Abstract
Aims
A modular interdisciplinary platform was developed to investigate the economic impact of oseltamivir treatment by dosage regimen under simulated influenza pandemic scenarios.
Methods
The pharmacology module consisted of a pharmacokinetic distribution of oseltamivir carboxylate daily area under the concentration–time curve at steady state (simulated for 75 mg and 150 mg twice daily regimens for 5 days) and a pharmacodynamic distribution of viral shedding duration obtained from phase II influenza inoculation data. The epidemiological module comprised a susceptible, exposed, infected, recovered (SEIR) model to which drug effect on the basic reproductive number (R0), a measure of transmissibility, was linked by reduction of viral shedding duration. The number of infected patients per population of 100 000 susceptible individuals was simulated for a series of pandemic scenarios, varying oseltamivir dose, R0 (1.9 vs. 2.7), and drug uptake (25%, 50%, and 80%). The number of infected patients for each scenario was entered into the health economics module, a decision analytic model populated with branch probabilities, disease utility, costs of hospitalized patients developing complications, and case‐fatality rates. Change in quality‐adjusted life years was determined relative to base case.
Results
Oseltamivir 75 mg relative to no treatment reduced the median number of infected patients, increased change in quality‐adjusted life years by deaths averted, and was cost‐saving under all scenarios; 150 mg relative to 75 mg was not cost effective in low transmissibility scenarios but was cost saving in high transmissibility scenarios.
Conclusion
This methodological study demonstrates proof of concept that the disciplines of pharmacology, disease epidemiology and health economics can be linked in a single quantitative framework.
Keywords: epidemiology, health economics, influenza, interdisciplinary pharmacometrics, oseltamivir, pharmacokinetics/pharmacodynamics
What is Already Known about this Subject
To date, modelling of influenza has been conducted in discrete discipline areas.
The discrete pharmacology, epidemiology and health economic models are not linked and make assumptions about the adjacent disciplines that are inappropriate.
There are no epidemiological or health economic models which have taken into account between subject variability in the pharmacology of influenza treatments.
What this Study Adds
This study provides the first integrated interdisciplinary framework to understand the cost‐utility of antiviral therapy under various influenza pandemic scenarios linking drug pharmacokinetics/pharmacodynamics, epidemiological and health economics endpoints.
This quantitative framework was able to show that oseltamivir reduced the median number of infected individuals, increased quality‐adjusted life years by deaths averted, and was cost‐saving under most pandemic scenarios.
Given the growing need to justify pricing of medicines to society and payer, the methodology of interdisciplinary pharmacometrics can be applied across all disease areas where the pharmacokinetics/pharmacodynamics, clinical or epidemiological endpoints of interest can eventually be linked to health economic value.
Introduction
Influenza is a common transmissible viral respiratory illness that, in susceptible individuals, can be associated with substantial morbidity and mortality due to complications such as pneumonia and bronchitis. Influenza tends to spread rapidly in seasonal epidemics and in some cases extensive spread results in a pandemic.
A very relevant and essential public health topic is influenza pandemic containment and how to apply strategies to mitigate the impact of a pandemic in a timely manner 1, 2. To date, however, even the most sophisticated mathematical modelling approaches, which are important for informing influenza pandemic planning, do not consider basic features of antiviral pharmacology. These approaches consider drug effect as either on or off in terms of altering transmission 1, 2. There has been very little consideration of variability in pharmacokinetics (PK) or drug response/pharmacodynamics (PD) 1, 2. Furthermore, such models have not linked drug PK/PD to epidemiological or health economics endpoints. An integrated framework linking these modules is required to better understand the cost‐utility of current and emerging antiviral therapies, and their application and optimal deployment to manage influenza pandemics.
The current antiviral cornerstone of influenza pandemic preparedness is the neuraminidase inhibitor oseltamivir 3. Oseltamivir is an oral prodrug that is extensively metabolized by hepatic carboxylesterase 1A1 to oseltamivir carboxylate (OC). Once in circulation, OC is predominately cleared by the kidney via glomerular filtration and renal secretion. Following oral dosing, plasma oseltamivir concentrations decline rapidly with an apparent elimination half‐life (t1/2) of 1–3 h, while OC has a t1/2 of 6–10 h 4. OC inhibits the production of influenza virus (viral shedding) from infected host cells 4 thereby reducing the duration of infection and hastening resolution of signs and symptoms of infection.
Whilst there is general agreement that oseltamivir in adults with influenza accelerates time to clinical symptom alleviation, there are divergent views expressed in the literature on whether oseltamivir reduces risk of lower respiratory tract complications, and admittance to hospital 5, 6.
Recently, Rayner and colleagues have identified the PK/PD determinants of oseltamivir efficacy, showing an area under the concentration–time curve (AUC) relationship with time to cessation of viral shedding and time to resolution of influenza symptoms 7. The AUC breakpoints were similar for virological and clinical endpoints, indicating that the PD effects of oseltamivir on viral shedding and symptoms were synchronized by drug exposure.
In epidemiology, the basic reproductive number (R0) is a fundamental concept describing transmissibility of an infectious disease in a given population. Qualitatively, R0 is defined as the number of secondary infections produced from a primary infective source 8. Quantitatively, R0 can be captured implicitly within a simple transmission model called the susceptibility, infectivity and recovered model, which describes the progression of an epidemic/pandemic over time in a population 9.
Modelling approaches, such as decision analytic models, are commonly employed in health economic studies to estimate the cost‐utility of various interventions and health outcomes. The cost‐utility analysis is a specific type of cost‐effectiveness analysis in which effectiveness is measured in terms of quality‐adjusted life‐years (QALY). The QALY is calculated by using utility values to adjust the duration of time in a particular health state for the quality of an individual's life in that health state. A recent review reported that most antiviral economic studies used static models, which are incapable of incorporating the dynamic nature of the viral transmission process 10.
The current work describes a modular approach to link oseltamivir PK/PD 7, influenza epidemiology via a variant of the susceptibility, infectivity and recovered model, and a health economic decision analytic model. Our motivation was driven by: (i) public health considerations: to understand the impact of oseltamivir pharmacological variability on patient outcomes, health utilization and economics under different simulated pandemic scenarios; and (ii) production of a proof of concept framework: to integrate drug PK/PD, dynamic disease transmission, and health economic data. To our knowledge, linking of the adjacent disciplines of pharmacology, epidemiology and health economics has not to date been successfully implemented in a single quantitative framework.
Methods
Pharmacology (PK/PD) module
The PK/PD relationships were previously generated based on data from 140 subjects collected from two phase II inoculation studies; each study was approved by an ethics committee and conducted in accordance with the Declaration of Helsinki; all participants gave their informed consent prior to inclusion. For a full description of the study designs, population, and PK/PD analysis see 7, 11. A three‐group (placebo, low and high exposure) OC AUC relationship with time to cessation of viral shedding (Figure 1A) and resolution of composite symptoms (Figure 1B) have been reported. An AUC breakpoint of >14 180 ng.h ml−1 was identified as a common PK/PD threshold of interest 5 based on enhanced cessation of viral shedding and resolution of influenza symptoms (Figure 1A,B). A previously published oseltamivir population PK model of 390 healthy and infected subjects ranging from 1–78 years across a dose range of 20–1000 mg was used to describe OC exposure. The final covariate model from this population PK analysis included a relationship between weight and creatinine clearance on OC clearance and weight on the OC central volume of distribution. All covariates had a fitted allometric exponent 11. From this final covariate model, we simulated oseltamivir PK parameter profiles in 5000 70‐kg adult patients aged 18–65 with normal renal function receiving the standard 75 mg twice daily (BID) and 150 mg BID oseltamivir for 5 days. The population pharmacokinetic model structure consisted of a two‐compartment model with first‐order absorption of oseltamivir and first‐order conversion of oseltamivir to OC and a one‐compartment model with first‐order elimination of OC. The AUCs for each patient were quantified and the proportion of patients with OC AUCs above the identified PK/PD viral shedding threshold (14 180 ng.h ml−1) was calculated for each dosing regimen. Additional Monte Carlo simulations were conducted for each dosage regimen, sampling from the oseltamivir population AUC distributions (Figure 1C) to construct a density distribution of the population fraction achieving target attainment for each regimen. For each patient at a given oseltamivir dose, an individual duration of viral shedding (Tshed; ϒ) value was assigned from a log‐normal Tshed distribution based on inoculation study data 7 (see Table 1).
Figure 1.
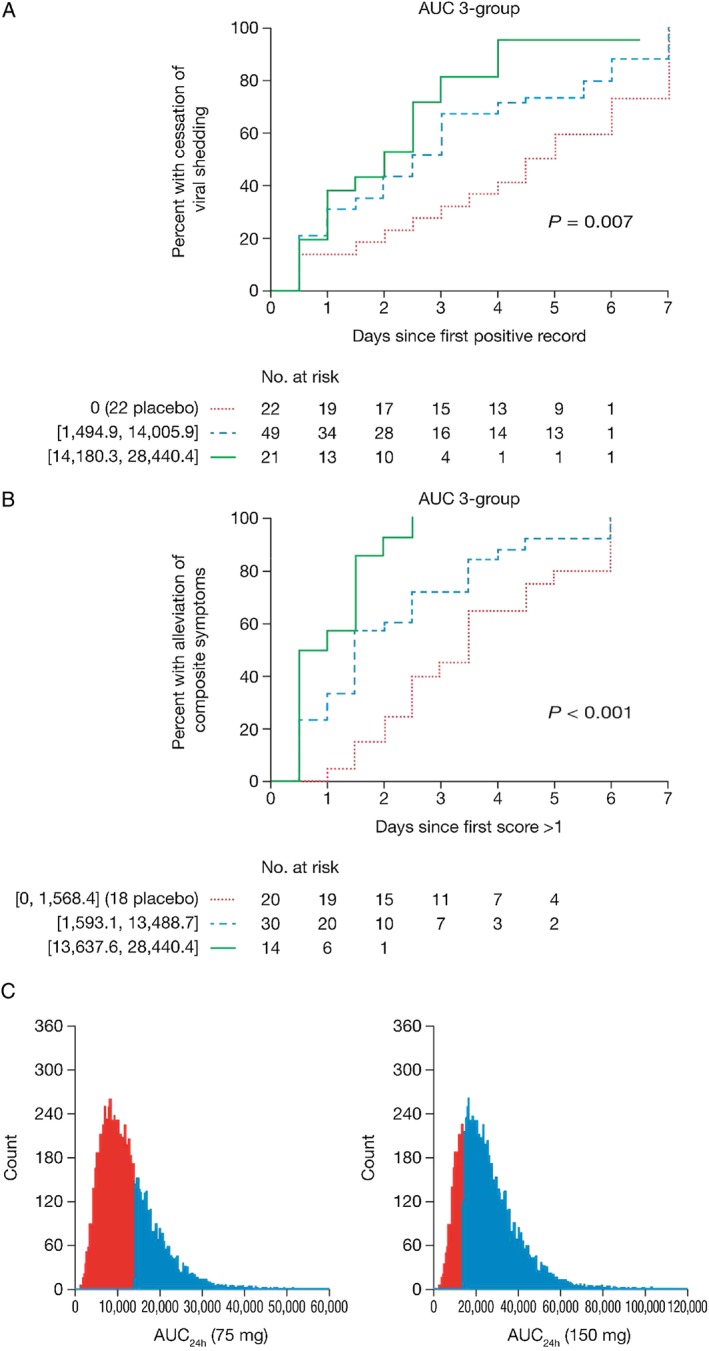
Pharmacology (pharmacokinetics/pharmacodynamics) module. Figure 1A and B were adopted from Rayner et al. 7 and show the relationship between oseltamivir area under the concentration‐time curve (AUC) and time to cessation of viral shedding and resolution of symptoms, respectively, from phase II data. A cut‐off AUC of 14 180 ng.h ml−1 separated low (blue dashed line) and high oseltamivir exposed (green solid line) patients in the three‐group relationship (AUC breakpoints are shown in brackets next to number of patients carrying over); red dotted line shows unexposed patients (placebo). Figure 1C shows the proportion simulated above and below the cut‐off AUC at two dosage regimens (75 and 150 mg twice daily for 5 days) using an established pop pharmacokinetic (PK) model (Kamal et al. 9). Dark red = ‘low’ AUC group; light blue = high ‘AUC’ group
Table 1.
Input parameters used in the pharmacology–epidemiology modules
| Descriptor | Value |
|---|---|
| Population size (In) | 100 000 cases |
| Latency period (1/κ) | 1 day |
| FAUChigh (150 mg BID) | 0.795 (0.095) |
| FAUChigh (75 mg BID) | 0.326 (0.048) |
| Tshed(0) (No treatment) | 6 (2.5) days |
| Tshed(low) (AUC 0–14 180 ng.h ml−1) | 3 (0.58) days |
| Tshed(high) (AUC > 14 180 ng.h ml−1) | 1.9 (0.51) days |
| β, Moderate infectivity | 0.21 days−1 |
| β, High infectivity | 0.41 days−1 |
κ, the delay rate between exposure to influenza and symptom development; FAUChigh, the mean (SD) fraction of the simulated population receiving oseltamivir with an AUC >14 180 ng.h ml−1; Tshed(0), the duration of viral shedding under no treatment; Tshed(low), the mean (SD) duration of viral shedding if OC AUC is <14 180 ng.h ml−1; Tshed(high), the mean (SD) duration of viral shedding if OC AUC is greater than 14 180 ng.h ml−1 β, the rate of infectivity; AUC, area under the concentration–time curve; BID, twice daily
Epidemiology module
To link oseltamivir PK/PD to influenza epidemiology, we used a stochastic susceptible, exposed, infected, recovered (SEIR) epidemiological model 12, adapted to incorporate the impact of antiviral therapy (Figure 2A).
Figure 2.
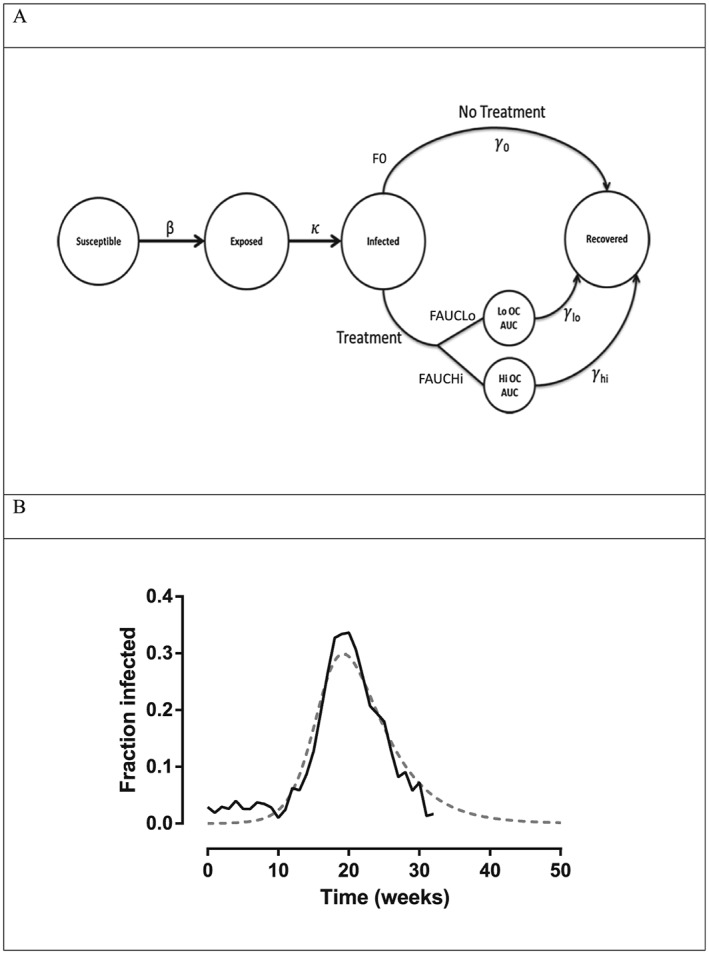
Epidemiology module. Figure 2A shows the SEIR (susceptible, exposed, infected, recovered) influenza epidemiology model adapted to account for oseltamivir treatment effect. Figure 2B shows fit of the SEIR model to 2007–2008 Influenza Epidemic Data from the Midwestern USA (http://www.cdc.gov/flu/weekly/pastreports.htm). The fitting produced parameter values of β = 0.73, γ = 4.1 day, and 1/κ = 1 day. The grey dotted line represents model fitted function; the solid black line represents actual data. AUC, area under the concentration‐time curve; OC, oseltamivir carboxylate
The differential equations describing the SEIR model are:
| (1) |
| (2) |
| (3) |
| (4) |
where S is the number of individuals in the population who are susceptible to influenza, E represents the number of individuals exposed and in the latent stage of influenza infection, I represents the number of infected individuals, and R represents individuals who have recovered from infection and are immune to re‐infection. The parameter β governs infectivity, and is a composite of both frequency of individual interactions (population density and social behaviours), and the probability that an interaction will result in a successful influenza infection in a susceptible individual (infectiousness). κ represents the transit time from E to I (delay time between influenza exposure and development of symptoms), which is assumed to be 1 day 13 while γ governs the disease recovery rate in the population. F 0 represents the fraction of the simulated population not receiving therapy, F AUClow is the fraction of the population receiving oseltamivir with an AUC ≤ 14 180 ng.h ml−1, and F AUChigh is the fraction of the population receiving oseltamivir with an AUC > 14 180 ng.h ml−1, obtained from the pharmacology module. N equals the total population (assumed to be 100 000). Because each simulation was limited to a single influenza season (1 year), population birth and death rates were not incorporated into the model. Initial conditions for each compartment were as follows: S = 100 000; E = 0; I = 1; R = 0.
As oseltamivir acts to reduce duration of illness by inhibiting Tshed, the recovery rate γ in the SEIR model is inversely related to Tshed (Tshed = 1/γ). The R0 or transmissibility can be expressed in approximate terms 12 as R0 ≈ infectivity rate/rate of recovery ≈ β/γ ≈ β × Tshed and hence empirically by reducing Tshed, oseltamivir reduces R0.
To confirm that the SEIR model provided results that were in general agreement with prior experience, the SEIR model (without incorporation of antiviral therapy) was validated using data from a previous influenza outbreak in the Midwestern USA (2007–2008 seasons) extracted from the Centers for Disease Control and Prevention influenza seasonal summary database (http://www.cdc.gov/flu/weekly/pastreports.htm). In the Midwestern USA, 20 263 patients were tested for influenza, with 4970 confirmed with an influenza infection. The structural model validation was achieved by loading the SEIR structural model (without antiviral therapy) and the data from the Midwestern influenza outbreak into Berkeley Madonna Software. Using the model fitting procedure available in Berkeley Madonna, which uses a weighted sum of squared differences approach to achieve minimization, the attack rate and viral shedding rate was estimated. These values were consistent with those used in our simulations and provided a satisfactory fit with the original data set.
Using the model, simulations were then undertaken to evaluate the impact of oseltamivir treatment regimens under a number of pandemic scenarios; input parameters used in the simulations are provided in Table 1. The simulation scenarios evaluated were stratified by: treatment (no treatment, oseltamivir 75 mg and 150 mg BID for 5 days); the percent drug uptake, i.e., percentage of the infected population who had an uptake of oseltamivir (25%, 50%, and 80%); and transmissibility (R0 of approximately 1.9 and 2.7) 14. For each update scenario, 100% adherence to therapy was assumed. Parameters for β were adjusted to achieve the requisite R0 number as outlined in Table 1. For each of the above, 1000 Monte Carlo simulations (pandemics) were conducted to provide the median attack rate (number of infected cases per total population of 100 000). Each simulation was run across a period of 1 year i.e. an entire flu season. All pharmacometric and epidemiological simulations were conducted in Berkley Madonna version 8.3.18.
Health economics module
The number of infected individuals obtained from each simulated pandemic scenario, from the epidemiology module (SEIR model), were entered into a decision analytic model, i.e. a decision tree (See Figure 3). Each branch of the decision model represents a possible decision or occurrence which is mutually exclusive. A cost‐utility analysis was undertaken based on the US population of healthy adults, aged 18–64 years, from both a payer and societal perspective. The payer perspective included only direct costs, whereas the societal perspective included both direct and indirect costs. Total costs and QALYs were determined over a 1‐year time period. These two outcomes were combined to calculate incremental cost‐effectiveness ratios (ICERs), a commonly used metric in cost‐utility analysis that is constructed by dividing the difference in cost between two interventions by the difference in effectiveness between two interventions. ICERs are a useful way of describing the increased cost required to yield one more unit of effectiveness when implementing one strategy over another. QALYs were calculated by multiplying the life‐years (LY) by a utility, a value that describes the patient's quality of life in a certain health state ranging from 0 to 1 with 0 being death and 1 being perfect health. The number of LYs gained by a therapeutic intervention was determined by the number of deaths averted (one LY was lost per death from a population of 100 000 individuals). The expected value of utility was determined from the utility of all the health state events in Figure 3.
Figure 3.
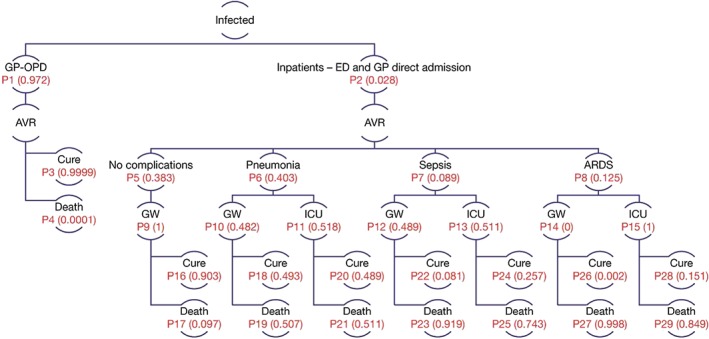
Health economics module. The number of infected‐influenza patients per population of 100 000 (calculated from the epidemiology module) entered a decision analytic model. Patients received treatment in an outpatient or inpatient setting. Inpatients admitted into a general ward (GW), or an intensive care unit (ICU) may experience pneumonia, sepsis, or acute respiratory distress syndrome (ARDS). The branch probabilities (P1‐P29) are shown for the base case (see Table 2 for data source). AVR, antiviral treatment; ED, emergency department; GP‐OPD, general practice or outpatient department
While there is no consensus as to the optimal ICER threshold in the USA, experts in a recent commentary advocated for a threshold of $100 000 to $150 000 per QALY. For the purpose of this study, we assumed that a gain of one QALY was valued at 100 000 USD 15. Therefore, an ICER >100 000 USD per QALY indicated the new intervention was not cost‐effective. Because of the difficulty in interpreting an ICER <0 (∆C < 0 and ∆E > 0), in these instances we simply indicate that cost‐saving has occurred.
An infected individual entered the health economics (HE) model either as an outpatient or inpatient (Figure 3). Inpatients admitted into a general ward or an intensive care unit could experience pneumonia, sepsis, or acute respiratory distress syndrome (ARDS) 16, 17, 18. We assumed that patients could only experience one influenza‐related complication within 1 year. The infected patient either recovered from the infection or died. It was also assumed that all patients were 100% adherent to treatment received, and that oseltamivir reduced the time of symptom alleviation by 21 hours and had no direct effects on influenza complications or hospitalization rate 19, 20, 21.
Data inputs for the HE model (Table 2) such as branch probabilities, direct medical costs (medication and hospitalization), direct nonmedical costs (transportation to and from hospital), length of hospitalization, case‐fatality rates from influenza complications and health state utilities (which are used in the construction of the QALY) were obtained from the published literature 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42 and Healthcare Cost and Utilization Project Nationwide Inpatient Sample database. Where possible, data related to the 2009 pandemic H1N1 influenza was used. All costs were converted to 2013 USD using the Consumer Price Index 43. Indirect costs, defined as the costs attributed to daily work productivity loss by age, were determined by the approach of Meltzer et al. 36.
Table 2.
Input parameters, values and data sources used in the health economics module
| Parameters | Base–case value | Source(s) | |
|---|---|---|---|
| Probabilities | |||
| Medical care received | |||
| Outpatient visit | 0.972 | 22 | |
| Inpatient | 0.028 | 22 | |
| Channels of inpatient admission | |||
| Through ED visit | 0.778 | 23 | |
| Through outpatient visit | 0.222 | 23 | |
| Complication associated with influenza | |||
| No complications | 0.383 | ||
| Pneumonia | 0.403 | 16, 17, 18 | |
| Sepsis | 0.089 | 17, 18 | |
| ARDS | 0.125 | 17, 18 | |
| Type of hospitalization | |||
| No complication | |||
| ICU | 0 | ||
| GW | 1 | Assumed | |
| Pneumonia | |||
| ICU | 0.518 | 16 | |
| GW | 0.482 | ||
| Sepsis | |||
| ICU | 0.511 | 24 | |
| GW | 0.489 | ||
| ARDS | |||
| ICU | 1 | 18 | |
| GW | 0 | ||
| Probable outcome from the medical care received | |||
| GP | |||
| Cure | 0.9999 | ||
| Death | 0.0001 | 25 | |
| No complication | |||
| In GW | |||
| Cure | 0.903 | ||
| Death | 0.097 | 17, 18 | |
| Pneumonia | |||
| In GW | |||
| Cure | 0.493 | 17, 18 | |
| Death | 0.507 | ||
| In ICU | |||
| Cure | 0.489 | ||
| Death | 0.511 | 17, 18 | |
| Sepsis | |||
| In GW | |||
| Cure | 0.081 | 17, 18 | |
| Death | 0.919 | ||
| In ICU | |||
| Cure | 0.257 | ||
| Death | 0.743 | 17, 18 | |
| ARDS | |||
| In GW | |||
| Cure | 0.002 | 17, 18 | |
| Death | 0.998 | ||
| In ICU | |||
| Cure | 0.151 | ||
| Death | 0.849 | 17, 18 | |
| Costs (USD, year of costing: 2013) | |||
| Direct medical care costs | |||
| Oseltamivir | 132.77 | 26 | |
| Over the counter medications | 16.95 | 27 | |
| GP visit | 169~ | 22 | |
| ED visit | 551 | 28 | |
| Hospitalization | |||
| GW | |||
| No complication | 17 260 | 29 | |
| Pneumonia | 18 966 | 29 | |
| Sepsis | 23 771 | 29 | |
| ARDS | 45 330 | 30 | |
| ICU | |||
| Pneumonia | 22 771 | 31 | |
| Sepsis | 44 958 | 32 | |
| ARDS | 128 860 | 30 | |
| Direct nonmedical care cost | |||
| Transportation (per visit) | 2.83 | 33, 34 | |
| Indirect costs (daily productivity loss by age) | |||
| Age 18–64 | 146.04 | 35 | |
| Productivity loss (days lost) = length of stay plus days of convalescence | |||
| GP visit | 2.0 | 22, 36 | |
| Hospitalization | |||
| GW | |||
| No complication | 7.4 | 29, 36 | |
| Pneumonia | 8.4 | 29, 36 | |
| Sepsis | 10.5 | 29, 36 | |
| ARDS | 13.0 | 29, 36 | |
| ICU | |||
| Pneumonia | 9.7 | 31, 36 | |
| Sepsis | 14.4 | 32, 36 | |
| ARDS | 17.0 | 30, 36 | |
| Length of stay (days) | |||
| GP visit | 1.0 | NA | 22 |
| Hospitalization | |||
| GW | |||
| No complication | 6.4 | 29 | |
| Pneumonia | 7.4 | 29 | |
| Sepsis | 9.5 | 29 | |
| ARDS | 12.0 | 30 | |
| ICU | |||
| Pneumonia | 8.7 | 31 | |
| Sepsis | 13.4 | 32 | |
| ARDS | 16.0 | 30 | |
| Utilities (95% CI) | |||
| Baseline average quality of life | 0.96 | (0.92–1.00) | 37, 38, 39 |
| Quality of life during illness with influenza | 0.81 | (0.70–0.90) | 37, 40 |
| Pneumonia | 0.63 | 41 | |
| Sepsis in hospital ward | 0.59 | 42 | |
| Sepsis in ICU | 0.10 | (0.08–0.15) | 39, 40, 41, 42 |
| ARDS in hospital ward | 0.59 | 42 | |
| ARDS intubated in ICU | 0.10 | (0.08–0.15) | 42 |
| Recovery from severe influenza, for patients who received inpatient ICU care | 0.90 | (0.85–0.95) | 37 |
Four pandemic scenarios were assessed in the HE model: (i) high transmissibility and high severity; (ii) low transmissibility and low severity; (iii) high transmissibility and low severity; and (iv) low transmissibility and high severity. Within each scenario, three interventions were assessed (no treatment, oseltamivir 75 mg and oseltamivir 150 mg BID for 5 days). Drug uptake was varied as described in the epidemiological module (25%, 50%, and 80%). The β values for transmissibility were obtained from the epidemiological module (Table 1) whereas severity of illness (proxy for virulence of disease) was based on health care utilization; low severity was based on 2009 H1N1 pandemic experience 44; and the high severity scenario involved doubling the probability of hospitalization 45 for the low severity scenario. All HE modelling was performed using Microsoft Excel and R (R Development Core Team (2008). R: A language and environment for statistical computing. R Foundation for Statistical Computing, http://www.R‐project.org._)
Sensitivity analysis
As the magnitude and effect of the outcome of this proof of concept quantitative framework is dependent on the point estimate of Tshed used in our simulations, a sensitivity analysis around the change in viral shedding in terms of infected patients and the subsequent impact on the HE model was warranted. Using the data provided by Rayner et al. 7, the 25th and 75th percentiles of viral shedding in each of the OC AUC exposure groups defined by the classification and regression analysis were calculated. All dosing scenarios described above were then repeated, using these upper and lower bounds of viral shedding to provide upper and lower bounds on number of patients infected (see Table 2). The number of infected patient at 25% Tshed, 50% Tshed and 75% Tshed were then used as inputs into the current HE model for comparison.
Results
Pharmacology (PK/PD) module
The PK/PD target threshold, which separated low and high oseltamivir exposure groups in the time to event relationships 7 between OC AUC and cessation of influenza viral shedding (Figure 1A) and resolution of influenza symptoms (Figure 1B) was 14 180 ng.h ml−1 7. The simulated AUC density distributions for the 75 mg and 150 mg BID dosing regimens are shown in Figure 1C. Simulation results determined that the proportion of patients in a population with OC AUCs above the PK/PD threshold was 0.326 and 0.795 when treated with 75 mg and 150 mg BID regimens, respectively (Table 1). As shown in Table 1, the distribution of duration of viral shedding (Tshed) data obtained from the influenza inoculation studies 7 was as follows: for patients above the PK/PD threshold, the Tshed distribution with mean (standard deviation; SD) ϒhigh was 1.9 (0.51) days, for those less than the PK/PD target the mean (SD) ϒlow was 3 (0.58) days, and for patients not receiving drug the mean (SD) ϒ0 was 6 (2.5) days.
Epidemiology module
A schematic of the epidemiological SEIR model adapted to incorporate oseltamivir PK/PD is shown in Figure 2A (see Methods). Results of the SEIR model external validation to epidemic data gathered from a previous influenza outbreak in the Midwestern USA (2007–2008 seasons) is shown in Figure 2B. As shown, the model was adequately fitted to the extracted data, capturing the fraction of the population infected over the duration of the influenza outbreak.
The SEIR model was then used to simulate the median number of individuals infected (per population of 100 000) under different pandemic and treatment intervention scenarios. Results of these simulations are shown in Table 3. A pandemic, by definition, requires an R0 > 1 8, 12. As shown, in the low transmissibility scenario (R0 = 1.9), under no antiviral treatment, approximately 37 000 individuals would be infected, with that number decreasing to ~5000 under oseltamivir 75 mg BID treatment and 25% drug uptake. As expected, increasing the proportion of the infected population receiving antiviral treatment (drug uptake) had a significant impact on the incidence of infection in the population in both low and high (R0 = 2.7) transmissibility pandemic scenarios. A higher dose of oseltamivir, 150 mg BID, demonstrated a greater improvement in reducing the number of infected individuals as the percentage of the population treated increased, particularly in the high transmissibility scenario.
Table 3.
Output of the combined pharmacology–epidemiology modules. Median number of infected individuals per population of 100 000 by therapeutic intervention, transmissibility (R0), and % of infected individuals treated with oseltamivira
| % treated | No treatment | 75 mg BID | 150 mg BID |
|---|---|---|---|
| Low transmissibility (R 0 = 1.9) | |||
| 37 068 | |||
| 25% | ‐‐ | 7846 (7846–20 354) | 5311 (5311–1,6303) |
| 50% | ‐‐ | 2252 (2252–12 279) | 1357 (1357–7202) |
| 80% | ‐‐ | 1349 (1349–8504) | 741 (741–4891) |
| High transmissibility (R 0 = 2.7) | |||
| 67 512 | |||
| 25% | ‐‐ | 60 397 (60397–71 149) | 53 032 (53032–59 498) |
| 50% | ‐‐ | 41 331 (41331–55 660) | 31 700 (31700–37 024) |
| 80% | ‐‐ | 20 941(20941–40 616) | 12 881 (12881–14 665) |
BID, twice daily; R0, the basic reproductive number.
median (25th and 75th % of viral shed) simulation are reported in this table. Note: 25th and 50th percentile identical given right skewed distribution.
HE module
The median number of infected individuals (per population of 100 000) for each pandemic scenario (Table 3) was entered into a decision analytic model and became part of the economic analysis. The final HE decision analytic model is shown in Figure 3. Data inputs (and literature sources) for the HE model such as branch probabilities, direct medical costs (medication and hospitalization), direct nonmedical costs (transportation to and from hospital), length of hospitalization, case‐fatality rates from influenza complications, and health state utilities are shown in Table 2. As shown in the table, the vast majority of influenza‐infected patients in a population present as outpatients (>97%), and of the 3% who present as inpatients, 40% present with pneumonia as an influenza complication followed by (ARDS; 12%) and sepsis (8%).
HE simulation scenarios and treatment comparison
Results of the HE analysis are shown in Table 4. As shown, across all pandemic scenarios, 75 mg BID oseltamivir was cost‐saving relative to no treatment, i.e., ∆C (incremental cost) <0 and ∆E (incremental effectiveness) >0 (see Methods, Equation 5).
Table 4.
Health economics module output: Effect of oseltamivir treatment intervention at 50% drug uptake on cost, life‐years (LY) and quality‐adjusted LY (QALY) by pandemic scenario. A cost per QALY gained of <0 indicates the new intervention is cost‐saving, a cost per QALY gained of >0 and <100 000 USD indicates the new intervention is cost effective, and a cost per QALY gained >100 000 USD indicates the new intervention is not cost effective
| Payer perspective | Societal perspective | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comparators (Treatment vs. baseline) | Costs (A) (payer) | Costs (B) (payer – Baseline) | Costs (A) (societal) | Costs (B) (societal ‐ Baseline) | Death (A) | Death (B) | Δ Death (A–B) | Δ LYs (A–B) | Δ QALYs (A–B) | Cost per LY gained | Cost per QALY gained | Cost per LY gained | Cost per QALY gained |
| Low transmissibility and low severity | |||||||||||||
| 75 mg (A) vs. no treatment (B) | 9 225 251 | 42 578 018 | 12 998 947 | 106 995 703 | 27 | 439 | –412 | 399 | 430 | Cost‐saving | Cost‐saving | Cost‐saving | Cost‐saving |
| 150 mg (A) vs. 75 (B) mg | 14 835 713 | 9 225 251 | 17 109 649 | 12 998 947 | 16 | 27 | –11 | 10 | 11 | 546 753 | 515 260 | 400 598 | 377 524 |
| High transmissibility and high severity | |||||||||||||
| 75 mg (A) vs. no treatment (B) | 94 961 869 | 144 271 547 | 171 053 550 | 272 957 742 | 974 | 1591 | –617 | 598 | 629 | Cost‐saving | Cost‐saving | Cost‐saving | Cost‐saving |
| 150 mg (A) vs. 75 mg(B) | 81 019 150 | 94 961 869 | 139 379 855 | 171 053 550 | 747 | 974 | –227 | 220 | 227 | Cost‐saving | Cost‐saving | Cost‐saving | Cost‐saving |
| Low transmissibility and high severity | |||||||||||||
| 75 mg (A) vs. no treatment (B) | 11 450 971 | 79 213 439 | 15 596 974 | 149 869 617 | 53 | 874 | –821 | 795 | 828 | Cost‐saving | Cost‐saving | Cost‐saving | Cost‐saving |
| 150 mg (A) vs. 75 mg (B) | 16 176 877 | 11 450 971 | 18 675 157 | 15 596 974 | 32 | 53 | –21 | 20 | 21 | 231 280 | 223 797 | 150 642 | 145 768 |
| High transmissibility and low severity | |||||||||||||
| 75 mg (A) vs. no treatment (B) | 54 113 197 | 77 547 403 | 123 371 900 | 194 871 423 | 489 | 799 | –310 | 300 | 330 | Cost‐saving | Cost‐saving | Cost‐saving | Cost‐saving |
| 150 mg (A) vs. 75 mg (B) | 49 689 085 | 54 113 197 | 102 809 041 | 123 371 900 | 375 | 489 | –114 | 110 | 117 | Cost‐saving | Cost‐saving | Cost‐saving | Cost‐saving |
All costs are expressed in 2013 USD.
A, the alternative intervention; B, the baseline intervention.
The decrease in costs (ΔC) was driven by savings from direct medical and nonmedical costs and indirect nonmedical costs (through fewer work days lost) which offset the increased drug costs. As shown in Table 4, and using the low transmissibility and low severity pandemic scenario as an example, when comparing oseltamivir 75 mg vs. no treatment, costs from the payer perspective (which include direct costs only) were substantially lower (9.2 million US dollars [USD] vs. 42.6 million USD at baseline). From the societal perspective, which includes indirect costs in addition to direct costs, there was also a large decrease in costs (13 million USD vs. 107 million USD at baseline). The increase in QALYs (ΔE) for the 75 mg oseltamivir treatment strategy relative to no treatment was primarily driven by a reduction in mortality. As shown in Table 4, again using the low transmissibility and low severity scenario, a gain in QALYs (ΔQALY) of 430 was seen with the oseltamivir 75 mg intervention relative to no treatment. This value was driven by the number of deaths averted downstream in Figure 3 by the 75 mg BID intervention (Δ Death = 412 for this scenario). As shown in Table 4, the total number of deaths per population of 100 000 was dependent on the intervention, and ranged from as low as 16 to as high as 1591 deaths, equivalent to mortality rates of approximately ~0.016% and 1.6% respectively, and is consistent with the reported range of mortality of known influenza pandemics 46. In the base case under no treatment low transmissibility and low severity), death due to hospitalization with pneumonia constituted the highest percentage (47%) of total deaths from complications of influenza, followed by ARDS (27%) and sepsis (15.5%).
When compared with oseltamivir 75 mg at 50% drug uptake (Table 4), the new intervention using 150 mg was not cost‐effective in low transmissibility scenarios; however, it was cost‐saving in the high transmissibility scenarios.
Sensitivity analysis
The simulations from the sensitivity analysis using the 25th or 75th percentile of viral shedding on the epidemiological model are presented in Table 3. When the epidemiological data were linked to the HE model, only minimal changes in the overall HE conclusions were observed. The only significant change was with the 75th percentile viral shedding in the low transmissibility and high severity scenario. All HE outcomes were the same, except for the 150 mg dose compared to the 75 mg dose in the low transmissibility and high severity scenario, which now achieved cost‐savings from a payer and societal perspective (data not shown). This demonstrates that our quantitative framework is robust to possible intrinsic variability in viral shedding, yet is able to detect and translate meaningful antiviral exposure response without impacting the health economic conclusions.
Discussion
We developed a modular interdisciplinary pharmacometric platform that was able to show that oseltamivir reduced the median number of infected individuals, increased QALYs by preventing deaths, and was cost‐saving under most pandemic scenarios. The pharmacology–epidemiology modules aimed to translate variability in the exposure (PK)–response (PD) effect of oseltamivir on influenza viral shedding and then demonstrate the indirect benefits achieved by altering disease transmissibility at a population level. The individual and population outcomes were translated into improvements in the QALY and cost‐utility (captured by the ICER) allowing insight into the health economic impact of various interventions under pandemic scenarios.
The positive economic impact of oseltamivir was mostly driven by reducing the number of infected patients (per 100 000 population) entering the decision analytic model (Figure 3), thereby reducing the number of individuals who otherwise may have gone forward to develop influenza complications and hence averting deaths downstream (increased QALY). From a payer perspective, direct medical costs were decreased through less health care use (Figure 3), and from a societal perspective, the decrease in indirect costs was driven mainly by fewer days lost from work. As shown in Table 4, increased dose (150 mg) relative to standard dose (75 mg) may have some economic impact in high transmissibility scenarios, but not low transmissibility at 50% drug uptake (drug uptake is a proxy for the ability to stockpile and distribute drug to susceptible individuals). The exception is at 25% drug uptake (data not shown) where the 150 mg dose may have a favourable ICER, suggesting that in low transmissibility scenarios, treatment of a smaller portion of infected individuals with a higher dose may have some economic value.
The integrated platform developed in the current study may also be used to investigate the health economic impact of other antivirals under development once a PK/PD readout has been achieved in early development. This would allow early consideration of novel antiviral compounds for pandemic planning, such as a haemagglutinin monoclonal antibody that inhibits viral entry into the host cell 47. Such a therapeutic modality is being developed by different organizations to treat severe influenza, and hence may potentially act on the lower (distal) parts of the decision analytic model by reducing hospitalization duration once admitted to the intensive care unit, where oseltamivir acts proximally by reducing the number of infected individuals that enter the decision analytic model (Figure 3). As such, combining therapeutic modalities may be complementary from a health economic and pandemic perspective by addressing both transmissibility and severity (virulence). Other applications of the platform include conducting threshold analysis on drug pricing, which would lead to a desired ICER.
Certain competing factors may have confounded our estimation of the economic impact of oseltamivir. Factors leading to underestimation include: (i) capturing only treatment effect of oseltamivir on recovery rate (γ) and not the effect on the rate of infectivity, β (Figure 2A), via its documented effects on prophylaxis 48; and (ii) employing a conservative assumption of oseltamivir having no effect on reducing influenza complications, despite some evidence suggesting positive effects 5. By contrast, factors that may have led to overestimation include: (i) the univariate nature of our analysis with respect to intervention, where only the antiviral effect was considered without incorporation of other pandemic mitigating factors such as school/airport closure, social distancing, mask wearing and vaccination; and (ii) use of inoculation data that involved administration of drug immediately after infection, thereby lacking consideration of the effect of time to treatment 49 (the effectiveness of oseltamivir is highest at early treatment times relative to infection). We sourced PK/PD data from phase II inoculation studies because they employed the widest range of oseltamivir doses and had well characterized PK/PD relationships 7, although emerging work 50 is also showing some PK/PD relationships from phase III data (seasonal influenza), where significant differences could not be revealed by comparing dose groups alone. It is important to also note that different opinions exist on the potential effect of doubling an oseltamivir dose 7, 51, 52, 53, 54, 55, 56; some have also noted improved viral shedding in hospitalized adults infected with influenza B with higher doses 51, whereas others have failed to identify the superiority of double dosing in avian and severe influenza 52
Numerous oseltamivir population PK models have been developed to evaluate PK for specific purposes including impact of obesity, pregnancy, end‐stage renal dysfunction, ontogeny in infants younger than 1 year and the impact of probenecid to incite a drug–drug interaction 11, 57, 58, 59, 60, 61, 62. Whilst fit for their specific purpose, such models were too narrow for application in this program. The population PK model used in this framework had the greatest patient number, and age, weight, creatinine clearance and dose range. Thus enabling suitable populations of interest to be robustly simulated to evaluate OC exposure in this framework.
The situation of administering drug immediately after infection is an ideal scenario, and is likely to deviate from the reality of a pandemic. In the current methodological study, linkage of the pharmacoepidemiology and HE modules is demonstrated deterministically, i.e. median predictions of the number of infected individuals (Table 2) from the pharmacoepidemiological modules form the input into the HE module. This approach was undertaken because currently available epidemiological models are not able to provide the full variance–covariance matrix between parameters due to paucity of observational data of influenza seasons to build such models robustly. Nevertheless, it was reassuring that our model was able to more than adequately describe the previous influenza outbreak in the Midwestern USA (2007–2008 seasons) extracted from the Centers for Disease Control.
To address some of these limitations in future work, this iteration is amenable to improvement due to the modular nature of the platform. For example, to overcome the limitations of the SEIR model, which is well stirred with regards to susceptibility and infectiousness of influenza, an agent‐based epidemiology model (ABM) could be used instead. The ABM would account for the unique contact structures in a population 1, 12 allowing for more heterogeneous representation of infectiousness (β in Figure 2A). Some of the present authors have begun steps towards the integration of an ABM 63. Future efforts may also involve exploring the potential health economic impact of alternate dosing scenarios (e.g. half‐dose, triple dose). It is worth noting that considering adverse events and disease complications brings the HE model closer to clinical reality. However, it should be highlighted that the focus of the current study is the demonstration of proof‐of‐concept of linking PK/PD information to the HE model through the epidemiological model. However, those factors will be considered in our future work along with the use of the more sophisticated ABM model.
The model also did not consider emergence of resistance during an influenza season, which might result in decreased viral clearance, and therefore potentially reduced impact of oseltamivir. Neither did it consider the acquisition of resistance mutations that may also result in a cost to infectivity that may reduce the potential for transmission. There is a paucity of data to support or describe the rate and extent of resistance emergence within an influenza season to support assumptions for such scenarios. The spatial and time aspects of emergence of resistance (e.g. arising in a specific geography mid‐way through an epidemic) are more appropriately explored using ABM approaches.
Despite these limitations, we have successfully demonstrated proof of concept that relevant endpoints can be linked across adjacent disciplines (in this case drug AUC, viral shedding, and their respective variabilities, R0 and ICER). Many have, through use of meta‐analysis of randomized clinical trial data or retrospective analysis of pandemic data, advocated use of neuraminidase inhibitors as one of several strategies to contain a pandemic 5, 64, and this is reflected in current guidelines 3. While we recognize that further work is needed to quantify the health economic impact of oseltamivir more rigorously in a given pandemic scenario, the potential utility of interdisciplinary pharmacometric methodology in beginning to solve multilayered problems should not be understated. Given the growing need to justify pricing of medicines to society and payer, the authors favour greater application of interdisciplinary techniques across all disease areas where the PK/PD, clinical, or epidemiological endpoints of interest can eventually be linked to health economic value. We advocate that this approach will bring together the developer, payer and regulator earlier in the drug development process to facilitate accelerated access to affordable medicines.
Conclusion
Oseltamivir 75 mg relative to no treatment reduced the median number of infected patients, increased ΔQALY by deaths averted, and was cost‐saving under all simulated pandemic scenarios, while 150 mg relative to 75 mg was not cost effective in low transmissibility scenarios but was cost saving in high transmissibility scenarios. This methodological study demonstrates proof of concept that the disciplines of pharmacology, disease epidemiology and health economics can be linked in a single quantitative framework.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: M.A.K., P.F.S., K.N., G.D., S.T. and C.R.R. all had support from Roche for the submitted work; M.A.K. was an employee of Roche in the previous 3 years; P.S., K.N., G.D., S.T. and C.R. are employees of d3 Medicine, which is a strategic advisory company in drug development and advised multiple pharmaceutical and biotechnology companies, including Roche in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
This work was supported by funding from F. Hoffmann‐La Roche Ltd. Support for third‐party writing assistance for this manuscript was provided by F. Hoffmann‐La Roche Ltd.
Kamal, M. A. , Smith, P. F. , Chaiyakunapruk, N. , Wu, D. B. C. , Pratoomsoot, C. , Lee, K. K. C. , Chong, H. Y. , Nelson, R. E. , Nieforth, K. , Dall, G. , Toovey, S. , Kong, D. C. M. , Kamauu, A. , Kirkpatrick, C. M. , and Rayner, C. R. (2017) Interdisciplinary pharmacometrics linking oseltamivir pharmacology, influenza epidemiology and health economics to inform antiviral use in pandemics. Br J Clin Pharmacol, 83: 1580–1594. doi: 10.1111/bcp.13229.
[Correction note: This article was first published online on the 20th of February 2017. Minor grammatical corrections were made in the abstract, Introduction and Methods sections on the 22nd of March 2017 prior to issue publication.]
Contributor Information
Patrick F. Smith, Email: patrick.smith@d3medicine.com
Nathorn Chaiyakunapruk, Email: nathorn.chaiyakunapruk@monash.edu.
Carl M. Kirkpatrick, Email: carl.kirkpatrick@monash.edu
References
- 1. Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature 2006; 442: 448–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Longini IM Jr, Nizam A, Xu S, Ungchusak K, Hanshaoworakul W, Cummings DA, et al. Containing pandemic influenza at the source. Science 2005; 309: 1083–1087. [DOI] [PubMed] [Google Scholar]
- 3. WHO Guidelines for Pharmacological Management of Pandemic Influenza A(H1N1) 2009 and other Influenza Viruses. 2010. Available at http://www.who.int/csr/resources/publications/swineflu/h1n1_guidelines_pharmaceutical_mngt.pdf (last accessed 2 October 2016). [PubMed]
- 4. Tamiflu Summary of Product Characteristics (SmPC) . 2011. Available at http://www.medicines.org.uk/emc/ (last accessed 2 October 2016).
- 5. Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta‐analysis of randomised controlled trials. Lancet 2015; 385: 1729–1737. [DOI] [PubMed] [Google Scholar]
- 6. Jefferson T, Jones M, Doshi P, Spencer EA, Onakpoya I, Heneghan CJ. Oseltamivir for influenza in adults and children: A systematic review of clinical study reports and summary of regulatory comments. BMJ 2014; 348: g2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rayner CR, Bulik CC, Kamal MA, Reynolds DK, Toovey S, Hammel JP, et al. Pharmacokinetic‐pharmacodynamic determinants of oseltamivir efficacy using data from phase 2 inoculation studies. Antimicrob Agents Chemother 2013; 57: 3478–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dietz K. The estimation of the basic reproduction number for infectious diseases. Stat Methods Med Res 1993; 2: 23–41. [DOI] [PubMed] [Google Scholar]
- 9. Kermack WO, McKendrick AG. A contribution to the mathematical theory of epidemics. Proc R Soc Lond A 1927; 115: 700–721. [Google Scholar]
- 10. Pradas‐Velasco R, Antonanzas‐Villar F, Martinez‐Zarate MP. Dynamic modelling of infectious diseases: an application to the economic evaluation of influenza vaccination. Pharmacoeconomics 2008; 26: 45–56. [DOI] [PubMed] [Google Scholar]
- 11. Kamal MA, Van Wart SA, Rayner CR, Subramoney V, Reynolds DK, Bulik CC, et al. Population pharmacokinetics of oseltamivir: pediatrics through geriatrics. Antimicrob Agents Chemother 2013; 57: 3470–3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murillo LN, Murillo MS, Perelson AS. Towards multiscale modeling of influenza infection. J Theor Biol 2013; 332: 267–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carrat F, Vergu E, Ferguson NM, Lemaitre M, Cauchemez S, Leach S, et al. Timelines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol 2008; 167: 775–785. [DOI] [PubMed] [Google Scholar]
- 14. Colizza V, Barrat A, Barthelemy M, Valleron AJ, Vespignani A. Modeling the worldwide spread of pandemic influenza: baseline case and containment interventions. PLoS Med 2007; 4: e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn't it increase at the rate of inflation? Arch Intern Med 2013; 163: 1637–1641. [DOI] [PubMed] [Google Scholar]
- 16. Jain S, Benoit SR, Skarbinski J, Bramley AM, Finelli L. Influenza‐associated pneumonia among hospitalized patients with 2009 pandemic influenza A (H1N1) virus – United States, 2009. Clin Infect Dis 2012; 54: 1221–1229. [DOI] [PubMed] [Google Scholar]
- 17. Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med 2009; 361: 1935–1944. [DOI] [PubMed] [Google Scholar]
- 18. Skarbinski J, Jain S, Bramley A, Lee EJ, Huang J, Kirschke D, et al. Hospitalized patients with 2009 pandemic influenza A (H1N1) virus infection in the United States – September–October 2009. Clin Infect Dis 2011; 52: S50–S59. [DOI] [PubMed] [Google Scholar]
- 19. Muthuri SG, Venkatesan S, Myles PR, Leonardi‐Bee J, Al Khuwaitir TS, Al Mamun A, et al. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09virus infection: a meta‐analysis of individual participant data. Lancet Respir Med 2014; 2: 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burch J, Paulden M, Conti S, Stock C, Corbett M, Welton NJ, et al. Antiviral drugs for the treatment of influenza: a systematic review and economic evaluation. Health Technol Assess 2009; 13: 1–265, iii–iv. [DOI] [PubMed] [Google Scholar]
- 21. Hsu J, Santesso N, Mustafa R, Brozek J, Chen YL, Hopkins JP, et al. Antivirals for treatment of influenza: a systematic review and meta‐analysis of observational studies. Ann Intern Med 2012; 156: 512–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Molinari NA, Ortega‐Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007; 25: 5086–5096. [DOI] [PubMed] [Google Scholar]
- 23. Gonzalez M K, Bauhoff S C, Blanchard J, Abir M, Iyer N, Smith A, et al The evolving role of emergency departments in the United States. 2013. Available at http://www.rand.org/content/dam/rand/pubs/research_reports/RR200/ RR280/RAND_RR280.pdf. [PMC free article] [PubMed]
- 24. Angus DC, Linde‐Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29: 1303–1310. [DOI] [PubMed] [Google Scholar]
- 25. Presanis AM, Lipsitch M, De Angelis D, Swine Flu Investigation Team , New York City Department of Health and Mental Hygiene , Hagy A, et al. The severity of pandemic H1N1 influenza in the United States, from April to July 2009: a Bayesian analysis. PLoS Med 2009; 6: e1000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. PDR Red Book: Pharmacy's Fundamental Reference. Montvale, New Jersey: Thompson Healthcare Inc., 2013. [Google Scholar]
- 27. Lee BY, Bacon KM, Donohue JM, Wiringa AE, Bailey RR, Zimmerman RK. From the patient perspective: the economic value of seasonal and H1N1 influenza vaccination. Vaccine 2011; 29: 2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Caldwell N, Srebotnjak T, Wang T, Hsia R. "How much will I get charged for this?" Patient charges for top ten diagnoses in the emergency department. PLoS One 2013; 8: e55491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. The National (Nationwide) Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP). 2009. Available at www.hcup‐us.ahrq.gov/nisoverview.jsp (last accessed 12 September 2013).
- 30. Wiesen J, Komara JJ, Walker E, Wiedemann HP, Guzman JA. Relative cost and outcomes in the intensive care unit of acute lung injury (ALI) due to pandemic influenza compared with other etiologies: a single‐center study. Ann Intensive Care 2012; 2: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yu H, Rubin J, Dunning S, Li S, Sato R. Clinical and economic burden of community‐acquired pneumonia in the Medicare fee‐for‐service population. J Am Geriatr Soc 2012; 2137–2143. [DOI] [PubMed] [Google Scholar]
- 32. MacLaren R, Bond CA, Martin SJ, Fike D. Clinical and economic outcomes of involving pharmacists in the direct care of critically ill patients with infections. Crit Care Med 2008; 36: 3184–3189. [DOI] [PubMed] [Google Scholar]
- 33. Internal Revenue Service – Standard Mileage Rates for 2013. 2013. Available at http://www.irs.gov/uac/Newsroom/2013‐Standard‐Mileage‐Rates‐Up‐1‐Cent‐per‐Mile‐for‐Business,‐Medical‐and‐Moving (last accessed 24 September 2013).
- 34. Center of Disease Prevention – Distance to Nearest Hospital Files, NAMCS and NHAMCS. 2009. Available at http://www.cdc.gov/nchs/data/ahcd/distance_to_nearest_hospital_file.pdf (last accessed 24 September 2013).
- 35. United States Bureau of Labor Statistics – Median weekly earnings by age, sex, race and Hispanic or Latino ethnicity, first quarter 2013. 2013. Available at http://www.bls.gov/opub/ted/2013/ted_20130419.htm (last accessed 23 September 2013).
- 36. Meltzer MI, Cox NJ, Fukuda K. The economic impact of pandemic influenza in the United States: priorities for intervention. Emerg Infect Dis 1999; 5: 659–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Khazeni N, Hutton DW, Garber AM, Owens DK. Effectiveness and cost‐effectiveness of expanded antiviral prophylaxis and adjuvanted vaccination strategies for an influenza A (H5N1) pandemic. Ann Intern Med 2009; 151: 840–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fryback DG, Dasbach EJ, Klein R, Klein BE, Dorn N, Peterson K, et al. The Beaver Dam Health Outcomes Study: initial catalog of health‐state quality factors. Med Decis Making 1993; 13: 89–102. [DOI] [PubMed] [Google Scholar]
- 39. The U.S. Census Bureau. 2007. Available at http://www.census.gov/ (last accessed 27 September 2013).
- 40. Turner DA, Wailoo AJ, Cooper NJ, Sutton AJ, Abrams KR, Nicholson KG. The cost‐effectiveness of influenza vaccination of healthy adults 50‐64 years of age. Vaccine 2006; 24: 1035–1043. [DOI] [PubMed] [Google Scholar]
- 41. Song Y, Tai JH, Bartsch SM, Zimmerman RK, Muder RR, Lee BY. The potential economic value of a Staphylococcus aureus vaccine among hemodialysis patients. Vaccine 2012; 30: 3675–3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Angus DC, Musthafa AA, Clermont G, Griffin MF, Linde‐Zwirble WT, Dremsizov TT, et al. Quality‐adjusted survival in the first year after the acute respiratory distress syndrome. Am J Respir Crit Care Med 2001; 163: 1389–1394. [DOI] [PubMed] [Google Scholar]
- 43. United States Bureau of Labor Statistics – CPI Inflation Calculator. 2013. Available at http://www.bls.gov/data/inflation_calculator.htm (last accessed 23 September 2013).
- 44. Girard MP, Tam JS, Assossou OM, Kieny MP. The 2009 A (H1N1) influenza virus pandemic: a review. Vaccine 2010; 28: 4895–4902. [DOI] [PubMed] [Google Scholar]
- 45. Lee BY, McGlone SM, Bailey RR, Wiringa AE, Zimmer SM, Smith KJ, et al. To test or to treat? An analysis of influenza testing and antiviral treatment strategies using economic computer modelling. PLoS One 2010; 5: e11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Potter CW. A History of Influenza. J Appl Microbiol 2001; 91: 572–579. [DOI] [PubMed] [Google Scholar]
- 47. Chen Z, Wang J, Bao L, Guo L, Zhang W, Xue Y, et al. Human monoclonal antibodies targeting the haemagglutinin glycoprotein can neutralize H7N9 influenza virus. Nat Commun 2015; 6: 6714. [DOI] [PubMed] [Google Scholar]
- 48. Jackson RJ, Cooper KL, Tappenden P, Rees A, Simpson EL, Read RC, et al. Oseltamivir, zanamivir and amantadine in the prevention of influenza: a systematic review. J Infect 2011; 62: 14–25. [DOI] [PubMed] [Google Scholar]
- 49. Aoki FY, Macleod MD, Paggiaro P, Carewicz O, El Sawy A, Wat C, et al. Early administration of oral oseltamivir increases the benefits of influenza treatment. J Antimicrob Chemother 2003; 51: 123–129. [DOI] [PubMed] [Google Scholar]
- 50. Bulik C, Rayner C, Hammel J, Smith PF, Forrest A, Van Wart SA, et al Pharmacokinetic‐pharmacodynamic (PK‐PD) evaluation of the impact of oseltamivir on influenza viral endpoints. Abstr. A‐010. 54th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), Washington DC, USA. 2014. Available at http://www.icaaconline.com/php/icaac2014abstracts/data/index.htm (last accessed 23 May 2014).
- 51. Lee N, Hui DS, Zuo Z, Ngai KL, Lui GC, Wo SK, et al. A prospective intervention study on higher‐dose oseltamivir treatment in adults hospitalized with influenza A and B infections. Clin Infect Dis 2013; 57: 1511–1519. [DOI] [PubMed] [Google Scholar]
- 52. South East Asia Infectious Disease Clinical Research Network . Effect of double dose oseltamivir on clinical and virological outcomes in children and adults admitted to hospital with severe influenza: double blind randomised controlled trial. BMJ 2013; 346: f3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jefferson T, Jones M, Doshi P, Del Mar CB, Hama R, Thompson MJ, et al Regulatory information on trials of oseltamivir (Tamiflu) and zanamivir (Relenza) for influenza in adults and children updated. Cochrane Summaries (2014).
- 54. Influenza antiviral medications: Summary for clinicians. CDC. Centers for Disease Control and Prevention 2014.
- 55. WHO guidelines for pharmacological management of pandemic (H1N1) 2009 influenza and other influenza viruses. WHO, 2010. [PubMed]
- 56. Roos R. WHO director replies to BMJ critique of pandemic actions. CIDRAP. University of Minnesota 2010.
- 57. Kamal MA, Brennan BJ, Subramoney V, Lien YT, Morcos PN, Frey N, et al. Identification of new oral dosing regimens for the neuraminidase inhibitor oseltamivir in patients with moderate and severe renal impairment. Clin Pharmacol Drug Dev 2015; 4: 326–336. [DOI] [PubMed] [Google Scholar]
- 58. Chairat K, Jittamala P, Hanpithakpong W, Day NP, White NJ, Pukrittayakamee S, et al. Population pharmacokinetics of oseltamivir and oseltamivir carboxylate in obese and non‐obese volunteers. Br J Clin Pharmacol 2016; 81: 1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kamal MA, Lien KY, Robson R, Subramoney V, Clinch B, Rayner CR, et al. Investigating clinically adequate concentrations of oseltamivir carboxylate in end‐stage renal disease patients undergoing hemodialysis using a population pharmacokinetic approach. Antimicrob Agents Chemother 2015; 59: 6774–6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pillai VC, Han K, Beigi RH, Hankins GD, Clark S, Hebert MF, et al. Population pharmacokinetics of oseltamivir in non‐pregnant and pregnant women. Br J Clin Pharmacol 2015; 80: 1042–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kamal MA, Acosta EP, Kimberlin DW, Gibiansky L, Jester P, Niranjan V, et al. The posology of oseltamivir in infants with influenza infection using a population pharmacokinetic approach. Clin Pharmacol Ther 2014; 96: 380–389. [DOI] [PubMed] [Google Scholar]
- 62. Rayner CR, Chanu P, Gieschke R, Boak LM, Jonsson EN. Population pharmacokinetics of oseltamivir when coadministered with probenecid. J Clin Pharmacol 2008; 48: 935–947. [DOI] [PubMed] [Google Scholar]
- 63. Fidler M, Murillo M, Rayner C, Kamal MA, Smith PF. Abstr. Modeling the Spread of Pandemic Influenza in the United States: Impact of Antiviral Interventions, Pharmacology, and Resistance. Fifth American Conference on Pharmacometrics 2014.
- 64. Michiels B, Van Puyenbroeck K, Verhoeven V, Vermeire E, Coenen S. The value of neuraminidase inhibitors for the prevention and treatment of seasonal influenza: a systematic review of systematic reviews. PLoS One 2013; 8: e60348. [DOI] [PMC free article] [PubMed] [Google Scholar]


