Abstract
Aims
Venous thromboembolism is an important cause of postoperative morbidity and mortality in bariatric surgery. Studies of direct oral anticoagulants (DOACs) are not available in this surgical field. The objective of this phase 1 clinical trial was to investigate pharmacokinetic and pharmacodynamic (PK/PD) parameters of rivaroxaban in bariatric patients.
Methods
In this single‐centre study, obese patients received single oral doses of rivaroxaban (10 mg) 1 day prior to and 3 days after bariatric surgery. PK and PD parameters were assessed at baseline and during 24 h after drug ingestion.
Results
Six Roux‐en‐Y gastric bypass patients and six sleeve gastrectomy patients completed the study. Mean rivaroxaban area under plasma concentration–time curve, peak plasma concentration, time to peak plasma concentration and terminal half‐life were 971.9 μg·h l–1 (coefficient of variation: 10.6), 135.3 μg l–1 (26.7), 1.5 h and 13.1 h (34.1) prior to and 1165.8 (21.9), 170.0 (15.9), 1.5 and 8.9 (44.6) postsurgery for SG patients and 933.7 μg·h l–1 (22.3), 136.5 μg l–1 (10.7), 1.5 h und 13.8 h (46.6) prior to and 1029.4 (7.4), 110.8 (31.8), 2.5 and 15 (60.0) postsurgery for Roux‐en‐Y gastric bypass patients, respectively. Prothrombin fragments (F1 + 2) decreased during the first 12 hours and increased thereafter in the pre‐ and the postbariatric setting. Thrombin–antithrombin complexes dropped within 1–3 h in the prebariatric setting and remained low after surgery until they increased at 24 h postdose. Rivaroxaban was well tolerated and no relevant safety issues were observed.
Conclusions
Bariatric surgery does not appear to alter PK of rivaroxaban in a clinically relevant way. Effective prophylactic postbariatric anticoagulation is supported by changes in PD.
Keywords: anticoagulation, bariatric surgery, pharmacodynamics, rivaroxaban, Roux‐en‐Y gastric bypass, sleeve gastrectomy
What is Already Known About this Subject
Venous thromboembolism represents a significant cause of morbidity and mortality after bariatric surgery.
Thrombosis prophylaxis with rivaroxaban is established in the perioperative setting of orthopaedic patients (hip and knee arthroplasty).
To date, direct oral anticoagulants have not been systematically investigated in bariatric patients.
What this Study Adds
This study represents the first systematic pharmacokinetic/pharmacodynamic investigation of prophylactic rivaroxaban doses in bariatric patients.
Single doses of 10 mg rivaroxaban resulted in similar systemic drug exposures prior to and after bariatric surgery, independent of the bariatric procedure performed.
Effective prophylactic anticoagulation is supported by the pharmacodynamic results of this trial.
Tables of Links
| TARGETS |
|---|
| Enzymes 2 |
| Coagulation factor X |
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http: //www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 1, and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 2.
Introduction
The prevalence of obesity as well as morbid obesity is increasing worldwide and therefore becoming a growing medical and socioeconomic burden 3, 4, 5. Bariatric surgery leads to the most sustained reduction of weight and associated comorbidities, but patients undergoing bariatric surgery are at increased risk of venous thromboembolic events (VTE) 6. Obesity is an independent risk factor for the development of venous thromboembolism itself and the association between obesity and VTE after bariatric surgery is well established 7, 8, 9. The incidence of symptomatic deep vein thrombosis (DVT) and pulmonary embolism (PE) ranges from 0 to 5.4% and 0 to 6.4%, respectively, but the true incidence remains uncertain 10. Although the overall incidence is low, VTE represents a significant cause of morbidity and mortality after surgery. Even with aggressive prophylaxis, VTE cannot be fully prevented 11, 12, 13. The American Society of Metabolic and Bariatric Surgeons and the American College of Chest Physicians recommend prophylaxis against DVT for all bariatric surgery patients 14. Routine prophylactic perioperative use of low‐molecular weight heparins (LMWHs), intermittent pneumatic compression devices and early mobilization are currently the major accepted measures to prevent VTE, particularly in high‐risk groups [body mass index (BMI) >50 kg/m2], advanced age, history of previous VTE, obesity hypoventilation syndrome, open and revisional surgery 13, 15. In clinical practice, physicians lack guidelines supporting their therapeutic decisions regarding LMWH dosing in the field of bariatric surgery. In summary, there exists currently no robust evidence to provide guidance regarding type, dose and duration of antithrombotic prophylaxis after bariatric surgery 15. Due to the fact that most postdischarge VTE events occur within the first 30 days after surgery, extended VTE prophylaxis should be considered, but the specific duration of chemical prophylaxis is still a matter of discussion 12.
Direct oral anticoagulants (DOACs) are a new class of anticoagulants, whose application is more convenient compared to LMWH. DOACs allow effective and safe anticoagulation and their monitoring is usually not required.
Rivaroxaban is the first oral direct factor Xa inhibitor marketed. It was initially approved for the prevention of venous thromboembolism in patients after elective hip and knee replacement surgery. Rivaroxaban is generally well tolerated and demonstrates a predictable, dose‐dependent pharmacology profile up to 24 h after single dose application. The 10 mg dose of rivaroxaban has a high oral bioavailability (80–100%) irrespective of food intake, a rapid onset of action and the maximum plasma level is achieved 2–4 h after oral administration 16, 17. Prophylaxis with rivaroxaban had a significantly higher efficacy in VTE prophylaxis as compared with enoxaparin after hip and knee replacement surgery with similar rates of bleeding 18, 19, 20, 21. Friedman et al. 22 compared the efficacy of rivaroxaban in orthopaedic surgery patients with BMI ≥40 kg/m2 vs. <40 kg/m2 (posthoc subanalysis of a group of 12 355 patients) and found no difference in the incidence rates of DVT, PE or bleedings. Since age, sex or body weight 23 do not seem to alter pharmacokinetics (PK) and pharmacodynamics (PD) to a clinically relevant degree, the current recommendation for prophylaxis is 10 mg rivaroxaban once daily in all patients.
However, as pointed out in an editorial by Duffull 24, rivaroxaban PK/PD studies indicate a high degree of between‐subject variability in the drug concentration–time profile 23. Additionally, the effects of bariatric surgery on PK and PD parameters of DAOCs have not been sufficiently investigated to date, and there is no approved dosing recommendation for obese patient in the perioperative setting. With this clinical trial, we close part of this knowledge gap and lay ground for a broader investigation of rivaroxaban in morbidly obese patients, especially in the perioperative setting.
Methods
This single centre open‐label, nonrandomized phase 1 clinical trial was designed to investigate the single dose PK and PD parameters of rivaroxaban when administered to 12 patients undergoing a planned bariatric surgical procedure [six Roux‐en‐Y gastric bypass (RYGB) and six sleeve gastrectomy (SG) patients] in the framework of a pilot study.
The trial was approved by the Independent Ethics Committee of Bern, Switzerland, and the Swiss competent authority, Swissmedic. All patients gave written informed consent, and the trial was conducted according to the Declaration of Helsinki, the Good Clinical Practice guideline and local laws and regulations. The study was registered in the ClinicalTrials.gov registry with the identifier number NCT02438098.
Inclusion and exclusion criteria
Eligible patients were men and women, aged 18 years or older, with a BMI ≥35 kg/m2 with planned elective primary laparoscopic bariatric surgery (RYGB or SG). Main exclusion criteria were a history of active bleeding or a high‐risk for bleeding, a clinical indication for long‐term anticoagulation, and evidence of a thrombosis or PE in the personal history. The decision to perform bariatric surgery was taken independent of this trial.
Study procedure
Enrolled patients received a single oral dose of 10 mg rivaroxaban (Xarelto; Bayer Pharma AG, Germany) 1 day prior to and 3 days after surgery under nonfasting conditions. Venous blood samples were taken to assess the PK and PD parameters on both of these days. Blood samples were taken predose (Baseline) and 1, 2, 3, 4, 6, 8, 12 and 24 h post rivaroxaban administration.
After surgery, use of intermittent pneumatic compression as thrombosis prophylaxis and early mobilization were applied as standard of care. LMWH was started postoperatively 6 h after closure of the surgical site provided stable haemostasis had been achieved. Patients with BMI <50 kg/m2 received 40 mg of subcutaneous enoxaparin (Clexane), those with BMI ≥50 kg/m2 received 60 mg, respectively. Prophylaxis with LMWH was paused on day 3, when rivaroxaban was investigated.
On the 1st postoperative day, a gastrographin image series was performed to exclude a postoperative leak. Patients were discharged on day 4 after the surgical intervention. The last study visit was at day 30 ± 7, to collect safety data.
Study endpoints
Primary study endpoints were the single dose PK parameters of rivaroxaban after oral administration prior to and after RYGB and SG. Secondary endpoints were PD parameters as assessed by thrombin–antithrombin complexes (TAT), Prothrombin fragments 1 and 2 (F1 + 2) and D‐dimers. Safety endpoints were mortality, clinically evident proximal or distal DVT, PE and all bleeding events.
Sample analysis
Anti‐Xa activity of heparins and rivaroxaban was measured using the CE labelled chromogenic anti‐FXa assay Biophen Heparin 6 (Hyphen BioMed, Neuilly‐sur Oise, France). This is a one‐stage assay that utilizes endogenous antithrombin. It is an automated kinetic method during which a constant amount of exogenously added bovine FXa is inhibited by anticoagulants in the sample to be tested. Noninhibited FXa cleaves a FXa‐specific chromogenic substrate, producing a yellow signal that is detected at 405 nm. The measured anti‐Xa activity was converted to units anti‐Xa ml–1 (LMWH) or ng ml–1 (rivaroxaban) by the appropriate commercial calibrators, respectively. As for rivaroxaban, the performance of this assay has been evaluated against the standard high‐performance liquid chromatography with mass spectrometry method and results were comparable 25.
Prothrombin time was measured using Innovin (Siemens, Marburg, Germany) as the reagent, the assay was calibrated with a commercial kit containing four defined lyophilized plasmas (Siemens), the results are the average of duplicate measurements. Activated partial thromboplastin time is measured with Pathromtin SL (Siemens), the results are the average of duplicate measurements. Coagulation and chromogenic assays were performed on a Behring Coagulation System and a CS‐5100 automated analyser (Siemens), respectively 26.
Prothrombin activation fragments 1 + 2 (F1+2) and TAT were measured by a quantitative sandwich enzyme immunoassay, according to the protocol of the manufacturers (Enzygnost TAT micro and Enzygnost F1+2 micro; Siemens). The absorbance was measured using a microtiter plate reader at 492 nm 27. D‐dimers concentrations were determined by an automated quantitative immunoassay, according to the manufacturer's instructions (INNOVANCE D‐dimer; Siemens).
Safety and tolerability
Prior to the application of the study drug, every patient received an extensive evaluation including clinical chemistry, haematology and coagulation analyses, an electrocardiogram and clinical workup. After the application of the study medication, safety and tolerability were closely monitored during the first 24 h by measuring vital signs and specifically asking for untoward symptoms. Adverse events were monitored throughout the study to the final visit at 30 (±7) days postoperation. Each adverse event was classified according to its severity and seriousness.
Statistics
Demographics and relevant baseline variables are summarized for the per protocol set in tabular form. Data are stratified by type of surgery (RYGB, SG). Categorical data are presented as frequencies and percentages. For continuous variables, total number of measurements, mean and standard deviation are presented. Per protocol, only descriptive statistical analyses were foreseen.
PK and PD analysis
PK parameters were assessed prior to and after surgery by measuring rivaroxaban concentrations at nine different time points: prior to administration of study medication and 1, 2, 3, 4, 6, 8, 12 and 24 h thereafter. Noncompartmental PK parameters have been calculated using the R package DescTools (DescTools: Tools for descriptive statistics. A. Signorelli et al. 2015. R package version 0.99.18, http://CRAN.R‐project.org/package=DescTools). For the calculation of the area under plasma concentration–time curve (AUC), a spline‐interpolation was used.
For both surgical procedures, the following PK endpoints are presented: AUC; Cmax: peak plasma concentration; t1/2: terminal half‐life; Vz/f: = (Dose/C0)/bodyweight: apparent volume of distribution during the terminal phase divided by total body weight (in kg); tmax: time to peak plasma concentration. For some patients tmax could not be determined, since its values were the same for two points of time. These measurements were not included in the analysis of tmax. Cmax and tmax are presented in tabular and graphical form.
For D‐dimers, prothrombin fragments (F1+2) and TAT, maximal concentration Cmax and time to maximal concentration tmax is presented in tabular form. Two patients (ID 7 prior to surgery and ID 12 after surgery) are only partially included in the analysis since no valid PD results were obtained due to technically difficult blood sampling. For the assessment of the PD parameters, measurements at the following points of time were used: 0, 1, 3, 12 and 24 h after the application of the study medication. PK/PD data were only generated and analysed if the patient in fact received the study treatment.
Results
Study population
Between 19th July 2015 and 25th November 2015, 13 patients were enrolled into the study; one patient was withdrawn prior to the second application of rivaroxaban for safety reasons. Of the remaining 12 patients, six patients had SG and six were treated with RYGB surgery. Mean age was 39 years for both groups, and the proportion of male patients was 50 and 33% in the SG and in the RYGB group, respectively. Mean BMI was higher in the SG group (44.6 kg/m2) than in the RYGB group (38.5 kg/m2). All patients were of Caucasian origin (Table 1).
Table 1.
Baseline characteristics in the per protocol set
| Sleeve gastrectomy | Roux‐en‐Y gastric bypass | |
|---|---|---|
| n | 6 | 6 |
| Age (years), median (range) | 37.7 (24–51) | 39.0 (28–51) |
| Sex = male (%) | 3 (50.0) | 1 (16.7) |
| Weight median (range) | 137.0 (112–153) | 101.5 (96–120) |
| Height, median (range) | 172.5 (167–190) | 167.5 (156–171) |
| Body mass index, median (range) | 44.6 (38.3–50.6) | 38.2 (35.4–42.5) |
| ASA (%) | ||
| 2 | 1 (16.7) | 2 (33.3) |
| 3 | 3 (50) | 4 (66.7) |
| 4 | 2 (33.3) | 0 (0.0) |
| eGFR (%) | ||
| 71 | 1 (16.7) | 0 (0.0) |
| 80 | 1 (16.7) | 1 (16.7) |
| >90 | 4 (66.7) | 5 (83.3) |
| Ethnicity = Caucasian (%) | 6 (100.0) | 6 (100.0) |
ASA, American Society of Anesthesiologists physical status classification system; eGFR, estimated glomerular filtration rate
PK
Single application of 10 mg rivaroxaban resulted in a rivaroxaban area under the curve (AUC) of 933.7 μg·h l–1 (prebariatric assessment) and 1029.4 μg·h l–1 (postbariatric) in the RYGB group and of 971.9 μg·h l–1 (prebariatric) and 1165.8 μg·h l–1 (postbariatric) in the SG group, respectively. Cmax prior to bariatric surgery was similar in both groups (136.5 μg l–1 in patients RYGB vs. 135.3 μg l–1 in SG patients), whereas after the bariatric intervention Cmax was lower in RYGB patients (110.8 μg l–1) and higher in patients after SG (170 μg l–1). Mean tmax was slightly delayed after bariatric intervention in the RYGB group (1.5 vs. 2.5 h) but not in the SG group (1.5 h). However, the range was similar for both groups and both assessments (pre‐ and postbariatric). Half‐life of rivaroxaban was similar in both groups prior to and after bariatric surgery (Table 1). PK curves of the two different surgical procedures are displayed in Figure 1. PK parameters are summarized in Table 2.
Figure 1.
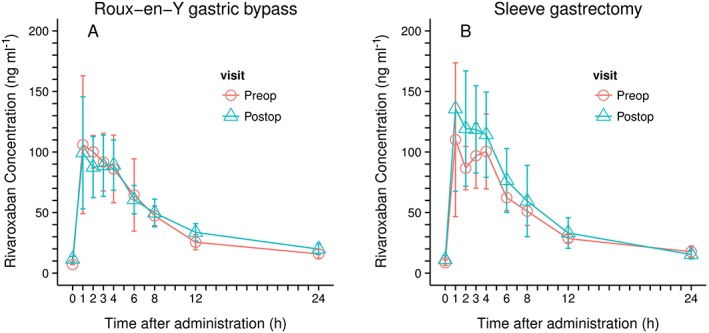
Rivaroxaban concentration: raw data by type of surgery; left, Roux‐en‐Y gastric bypass; right, sleeve gastrectomy
Table 2.
Pharmacokinetic parameters for all patients (summarized), and Roux‐en‐Y gastric bypass as well as sleeve gastrectomy patients (separated); prior to and after surgery the geometric mean and the coefficient of variation is presented. For tmax the median and the range is presented. The ratio prior to surgery/after surgery is presented together with its 95% confidence interval
| Patients | Parameters | Before surgery | After surgery | Ratio prior to surgery/after surgery |
|---|---|---|---|---|
| All patients pooled | AUC (μg·h l–1) | 952.6 / 16.8 | 1095.5 / 16.8 | 0.87 [0.77;0.98] |
| Cmax (μg l–1) | 135.9 / 19.3 | 137.3 / 33.3 | 0.99 [0.79;1.24] | |
| t1/2 (h) | 13.5 / 38.8 | 11.6 / 58.7 | 1.16 [0.82;1.64] | |
| VZ/f (l kg–1) | 47.9 / 22.3 | 44.4 / 26.1 | 1.08 [0.99;1.18] | |
| Tmax (h) | 1.5 (0.9–4) | 2 (1–4) | NA | |
| Roux‐en‐Y gastric bypass | AUC (μg · h l–1) | 933.7 / 22.3 | 1029.4 / 7.4 | 0.91 [0.75;1.09] |
| Cmax (μg l–1) | 136.5 / 10.7 | 110.8 / 31.8 | 1.23 [0.91;1.66] | |
| t1/2 (h) | 13.8 / 46.6 | 15 / 60.0 | 0.92 [0.57;1.48] | |
| VZ/f (l kg–1) | 55.3 / 22.5 | 52.7 / 20.8 | 1.05 [0.91;1.21] | |
| tmax (h) | 1.5 (0.9–4) | 2.5 (1–4) | NA | |
| Sleeve gastrectomy | AUC (μg h l–1) | 971.9 / 10.6 | 1165.8 / 21.9 | 0.83 [0.68;1.02] |
| Cmax (μg l–1) | 135.3 / 26.7 | 170.0 / 15.9 | 0.8 [0.59;1.08] | |
| t1/2 (h) | 13.1 / 34.1 | 8.9 / 44.6 | 1.47 [0.82;2.64] | |
| VZ/f (l kg–1) | 41.5 / 9.5 | 37.4 / 18.1 | 1.11 [0.95;1.29] | |
| tmax (h) | 1.5 (1–4) | 1.5 (1–4) | NA |
AUC, area under the plasma–concentration time curve from time 0 to infinity; Cmax, peak plasma concentration; t1/2, terminal half‐life; Vz/f, (Dose/C0)/bodyweight apparent volume of distribution during the terminal phase divided by total body weight (in kg); tmax, time to peak plasma concentration
PD
PD effects of rivaroxaban has been evaluated by the assessment of TAT, prothrombin fragments F1+2 and D‐dimers.
TAT decreased in the preoperative setting within the first 1–3 h after the application of rivaroxaban. Values significantly dropped within 1 h from a median TAT concentration of 10.6 to 2.6 ng ml–1 and from 13.7 to 2.8 ng ml–1 for the RYGB and the SG group, respectively, and this effect was maintained for at least 12 hours after the application of rivaroxaban. After 24 hours, TAT values increased slightly but were still lower than those values prior to the application of rivaroxaban for both groups in the preoperative setting (Figure 2, Figure S1, Table S1).
Figure 2.
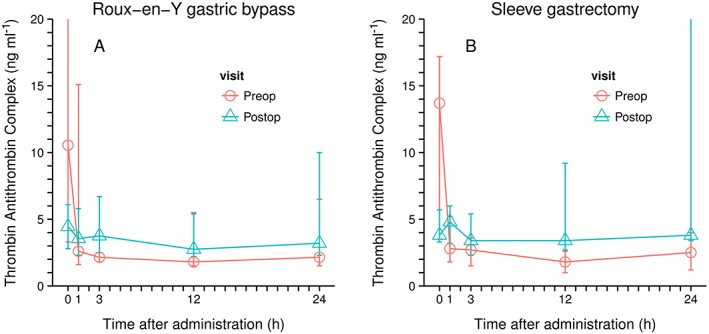
Thrombin–antithrombin complex concentrations (median and range); left Roux‐en‐Y Gastric bypass; n = 6; right, sleeve gastrectomy, n = 5
Postoperatively, TAT values were already decreased prior to the application of rivaroxaban, due to the fact that patients received prophylactic LMWH the day before as part of standard of care. However, a further slight decrease in these values was observed both 3 and 12 h after the application of rivaroxaban on Study Day 3. After 24 h, TAT values increased similar to the increase observed in the assessment taken prior to the surgical intervention (Figure 2, Figure S1, Table S1).
Similar to TAT, F1+2 are characterized by a relevant drop after the application of rivaroxaban. Decrease of concentration is most prominent 12 h after the application of rivaroxaban (reduction of median F1+2 concentration within 12 hours from 269 to 119 pmol l–1 and from 212 to 71 pmol l–1 for the RYGB and the SG group, respectively) whereas values rise towards the initial level after 24 h (Figure 3, Figure S2, Table S1). The dynamic changes observed with F1+2 was similar in the pre‐ and postoperative setting.
Figure 3.
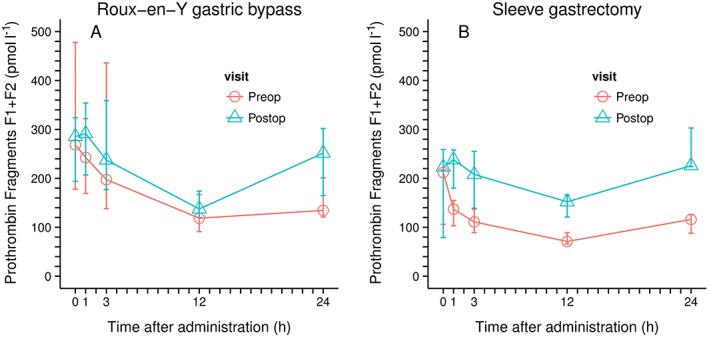
Prothrombin activation fragments F1 + 2 concentrations (median and range); left Roux‐en‐Y Gastric bypass; n = 6; right, sleeve gastrectomy, n = 5
D‐dimers decrease slightly during the first 12 hours after the application of rivaroxaban (reduction of median D‐dimer concentrations from 708 to 657 and from 629 ng ml–1 to 572 ng ml–1 over 12 h for the RYGB and the SG group, respectively) and increase to the initial D‐dimer level 24 h after the application of rivaroxaban. In the postoperative setting, D‐dimer values are generally higher than in the preoperative assessment but the dynamic changes observed are comparable to the preoperative setting (Figure 4, Figure S3, Table S1).
Figure 4.
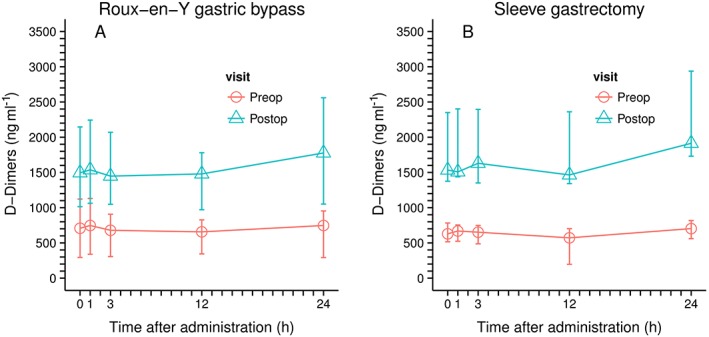
D‐Dimer concentrations (median and range); left Roux‐en‐Y Gastric bypass; n = 6; right, sleeve gastrectomy, n = 5
The PD parameters of two patients with SG (one patients in the presurgical and one in the postsurgical group) were excluded from further analysis due to false positive values that have been attributed to technical problems during the collection of the blood sample.
Safety and tolerability
All recorded adverse events and serious adverse events are listed in Table 3 together with the safety measures taken. There was only one serious adverse event. This patient suffered from a jejunal obstruction after RYGB that was unrelated to the study intervention but required surgical revision. This patient was withdrawn from the study and from the per protocol analysis set. Only in two events was the relationship to the study medication rated as possible, and both events were assessed as mild and moderate in intensity (Table 3).
Table 3.
Adverse events and safety measures taken
| ID | Diagnosis | Symptoms | AE grade | SAE | Relationship to study drug | Change of study intervention |
|---|---|---|---|---|---|---|
| 1 | Head ache | Headache | Mild | No | Unlikely | No change |
| 2 | Granuloma liver | Mild | No | Unlikely | No change | |
| 3 | Headache | Headache | Mild | No | Unlikely | No change |
| 3 | Coprostasis | Abdominal pain | Mild | No | Unlikely | No change |
| 5 | Nausea | Nausea | Mild | No | Unlikely | No change |
| 6 | Jejunal obstruction | Abdominal pain | Severe | Yes | Unlikely | Withdrawn |
| 6 | Superficial surgical site infection | Moderate | No | Unlikely | No change | |
| 6 | Deep surgical site infection | Moderate | No | Unlikely | No change | |
| 7 | Headache | Headache | Mild | No | Unlikely | No change |
| 8 | Headache | Headache | Mild | No | Unlikely | No change |
| 9 | Nausea | Moderate | No | Unlikely | No change | |
| 9 | Dizziness | Moderate | No | Unlikely | No change | |
| 9 | Hematoma of abdominal wall near incision | Mild | No | Possible | No change | |
| 9 | Low haemoglobin (72 g l–1) | Moderate | No | Possible | No change | |
| 9 | Impaired oesophagogastral transit of gastrographin | Vomiting | Mild | No | Unlikely | No change |
| ID | Hospitalization prolonged | Drug therapy | Other action taken | Death | Life threatening | ||
|---|---|---|---|---|---|---|---|
| 1 | No | No | No | ||||
| 2 | No | No | No | ||||
| 3 | No | No | No | ||||
| 3 | No | Yes | Metamizole | No | |||
| 5 | No | Yes | Metoclopramide | No | |||
| 6 | Yes | No | Yes | Reintervention | No | Yes | |
| 6 | No | No | Yes | Vacuum therapy of laparatomy wound | |||
| 6 | No | No | Yes | Drainage | |||
| 7 | No | Yes | Paracetamol | No | |||
| 8 | No | Yes | Paracetamol | No | |||
| 9 | No | Yes | Metoclopramide | No | |||
| 9 | No | No | No | ||||
| 9 | No | No | No | ||||
| 9 | No | Yes | Ferrinject (ferric carboxymaltose) | No | |||
| 9 | No | No | No | ||||
AE, adverse event; SAE, serious adverse event
Discussion
Single doses of 10 mg rivaroxaban resulted in similar systemic exposures, as measured by AUC, both prior to and after bariatric surgery, regardless of the type of bariatric procedure performed. In contrast to what might have been expected, the AUC values of both surgical groups were higher in the postoperative setting, compared to the preoperative setting. Maximum concentrations (Cmax) were higher in the SG group postoperatively and lower in the RYGB group compared to the presurgical assessment. However, this effect is less pronounced than what has been observed with different 10 mg galenic formulations of rivaroxaban and lies in the expected range of variation of other nonobese patient groups 17, 28. In the postoperative setting of RYGB patients tmax is slightly delayed, but the range remains unaffected. Overall, AUC of 10 mg rivaroxaban in this obese study population (prior to surgery 952.6 μg·h l–1, after surgery 1095.5 μg·h l–1) was similar to the AUC in healthy individuals with normal BMI (1020/14.9 μg·h l–1) and patients after total hip replacement surgery (1170 μg·h l–1) that have been exposed to the same dose and formulation of rivaroxaban supporting the finding that the AUC is not affected in a significant way by bariatric surgery 17, 29.
Prophylactic doses of rivaroxaban administered prior to the bariatric surgery led to a rapid PD response with a significant (>70%) median decrease of TAT within 1 h after the exposition to the anticoagulant. In the postoperative groups the initial drop of TAT was less pronounced since the patients already received LMWH the day prior to the application of rivaroxaban as part of the standard prophylactic treatment. TAT levels 24 h after the exposition to rivaroxaban did not return to normal as compared to preoperative levels prior to the ingestion of rivaroxaban but to a range observed 1–3 h after the application of rivaroxaban.
Additionally, the PD effects as measured by prothrombin activation fragments is characterized by a significant (>55%) median drop of F1+2 value within the timeframe of 12 h. After 24 h, prothrombin activation fragments remained below the levels measured prior to the administration of rivaroxaban in the preoperative groups, whereas, in the postoperative group, F1+2 levels were equal to the levels measured prior to the administration of rivaroxaban, probably reflecting the effect of previously administered LMWH.
The delayed response of F1+2 compared to TAT is explained by its longer half‐life (about 90 min) compared to TAT (about 10 min) 30. For D‐dimers, which are characterized by an even longer half‐life (around 8–12 h), only a slight decrease of concentration could be observed 12 h after rivaroxaban ingestion.
With the exception of baseline levels of F1+2 and particularly TAT, PD values in the postsurgical analyses were higher compared to the presurgical investigations as a consequence of the procoagulant effect of the surgical intervention. This observation may indicate that the same dose of anticoagulant is slightly less effective in controlling the postoperatively increased procoagulant state.
Kubitza et al. 31 investigated PK, PD and the safety profile of 10 mg single dose rivaroxaban administration in different body weight groups. Interestingly, AUC values were stable across all weight groups: 1172 μ·h l–1 in female patients ≤50 kg, 1029 μ·h l–1 in patients weighing 70–80 kg, and 1155 μ·h l–1 in the >120 kg but <150 kg weight group. The results of our study indicate, too, that neither increased body weight nor the bariatric intervention significantly affect the PK and PD parameters of the drug (prebariatric AUC 952.6 μ·h l–1, post bariatric AUC 1095.5 μ·h l–1). The most probable explanation for this observation is the low volume of distribution of rivaroxaban. In fact, rivaroxaban is extensively bound to plasma proteins und has a relatively low tissue affinity 31. In the trial of Kubitza et al. 31, women in the ≤50 kg weight group showed an increased Cmax (178 μg l–1) whereas rivaroxaban AUCs were similar in all groups. Our results demonstrate a higher Cmax (170 μg l–1) and an increased interindividual variability of postoperative rivaroxaban plasma levels in the SG group but a slightly decreased Cmax (110 μ l–1) and an increased tmax (plus 1 h, range unaffected) in the RYGB group, again with similar AUCs in both surgical groups prior to and after the bariatric intervention. Reasons for these observations may be an increased variability in gastric passage time in patients who had bariatric surgery directly affecting the stomach, and alterations in the site of drug absorption in RYGB patients as a consequence of the partially bypassed stomach and the bypassed duodenum. However, these observations are within the known variations of rivaroxaban PK parameters.
Overall, prophylactic application of rivaroxaban in bariatric patients resulted in PK results comparable to those reported from prior trials and the assessment of PD parameters supports the clinical effectiveness of a 10 mg rivaroxaban dose in obese patients.
The data obtained from our trial support the up to now published results and also expand our understanding of the clinical pharmacology of rivaroxaban, specifically showing that the PK and PD properties remain unaltered after SG and RYGB.
This clinical trial is the first systematic investigation of rivaroxaban in bariatric surgery patients. It shows that there were no relevant alterations in the clinical pharmacology profile of rivaroxaban in the postoperative setting compared to results obtained prior to the surgical intervention. Single doses of 10 mg rivaroxaban showed an unremarkable safety profile without clinically relevant signs of bleeding after bariatric surgery and there was no thrombotic event observed during this clinical trial.
Limitations of this phase 1 clinical trial are the relatively small sample size and the single applications of rivaroxaban. However, it is important to note that rivaroxaban does not have significant accumulation after multiple doses, so that the single‐dose profile is predictive of the multiple dose profile in patients without impaired renal function.
Another limitation is the short interval between the surgical intervention and the application of rivaroxaban. Although this takes into account the timeframe at interest for a prophylactic postoperative anticoagulation, it is not known whether PK parameters remain unchanged over the following period of weight loss and postsurgical functional adaptations of the gastrointestinal tract.
In conclusion, single doses of 10 mg rivaroxaban had a favourable PK, PD and safety profile in this limited bariatric surgery collective. The results of this study will help to design larger trials with clinical endpoints in this particular patient population with the final goal of safe and efficacious use of rivaroxaban in morbidly obese patients.
Competing Interests
Dino Kröll, Guido Stirnimann, Andreas Vogt, Desirée Lin Lee Lai, Yves Michael Borbély, Julia Altmeier, Sabine Schädelin, Daniel Candinas, Philipp Christoph Nett declare that they have no conflict of interest. Lorenzo Alberio has received travel grants and consultancy fees from Bayer; he is member of the Swiss Advisory Board for the clinical use of Rivaroxaban in VTE and of the working group RIVAMOS 25. There are no commercial interests related to the subject of this manuscript. Financial or material support: This clinical trial has been supported by a grant of Bayer (Schweiz) AG, Medical Department, Grubenstrasse 6, CH 8045 Zürich, Switzerland.
The authors would like to thank Kenneth Todd Moore for review and proofreading of the manuscript and Christiane Gerschheimer (Central Haematology Laboratory, CHUV, Lausanne, Switzerland) for her skilled laboratory support.
Contributors
Wrote manuscript: D.K., G.S., L.A., S.S. Designed research: D.K., G.S., L.A., S.S. Performed research: D.K., Y.B., J.A., P.N., D.C., A.V., D.L. Analysed data: D.K., G.S., L.A., S.S. Critical review of manuscript: P.N., Y.B., D.C., A.V., D.L., J.A.
Supporting information
Figure S1 PD data TAT
Figure S2 PD data F1+2
Figure S3 PD data D‐dimers
Table S1 PD Data
Kröll, D. , Stirnimann, G. , Vogt, A. , Lai, D. L. L. , Borbély, Y. M. , Altmeier, J. , Schädelin, S. , Candinas, D. , Alberio, L. , and Nett, P. C. (2017) Pharmacokinetics and pharmacodynamics of single doses of rivaroxaban in obese patients prior to and after bariatric surgery. Br J Clin Pharmacol, 83: 1466–1475. doi: 10.1111/bcp.13243.
Contributor Information
Dino Kröll, Email: dino.kroell@insel.ch.
Lorenzo Alberio, Email: lorenzo.alberio@chuv.ch.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 2016; 44 (D1): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 2015; 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. GBD 2013 Risk Factors Collaborators , Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet 2015; 386: 2287–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eckel RH, Alberti KG, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2010; 375: 181–183. [DOI] [PubMed] [Google Scholar]
- 5. Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta‐analysis. Am J Med 2009; 122: 248–256e5. [DOI] [PubMed] [Google Scholar]
- 6. Allman‐Farinelli MA. Obesity and venous thrombosis: a review. Semin Thromb Hemost 2011; 37: 903–907. [DOI] [PubMed] [Google Scholar]
- 7. Stein PD, Matta F, Goldman J. Obesity and pulmonary embolism: the mounting evidence of risk and the mortality paradox. Thromb Res 2011; 128: 518–523. [DOI] [PubMed] [Google Scholar]
- 8. Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta‐analysis. Circulation 2008; 117: 93–102. [DOI] [PubMed] [Google Scholar]
- 9. Juhan‐Vague I, Alessi MC, Mavri A, Morange PE. Plasminogen activator inhibitor‐1, inflammation, obesity, insulin resistance and vascular risk. J Thromb Haemost 2003; 1: 1575–1579. [DOI] [PubMed] [Google Scholar]
- 10. Escalante‐Tattersfield T, Tucker O, Fajnwaks P, Szomstein S, Rosenthal RJ. Incidence of deep vein thrombosis in morbidly obese patients undergoing laparoscopic roux‐en‐Y gastric bypass. Surg Obes Relat Dis 2008; 4: 126–130. [DOI] [PubMed] [Google Scholar]
- 11. Arnold DM, Kahn SR, Shrier I. Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of thromboprophylaxis guidelines. Chest 2001; 120: 1964–1971. [DOI] [PubMed] [Google Scholar]
- 12. Winegar DA, Sherif B, Pate V, DeMaria EJ. Venous thromboembolism after bariatric surgery performed by bariatric surgery Center of Excellence Participants: analysis of the bariatric outcomes longitudinal database. Surg Obes Relat Dis Surgery 2011; 7: 181–188. [DOI] [PubMed] [Google Scholar]
- 13. Jamal MH, Corcelles R, Shimizu H, Kroh M, Safdie FM, Rosenthal R, et al. Thromboembolic events in bariatric surgery: a large multi‐institutional referral center experience. Surg Endosc 2015; 29: 376–380. [DOI] [PubMed] [Google Scholar]
- 14. American Society for Metabolic and Bariatric Surgery Clinical Issues Committee . ASMBS updated position statement on prophylactic measures to reduce the risk of venous thromboembolism in bariatric surgery patients. Surg Obes Relat Dis 2013; 9: 493–497. [DOI] [PubMed] [Google Scholar]
- 15. Finks JF, English WJ, Carlin AM, Krause KR, Share DA, Banerjee M, et al. Predicting risk for venous thromboembolism with bariatric surgery: results from the Michigan bariatric surgery collaborative. Ann Surg 2012; 255: 1100–1104. [DOI] [PubMed] [Google Scholar]
- 16. Bayer Pharma AG . Xarelto® (rivaroxaban) summary of product characteristics. Available at http: //wwwemaeuropaeu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/000944/WC500057108pdf. 2013 (last accessed 2 August 2016).
- 17. Kubitza D, Becka M, Voith B, Zuehlsdorf M, Wensing G. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59‐7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther 2005; 78: 412–421. [DOI] [PubMed] [Google Scholar]
- 18. Turpie AG, Lassen MR, Davidson BL, Bauer KA, Gent M, Kwong LM, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet 2009; 373: 1673–1680. [DOI] [PubMed] [Google Scholar]
- 19. Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008; 358: 2765–2775. [DOI] [PubMed] [Google Scholar]
- 20. Lassen MR, Ageno W, Borris LC, Lieberman JR, Rosencher N, Bandel TJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008; 358: 2776–2786. [DOI] [PubMed] [Google Scholar]
- 21. Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Muntz J, et al. Extended duration rivaroxaban versus short‐term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double‐blind, randomised controlled trial. Lancet 2008; 372: 31–39. [DOI] [PubMed] [Google Scholar]
- 22. Friedman RJ, Hess S, Berkowitz SD, Homering M. Complication rates after hip or knee arthroplasty in morbidly obese patients. Clin Orthop Relat Res 2013; 471: 3358–3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mueck W, Eriksson BI, Bauer KA, Borris L, Dahl OE, Fisher WD, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban – an oral, direct factor Xa inhibitor‐‐in patients undergoing major orthopaedic surgery. Clin Pharmacokinet 2008; 47: 203–216. [DOI] [PubMed] [Google Scholar]
- 24. Duffull SB. Is the ideal anticoagulant a myth? Expert Rev Clin Pharmacol 2012; 5: 231–236. [DOI] [PubMed] [Google Scholar]
- 25. Asmis LM, Alberio L, Angelillo‐Scherrer A, Korte W, Mendez A, Reber G, et al. Rivaroxaban: quantification by anti‐FXa assay and influence on coagulation tests: a study in 9 Swiss laboratories. Thromb Res 2012; 129: 492–498. [DOI] [PubMed] [Google Scholar]
- 26. Zurcher M, Sulzer I, Barizzi G, Lammle B, Alberio L. Stability of coagulation assays performed in plasma from citrated whole blood transported at ambient temperature. Thromb Haemost 2008; 99: 416–426. [DOI] [PubMed] [Google Scholar]
- 27. Chilver‐Stainer L, Lammle B, Alberio L. Titre of anti‐heparin/PF4‐antibodies and extent of in vivo activation of the coagulation and fibrinolytic systems. Thromb Haemost 2004; 91: 276–282. [DOI] [PubMed] [Google Scholar]
- 28. Mueck W, Stampfuss J, Kubitza D, Becka M. Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin Pharmacokinet 2014; 53: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mueck W, Borris LC, Dahl OE, Haas S, Huisman MV, Kakkar AK, et al. Population pharmacokinetics and pharmacodynamics of once‐ and twice‐daily rivaroxaban for the prevention of venous thromboembolism in patients undergoing total hip replacement. Thromb Haemost 2008; 100: 453–461. [PubMed] [Google Scholar]
- 30. Chandler WL, Velan T. Estimating the rate of thrombin and fibrin generation in vivo during cardiopulmonary bypass. Blood 2003; 101: 4355–4362. [DOI] [PubMed] [Google Scholar]
- 31. Kubitza D, Becka M, Zuehlsdorf M, Mueck W. Body weight has limited influence on the safety, tolerability, pharmacokinetics, or pharmacodynamics of rivaroxaban (BAY 59‐7939) in healthy subjects. J Clin Pharmacol 2007; 47: 218–226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 PD data TAT
Figure S2 PD data F1+2
Figure S3 PD data D‐dimers
Table S1 PD Data


