Abstract
We describe using a polyphasic approach that combines proteomic by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF) analysis, genomic data and phenotypic characterization the features of Lactococcus garvieae strain M14 newly isolated from the fermented milk (known as raib) of an Algerian cow. The 2 188 835 bp containing genome sequence displays a metabolic capacity to form acid fermentation that is very useful for industrial applications and encodes for two bacteriocins responsible for its eventual bioprotective properties.
Keywords: Fermented milk, genome, lactic acid bacteria, Lactococcus garvieae, sequencing
Introduction
Lactococcus garvieae is a lactic acid bacteria (LAB) that has commonly been used in the manufacture of many varieties of cheese and other fermented milk products [1], [2] as well as meat products [3]. The ability of some LAB to produce proteins with bactericidal properties called bacteriocins led to their potential utilization as biopreservatives in the food industry against a range of pathogenic bacteria, including Listeria sp. and Clostridium sp. [4], [5]. Also, because of bacteriocins, some LAB are thought to act as bioprotective organisms that play a major role in the composition of the microbiota [6]. First isolated from cases of bovine mastitis [7], L. garvieae has gained recognition as a potential pathogen of various fish species, including rainbow trout [8]. Moreover, L. garvieae has been involved in many clinical cases including infective endocarditis associated with septicaemia, spondylodiscitis and urinary and skin infections [9], [10], [11], [12], [13], [14], [15]. Genomic interspecies microarray hybridization and pan-genome comparative analysis of the pathogenic strain Lg2 and the nonvirulent strain YT-3 identified genes encoding for host colonization and the development of pathogenesis including a capsule gene cluster and genes encoding for a myosin cross-reactive antigen and haemolysin [16], [17]. The phenotypic diversity of L. garvieae seems to be related to the specific animal host they colonize [18], [19]. Altogether, the analysis of L. garvieae genomes isolated from a dairy product and its comparison with pathogenic isolates seems to be necessary to clarify the global genetic variations that may justify, at least in part, the observed phenotypic differences.
In this work, we have isolated and identified a new strain of Lactococcus garvieae from the fermented milk product of an Algerian cow, known as raib, as a part of the study of LAB and revealed their antibacterial activity. A total of 47 different bacterial species including Lactococcus and Streptococcus spp. as identified by API 50CHL and mass spectrometry were isolated from milk product specimens (unpublished data). Lactococcus spp. was the only species with antibacterial effect as shown by the agar well-diffusion assay. We accomplished deep studies including phenotypic, polyphasic taxonomy, genotypic and phylogenetic analyses.
Here we provide a set of features for the identified Lactococcus garvieae strain M14, together with the description of the complete genome sequence and annotation. We present a comparative genomic analysis of all available sequences of closely related species to L. garvieae. This integrative approach dealing with a large data set has the potential to explore the relationship between the presence of L. garvieae in dairy and food safety in order to recognize and prevent a potential hazard for consumers.
Materials and Methods
Sample collection
Raw cow's milk samples were collected from a farm in Guelma, in the east of Algeria, in sterile glass bottles and transported in an isotherm container to the laboratory. A total of 200 mL of milk samples was allowed to clot at room temperature to promote the development of endogenous lactic flora according to Zadi-Karam and Karam [20].
Strain isolation
M17 agar media (Sigma-Aldrich, St. Louis, MO, USA) prepared in accordance with manufacturer's instructions was used for the growth of the LAB strain screened in this investigation. Thus, 0.1 mL of fermented milk sample was plated onto M17 to promote the bacterial flora cultivation in aerobic conditions by incubation at 37°C for 24 hours. Lactococcus garvieae strain M14 was then isolated and stored at −20°C until further use.
Phenotypic, genotypic and phylogenetic analyses
We used 16S ribosomal RNA gene sequencing (16S rRNA) to provide genus and species identification for the isolate [21] and taxonomic classification of strain M14. A comparison of nucleotides query sequences against the nucleotide sequence database was also performed using BLAST (Basic Local Alignment Search Tool). The 16S rRNA sequences of all Lactococcus strains with draft genome were searched within the scaffolds using the RNAmmer server [22]. The phylogenetic tree, based on almost complete 16S rRNA gene sequences with a minimum length of 1517 nucleotides, was reconstructed using distance matrix (neighbour joining) within the MEGA 5 software [23]. Sequences were aligned using Clustal X 2.0 [24].
Different temperatures (room temperature, 28, 37 and 45°C) were tested to determine the growth temperature range and the optimal growth temperature for the strain. Growth of the strain was tested on 5% sheep's blood agar under anaerobic and microaerophilic conditions using the GENbag anaer and GENbag microaerosystems respectively (bioMérieux, Marcy l'Etoile, France) and in aerobic conditions, with or without 5% CO2.
L. garvieae strain M14 morphology was characterized by transmission electron microscopy (TEM) using a Morgani 268D (Philips, Amsterdam, The Netherlands) spectrometer with operating voltage of 60 kV. Polyphasic taxonomic identification by manufactured kits is widely used; the API 50CH carbohydrate fermentation strips and API ZYM enzyme test system (bioMérieux) were used to determine the biochemical profile of strain M14 according to the manufacturer's instructions.
Protein mass spectroscopy analysis was carried out by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF) as previously described [25] using a Microflex spectrometer (Bruker Daltonics, Leipzig, Germany). Twelve distinct deposits were made for strain M14 from 12 isolated colonies.
The 12 collected spectra for M14 were imported into the MALDI BioTyper 2.0 software (Bruker) and analysed by standard pattern matching (with default parameter settings) against 7.289 bacterial spectra including 26 spectra from three L. garvieae species, used as reference data, in the BioTyper database. Interpretation of scores as established by Bruker was as follows: a score >1.9 to a validly published species enabled the identification at the species level, a score >1.7 but <1.9 enabled the identification at the genus level and a score <1.7 did not enable any identification.
Growth conditions and genomic DNA preparation
L. garvieae was grown on 5% sheep's blood–enriched Columbia agar (bioMérieux) at 37°C in aerobic atmosphere. Bacteria grown on three petri dishes were collected and resuspended in 4 × 100 μL of Tris-EDTA (TE) buffer. Then 200 μL of this suspension was diluted in 1 mL TE buffer for lysis treatment. After a lysozyme incubation of 30 minutes at 37°C, lysis was performed with lauryl sarcosyl by 1% final and RNAse A treatment at 50 μG/μL final concentration during 1 hour at 37°C, followed by an overnight proteinase K incubation at 37°C. Extracted DNA was then purified using three successive phenol–chloroform extractions and ethanol precipitation at −20°C overnight. After centrifugation, the DNA was resuspended in 70 μL TE buffer. The yield and concentration were measured by the Quant-it Picogreen kit (Invitrogen; Life Technologies, Carlsbad, CA, USA) on the Genios-Tecan fluorometer at 113 ng/μL.
Genome sequencing and assembly
Genomic DNA (gDNA) of L. garvieae was sequenced using MiSeq Technology sequencer (Illumina, San Diego, CA, USA) with the mate pair strategy. The gDNA was barcoded in order to be mixed with 11 other projects with the Nextera Mate Pair sample prep kit (Illumina). The gDNA was quantified by a Qubit assay with the high sensitivity kit (Life Technologies, Carlsbad, CA, USA) to 40.8/μL. The mate pair library was prepared with 1 μg of genomic DNA using the Nextera mate pair Illumina guide. The genomic DNA sample was simultaneously fragmented and tagged with a mate pair junction adapter. The profile of the fragmentation was validated on an Agilent 2100 BioAnalyzer (Agilent Technologies, Santa Clara, CA, USA) with a DNA 7500 lab chip. The DNA fragments exhibited a mean of 4.5 kb (4486 pb). No size selection was performed, and only 308.9 ng of tagmented fragments were circularized. The circularized DNA was mechanically sheared to small fragments with an optimal size of 652 bp on the Covaris device S2 in microtubes (Covaris, Woburn, MA, USA). The library profile was visualized on a High Sensitivity Bioanalyzer LabChip (Agilent Technologies). The libraries were normalized at 2 nM and pooled. After a denaturation step and dilution at 10 pM, the pool of libraries was loaded onto the reagent cartridge and then onto the instrument along with the flow cell. Automated cluster generation and sequencing run were performed in a single 42-hour run in a 2 × 251 bp read length. Total information of 8.6 Gb was obtained from a 950K/mm2 cluster density with a cluster passing quality control filters of 93.12% (18 182 000 clusters). Within this run, the index representation for L. garvieae was determined to 9.73%. The whole genome shotgun strategy using Illumina sequencing technology gave 3 294 808 reads. Illumina reads were trimmed using Trimmomatic [26], then assembled with Spades software [27], [28]. Contigs obtained were combined together by SSpace [29] and Opera software 1.2 [30] helped by GapFiller 1.10 [31] to reduce the set. Some manual refinements using CLC Genomics 7 software (CLC Bio, Aarhus, Denmark) and homemade tools in Python improved the genome.
Genome annotation
Noncoding genes and miscellaneous features were predicted using RNAmmer [22], ARAGORN [32], Rfam [33], PFAM [34] and Infernal [35]. Coding DNA sequences were predicted using Prodigal [36], and functional annotation was achieved using BLAST+ [37] and HMMER3 [38] against the UniProtKB database [39]. The KEGG orthology [40] annotation was done by the KAAS online server [41] using the SBH method. The pathways in which each gene might be involved were derived from the best KO hit. Gene functions were assigned by the Clusters of Orthologous Groups (COGs) database [42], [43]. The bacteriocin database of the Unité des Maladies Infectieuses et Tropicales Emergentes (URMITE), known as the BUR database, was used to annotate genes encoding for bacteriocins [44].
Results and Discussion
Organism classification and features
The sequenced 16S rRNA gene of strain M14 was deposited in the GenBank database under the accession number LK985397. A BLAST search against the nucleotide database showed that strain M14 was most closely related to Lactococcus species, with a gene sequence identity value of 99.7% with Lactococcus garvieae YT-3. On the basis of the comparative sequence analysis of 16S rRNA gene sequence, strain M14 belongs to the already described species L. garvieae [45], [46].
A neighbour-joining phylogenetic tree, based on almost-complete 16S rRNA gene sequences of Lactococcus garvieae M14 strain and closely related species, is shown in Fig. 1. Sequences of the closest species including Lactococcus garvieae strains and strains of Lactococcus lactis subsp. lactis (NR_103918), Lactococcus lactis subsp. cremoris (NR_074949), Lactobacillus rhamnosus GG (NR_102778), Lactobacillus sakei subsp. sakei 23K (NR_075042), Lactobacillus plantarum WCFS1 (NR_075041), Lactobacillus fermentum IFO 3956 (NR_075033), Lactobacillus salivarius UCC118 (NR_074589) and Lactobacillus ruminis ATCC 27782 (NR_102839) were aligned with the 16S rRNA gene sequence of the strain M14. The strain M14 formed together with Lactococcus garvieae strains, and a common lineage with the L. lactis species was supported by a high bootstrap value of 99% (Fig. 1).
Fig. 1.
Phylogenetic tree highlighting position of Lactococcus garvieae strain M14 (LK985397) relative to other phylogenetically close strains within genus Lactococcus and Lactobacillus, with Lysinibacillus sphaericus as outgroup. Numbers at nodes are percentages of bootstrap values obtained by repeating analysis 500 times to generate majority consensus tree. Scale bar = 2% nucleotide sequence divergence.
Growth occurred for all of the tested temperatures, but optimal growth was observed at 37°C. The colonies were 1 to 6 mm in diameter and moderately opaque in facultative anaerobic conditions on enriched-blood Columbia agar (bioMérieux) and appeared whitish in colour at 28°C. The motility test was negative. Gram staining showed Gram-positive nonsporulating cocci. Cells grown on agar range in length from 0.79 to 0.93 μm (mean, 0.86 μm) and diameter from 0.59 to 0.63 μm (mean, 0.61 μm) as determined by negative staining TEM micrograph.
Strain M14 did neither have catalase nor oxidase activity. Using the API 50CH system, a positive reaction was observed for d-ribose, d-glucose, d-fructose, d-mannose, d-galactose, d-mannitol, amygdalin, arbutin, N-acetylglucosamine, esculin, salicin, d-cellobiose, d-lactose, d-saccharose and d-trehalose. Negative reactions were observed for glycerol, erythritol, d-arabinose, l-arabinose, d-xylose, l-xylose, d-adonitol, methyl-βd-xylopyranoside, l-sorbose, l-rhamnose, dulcitol, inositol, d-sorbitol, methyl-αd-mannopyranoside, methyl-αd-glucopyranoside, d-maltose, d-melibiose, inulin, d-melezitose, d-raffinose, starch, glycogen, xylitol, gentiobiose, d-turanose, d-lyxose, d-tagatose, d-fucose, l-fucose, d-arabitol and gluconate. Using the API ZYM system, negative reactions were observed for alkaline phosphatase, cystine arylamidase (proteases), trypsin, α-galactosidase (melibiase), β-glucosidase (cellulase), α-mannosidase and α-fucosidase, and positive reactions were observed for esterase, esterase lipase, lipase, leucine and valine arylamidase, α-chemotrypsin, acid phosphatase, β-galactosidase, β-glucuronidase and α- and β-glucosidase. The urease reaction, nitrate reduction and indole production were negative. When compared to the phylogenetically close species from Lactococcus and Lactobacillus [17], [47], [48], [49], [50], [51], [52], [53], L. garvieae strain M14 exhibited the phenotypic differences detailed in Table 1. L. garvieae was susceptible to amoxicillin, imipenem, piperacillin, ciprofloxacin, ceftriaxone, erythromycin, vancomycin, nitrofurantoin, nitrofurantoin, metronidazole and rifampicin but resistant to cefoxitin and cotrimoxazole.
Table 1.
Differential phenotypic characteristics between Lactococcus garvieae strain M14 and phylogenetically close speciesa
| Characteristic | L. garvieae M14 DSM 29394 | L. garvieae YT-3 DSM 6783 | L. lactis subsp. lactis DSM 20481 | L. lactis subsp. cremoris DSM 20069 | L. rhamnosus DSM 20021 | L. sakei subsp. sakei DSM 20017 | L. plantarum DSM 20174 | L. fermentum DSM 20052 | L salivarius DSM 20555 |
|---|---|---|---|---|---|---|---|---|---|
| Gram stain | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive |
| Cell shape | Cocci | Cocci | Rod | Rod | Rod | Rod | Rod | Rod | Rod |
| l-Arabinose | − | − | − | − | − | − | + | − | − |
| d-Ribose | + | + | + | − | + | + | + | + | − |
| d-Glucose | + | + | + | + | + | + | + | + | + |
| d-Galactose | + | + | + | + | + | + | + | + | + |
| d-Mannitol | + | + | + | + | + | − | + | − | + |
| Amygdalin | + | + | + | − | + | − | + | − | − |
| Arbutin | + | + | + | − | + | − | + | − | − |
| Esculin | + | + | + | + | + | + | + | + | + |
| d-Cellobiose | + | + | + | + | + | − | + | + | − |
| d-Lactose | + | − | + | − | + | − | + | + | + |
| Inulin | − | − | + | − | − | − | + | − | − |
| d-Melezitose | − | − | − | − | + | − | + | − | − |
| Glycogen | − | − | − | − | − | − | − | − | − |
| Alkaline phosphatase | − | − | − | − | + | − | − | − | − |
| Acid phosphatase | + | + | − | + | + | − | − | − | + |
| α-Glucosidase | + | + | + | − | + | − | − | + | + |
| N-acetyl-β-glucosaminidase | − | − | + | − | + | − | − | + | − |
| α-Mannosidase | − | − | + | − | − | − | + | + | − |
DSMZ, Deutsche Sammlung von Mikroorganismen.
The type strains of related lactic acid bacteria species were obtained from the DSMZ culture collection (Braunschweig, Germany). All strains were cultured according to recommendations given in the DSMZ catalogue of strains.
Extended features descriptions
MALDI-TOF analysis results of strain M14 showed scores ranging from 2.177 to 2.343 with Lactococcus spp., suggesting that our isolate was a member of Lactococcus species yet not a known strain. We incremented our database with the spectrum of strain M14 (Fig. 2a). Spectrum differences with others of phylogenetically close species are shown in Fig. 2b. The gel view displays the raw spectra of loaded spectrum files arranged in a pseudo-gel-like look. The x-axis records the m/z value. The left y-axis displays the running spectrum number originating from subsequent spectra loading. The peak intensity is expressed by a greyscale scheme code. The colour bar and the right y-axis indicate the relation between the colour a peak is displayed with and the peak intensity in arbitrary units. The compared species are indicated on the left.
Fig. 2.
(a) Reference mass spectrum from Lactococcus garvieae strain M14 and (b) gel view comparing L. garvieae M14 spectra with Lactococcus species (Lactococcus lactis subsp. lactis, Lactococcus lactis subsp. cremoris and two strains of Lactococcus garvieae) and with Lactobacillus species (Lactobacillus sakei subsp. sakei, Lactobacillus salivarus, Lactobacillus ruminis, Lactobacillus rhamnosus, Lactobacillus plantarum and Lactobacillus fermentum).
Genome project history
Lactococcus garvieae is commonly used in the industry of dairy products manufactured from raw milk. Moreover, the M14 strain has shown bactericidal effects against several bacteria (unpublished data) which indicate the eventual role played by L. garvieae in the composition of the gut microbiota. The sequencing of this strain is part of a study of the human digestive flora aiming at describing the force balance that shapes its composition, including antibacterial potency. Indeed, L. garvieae strain M14 was the 46th genome from the genus Lactococcus and the 16th genome of L. garvieae sp. The European Molecular Biology Laboratory (EMBL) accession number is CCXC01000001–CCXC01000013 and consists of 13 contigs without gaps, including four contigs that have been assigned to four plasmids (Table 2).
Table 2.
Project information
| MIGS ID | Property | Term |
|---|---|---|
| MIGS-31 | Finishing quality | High-quality draft |
| MIGS-28 | Libraries used | 1 mate paired |
| MIGS-29 | Sequencing platforms | MiSeq Illumina |
| MIGS-31.2 | Sequencing coverage | 110 |
| MIGS-30 | Assemblers | Spades |
| MIGS-32 | Gene calling method | Prodigal |
| GenBank ID | CCXC01000001–CCXC01000013 | |
| GenBank Date of Release | October 2014 | |
| MIGS-13 | Source material identifier | M14 |
| Project relevance | Potential probiotic and biopreservative |
MIGS, minimum information about a genome sequence.
Genome properties
The draft genome of L. garvieae M14 consists of 13 contigs of sizes ranging between 889 and 1 512 971 bp. The genome of M14 is composed of a single linear chromosome (2 188 835 bp; 37.69% G+C content) and four plasmids ranging in size from 42 306 to 1095 bp, including one circular plasmid (Fig. 3, Table 3, Table 4). The chromosome contains 91 predicted RNA including five rRNA (one 16S, one 23S and three 5S), 45 tRNA, one tmRNA and 40 miscellaneous RNA and 2214 protein-coding genes which represent 1 934 957 (88.40% of the genome). A total of 1515 genes (68.42%) were assigned a putative function (by COGs) [42], [43]. We found that 23.63% of the genes encode for information storage and processing (J, A, K, L and B categories), 21.11% were involved in cellular processes and signaling (D, V, T, M, N, U, O and X categories), 40.29% participate in metabolism (C, G, E, F, H, I, P and Q categories) and 14.97% were poorly characterized (R and S categories). The distribution of genes into COGs functional categories is presented in Fig. 4.
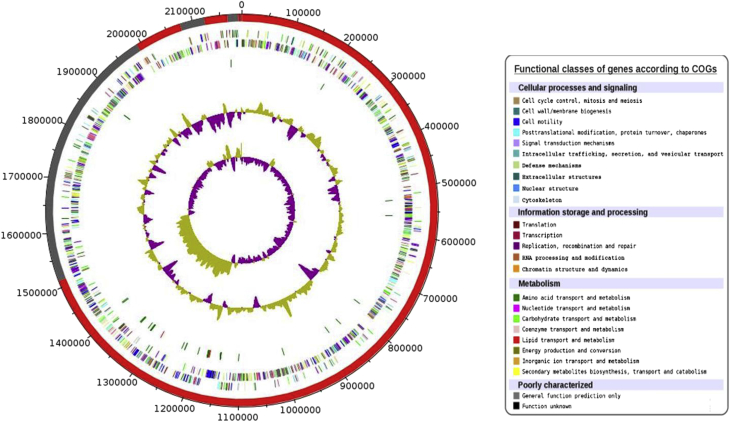
Fig. 3.
Circular representation of Lactococcus garvieae strain M14 genome. Circles from outside to center: Contigs (red/grey), genes colored according to categories determined in COGs on forward and reverse strands (two circles), rRNAs red, tRNA green, GC content and GC skew (green/purple).
Table 3.
Nucleotide content and gene count levels of genome
| Attribute | Value | % of totala |
|---|---|---|
| Genome size (bp) | 2 188 835 | 100.00 |
| DNA coding region (bp) | 1 934 957 | 88.40 |
| DNA G+C content (bp) | 827 233 | 37.79 |
| Total genes | 2264 | 100.00 |
| rRNA | 5 | 0.21 |
| tRNA | 45 | 1.90 |
| tmRNA | 1 | 0.04 |
| miscRNA | 40 | 1.69 |
| Protein-coding genes | 2214 | 97.79 |
| Genes with function prediction | 1651 | 72.92 |
| Genes assigned to COGs | 1515 | 68.42 |
COGs, Clusters of Orthologous Groups database.
Total is based on either size of genome (bp) or total number of protein-coding genes in annotated genome.
Table 4.
Nucleotide content and gene count levels of plasmids
| Attribute | Value | % of totala |
|---|---|---|
| Size (bp) | 64 869 (42 306; 16 485; 4983; 1095)b | 100 |
| DNA coding region (bp) | 55 608 | 85.72 |
| DNA G+C content (bp) | 22 256 | 34.31 |
| Total genes | 75 | 100 |
| rRNA | 0 | 0 |
| Protein-coding genes | 75 | 100 |
| Genes with function prediction | 20 | 35.09 |
| Genes assigned to COGs | 10 | 17.54 |
COGs, Clusters of Orthologous Groups database.
Total is based on either size of plasmids (bp) or total number of protein-coding genes in annotated sequences.
Sizes are indicated in parentheses.
Fig. 4.
Functional classification of genes encoded by Lactococcus garvieae M14 and its comparison with Lactococcus garvieae YT-3 and Lactococcus garvieae Lg2. Protein-coding sequences are classified according to COGs categories. COGs, Clusters of Orthologous Groups (COGs) database.
Genome comparison
When comparing L. garvieae M14 with nine Lactobacillus species and two Lactococcus strains that have similar 16S rRNA sequences, we found that the genome sequence of L. garvieae M14 is smaller than those of Lactobacillus plantarum WCFS1, Lactobacillus rhamnosus GG, Lactococcus lactis subsp. cremoris SK11 and Lactococcus lactis subsp. lactis Il1403 (3.35, 3.01, 2.60 and 2.37 MB respectively), but larger than those of Lactobacillus salivarius UCC118, Lactobacillus fermentum IFO 3956, Lactobacillus ruminis ATCC 27782, L. garvieae Lg2, L. garvieae YT-3, and Lactobacillus sakei subsp. sakei 23K (2.13, 2.10, 2.07, 1.96, 1.95 and 1.88 Mb respectively) (Table 5). The G+C content of L. garvieae M14 is smaller than those of L. fermentum IFO 3956, L. rhamnosus GG, L. plantarum WCFS1, L. ruminis ATCC 27782, L. sakei subsp. sakei 23K, L. garvieae YT-3 and L. garvieae Lg2 (51.47, 46.69, 44.42, 43.47, 41.26, 38.83 and 38.76% respectively), but larger than those of L. lactis subsp. cremoris SK11, L. lactis subsp. lactis Il1403 and L. salivarius UCC118 (35.78, 35.33 and 33.04% respectively) (Table 5). The gene content of L. garvieae M14 is smaller than those of L. plantarum WCFS1, L. rhamnosus GG and L. lactis subsp. cremoris SK11 and L. lactis subsp. lactis Il1403 (3063, 2944, 2504 and 2277 respectively), but larger than those of L. salivarius UCC118, L. sakei subsp. sakei 23K, L. ruminis ATCC 27782 and L. fermentum IFO 3956 (2014, 1885, 1862 and 1843 respectively) (Table 5). The proportion of gene count (in percentage) related to each COGs category was similar among the studied strains of L. garvieae. However, the distribution of genes into COGs category was not entirely similar in all the compared genomes (Fig. 4). L. garvieae M14 have an important number of genes participating in carbohydrate transport and metabolism (175 genes), yet less important than those of L. plantarum WCFS1 and L. rhamnosus GG (267 and 263 genes respectively). L. garvieae M14 and L. rhamnosus GG have the highest number of genes involved in defence mechanisms (51 and 63 genes respectively), compared to the other analysed genomes (37 genes in average). Unlike L. garvieae YT-3, L. garvieae strain M14 possesses plasmids the sequences of which were closely related to L. garvieae strain 21881 plasmid pGL5, L. garvieae strain IPLA31405 plasmid plG42, L. lactis plasmid pSRQ900 and L. lactis strain MJC15 plasmid pCD4 sequences. The genes of the plasmids encode for a type IV secretory pathway and for proteins with hypothetical functions.
Table 5.
General genome features
| Strain | Size (Mb) | G+C% | Gene content |
|---|---|---|---|
| Lactobacillus plantarum WCFS1 | 3.35 | 44.42 | 3063 |
| Lactobacillus rhamnosus GG | 3.01 | 46.69 | 2944 |
| Lactococcus lactis subsp. cremoris SK11 | 2.60 | 35.78 | 2504 |
| Lactococcus lactis subsp. lactis Il1403 | 2.37 | 35.33 | 2277 |
| Lactococcus garvieae M14 | 2.19 | 37.79 | 2264 |
| Lactobacillus salivarius UCC118 | 2.13 | 33.04 | 2014 |
| Lactobacillus fermentum IFO | 2.10 | 51.47 | 1843 |
| Lactobacillus ruminis ATCC 27782 | 2.07 | 43.47 | 1862 |
| Lactococcus garvieae Lg2 | 1.96 | 38.76 | 1968 |
| Lactococcus garvieae YT-3 | 1.95 | 38.83 | 1947 |
| Lactobacillus sakei subsp. sakei 23K | 1.88 | 41.26 | 1885 |
Energy metabolism and transporters
The coding DNA sequences annotated by the COGs database revealed that as much as 10.5% of the genomes corresponded to genes involved in carbohydrate transport and metabolism. Like all obligately homofermentative strains, L. garvieae M14 was found to possess the fructose bisphosphate aldolase (EC 4.1.2.13) in its genome, which is a key enzyme of the glycolysis pathway, whereas it lacks the phosphoketolase enzyme (EC 4.1.2.9) of the pentose phosphate pathway, present only in heterofermentative bacteria genomes. All the genes required for the degradation of the glucose to pyruvate are present in the genome, as well as the lactate dehydrogenase gene which allows the conversion of pyruvate into lactic acid. Several enzymes acting on pyruvate, including α-acetolactate synthase, pyruvate-formate lyase, lactate dehydrogenase and pyruvate oxidase, have also been identified in the strain M14 genome. Further, genome examination indicates that some enzymes needed for the full citrate cycle and for the gluconeogenesis are missing. The phosphotransferase system (PTS) for fructose, galactose, mannose, maltose, lactose, sucrose, trehalose, mannitol and cellobiose were present in the genome, while PTS for xylose, gluconate and ribose were absent. On the basis of its metabolic profile, L. garvieae M14 produces primarily lactic acid from hexoses using glycolysis. This ability of homolactic fermentation is useful for industrial applications.
Defense mechanism
We identified in the genome of L. garvieae M14 several phage-related genes and 51 proteins involved in defence features including a glycopeptide antibiotics resistance protein and two bacteriocins that are localized in the chromosome. The first bacteriocin has a length of 64 amino acids, and the use of the BUR database allowed us to identify a very similar bacteriocin sequence in the genomes of L. garvieae strains YT-3, Lg2 and TRF1. These sequences have been previously annotated as encoding for hypothetical proteins in the genomes of L. garvieae strains YT-3 and Lg2. The second bacteriocin has a length of 184 amino acids and corresponds to a colicin V, also found in the genome of the strain Lg2. Garviecin L1-5 was the first bacteriocin detected in a Lactococcus garvieae strain [54]. It has been shown to inhibit the growth of species relatively closely related to the producer but also of the human pathogen Listeria monocytogenes. Altogether, the production of bacteriocins gives L. garvieae strains a competitive advantage within their environment, allowing them to directly inhibit other bacteria and proliferate.
Conclusions
We have presented the phenotypic, phylogenetic and genomic analyses that allowed the description of Lactococcus garvieae strain M14. This new bacterial strain, isolated from a fermented milk sample from an Algerian cow, is essential in the manufacture of dairy products and seems to play a major role as a biopreservative in the food industry. If the infectious risk is definitively ruled out, it may be a potential probiotic.
Acknowledgements
The authors thank the Xegen Company (http://www.xegen.fr/) for automating the genomic annotation process. VM was supported by a Chairs of Excellence program from the Centre National de la Recherche Scientifique (CNRS). This study was funded by the Fondation Méditerranée Infection.
Conflict of Interest
None declared.
References
- 1.El-Baradei G., Delacroix-Buchet A., Ogier J.C. Biodiversity of bacterial ecosystems in traditional Egyptian Domiati cheese. Appl Environ Microbiol. 2007;73:1248–1255. doi: 10.1128/AEM.01667-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Baradei G., Delacroix-Buchet A., Ogier J.C. Bacterial biodiversity of traditional Zabady fermented milk. Int J Food Microbiol. 2008;121:295–301. doi: 10.1016/j.ijfoodmicro.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 3.Aquilanti L., Santarelli S., Silvestri G., Osimani A., Petruzzelli A., Clementi F. The microbial ecology of a typical Italian salami during its natural fermentation. Int J Food Microbiol. 2007;120:136–145. doi: 10.1016/j.ijfoodmicro.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Schoster A., Kokotovic B., Permin A., Pedersen P.D., Bello F.D., Guardabassi L. In vitro inhibition of Clostridium difficile and Clostridium perfringens by commercial probiotic strains. Anaerobe. 2013;20:36–41. doi: 10.1016/j.anaerobe.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Monteagudo-Mera A., Rodríguez-Aparicio L., Rúa J., Martínez-Blanco H., Navasa N., García-Armesto M.R. In vitro evaluation of physiological probiotic properties of different lactic acid bacteria strains of dairy and human origin. J Funct Foods. 2012;4:531–541. [Google Scholar]
- 6.Bernbom N., Licht T.R., Brogren C.-H., Jelle B., Johansen A.H., Badiola I. Effects of Lactococcus lactis on composition of intestinal microbiota: role of nisin. Appl Environ Microbiol. 2006;72:239–244. doi: 10.1128/AEM.72.1.239-244.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins M.D., Farrow J.A.E., Phillips B.A., Kandler O. Streptococcus garvieae sp. nov. and Streptococcus plantarum sp. nov. J Gen Microbiol. 1983;129:3427–3431. doi: 10.1099/00221287-129-11-3427. [DOI] [PubMed] [Google Scholar]
- 8.Vendrell D., Balcázar J.L., Ruiz-Zarzuela I., de Blas I., Gironés O., Múzquiz J.L. Lactococcus garvieae in fish: a review. Comp Immunol Microbiol Infect Diss. 2006;29:177–198. doi: 10.1016/j.cimid.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Fihman V., Raskine L., Barrou Z., Kiffel C., Riahi J., Bercot B. Lactococcus garvieae endocarditis: identification by 16S rRNA and sodA sequence analysis. J Infect. 2006;52:e3–e6. doi: 10.1016/j.jinf.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Chan J.F., Woo P.C., Teng J.L., Lau S.K., Leung S.S., Tam F.C. Primary infective spondylodiscitis caused by Lactococcus garvieae and a review of human L. garvieae infections. Infection. 2011;39:259–264. doi: 10.1007/s15010-011-0094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C.Y., Shie H.S., Chen S.C., Huang J.P., Hsieh I.C., Wen M.S. Lactococcus garvieae infections in humans: possible association with aquaculture outbreaks. Int J Clin Pract. 2007;61:68–73. doi: 10.1111/j.1742-1241.2006.00855.x. [DOI] [PubMed] [Google Scholar]
- 12.Vinh D.C., Nichol K.A., Rand F., Embil J.M. Native-valve bacterial endocarditis caused by Lactococcus garvieae. Diagn Microbiol Infect Dis. 2006;56:91–94. doi: 10.1016/j.diagmicrobio.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Wilbring M., Alexiou K., Reichenspurner H., Matschke K., Tugtekin S.M. Lactococcus garvieae causing zoonotic prosthetic valve endocarditis. Clin Res Cardiol. 2011;100:545–546. doi: 10.1007/s00392-011-0286-3. [DOI] [PubMed] [Google Scholar]
- 14.Li W.K., Chen Y.S., Wann S.R., Liu Y.C., Tsai H.C. Lactococcus garvieae endocarditis with initial presentation of acute cerebral infarction in a healthy immunocompetent man. Intern Med. 2008;47:1143–1146. doi: 10.2169/internalmedicine.47.0795. [DOI] [PubMed] [Google Scholar]
- 15.Yiu K.H., Siu C.W., To K.K., Jim M.H., Lee K.L., Lau C.P. A rare cause of infective endocarditis; Lactococcus garvieae. Int J Cardiol. 2007;114:286–287. doi: 10.1016/j.ijcard.2005.11.092. [DOI] [PubMed] [Google Scholar]
- 16.Aguado-Urda M., Lopez-Campos G.H., Fernandez-Garayzabal J.F., Martin-Sanchez F., Gibello A., Dominguez L. Analysis of the genome content of Lactococcus garvieae by genomic interspecies microarray hybridization. BMC Microbiol. 2010;10:79. doi: 10.1186/1471-2180-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morita H., Toh H., Oshima K., Yoshizaki M., Kawanishi M., Nakaya K. Complete genome sequence and comparative analysis of the fish pathogen Lactococcus garvieae. PLoS One. 2011;6:e23184. doi: 10.1371/journal.pone.0023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyauchi E., Toh H., Nakano A., Tanabe S., Morita H. Comparative genomic analysis of Lactococcus garvieae strains isolated from different sources reveals candidate virulence genes. Int J Microbiol. 2012;2012:728276. doi: 10.1155/2012/728276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawanishi M., Yoshida T., Yagashiro S., Kijima M., Yagyu K., Nakai T. Differences between Lactococcus garvieae isolated from the genus Seriola in Japan and those isolated from other animals (trout, terrestrial animals from Europe) with regard to pathogenicity, phage susceptibility and genetic characterization. J Appl Microbiol. 2006;101:496–504. doi: 10.1111/j.1365-2672.2006.02951.x. [DOI] [PubMed] [Google Scholar]
- 20.Zadi-Karam H and Karam N-E. Lactic acid bacteria from camel milk. Poster session presented at: 12th Annual International French Congress of Rencontres recherches ruminants; 2005 Dec 7–8; Paris, France.
- 21.Fox G.E., Magrum L.J., Balch W.E., Wolfe R.S., Woese C.R. Classification of methanogenic bacteria by 16S ribosomal RNA characterization. Proc Natl Acad Sci U S A. 1977;74:4537–4541. doi: 10.1073/pnas.74.10.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagesen K., Hallin P., Rodland E.A., Staerfeldt H.H., Rognes T., Ussery D.W. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007;35:3100–3108. doi: 10.1093/nar/gkm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larkin M.A., Blackshields G., Brown N.P., Chenna R., McGettigan P.A., McWilliam H. Clustal W and clustal x version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 25.Seng P., Drancourt M., Gouriet F., La Scola B., Fournier P.E., Rolain J.M. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Infect Dis. 2009;49:543–551. doi: 10.1086/600885. [DOI] [PubMed] [Google Scholar]
- 26.Lohse M., Bolger A.M., Nagel A., Fernie A.R., Lunn J.E., Stitt M. RobiNA: a user-friendly, integrated software solution for RNA-Seq-based transcriptomics. Nucleic Acids Res. 2012;40(Web Server issue):W622–W627. doi: 10.1093/nar/gks540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nurk S., Bankevich A., Antipov D., Gurevich A.A., Korobeynikov A., Lapidus A. Assembling single-cell genomes and mini-metagenomes from chimeric MDA products. J Comput Biol. 2013;20:714–737. doi: 10.1089/cmb.2013.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bankevich A., Nurk S., Antipov D., Gurevich A.A., Dvorkin M., Kulikov A.S. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boetzer M., Henkel C.V., Jansen H.J., Butler D., Pirovano W. Scaffolding pre-assembled contigs using SSPACE. Bioinformatics. 2011;27:578–579. doi: 10.1093/bioinformatics/btq683. [DOI] [PubMed] [Google Scholar]
- 30.Gao S., Sung W.K., Nagarajan N. Opera: reconstructing optimal genomic scaffolds with high-throughput paired-end sequences. J Comput Biol. 2011;18:1681–1691. doi: 10.1089/cmb.2011.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boetzer M., Pirovano W. Toward almost closed genomes with GapFiller. Genome Biol. 2012;13:R56. doi: 10.1186/gb-2012-13-6-r56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laslett D., Canback B. ARAGORN, a program to detect tRNA genes and tmRNA genes in nucleotide sequences. Nucleic Acids Res. 2004;32:11–16. doi: 10.1093/nar/gkh152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griffiths-Jones S., Bateman A., Marshall M., Khanna A., Eddy S.R. Rfam: an RNA family database. Nucleic Acids Res. 2003;31:439–441. doi: 10.1093/nar/gkg006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Punta M., Coggill P.C., Eberhardt R.Y., Mistry J., Tate J., Boursnell C. The Pfam protein families database. Nucleic Acids Res. 2012;40(Database issue):D290–D301. doi: 10.1093/nar/gkr1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nawrocki E.P., Kolbe D.L., Eddy S.R. Infernal 1.0: inference of RNA alignments. Bioinformatics. 2009;25:1335–1337. doi: 10.1093/bioinformatics/btp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hyatt D., Chen G.L., Locascio P.F., Land M.L., Larimer F.W., Hauser L.J. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010;11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Camacho C., Coulouris G., Avagyan V., Ma N., Papadopoulos J., Bealer K. BLAST+: architecture and applications. BMC Bioinform. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eddy S.R. Accelerated profile HMM searches. Nucleic Acids Res. 2011;7:e1002195. doi: 10.1371/journal.pcbi.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.UniProt Consortium Ongoing and future developments at the Universal protein Resource. Nucleic Acids Res. 2011;39(Database issue):D214–D219. doi: 10.1093/nar/gkq1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanehisa M., Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moriya Y., Itoh M., Okuda S., Yoshizawa A.C., Kanehisa M. KAAS: an automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 2007;35(Web Server issue):W182–W185. doi: 10.1093/nar/gkm321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tatusov R.L., Galperin M.Y., Natale D.A., Koonin E.V. The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000;28:33–36. doi: 10.1093/nar/28.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tatusov R.L., Koonin E.V., Lipman D.J. A genomic perspective on protein families. Science. 1997;278(5338):631–637. doi: 10.1126/science.278.5338.631. [DOI] [PubMed] [Google Scholar]
- 44.Drissi F., Buffet S., Raoult D., Merhej V. Common occurrence of antibacterial agents in human intestinal microbiota. Nucleic Acids Res. 2015;6:441. doi: 10.3389/fmicb.2015.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stackebrandt E., Ebers J. Taxonomic parameters revisited: tarnished gold standards. Microbiol Today. 2006;33:152–155. [Google Scholar]
- 46.Tindall B.J., Rossello-Mora R., Busse H.J., Ludwig W., Kampfer P. Notes on the characterization of prokaryote strains for taxonomic purposes. Int J Syst Evol Microbiol. 2010;60(pt 1):249–266. doi: 10.1099/ijs.0.016949-0. [DOI] [PubMed] [Google Scholar]
- 47.Schleifer K.H., Kraus J., Dvorak C., Kilpper-Bälz R., Collins M.D., Fischer W. Transfer of Streptococcus lactis and related streptococci to the genus Lactococcus gen. nov. Syst Appl Microbiol. 1985;6:183–195. [Google Scholar]
- 48.Morita H., Toh H., Oshima K., Murakami M., Taylor T.D., Igimi S. Complete genome sequence of the probiotic Lactobacillus rhamnosus ATCC 53103. J Bacteriol. 2009;191:7630–7631. doi: 10.1128/JB.01287-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chaillou S., Champomier-Verges M.C., Cornet M., Crutz-Le Coq A.M., Dudez A.M., Martin V. The complete genome sequence of the meat-borne lactic acid bacterium Lactobacillus sakei 23K. Nat Biotechnol. 2005;23:1527–1533. doi: 10.1038/nbt1160. [DOI] [PubMed] [Google Scholar]
- 50.Kleerebezem M., Boekhorst J., van Kranenburg R., Molenaar D., Kuipers O.P., Leer R. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc Natl Acad Sci U S A. 2003;100:1990–1995. doi: 10.1073/pnas.0337704100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morita H., Toh H., Fukuda S., Horikawa H., Oshima K., Suzuki T. Comparative genome analysis of Lactobacillus reuteri and Lactobacillus fermentum reveal a genomic island for reuterin and cobalamin production. DNA Res. 2008;15:151–161. doi: 10.1093/dnares/dsn009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Claesson M.J., Li Y., Leahy S., Canchaya C., van Pijkeren J.P., Cerdeno-Tarraga A.M. Multireplicon genome architecture of Lactobacillus salivarius. Proc Natl Acad Sci U S A. 2006;103:6718–6723. doi: 10.1073/pnas.0511060103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Forde B.M., Neville B.A., O'Donnell M.M., Riboulet-Bisson E., Claesson M.J., Coghlan A. Genome sequences and comparative genomics of two Lactobacillus ruminis strains from the bovine and human intestinal tracts. Microb Cell Fact. 2011;10(Suppl. 1):S13. doi: 10.1186/1475-2859-10-S1-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Villani F., Aponte M., Blaiotta G., Mauriello G., Pepe O., Moschetti G. Detection and characterization of a bacteriocin, garviecin L1-5, produced by Lactococcus garvieae isolated from raw cow's milk. J Appl Microbiol. 2001;90:430–439. doi: 10.1046/j.1365-2672.2001.01261.x. [DOI] [PubMed] [Google Scholar]