Abstract
Vγ9Vδ2 T cells are activated by phosphoantigens, such as isopentenyl pyrophosphate (IPP), which is generated in the mevalonate pathway of antigen-presenting cells. IPP is released in the extracellular microenvironment via unknown mechanisms. Here we show that the ATP-binding cassette transporter A1 (ABCA1) mediates extracellular IPP release from dendritic cells (DC) in cooperation with apolipoprotein A-I (apoA-I) and butyrophilin-3A1. IPP concentrations in the supernatants are sufficient to induce Vγ9Vδ2 T cell proliferation after DC mevalonate pathway inhibition with zoledronic acid (ZA). ZA treatment increases ABCA1 and apoA-I expression via IPP-dependent LXRα nuclear translocation and PI3K/Akt/mTOR pathway inhibition. These results close the mechanistic gap in our understanding of extracellular IPP release from DC and provide a framework to fine-tune Vγ9Vδ2 T cell activation via mevalonate and PI3K/Akt/mTOR pathway modulation.
γδT cells are activated by phosphoantigens, and ABCA1 is involved in cholesterol transport. Here the authors link these ideas to show that ABCA1, apoA-I and BTN3A1 regulate extracellular phosphoantigen release by dendritic cells, and implicate ABCA1 in mevalonate-mediated activation of Vγ9Vδ2 T cells.
Vγ9Vδ2 T cells are activated by phosphoantigen that are generated in the mevalonate and non-mevalonate pathway of microbial pathogens. The mevalonate pathway of mammalian cells also generates phosphoantigens such as isopentenyl pyrophosphate (IPP), which activate Vγ9Vδ2 T cells almost as efficiently as microbial phosphoantigens1,2. Vγ9Vδ2 T cells recognize tumour cells that release mevalonate pathway–derived phosphoantigens as IPP1. In addition, Vγ9Vδ2 T cells are specifically activated by antigen-presenting cells, such as dendritic cells (DC), particularly when intracellular IPP generation is boosted with zoledronic acid (ZA), which is an inhibitor of the farnesyl pyrophosphate synthase (FPPS) in the mevalonate pathway3,4,5. How IPP is released in the extracellular microenvironment and delivered to Vγ9Vδ2 T cells is unknown.
CD277/butyrophilin-3A1 (BTN3A1) is a type I glycoprotein that has a central function in phosphoantigen-induced Vγ9Vδ2 T cell activation (reviewed in Harly et al.)4. Two mechanisms have been hypothesized: the ‘allosteric model' proposed by Harly et al.6 and ‘antigen-presenting model' by Vavassori et al.7 In the first model, the interaction between intracellular phosphoantigens and the intracellular B30.2 domain of BTN3A1 induces conformational changes of the extracellular domain, which are sensed by Vγ9Vδ2 T cells and cause their activation. In Vavassori's model, intracellular phosphoantigens are exported to the extracellular microenvironment by an unidentified plasma-membrane-associated transporter and presented to Vγ9Vδ2 T cells by the BTN3A1 extracellular domain. Most data currently available are in favour of the allosteric model8,9, but it is currently unknown whether BTN3A1 is also involved in extracellular phosphoantigen release.
Ecto-F1-ATPase and apolipoprotein A-I (apoA-I) are additional important molecules involved in Vγ9Vδ2 T cell activation10,11,12,13. F1-ATPase can bind both apoA-I and triphosphoric acid 1-adenosin-59-yl ester 3-(3-methylbut-3-enyl) ester (ApppI), which is an adenylated derivative of IPP that accumulates in ZA-treated cells. IPP can be released from ApppI by nucleotide pyrophosphatase causing the activation of Vγ9Vδ2 T cells11. Soluble apoA-I can improve Vγ9Vδ2 T cell recognition of tumour cells, which overproduce IPP (that is, after ZA treatment), whereas low levels of circulating apoA-I in chronic inflammation are associated with a low activation state of Vγ9Vδ2 T cells11,12,13.
apoA-I is required for the assembly of nascent high-density lipoprotein (HDL) particles, which mediate the reverse cholesterol transport14. apoA-I binds the extracellular domain of the ATP-binding cassette transporter A1 (ABCA1). ABCA1 is a member of the ABC transmembrane transporter family, which is abundant in the liver, the gastrointestinal tract and macrophages. ABCA1 effluxes cholesterol and phospholipids to lipid-poor/lipid-free apoA-I. During the HDL assembly process, ABCA1 cooperates with the ATP-binding cassette transporter G1 (ABCG1) and the scavenger receptor-BI (SR-BI), which are two transporters that are involved in the delivery of cholesterol and choline-rich phospholipids to HDL14. Interestingly, ABCA1 also effluxes α-tocopherol15, which is a molecule that contains multiple isoprenoid units; however, it has never been reported whether ABCA1 or any other ABC transporter are involved in the extracellular release of IPP or other isoprenoids.
On the basis of these observations, here we test the hypothesis that ABCA1, apoA-I, and BTN3A1 are involved in extracellular IPP release. We demonstrate that ABCA1, in cooperation with apoA-I and BTN3A1, has a major function in extracellular IPP release from ZA-treated DC. Supernatants from ZA-treated DC contain sufficient IPP to induce Vγ9Vδ2 T cell proliferation even after removal of ZA-treated DC. These data show that ABCA1 has a function that may alter our understanding of the relationship between lipid metabolism and immune function.
Results
Soluble IPP-induced Vγ9Vδ2 T cell proliferation
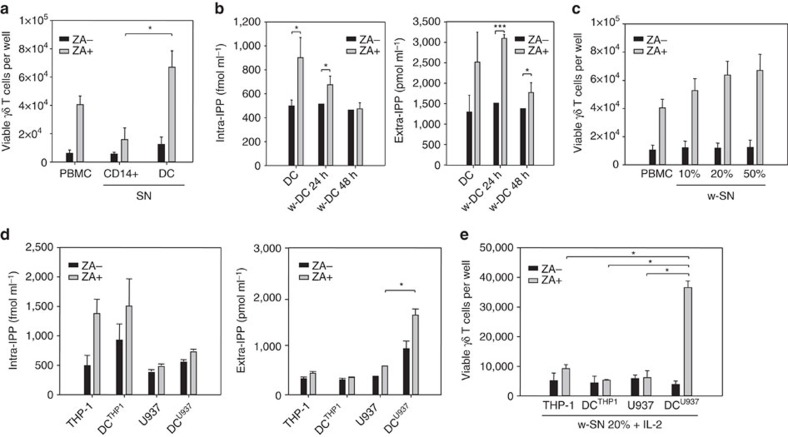
Figure 1a shows the proliferation of Vγ9Vδ2 T cells after 7-day stimulation of peripheral blood mononuclear cells (PBMC) from healthy donors with supernatants obtained from autologous monocytes (CD14+) or DC that are left untreated or treated with ZA for 24 h. As previously reported, supernatants from ZA-treated DC induced significant Vγ9Vδ2 T cell proliferation3.
Figure 1. Vγ9Vδ2 T cell proliferation is induced by extracellular IPP.
(a) Vγ9Vδ2 T cell proliferation after 7-day PBMC stimulation with ZA or supernatant (SN) retrieved from untreated (ZA-) and ZA-treated (ZA+) CD14+ cells and DC. Supernatant from ZA-treated DC induced significantly higher Vγ9Vδ2 T cell proliferation than PBMC stimulation with supernatant from ZA-treated CD14+ cells (*P<0.05; Wilcoxon–Mann–Whitney). The bars represent the mean value±s.e.m. of eight experiments. (b) Intracellular IPP concentrations in untreated (ZA-) and ZA-treated (ZA+) washed DC (w-DC) and extracellular IPP concentrations in corresponding SN after washing and incubation for 24 and 48 h without further ZA stimulation. ZA-treated w-DC released significant IPP amounts for 24 h after ZA removal. The bars represent the mean value±s.e.m. of ten experiments (*P<0.05; *P<0.001; ANOVA). (c) Vγ9Vδ2 T cell proliferation after 7-day PBMC stimulation with ZA or with serial dilutions of supernatants (w-SN) retrieved from untreated (ZA-) and ZA-treated (ZA+) w-DC. The bars represent the mean value±s.e.m. of eight experiments. (d) Intracellular and extracellular IPP levels generated in untreated (ZA-) and ZA-treated (ZA+) THP-1 cells, DCTHP1, U937 cells and DCU937. ZA-treated THP-1 cells and DCTHP1 generated high amounts of intracellular IPP, which was not released in the supernatant. Conversely, ZA-treated DCU937 released extracellular IPP amounts that were close to the extracellular IPP amounts released by ZA-treated DC. Bars represent the mean value±s.e.m. of eight experiments (*P<0.05; ANOVA). (e) Vγ9Vδ2 T cell proliferation after 7-day PBMC stimulation with supernatant obtained from untreated (ZA-) and ZA-treated (ZA+) THP-1 cells, DCTHP1, U937 cells and DCU937. Only SN from ZA-treated DCU937 induced Vγ9Vδ2 T cell proliferation. The bars represent the mean value±s.e.m. of five experiments (*P<0.05; Wilcoxon–Mann–Whitney). ANOVA, analysis of variance.
In a second set of experiments, DC were incubated for 24 h with or without ZA and washed to clear supernatants from any residual ZA. Washed DC were re-plated for an additional 24 and 48 h in the absence of any other treatment, and intracellular and extracellular IPP levels were evaluated as previously reported (Fig. 1b)3. ZA-treated washed DC released significant amounts of IPP for the first 24 h and their supernatants induced a dose-dependent proliferation of Vγ9Vδ2 T cells (Fig. 1c).
The same set of experiments was performed using unmanipulated or DC-like cells that were generated from the acute monocytic leukaemia THP-1 cell line (DCTHP1) and the histiocytic lymphoma U937 cell line (DCU937)16,17,18. DCTHP1 and DCU937 have been shown to perform in many functional assays as well as the conventional counterparts generated from CD14+ cells. ZA-treated THP-1 cells and DCTHP1 produced significant amounts of intracellular IPP, such as ZA-treated DC (Fig. 1b), whereas U937 cells and DCU937 produced lower amounts, which were unaffected by ZA treatment (Fig. 1d). Extracellular IPP release showed an opposite pattern: THP-1 cells and DCTHP1 did not release extracellular IPP even after ZA treatment, whereas ZA-treated DCU937 released intermediate levels (Fig. 1d) that were similar to the levels released by ZA-treated DC (Fig. 1b). The different IPP concentrations in the supernatants translated into a different ability to induce Vγ9Vδ2 T cell proliferation (Fig. 1e).
Increased ABCA1 and apoA-I expression after ZA treatment
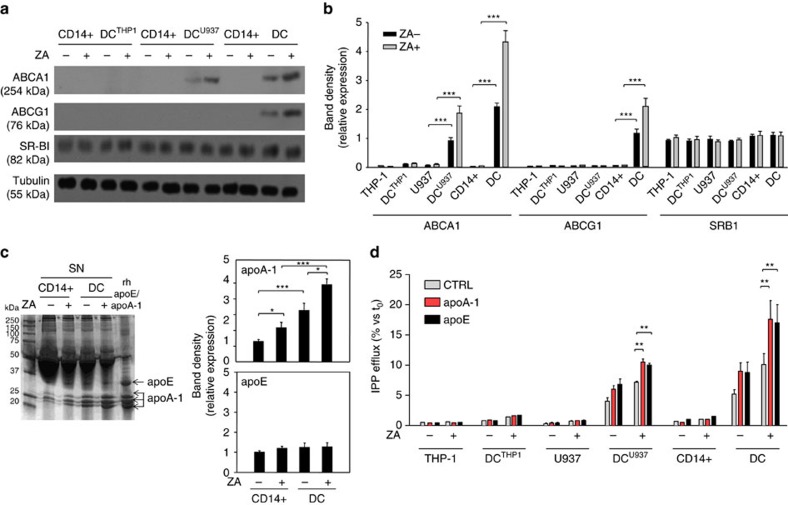
ABCA1, ABCG1 and SR-BI expression were investigated under baseline conditions and after ZA treatment (Fig. 2a). THP-1, U937 and CD14+ cells exhibited no detectable ABCA1 or ABCG1 levels, irrespective of ZA treatment. ZA-treated DCTHP1 also exhibited no expression, whereas untreated DCU937 and DC already exhibited detectable ABCA1 expression, which increased after ZA treatment. DC were the only cells to show detectable ABCG1 expression, which was upregulated by ZA treatment. SR-BI expression was uniformly detectable in all cell preparations and unaffected by ZA treatment. Pooled data with statistical analyses are shown in Fig. 2b.
Figure 2. ZA treatment increases ABCA1 and apoA-1 expression.
(a) Western blot analysis of ABCA1, ABCG1 and SR-BI expression in untreated (ZA-) and ZA-treated (ZA+) THP-1 cells, U937 cells, CD14+ cells and corresponding DC subsets (DCTHP1, DCU937, DC). ABCA1 was already detectable in DCU937 and DC and its expression was upregulated by ZA. β-tubulin was employed to check the equal protein loading per lane. The results are obtained from one representative experiment of three experiments. (b) Pooled data obtained by densitometric analysis with ImageJ software (http://imagej.nih.gov/ij/). The results are expressed as arbitrary units (THP-1 cells and DCTHP1, n=3; U937 cells and DCU937, n=3; CD14+ cells and DC, n=14) (***P<0.001; ANOVA). (c) Supernatants from untreated (ZA-) or ZA-treated (ZA+) CD14+ cells and DC were resolved by SDS–PAGE and stained with Coomassie brilliant blue. A concentration of 1 μg ml−1 recombinant human apoE or apoA-I were employed as standards. Densitometric analyses of bands identified as apoE or apoA-I by MALDI-TOF/MS were performed with ImageJ software. The results are expressed as arbitrary units. The gel is representative of one out of the three experiments (*P<0.05, ***P<0.001; ANOVA). (d) Extracellular IPP released in the supernatants retrieved from untreated (ZA-) or ZA-treated (ZA+) CD14+ cells, U937 cells, THP-1 cells and corresponding DC subsets (DC, DCU937, DCTHP1) in the absence (CTRL) or presence of human recombinant 1 μg ml−1 apoA-I or apoE added during the last 24 h of incubation. apoA-I and apoE increased the IPP release in DC and DCU937 but did not increase the IPP release in DCTHP1. The bars represent the mean value±s.e.m. of three experiments (**P<0.01; ANOVA). ANOVA, analysis of variance. MALDI-TOF/MS, matrix-assisted laser desorption ionization-time-of-flight/mass spectrometry. SDS-PAGE: sodium dodecyl sulfate-polyacrilammide gel electophoresis.
apoA-I binds to the extracellular domain of ABCA1 to load cholesterol and phospholipids that are released in the reverse cholesterol transport. apoE is also involved in the same process but it binds to ABCG1. We performed a matrix-assisted laser desorption ionization-time-of-flight/mass spectrometry (MALDI-TOF/MS) analysis to investigate whether apoA-I and apoE were present in the supernatant of CD14+ cells and DC (Supplementary Table 1). Two bands of 36.1 and 30.7 kD, which correspond to apoE and apoA-I, were identified (Fig. 2c, left; Supplementary Fig. 1; Supplementary Table 1). ZA treatment did not modify apoE levels, whereas it increased apoA-I expression (Fig. 2c, right). These data indicate that ZA-induced mevalonate pathway inhibition increases the extracellular release of both IPP and apoA-I in both CD14+ and DC.
To further explore the relationship among IPP, apoA-I, and apoE, extracellular IPP was quantified in the supernatant of untreated and ZA-treated THP-1 cells, U937 cells, CD14+ cells, DCTHP1, DCU937, and DC after incubation with recombinant human apoA-I and apoE and pulsing with [14C]-IPP. As shown in Fig. 2d, exogenously added apoA-I and apoE promoted extracellular IPP release only in cells with detectable ABCA1 expression, that is, untreated and ZA-treated DCU937 and DC.
To further build on the relationship between IPP and ABCA1, we correlated intracellular and extracellular IPP levels with ABCA1, ABCG1 and SR-B1 expression in a series of haematologically derived neoplastic and non-neoplastic cells (Supplementary Fig. 2A, Supplementary Fig. 2B). A strong correlation was detected between ABCA1 levels and extracellular IPP levels (r2=0.98), whereas no correlation between intracellular IPP levels and ABCA1 levels was identified (Supplementary Fig. 2C). ZA treatment upregulated ABCA1 expression only in cells with detectable ABCA1 under baseline conditions, such as the myeloma cell line SKMM1, primary multiple myeloma cells (CD138+ MM) and bone marrow stromal cells (BMSC) derived from multiple myeloma patients (Supplementary Fig. 3).
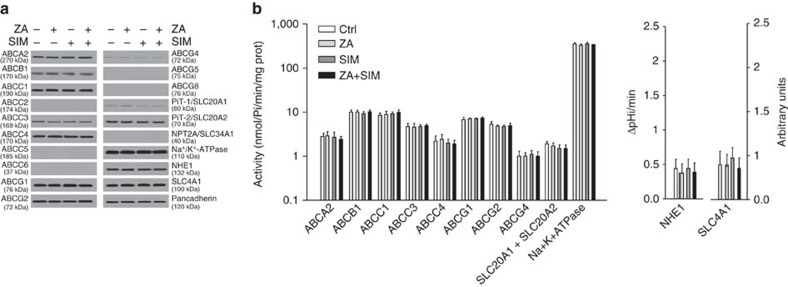
To eliminate the possibility that ZA-induced ABCA1 upregulation was not merely due to modifications of cholesterol content in the plasma-membrane and associated with non-specific changes in other membrane proteins, we compared the expression levels and activities of unrelated transporters and channels in untreated and DC treated with ZA and/or simvastatin. Simvastatin is a selective inhibitor of 3-β-hydroxy-β-methylglutaryl coenzyme A reductase (HMGR) upstream to FPPS in the mevalonate pathway that strongly reduces cholesterol synthesis3. The results in Fig. 3 indicate that neither the expression nor the activity of other ABC transporters that are involved in cholesterol and lipid metabolites efflux (ABCA2, ABCB1, ABCC1, ABCC2, ABCC3, ABCC4, ABCC5, ABCC6, ABCG2, ABCG4, ABCG5, ABCG8), phosphate transporters potentially involved in pyrophosphate transport (PiT-1/SLC20A1, PiT-2/SLC20A2, NPT2A/SLC34A1), and other ubiquitous and unrelated pumps or channels (Na+/K+-ATPase, Na+/H+ exchanger 1/NHE1, Cl−/HCO3− transporter/SLC4A1) were modified by ZA or simvastatin treatment.
Figure 3. ZA treatment does not affect other transporters in ZA and SIM-treated DC.
Levels of the indicated plasma-membrane-associated proteins are evaluated by western blotting in biotinylated extracts from untreated or 1 μM ZA and/or simvastatin (SIM) treated DC. Pancadherin is employed as a control of equal protein loading. The blots are representative of one out of the three experiments. (b) Activities of the indicated plasma-membrane-associated proteins are measured after immunopurification from the biotinylated extracts of DC treated, as shown in a. The results are expressed as nmoles hydrolysed phosphate (Pi)/min/mg proteins according to the previously prepared titration curve. The NHE1 activities and SLC4A1 activities are fluorimetrically measured, and the results are expressed as ΔpHi/min and arbitrary units, respectively. The bars represent the mean±s.e.m. of three experiments. None of the differences is statistically significant.
IPP is released by ABCA1 in cooperation with BTN3A1
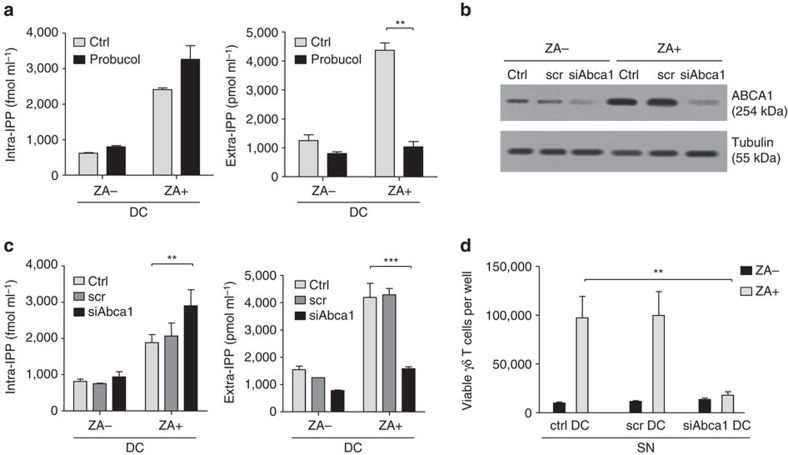
Probucol is a functional ABCA1 inhibitor19. Probucol incubation of untreated and ZA-treated DC for 24 h marginally increased intracellular IPP levels and completely abrogated ZA-induced extracellular IPP release (Fig. 4a).
Figure 4. ABCA1 has a pivotal function in extracellular IPP release.
(a) Intracellular and extracellular IPP levels generated by untreated (ZA-) and ZA-treated (ZA+) DC incubated for 24 h in fresh medium (CTRL) or with 10 μM probucol to inhibit ABCA1 function. Probucol marginally increased intracellular IPP but significantly decreased extracellular IPP release in ZA-treated DC (**P<0.01; ANOVA). The bars represent the mean±s.e.m. of four experiments. (b) Western blot analysis of ABCA1 expression in untreated (ZA-) and ZA-treated (ZA+) DC after Abca1-silencing with siRNA (scrambled [scr] non-targeting siRNA; siAbca1: Abca1-silencing siRNA). The blots are representative of one out of the three experiments. β-tubulin was employed as the control of equal protein loading. (c) Intracellular and extracellular IPP levels in DC incubated for 48 h in fresh medium (CTRL) or in the presence of scr or Abca1-silencing siRNA (siAbca1). ZA (1 μM) was added in the last 24 h. Extracellular IPP release is significantly lower in Abca1-silenced ZA-treated DC. Bars represent the mean±s.e.m. of six experiments (**P<0.01; ***P<0.001; ANOVA). (d) Vγ9Vδ2 T cell proliferation after 7-day PBMC stimulation with supernatants from DC treated as reported in c. Supernatant from Abca1-silenced ZA-treated DC is unable to induce Vγ9Vδ2 T cell proliferation (**P<0.01; Wilcoxon–Mann–Whitney). The bars represent the mean±s.e.m. of six experiments. ANOVA, analysis of variance.
To confirm the role of ABCA1, siRNA were employed to silence Abca1 in untreated and ZA-treated DC. The efficacy and specificity are shown in Fig. 4b. As expected, Abca1-silencing marginally increased intracellular IPP generation, whereas it completely abrogated ZA-induced extracellular IPP release (Fig. 4c). The inability to induce Vγ9Vδ2 T cell proliferation provided the functional demonstration that supernatants from ZA-treated Abca1-silenced DC were devoid of IPP (Fig. 4d). These results indicate that ABCA1 has a major role in the extracellular IPP release from ZA-treated DC.
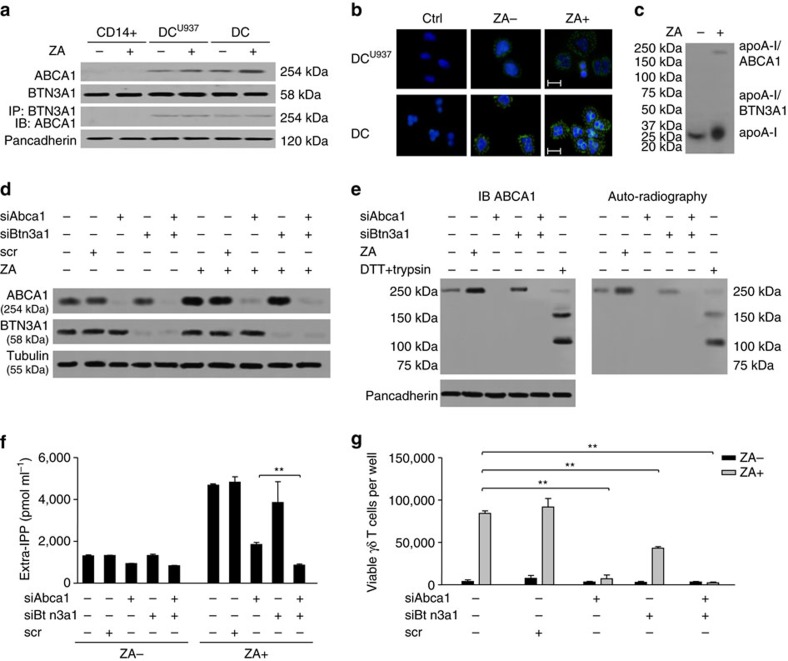
BTN3A1 has been reported to be involved in the phosphoantigen-induced activation and recognition of target cells by Vγ9Vδ2 T cells5,6. To explore any relationship between ABCA1 and BTN3A1, BTN3A1 expression was investigated by flow cytometry in CD14+ cells, DCTHP1, DCU937, and DC without detecting any difference under baseline conditions or after ZA treatment (Supplementary Fig. 2D). BTN3A1 and ABCA1 were co-immunoprecipitated in DCU937 and DC, which were the only cells to express detectable amounts of ABCA1 (Fig. 5a). ZA treatment increased ABCA1 expression but did not modify BTN3A1 expression. The physical interaction between ABCA1 and BTN3A1 was confirmed in DCU937 and DC by the proximity ligation assay (PLA) (Fig. 5b). Next, we tested the presence of interactions between apoA-I and ABCA1 or BTN3A1. After crosslinking of apoA-I to cell surface proteins and immunoprecipitation with anti-apoA-I antibody, we found that apoA-I and ABCA1 are physically associated as previously reported20, whereas no interaction was detected between apoA-I and BTN3A1 (Fig. 5c).
Figure 5. ABCA1 interactions with BTN3A1, apoA-I and IPP.
(a) ABCA1 and BTN3A1 co-immunoprecipitate in untreated and ZA-treated DCU937 and DC but not in CD14+ cells, and ZA treatment does not modify BTN3A1 expression. Pancadherin is employed as a control of equal protein loading (one out of three blots). (b) PLA of ABCA1-BTN3A1 interaction by confocal laser-scanning microscopy (ocular lens: × 10; objective: × 63). Ctrl: cells without primary antibodies. Scale bar, 10 μm; blue: nuclear staining (DAPI); green: ABCA1/BTN3A1 interaction (one out of the three experiments). (c) apoA-I is physically associated with ABCA1, not with BTN3A1. The expected molecular weight of apoA-I, ABCA1/apoA-I and BTN3A1/apoA-I are shown (one out of the three experiments). (d) ABCA1 and BTN3A1 expression in DC after incubation with siRNA for Abca1 (siABca1), Btn3a1 (siBtn3a1) or with scrambled non-targeting siRNA (scr). β-tubulin was employed as control of equal protein loading (n=3). (e) IPP is physically associated with ABCA1 in untreated and ZA-treated DC. The two bands of 150 and 100 kDa in the dithiothreitol (DTT)- and trypsin-treated DC lane are detected with an antibody specific for the N-terminal extracellular loop of ABCA1 in immunoblotting (IB; left). Autoradiography signal of the IPP-ABCA1 interaction (right). Pancadherin is employed as a control of equal protein loading (one out of three experiments). (f) Extracellular IPP release in Abca1- and/or Btn3a1-silenced DC left untreated (ZA-) or after ZA treatment (ZA+). The bars represent the mean±s.e.m. of three experiments (**P<0.001, Wilcoxon–Mann–Whitney). (g) Vγ9Vδ2 T cell proliferation after 7-day PBMC stimulation with supernatants from Abca1- and/or Btn3a1-silenced DC. The bars represent the mean±s.e.m. of three experiments (**P<0.01; Wilcoxon–Mann–Whitney).
To determine the reciprocal contributions to extracellular IPP release, BTN3A1 and ABCA1 were alternatively or concurrently silenced in untreated and ZA-treated DC. The efficacy and specificity of siRNA are shown in Fig. 5d: untreated DC (ZA−) expressed detectable levels of ABCA1, which further increased in ZA-treated DC (ZA+) and disappeared in untreated and ZA-treated Abca1-silenced and Abca1+Btn3a1-silenced DC, whereas ABCA1 expression was not suppressed in Btn3a1-silenced DC. To determine whether IPP was physically associated with ABCA1, DC were treated as shown in Fig. 5d, radiolabelled with 1 μM [14C]-IPP, and chemically crosslinked after extensive washing. ABCA1 was immunoprecipitated by plasma-membrane extracts, resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and probed with an anti-ABCA1 antibody that recognizes the N-terminal extracellular loop (Fig. 5e, left) or subjected to autoradiography (Fig. 5e, right). As internal control, biotinylated plasma-membrane extracts from ZA-treated DC were subjected to limited digestion to obtain distinct extracellular and intracellular ABCA1 fragments21,22. Autoradiography showed a positive signal in untreated DC coincidental with the expected ABCA1 molecular weight, which was increased in ZA-treated DC. The radioactive signal was still present in Btn3a1-silenced DC, whereas it was not detectable in Abca1-silenced and Abca1/Btn3a1-silenced DC. In dithiothreitol+trypsin-treated DC, the radioactive signal was coincidental with the 100 kDa band and, to a lesser extent, with the 150 kDa band, which suggests that IPP is physically associated with the N-terminal extracellular domain of ABCA1.
The functional consequences of Abca1-silencing and Btn3a1-silencing are shown in Fig. 5f,g. Minimal variations of extracellular IPP levels were induced when Abca1 and/or Btn3a1 were silenced in the absence of ZA-treatment (Fig. 5f). Conversely, ZA-treated Abca1-silenced DC showed a significant reduction in the ability to release extracellular IPP, whereas ZA-treated Btn3A1-silenced DC were only marginally affected. When both genes were silenced, further reduction in extracellular IPP release was observed (Fig. 5f). As expected, Vγ9Vδ2 T cell proliferation induced by supernatants from Abca1- and/or Btn3A1-silenced ZA-treated DC matched the extracellular IPP concentrations (Fig. 5g). These data indicate that ABCA1 plays a major role in extracellular IPP release; this function is facilitated by physical interactions with IPP, apoA-I and BTN3A1.
ZA increases IPP efflux via LXRα-mediated Abca1 activation
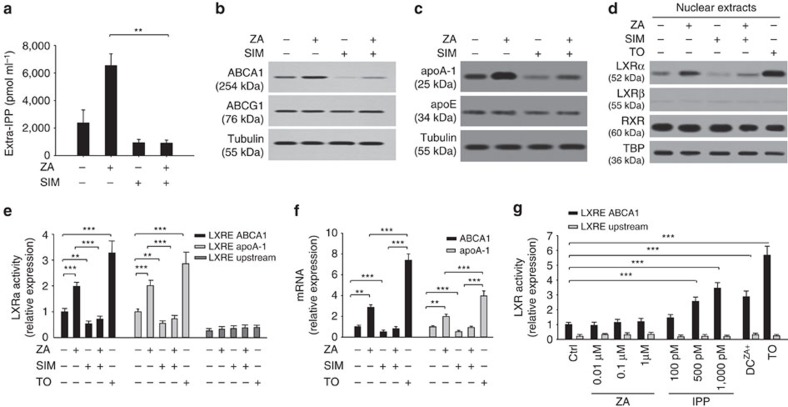
Simvastatin inhibits the mevalonate pathway upstream to FPPS3. As expected, simvastatin decreased extracellular IPP release in both untreated and ZA-treated DC (Fig. 6a). Interestingly, simvastatin also reduced ABCA1 (Fig. 6b) and apoA-I expression (Fig. 6c). These data confirm the close relationship between the mevalonate pathway, ABCA1, apoA-I and extracellular IPP release.
Figure 6. Regulation of ABCA1 and apoA-I expression via LXRα nuclear translocation.
(a) Extracellular IPP release in DC left untreated or incubated with 1 μM ZA and/or simvastatin (SIM, 1 μM). Simvastatin abrogated ZA-induced extracellular IPP release. Bars represent the mean±s.e.m. of four experiments (**P<0.01; ANOVA). (b) ABCA1 expression in experimental conditions as in a. ZA increased ABCA1 expression, whereas simvastatin showed the opposite effect and abrogated ZA-induced ABCA1 upregulation. (c) apoA-I expression in experimental conditions, as shown in a. ZA increased apoA-1 expression, whereas simvastatin exhibited the opposite effect and abrogated ZA-induced apoA-I upregulation. apoE was unaffected by ZA and/or simvastatin treatments. (d) LXRα, LXRβ and RXR levels were measured in nuclear extracts from DC incubated in the absence (−) or presence (+) of ZA and/or simvastatin. The LXRα activator TO-901317 (TO, 100 nM for 24 h) was employed as a positive control. ZA increased LXRα protein levels and this effect was antagonized by SIM. LXRβ and RXR were unaffected by ZA and/or simvastatin treatment. β-tubulin and TBP are employed as controls of equal protein loading as indicated. The blots are representative of one out of thee experiments (b–d). (e) Activity of Abca1 and apoA-I promoters in the experimental conditions, as shown in d. ZA increased, whereas simvastatin decreased and antagonized the ZA-induced LXRα transcriptional activity of Abca1 and apoA-I promoters. The bars represent the mean±s.e.m. of three experiments (**P<0.01; ***P<0.001; ANOVA). (f) Abca1 and apoA-I mRNA levels in the experimental conditions, as shown in d. ZA increased the Abca1 and apoA-I mRNA levels, whereas simvastatin had the opposite effect and antagonized ZA-induced upregulation. The bars represent the mean±s.e.m. of three experiments (**P<0.01; ***P<0.001; ANOVA). (g) Evaluation of LXRα binding to LRE in the Abca1 promoter. ZA did not modify LXRα transcriptional activity, whereas IPP induced a dose-dependent upregulation of LXRα-dependent Abca1 transcription. LXRα transcriptional activity in ZA-treated DC and TO-induced LXRα transcriptional activity are reported as positive internal controls. The bars represent the mean±s.e.m. of four experiments (*P<0.001; ANOVA). ANOVA, analysis of variance.
ABCA1 and apoA-I expressions are controlled by the liver X receptor (LXR)/retinoic acid X receptor (RXR) transcription factor receptor family23,24. We investigated whether ZA and simvastatin regulated ABCA1 and apoA-I expression via these transcription factors. Untreated DC expressed both LXRα/β and RXR in nuclear extracts (Fig. 6d). ZA treatment increased LXRα protein levels, whereas LXRβ and RXR intracellular distribution was unaffected (Fig. 6d). LXRα bound to Abca1 and apoA-I promoters was increased (Fig. 6e), leading to increased Abca1 and apoA-I mRNA levels (Fig. 6f). These ZA-induced effects were neutralized by simvastatin (Fig. 6d–f). These data indicate that ZA-induced LXRα activity is crucial to increase ABCA1 and apoA-I expression in DC and promotes extracellular IPP release.
To reinforce the role of LXRα, the experiments mentioned above were repeated using THP-1 cells, which are unable to release extracellular IPP, even after DC differentiation and ZA treatment. Unlike DC, LXRα and LXRβ failed to translocate into the nucleus after ZA treatment in THP-1 cells and DCTHP1 (Supplementary Fig. 4A). As expected, Abca1 and apoA-I mRNA levels remained unchanged by ZA treatment (Supplementary Fig. 4B). These data confirm that LXRα is critical to induce Abca1 and apoA-I transcription and protein expression after ZA-induced IPP accumulation.
To determine whether ZA or IPP was responsible for LXRα activation, genomic DNA was extracted from DC and challenged with increasing ZA and IPP concentrations in the presence of the human recombinant LXRα transcription protein. Only IPP was able to induce a dose-dependent LXRα binding to the LXR responsive element (LRE) in the Abca1 promoter (Fig. 6g). Optimal LXRα activation was induced by 500 pM IPP, which falls in the range of concentrations detected in the supernatants of ZA-treated DC (Fig. 6g). We tested whether other isoprenoids generated in the mevalonate pathway, such as FPP and GGPP, can also induce LXRα activation and we found that the former had no effect, whereas the latter downregulated LXRα activation (Supplementary Fig. 5) as previously reported25.
Next, we investigated whether ZA and/or IPP also increased the enzyme activity of ABCA1 in addition to its expression. Identical aliquots of ABCA1 were immunopurified from the plasma membrane of DC and incubated with increasing concentrations of ZA (Supplementary Fig. 6A) or IPP (Supplementary Fig. 6B). The experimental conditions were established to enable any change in ATPase activity to be referred to differences in enzyme activity instead of protein expression. The results indicate that neither ZA nor IPP were able to directly upregulate ABCA1 ATPase-activity (Supplementary Fig. 6A,B). Next, we compared the ATPase activity of ABCA1 immunopurified from the plasma membrane of untreated and ZA-treated CD14+ cells and DC. Untreated CD14+ cells exhibited the lowest ATPase activity, which remained unaffected by ZA treatment, whereas untreated DC revealed intermediate values, which almost doubled in ZA-treated DC (Supplementary Fig. 6C). The results suggest that the increased ATPase activity in ZA-treated DC is more likely due to increased ABCA1 protein levels than to the direct effect exerted by ZA or IPP on its enzymatic activity.
PI3K/AkT/mTOR pathway regulates IPP release via LXRα/Abca1
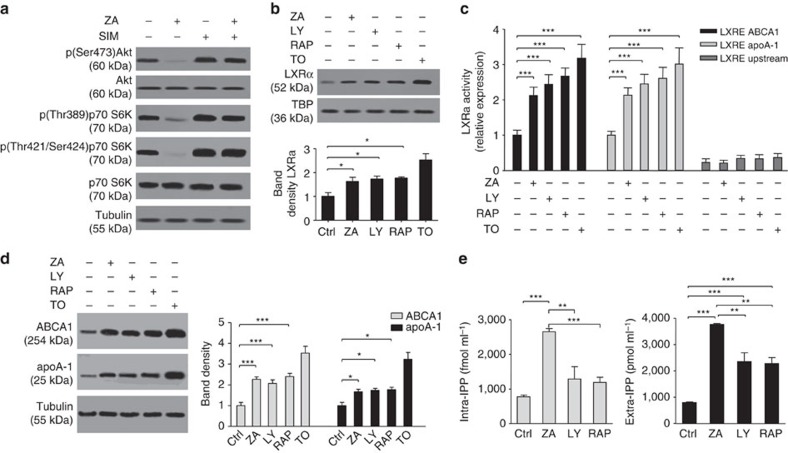
Akt suppresses cholesterol efflux by the activation of the mammalian target of rapamycin (mTOR) complex 1 TORC1, whereas the inhibition of mTORC1 by rapamycin promotes cholesterol efflux to apoA-I in an ABCA1-dependent manner26. The phosphoinositide 3-kinase (PI3K)/Akt/mTOR signalling pathway is also regulated by the Ras prenylation state, which is dependent on the mevalonate pathway in cancer cells and activated T cells27,28. To investigate the involvement of the PI3K/Akt/mTOR signalling pathway in ABCA1/apoA-I-mediated extracellular IPP release, DC were incubated with specific PI3K (LY294002) and mTOR (rapamycin) inhibitors (refer to Methods and Supplementary Fig. 7 for technical details). Untreated DC showed detectable phosphorylation activity of both Akt and mTOR, which was very effectively blocked by ZA treatment. Akt and mTOR phosphorylation activities were fully recovered by simvastatin treatment in ZA-treated DC (Fig. 7a), although simvastatin also induces intracellular isoprenoid deprivation and Ras deprenylation29,30. A possible explanation is that isoprenoid deprivation recovers the activity of the PI3K/Akt/mTOR pathway via induction of the endoplasmic reticulum (ER) stress and unfolded protein responses (UPR), as previously reported31. In line with this hypothesis, we have demonstrated that several ER-stress-related genes are significantly upregulated in ZA+simvastatin-treated compared with untreated DC (Supplementary Data 1), as previously reported with ZA or simvastatin in other cell types32,33.
Figure 7. The PI3K/AkT/mTOR pathway regulates IPP release via LXRα/Abca1 activation.
(a) Targeting the Mev pathway fine-tunes the PI3K/Akt/mTOR signalling pathway. DC were left untreated or incubated with 1 μM ZA or simvastatin (SIM). ZA decreased, whereas simvastatin increased the signalling activity of the PI3K/Akt/mTOR pathway. (b) DC were grown for 24 h in the absence (−) or presence (+) of ZA (1 μM), the PI3K inhibitor LY294002 (LY, 200 μM for 24 h ), the mTOR inhibitor rapamycin (RAP, 20 nM for 24 h). The LXRα activator TO-901317 (TO, 100 nM for 24 h) was included as positive control. PI3K/mTOR inhibitors increased LXRα nuclear translocation, such as ZA. Pooled data are obtained by densitometric analysis with ImageJ software (http://imagej.nih.gov/ij/). The results are expressed as arbitrary units (n=3) (*P<0.05; ANOVA). (c) Evaluation of LXRα binding to LRE sequences in the Abca1 and apoA-1 promoters by ChIP assay. ZA, LY294002 and rapamycin increased Abca1 and apoA-1 promoters activity. The bars represent the mean±s.e.m. of three experiments (***P<0.001; ANOVA). (d) Western blot analysis of ABCA1 and apoA-1 expression in experimental conditions, as shown in b. PI3K and mTOR inhibition mimicked ZA treatment and upregulated ABCA1 and apoA-1 expression. β-tubulin and TBP were employed as a control of equal protein loading, as indicated. All blots are representative of out the of three experiments. Pooled data are obtained by densitometric analysis with ImageJ software (http://imagej.nih.gov/ij/). The results are expressed as arbitrary units (n=3). (*P<0.05, ***P<0.001; ANOVA). (e) Intracellular and extracellular IPP levels in DC after incubation in experimental conditions, as shown in b. As expected, PI3K/Akt/mTOR inhibition only increased extracellular IPP. The bars represent the mean±s.e.m. of three experiments (**P<0.01, ***P<0.001; ANOVA). ANOVA, analysis of variance.
PI3K and mTOR inhibitors mimicked the effect of ZA by significantly increasing nuclear LXRα protein levels (Fig. 7b), LXRα transcriptional activity (Fig. 7c), and ABCA1 and apoA-I protein expression (Fig. 7d). Unlike ZA, LY294002 and rapamycin did not increase intracellular IPP levels as these inhibitors do not target the mevalonate pathway (Fig. 7e, left). However, both inhibitors increased extracellular IPP release as they upregulated ABCA1 and apoA-I protein expression. However, LY294002 and rapamycin did not achieve ZA-induced extracellular IPP values as they did not induce intracellular IPP accumulation (Fig. 7e, right).
Discussion
In this study, we confirm that soluble IPP can induce Vγ9Vδ2 T cell activation3,34 and demonstrate that the plasma-membrane-associated transporter ABCA1 plays a major role in the extracellular IPP release from ZA-treated DC and other cells. apoA-1 and BTN3A1 are also important contributors to this process, which ultimately causes the activation of Vγ9Vδ2 T cells. Although other metabolites downstream to IPP can also activate Vγ9Vδ2 T cells (that is, DMAPP, GPP, FPP and GGPP)35, none of these molecules achieved mitogenic concentrations in untreated DC and their levels further decreased after ZA treatment. These data exclude a contribution of these molecules in ZA-induced Vγ9Vδ2 T cell activation.
Cells that are unable to release extracellular IPP in baseline conditions or after ZA-induced intracellular IPP accumulation do not express ABCA1; contrariwise, cells with a high capacity to release intracellular IPP express ABCA1 and further up-regulate its expression after ZA treatment. IPP is physically associated with the N-terminal extracellular domain of ABCA1 and this association is strengthened in ZA-treated cells that have increased intracellular ABCA1 and IPP levels.
ABCA1 upregulation by ZA treatment is associated with an increased apoA-1 concentration in the supernatants. The mutual interaction between ABCA1 and apoA-1 was confirmed by the ability of exogenously added apoA-I to facilitate extracellular IPP release, but only in cells that already express ABCA1 in baseline conditions or after ZA treatment. We cannot exclude a priori that other transporters are involved in IPP efflux. However, the unique relationship between ABCA1 upregulation and intracellular IPP accumulation was corroborated by the observation that ZA and simvastatin treatment, which increase IPP and decrease IPP respectively, do not modify the expression of other ABC transporters, phosphate transporters or other ubiquitous and unrelated pumps or channels. These results also eliminated the possibility that ABCA1 expression is upregulated merely because the cholesterol content of plasma membranes or the intracellular concentration of other mevalonate pathway-related metabolites are modified by ZA or simvastatin.
The experiments in which ABCA1 was functionally inhibited with probucol, or protein expression specifically silenced with siRNA, provided direct evidence that ABCA1 plays a major role in extracellular IPP release. Both treatments only slightly increased intracellular IPP concentrations in untreated and ZA-treated DC, probably because downstream enzymes in the mevalonate pathway like IPP isomerase and prenyl transferases intervene to handle IPP accumulation in the absence of ABCA1 upregulation. More importantly, probucol and Abca1-silencing abrogated extracellular IPP release. IPP deprivation in the supernatants from ABCA1-inhibited DC was functionally confirmed by the inability to induce Vγ9Vδ2 T cell proliferation.
BTN3A1 is also involved in extracellular IPP release. BTN3A1 is an immunoglobulin superfamily protein playing a key role in Vγ9Vδ2 T cell activation and target recognition by Vγ9Vδ2 T cells4,6,7,36,37,38,39. The allosteric/sensor model anticipates that intracellular interactions between the phosphoantigens domain and the B30.2 domain of BTN3A1 induce conformational changes of the transmembrane domain that are sensed by Vγ9Vδ2 T cells, which cause their activation. The presentation model predicts that BTN3A1 functions as an antigen-presenting molecule for Vγ9Vδ2 T cells by extracellularly binding phosphoantigens7. The two models are not mutually exclusive and both models contemplate the possible contribution of a second protein that has yet to be characterized4,40. In the allosteric/sensor model, the unknown protein is expected to facilitate intracellular interactions between phosphoantigens and the B30.2 domain, whereas the unknown protein in the presentation model is expected to facilitate the release of endogenous phosphoantigens and the subsequent binding to extracellular BTN3A1. It has been proposed that this hypothetical transporter only intervenes when intracellular IPP exceeds a critical threshold40. Our results unveils a new function of BTN3A1 by showing its contribution to extracellular IPP release in cooperation with ABCA1 and apoA-I. BTN3A1 is physically associated with ABCA1 but is not associated with apoA-I. BTN3A1 expression is not required to maintain the association between ABCA1 and IPP; it is not correlated with intracellular IPP concentrations and/or extracellular IPP release and is not affected by ZA treatment. Btn3a1-silenced ZA-treated DC are only marginally penalized in the ability to release extracellular IPP compared with Abca1-silenced or Btn3a1/Abca1-double silenced DC. Remarkably, ZA-treated THP-1 cells and DCTHP1 accumulate higher amounts of intracellular IPP than ZA-treated DC (approximately 1,500 versus 900 fmol ml−1). In the absence of ABCA1 and despite identical BTN3A1 expression, DCTHP1 fail to release extracellular IPP and activate Vγ9Vδ2 T cells. These data indicate that ABCA1 serves a major role in extracellular IPP release from ZA-treated DC; this function is fine-tuned by one-to-one physical interactions of ABCA1 with IPP, apoA-1 and BTN3A1. The functional hierarchy of these molecules may be very different to drive Vγ9Vδ2 T cell activation and target cell recognition once IPP has been released in the microenvironment. In the absence of ZA-treated cells, it is possible that IPP internalization in selected cell subsets and the subsequent interaction with the intracellular B30.2 domain of BTN3A1 play a more critical role than ABCA1 and apoA-I to induce Vγ9Vδ2 T cell activation.
We have deciphered two mechanisms that underlie ZA-induced ABCA1 and apoA-1 upregulation. In vitro chromatin immunoprecipitation (ChIP) experiments indicated that IPP induces a dose-dependent LXRα binding to the LRE of the Abca1 promoter. The degree of DNA binding of purified LXRα incubated with 500 pM IPP was similar to the degree of DNA binding that was obtained in nuclear extracts of ZA-treated DC, the intracellular IPP levels of which range between 500 and 1,000 pM. Thus, the first mechanism is initiated by intracellular IPP accumulation due to ZA-induced FPPS inhibition, which causes LXRα activation, upregulation of Abca1/apoA-I transcription and increased ABCA1 and apoA-I protein expression. IPP-induced LXRα activation in our model was highly specific and cannot be reproduced with FPP or GGPP: the former had no effect, whereas GGPP downregulated LXRα activation as previously reported25. The antagonistic effect exerted by simvastatin confirmed the central role of IPP accumulation. By inhibiting HMGCR upstream to FPPS, simvastatin prevents ZA-induced intracellular IPP accumulation, ABCA1 and apoA-I upregulation, extracellular IPP release and Vγ9Vδ2 T cell activation. Unlike LXRα, DC express very low levels of LXRβ41, which remains unmodulated by ZA or simvastatin. Thus, the LXRα/LXRβ nuclear ratio is critical to fine-tune not only intracellular cholesterol concentrations in resting and activated conventional T cells to satisfy their metabolic requirements27 but also to regulate extracellular IPP release in DC and the subsequent activation of Vγ9Vδ2 T cells. It is also possible that phosphoantigens are internalized from the microenvironment via fluid phase endocytosis o energy-dependent mechanism42, especially when extracellular concentrations are much higher than intracellular concentrations, as in the supernatants from ZA-treated cells after removal of these cells. In this case, LXRα activation, ABCA1, and apoA-I upregulation may be regulated by internalization of extracellular IPP.
The second mechanism is dependent on ZA-induced inhibition of the PI3K/Akt/mTOR signalling pathway26,30,43. This mechanism has been described in tumour cells and associated with anti-tumour ZA activity32,44. LXRα is also regulated by the PI3K/Akt/mTOR pathway with different outcomes according to the cell type26,45. In DC, ZA-induced inhibition of the PI3K/Akt/mTOR signalling pathway increased LXRα-induced transcription of Abca1 and apoA-I, which causes enhanced protein expression, increased extracellular IPP release and Vγ9Vδ2 T cell activation. Similar results were obtained by targeting the PI3K/Akt/mTOR pathway with inhibitors other than ZA, which also upregulated ABCA1 and apoA-1. These inhibitors, however, caused inferior extracellular IPP release as they do not target the mevalonate pathway and do not induce intracellular IPP accumulation.
The mechanism beyond ZA-induced PI3K/Akt/mTOR inhibition is likely due to intracellular isoprenoid deprivation instead of IPP accumulation. ZA decreases GTP-bound Ras and downstream Ras-regulated signalling pathways29,30. As activity of the PI3K/Akt/mTOR pathway is upregulated by Ras, Ras deprenylation is expected to downregulate PI3K/Akt/mTOR signalling and increase LXRα activation28. Simvastatin also induces intracellular isoprenoid deprivation and Ras deprenylation, but it unexpectedly restored the activity of the PI3K/Akt/mTOR signalling in ZA-treated DC. A possible explanation is that the concurrent ZA and simvastatin treatments are so stressful to induce the expression of ER-stress-related and UPR genes, which causes the recovery of PI3K/Akt/mTOR signalling31.
Our results indicate that ABCA1 serves a pivotal role in extracellular IPP release in cooperation with apoA-I and BTN3A1. ABCA1 is upregulated by ZA via LXRα transcriptional activation that is induced by intracellular IPP accumulation and inhibition of the PI3K/Akt/mTOR signalling pathway. The supernatants of ZA-treated DC contain sufficient IPP concentrations to induce Vγ9Vδ2 T cell activation also in the absence of DC, but the manner in which soluble IPP engages Vγ9Vδ2 T cells remains to be determined (see Fig. 8 for a schematic representation). Thus, Vγ9Vδ2 T cell activation can be regulated not only with drugs that target the mevalonate pathway, such as ZA and simvastatin but also with drugs that target the PI3K/Akt/mTOR signalling pathway. Moreover, our data credit ABCA1 with a novel immune function that is linked to the activation of Vγ9Vδ2 T cells and contributes new perspectives for understanding the relationship between lipid metabolism and immune functions.
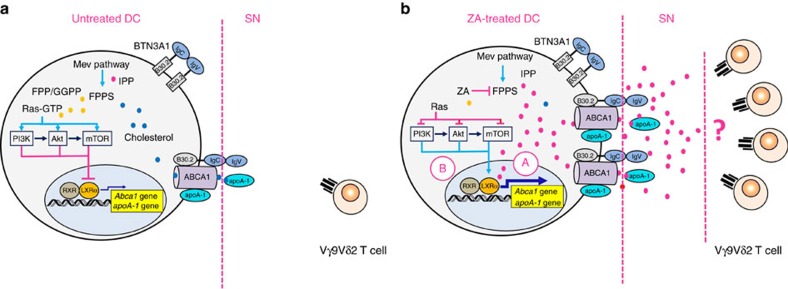
Figure 8. Mechanisms involved in IPP release from untreated and ZA-treated DC.
(a): Mevalonate (Mev) pathway of untreated DC generates isoprenoids (IPP: red circles; FPP/GGPP: yellow circles) and cholesterol (blue circles). Ras is prenylated in untreated DC (Ras-GTP) and causes the activation of the PI3K/Akt/mTOR signalling pathway and suppression of LXRα transcriptional activity. ABCA1 and apoA-I expression is finalized to tune the intracellular concentrations of cholesterol and other mevalonate pathway metabolites that were generated in physiological conditions. The supernatant obtained from untreated DC is unable to induce the proliferation of Vγ9Vδ2 T cells as extracellular IPP concentrations are below a critical threshold. (b) Farnesyl pyrophosphate synthase (FPPS) is inhibited in ZA-treated DC, which causes intracellular IPP accumulation (red circles), and isoprenoid (yellow circles) and cholesterol (blue circles) deprivation. Intracellular IPP induces LXRα nuclear translocation (a), which enhances Abca1 and apoA-1 gene transcription and upregulation of ABCA1 and apoA-I expression. Isoprenoid deprivation concurrently causes Ras deprenylation (Ras), which relieves the inhibition operated by the PI3K/Akt/mTOR pathway on LXRα nuclear translocation (b). Thus, two mechanisms are operative in ZA-treated DC, which causes upregulation of ABCA1 and apoA-1 expression and extracellular IPP and apoA-I release. The supernatant from ZA-treated DC contains sufficient amounts of IPP and apoA-I to induce the activation of Vγ9Vδ2 T cells. The question mark in the right panel indicates that the mechanisms by which soluble IPP released in the supernatant from ZA-treated DC induces Vγ9Vδ2 T cell activation remain to be elucidated.
Methods
Chemicals
Plasticware for cell cultures was obtained from Falcon (Becton Dickinson, Mountain View, CA). ZA was a gift from Novartis (Basel, Switzerland). Simvastatin was purchased from Calbiochem (Billerica, MA). The electrophoresis reagents were obtained from Bio-Rad Laboratories (Hercules, CA). The protein content of cell lysates was assessed with the BCA kit from Sigma Chemical Co (St Louis, MO). If not specified, all other reagents were purchased from Sigma Chemical Co.
Cells
Peripheral blood samples were drawn from healthy blood donors; the samples were provided by the local Blood Bank (Fondazione Strumia). Peripheral blood and bone marrow samples from multiple myeloma patients and B-cell chronic lymphocytic leukaemia patients who carried mutated or unmutated immunoglobulin heavy-chain variable regions were collected after informed consent and approval by the local Institutional Review Board (Comitato Etico Interaziendale A.O.U. Città della Salute e della Scienza di Torino—A.O. Ordine Mauriziano—A.S.L. TO1) were received (DG 767/2015). After isolation on a Ficoll-Hypaque density gradient, PBMC from healthy donors were kept unfractionated or processed to purify CD14+ cells and generate different antigen-presenting cell (APC) preparations. When PBMC from chronic lymphocytic leukaemia patients contained less than 90% of CD19+/CD5+ cells, cells were purified by negative selection using a magnetic bead-activated cell sorting with a B cell Isolation Kit II (Miltenyi Biotec, Bologna, Italy). Bone marrow mononuclear cells (BMMC) from multiple myeloma and chronic lymphocytic leukaemia patients were left unfractionated to generate BMSC or were processed to purify CD138+ multiple myeloma cells. Control BMMC from healthy donors were purchased from Stem Cell Technology (Peschiera Borromeo, Italy) to generate control BMSC. SKMM1, which is a multiple myeloma cell line, THP-1 and U937 cells, which are two monocytic leukaemia cell lines, and A549 cells, which is a non-small cell lung cancer cell line, were provided by the ATCC (Manassas, VA). The standard culture medium was RPMI 1640 (Euroclone, Milano, Italy) or HAM F12 medium (Euroclone) for A549 cells, which contain 10% fetal calf serum (FCS) (Euroclone), 2 mM L-glutamine, 100 U ml−1 penicillin, and 100 mg ml−1 streptomycin. Commercial cell lines were authenticated by microsatellite analysis using the PowerPlex kit (Promega Corporation, Madison, WI; last authentication: December 2015). The cells were checked for Mycoplasma spp. contamination by PCR every 3 weeks, and the contaminated cells were discharged.
Dendritic cell generation
CD14+ cells were purified using CD14 MicroBeads and LS columns (Miltenyi Biotec) and incubated for 24 h in 24-well plates at 1 × 106 per ml in the presence or absence of 1 μM ZA. DC were generated as previously reported3. Purified CD14+ cells were cultured in a standard culture medium at 0.5–1.5 × 106 cells per ml that were supplemented with GM-CSF (1000 U ml−1) and IL-4 (500 U ml−1) in flat-bottomed 24-well plates for 24 h. After 24 h, DC were left untreated (ZA-) or treated for an additional 24 h with 1 μM ZA (ZA+). To clear supernatants from any residual ZA, the DC were washed after incubation with ZA and re-plated with fresh medium for an additional 24 and 48 h (washed DC). DCTHP1 and DCU937 were generated with the same procedure as previously described from THP-1 and U937 cells. The DC phenotype was verified by cytofluorimetric analysis as follows.
In selected experiments, supernatants obtained from different cell cultures were collected and saved for a subsequent quantification of intracellular IPP and extracellular IPP levels and apoA-1 and apoE levels and to investigate their ability to induce autologous Vγ9Vδ2 T cell proliferation.
Bone marrow stromal cells generation
BMSC were generated by seeding 1 × 106 BMMC/well in 24-well plates in a DMEM medium that was supplemented with 10% FCS and replaced every 3 days with fresh medium. After 4 days, non-adherent cells were washed off and adherent cells—predominantly fibroblast-like cells—were grown in DMEM medium with 10% FCS until confluence (2–3 weeks). The BMSC phenotype was determined by cytofluorimetric analysis as follows.
Immune-phenotyping
Phenotypic characterization of DC, Vγ9Vδ2 T cells, myeloma cells and BMSC cells was performed with multicolour flow cytometry. The following anti-human monoclonal-antibody combinations were employed in the characterization: (a) Vγ9Vδ2 T cells: anti-TCR Vγ9 (clone B6, BD Pharmingen, dilution 1/50), anti-CD3 (clone BW264/56, Miltenyi Biotec, dilution 1/11); (b) DC: anti-CD80 (clone 2D10, Miltenyi Biotec, dilution 1/11), anti-CD86 (clone FM95, Miltenyi Biotec, dilution 1/11), anti-HLA-DR (clone AC122, Miltenyi Biotec, dilution 1/11); (c) myeloma cells: anti-CD138 (clone 44F9, Becton Dickinson, dilution 1/20); anti-CD38 (clone IB6, Becton Dickinson, dilution 1/20); and (d) BMSC cells: anti-CD44 (clone G44-26, Becton Dickinson, dilution 1/20), anti-CD105 (clone 266, Becton Dickinson, dilution 1/20), anti-CD11a (clone HI111, Becton Dickinson, dilution 1/20). Three- and four-colour flow cytometry were performed with the appropriate combinations of fluorescein isothiocyanate (FITC), r-phycoerythrin-(PE), Tricolour-(Tri), Peridinin Chlorophyll Protein Complex (PerCP) or allophycocyanin-(APC) conjugated antibodies, a FACScan cell sorter, and CELLQuest software (Becton Dickinson). Gating strategies are reported in the Supplementary Fig. 8.
Vγ9Vδ2 T cell activation and proliferation
PBMC were plated for 7 days at 106 cells per ml in 96-wells round-bottomed plates at 37 °C in a humidified atmosphere of 5% CO2 in air in the presence of 10 IU ml−1 IL-2 (Eurocetus, Milano, Italy) and 1 μM ZA (kindly provided by Novartis Pharma, Origgio, Italy). The standard culture medium was RPMI 1640 (Eurobio, Les Ulis, France), containing 10% FCS (Mascia Brunelli, Milano, Italy), 2 mM L-glutamine (Eurobio, Les Ulis, France), 100 U ml−1 penicillin (Eurobio, Les Ulis, France) and 100 mg ml−1 streptomycin (Eurobio, Les Ulis, France)3. Total counts of viable Vγ9Vδ2 T cells on day 7 were evaluated by multiplying total counts of viable cells per well, identified with the trypan blue staining assay, by the percentage of Vγ9Vδ2 T cells identified by flow cytometry46.
Quantification of intracellular and extracellular IPP
After 24 h culture, 1 × 106 cell per ml were incubated for another 24 h with 1 μCi of [3H]acetate (3,600 mCi mmol−1; Amersham International, Piscataway, NJ) to measure the intracellular IPP synthesis. 300 μl cells lysate or culture supernatant was diluted 1:2 into an ice-cold acetonitrile solution containing 100 mM NaVO4 and 250 mM NaF, and centrifuged at 1,200g for 5 min at 4 °C. After lyophilization under vacuum, samples were resuspended in 20 μl dimethylhexylamine and separated by thin layer chromatography (TLC; LK6D Whatmann silica gel Merck, Darmstadt, Germany), using 1:1 solution of 50% methanol/0.8 M ammonium formate, pH 7.4, containing 2 mM dimethylhexylammine as running buffer. Gels were exposed to an iodine-saturated atmosphere for 2 h, and the spot that corresponds to IPP was quantified by liquid scintillation counting (UltimaGold; PerkinElmer, Waltham, MA). According to the titration curve, the results are expressed as fmoles ml−1 for intracellular IPP or pmoles ml−1 for extracellular IPP.
Efflux of exogenous [14C]-IPP
To measure the efflux of an exogenous pulse of IPP, 1 × 106 cell ml−1 were labelled for 1 h with 0.02 μCi of [14C] IPP (50 mCi mmol−1; Amersham International),washed five times with PBS and left for 24 h in fresh medium. After this incubation time, supernatants were collected and underwent the same analytical process that was used to measure endogenously generated extracellular IPP. The amount of [14C]IPP that was present in the culture medium at t0 is considered to be 100%; the amount of [14C]IPP that was measured after incubation is expressed as a percentage relative to the amount measured at t0.
MALDI-TOF/MS analysis
Fifty μl of the culture medium of 1 × 106 CD14+ or DC were resolved by 12% SDS–PAGE, then stained using colloidal Coomassie brilliant blue (18% v-v ethanol, 15% w-v ammonium sulphate, 2% v-v phosphoric acid, 0.2% w/v Coomassie brilliant blue G-250). Gel slices from Coomassie-stained gels aligned to the recombinant human apoE or apoA-I, which were employed as standards, were excised and digested as previously described47. Gel slices were destained in 50% v-v acetonitrile in 5 mM ammonium bicarbonate, dried using pure acetonitrile and rehydrated for 45 min at 4 °C with digestion buffer that contains 10 ng μl−1 trypsin in 5 mM ammonium bicarbonate. Digestion proceeded overnight at 37 °C. The mass spectrometry analysis of peptides was performed using a matrix-assisted laser desorption ionization-time-of-flight/mass spectrometry device (MALDI micro MX; Waters, Milford, MA, USA) that was equipped with a delayed extraction unit that operated in reflectron mode. The peptide solution was prepared with equal volumes of saturated a-cyano-4-hydroxycinnamic acid solution in 40% v/v acetonitrile-0.1% v/v trifluoroacetic acid. The MALDI-TOF was calibrated with a mix of PEG (PEG 1000, 2000 and 3000 at a ratio of 1:2:2) and mass spectra were acquired in the positive-ion mode. Peak lists were generated by ProteinLynx Global Server 2.2.5 (Waters, Milford, MA, USA) using the following parameters: external calibration with lock mass using a mass of 2465.1989 Da for ACTH (adrenocorticotropic hormone), background subtract type adaptive that combined all scans, and de-isotoping with a threshold of 1%. The 25 most intense masses were employed for database searches against the SWISSPROT database using the free search program MASCOT (http://www.matrixscience.com). The following parameters were employed in the searches: taxa Homo sapiens, trypsin digest, one missed cleavage by trypsin, methionine oxidation as variable modification and allowed maximum error of 100 p.p.m.
Western blotting
The cells were lysed in a MLB buffer (125 mM Tris-HCl, 750 mM NaCl, 1% v/v NP40, 10% v/v glycerol, 50 mM MgCl2, 5 mM EDTA, 25 mM NaF, 1 mM NaVO4, 10 μg ml−1 leupeptin, 10 μg ml−1 pepstatin, 10 μg ml−1 aprotinin, 1 mM phenylmethylsulphonyl fluoride, pH 7.5), sonicated and centrifuged at 13,000g for 10 min at 4 °C. Plasma-membrane-associated proteins were isolated by the biotinylation method, using the Surface Protein Isolation Kit (Fisher Scientific Inc., Rockford, IL)48. Twenty microgram of proteins from cell lysates or biotinylated extracts were subjected to western blotting and probed with the following antibodies: anti-ABCA1 (HJI, Abcam, Cambridge, UK, dilution 1/500), anti-ABCA2 (ab91571, Abcam, dilution 1/250), anti-ABCB1 (C219, Novus Biologicals, Littleton, CO, dilution 1/250), anti-ABCC1 (IU2H10, Abcam, dilution 1/100), anti-ABCC2 (M2 III-6, Abcam, dilution 1/50), anti-ABCC3 (sc-5776, Santa Cruz Biotechnology Inc., Santa Cruz, CA, dilution 1/500), anti-ABCC4 (ab77184, Abcam, dilution 1/250), anti-ABCC5 (sc-5781, Santa Cruz Biotechnology Inc., dilution 1/500), anti-ABCC6 (ab54826, Abcam, dilution 1/500), anti-ABCG1 (sc-11150, Santa Cruz Biotechnology Inc., dilution 1/1,000), anti-ABCG2 (sc-25882, Santa Cruz Biotechnology Inc., dilution 1/100), anti-ABCG4 (ab101528, Abcam, dilution 1/250), anti-ABCG5 (EPR6203, Abcam, dilution 1/500), anti-ABCG8 (ab126493, Abcam, dilution 1/500), anti-SR-BI (NB400-104, Novus Biologicals, dilution 1/1,000), anti-BTN3A1 (25221-1-AP, Proteintech, Chicago, IL, dilution 1/500), anti-apoA-I (SAB1104904, Sigma Chemical Co., dilution 1/1,000), anti-apoE (AV54283, Sigma Chemical Co., dilution 1/1,000), anti-PiT-1/SLC20A1 (ab58181, Abcam, dilution 1/200), anti-PiT-2/SLC20A2 (ab155259, Abcam, dilution 1/200), anti-NPT2A/SLC34A1 (ab151129, Abcam, dilution 1/500), anti-Na+/K+-ATPase (M7-PB-E9, Sigma Chemical Co., dilution 1/2,000), anti-Na+/H+ exchanger 1/NHE1 (sc-136239, Santa Cruz Biotechnology Inc., dilution 1/500), anti-Cl-/HCO3- transporter/SLC4A1 (EPR1426, Abcam, dilution 1/1,000), anti-phospho(Ser473)Akt (6F5, Millipore, Billerica, MA, dilution 1/1,000), anti-Akt (SKB1, Millipore, dilution 1/500), anti-phospho(Thr389)-p70 S6K (#9205, Cell Signalling Technology, Danvers, MA, dilution 1/1,000), anti-phospho(Thr421/Ser424)-p70 S6K (#9204, Cell Signalling Technology, dilution 1/1,000), and anti-p70 S6K (#9202, Cell Signalling Technology, dilution 1/1,000). Anti-β-tubulin (sc-5274, Santa Cruz Biotechnology Inc., dilution 1/1,000) and anti-pancadherin (sc-1499, Santa Cruz Biotechnology Inc., dilution 1/500) were employed as a control of equal protein loading in whole cell and plasma-membrane extracts, followed by a peroxidase-conjugated secondary antibody. The proteins were detected by enhanced chemiluminescence (Bio-Rad Laboratories).
In the co-immunoprecipitation assays, 100 μg of proteins from biotinylated plasma-membrane extracts, which were prepared as previously described, were immunoprecipitated with the anti-BTN3A1 antibody, using the PureProteome protein A and protein G Magnetic Beads (Millipore). The immunoprecipitated proteins were separated by SDS–PAGE and probed with the anti-ABCA1 or apoA-I antibodies. An anti-pancadherin antibody was employed as the control for equal plasma-membrane protein loading.
To evaluate LXRα, LXRβ and RXR nuclear translocations, nuclear extracts were prepared using the Nuclear Extract kit (Active Motif, La Hulpe, Belgium). Ten microgram of nuclear proteins were resolved by SDS–PAGE and probed with anti-LXRα (61175, Active Motif, dilution 1/500), anti-LXRβ (ABN65, Millipore, dilution 1/500), anti-RXR (ab24363, Abcam, dilution 1/2,000) antibodies or with an anti-TFIID/TATA box-binding protein (TBP) antibody (58C9, Santa Cruz Biotechnology Inc., dilution 1/250) as the equal protein loading control.
The images of uncropped gels reported in main figures and Supplementary Figures are shown in Supplementary Figs 9 and 10.
Abca1 and Btn3a1 silencing
Two × 105 DC were transfected with Accell Human siRNA ABCA1, Accell Human BTN3A1 siRNA or 19–25 nucleotide non-targeting scrambled siRNAs (from Thermo Scientific Open Biosystems, Waltham, MA) per the manufacturer's instructions. The efficacy of silencing was verified by western blotting, as previously described.
Proximity ligation assay
The presence of ABCA1-BTN3A1 interactions was assessed with the PLA method using the DuoLink In Situ kit (Sigma Chemical Co.) per the manufacturer's instructions. A total of 1 × 104 cells were seeded on sterile coverglass, washed with PBS, fixed for 10 min at room temperature with 4% v/v paraformaldehyde, washed twice with PBS, and incubated 30 min at 37 °C in a humidified chamber with 40 μl of the kit-provided Blocking solution. A mouse anti-human ABCA1 (HJI, Abcam) and rabbit anti-human BTN3A1 (HPA012565, Sigma Chemical Co.) antibodies were added at 1:50 final dilution. Samples were maintained at 4 °C overnight. Slides were washed once with the Wash buffer A for 5 min at room temperature, and incubated 1 h at 37 °C with the respective PLA probes diluted 1:5 into 40 μl diluent antibody solution. Samples were washed twice with wash buffer A for 5 min at room temperature and incubated for 30 min at 37 °C with 40 μl ligation solution diluted 1:5 into RNAse/DNAse-free water and containing 25 mU ligase. After two washes at the rate of 2 min per each at room temperature, amplification was performed by adding 40 μl amplification solution that was diluted 1:5 into RNAse/DNAse-free water and contained 125 mU polymerase. Samples were maintained for 2 h at 37 °C, washed twice with undiluted wash buffer B for 10 min, once with wash buffer B, diluted 1:100 for 1 min, and mounted with the DuoLink In Situ Mounting medium, which contained 4′,5-diamidino-2 phenylindole dyhydrochloride (DAPI) to counterstain cells' nuclei. Cells were examined using a Leica TCS SP2 AOP confocal laser-scanning microscope (Leica Microsystem, Wetzlar, Germany). A minimum of five fields were examined for each experimental condition.
Crosslinking assay
Crosslinking of apoA-I to cell surface proteins was performed as previously reported20. After labelling for 2 h at 37 °C with 5 μg ml−1 [125I]-apoA-I and chemically crosslinking with dithiobis(succinimidyl propionate) (DSP), cells were lysed and subjected to immunoprecipitation with an anti-apoA-I antibody (SAB1104904, Sigma Chemical Co., dilution 1/100). Samples were resolved by SDS–PAGE, and blotted proteins were detected by autoradiography (Kodak BioMax MR-1, PerkinElmer).
IPP-ABCA1 interaction
IPP-ABCA1 interaction was measured in the cells labelled with 1 μM [14C]-IPP for 1 h. After extensively washing (five washing steps of 5 min with PBS) to remove any traces of residual [14C]-IPP in the extracellular medium, the cells were grown in fresh medium for 1 h and fixed with DSP. The surface proteins were isolated with the Surface Protein Isolation Kit (Fisher Scientific Inc. A total of 500 μg of surface proteins were subjected to immunoprecipitation with an anti-ABCA1 antibody (HJI, Abcam, dilution 1/50). As an internal control, cells were incubated 5 min with 2 mM dithiothreitol, and the biotinylated extracts were subjected to limited digestion with 10 μg ml−1trypsin, as reported21,22. Immunopurified ABCA1 was resolved by SDS–PAGE, and run in duplicate on the same gel. One part of the gel was probed with the anti-ABCBA antibody; the second part was dried and subjected to autoradiography to visualize the [14C]-IPP signal.
Quantitative real time-PCR and PCR array
Total RNA was reverse-transcribed using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories). The qRT-PCR was performed with the IQ SYBR Green Supermix (Bio-Rad). The same cDNA preparation was employed to quantify the genes of interest and the housekeeping gene S14. The primer sequences, which were designed using the qPrimerDepot database (http://primerdepot.nci.nih.gov/), were as follows: for Abca1: 5′-CAGAGCTCACAGCAGGGAC-3′; 5′-CTTCTCCGGAAGGCTTGTC-3′; for apoA-I: 5′-CCCAGTTGTCAAGGAGCTTT-3′, 5′-TGGATGTGCTCAAAGACAGC-3′; for S14: 5′-CGAGGCTGATGACCTGTTCT-3′; 5′-GCCCTCTCCCACTCTCTCTT-3′. The relative quantification was performed by comparing each PCR product with the housekeeping PCR product S14, using the Bio-Rad Software Gene Expression Quantitation (Bio-Rad). PCR arrays were generated using 1 μg cDNA and Human Unfolded Protein Response Plus RT2 Profiler PCR Array (Bio-Rad Laboratories) according to the manufacturer's instructions. Data analysis was performed using the PrimePCR Analysis Software (Bio-Rad Laboratories).
Chromatin Immunoprecipitation
ChIP samples were prepared as described by Campia et al.49, using a ChIP-tested anti-LXRα antibody (61175, Active Motif, dilution 1/50). The putative Liver X Receptor Response Element (LRE) sites on Abca1 and apoA-I human promoters were validated with the Matinspector Software (https://www.genomatix.de/matinspector.html). Primer sequences were as follows: for Abca1 promoter: 5′-GGAGAGCACAGGCTTTGACC-3′; 5′-CTCTCGCGCAATTACGGG-3′; for apoA-I promoter: 5′-AACTGCCCACACACTCCCAT-3′; 5′-TCCTTCTCGCAGTCTCTAAGCA-3′. Primers used as negative internal controls for a nonspecific 10 000 bp upstream sequence, were as follows: 5′-GTGGTGCCTGAGGAAGAGAG-3′ and 5′-GCAACAAGTAGGCACAAGCA-3′. The immunoprecipitated products were amplified by qRT-PCR.
In in vitro ChIP experiments, total DNA from 1 × 106 DC was extracted using the GenElute Mammalian Genomic DNA Miniprep Kit (Sigma Chemical Co.), sonicated, incubated with 1 ng of recombinant human LXRα protein (Abcam), immunoprecipitated with the anti-LXRα antibody and processed as previously described.
Activity of plasma-membrane-associated transporter and channels
Plasma-membrane vesicles enriched for ATP-binding cassette transporters were prepared as detailed elsewhere with minor modifications50. Cells were washed with Ringer's solution (148.7 mM NaCl, 2.55 mM K2HPO4, 0.45 mM KH2PO4, 1.2 mM MgSO4; pH 7.4), lysed on crushed ice with lysis buffer (10 mM Hepes/Tris, 5 mM EDTA, 5 mM EGTA, 2 mM dithiothreitol; pH 7.4) supplemented with 2 mM phenylmethylsulfonyl fluoride, 1 mM aprotinin, 10 μg ml−1 pepstatin, 10 μg ml−1 leupeptin, and subjected to nitrogen cavitation at 1200, p.s.i for 20 min. Samples were centrifuged at 300g for 10 min, diluted 1:4 in the pre-centrifugation buffer (10 mM Tris/HCl, 25 mM sucrose; pH 7.5), overlaid on a sucrose cushion (10 mM Tris/HCl, 35% w/v sucrose, 1 mM EDTA; pH 7.5) and centrifuged at 14,000g for 10 min. The interface was collected, diluted 1:5 in the centrifugation buffer (10 mM Tris/HCl, 250 mM sucrose; pH 7.5) and subjected to a third centrifugation at 100,000 × g for 45 min. The vesicle pellet was resuspended in 0.5 ml centrifugation buffer and stored at −80 °C until the use, after the quantification of the protein content. Proteins were extracted by non-denaturing immunoprecipitation using the previously indicated antibodies. The ATPase activity of immunopurified ABCA1, ABCA2, ABCB1, ABCC1, ABCC3, ABCC4, ABCG1, ABCG2 and ABCG4 was measured with a spectrophotometric method51. Samples (containing 25 μg protein) were incubated for 30 min at 37 °C with 50 μl of the reaction mix (25 mM Tris/HCl, 3 mM ATP, 50 mM KCl, 2.5 mM MgSO4, 3 mM dithiothreitol, 0.5 mM EGTA, 2 mM ouabain, 3 mM NaN3; pH 7.0). In each set of experiments, a blank containing 0.5 mM Na3VO4 was included52. The reaction was stopped by adding 0.2 ml ice-cold stopping buffer (0.2% w/v ammonium molybdate, 1.3% v/v H2SO4, 0.9% w/v SDS, 2.3% w/v trichloroacetic acid, 1% w/v ascorbic acid). After a 30 min incubation at room temperature, the absorbance of the phosphate hydrolyzed from ATP was measured at 620 nm, using a Packard EL340 microplate reader (Bio-Tek Instruments, Winooski, MA). The absorbance was converted into μmol hydrolyzed phosphate per min per mg proteins, according to the titration curve previously prepared. The activity of PiT-1/SLC20A1 and PiT-2/SLC20A2 (ref. 50), and Na+/K+-ATPase53 were spectrophotometrically measured. The results are expressed as nmoles of hydrolysed phosphate per min per mg proteins according to the previously prepared titration curve. The NHE1 and SLC4A1 activities were fluorimetrically measured54,55, and the results are expressed as ΔpHi/min and arbitrary units, respectively.
Statistical analysis
The results are expressed as the mean±s.e.m. Differences between the groups were evaluated with a one-way analysis of variance, a Wilcoxon–Mann–Whitney non-parametric test for paired or unpaired samples as appropriate and was considered to be statistically significant for P values <0.05. Correlation analyses were performed with the non-parametric Spearman Rank Order test with a cutoff P value <0.05. The GraphPad software was used for the statistical analyses; its symbology has been adopted to define the statistical significance (ns=P>0.05; *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001). The sample size was calculated with the G*Power software (www.gpower.hhu.de), with the setting P<0.05 as the significance level and 0.80 as the power of the study.
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and its Supplementary Information files.
Additional information
How to cite this article: Castella, B. et al. The ATP-binding cassette transporter A1 regulates phosphoantigen release and Vγ9Vδ2 T cell activation by dendritic cells. Nat. Commun. 8, 15663 doi: 10.1038/ncomms15663 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary Figures and Supplementary Table
Expression of UPR genes after ZA and simvastatin treatment. The expression of ER stress-related genes was measured in untreated DC and DC treated with ZA (1 μM) and simvastatin (SIMV; 1 μM) for 24 h. Tunicamycin (Tun) (1 μM) was included as an inducer of ER stress. Total mRNA was extracted, retro-transcribed and amplified with specific primers for ER stress-related genes, which are contained in the Human Unfolded Protein Response Plus RT2 Profiler(tm) PCR Array (Bio-Rad Laboratories). Data analysis was performed using the PrimePCR(tm) Analysis Software (Bio-Rad Laboratories). Fold-Change (2^(- Delta Delta Ct)) is the normalized gene expression (2^(- Delta Ct)) in treated DC, which divided the normalized gene expression (2^(- Delta Ct)) in untreated DC (n= 4), where Ct is the threshold cycle in qRT-PCR. Fold-change values greater than one indicate up-regulation, whereas fold-change values less than one indicate down-regulation. The P values are calculated based on Student's t test of the replicate 2^(- Delta Ct) values for each gene. P < 0.05 was considered to be significant. ERAD: ER-associated degradation; ERQC: ER-quality control.
Acknowledgments
This study was supported by the Italian Association for Cancer Research (AIRC) (MFAG11475 and IG15232 to CR), (5 x mille n.12182 and IG 15257 to FN), (IG 13119 and IG 16985 to MM); PRIN 2010NECHBX_002 to MM; Compagnia San Paolo-University of Turin to FN; Progetti Ateneo (2014-PC_METHAIMMUNOTHER and 2011-RETHE to FN); Fondazione Ricerca Molinette to F.N. B.C., M.F. and J.K. are post-doc research fellows supported by PRIN (BC), AIRC (MF), and Fondazione Umberto Veronesi (JK). We are grateful to Prof. Amalia Bosia and Prof. Dario Ghigo (Dipartimento di Oncologia, Università di Torino) for their fruitful discussions and to Mr. Costanzo Costamagna (Dipartimento di Oncologia, Università di Torino) for his technical assistance. We are also grateful to Mr. Andrew Martin Garvey, BAHons LTCL(TESOL) PGDip MA for his editorial assistance.
Footnotes
The authors declare no competing financial interests.
Author contributions B.C., C.R. and M.M. designed the study, analysed the data and drafted the manuscript. B.C. and J.K. performed the experiments and analysed the data. P.S., G.M. and M.F. assisted with the experiments and analysed the data. N.M. and D.C. conceived the working hypothesis, contributed to the data analysis and revised the manuscript. B.C., F.N., C.R. and M.M. interpreted the data, revised the manuscript and obtained funding. B.C., C.R. and M.M. supervised the study and finalized the submission.
References
- Gober H. J. et al. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J. Exp. Med. 197, 163–168 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs A. J., Thompson K., Gordon S. & Rogers M. J. Molecular mechanisms of action of bisphosphonates: current status. Clin. Cancer Res. 12, 6222s–6230s (2006). [DOI] [PubMed] [Google Scholar]
- Castella B. et al. Immune modulation by zoledronic acid in human myeloma: an advantageous cross talk between Vγ9Vδ2 T cells, αβ CD8+ T cells, regulatory T cells, and dendritic cells. J. Immunol. 187, 1578–1590 (2011). [DOI] [PubMed] [Google Scholar]
- Harly C., Peigné C. M. & Scotet E. Molecules and mechanisms implicated in the peculiar antigenic activation process of human Vγ9Vδ2 T Cells. Front. Immunol. 5, 657 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore F. et al. Enhanced ability of dendritic cells to stimulate innate and adaptive immunity on short-term incubation with zoledronic acid. Blood 110, 921–927 (2007). [DOI] [PubMed] [Google Scholar]
- Harly C. et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T cell subset. Blood 120, 2269–2279 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavassori S. et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human γδ T cells. Nat. Immunol. 14, 908–916 (2013). [DOI] [PubMed] [Google Scholar]
- Gu S., Nawrocka W. & Adams E. J. Sensing of pyrophosphate metabolites by Vγ9Vδ2 T Cells. Front Immunol 5, 688 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palakodeti A. et al. The molecular basis for modulation of human Vγ9Vδ2 T cell responses by CD277/butyrophilin-3 (BTN3A)-specific antibodies. J. Biol. Chem. 287, 32780–32790 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vantourout P. et al. Specific requirements for Vgamma9Vdelta2 T cell stimulation by a natural adenylated phosphoantigen. J. Immunol. 183, 3848–3857 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotet E. et al. Tumor recognition following Vgamma9Vdelta2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity 22, 71–80 (2005). [DOI] [PubMed] [Google Scholar]
- Mookerjee-Basu J. et al. F1-adenosine triphosphatase displays properties characteristic of an antigen presentation molecule for Vgamma9Vdelta2 T cells. J. Immunol. 184, 6920–6982 (2010). [DOI] [PubMed] [Google Scholar]
- Champagne E., Martinez L. O., Vantourout P., Collet X. & Barbaras R. Role of apolipoproteins in gammadelta and NKT cell-mediated innate immunity. Immunol. Res. 33, 241–255 (2005). [DOI] [PubMed] [Google Scholar]
- Hafiane A. & Genest J. HDL, atherosclerosis, and emerging therapies. Cholesterol 2013, 891403 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichiri M. et al. ATP-binding cassette transporter A1 is involved in hepatic alpha-tocopherol secretion. J. Nutr. Biochem. 21, 451–456 (2010). [DOI] [PubMed] [Google Scholar]
- Berges C. et al. A cell line model for the differentiation of human dendritic cells. Biochem. Biophys. Res. Commun. 333, 896–907 (2005). [DOI] [PubMed] [Google Scholar]
- Chan W. K., Cheung C. C., Law H. K., Lau Y. L. & Chan G. C. Ganoderma lucidum polysaccharides can induce human monocytic leukemia cells into dendritic cells with immuno-stimulatory function. J. Hematol. Oncol. 1, 9 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos G. G. et al. Progress on the development of human in vitro dendritic cell based assays for assessment of the sensitizing potential of a compound. Toxicol. Appl. Pharmacol. 236, 372–382 (2009). [DOI] [PubMed] [Google Scholar]
- Yamamoto S. et al. Pharmacologic suppression of hepatic ATP-binding cassette transporter 1 activity in mice reduces high-density lipoprotein cholesterol levels but promotes reverse cholesterol transport. Circulation 124, 1382–1390 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedhachalam C. et al. ABCA1-induced cell surface binding sites for apoA-I. Arterioscler. Thromb. Vasc. Biol. 27, 1603–1609 (2007). [DOI] [PubMed] [Google Scholar]
- Takahashi K., Kimura Y., Kioka N., Matsuo M. & Ueda K. Purification and ATPase activity of human ABCA1. J. Biol. Chem. 281, 10760–10768 (2006). [DOI] [PubMed] [Google Scholar]
- Hozoji M., Kimura Y., Kioka N. & Ueda K. Formation of two intramolecular disulfide bonds is necessary for apoA-I-dependent cholesterol efflux mediated by ABCA1. J. Biol. Chem. 284, 11293–11300 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costet P., Luo Y., Wang N. & Tall A. R. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J. Biol. Chem. 275, 28240–28245 (2000). [DOI] [PubMed] [Google Scholar]
- Lee J., Tauscher A., Seo D. W., Oram J. F. & Kuver R. Cultured gallbladder epithelial cells synthesize apolipoproteins A-I and E. Am. J. Physiol. Gastrointest. Liver Physiol. 285, G630–G641 (2003). [DOI] [PubMed] [Google Scholar]
- Gan J. et al. Dual mechanisms of ABCA1 regulation by geranylgeranyl pyrophosphate. J Biol Chem. 276, 48702–48708 (2001). [DOI] [PubMed] [Google Scholar]
- Dong F., Mo Z., Eid W., Courtney K. C. & Zha X. Akt inhibition promotes ABCA1-mediated cholesterol efflux to apoA-I through suppressing mTORC1. PLoS One 9, e113789 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurnher M. & Gruenbacher G. T lymphocyte regulation by mevalonate metabolism. Sci. Signal. 8, re4 (2015). [DOI] [PubMed] [Google Scholar]
- Okkenhaug K. Signaling by the phosphoinositide 3-kinase family in immune cells. Annu. Rev. Immunol. 31, 675–704 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riganti C. et al. Zoledronic acid restores doxorubicin chemosensitivity and immunogenic cell death in multidrug-resistant human cancer cells. PLoS ONE 8, e60975 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Moriceau G. et al. Zoledronic acid potentiates mTOR inhibition and abolishes the resistance of osteosarcoma cells to RAD001 (Everolimus): pivotal role of the prenylation process. Cancer Res. 70, 10329–10334 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Yang X. & Zhang J. Bridges between mitochondrial oxidative stress, ER stress and mTOR signaling in pancreatic β cells. Cell Signal 28, 1099–1104 (2016). [DOI] [PubMed] [Google Scholar]
- Lan Y. C. et al. Zoledronic acid-induced cytotoxicity through endoplasmic reticulum stress triggered REDD1-mTOR pathway in breast cancer cells. Anticancer Res. 33, 3807–3814 (2013). [PubMed] [Google Scholar]
- Ghavami S. et al. Apoptosis, autophagy and ER stress in mevalonate cascade inhibition-induced cell death of human atrial fibroblasts. Cell Death Dis. 3, e330 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y. et al. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature 375, 155–158 (1995). [DOI] [PubMed] [Google Scholar]
- Gruenbacher G. et al. Stress-related and homeostatic cytokines regulate Vγ9Vδ2 T cell surveillance of mevalonate metabolism. Oncoimmunology 3, e953410 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D. A. et al. Activation of human γδ T cells by cytosolic interactions of BTN3A1 with soluble phosphoantigens and the cytoskeletal adaptor periplakin. J Immunol. 194, 2390–2398 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom A. et al. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vγ9Vδ2 T cells. Immunity 40, 490–500 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaño F. et al. Vγ9Vδ2 TCR-activation by phosphorylated antigens requires butyrophilin 3 A1 (BTN3A1) and additional genes on human chromosome 6. Eur. J. Immunol. 44, 2571–2576 (2014). [DOI] [PubMed] [Google Scholar]
- Wang H. & Morita C. T. Sensor function for butyrophilin 3A1 in prenyl pyrophosphate stimulation of human Vγ2Vδ2 T Cells. J. Immunol. 195, 4583–4594 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Libero G., Lau S. Y. & Mori L. Phosphoantigen presentation to TCR γδ cells, a cnundrum getting less gray zones. Front. Immunol. 5, 679 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyeregger R. et al. Liver X receptors regulate dendritic cell phenotype and function through blocked induction of the actin-bundling protein fascin. Blood 109, 4288–4295 (2007). [DOI] [PubMed] [Google Scholar]
- Kilcollins A. M., Li J., Hsiao C. H. & Wiemer A. J. HMBPP analog prodrugs bypass energy-dependent uptake to promote efficient BTN3A1-mediated malignant cell lysis by Vgamma9Vdelta2 T lymphocyte effectors. J. Immunol. 197, 419–428 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakiewicz R. D., Schieferl S. M., Gingras A. C., Sonenberg N. & Comb M. J. mu-Opioid receptor activates signaling pathways implicated in cell survival and translational control. J. Biol. Chem. 273, 23534–23541 (1998). [DOI] [PubMed] [Google Scholar]
- Li S. & De Souza P. Ras isoprenylation and pAkt inhibition by zoledronic acid and fluvastatin enhances paclitaxel activity in T24 bladder cancer cells. Cancers (Basel) 3, 662–674 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwahng S. H., Ki S. H., Bae E. J., Kim H. E. & Kim S. G. Role of adenosine monophosphate-activated protein kinase-p70 ribosomal S6 kinase-1 pathway in repression of liver X receptor-alpha-dependent lipogenic gene induction and hepatic steatosis by a novel class of dithiolethiones. Hepatology 49, 1913–1925 (2009). [DOI] [PubMed] [Google Scholar]
- Mariani S. et al. Effector gammadelta T cells and tumor cells as immune targets of zoledronic acid in multiple myeloma. Leukemia 19, 664–670 (2005). [DOI] [PubMed] [Google Scholar]
- Mandili G. et al. Characterization of the protein ubiquitination response induced by Doxorubicin. FEBS J. 279, 2182–2191 (2012). [DOI] [PubMed] [Google Scholar]
- De Boo S. et al. iNOS activity is necessary for the cytotoxic and immunogenic effects of doxorubicin in human colon cancer cells. Mol. Cancer 8, 108 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campia I. et al. Digoxin and ouabain induce the efflux of cholesterol via liver X receptor signalling and the synthesis of ATP in cardiomyocytes. Biochem. J. 447, 301–311 (2012). [DOI] [PubMed] [Google Scholar]
- Litman T., Zeuthen T., Skovsgaard T. & Stein W. D. Competitive, non-competitive and cooperative interactions between substrates of P-glycoprotein as measured by its ATPase activity. Biochim. Biophys. Acta. 1361, 169–176 (1997). [DOI] [PubMed] [Google Scholar]
- Kopecka J. et al. Insights in the chemical components of liposomes responsible for P-glycoprotein inhibition. Nanomedicine 10, 77–87 (2014). [DOI] [PubMed] [Google Scholar]
- Beck L. et al. The phosphate transporter PiT1 (Slc20a1) revealed as a new essential gene for mouse liver development. PLoS ONE 5, e9148 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bełtowski J., Jamroz-Wiśniewska A., Nazar J. & Wójcicka G. Spectrophotometric assay of renal ouabain-resistant Na+-ATPase and its regulation by leptin and dietary-induced obesity. Acta Biochim. Pol. 51, 1003–1014 (2004). [PubMed] [Google Scholar]
- Miraglia E. et al. Na1/H1 exchanger activity is increased in doxorubicin-resistant human colon cancer cells and its modulation modifies the sensitivity of the cells to doxorubicin. Int J Cancer. 115, 924–929 (2005). [DOI] [PubMed] [Google Scholar]
- Nagarajan S. et al. Mechanical perturbations trigger endothelial nitric oxide synthase activity in human red blood cells. Sci. Rep. 6, 26935 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures and Supplementary Table
Expression of UPR genes after ZA and simvastatin treatment. The expression of ER stress-related genes was measured in untreated DC and DC treated with ZA (1 μM) and simvastatin (SIMV; 1 μM) for 24 h. Tunicamycin (Tun) (1 μM) was included as an inducer of ER stress. Total mRNA was extracted, retro-transcribed and amplified with specific primers for ER stress-related genes, which are contained in the Human Unfolded Protein Response Plus RT2 Profiler(tm) PCR Array (Bio-Rad Laboratories). Data analysis was performed using the PrimePCR(tm) Analysis Software (Bio-Rad Laboratories). Fold-Change (2^(- Delta Delta Ct)) is the normalized gene expression (2^(- Delta Ct)) in treated DC, which divided the normalized gene expression (2^(- Delta Ct)) in untreated DC (n= 4), where Ct is the threshold cycle in qRT-PCR. Fold-change values greater than one indicate up-regulation, whereas fold-change values less than one indicate down-regulation. The P values are calculated based on Student's t test of the replicate 2^(- Delta Ct) values for each gene. P < 0.05 was considered to be significant. ERAD: ER-associated degradation; ERQC: ER-quality control.
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the paper and its Supplementary Information files.