Abstract
Aims
This study aimed to investigate whether cabotegravir (CAB), an integrase inhibitor in development for treatment and prevention of human immunodeficiency virus‐1, influences the pharmacokinetics (PK) of a levonorgestrel (LNG) and ethinyl oestradiol (EO)–containing oral contraceptive (OC) in healthy women.
Methods
In this open‐label, fixed‐sequence crossover study, healthy female subjects received LNG 0.15 mg/EO 0.03 mg tablet once daily Days 1–10 alone and with oral CAB 30 mg once daily Days 11–21. At the end of each treatment period, subjects underwent predose sampling for concentrations of follicle‐stimulating hormone, luteinizing hormone, and progesterone and serial PK sampling for plasma LNG, EO, and CAB concentrations.
Results
Twenty women were enrolled, and 19 completed the study. One subject was withdrawn due to an adverse event unrelated to study medications. Geometric least squares mean ratios (90% confidence interval) of LNG + CAB vs. LNG alone for LNG area under the plasma concentration–time curve over the dosing interval of duration τ and maximum observed plasma concentration were 1.12 (1.07–1.18) and 1.05 (0.96–1.15), respectively. Geometric least squares mean ratio (90% confidence interval) of EO + CAB vs. EO alone for EO area under the plasma concentration–time curve over the dosing interval of duration τ and maximum observed plasma concentration were 1.02 (0.97–1.08) and 0.92 (0.83–1.03), respectively. Steady‐state CAB PK parameters were comparable to historical values. There was no apparent difference in mean luteinizing hormone, follicle‐stimulating hormone, and progesterone concentrations between periods. No clinically significant trends in laboratory values, vital signs, or electrocardiography values were observed.
Conclusions
Repeat doses of oral CAB had no significant effect on LNG/EO PK or pharmacodynamics, which supports CAB coadministration with LNG/EO OCs in clinical practice.
Keywords: cabotegravir, contraceptive, ethinyl oestradiol, levonorgestrel, pharmacokinetics
What is Already Known about this Subject
In vitro and clinical data suggest that cabotegravir (CAB) is unlikely to cause or be subject to clinically significant drug interactions with the components of hormonal contraceptives. However, before this present study, it was unknown if CAB would impact the pharmacokinetic (PK) profile of levonorgestrel/ethinyl oestradiol (LNG/EO) in healthy female volunteers.
What this Study Adds
Repeat doses of oral CAB had no significant effect on the PK of LNG/EO, and steady‐state CAB PK parameters were similar to previous estimates.
This supports coadministration of CAB with LNG/EO–containing oral contraceptives.
Metabolic and excretory pathways of oral and long‐acting injectable CAB are comparable, supporting extrapolation of these results to the long‐acting formulation of CAB.
Tables of Links
| LIGANDS |
|---|
| Ethinyl oestradiol |
| Follicle‐stimulating hormone |
| Levonorgestrel |
| Luteinizing hormone |
Introduction
Cabotegravir (CAB) is an investigational integrase strand transfer inhibitor in development for the treatment and prevention of human immunodeficiency virus (HIV)‐1 infection. Cabotegravir is formulated as a long‐acting injectable and amenable to monthly or less frequent dosing 3, 4. As both an HIV‐1 treatment and prevention modality, CAB is expected to be coadministered with hormonal contraceptives in HIV‐infected and uninfected women. A critical component of care for HIV‐infected women of childbearing potential includes the incorporation of effective contraceptive methods to reduce the risk of unintended pregnancy and mother‐to‐child transmission 5. Combined oral contraceptives are also commonly used by uninfected women who may seek preventative strategies for both pregnancy and HIV acquisition (i.e., pre‐exposure prophylaxis or PrEP).
In vitro and clinical studies demonstrate CAB has low potential to be a significant perpetrator or victim of clinically significant drug interactions. Cabotegravir is primarily metabolized by uridine diphosphate glucuronosyltransferase (UGT) 1A1 with a minor contribution by UGT 1A9 6. At clinically relevant concentrations, CAB does not inhibit or induce the major cytochrome P450 (CYP) or UGT enzymes in vitro and had no significant effect on the pharmacokinetics (PK) of midazolam, a sensitive CYP3A4 probe substrate 7.
Levonorgestrel/ethinyl oestradiol (LNG/EO) is a popular monophasic combined oral contraceptive that contains a fixed‐dose combination of 0.15 mg LNG and 0.03 mg EO, a synthetic progestin and oestrogen, respectively 8. Combined oral contraceptives such as LNG/EO inhibit ovulation by suppressing the release of follicle stimulating hormone (FSH) and luteinizing hormone (LH) via a negative feedback mechanism on the hypothalamus and pituitary gland. A balance of both the oestrogen and progestin components of OC must be maintained in order to inhibit ovulation and prevent pregnancy effectively. EO and LNG are primarily metabolized by the CYP pathway and are impacted by agents that induce or inhibit these metabolizing enzymes. Coadministration with agents known to induce this metabolic pathway may reduce plasma concentrations of these exogenous hormones, permitting escape ovulation and potentially resulting in contraceptive failure 9, 10. Since EO and LNG are not known inhibitors or inducers of CYP or glucuronyl transferase activity, it is unlikely that either would impact the PK of CAB.
Given the widespread use of hormonal contraceptives and the expected need for coadministration with CAB in both HIV‐infected and uninfected women, and because of uncertainties implicit in extrapolating from in vitro and probe data, the primary objective of this present study was to confirm the lack of effect of CAB on the PK of LNG/EO in healthy women.
Methods
Study design
This was an open‐label, single‐centre, fixed‐sequence crossover study in healthy, HIV‐negative women between the ages of 18 and 45 years, inclusive (NCT02159131; EudraCT Number 2014–001334‐28; Figure 1). Women were eligible to participate if they had a body mass index of 18–30 kg m–2 and body weight ≥50 kg and <114 kg. In addition to once daily LNG/EO, women of childbearing potential were required to use an additional form of effective nonhormonal contraception throughout the study and follow‐up period. Pregnant or lactating women were excluded. Women were ineligible if they had current or chronic history of liver disease, known hepatic or biliary abnormalities, or a positive pre‐study hepatitis B surface antigen or hepatitis C antibody test within 3 months of screening. Potential subjects with a positive alcohol and/or drug test at screening, history of regular alcohol consumption within 6 months of the study (defined as an average weekly intake >14 units), or current or recent use of tobacco‐containing products or subjects who required use of prescription or nonprescription drugs, including vitamins, herbal and dietary supplements within 7 days (or 14 days if the drug was a potential enzyme inducer) or five half‐lives (whichever was longer) before the first dose of study drug, were excluded.
Figure 1.
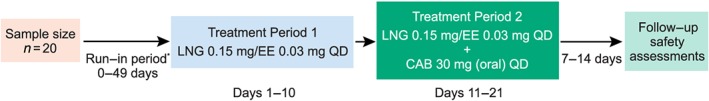
Study design. *Run‐in period was omitted for subjects stabilized on LNG 0.15 mg/EO 0.03 mg and/or could be extended for up to 49 days to synchronize dosing days. No LNG 0.15 mg/EO 0.03 mg was given 7 days prior to Day 1, treatment period 1. EO, ethinyl oestradiol; LNG, levonorgestrel; QD = once daily
Screening assessments were performed within 30 days before Day 1 of the study. Consenting subjects who were not already on a stable regimen of LNG/EO were required to switch to LNG 0.15 mg/EO 0.03 mg once daily for a minimum of one cycle of 21 days to evaluate tolerability before proceeding to the treatment phase of the study. The study consisted of two treatment periods within a single 28‐day cycle. In treatment period 1, subjects received LNG 0.15 mg/EO 0.03 mg alone once daily on Days 1–10. Following PK sampling on Days 10–11, subjects continued to receive LNG 0.15 mg/EO 0.03 mg once daily but in combination with one CAB 30 mg tablet orally once daily on Days 11–21. Subjects were instructed to take study drug at the same designated time each day without regard for food except for intensive PK sampling days (Day 10 and Day 21), when subjects were required to fast for 6 hours before and 4 hours after the study drug dose, which was administered under supervision within the drug research unit. Subjects returned to the unit for an outpatient visit 7–14 days after the last dose of study drug for follow‐up safety assessments. The study was performed at the Quintiles Drug Research Unit at Guy's Hospital in London, UK. Subjects were resident in the research unit for intensive PK sampling on Days 8–11 and Days 19–22.
Written informed consent was obtained from each subject before the performance of any study‐specific procedures. The study was conducted in compliance with Good Clinical Practice and in agreement with the Declaration of Helsinki principles. The protocol and all amendments were reviewed and approved by the Office for Research Committees, Northern Ireland.
PK assessments and analytical methods
Plasma concentrations of LNG and EO were determined predose and at 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12, and 24 hours postdose at the end of each treatment period (Day 10 and Day 21). Predose concentrations of LNG/EO were also assessed on Day 9 and Day 20. CAB concentrations were determined predose on Day 20 and Day 21 and then again at 1, 2, 3, 4, 6, 8, 12, and 24 hours postdose on Day 21.
Plasma samples were analysed for CAB and LNG/EO by Covance Laboratory Inc (Madison, Wisconsin, USA) using a validated analytical method based on protein precipitation, followed by high‐performance liquid chromatography–tandem mass spectrometry analysis for CAB and a validated liquid–liquid extraction with derivatization followed by high‐performance liquid chromatography–tandem mass spectrometry for LNG/EO. The lower limit of quantification for CAB, LNG, and EO was 25 ng ml–1, 150 pg ml–1, and 7.50 pg ml–1, respectively. The CAB analytical method was validated over the range of 25–25 000 ng ml–1. The LNG/EO method was validated over the range of 150–10 000 pg ml–1 and 7.50–500 pg ml–1 for LNG and EO, respectively. Quality control (QC) samples, prepared at three different concentrations for each analyte, were analysed with each sample batch against separately prepared calibration standards. For the analysis to be acceptable, two‐thirds of all the undiluted QC samples and half at each QC concentration could deviate no more than 15% from the nominal concentration. Additionally, incurred sample repeatability was assessed to ensure that original and re‐assay values were within 20% of each other. The analytical runs met all the predefined acceptance criteria.
Plasma CAB, EO, and LNG concentration–time data were analysed by noncompartmental methods using WinNonlin (version 5.2; Pharsight, Cary, NC, USA) and the following steady‐state PK parameters were estimated: area under the plasma concentration–time curve over the dosing interval of duration τ (AUC0–τ), maximum observed plasma concentration (Cmax), concentration at the end of dosing interval (i.e., trough concentration; Cτ), and time of Cmax (tmax). Calculations were based on the actual sampling times recorded during the study.
Pharmacodynamic and safety assessments
Blood samples to measure LH, FSH, and progesterone levels were collected at screening and predose on Days 1, 10, 11, 21, and 22.
Safety assessments including vital signs, 12‐lead electrocardiograms, haematology, and clinical chemistry laboratory tests were performed periodically throughout the study. Adverse events (AEs) and serious AEs were recorded from the start of study treatment through the follow‐up visit (7–14 days after the last dose of study drug). All subjects who enrolled in the study and received at least one dose of study drug were included in the safety analysis. AEs were graded using the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events Version 1.0, August 2009. Demographic and safety data were summarized descriptively.
Statistical analysis
A sample size of 16 subjects was expected to provide >90% power to demonstrate lack of interaction under the bioequivalence limit of 0.8–1.25, assuming a within subject variability of 15% and a true ratio of 1. Twenty female subjects were recruited in order to have at least 16 subjects complete all assessments.
Following loge‐transformation, AUC0–τ, Cmax, and Cτ of EO and LNG were analysed using a mixed effects model with treatment as a fixed effect and subject as a random effect. The effect of coadministration with CAB was estimated by exponentiating the difference in least squares means (LNG/EO plus CAB – LNG/EO alone) and the corresponding 90% confidence interval (CI) to obtain the point estimate and CI for the ratio of means (LNG/EO plus CAB:LNG/EO alone). Lack of effect was to be confirmed if the 90% CIs for AUC0–τ and Cmax geometric least squares (GLS) mean ratios for both LNG and EO were within the bioequivalence bounds of 0.8 and 1.25 11.
Statistical analyses were performed using SAS (Version 9; SAS Institute, Cary, NC, USA).
Results
A total of 20 female subjects of childbearing potential were enrolled and received at least one dose of LNG 0.15 mg/EO 0.03 mg and CAB. Of the 20 subjects, 19 completed dosing and all critical assessments. One subject was withdrawn from the study due to an AE prior to collection of all PK assessments and therefore was not included in the statistical analysis. The majority of subjects were white (18/20; 90%) with a mean (range) age and body mass index of 26.5 (18–39) years and 24.5 (20.2–30.0) kg m–2, respectively.
The mean (standard deviation) concentration–time profiles of LNG and EO are shown in Figure 2A and 2B, respectively, and the geometric mean (95% CI) PK parameters are shown in Table 1.
Figure 2.
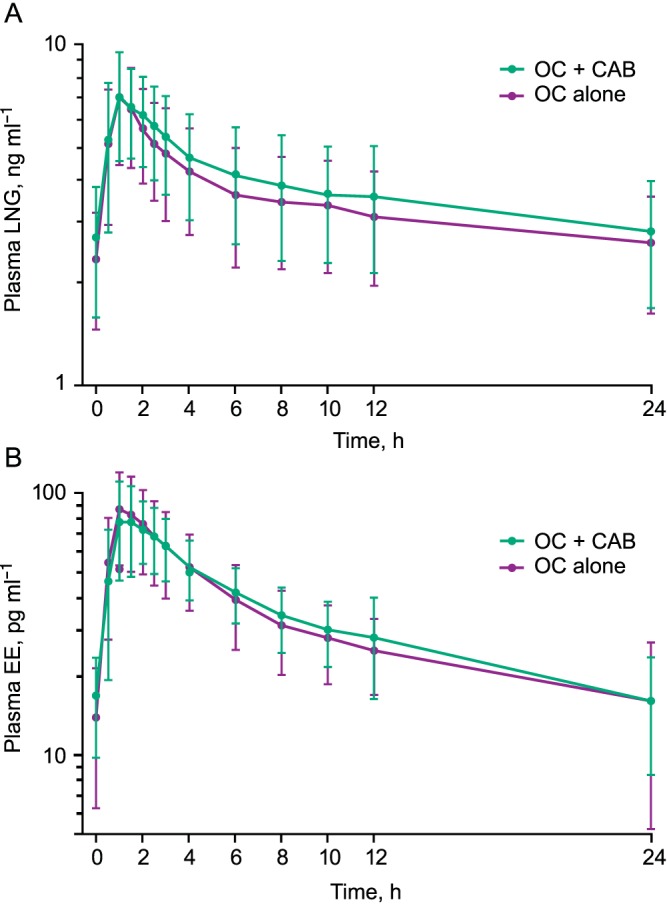
Mean plasma concentration‐time profiles for (A) LNG in treatment period 1 (OC alone) and treatment period 2 (OC + CAB) and (B) EO in treatment period 1 (OC alone) and treatment period 2 (OC + CAB). Error bars represent standard deviation. CAB, cabotegravir; EO, ethinyl oestradiol; LNG, levonorgestrel; OC, oral contraceptive
Table 1.
Summary of LNG and EO PK parameters
| Plasma PK parameter | Geometric mean (95% CI) | GLS mean ratio (90% CI) | |
|---|---|---|---|
| OC alone (n = 19) | OC + CAB (n = 19) | (OC + CAB/OC alone) | |
| LNG | |||
| AUC 0–τ (ng × h ml –1 ) | 77.4 (64.5, 92.9) | 87.0 (72.1, 104.9) | 1.12 (1.07, 1.18) |
| C max (ng ml –1 ) | 6.86 (5.84, 8.06) | 7.20 (6.28, 8.26) | 1.05 (0.96, 1.15) |
| C τ (ng ml –1 ) | 2.41 (1.98, 2.95) | 2.59 (2.09, 3.22) | 1.07 (1.01, 1.15) |
| t max a (h) | 1.00 (0.5–2.5) | 1.00 (0.5–3.0) | ND |
| EO | |||
| AUC 0–τ (pg × h ml –1 ) | 773b (656, 911) | 800c (698, 916) | 1.02 (0.97, 1.08) |
| C max (pg ml –1 ) | 86.2 (72.4, 102.7) | 79.5 (68.0, 92.8) | 0.922 (0.83, 1.03) |
| C τ (pg ml –1 ) | 16.0b (12.5, 20.4) | 15.7c (12.9, 19.0) | 1.00 (0.92, 1.10) |
| t max a (h) | 1.00 (0.5–2.5) | 1.5 (0.5–2.6) | ND |
EO was not quantifiable at 24 h post dose for two subjects receiving OC alone and one subject receiving OC + CAB; therefore, EO AUC0–τ and Cτ were not reported for these cases. AUC0–τ, area under the plasma concentration–time curve over the dosing interval of duration τ; CAB, cabotegravir; Cmax, maximum observed plasma concentration; Cτ, concentration at the end of dosing interval; EO, ethinyl oestradiol; GLS, geometric least squares; LNG, levonorgestrel; ND, not determined; OC, oral contraceptive; PK, pharmacokinetic; tmax, time of Cmax.
Median (range)
n = 17
n = 18
LNG and EO AUC0–τ, Cmax, and Cτ following repeat doses of CAB were comparable to values observed following LNG/EO alone (Table 1). The GLS mean ratio (OC with CAB/OC alone) and 90% CI for each of these parameters fell within the predefined bounds of bioequivalence (0.8–1.25).
Steady‐state CAB PK parameters were estimated following coadministration with LNG/EO. The CAB geometric mean (95% CI) AUC0–τ, Cmax, and Cτ were 133 (121, 148) h × μg ml–1, 7.81 (7.13, 8.56) μg ml–1, and 4.33 (3.87, 4.86) μg ml–1, respectively. Median (range) tmax was 3.00 (1.0–4.2) h.
There were no consistent differences in mean FSH, LH, or progesterone concentrations between treatment periods (Figure 3). The mean maximum observed LH and FSH concentrations in treatment period 1 (LNG/EO alone) were 3.2 mIU ml–1 and 1.8 mIU ml–1, respectively, compared to 1.6 mIU ml–1 and 1.3 mIU ml–1, respectively, in treatment period 2 (LNG/EO + CAB). Mean progesterone levels were below 6 ng ml–1 at all sampled time points within treatment periods 1 and 2.
Figure 3.
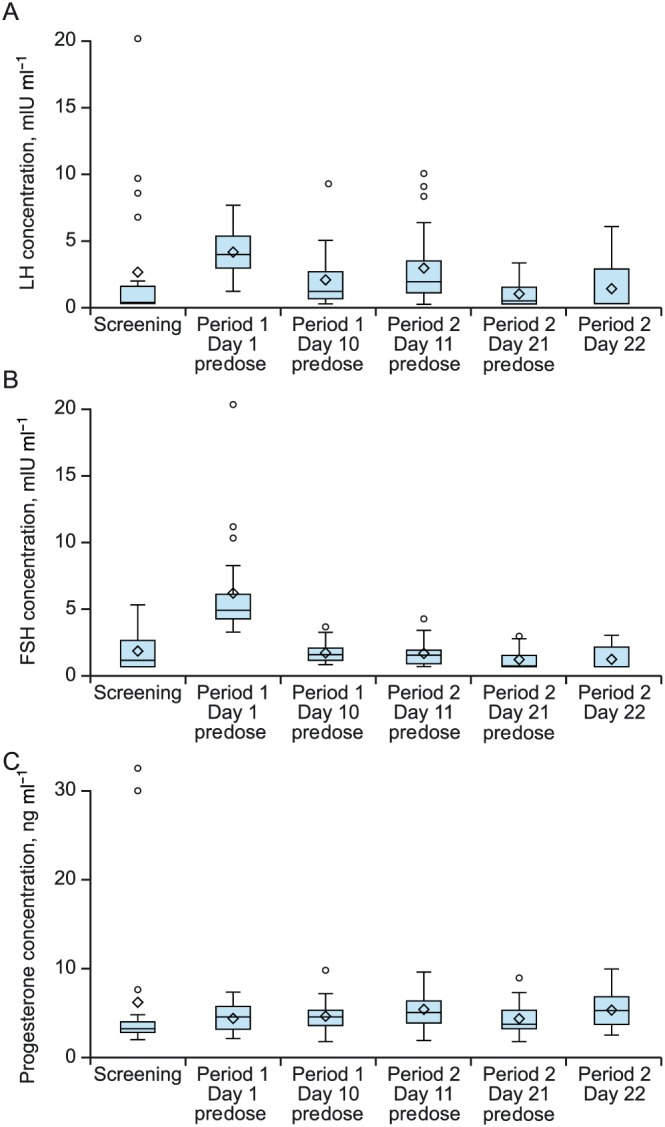
Predose (A) luteinizing hormone, (B) follicle‐stimulating hormone, and (C) progesterone concentration by day and treatment period. The diamond represents the mean, middle hash line is median, and upper quartile and lower quartile are the top and bottom box lines. Lines outside of box are minimum and maximum if no value was above or below 1.5 times the interquartile range. If there are any values over 1.5 times the interquartile range, these values are presented as individual points. FSH, follicle‐stimulating hormone; LH, luteinizing hormone
Thirteen subjects (65%) reported at least one AE. All AEs were of mild‐to‐moderate intensity and occurred at similar rates between treatment period 1 (40%) and treatment period 2 (45%). The most commonly reported AEs were headache (n = 6 [30%]), and nausea (n = 5 [25%]). One subject developed a migraine of Grade 2 intensity that was attributed to study drug by the investigator. The migraine occurred 5 days after the last dose of CAB and resolved in approximately 28 h. There were no Grade 3/4 treatment‐emergent laboratory abnormalities reported in the study. One subject was withdrawn due to an AE of urinary tract infection that required treatment with antibiotics. The AE was not attributed to study drug. There were no serious AEs, deaths, pregnancies, or clinically significant changes in laboratory abnormalities, vital signs, or electrocardiography values observed (data not shown).
Discussion
Coadministration of repeat dose CAB and the oral contraceptive LNG 0.15 mg/EO 0.03 mg had no significant effect on the PK profiles of LNG and EO. The GLS mean ratios and 90% CIs for the differences in steady‐state AUC0–τ, Cmax, and Cτ between test (LNG/EO + CAB) and reference (LNG/EO alone) treatments were each within the limits of 0.80–1.25, confirming a lack of pharmacokinetically significant interaction between CAB and LNG or EO. These findings are consistent with in vitro and clinical drug interaction studies, which suggested that CAB has minimal interaction liability 7, 12, 13. In addition, steady‐state CAB plasma PK parameters observed in this study were comparable to historical values, suggesting that LNG/EO had no impact on CAB exposure, as expected 12, 13.
The pharmacodynamic assessments (predose concentrations of FSH, LH, and progesterone) support the PK results. The LNG 0.15 mg/EO 0.03 mg OC inhibits ovulation by suppressing the release of gonadotropins, FSH and LH. Adequate concentrations of the synthetic oestrogen and progestin components of OC lead to negative feedback inhibition of the release of FSH and LH, thereby inhibiting ovulation and preventing pregnancy. Women stabilized on an OC such as LNG/EO would be expected to maintain suppressed levels of FSH, LH, and progesterone throughout the 21 days of treatment. In this study, no apparent differences in predose concentrations of FSH, LH, or progesterone were observed between treatment periods (Day 10,11 vs Day 21,22). Although of limited frequency, the PD assessments in conjunction with the lack of PK interaction between CAB and LNG/EO suggest that contraceptive efficacy should be maintained during coadministration of CAB and LNG/EO.
Overall, the combination of CAB and LNG/EO demonstrated an acceptable tolerability and safety profile in this study. All AEs were reported as mild to moderate in intensity and only 1 AE was considered by the investigator to be drug‐related (Grade 2 migraine).
Given the absorption rate limited kinetics and associated prolonged apparent terminal phase plasma half‐life of injectable CAB LA (~20–40 days), this drug–drug interaction study was performed using the oral tablet formulation (t1/2 of approximately 40 h) to facilitate enrolment and timely study completion, while limiting prolonged exposure to the long‐acting investigational product in healthy women of reproductive potential 3. The CAB 30 mg oral tablet is the formulation currently used in Phase 2 studies as a short‐term oral lead‐in therapy (30 mg once daily) to assess safety and tolerability prior to injection, and provides average plasma concentrations in excess of those maintained by therapeutic dosing of long‐acting CAB. Because the pathways of metabolism and excretion are comparable between the oral and injectable formulation, it is expected that the results of this drug interaction study can be extrapolated to long‐acting CAB. Combined and progestin‐only contraceptives are also available as long‐acting injectables and are administered every 1–3 months depending on product and formulation. Progestin‐only products such as norethisterone enanthate and depot medroxyprogesterone acetate are commonly prescribed, specifically in sub‐Saharan Africa, where it is estimated that approximately one‐third of contraceptive use is via injectable formulations 14, 15. Although the metabolic pathways for such products are complex and vary somewhat from EO and LNG, the current results, in conjunction with CAB being neither a significant inhibitor nor inducer of CYP or UGT isoenzymes at clinically relevant concentrations, support CAB use with caution with other combination or progestin‐only oral or injectable contraceptives. The potential to coordinate the administration of long‐acting CAB injections for either the treatment or prevention of HIV‐1 with administration of a long‐acting injectable contraceptive may be an attractive combination for women of child‐bearing potential seeking treatment or preventative options that do not require daily or coitally dependent administration.
In conclusion, this study demonstrates that coadministration of repeat dose CAB and the OC LNG 0.15 mg/EO 0.03 mg had no significant effect on the PK profile of LNG and EO or PD effects. These data provide confidence that CAB can be administered in combination with LNG and EO‐containing OCs without clinically significant drug–drug interactions and indicate contraceptive efficacy should be maintained.
Competing Interests
There are no competing interests to declare.
This study was sponsored by ViiV Healthcare and operationalized by GlaxoSmithKline. The authors wish to thank the subjects who took part in this study and the study team at Quintiles, London, UK. The authors would also like to thank Steve Piscitelli and Gary Bowers for their contributions to this study. The authors wish to acknowledge the following individual for editorial assistance during the development of this manuscript: Diane Neer. Funding for this work was provided by ViiV Healthcare.
S.F., E.G., Y.L., A.B., W.S., and P.P. were employees of GSK at the time of study conduct., C.T. was a post‐doctoral fellow on assignment at GlaxoSmithKline at the time of study conduct. C.T., A.B., W.S., and P.P. are currently employed by and own stock in ViiV Healthcare/GlaxoSmithKline. S.F., Y.L., and C.H. are currently employed by Parexel International. E.G. is currently employed by PPD.
Contributors
E.G. and A.B. contributed to the development of the study design, data analysis and interpretation, and preparation of this manuscript. A.B. served as the medical monitor. C.T., S.F., Y.L., C.H., and W.S. contributed to study design, data analysis and interpretation, and preparation of this manuscript. P.P. contributed to data analysis, interpretation, and preparation of this manuscript. J.R. served as the principal investigator and was responsible for the clinical care of the study subjects. J.R. contributed to the protocol development and preparation of this manuscript. All listed authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors.
Trezza, C. , Ford, S. L. , Gould, E. , Lou, Y. , Huang, C. , Ritter, J. M. , Buchanan, A. M. , Spreen, W. , and Patel, P. (2017) Lack of effect of oral cabotegravir on the pharmacokinetics of a levonorgestrel/ethinyl oestradiol‐containing oral contraceptive in healthy adult women. Br J Clin Pharmacol, 83: 1499–1505. doi: 10.1111/bcp.13236.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44: D1054–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 2015; 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spreen W, Ford SL, Chen S, Wilfret D, Margolis D, Gould E, et al. GSK1265744 pharmacokinetics in plasma and tissue after single‐dose long‐acting injectable administration in healthy subjects. J Acquir Immune Defic Syndr 2014; 67: 481–486. [DOI] [PubMed] [Google Scholar]
- 4. Spreen W, Williams P, Margolis D, Ford SL, Crauwels H, Lou Y, et al. Pharmacokinetics, safety, and tolerability with repeat doses of GSK1265744 and rilpivirine (TMC278) long‐acting nanosuspensions in healthy adults. J Acquir Immune Defic Syndr 2014; 67: 487–492. [DOI] [PubMed] [Google Scholar]
- 5. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV‐1‐infected adults and adolescents. Department of Health and Human Services. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. (last accessed 28 March 2016 [HIV‐Infected Women; pg I20–26]).
- 6. Bowers GD, Culp A, Reese MJ, Tabolt G, Moss L, Piscitelli S, et al. Disposition and metabolism of cabotegravir: a comparison of biotransformation and excretion between different species and routes of administration in humans. Xenobiotica 2016; 46: 147–162. [DOI] [PubMed] [Google Scholar]
- 7. Reese MJ, Bowers GD, Humphreys JE, Gould EP, Ford SL, Webster LO, et al. Drug interaction profile of the HIV integrase inhibitor cabotegravir: assessment from in vitro studies and a clinical investigation with midazolam. Xenobiotica 2016; 46: 445–456. [DOI] [PubMed] [Google Scholar]
- 8. Microgynon, Summary of Product Characteristics. Available at http://www.medicines.org.uk/EMC/medicine/1827/SPC/Microgynon+30. Updated November 18, 2014 (last accessed 20 May 2016).
- 9. Scarsi KK, Darin KM, Nakalema S, Back DJ, Byakika‐Kibwika P, Else LJ, et al. Unintended pregnancies observed with combined use of the levonorgestrel contraceptive implant and efavirenz‐based antiretroviral therapy: a three‐arm pharmacokinetic evaluation over 48 weeks. Clin Infect Dis 2016; 62: 675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crawford P, Chadwick DJ, Martin C, Tjia J, Back DJ, Orme M. The interaction of phenytoin and carbamazepine with combined oral contraceptive steroids. Br J Clin Pharmacol 1990; 30: 892–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schuirmann DJ. A comparison of the two one‐sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm 1987; 15: 657–680. [DOI] [PubMed] [Google Scholar]
- 12. Ford SL, Gould E, Chen S, Lou Y, Dumont E, Spreen W, et al. Effects of etravirine on the pharmacokinetics of the integrase inhibitor S/GSK1265744. Antimicrob Agents Chemother 2013; 57: 277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ford SL, Gould E, Chen S, Margolis D, Spreen W, Chauwels H, et al. Lack of pharmacokinetic interaction between rilpivirine and integrase inhibitors dolutegravir and GSK1265744. Antimicrob Agents Chemother 2013; 57: 5472–5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ross JA, Agwanda AT. Increased use of injectable contraception in sub‐Saharan Africa. Afr J Reprod Health 2012; 16: 68–80. [PubMed] [Google Scholar]
- 15. World Health Organization , US Agency for International Development , Family Health International . Community‐based health workers can safely and effectively administer injectable contraceptives: conclusions from a technical consultation. 2009. Available at http://www.who.int/reproductivehealth/publications/family_planning/WHO_CBD_brief/en/ (last accessed 20 May 2016).


