 (48.8%) | 20/40 (50.0%) | 0.890 | |
| Any productivity lossc | 23/46 (50.0%) | 18/21 (85.7%) | 0.007 | 30/50 (60.0%) | 11/17 (64.7%) | 0.731 | |
| Absenteeismc | 4/46 (8.7%) | 6/21 (28.6%) | 0.060 | 7/50 (14.0%) | 3/17 (17.7%) | 0.706 | |
| Presenteeismc | 29/61 (47.5%) | 23/27 (85.2%) | 0.001 | 41/68 (77.3%) | 11/20 (55.0%) | 0.672 | |
| % absenteeismb | 0 (0, 0) | 0 (0, 33.33) | 0.019 | 0 (0, 0) | 0 (0,0) | 0.780 | |
| % presenteeismb | 0 (0, 20) | 30 (10, 50) | <0.0001 | 10 (0, 30) | 10 (0, 40) | 0.642 | |
| % work productivity loss | 2.54 (0, 20) | 50 (20, 64) | <0.0001 | 10 (0, 40) | 20 (0, 40) | 0.602 | |
| % activity impairmentb | 10 (0, 30) | 30 (20, 60) | <0.0001 | 20 (0, 50) | 10 (0, 30) | 0.237 | |
| Hours missed due to health problemsb; range | 0 (0, 0); 0–24 | 0 (0,8); 0–48 | 0.006 | 0 (0, 0); 0–48 | 0 (0, 0); 0–24 | 0.468 | |
WPAI, work productivity and activity impairment. Data given as bmedian (Q1, Q2), cNo. (%), Chi2 test or Fisher’s exact test. Italic face indicates significant.
Severe asthma and triple-controller therapy
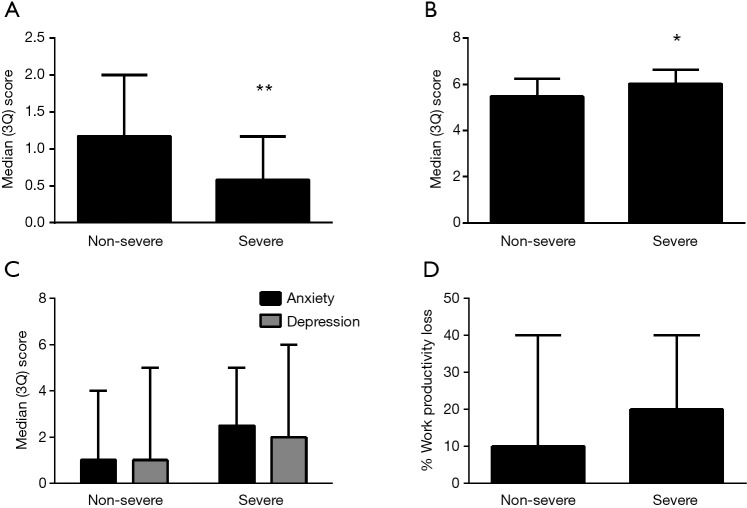
Based on the pre-specific alternative definition of severe asthma, 14.8% of subjects (n=56) were defined as severe asthma with treatment of Step 4/5, but only 13 (3.4%) met the ERS/ATS definition of severe refractory asthma in our study. There were similar lung function and GINA asthma control assessment, but the patients in the severe asthma group had better asthma control by ACQ [0.58 (0, 1.17) vs. 1.17 (0.5, 2.0), P<0.0001] and better quality of life scores (P=0.001) compared with the non-severe asthma group (Figure 3, Table 5). There was no difference in HADS scores [2.5 (1.0, 5.0) vs. 1.0 (0, 4.0), P=0.056, for HADS-A and 2.0 (0, 6.0) vs. 1.0 (0, 4.0), P=0.251, for HADS-D]. Health-related work productivity loss was equivalent in both severe and non-severe groups (Figure 3). In terms of comorbidities, the percentage of patients with nasal polyps and COPD in those with more severe asthma was increased in comparison to non-severe asthma (12.5% vs. 3.4%, P=0.006; 12.5% vs. 4.4%, P=0.015, respectively). Other comorbidities were similar between the two groups (data not shown). The levels of eosinophils in sputum and peripheral blood in the severe asthma group was significantly lower compared to the non-severe asthma group [0% (0, 0.75) vs. 0.6% (0, 4.5), P=0.026; 0.17×109/L (0.07, 0.31) vs. 0.2×109/L (0.1, 0.4), P=0.036, respectively] (Table 6, Figure 1). Most of the patients with severe asthma had a paucigranulocytic phenotype (73.7%) with only 15.8% exhibiting an eosinophilic phenotype.
Figure 3.
Asthma control score (A), quality of life (B), psychological symptoms (C) and work productivity (D) in non-severe and severe asthma. *, P<0.001; **, P<0.0001.
Table 5. Characteristics of patients with severe and non-severe asthma.
| Variables | Non-severe asthma | Severe asthma | P value |
|---|---|---|---|
| N | 323 | 56 | |
| Agea | 47.20 (13.89) | 48.15 (14.39) | 0.639 |
| Male/Female | 142/181 | 21/35 | 0.367 |
| Smokingc: never | 183/296 (61.8) | 34/48 (70.8) | 0.099 |
| Pack years (ex/current smokers)b | 19.5 (7.5, 31.5) | 21.9 (7.1, 37.75) | 0.731 |
| Atopyc | 57/95 (60.0) | 21/32 (65.6) | 0.572 |
| Pre-β2 agonist spirometrya (N) | 321 | 54 | |
| FEV1% predicted | 70.73(23.24) | 75.92 (23.35) | 0.131 |
| FVC % predicted | 87.76 (20.71) | 91.85 (16.75) | 0.170 |
| FEV1/FVC | 66.23 (14.36) | 67.87 (14.58) | 0.463 |
| Post-β2 agonist spirometrya (N) | 293 | 47 | |
| FEV1% predicted | 78.38 (23.61) | 79.96 (22.79) | 0.669 |
| FVC % predicted | 93.84 (20.31) | 94.40 (15.17) | 0.859 |
| FEV1/FVC | 68.78 (14.10) | 69.55 (15.24) | 0.730 |
| Asthma control | |||
| Uncontrolled Asthma (ACQ score ≥1.5)c | 138/317 (43.5) | 9/56 (16.1) | <0.0001 |
| ACQ6 scoreb | 1.17 (0.5, 2.0) | 0.58 (0, 1.17) | <0.0001 |
| Exacerbation history | |||
| ≥1 severe exacerbation in past year requiring OCS | 111/321 (34.6) | 22/56 (39.3) | 0.496 |
| Hospital admission | 87/323 (26.9) | 26/56 (46.4) | 0.101 |
| ICU admission | 3/323 (0.9) | 0/56 | 1.000 |
| ER visit | 37/320 (11.6) | 10/55 (18.2) | 0.171 |
| Unscheduled doctor visit | 85/321 (26.5) | 18/56 (32.1) | 0.380 |
| Respiratory medications | |||
| OCS usec | 4/323 (1.2) | 2/56 (3.6) | 0.217 |
| ICS/LABA usec | 103/322 (32.0) | 56/56 (100.0) | <0.0001 |
| ICS dose (BDPmcg/day)b | 400 (200, 800) | 650 (400, 1,000) | 0.0002 |
| Long acting anticholinergicc | 7/323 (2.2) | 1/56 (1.8) | 1.0 |
| Leukotriene modifierc | 21/322 (6.5) | 49/56 (87.5) | <0.0001 |
| Theophyllinec | 14/323 (4.3) | 35/56 (62.5) | <0.0001 |
ACQ, Asthma Control Questionnaire; BDP, beclomethasone dipropionate; ER, emergency room; ICS, Inhaled corticosteroid; ICU, intensive care unit; LABA, long-acting beta2-agonist; OCS, oral corticosteroid. Data given as amean (SD), bmedian (Q1, Q2), cNo. (%). Italic face indicates significant.
Table 6. Blood and sputum biomarkers in severe and non-severe patients.
| Variables | Non-severe asthma | Severe asthma | P value |
|---|---|---|---|
| Sputum cell count | |||
| Sputumb (N) | 198 | 38 | |
| Eosinophils (%) | 0.6 (0, 4.5) | 0 (0, 0.75) | 0.026 |
| Neutrophils (%) | 4.4 (0.3, 35.75) | 13.25 (2.75, 36.75) | 0.069 |
| Macrophage (%) | 80.38 (46.50, 93.50) | 70.63 (51.25, 92.0) | 0.600 |
| Lymphocyte (%) | 1.0 (0.25, 2.50) | 0.5 (0.25, 1.30) | 0.320 |
| Sputum absolute cell countb (N) | N=148 | N=36 | |
| Eosinophils, ×104/mL | 0.24 (0, 4.59) | 0 (0, 0.27) | 0.020 |
| Neutrophils, ×104/mL | 3.53 (0.47, 13.89) | 7.68 (1.26, 16.40) | 0.197 |
| Macrophage, ×104/mL | 43.78 (18.96, 273.33) | 24.19 (16.98, 39.26) | 0.017 |
| Lymphocyte, ×104/mL | 0.8 (0.16, 5.39) | 0.32 (0.11, 0.67) | 0.022 |
| Sputum inflammatory phenotypec | 0.307 | ||
| Eosinophilic | 57 (28.8%) | 6 (15.8%) | |
| Neutrophilic | 23 (11.6%) | 4 (10.5%) | |
| Paucigranulocytic | 115 (58.1%) | 28 (73.7%) | |
| Mixed | 3 (1.5%) | 0 | |
| Full blood countb (N) | 296 | 53 | |
| White cell count, ×109/L | 7.0 (5.74, 8.50) | 6.10 (5.10, 7.85) | 0.025 |
| Eosinophilis, ×109/L | 0.20 (0.10, 0.40) | 0.17 (0.07, 0.31) | 0.036 |
| Monocytes, ×109/L | 0.40 (0.30, 0.52) | 0.35 (0.27, 0.60) | 0.184 |
| Basophils, ×109/L | 0.03 (0, 0.05) | 0.02 (0.01, 0.04) | 0.756 |
| Lymphocytes, ×109/L | 2.0 (1.64, 2.50) | 1.69 (1.32, 2.0) | 0.0001 |
| Neutrophils, ×109/L | 3.90 (3.12, 5.14) | 3.66 (3.12, 5.02) | 0.597 |
| Platelets, ×109/L | 210.5 (168.5, 252.5) | 169.0 (131.0, 220.0) | 0.0009 |
| Red cell count, ×109/L | 4.76 (4.43, 5.18) | 4.66 (4.37, 4.96) | 0.076 |
| Serum biomarkers (N) | 139 | 42 | |
| Serum IgEb, IU/mL | 116.45 (41.36, 303.49) | 95.48 (30.89, 306.37) | 0.612 |
Data given as bmedian (Q1, Q2), cNo. (%). Italic face indicates significant.
As the first-line controller therapy, ICS plus LABA was used in all patients with severe asthma. To achieve better asthma control, the greater proportion of patients with severe asthma took the second-line controller therapies such as leukotriene modifier (87.5% vs. 6.5%, P<0.0001) and theophylline (62.5% vs. 4.3%, P<0.0001), but not LAMA or anti-IgE compared with non-severe asthma. This suggests an approach where a third controller is added when there is insufficient control from two agents, which is consistent with a more severe form of asthma. The use of triple controller therapy indicated a high risk of ATS/ERS-defined severe asthma [OR =9.34; 95% CI: (2.53, 34.54), P=0.001] when data were adjusted for age, gender, smoking and site. The use of triple controller therapy in severe asthma was associated with better asthma control and quality of life and lower airway inflammation.
Discussion
In this study, patients with asthma from three centres underwent systematic evaluation of their condition. Although there was a relatively low proportion of severe asthma based on ERS/ATS criteria, two of five patients with asthma in China were uncontrolled, with an increased illness burden, impaired work productivity and airway eosinophilic inflammation. The use of triple controller therapy was associated with improved control and reduced eosinophilia, suggesting it may be a promising treatment approach in persistent asthma.
In our study, 39.4% of patients had uncontrolled asthma. This agrees with the Asthma Insight and Management survey which showed 42.0% of patients with uncontrolled asthma in China (2), however is different to the first national survey from China which found that 26.3% of patients were uncontrolled (14,15). Some issues that can explain this inconsistency include study design, tools assessing asthma control, population and regional variation. The proportion of patients with uncontrolled asthma was substantially greater in China, which led to greater health-care utilization such as exacerbations, severe exacerbations, more intensive therapy and unscheduled doctor visits.
We also found that psychological disturbance was important in the Chinese population. We identified 15.4% (n=57) of patients with psychological symptoms, which is similar to other studies (16,17). There was a higher prevalence of psychological symptoms in patients with uncontrolled or severe asthma, which is also similar to other studies (18). The mechanisms that underlie the relationship between psychological symptoms and asthma remain unclear (19). We recently found that anxiety symptoms are associated with greater perceived dyspnea intensity in asthma during bronchoconstriction (16); and that depression and high stress were associated with reduced bronchodilator response (20,21), and altered pattern of inflammation (22), which could be involved in therapy resistance. The relationship between psychological disturbance and asthma control needs further study.
Productivity loss is the opportunity cost due to foregone labor. It is described as absenteeism (the withdrawal of labor) or presenteeism (inefficiency of labor due to impairment). Few studies have reported on the relationship between asthma and productivity loss in the Chinese population. Su et al. identified that 28.6% of employed patients reported work loss during the previous year (15). In our study, we found that 14.9% of patients reported absenteeism and 59.1% reported presenteeism. We also found that productivity loss due to both absenteeism and presenteeism was associated with worse asthma control. By comparison, a prospective cross-sectional study from Canada found that 16.3% of employed adults reported absenteeism and 45.7% reported presenteeism from asthma, but they did not find that productivity loss due to absenteeism was associated with asthma control (23). This difference may be explained by different population characteristics and social circumstances. Interestingly, more severe asthma requiring treatment with triple controller therapy had no apparent effect on work productivity impairment. This is possibly because better asthma control was achieved using triple-controller therapy.
The definition of severe refractory asthma from the ERS/ATS Task Force Report (3) requires high dose ICS plus a second controller and/or systemic corticosteroids. However, our study found that triple controller therapy, rather than high dose ICS, was more frequently taken in the Chinese population if optimal asthma control was not achieved, as reported in other studies from China (14,15). The second-line therapies that are added to moderate dose ICS and LABA include LTRA, theophylline or LAMA. Our results suggest that this approach can be effective, as it was associated with better asthma control and reduced levels of airway eosinophilia. This approach may allow patients to maintain moderate ICS dose (4) while at the same time achieve improved asthma control (5,24-26). A consequence of this approach, however, relates to the definition of severe asthma. This approach does not satisfy the ERS/ATS guideline definition of severe asthma and raises the question of whether patients on triple-controller therapy can be defined as severe asthma.
The use of triple controller therapy for asthma in China may be due to the perceived side effects of ICS (27,28). Alternatively, LTRA may be used in those with comorbid rhinitis (25), and, theophylline as a bronchodilator, may be used for its anti-inflammatory effects (29). When theophylline was added to moderate ICS plus LABA it improved small airway function, airway inflammation and reduced asthma exacerbations in Chinese patients (5). These results suggest that triple controller therapy may be a useful option in more severe asthma, and that it is also a form of severe asthma.
We assessed the inflammatory phenotype of asthma using induced sputum, and found the distribution of phenotypes in this Chinese population with asthma to be similar to studies from western countries (30). Eosinophil % in both sputum and peripheral blood related with poor asthma control.
Conclusions
In conclusion, we found that, although there was a relatively low proportion of severe asthma based on ERS/ATS criteria, two of five patients with asthma in China were uncontrolled. Uncontrolled asthma had more psychological symptoms, work productivity and activity impairment as well as eosinophilic airway inflammation. Substantial gain in asthma control was apparent in patients treated with triple-controller therapy. This may represent a form of severe asthma, and our study has implications for the definition and systematic assessment of severe asthma.
Acknowledgements
All authors thank Ms. Heather Powell for statistical analyses. The Severe Asthma Web-based Database was funded by the Thoracic Society of Australia and New Zealand (TSANZ).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.GINA. Global Initiative for Asthma. Global strategy for asthma management and prevention. Updated 2015. Available online: www.ginasthma.org
- 2.Thompson PJ, Salvi S, Lin J, et al. Insights, attitudes and perceptions about asthma and its treatment: findings from a multinational survey of patients from 8 Asia-Pacific countries and Hong Kong. Respirology 2013;18:957-67. 10.1111/resp.12137 [DOI] [PubMed] [Google Scholar]
- 3.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014;43:343-73. 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 4.Wang K, Tian P, Fan Y, et al. Assessment of second-line treatments for patients with uncontrolled moderate asthma. Int J Clin Exp Med 2015;8:19476-80. [PMC free article] [PubMed] [Google Scholar]
- 5.Nie H, Zhang G, Liu M, et al. Efficacy of theophylline plus salmeterol/fluticasone propionate combination therapy in patients with asthma. Respir Med 2013;107:347-54. 10.1016/j.rmed.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 6.Ohta K, Ichinose M, Nagase H, et al. Japanese Guideline for Adult Asthma 2014. Allergol Int 2014;63:293-333. 10.2332/allergolint.14-RAI-0766 [DOI] [PubMed] [Google Scholar]
- 7.British Thoracic S, Scottish Intercollegiate Guidelines N. British guideline on the management of asthma. Thorax 2014;69 Suppl 1:1-192. [PubMed] [Google Scholar]
- 8.Juniper EF, Bousquet J, Abetz L, et al. Identifying 'well-controlled' and 'not well-controlled' asthma using the Asthma Control Questionnaire. Respir Med 2006;100:616-21. 10.1016/j.rmed.2005.08.012 [DOI] [PubMed] [Google Scholar]
- 9.Juniper EF, Buist AS, Cox FM, et al. Validation of a standardized version of the Asthma Quality of Life Questionnaire. Chest 1999;115:1265-70. 10.1378/chest.115.5.1265 [DOI] [PubMed] [Google Scholar]
- 10.Zhen JP. Textbook of pulmonary function testing. 1st ed. Guangzhou, Guangdong Province, China: Guangdong Science and Technology Publishing House, 2007. [Google Scholar]
- 11.Wang G, Baines KJ, Fu JJ, et al. Sputum mast cell subtypes relate to eosinophilia and corticosteroid response in asthma. Eur Respir J 2016;47:1123-33. 10.1183/13993003.01098-2015 [DOI] [PubMed] [Google Scholar]
- 12.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 13.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993;4:353-65. 10.2165/00019053-199304050-00006 [DOI] [PubMed] [Google Scholar]
- 14.Su N, Lin J, Liu G, et al. An epidemiological survey of current asthma control status in China. Zhonghua Nei Ke Za Zhi 2014;53:601-6. [PubMed] [Google Scholar]
- 15.Su N, Lin J, Chen P, et al. Evaluation of asthma control and patient's perception of asthma: findings and analysis of a nationwide questionnaire-based survey in China. J Asthma 2013;50:861-70. 10.3109/02770903.2013.808346 [DOI] [PubMed] [Google Scholar]
- 16.Li HL, He XL, Liang BM, et al. Anxiety but not depression symptoms are associated with greater perceived dyspnea in asthma during bronchoconstriction. Allergy Asthma Proc 2015;36:447-57. 10.2500/aap.2015.36.3897 [DOI] [PubMed] [Google Scholar]
- 17.Ciprandi G, Schiavetti I, Rindone E, et al. The impact of anxiety and depression on outpatients with asthma. Ann Allergy Asthma Immunol 2015;115:408-14. 10.1016/j.anai.2015.08.007 [DOI] [PubMed] [Google Scholar]
- 18.Vamos M, Kolbe J. Psychological factors in severe chronic asthma. Aust N Z J Psychiatry 1999;33:538-44. 10.1080/j.1440-1614.1999.00591.x [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Zhang X, Zheng J, et al. Co-morbid psychological dysfuntion is associated with a higer risk of asthma exacerbations: a systematic review and meta-analysis. J Thorac Dis 2016;8:1257-68 10.21037/jtd.2016.04.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han YY, Forno E, Marsland AL, et al. Depression, Asthma, and Bronchodilator Response in a Nationwide Study of US Adults. J Allergy Clin Immunol Pract 2016;4:68-73.e61. 10.1016/j.jaip.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brehm JM, Ramratnam SK, Tse SM, et al. Stress and Bronchodilator Response in Children with Asthma. Am J Respir Crit Care Med 2015;192:47-56. 10.1164/rccm.201501-0037OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu M, Liang Z, Wang T, et al. Th1/Th2/Th17 cells imbalance in patients with asthma with and without psychological symptoms. Allergy Asthma Proc 2016;37:148-56. 10.2500/aap.2016.37.3928 [DOI] [PubMed] [Google Scholar]
- 23.Sadatsafavi M, Rousseau R, Chen W, et al. The preventable burden of productivity loss due to suboptimal asthma control: a population-based study. Chest 2014;145:787-93. 10.1378/chest.13-1619 [DOI] [PubMed] [Google Scholar]
- 24.Lipworth BJ. Emerging role of long acting muscarinic antagonists for asthma. Br J Clin Pharmacol 2014;77:55-62. 10.1111/bcp.12123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang HP, Jia CE, Lv Y, et al. Montelukast for prevention and treatment of asthma exacerbations in adults: Systematic review and meta-analysis. Allergy Asthma Proc 2014;35:278-87. 10.2500/aap.2014.35.3745 [DOI] [PubMed] [Google Scholar]
- 26.Evans DJ, Kew KM, Anderson DE, et al. Long-acting muscarinic antagonists (LAMA) added to inhaled corticosteroids (ICS) versus higher dose ICS for adults with asthma. Cochrane Database Syst Rev 2015;(7):CD011437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: A systematic review and meta-analysis. Arch Intern Med 1999;159:941-55. 10.1001/archinte.159.9.941 [DOI] [PubMed] [Google Scholar]
- 28.Boulet LP. Perception of the role and potential side effects of inhaled corticosteroids among asthmatic patients. Chest 1998;113:587-92. 10.1378/chest.113.3.587 [DOI] [PubMed] [Google Scholar]
- 29.Barnes PJ. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol 2013;131:636-45. 10.1016/j.jaci.2012.12.1564 [DOI] [PubMed] [Google Scholar]
- 30.Zhang XY, Simpson JL, Powell H, et al. Full blood count parameters for the detection of asthma inflammatory phenotypes. Clin Exp Allergy 2014;44:1137-45. 10.1111/cea.12345 [DOI] [PubMed] [Google Scholar]