Abstract
Aims
Dipeptidyl peptidase 4 inhibitors (DPP4is) are suggested as a second‐ and third‐line antidiabetic treatment for type 2 diabetes. Previous studies assessed only the cardiovascular effects of DPP4is as a second‐line treatment, included sulphonylurea as the only comparator, and yielded inconclusive results on the risk of heart failure. The present study therefore evaluated the comparative cardiovascular risks of DPP4is with other second‐ and third‐line antidiabetic drugs.
Methods
Based on a large nationwide diabetic cohort, 113 051 patients with type 2 diabetes newly on metformin‐based dual or triple therapy were identified in 2009–2011 and followed until 2013, or death if this occurred sooner. Primary interest targeted hospitalizations for ischaemic stroke, myocardial infarction and heart failure. Secondary outcomes were hypoglycaemia and all‐cause mortality. Cox proportional hazards models were performed to assess time‐to‐event hazard ratio between propensity score‐matched antidiabetic treatment groups.
Results
DPP4is as a second‐line add‐on to metformin had a significantly lower stroke risk [hazard ratio (HR) 0.817 (95% confidence interval 0.687, 0.971)] and all‐cause mortality [HR 0.825 (0.687, 0.992)] than those for sulphonylurea. DPP4is as a third‐line add‐on to metformin and sulphonylurea combined dual therapy had a significantly lower risk for stroke [HR 0.826 (0.740, 0.923)] and all‐cause mortality [HR 0.784 (0.701, 0.878)] than those for acarbose, and significantly lower risks for stroke [HR 0.653 (0.542, 0.786)], heart failure [HR 0.721 (0.568, 0.917)] and all‐cause mortality [HR 0.689 (0.594, 0.703)] than those for meglitinide.
Conclusions
DPP4is as a second‐ or third‐line add‐on treatment provided cardiovascular benefits and posed no increased risks for heart failure, hypoglycaemia or death.
Keywords: cardiovascular diseases, dipeptidyl peptidase 4 inhibitors, hypoglycaemia, type 2 diabetes
What is Already Known About this Subject
Dipeptidyl peptidase 4 inhibitors (DPP4is) are recommended as a second‐ and third‐line add‐on treatment with other antidiabetic drugs. With their high price, DPP4is are commonly prescribed as a third‐line add‐on to combination therapy with metformin and suphonylurea for those with inadequate glycaemic control.
Current evidence suggests only that DPP4is have cardiovascular effects as a second‐line treatment, describing their relative effects compared with sulphonylurea, and yields inconclusive results on the risk of heart failure.
In April 2016, the Food and Drug Administration in the United States warned of the potential increased risk of heart failure with use of DPP4is, and suggested that a longitudinal evaluation be carried out.
What this Study Adds
This large population‐based longitudinal study confirms previous evidence of the cardiovascular benefits of DPP4is as a second‐line add‐on treatment to metformin and adds evidence about the favourable cardiovascular outcomes of DPP4is as a third‐line add‐on treatment to the metformin and sulphonylurea dual regimen.
The use of DPP4is as a second‐ or third‐line treatment provides cardiovascular benefits, reduces all‐cause mortality and poses no increased risk of heart failure or hypoglycaemia over those of other antidiabetic drugs (i.e. acarbose, meglitinide and thiazolidinediones).
DPP4is as a second‐ or third‐line treatment in type 2 diabetes patients who have inadequate glycaemic control under metformin monotherapy might alter the future risks of developing cardiovascular diseases.
Tables of Links
| TARGETS |
|---|
| Enzymes 2 |
| Dipeptidyl peptidase 4 |
Introduction
With the growing prevalence of type 2 diabetes mellitus (T2DM) worldwide, this disease now contributes considerably to morbidity and mortality 3, 4. Cardiovascular diseases (CVDs) are the leading causes of mortality in patients with T2DM 5. Antidiabetic drugs are used to improve glycaemic control and to help to reduce further the risk of developing CVDs 6. Dipeptidyl peptidase 4 inhibitors (DPP4is), newly available antidiabetic drugs, are recommended as second‐ and third‐line add‐on treatment to other antidiabetic drugs 7. With their high price, DPP4is are commonly prescribed as a third‐line add‐on for those with inadequate glycaemic control on combination therapy with metformin (Met) and sulphonylurea (SU). In addition to improved glycaemic control, glucagon‐like peptide‐1 (GLP‐1)‐induced myocardial protection has been proposed for cardiovascular protection by DPP4is 8. As a second‐line add‐on therapy to metformin, DPP4is were associated with a lower risk for major adverse cardiovascular events (MACEs) 9, 10, 11, 12, 13, 14 and all‐cause mortality 12, 15 compared with SU. In addition, a large observational study of 127 555 T2DM patients in Italy showed a significantly lower risk of heart failure (HF) with use of DPP4is as compared with SU 14 However, the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction 53 (SAVOR‐TIMI 53) trial 16 and a recent large observational study from the US 17 found an increased risk of HF with DPP4is. A recent study by Ou et al. 18, based on the 2009–2013 Taiwan National Health Insurance Research Database (NHIRD), showed that, compared with users of a combination of Met and SU (Met + SU), Met + DPP4i users had significantly lowered risks for all‐cause mortality, stroke, and hypoglycaemia, but there was no effect on the risks for myocardial infarction (MI) and HF 18. However, most previous studies have focused only on DPP4is as a second‐line add‐on 12, 15, 18, 19, 20, included SU as the sole comparator 12, 15, 18, 20 and assessed only hypoglycaemia as a safety endpoint 18. No studies study has assessed the cardiovascular outcomes of DPP4is as a third‐line treatment. In addition, although DPP4is appear to provide cardiovascular benefits (i.e. a low risk for MACEs 9, 10, 11, 12, 13, 14, 20 and stroke 18), previous study findings on the HF risk with use of DPP4is have been inconsistent 16, 17, 18, 19, 21. In April 2016, the US Food and Drug Administration (FDA) warned of the potentially increased risk of HF with the use of two DPP4i drugs – saxagliptin and alogliptin – and urged healthcare professionals and patients to report cardiovascular events involving DPP4is 22. However, Taiwan's FDA has no regulatory labelling on DPP4is with regard to their potentially elevated risk of HF. This is, in part, because of a lack of local data to confirm this safety concern in the Taiwanese population, which highlights the need for research in this area.
Against this background, the present study sought to utilize a nationwide longitudinal cohort of diabetic patients to conduct a comprehensive evaluation on cardiovascular risks and safety issues (i.e. hypoglycaemia) with the use of DPP4is, compared with other second‐ and third‐line antidiabetic treatments, including SU, meglitinide (MEG), thiazolidinediones (TZD) and acarbose.
Materials and methods
The Institutional Review Board (IRB) of the National Cheng Kung University Hospital approved the study before commencement (A‐ER‐103‐298). Patients’ informed consents were waived by the IRB because the study was retrospective and based on secondary data (i.e. the NHIRD), in which people had been de‐identified.
Data source
We utilized the Longitudinal Cohort of Diabetes Patients (LHDB) 1996–2013 from the NHIRD. Taiwan's NHIRD is population based and derived from the claims data from the National Health Insurance (NHI) programme, a mandatory‐enrolment, single‐payment system that covers over 99% of Taiwan's population 23. The LHDB is a valid national dataset that consists of a random sample of 120 000 de‐identified all diabetes incident cases from each calendar year, who were tracked back to 1996 and followed up to 2013 to establish a longitudinal cohort. The LHDB is most representative of Taiwan's diabetic population and provides a research opportunity to evaluate the long‐term health outcomes of patients.
Cohort
From the LHDB, we selected patients aged ≥20 years with a diagnosis of T2DM [International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) = 250.0X‐250.9X, where X = 0 or 2] during 2009–11. Study subjects treated with two and three antidiabetic drugs were classified into dual and triple therapy cohorts, respectively. Pharmacotherapy for T2DM is typically based on a stepped‐up scheme, where patients begin with monotherapy, then dual and triple therapy, and finally rely on a combination injectable therapy (e.g., Met+DPP4i+insulin) 24. More add‐on drugs imply more severe diabetes cases. So, patients treated with dual therapy are likely to be different from those on triple therapy. With this in mind, we stratified patients by the number of antidiabetic drugs they were on (i.e. dual or triple) and analysed them separately.
For the dual therapy cohort, we first selected the cases with stable use of Met. Stable Met users were defined as those who had more than three consecutive Met refills during 2009–2011 and any gaps between two consecutive refills of fewer than 30 days. The definition of ‘three consecutive refills’ was applied because, for a chronic disease population such as diabetes in Taiwan, when a patient has had three or more consecutive refills on a certain medication, he or she is considered to be stable on this treatment and likely to continue with it. This criterion has been used in previous diabetes studies to define the stable use of antidiabetic medications 25, 26, 27. The date of the first claim for addition of the second‐line antidiabetic drug to Met in 2009–2011 was defined as the index date. Patients with a history of dual antidiabetic therapy at 180 days before the index date were excluded, to ensure that only new dual therapy users during 2009–2011 were included. For the triple therapy cohort, we first assessed the pattern of Met‐based triple regimens. We found that Met and SU (Met + SU) combination regimens, including Met + SU + DDP4i, Met + SU + acarbose (ACA), Met + SU+ TZDs, and Met + SU + MEG, were most commonly prescribed. We therefore selected the cases on Met + SU‐based triple therapies in the analysis. We first identified stable Met + SU combination users in 2009–2011 (i.e. the patients who had been stable on a combination regimen of Met + SU during 2009–2011). As mentioned above, ‘stable’ use was defined as ‘ more than three consecutive refills on a Met + SU combination regimen during 2009–2011 and any gaps between two consecutive refills of fewer than 30 days’. The date of the first claim for addition of a third‐line antidiabetic treatment to the Met + SU regimen in 2009–2011 was then defined as the index date. We excluded those with a history of triple therapy before the index date (naïve to the third‐line add‐on drug in the 180 days prior to the index date), so only new triple therapy users during 2009–2011 were included.
Exposure to antidiabetic drugs
Medication utilization was identified in the NHIRD. The Anatomical Therapeutic Chemical (ATC) Classification System was used for classification of the active ingredients of drugs. Dual therapy users were classified into five mutually exclusive groups according to the first second‐line add‐on antidiabetic drug to Met in 2009–2011, including SU, DPP4is, TZDs, ACA and MEG. Triple therapy users were classified into four mutually exclusive groups according to the first third‐line add‐on antidiabetic drug to the Met + SU dual regimen during 2009–2011, including DPP4is, TZDs, ACA and MEG. As the main interest of the present study was to assess the comparative effects between DPP4is and other oral antidiabetic agents (OADs), insulin use was not analysed. The clinical use/placement of DPP4is in Taiwan is likely to be comparable with that of OADs rather than insulin. Prescribers there are likely to make a selection between DPP4is and other OADs, but not insulin, in treating diabetes. A previous study in the Taiwanese population with T2DM showed that the patients treated with insulin had already used 2.7 ± 0.7 OADs before insulin treatment 28. Insulin therapy in Taiwan is often administered to patients with acute medical conditions or treatment failure of three OADs on their maximum doses. Therefore, insulin users are likely to be those with advanced diabetes who required more intensive glycaemic control (i.e. using insulin) compared with those with OADs only. Insulin users at baseline may be clinically different from those with OADs, and the inclusion of these insulin users may have confounded our findings. Therefore, the potential effect of exposure to insulin was not analysed in the present study. When using observational data, propensity score (PS) matching is commonly used to reduce the bias due to a lack of distribution overlap and that due to different density weighting 29. A PS is generally defined as the probability of study participants receiving a treatment based on observed characteristics 30. PS and matching algorithm allow scholars to reconstruct counterfactuals using observation data and to estimate the causal effect 31. In the present study, we adapted the PS matching using the nearest neighbour approach without replacement within a caliper of 0.025 on the PSs at a fixed ratio of 1:1 30, to match the two comparison treatment groups (i.e. Met + SU vs. Met + DPP4i). A previous study on variables selection for PS suggested that it is preferable to include either the variables that affect the outcome or those that affect both treatment selection and the outcome 32. The PSs were computed by using a logistic regression model, where treatment status (i.e. receipt of Met + SU vs. Met + DPP4i) was a dependent variable and there was a list of independent variables which were considered to be associated with the selection of OAD and cardiovascular outcomes of interest, including demographics (i.e. age, gender), comorbidity history [hypertension, hyperlipidaemia, coronary artery disease (CAD), a composites score – Charlson comorbidity index 33], diabetic complications (measured via the adapted diabetes complication severity index 34), CVD history (stroke, MI, HF),and CVD medication history (α‐blockers, β‐blockers, angiotensin receptor blockers (ARBs), angiotensin‐converting enzyme inhibitors (ACEIs), antiplatelet agents, anticoagulants, diuretics, digoxin, and nitrates). For dual therapies, the Met + SU group served as a reference group for PS matching because this combination is the most conventional and commonly used regimen 25. As Met + SU + DPP4i is the most commonly prescribed triple regimen in patients receiving triple therapy in Taiwan 25, we selected, in PS matching, patients with the Met + SU + DPP4i to form three study groups that has PS distributions comparable to each of the three triple therapy groups (i.e. Met + SU + TZDs, Met + SU + ACA and Met + SU + MEG).
Study outcomes
The primary outcomes of interest were MACEs [a composite outcome of nonfatal hospitalizations for ischaemic stroke (ICD‐9: 430–438), MI (ICD‐9: 410, 412) and HF (ICD‐9: 428)] and individual CVD components (i.e. nonfatal stroke, MI and HF). The safety endpoint was an emergency room visit or hospitalization for hypoglycaemia (ICD‐9: 250.8, 251.0, 251.1, 251.2). Mortality status was ascertained from inpatient files from the NHIRD, which indicate if the patient expired during the hospitalization (coded as ‘4’), if the patient was in the acute stage of their illness when discharged (coded as ‘A’), or if the patient was discharged against medical advice (coded as ‘5’), which mostly occurs in Taiwan when there is no further effective treatment for sustaining life and the patient and/or the family wishes to bring the patient home for comfort. Additionally, we used the disenrollment records from the NHIRD registration files of beneficiaries to confirm mortality. As NHI enrolment is mandatory for all residents of Taiwan, the most common reason that can allow disenrollment is death. A previous validation study in Taiwan indicated that the death records in the NHIRD were in high accordance with those in the catastrophic illness registry (CIR) and the in‐hospital electronic medical records (EMR) 35. The CIR and EMR both had death records and thus served as a gold standard in the validation study 35.
Statistics
Our primary analysis was performed as an ‘intent‐to‐treat’ analysis, where all patients were followed from the index date until death, withdrawal from the NHI programme or the end of 2013 – whichever came first. The crude incidence rate of events (i.e. CVD) was calculated as the total number of events over the follow‐up period, divided by person‐years at risk. The person‐years at risk was defined as the sum of patients from the index date (i.e. the start date of dual or triple therapy) to the diagnosis of the first event, dropout from the NHI programme, death, or 31 December 2013, whichever came first. Cox proportional hazards regression was applied to evaluate the time to event between two matched groups. The graph of the log[−log(survival)] vs. the log of survival time graph (using the PROC LIFETEST function in SAS, SAS® software, version 9.4 SAS Institute, Cary, NC, USA) resulted in parallel curves, implying that the variables in the Cox model satisfied the proportional hazard assumption 36. Hazard ratios (HRs) and their 95% confidence intervals (CIs) were computed. Subgroup analyses were conducted for the patients with or without a history of CVD (including stroke, MI, HF and CAD). Several sensitivity analyses were conducted. First, we modified the PS‐matching ratio to 4:1 (controls to cases). Typically, increasing the number of matched controls up to a ratio of about 4/1 improves the power of the study. However, such a rise is not linear; beyond a ratio of about 4/1 37, there is little improvement in the power of the results beyond that of increasing the number of controls 38. Boosting the ratio of controls to cases affects the confidence interval (the precision of the results) and increases the power of the study to find an association 39. Secondly, we added Met persistent use (medication possession ratio, MPR ≥ 0.7) as one of inclusion criteria in the follow‐up, to eliminate the potential bias of non‐Met adherence. Thirdly, we applied ‘on treated’ analysis, where patients were censored (the observation was ended) at 90 days after they were switched to another antidiabetic drug or had one added on, or discontinued treatment. Fourthly, we adjusted for potential competing causes of death in the Cox model analyses by using Fine and Gray's method 40, 41. A P value of <0.05 was considered statistically significant. SAS version 9.4 was utilized for analysis.
Results
A total of 113 051 patients with T2DM newly on metformin‐based therapies (68 967 dual therapy users, 44 084 triple therapy users) were identified (Figure 1). Among patients on dual therapy, Met + SU users accounted for the majority of patients (76.8%), following by Met + DPP4i users (9.0%). For triple therapies, Met + SU‐based triple regimens accounted for the majority of patients (n = 42 662, 96.77%), with most patients prescribed a DPP4i as a third‐line add‐on (40.27%), followed by ACA (27.60%). Table 1 shows the characteristics of the patients in the two matched dual therapy regimens. After matching, the Met + SU group had higher percentages of patients with a history of hyperlipidaemia and using an ACEI/ARB compared with the Met + DPP4i group. As shown in Table 2, there was no significant difference in patients' baseline characteristics between the two matched triple regimen user groups. After PS matching, the Met + DPP4i group had lower crude rates of MACEs, stroke, MI, death, and hypoglycemia compared with their matched Met + SU referents (Table 3). In addition, the Met + SU + DPP4i group had lower crude rates of MACEs, stroke, AMI, death and hypoglycaemia compared with their matched Met + SU + ACA or Met + SU + MEG referents. Tables 4 and 5 present the results from Cox model analyses of the comparative risks for CVD, all‐cause mortality and hypoglycaemia between the two matched dual regimens and between the two matched triple regimens, respectively. In the analyses that compared the dual therapy cohort, the Cox model also controlled for differences in patients' characteristics between the two matched groups (as shown in Table 1) as covariates. As there was no statistical difference in patients' characteristics between the two matched triple regimen groups (Table 2), the Cox model analyses for the triple regimens did not control for any covariates (only treatment status – e.g. receipt of Met + SU + DPP4i vs. Met + SU + TZDs in the model). Table 4 and Figure 2 indicate that, compared with Met + SU referents, only Met + DPP4i users had significantly lower risks for MACEs, stroke, all‐cause mortality and hypoglycaemia. Table 5 and Figure 3 show that Met + SU + DPP4i users had significantly lowered risks for MACEs, stroke and all‐cause mortality compared with their matched Met + SU + ACA referents. Met + SU + DPP4i users also had lower risks for MACEs, stroke, HF, all‐cause mortality and hypoglycaemia compared with their matched Met + SU + MEG referents. Subgroup analysis of patients with and without a history of CVD in dual and triple therapy cohorts also showed consistent trends (Tables 4 and 5). The sensitivity analyses for dual and triple therapies yielded results similar to those of the primary analysis (Tables S1 and S2).
Figure 1.
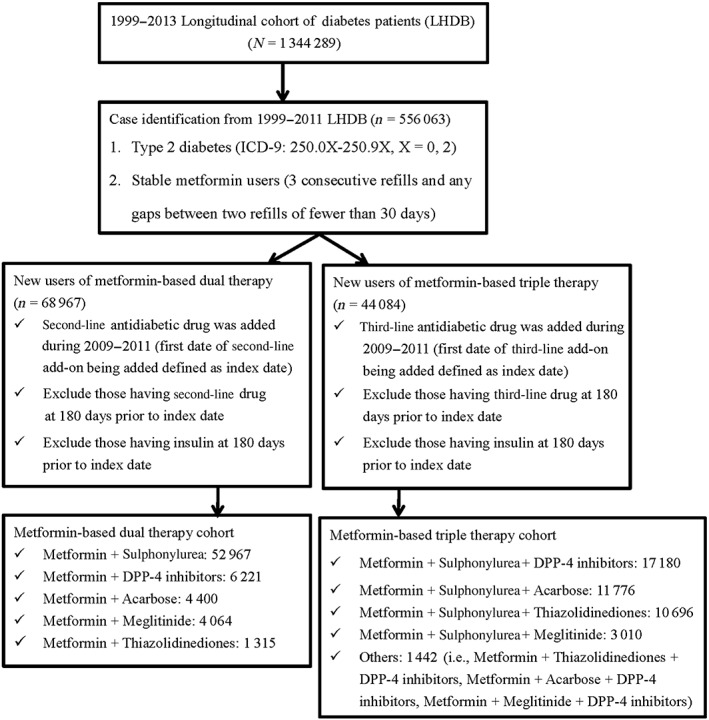
Flow chart of case selectionDPP‐4, dipeptidyl peptidase 4; ICD‐9, International Classification of Diseases, Ninth Revision
Table 1.
Baseline patient characteristics of metformin‐based dual regimen users
| Dual therapy cohort | Before PS matching | After PS matching | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
Met + SU (n = 52 967) |
Met + DPP4i (n = 5980) |
1:1 matched Met + SU |
Met + TZDs (n = 1315) |
1:1 matched Met + SU |
Met + ACA (n = 4386) |
1:1 matched Met + SU |
Met + MEG (n = 4050) |
1:1 matched Met + SU | |
| Person‐years (mean) | – | 3.20 | 3.42 | 3.62 | 3.40 | 3.32 | 3.37 | 3.25 | 3.30 |
| Age (mean ± SD) | 55.7 ± 12.3 | 57.2 ± 12.5 | 57.1 ± 12.4 | 57.6 ± 12.0 | 58.1 ± 12.7 | 58.1 ± 13.0 | 58.3 ± 12.3 | 59.1 ± 13.5 | 59.3 ± 12.7 |
| Male (%) | 57.41% | 52.41% | 51.76% | 54.90% | 52.32% | 52.19% | 50.71% | 56.0% | 55.2% |
| Comorbidity history | |||||||||
| Hypertension (%) | 51.86 | 57.41 | 58.91 | 61.90 | 62.89 | 59.69 | 61.88* | 58.00 | 58.90 |
| Hyperlipidaemia (%) | 44.57 | 55.74 | 57.99* | 59.70 | 62.66 | 55.65 | 58.76** | 47.01 | 48.12 |
| Stroke (%) | 6.54 | 8.24 | 8.55 | 7.76 | 8.14 | 9.03 | 9.26 | 12.41 | 13.01 |
| Heart failure (%) | 2.29 | 2.64 | 3.04 | 1.90 | 2.51 | 2.55 | 2.80 | 4.32 | 4.50 |
| CAD (%) | 10.63 | 15.64 | 16.51 | 13.46 | 14.98 | 17.76 | 18.56 | 15.20 | 16.51 |
| CCI (mean ± SD) | 2.9 ± 1.0 | 3.0 ± 1.5 | 3.1 ± 1.5 | 3.0 ± 1.4 | 3.1 ± 1.5 | 3.2 ± 1.5 | 3.3 ± 1.7 | 3.4 ± 1.8 | 3.4 ± 1.8 |
| aDCSI (mean ± SD) | 0.6 ± 1.1 | 0.8 ± 1.3 | 0.8 ± 1.4 | 0.7 ± 1.2 | 0.8 ± 1.3 | 0.8 ± 1.3 | 0.9 ± 1.4 | 0.9 ± 1.5 | 1.0 ± 1.6 |
| Diabetes duration (years) | 1.5 ± 2.6 | 4.1 ± 3.4 | 4.2 ± 3.5 | 4.0 ± 3.4 | 4.0 ± 3.6 | 3.5 ± 3.3 | 3.5 ± 3.5 | 3.2 ± 3.4 | 3.2 ± 3.5 |
| Metformin duration (years) (mean ± SD) | 0.6 ± 1.5 | 2.7 ± 2.6 | 2.6 ± 2.8 | 2.6 ± 2.7 | 2.4 ± 2.9 | 2.2 ± 2.6 | 2.1 ± 2.7 | 1.9 ± 2.6 | 1.9 ± 2.7 |
| Medication history | |||||||||
| α‐blocker (%) | 2.49 | 3.16 | 2.99 | 3.95 | 3.80 | 3.40 | 3.29 | 3.70 | 3.57 |
| β‐blocker (%) | 16.52 | 18.60 | 19.85 | 19.24 | 18.40 | 20.77 | 21.66 | 17.51 | 18.41 |
| Diuretics (%) | 12.96 | 10.84 | 11.09 | 12.62 | 11.94 | 14.59 | 15.48 | 15.02 | 15.52 |
| CCB (%) | 26.97 | 29.15 | 29.73 | 35.82 | 36.58 | 34.22 | 35.23 | 32.41 | 32.80 |
| AECI/ARB (%) | 28.66 | 43.48 | 45.38* | 46.08 | 49.05 | 41.81 | 43.87 | 36.80 | 37.73 |
| Lipid‐lowering agent (%) | 29.77 | 47.61 | 49.30 | 50.42 | 55.29* | 43.37 | 47.20*** | 34.24 | 35.92 |
| Antiplatelet agent (%) | 19.04 | 29.16 | 29.62 | 32.02 | 32.78 | 30.37 | 32.12 | 27.10 | 27.72 |
| Nitrates (%) | 2.52 | 4.83 | 5.30 | 3.73 | 4.11 | 4.79 | 4.90 | 3.83 | 4.31 |
| Anticoagulant (%) | 0.51 | 0.97 | 1.07 | 0.91 | 0.99 | 0.66 | 0.75 | 0.81 | 0.70 |
| Digoxin (%) | 1.29 | 1.49 | 1.77 | 1.14 | 0.91 | 1.28 | 1.39 | 2.32 | 2.41 |
Baseline patient characteristics were measured 1 year prior to the second‐line antidiabetic drug being added (index date). Diabetes duration: from diabetes diagnosis to the beginning of dual antidiabetic therapy
ACA, acarbose; ACEI/ARB, angiotensin II‐converting enzyme inhibitor/angiotensin receptor blocker; aDCSI, adapted diabetes complication severity index; CAD, coronary artery diseases; CCB, calcium channel blocker; CCI, Charlson Comorbidity Index; DPP4i, dipeptidyl peptidase‐4 inhibitor; MEG, meglitinide; Met, metformin; PS matching, propensity score 1:1 matching using the nearest neighbour technique; SD, standard deviation SU, sulphonylurea; TZD, thiazolidinedione
P < 0.05;
P < 0.01;
P < 0.001
Table 2.
Baseline patient characteristics of metformin‐based triple regimen users
| Triple therapy cohort | Before PS matching | After PS matching | |||||
|---|---|---|---|---|---|---|---|
|
Met + SU + DPP4i (n = 17 180) |
Met + SU + DPP4i (n = 10 475) | 1:1 matched Met + SU + TZDs |
Met + SU + DPP4i (n = 11 248) |
1:1 matched Met + SU + AC |
Met + SU + DPP4i (n = 3008) |
1:1 matched Met + SU + MG | |
| Person‐years (mean) | – | 3.32 | 3.60 | 3.29 | 3.43 | 3.20 | 3.38 |
| Age (mean ± SD) | 57.7 ± 11.5 | 57.0 ± 11.1 | 56.9 ± 11.6 | 58.0 ± 11.9 | 58.0 ± 11.7 | 60.0 ± 12.3 | 59.6 ± 11.9 |
| Male (%) | 53.49 | 55.04 | 54.30 | 54.99 | 55.07 | 54.36 | 54.79 |
| Comorbidity history | |||||||
| Hypertension (%) | 61.36 | 60.48 | 60.76 | 60.34 | 60.57 | 64.33 | 63.66 |
| Hyperlipidaemia (%) | 59.62 | 61.88 | 61.98 | 57.10 | 56.90 | 51.80 | 52.03 |
| Stroke (%) | 8.44 | 5.50 | 5.86 | 8.47 | 8.77 | 12.70 | 12.30 |
| Heart failure (%) | 2.65 | 1.56 | 1.57 | 2.70 | 2.78 | 4.69 | 4.26 |
| CAD (%) | 16.12 | 12.53 | 12.53 | 15.23 | 15.50 | 18.42 | 17.85 |
| CCI (mean ± SD) | 3.0 ± 1.4 | 3.0 ± 1.3 | 3.0 ± 1.4 | 3.1 ± 1.5 | 3.1 ± 1.5 | 3.5 ± 1.8 | 3.5 ± 1.9 |
| aDCSI (mean ± SD) | 1.0 ± 1.4 | 0.8 ± 1.2 | 0.8 ± 1.2 | 0.9 ± 1.5 | 0.9 ± 1.4 | 1.3 ± 2.0 | 1.3 ± 1.8 |
| Diabetes duration (years) | 6.1 ± 3.2 | 5.4 ± 3.2 | 5.4 ± 3.2 | 5.3 ± 3.3 | 5.3 ± 3.2 | 5.3 ± 3.2 | 5.2 ± 3.3 |
| Metformin duration (years) (mean ± SD) | 4.7 ± 3.0 | 4.1 ± 2.9 | 4.1 ± 2.9 | 3.9 ± 2.9 | 4.0 ± 2.8 | 3.9 ± 2.9 | 3.8 ± 2.8 |
| SU duration (years) (mean ± SD) | 5.1 ± 3.1 | 4.6 ± 3.1 | 4.6 ± 3.1 | 4.3 ± 3.1 | 4.4 ± 3.0 | 4.5 ± 3.1 | 4.4 ± 3.1 |
| Medication history | |||||||
| α‐blocker (%) | 3.86 | 2.91 | 2.90 | 3.81% | 3.98 | 4.92 | 4.69 |
| β‐blocker (%) | 20.27 | 18.00 | 17.66 | 19.64% | 19.67 | 19.78 | 20.68 |
| Diuretics (%) | 13.45 | 14.34 | 14.57 | 15.50% | 15.39 | 20.08 | 18.52 |
| CCB (%) | 34.51 | 36.04 | 35.70 | 36.43% | 36.21 | 40.39 | 40.86 |
| AECI/ARB (%) | 53.29 | 45.20 | 45.03 | 46.96% | 47.36 | 46.04 | 45.05 |
| Lipid‐lowering agent (%) | 55.06 | 52.69 | 52.49 | 48.95% | 48.67 | 43.85 | 43.55 |
| Antiplatelet agent (%) | 34.33 | 29.15 | 29.11 | 32.41% | 32.62 | 36.24 | 36.10 |
| Nitrates (%) | 4.89 | 2.60 | 2.80 | 4.19% | 4.17 | 5.39 | 4.92 |
| Anticoagulant (%) | 0.87 | 0.45 | 0.47 | 0.67% | 0.73 | 1.13 | 1.03 |
| Digoxin (%) | 1.70 | 1.24 | 1.31 | 1.77 | 1.80 | 2.13 | 1.96 |
Baseline patient characteristics were measured one year prior to the third‐line antidiabetic drug being added (index date). No statistical difference in baseline patient characteristics between each type of triple regimen and their PS‐matched referents. Diabetes duration: from diabetes diagnosis to the beginning of triple antidiabetic therapy
Baseline patient characteristics were measured 1 year prior to the second‐line antidiabetic drug being added (index date). Diabetes duration: from diabetes diagnosis to the beginning of dual antidiabetic therapy
ACA, acarbose; ACEI/ARB, angiotensin II‐converting enzyme inhibitor/angiotensin receptor blocker; aDCSI, adapted diabetes complication severity index; CAD, coronary artery diseases; CCB, calcium channel blocker; CCI, Charlson Comorbidity Index; DPP4i, dipeptidyl peptidase‐4 inhibitor; MEG, meglitinide; Met, metformin; PS matching, propensity score 1:1 matching using the nearest neighbour technique; SD, standard deviation SU, sulphonylurea; TZD, thiazolidinedione
Table 3.
Number and crude rate (per 1000 person‐years) of MACEs, death, and hypoglycaemia according to each type of metformin‐based dual and triple regimen user after propensity score matching
| Metformin‐based dual therapy cohort | Met + DPP4i (n = 5980) No. of events (crude rate) | 1:1 matched Met + SU No. of events (crude rate) | Met + TZDs (n = 1315) No. of events (crude rate) | 1:1 matched Met + SU No. of events (crude rate) | Met + ACA (n = 4368) No. of events (crude rate) | 1:1 matched Met + SU No. of events (crude rate) | Met + MEG (n = 4050) No. of events (crude rate) | 1:1 matched Met + SU No. of events (crude rate) |
|---|---|---|---|---|---|---|---|---|
| MACEs | 373 (19.52) | 464 (22.72) | 98 (20.57) | 109 (24.36) | 353 (24.25) | 380 (25.75) | 457 (34.71) | 412 (30.84) |
| Stroke | 228 (11.77) | 296 (14.28) | 64 (13.30) | 73 (16.11) | 230 (15.59) | 235 (15.62) | 301 (22.42) | 270 (19.85) |
| Heart failure | 136 (6.96) | 143 (6.79) | 25 (5.10) | 31 (6.75) | 103 (6.85) | 118 (7.72) | 171 (12.46) | 139 (10.00) |
| Myocardial infarction | 69 (3.50) | 95 (4.50) | 19 (3.87) | 17 (3.68) | 55 (3.64) | 73 (4.76) | 61 (4.39) | 72 (5.14) |
| Death | 94 (10.53) | 128 (11.84) | 51 (10.33) | 73 (15.70) | 246 (16.20) | 236 (15.23) | 405 (28.94) | 268 (18.98) |
| Hypoglycaemia | 50 (2.54) | 71 (3.36) | 15 (3.06) | 9 (1.94) | 37 (2.45) | 48 (3.12) | 105 (7.59) | 67 (4.79) |
| Metformin‐based triple therapy cohort | Met + SU+ DPP4i (n = 10 475) No. of events (crude rate) | 1:1 matched Met + SU+ TZDs No. of events (crude rate) | Met + SU+ DPP4i (n = 11 248) No. of events (crude rate) | 1:1 matched Met + SU+ ACA No. of events (crude rate) | Met + SU + DPP4i (n = 3008) No. of events (crude rate) | 1:1 matched Met + SU+ MEG No. of events (crude rate) | ||
|---|---|---|---|---|---|---|---|---|
| MACEs | 739 (21.24) | 785 (20.79) | 947 (25.59) | 1084 (28.08) | 297 (30.79) | 451 (44.39) | ||
| Stroke | 447 (12.67) | 520 (13.60) | 567 (15.07) | 707 (18.02) | 183 (18.62) | 290 (27.69) | ||
| Heart failure | 257 (7.20) | 249 (6.42) | 349 (9.16) | 349 (8.73) | 114 (11.42) | 165 (15.40) | ||
| Myocardial infarction | 131 (3.65) | 130 (3.33) | 171 (4.46) | 188 (4.67) | 49 (4.86) | 65 (5.95) | ||
| Death | 446 (12.37) | 470 (12.00) | 545 (14.11) | 687 (16.95) | 200 (19.70) | 331 (30.03) | ||
| Hypoglycaemia | 178 (4.98) | 211 (5.44) | 215 (5.62) | 264 (6.59) | 76 (7.58) | 142 (13.20) |
ACA, acarbose; DPP4i, dipeptidyl peptidase‐4 inhibitor, MEG, meglitinide; MACEs, major adverse cardiovascular events; Met, metformin; SU, sulphonylurea; TZDs; thiazolidinediones
Table 4.
Comparative risks for cardiovascular diseases, all‐cause mortality, and hypoglycaemia of metformin‐based dual regimens, and subgroup analyses stratified by patients' CVD history
| Met + DPP4i (n = 5980) vs. 1:1 matched Met + SU (reference) HR (95% CI) | Met + TZDs (n = 1315) vs. 1:1 matched Met + SU (reference) HR (95% CI) | Met + ACA (n = 4368) vs. 1:1 matched Met + SU (reference) HR (95% CI) | Met + MEG (n = 4050)vs. 1:1 matched Met + SU (reference) HR (95% CI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All patients (n = 11 960) | CVD history (n = 2758) | No CVD history (n = 9202) | All patients (n = 2630) | CVD history (n = 544) | No CVD history (n = 2086) | All patients (n = 8736) | CVD history (n = 2264) | No CVD history (n = 6508) | All patients (n = 81,00) | CVD history (n = 2140) | No CVD history (n = 5952) | |
| MACEs | 0.852 (0.743, 0.977) | 0.825(0.686, 0.992) | 0.929 (0.754, 1.146) | 0.845 (0.643, 1.110) | 0.921 (0.602, 1.409) | 1.079 (0.735, 1.584) | 0.940 (0.813, 1.087) | 1.217 (1.000, 1.482) | 1.014 (0.800, 1.287) | 1.124 (0.984, 1.284) | 1.185 (0.998, 1.407) | 1.161 (0.933, 1443) |
| Stroke | 0.817 (0.687, 0.971) | 0.812 (0.644, 1.024) | 0.887 (0.677, 1164) | 0.829 (0.593, 1.160) | 0.893 (0.547, 1459) | 1.014 (0.621, 1.655) | 0.994 (0.829, 1.192) | 1.227 (0.964, 1.562) | 0.902 (0.674, 1.207) | 1.128 (0.957, 1.329) | 1.155 (0.938, 1.423) | 1.090 (0.835, 1.423) |
| Heart failure | 1.012 (0.800, 1.281) | 0.896 (0.662, 1.213) | 1.045 (0.725, 1.508) | 0.759 (0.448, 1.286) | 0.639 (0.261, 1.565) | 1.067 (0.533, 2.139) | 0.885 (0.680, 1.153) | 1.060 (0.751, 1.497) | 1.574 (0.942, 2.632) | 1.245 (0.995, 1.557) | 1.355 (1.023, 1.793) | 1.495 (0.966, 2.243) |
| Myocardial infarction | 0.785 (0.575, 1.073) | 0.824 (0.532, 1.275) | 0.917 (0.562, 1.496) | 1.016 (0.528, 1.957) | 0.598 (0.231, 1543) | 1.769 (0.663, 4.719) | 0.769 (0.542, 1.091) | 1.158 (0.709, 1.892) | 0.825 (0.458, 1.485) | 0.851 (0.605, 1.196) | 0.854 (0.551, 1.322) | 1.210 (0.659. 2.223) |
| All‐cause mortality | 0.825 (0.687, 0.992) | 0.896 (0.678, 1.185) | 0.799 (0.621, 1.028) | 0.690 (0.482, 0.986) | 0.992 (0.516, 1.907) | 1.141 (0.693, 1.879) | 1.051 (0.897, 1.257) | 1.138 (0.869, 1.489) | 1.081 (0.845, 1.383) | 1.554 (1.331, 1.814) | 1.961 (1.553, 2.476) | 1.338 (1.085, 1.649) |
| Hypoglycaemia | 0.741(0.516, 0.901) | 0.665 (0.574, 0.943) | 0.895 (0.659, 0.917) | 1.603 (0.701, 3.664) | 2.487 (0.482, 12.824) | 1.198 (0.472, 3.037) | 0.780 (0.508, 1.198) | 0.833 (0.447, 1.553) | 0.523 (0.301, 0.910) | 1.581 (1.164, 2.148) | 1.777 (1.115, 2.832) | 1.531 (1.017, 2.304) |
ACA, acarbose; CI, confidence interval; CVD, cardiovascular disease; DPP4i, dipeptidyl peptidase 4 inhibitor; HR, hazard ratio; MACEs, major adverse cardiovascular events; MEG, meglitinide; Met, metformin; SU, sulphonylurea; TZDs; thiazolidinediones. Bolded words in Tables refers the values which are statistically significant (P < 0.05).
Table 5.
Comparative risks for cardiovascular diseases, all‐cause mortality and hypoglycaemia of metformin‐based triple regimens, and subgroup analyses stratified by patients' CVD history
| Met + SU + DPP4ia (n = 10 475) vs. 1:1 matched Met + SU + TZDs (reference) HR (95% CI) | Met + SU + DPP4ia (n = 11 248) vs. 1:1 matched Met + SU + ACA (reference) HR (95% CI) | Met + SU + DPP4ia (n = 3008) vs. 1:1 matched Met + SU + MEG (reference) HR (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All patients (n = 20 950) | CVD historyb (n = 3710) | No CVD historyb (n = 17 218) | All patients (n = 22 496) | CVD history (n = 5318) | No CVD history (n = 17 078) | All patients (n = 6016) | CVD history (n = 1768) | No CVD history (n = 4240) | |
| MACEs | 1.016 (0.919, 1.124) | 1.018 (0.867, 1.196) | 0.963 (0.844, 1.098) | 0.902 (0.827, 0.984) | 0.875 (0.776, 0.987) | 0.885 (0.780, 0.992) | 0.676 (0.583, 0.783) | 0.696 (0.579, 0.837) | 0.737 (0.585, 0.928) |
| Stroke | 0.924 (0.814, 1.049) | 0.907 (0.742, 1.108) | 0.933 (0.793, 1.099) | 0.826 (0.740, 0.923) | 0.822 (0.706, 0.957) | 0.807 (0.690, 0.943) | 0.653 (0.542, 0.786) | 0.669 (0.531, 0.843) | 0.721 (0.538, 0.967) |
| Heart failure | 1.120 (0.940, 1.333) | 1.172 (0.893, 1.537) | 1.033 (0.818, 1.304) | 1.037 (0.894, 1.203) | 0.933 (0.766, 1.136) | 1.178 (0.937, 1.482) | 0.721 (0.568, 0.917) | 0.694 (0.514, 0.938) | 0.880 (0.601, 0.982) |
| Myocardial infarction | 1.102 (0.864, 1.406) | 1.374 (0.935, 2.017) | 0.888 (0.643, 1.226) | 0.954 (0.775, 1.174) | 0.900 (0.684, 1.186) | 0.858 (0.620, 1.188) | 0.798 (0.551, 1.157) | 0.930 (0.660, 1.623) | 0.778 (0.642, 0.946) |
| All‐cause mortality | 0.948 (0.833, 1.079) | 0.718 (0.559, 0.922) | 0.963 (0.825, 1.125) | 0.784 (0.701, 0.878) | 0.708 (0.589, 0.851) | 0.788 (0.682, 0.911) | 0.689 (0.594, 0.703) | 0.645 (0.519, 0.708) | 0.629 (0.597, 0.797) |
| Hypoglycaemia | 0.911 (0.746, 1.113) | 0.915 (0.628, 1.333) | 0.885 (0.698, 1.122) | 0.843 (0.704, 1.010) | 0.973 (0.725, 1.307) | 0.748 (0.593, 0.942) | 0.757 (0.621, 0.836) | 0.784 (0.627, 0.871) | 0.757 (0.616, 0.801) |
ACA, acarbose; CI, confidence interval; CVD, cardiovascular disease; DPP4i, dipeptidyl peptidase 4 inhibitor; HR, hazard ratio; MACEs, major adverse cardiovascular events; MEG, meglitinide; Met, metformin; SU, sulphonylurea; TZD, thiazolidinedione
In each subgroup (e.g. Met + SU + DPP4i and its matched Met + SU + TZDs), the Met + SU + DPP4i group served as comparator for its matched triple regimen (e.g. Met + SU + TZDs). Therefore, an HR less than 1 implies a lower risk of the event of interest (e.g. CVD) with the use of the Met + SU + DPP4i regimen
A sum of 3710 (CVD history) and 17 218 (no CVD history) cases, adding up to 20 928, is not exactly equal to the initial total number of patients in this group (20 950). This is because, after the patients were stratified by CVD history, we conducted matching procedures for CVD history and non‐CVD history groups, separately. A reduction in the number of patients after matching in these stratified subgroups occurred mainly because some cases (i.e. 22) were not matched to another reference case (i.e. we were unable to find a similar reference patient) after they had been classified into a specific subgroup (i.e. CVD history or non‐CVD history group) and were then not included in the analyses.
Tables refers the values which are statistically significant (P < 0.05).
Figure 2.
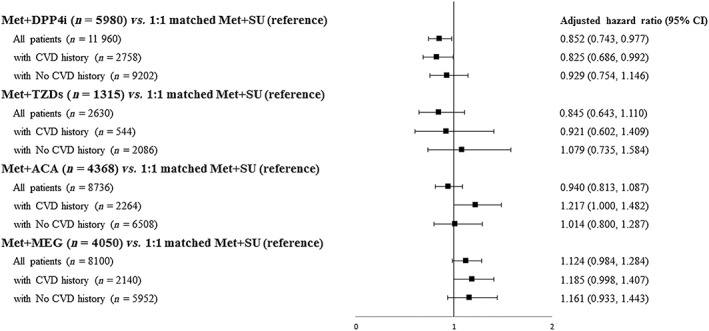
Comparative risks for major adverse cardiovascular events associated with metformin‐based dual regimens. ACA, acarbose; CI, confidence interval; CVD, cardiovascular disease; DPP‐4, dipeptidyl peptidase 4; MEG, meglitinide; Met, metformin; SU, sulphonylurea; TZD, thiazolidinedione
Figure 3.
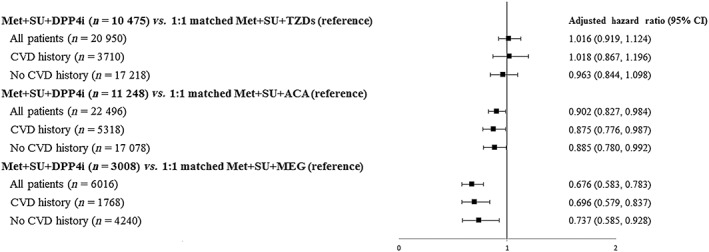
Comparative risks for major adverse cardiovascular events associated with metformin‐based triple regimens. ACA, acarbose; CI, confidence interval; CVD, cardiovascular disease; DPP‐4, dipeptidyl peptidase 4; MEG, meglitinide; Met, metformin; SU, sulphonylurea; TZD, thiazolidinedione
Discussion
This was the largest population‐based longitudinal cohort study comprehensively to evaluate the risks for cardiovascular events, hypoglycaemia and all‐cause mortality with use of DPP4is compared with other second‐ and third‐line antidiabetic drugs. We found that DPP4is as an add‐on (second‐ or third‐line) treatment to metformin yielded positive effects on cardiovascular outcomes and posed no increased risks for hypoglycaemia or death, compared with other conventional antidiabetic treatments. Our subgroup analyses further differentiated the effect of DPP4is for patients with and without a history of CVD, which eliminated potential bias from pre‐existing CVD conditions and provided an insight into the role of DPP4is for patients with and without a history of CVD, respectively.
Cardiovascular outcomes of DPP4is as a second‐line add‐on treatment
Our findings, based on real‐world patients, support those of several randomized clinical trials, in which DPP4i use appeared not to be associated with increased CVD risks and mortality 16, 42, 43, 44. In addition, our results are consistent with recent real‐world‐setting cohort studies showing favourable cardiovascular outcomes of DPP4is as a second‐line antidiabetic treatment. Several cohort studies from Denmark 12, the US 11 and the UK 15 have shown that DPP4is as a second‐line add‐on drug to Met had a significantly lower MACE risk (including stroke and MI) than that associated with SU. However, two Taiwanese cohort studies of cardiovascular outcomes of DPP4is as a second‐line add‐on provided inconsistent results. The study by Cheng et al., based on the 2009–2011 NHIRD, showed that, compared with Met + SU users, there was a significantly lower risk of MI only in Met + MEG and Met + ACA users, but not in Met + DPP4i users 20. Our study also assessed other dual regimens, but we did not observe the Met + ACA or Met + MEG regimen to provide better CVD benefits over the Met + SU regimen. The afore‐mentioned difference might be explained by different follow‐up periods, sample sizes and study methods. Cheng et al. 20 was limited by having a relatively shorter follow‐up time (i.e. median follow‐up for DPP4i users for MACEs: 0.59 years) and a more limited study sample (i.e. 2242 cases for DPP4i users), leading to lower rates of CVD events (i.e. crude rate of MACEs for DPP4i users: 0.16 per 1000 person‐years) compared with those in the present study. In addition, we applied PS matching on patients' baseline diabetic severity to adjust for potential confounding by indication, but Cheng et al. 20 did not. A recent study by Ou et al. 18, based on the 2009–2013 NHIRD showed that, compared with PS‐matched Met + SU referents, Met + DPP4i users had significantly lower all‐cause mortality and stroke risk, but there was no significant effect on MI and HF risks. Our results regarding the comparison in cardiovascular outcomes between Met + DPP4i and Met + SU use are consistent with those of Ou et al. 18. However, Ou et al. assessed only DPP4i and SU as second‐line add‐ons to metformin; they included no other antidiabetic drugs (e.g. TZDs) as add‐ons. In addition, there were some differences in the inclusion criteria and patient characteristics between our study and that by Ou et al. Ou et al. used a 1‐year longer time span for identifying study cases (2009–2012 in Ou et al. 18 vs. 2009–2011 in the present study). The stable Met user in the study by Ou et al. was defined by allowing a 90‐day gap between two consecutive refills 18, whereas we used a more limited gap of 30 days or fewer, which was considered stricter. As such, our selected cases were likely to be the patients with more stable treatment and better health outcomes compared with those in the study by Ou et al. These differences may have resulted in a smaller sample size and less severe T2DM in the patients analysed in our study. Additionally, the prevalence of comorbidities in the study by Ou et al. 18 was higher than that in our study, which may explain the higher rate of death in the study by Ou et al. 18.
Cardiovascular outcomes of DPP4is as a third‐line add‐on treatment
Clinically, DPP4is commonly serve as a third‐line treatment in countries such as Taiwan. The present study identified the most commonly used Met‐based triple regimens, where the combination of Met + SU + DPP4i was the most prescribed. Our findings showed that DPP4i as a third‐line add‐on to initial Met + SU dual therapy provided cardiovascular benefits and lowered all‐cause mortality over conventional antidiabetic drugs (i.e. ACA, MEG). Several studies have demonstrated that, compared with SU, DPP4is as a second‐line add‐on to Met are associated with a lower risk of stroke 12, 15, 17. The present study of triple antidiabetic regimens further showed that DPP4is as a third‐line add‐on treatment to Met + SU‐based therapy were associated with a significantly lower risk for stroke compared with ACA and MEG, and a nonstatistically significant trend towards being lower than that for the TDZs.
Several mechanisms have been proposed to explain the cardiovascular protective effects of DPP4is. These include GLP‐1 endothelial effects (i.e. enhancement of endothelial function 45); other, GLP‐1‐independent endothelial effects 46; and a potential anti‐inflammatory effect of GLP‐1 via reducing C reactive protective protein levels 47. Our results suggest that DPP4i use as a third‐line add‐on treatment might provide cardiovascular benefits over other antidiabetic drugs (e.g. ACA, MEG). Therefore, treatment decisions need to take the benefits and risks associated with antidiabetic drugs into consideration, with optimal treatment selected to alter CVD risks, especially for advanced T2DM patients (i.e. cases requiring triple therapies).
DPP4is and heart failure
The disruption of the neurohormonal regulatory mechanism has been hypothesized to explain the increased HF risk associated with DPP4i use 48. Several studies have assessed the association between DPP4i use and HF risk, although the results appeared to be inconclusive. Previous randomized controlled trials [i.e. Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR) 16 and Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care (EXAMINE) 21] and a US cohort study of Met‐based dual therapies 17 all showed an increased HF risk for DPP4i users, whereas the study by Kim et al. 11 found that DPP4i users (either on mono‐ or combination therapy) had a lower risk of HF than non‐DPP4i users, which was consistent with a recent large observational study from Italy, indicating a significantly lower HF risk with DPP4is compared with SU 19. In addition, the most recent placebo‐controlled trial reported that sitagliptin use was not associated with an increased HF risk 44. Consistent with previous observational studies 17, 19, 44, the present study did not find a significantly increased HF risk for Met + DPP4i users compared with Met + SU users. Additionally, we found that the patients taking a DPP4i as a third‐line treatment had a significantly lower HF risk compared with those on a MEG add‐on regimen. These results imply that DPP4is as a second‐ or third‐line antidiabetic treatment might be superior to other add‐ons (e.g. MEG) to alter the risk of HF for patients with T2DM.
Study limitations
First, we analysed only common combination triple regimens, which were Met + SU‐based triple therapies. Future studies might analyse other combinations (e.g. SU + ACA + DPP4i) to confirm our findings. However, the combinations of treatment regimens analysed in the present study have accounted for the majority of antidiabetic drug utilization patterns in T2DM. Second, due to the nature of an observational study, a potential confounding by indication could not be eliminated. Although PS matching might mitigate this concern, potential residual confounding by incomplete adjustment for unmeasured bias (i.e. lifestyle risk factors, physicians' behaviours) for study outcomes may still exist. Third, identifying CVD outcomes from Taiwan's NHIRD might suffer from misclassification. However, previous validation studies for the identification of CVD events (e.g. MI 49 and stroke 50) from the NHIRD showed high sensitivity and positive predictive values. Fourth, considering the Cox model that we utilized to examine the factors associated with ‘time to event’, the duration which a patient contributed to the analysis may vary by censored points. Study subjects would have different dosages and lengths of medication use, depending on censored points. However, the time‐varying dosages and lengths of medication use were not accounted for in our analyses. In addition, we did not further assess the dose–gradient relationship between medication use and CVD risk, mainly because not all study subjects had the same length of follow‐up. Fifth, GLP‐1 receptor agonists were only introduced into Taiwan's NHI formulary in 2011 (i.e. exenatide: May 2011, liraglutide: October 2012), and, thus, our study period (1999–2013) may not have been sufficiently long to capture its cardiovascular outcomes. In addition, in 2013, the utilization of and spending on GLP‐1 agonists in Taiwan accounted for only 1.8% and 0.8% of total antidiabetic drugs, respectively 25. In this regard, the present study may not have included a sufficient number of cases treated with GLP‐1 agonists to be compared with other antidiabetic drugs, so GLP‐1 agonists were not included in the analysis. Lastly, the study included only Taiwanese patients with T2DM. However, there is no apparent evidence indicating a difference in treatment efficacy regarding the use of DPP4is across ethnicities, so our study results may be applicable to other ethnicities and other health insurance settings. Moreover, as we also studied the role of DPP4is as a third‐line add‐on drug to Met + SU, such results may be beneficial for countries or healthcare settings in which a DPP4i is usually used in the later stages (i.e. as a third‐line treatment) as a result of its higher price, compared with other antidiabetic drugs.
Conclusions
The present study confirms the previous evidence on the cardiovascular outcomes of DPP4is as a second‐line treatment to Met and adds further evidence for the favourable clinical outcomes of DPP4is as a third‐line add‐on therapy. Additionally, our results suggest that DPP4is as a third‐line treatment, in addition to their second‐line role, might also provide cardiovascular benefits, reduce all‐cause mortality and pose no increased risk for HF or hypoglycaemia compared with other antidiabetic drugs. Therefore, adding DPP4is as a second‐ or third‐line treatment to T2DM patients with inadequate glycaemic control under Met monotherapy might alter future CVD risks.
Competing Interests
There are no competing interests to declare.
This work was supported by the Ministry of Science and Technology, Taiwan, grant [MOST 104–2320‐B‐006‐008‐MY3]. We gratefully thank National Cheng Kung University and its affiliated hospital for all their support.
Contributors
H.T.O. contributed substantially to the study concept and design, data acquisition, and analysis and interpretation of the data. K.C.C. contributed to data collection and the analysis. C.Y.L. and J.S.W. provided the clinical and statistical interpretation of the results. H.‐T.O. wrote the first draft of the manuscript, and K.C.C., C.Y.L. and J.S.W. critically revised it. All authors gave approval for the publication of the final version.
Supporting information
Table S1 Sensitivity analyses for comparative effectiveness and safety of metformin‐based dual therapy
Table S2 Sensitivity analyses for comparative effectiveness and safety of metformin‐based triple therapy
Ou, H.‐T. , Chang, K.‐C. , Li, C.‐Y. , and Wu, J.‐S. (2017) Comparative cardiovascular risks of dipeptidyl peptidase 4 inhibitors with other second‐ and third‐line antidiabetic drugs in patients with type 2 diabetes. Br J Clin Pharmacol, 83: 1556–1570. doi: 10.1111/bcp.13241.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44 (Database Issue): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 2015; 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization Diabetes Fact Sheet [online]. Available at http://www.who.int/mediacentre/factsheets/fs312/en/ (last accessed 23 March 2015).
- 4. Rydén L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, et al. ESC Guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2013; 34: 3035–3087. [DOI] [PubMed] [Google Scholar]
- 5. Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the US population, 1971–1993. Diabetes Care 1998; 21: 1138–1145. [DOI] [PubMed] [Google Scholar]
- 6. Palmer SC, Mavridis D, Nicolucci A, Johnson DW, Tonelli M, Craig JC, et al. Comparison of clinical outcomes and adverse events associated with glucose‐lowering drugs in patients with type 2 diabetes: a meta‐analysis. JAMA 2016; 316: 313–324. [DOI] [PubMed] [Google Scholar]
- 7. Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. American Association of Clinical Endocrinologists. AACE comprehensive diabetes management algorithm 2013. Endocr Pract 2013; 19: 327–336. [DOI] [PubMed] [Google Scholar]
- 8. Khan FZ, Heck PM, Hoole SP, Dutka DP. DPP‐4 inhibition by sitagliptin improves the myocardial response to dobutamine stress and mitigates stunning in a pilot study of patients with coronary artery disease. Circ Cardiovasc Imaging 2010; 3: 195–201. [DOI] [PubMed] [Google Scholar]
- 9. Frederich R, Alexander JH, Fiedorek FT, Donovan M, Berglind N, Harris S, et al. A systematic assessment of cardiovascular outcomes in the saxagliptin drug development program for type 2 diabetes. Postgrad Med 2010; 122: 16–27. [DOI] [PubMed] [Google Scholar]
- 10. Johansen OE, Neubacher D, von Eynatten M, Patel S, Woerle HJ. Cardiovascular safety with linagliptin in patients with type 2 diabetes mellitus: a pre‐specified, prospective, and adjudicated meta‐analysis of a phase 3 programme. Cardiovasc Diabetol 2012; 11: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim SC, Glynn RJ, Liu J, Everett BM, Goldfine AB. Dipeptidyl peptidase‐4 inhibitors do not increase the risk of cardiovascular events in type 2 diabetes: a cohort study. Acta Diabetol 2014; 51: 1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mogensen U, Andersson C, Fosbøl E, Schramm T, Vaag A, Scheller NM, et al. Cardiovascular safety of combination therapies with incretin‐based drugs and metformin compared with a combination of metformin and sulphonylurea in type 2 diabetes mellitus – a retrospective nationwide study. Diabetes Obes Metab 2014; 16: 1001–1008. [DOI] [PubMed] [Google Scholar]
- 13. Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369: 1317–1326. [DOI] [PubMed] [Google Scholar]
- 14. Reaven PD, Moritz TE, Schwenke DC, Anderson RJ, Criqui M, Detrano R, et al. Intensive glucose‐lowering therapy reduces cardiovascular disease events in veterans affairs diabetes trial participants with lower calcified coronary atherosclerosis. Diabetes 2009; 58: 2642–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morgan CL, Mukherjee J, Jenkins‐Jones S, Holden S, Currie C. Combination therapy with metformin plus sulphonylureas versus metformin plus DPP‐4 inhibitors: association with major adverse cardiovascular events and all‐cause mortality. Diabetes Obes Metab 2014; 16: 977–983. [DOI] [PubMed] [Google Scholar]
- 16. Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369: 1317–1326. [DOI] [PubMed] [Google Scholar]
- 17. Kannan S, Pantalone KM, Matsuda S, Wells BJ, Karafa M, Zimmerman RS. The risk of overall mortality and cardiovascular events in patients with type 2 diabetes on dual drug therapy including metformin: a large database study from Cleveland Clinic. J Diabetes 2015; 8: 279–285. [DOI] [PubMed] [Google Scholar]
- 18. Ou S‐M, Shih C‐J, Chao P‐W, Chu H, Kuo S‐C, Lee Y‐J, et al. Effects on clinical outcomes of adding dipeptidyl peptidase‐4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med 2015; 163: 663–672. [DOI] [PubMed] [Google Scholar]
- 19. Fadini GP, Avogaro A, Degli Esposti L, Russo P, Saragoni S, Buda S, et al. Risk of hospitalization for heart failure in patients with type 2 diabetes newly treated with DPP‐4 inhibitors or other oral glucose‐lowering medications: a retrospective registry study on 127,555 patients from the Nationwide OsMed Health‐DB Database. Eur Heart J 2015; 36: 2454–2462. [DOI] [PubMed] [Google Scholar]
- 20. Chang YC, Chuang LM, Lin JW, Chen ST, Lai MS, Chang CH. Cardiovascular risks associated with second‐line oral antidiabetic agents added to metformin in patients with Type 2 diabetes: a nationwide cohort study. Diabet Med 2015; 32: 1460–1469. [DOI] [PubMed] [Google Scholar]
- 21. White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369: 1327–1335. [DOI] [PubMed] [Google Scholar]
- 22. United States Food and Drug Administration . FDA Drug Safety Communication: FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin.
- 23. Cheng T‐M. Taiwan's new national health insurance program: genesis and experience so far. Health Aff 2003; 22: 61–76. [DOI] [PubMed] [Google Scholar]
- 24. American Diabetes Association 7 . Approaches to glycemic treatment. Diabetes Care 2016; 39 (Suppl. 1): S52–S9. [DOI] [PubMed] [Google Scholar]
- 25. Ou HT, Chang KC, Liu YM, Wu JS. Recent trends in the use of antidiabetic medications from 2008 to 2013: a nation‐wide population‐based study from Taiwan. J Diabetes 2017; 9: 256–266. [DOI] [PubMed] [Google Scholar]
- 26. Ou H‐T, Yang C‐Y, Wang J‐D, Hwang J‐S, Wu J‐S. Life expectancy and lifetime health care expenditures for type 1 diabetes: a nationwide longitudinal cohort of incident cases followed for 14 years. Value Health 2016; 19: 976–984. [DOI] [PubMed] [Google Scholar]
- 27. Hou W‐H, Chang K‐C, Li C‐Y, Ou H‐T. Dipeptidyl peptidase‐4 inhibitor use is not associated with elevated risk of severe joint pain in patients with type 2 diabetes: a population‐based cohort study. Pain 2016; 157: 1954–1959. [DOI] [PubMed] [Google Scholar]
- 28. Lin S‐D, Tsai S‐T, Tu S‐T, Su C‐C, Chen J‐F, Lu C‐H, et al. Glycosylated hemoglobin level and number of oral antidiabetic drugs predict whether or not glycemic target is achieved in insulin‐requiring type 2 diabetes. Prim Care Diabetes 2015; 9: 135–141. [DOI] [PubMed] [Google Scholar]
- 29. Heckman J, Ichimura H, Smith J, Todd P. Characterizing selection bias using experimental data: National Bureau of Economic Research 1998.
- 30. Dehejia RH, Wahba S. Propensity score‐matching methods for nonexperimental causal studies. Rev Econ Stat 2002; 84: 151–161. [Google Scholar]
- 31. Li M. Using the propensity score method to estimate causal effects: a review and practical guide. Organ Res Methods 2013; 16: 188–226. [Google Scholar]
- 32. Austin PC, Grootendorst P, Normand SLT, Anderson GM. Conditioning on the propensity score can result in biased estimation of common measures of treatment effect: a Monte Carlo study. Stat Med 2007; 26: 754–768. [DOI] [PubMed] [Google Scholar]
- 33. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 34. Chang H‐Y, Weiner JP, Richards TM, Bleich SN, Segal JB. Validating the adapted Diabetes Complications Severity Index in claims data. Am J Manag Care 2012; 18: 721–726. [PubMed] [Google Scholar]
- 35. Cheng C‐L, Chien H‐C, Lee C‐H, Lin S‐J, Yang Y‐HK. Validity of in‐hospital mortality data among patients with acute myocardial infarction or stroke in National Health Insurance Research Database in Taiwan. Int J Cardiol 2015; 201: 96–101. [DOI] [PubMed] [Google Scholar]
- 36. Hosmer DW, Lemeshow S, May S. Applied Survival Analysis: Regression Modeling of Time to Event Data (2nd edn). New Jersey: Wiley, 2008. [Google Scholar]
- 37. Kearney DJ, Crump C, Maynard C, Boyko EJ. A case–control study of endoscopy and mortality from adenocarcinoma of the esophagus or gastric cardia in persons with GERD. Gastrointest Endosc 2003; 57: 823–829. [DOI] [PubMed] [Google Scholar]
- 38. Wiebe DJ. Firearms in US homes as a risk factor for unintentional gunshot fatality. Accid Anal Prev 2003; 35: 711–716. [DOI] [PubMed] [Google Scholar]
- 39. Grimes DA, Schulz KF. Compared to what? Finding controls for case–control studies. Lancet 2005; 365: 1429–1433. [DOI] [PubMed] [Google Scholar]
- 40. Satagopan J, Ben‐Porat L, Berwick M, Robson M, Kutler D, Auerbach A. A note on competing risks in survival data analysis. Br J Cancer 2004; 91: 1229–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kohl M, Heinze G. PSHREG: A SAS macro for proportional and nonproportional substribution hazards regression with competing risk data. Vienna: Medical University of Vienna, 2013. [Google Scholar]
- 42. Gallwitz B, Rosenstock J, Rauch T, Bhattacharya S, Patel S, von Eynatten M, et al. 2‐year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double‐blind, non‐inferiority trial. Lancet 2012; 380: 475–483. [DOI] [PubMed] [Google Scholar]
- 43. White W, Pratley R, Fleck P, Munsaka M, Hisada M, Wilson C, et al. Cardiovascular safety of the dipetidyl peptidase‐4 inhibitor alogliptin in type 2 diabetes mellitus. Diabetes Obes Metab 2013; 15: 668–673. [DOI] [PubMed] [Google Scholar]
- 44. Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 373: 232–242. [DOI] [PubMed] [Google Scholar]
- 45. Takasawa W, Ohnuma K, Hatano R, Endo Y, Dang NH, Morimoto C. Inhibition of dipeptidyl peptidase 4 regulates microvascular endothelial growth induced by inflammatory cytokines. Biochem Biophys Res Commun 2010; 401: 7–12. [DOI] [PubMed] [Google Scholar]
- 46. Fadini GP, Avogaro A. Cardiovascular effects of DPP‐4 inhibition: beyond GLP‐1. Vascul Pharmacol 2011; 55: 10–16. [DOI] [PubMed] [Google Scholar]
- 47. Wu JD, Xu XH, Zhu J, Ding B, Du TX, Gao G, et al. Effect of exenatide on inflammatory and oxidative stress markers in patients with type 2 diabetes mellitus. Diabetes Technol Ther 2011; 13: 143–148. [DOI] [PubMed] [Google Scholar]
- 48. McMurray J. The Vildagliptin in Ventricular Dysfunction Diabetes trial (VIVIDD). European Society of Cardiology Heart Failure Association; 26 May 2013; Lisbon, Portugal; p. 99.
- 49. Cheng C‐L, Lee C‐H, Chen P‐S, Li Y‐H, Lin S‐J, Yang Y‐HK. Validation of acute myocardial infarction cases in the national health insurance research database in Taiwan. J Epidemiol 2014; 24: 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hsieh C‐Y, Chen C‐H, Li C‐Y, Lai M‐L. Validating the diagnosis of acute ischemic stroke in a National Health Insurance claims database. J Formos Med Assoc 2015; 114: 254–259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Sensitivity analyses for comparative effectiveness and safety of metformin‐based dual therapy
Table S2 Sensitivity analyses for comparative effectiveness and safety of metformin‐based triple therapy


