Abstract
Antibodies can prevent lentivirus infections in animals and may play a role in controlling viral burden in established infection. In preventing and particularly in controlling infection, antibodies likely function in the presence of large quantities of virus. In this study, we explored the mechanisms by which antibodies neutralize large inocula of human immunodeficiency virus type 1 (HIV-1) on different target cells. Immunoglobulin G (IgG) from HIV-infected patients was tested for neutralizing activity against primary R5 strains of HIV-1 at inocula ranging from 100 to 20,000 50% tissue culture infective doses. At all virus inocula, inhibition by antibody was enhanced when target cells for virus growth were monocyte-depleted, peripheral blood mononuclear cells (PBMCs) rather than CD4+ lymphocytes. However, enhanced inhibition on PBMCs was greatest with larger amounts of virus. Depleting PBMCs of natural killer (NK) cells, which express Fc receptors for IgG (FcγRs), abrogated the enhanced antibody inhibition, whereas adding NK cells to CD4+ lymphocytes restored inhibition. There was no enhanced inhibition on PBMCs when F(ab′)2 was used. Further experiments demonstrated that the release of β-chemokines, most likely through FcγR triggering of NK cells, contributed modestly to the antiviral activity of antibody on PBMCs and that antibody-coated virus adsorbed to uninfected cells provided a target for NK cell-mediated inhibition of HIV-1. These results indicate that Fc-FcγR interactions enhance the ability of antibody to neutralize HIV-1. Since FcγR-bearing cells are always present in vivo, FcγR-mediated antibody function may play a role in the ability of antibody to control lentivirus infection.
Passive infusion studies demonstrate that antibodies can prevent lentivirus infection in animals (2, 15-18, 26, 29, 33, 39, 44). Furthermore, correlative studies with humans and direct data from macaques indicate that antibodies may also play a role in controlling viral burden once infection is established (5, 13, 26).
The ability of antibody to prevent or modulate lentivirus infection has generally been associated with the antibody's capacity to neutralize cell-free virus in vitro (2, 16, 34, 47). Neutralizing activity has been measured by several methods that differ primarily by the inoculum of virus and the type of target cell or signal used to quantitate virus that escapes neutralization. These methods include the use of indicator cell lines that measure one-time infection of cells or the use of cell lines or primary lymphocytes that depend on multiple rounds of infection for an adequate antigen signal (30, 45). Although neutralization assays employing indicator cells and other cell lines have generated critical information about the mechanisms of virus neutralization, the use of primary lymphocytes provides conditions closer to those that occur in vivo.
With respect to primary lymphocytes, phytohemagglutinin (PHA)-stimulated peripheral blood mononuclear cells (PBMCs) have generally been used as target cells in neutralization assays (30, 40). However, unfractionated PBMCs contain natural killer cells and, if adherent cells are not depleted, monocytes as well. Both of these cell types express surface Fc receptors, which can interact with antibody and potentially impact its ability to inhibit virus replication.
The range of inocula that is generally encountered during natural infection is unknown; nor is it clear whether exposure to cell-free or cell-associated virus is primarily responsible for transmission. Based on the concentration of virus in blood and other fluids that might transmit infection—particularly upon exposure to individuals with acute infection—it is likely that protective antibodies will be required to act against large quantities of virus (10, 12, 20, 35, 36). Similarly, if antibodies have a role in controlling established infection, they must be active with high concentrations of virus.
In this study, we tested the hypothesis that the presence of Fc-receptor bearing target cells in neutralization assays would enhance the ability of antibody to inhibit human immunodeficiency virus type 1 (HIV-1). We found that antibody was more effective in inhibiting virus when the target cells were nonadherent PBMCs rather than purified CD4+ lymphocytes and that this enhanced neutralization was due to Fc-Fc receptor interactions. Furthermore, we found that increases in the amount of virus inoculum resulted in substantial reductions in neutralizing activity on CD4+ cells but had much less impact on PBMCs.
(This study was presented in part at AIDS Vaccine 2003, New York, N.Y., September 2003.)
MATERIALS AND METHODS
Virus.
HIV92US657, HIV92US712 and HIV91US005 (primary R5 strains of HIV-1) and HIV92HT599 (a primary X4 strain) were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program. Stocks of virus were made by collecting the supernatant fluid from infected PHA-stimulated primary PBMCs, and aliquots were stored at −80°C until used. Virus titer was determined by inoculating serial dilutions of virus stock on PHA-stimulated PBMCs in 96-well microtiter plates and measuring p24 antigen in supernatant fluid. The value of the 50% tissue culture infective dose (TCID50) was determined by the Kärber method (21).
Cells.
PBMCs were obtained by Ficoll-Hypaque centrifugation of whole blood, collected from healthy donors, in heparin sulfate. PBMCs were generally incubated for 48 h with 3 to 5 μg of PHA/ml. Nonadherent cells were then collected and either used as target cells for virus growth or used to separate CD4+ lymphocytes. CD4+ lymphocytes were purified by positive selection with magnetic beads according to the manufacturer's instructions (StemCell Technologies, Vancouver, B.C., Canada). In some experiments, NK cells were positively selected from PBMCs with anti-CD56 antibody-coated magnetic beads (StemCell Technologies). CD4+ and CD56+ cells were generally >90% pure when measured by flow cytometry. Since the CD4+ cells were separated from nonadherent PBMCs, they are likely to be almost exclusively lymphocytes and contain few monocytes; however, cells were not evaluated for either CD3 or CD14 expression.
Serum and IgG.
Serum samples collected from 10 HIV-infected patients were pooled for virus inhibition assays described below. All HIV-infected subjects had chronic infection. Sera were prescreened for neutralizing activity against HIV92US657 with PBMC target cells. Immunoglobulin G (IgG) was separated from serum samples after binding to protein G columns (Amersham Pharmacia, Piscataway, N.J.), elution from the columns, and pH neutralization according to the manufacturer's instructions. The purity of the IgG was assessed by polyacrylamide gel electrophoresis, and total protein content was determined by the Bradford method with a human IgG standard. Cytogam (MedImmune, Gaithersburg, Md.) was used as a source of HIV antibody-negative control IgG.
For some experiments, F(ab′)2 was made from patient or control IgG samples by pepsin digestion, followed by purification and concentration with Amicon centrifugal concentrators (Millipore, Bedford, Mass.). F(ab′)2 purity was verified by polyacrylamide gel electrophoresis.
Virus inhibition assay.
Various concentrations of virus were incubated with serum, IgG (0.5 mg/ml), or F(ab′)2 for 1 h and added to PHA-stimulated target cells in 96-well microtiter plates. Target cell numbers were 105 CD4+ lymphocytes or 3 × 105 monocyte-depleted PBMCs (which generally contained approximately 105 CD4+ lymphocytes). Four days later, cells were washed to remove the virus inoculum and antibody, and 3 days later (7 days postinfection), supernatant fluid was collected for measurement of p24 by enzyme-linked immunosorbent assay (ELISA) (Zeptometrix, Buffalo, N.Y.). In some experiments, the antibody and virus inoculum were removed 1 h after being added to cells. Postbinding inhibition experiments were performed by incubating IgG with HIV-1 at 37°C for 1 h and then adding the virus-antibody mixture to 105 CD4+ lymphocytes at 4°C to allow adsorption but limit viral fusion and entry. After 2 h at 4°C, unbound virus and antibody were washed off with cold phosphate-buffered saline (PBS), plates were brought to 37°C, and 3 × 105 NK cells from the same donor were added. Three and 7 days later, the concentration of p24 in supernatant fluid was determined. Virus inhibition was calculated by the following formula: percent inhibition = 100[1 − (p24p/p24c)], where p24p and p24c are the concentration of supernatant fluid p24 obtained from patient or control antibody sources, respectively.
Single-round virus inhibition assay.
A flow cytometric virus inhibition assay, modified from the methods of Mascola et al., was used to measure the effect of antibody and target cell type on a single round of infection (28). Briefly, PBMCs were stimulated with PHA (5 μg/ml) for 48 h, plastic-adherent cells were removed, and the remaining cells were stimulated for 72 h with interleukin-2 (20 U/ml). Alternatively, PBMCs were stimulated with both PHA and interleukin-2 for 48 h prior to removal of adherent cells. A portion of the PBMCs was used to select CD4+ lymphocytes as described above. HIV92US657 (at a final multiplicity of infection of 10,000 TCID50/100,000 CD4+ lymphocytes) was incubated with HIV-positive or -negative IgG (0.5 mg/ml) for 1 h, added to CD4+ lymphocytes or PBMCs, and further incubated for 24 h at 37°C. To limit virus infection to a single round, indinavir (National Institutes of Health AIDS Research and Reference Reagent Program) at a final concentration of 1 μM was added to cells just prior to addition of the virus-antibody mixture. Infected cells were collected, fixed with lysolecithin (20 μg/ml in 1% paraformaldehyde-PBS), washed, incubated in cold absolute methanol, and permeabilized in 0.1% NP-40. Cells were stained with a fluorescently labeled anti-p24 monoclonal antibody (KC57-RD1; Beckman-Coulter, Miami, Fla.) or isotype control for 30 min at room temperature, washed three times in PBS, resuspended in 2% paraformaldehyde overnight at 4°C in the dark, and read with a FACSCalibur flow cytometer (Becton-Dickinson Biosciences, San Jose, Calif.). Uninfected cells were stimulated, fixed, permeabilized, stained, and read in parallel. Virus inhibition was calculated by the formula noted above.
β-Chemokine measurements.
Macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and RANTES were measured in supernatant fluid 7 days postinfection by ELISA (BioSource, Camarillo, Calif.) according to the manufacturer's instructions. β-Chemokines were neutralized with goat anti-MIP-1α, anti-MIP-1β, and anti-RANTES IgG (R&D Systems, Minneapolis, Minn.) at final concentrations of 100, 100, and 20 μg/ml, respectively. Normal goat IgG was used as a control. In some experiments, recombinant MIP-1α (National Institutes of Health AIDS Research and Reference Reagent Program), MIP-1β (R&D Systems) and RANTES (R&D Systems) were added in combination to neutralization assays immediately after the addition of virus and HIV-positive or control IgG; the β-chemokines were replenished after the viral inoculum and antibody were washed off (4 days after infection).
RESULTS
Antibody inhibition of virus is dependent on both inoculum and target cell.
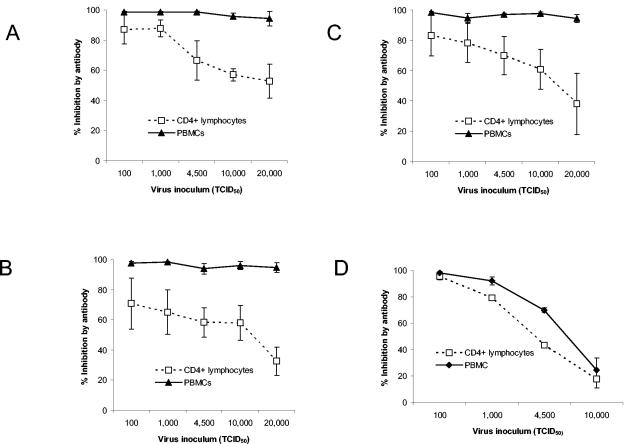
We simultaneously tested the effect of virus inoculum and target cell on the ability of antibody (0.5 mg/ml of IgG) to inhibit virus. At all inocula, inhibition of three primary R5 strains of HIV-1 (HIV92US657, HIV92US712, and HIV91US005) was greater with monocyte-depleted, PHA-stimulated PBMCs used as target cells than with PHA-stimulated CD4+ lymphocytes from the same donor (P < 0.01; Wilcoxon signed rank test) (Fig. 1). With increasing amounts of input virus, the difference in antibody inhibition between the two target cells was accentuated, and differences were statistically significant (P ≤ 0.05; Mann-Whitney U test) for inocula of >100 TCID50 for HIV92US657 and HIV92US712 (Fig. 1A and B) and >1,000 TCID50 for HIV91US005 (Fig. 1C). Enhanced inhibition was also observed with whole serum (data not shown), and the effect was the same with multiple target cell donors (Fig. 1). Enhanced neutralization on PBMCs also occurred with HIV92HT599, a primary X4 virus (P < 0.01; Wilcoxon signed rank test), although the effect was substantially less than that observed with the R5 strains, and statistical significance was not reached at any of the individual inocula (Fig. 1D).
FIG. 1.
Virus inhibition by antibody is dependent on virus inoculum and target cell. HIV antibody-positive or -negative polyclonal IgG (0.5 mg/ml) was incubated with different inocula of the R5 primary strains HIV92US657 (A), HIV92US712 (B), or HIV91US005 (C), or the X4 primary strain HIV92HT599 (D) for 1 h and added to either PHA-stimulated CD4+ lymphocytes or to PHA-stimulated PBMCs (from the same donor). Cells were washed 4 days later, and p24 was measured in supernatant fluid 3 days later (7 days after infection). The percentage of inhibition was calculated as described in Materials and Methods. Data represent the mean ± standard error (SE) of two to four independent experiments for each virus, performed with different target cell donors.
NK cells are responsible for the enhanced inhibition on PBMCs.
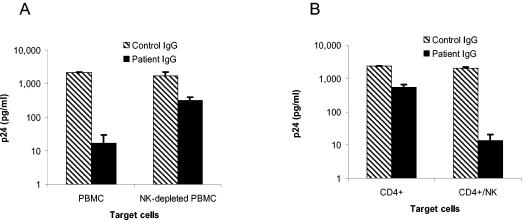
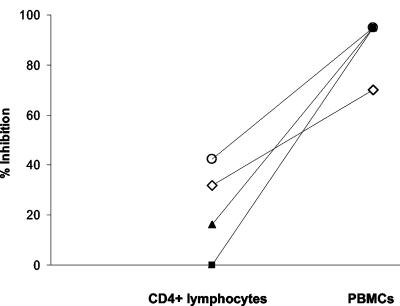
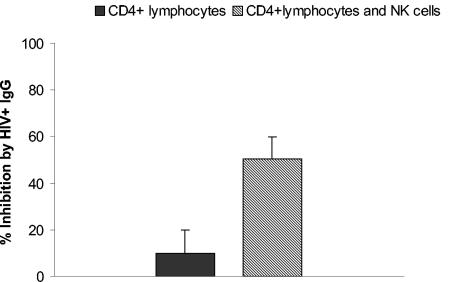
Nonadherent PBMCs contain NK cells, which express receptors for the Fc portion of IgG (FcγR) and could therefore interact with antibody to modulate antiviral activity. We determined the role of NK cells in inhibiting an inoculum of 4,500 TCID50 both by depleting NK cells from PBMCs with anti-CD56-coated magnetic beads and by repleting CD4+ lymphocytes with positively selected NK cells. Depleting NK cells completely abrogated the enhanced antiviral effect observed with PBMCs, bringing the level of inhibition down to that of CD4+ lymphocytes (Fig. 2A). As expected, adding NK cells to CD4+ lymphocytes from the same donor brought the level of inhibition back to that observed with PBMCs (Fig. 2B). Thus, NK cells are necessary for the enhanced inhibition of virus on PBMCs.
FIG. 2.
Enhanced virus inhibition by antibody on PBMCs is due to NK cells. Virus inhibition was measured using HIV92US657 at an inoculum of 4,500 TCID50 as described in Materials and Methods. Target cells for virus growth consisted of PHA-stimulated PBMCs or PBMCs from the same donor depleted of NK cells (A) and PHA-stimulated CD4+ lymphocytes alone or CD4+ lymphocytes to which NK cells from the same donor (ratio of NK to CD4+ cells = 1:3) were added (B). Data represent the mean plus SE from an experiment performed with two different donors and are representative of two independent experiments.
Fc is required for enhanced inhibition on PBMCs.
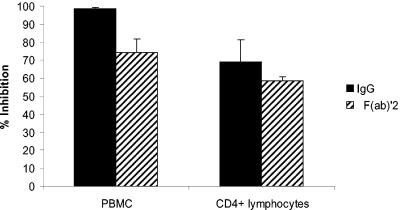
The role played by NK cells suggested that Fc-FcγR interactions might underlie enhanced virus inhibition. We therefore compared the antiviral activity of whole IgG with its F(ab′)2 on CD4+ lymphocytes and PBMCs. With an inoculum of 4,500 TCID50, there was a decrease in virus inhibition on PBMCs by F(ab′)2 compared with a similar molar concentration of whole IgG: mean inhibition was 74% by F(ab′)2 versus 99% by IgG (P = 0.05) (Fig. 3). On the other hand, there was little difference between F(ab′)2 and whole IgG when CD4+ lymphocytes were used as target cells: mean inhibition was 59 versus 69% for F(ab′)2 and IgG, respectively (P = 0.56) (Fig. 3). Furthermore, F(ab′)2 resulted in similar inhibition on both CD4+ lymphocytes (59%) and PBMCs (74%) (P = 0.13). Note that the similar amount of inhibition by F(ab′)2 and IgG on CD4+ lymphocytes indicates that F(ab′)2 binds to HIV antigens similarly to whole antibody; antigen-binding differences are therefore unlikely to account for the increased inhibition by whole IgG. We did not attempt to block FcγRs with anti-FcγR antibodies. Our findings indicate that enhanced virus inhibition by antibody on PBMCs requires an interaction between the Fc segment of IgG and FcγRs on NK cells.
FIG. 3.
Whole IgG, but not F(ab′)2, enhances virus inhibition on PBMC target cells. HIV92US657 (4,500 TCID50) was incubated with HIV antibody-positive or -negative polyclonal IgG (0.5 mg/ml) or similar molar concentrations of F(ab′)2 and added to PHA-stimulated PBMCs or CD4+ lymphocytes. Cells were washed 4 days later, and p24 was measured in supernatant fluid 3 days later (7 days after infection). The percentage of inhibition was calculated as described in Materials and Methods. Data represent mean plus SE of three separate experiments. There was significantly less virus inhibition on PBMCs by the F(ab′)2 than by whole IgG (P = 0.05).
Enhanced antibody inhibition on PBMCs occurs during the initial exposure of virus to antibody.
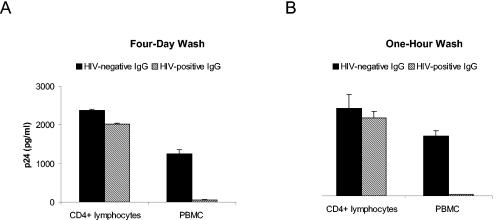
Since we initially chose to leave antibody on the target cells for 4 days prior to washing the cells, it was possible that the difference in inhibition between CD4+ cells and PBMCs was due to the effect of antibody on multiple rounds of infection and that infected cells were targets for antibody-dependent cellular cytotoxicity (AOCC) (13). To test this possibility, we incubated antibody with 4,500 TCID50 of virus for 1 h prior to adding the mixture to CD4+ or PBMC target cells and washed off the inoculum and antibody 1 h later. With the same donors, parallel experiments were performed, keeping the virus and antibody on the cells for 4 days. Very similar levels of inhibition were seen with either the 1-h or the 4-day washoff procedures for an R5 strain (Fig. 4) and the X4 strain (data not shown). Thus, the Fc-mediated enhancement of inhibition occurs after a very short exposure of antibody to virus and does not require antibody to interact with cells infected with virus that has escaped initial neutralization.
FIG. 4.
Enhanced antibody inhibition on PBMCs occurs during the initial exposure of virus to antibody. HIV antibody-positive or-negative polyclonal IgG (0.5 mg/ml) was incubated with 4,500 TCID50 of HIV92US657 for 1 h and added to either PHA-stimulated CD4+ lymphocytes or to PHA-stimulated PBMCs (from the same donor). Cells were then washed either 4 days (A) or 1 h (B) later, and p24 in supernatant fluid was measured 7 days after infection. Data represent the mean plus SE from an experiment performed with two different donors and are representative of two independent experiments.
To confirm that enhanced neutralization on PBMCs resulted from inhibition of an early event in HIV infection, a single-round assay, modified from Mascola et al., was used (28). This assay employs flow cytometry to measure the percentage of cells expressing p24 after a single round of infection. With CD4+ lymphocytes from four different donors, HIV-positive IgG inhibited an R5 virus (HIV92US657) by a mean of 23%. On the other hand, with PBMCs, there was complete inhibition of infected cells by HIV-positive IgG after one round of infection with three of the four donors and a 70% reduction with the fourth donor (Fig. 5).
FIG. 5.
Antibody neutralization of an R5 strain of HIV is enhanced on PBMCs in a single-round infection assay. The percentage of cells infected with HIV92US657 was determined by flow cytometry in a single-round infection assay as described in Materials and Methods. Using a forward versus side-scatter plot, an initial gate was placed around a cell population containing infected cells, as determined by comparing FL2 fluorescence of p24(KC57-RD1)-stained infected and uninfected cells. The percentage of infected cells within that gate was determined for all experimental conditions, and the percentage of inhibition was calculated as described in Materials and Methods. Data were obtained with four separate donors assayed in two independent experiments, and the diagonal lines connect data from each donor. Using PBMCs from three of the four donors, HIV-positive IgG resulted in complete inhibition of infected cells; for the figure, a value of 95% inhibition was arbitrarily assigned to these three donors.
Fc-FcγR interactions that enhance virus inhibition could occur as a result of the binding of antibody-coated virus to uninfected cells in a configuration that would allow Fc to bind to FcγRs on NK cells. To explore this possible mechanism, HIV-positive (or negative control) IgG was incubated with an R5 strain of HIV-1 at 37°C; 1 h later, the virus-antibody mixture was added to CD4+ lymphocytes at 4°C to allow adsorption but limit viral fusion and entry. After 2 h at 4°C, unbound virus and antibody were washed off, plates were brought to 37°C, and NK cells from the same donor were added. Under these conditions, HIV-positive IgG resulted in little or no inhibition of virus with CD4+ lymphocytes alone; however, when NK cells were added, HIV-positive IgG, but not HIV-negative IgG, resulted consistently in about 40% inhibition of virus (Fig. 6). Thus, antibody-coated virus adsorbed to uninfected cells provides a target for NK cell-mediated inhibition of HIV-1.
FIG. 6.
Antibody-coated virus adsorbed to uninfected cells provides a target for NK cell-mediated inhibition of HIV-1. HIV antibody- positive or -negative IgG was incubated with 10,000 TCID50 of HIV92US657 at 37°C; 1 h later, the virus-antibody mixture was added to CD4+ lymphocytes at 4°C. After 2 h at 4°C, unbound virus and antibody were washed off, plates were brought to 37°C, and NK cells from the same donor (NK to CD4 ratio = 3:1) were added. Three days later, p24 in supernatant fluid was measured, and inhibition was calculated as described in Materials and Methods. There was significant augmentation of virus inhibition by antibody when NK cells were added to CD4+ lymphocytes with adsorbed virus (P = 0.05). Data represent the mean plus SE of three independent experiments. Although not statistically significant, similar results were obtained 7 days postinfection with a virus inoculum of 10,000 TCID50 and 3 and 7 days postinfection with an inoculum of 4,500 TCID50.
β-Chemokines are produced by PBMCs in the presence of HIV-1 and specific antibody.
We have previously shown that antibody inhibition of HIV-1-infected cells may in part be due to FcγR-mediated release of β-chemokines from NK cells (13). To determine if β-chemokines played a role in the enhanced inhibition of HIV-1 on PBMC target cells, supernatant fluid obtained from CD4+ lymphocytes or PBMC cultures 7 days after infection and addition of IgG was assayed for MIP-1α, MIP-1β, and RANTES. In the presence of 4,500 TCID50 of an R5 strain of HIV-1 and IgG from either HIV-infected subjects or uninfected controls, there was little production of β-chemokines by CD4+ lymphocytes (Table 1). However, when PBMCs were used as target cells, β-chemokine levels were augmented by HIV-specific IgG compared with IgG from uninfected subjects. This augmentation was observed for MIP-1α, MIP-1β, and RANTES (Table 1). Similarly, when antibody-coated virus was adsorbed on uninfected cells at 4°C, β-chemokine production was augmented by HIV-positive IgG in the presence of NK cells (data not shown). Thus, under conditions where enhanced inhibition of virus by antibody is observed, β-chemokine production is augmented.
TABLE 1.
β-Chemokines are produced by PBMCs in the presence of HIV-specific IgGa
| Protein | PBMCs
|
CD4+ lymphocytes
|
||
|---|---|---|---|---|
| HIV-positive IgG | HIV-negative IgG | HIV-positive IgG | HIV-negative IgG | |
| MIP-1α | 908 ± 184 | 280 ± 58 | 233 ± 30 | 226 ± 36 |
| MIP-1β | 3,901 ± 321 | 1,408 ± 589 | 1,012 ± 224 | 402 ± 239 |
| RANTES | 4,485 ± 1,147 | 2,041 ± 320 | 806 ± 286 | 1,171 ± 205 |
HIV92US657 (4,500 TCID50) was incubated with HIV antibody-positive or -negative polyclonal IgG (0.5 mg/ml) and added to PHA-stimulated PBMCs or CD4+ lymphocytes; virus and IgG were washed off 4 days later. β-Chemokines were measured in supernatant fluid by ELISA 7 days postinfection; data are in picograms per milliliter and represent means ± SE of six experiments. Results with HIV-positive IgG on PBMCs (boldface type) are significantly higher (P ≤ 0.05) than for other conditions.
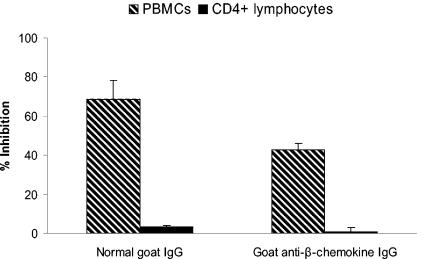
Further evidence of the influence of β-chemokines on enhanced neutralization was obtained by adding goat anti-MIP-1α, anti-MIP-1β, and anti-RANTES antibodies or normal goat IgG to CD4+ or PBMC target cells immediately prior to infection with an R5 strain and treatment with anti-HIV or control IgG. All three anti-β-chemokine antibodies were used in combination at final concentrations of 100, 100, and 20 μg/ml, respectively, and fresh antibodies were added after the 4-day washout (which removed the virus inoculum and the treatment IgG). The combination of anti-β-chemokine antibodies partially reduced the enhanced inhibition of virus on PBMCs but had no effect with CD4+ lymphocytes; however, only about 40% of the enhanced neutralization on PBMCs could be eliminated by the anti-β-chemokine antibodies (Fig. 7).
FIG. 7.
β-Chemokine neutralization partially reduces the enhanced inhibition of HIV-1 on PBMCs. Goat anti-MIP-1α, anti-MIP-1β, and anti-RANTES antibodies or normal goat IgG was added to CD4+ lymphocytes or PBMC target cells immediately prior to infection with 4,500 TCID50 of HIV92US657 and treatment with anti-HIV or control IgG. All three anti-β-chemokine antibodies were used in combination at final concentrations of 100 μg/ml (anti-MIP-1α and anti-MIP-1β) and 20 μg/ml (RANTES); fresh goat antibodies were added after the 4-day washout (which removed the virus inoculum and the treatment IgG). p24 in supernatant fluid was measured 3 days later (7 days after infection), and the percentage of inhibition was calculated as described in Materials and Methods. Data represent the mean plus SD from two separate experiments, each run in triplicate.
To establish directly that β-chemokines could enhance virus neutralization, recombinant MIP-1α, MIP-1β, and RANTES were added in combination to CD4+ lymphocytes or PBMCs immediately after an R5 strain of HIV and HIV-positive (or negative) IgG were added. The β-chemokines were added at concentrations about twice that observed on average after 7 days' incubation of virus and IgG on PBMCs (Table 1). Contrary to our expected result, virus neutralization was not increased on CD4+ lymphocytes in the presence of β-chemokines. On the other hand, there was a slight enhancement of neutralization on PBMCs in the presence of the β-chemokines, from 85.1% inhibition without β-chemokines to 91.5% inhibition with β-chemokines (P = 0.068; Wilcoxon signed rank test). Thus, with the experimental conditions used, the β-chemokines did not seem to augment antibody neutralization by blocking or down-regulating coreceptors but more likely affected NK or other cells in the PBMCs.
Finally, the role of β-chemokines in enhanced neutralization was explored by using HIV92HT599, an X4 strain of HIV. As noted above, neutralization of HIV92HT599 was enhanced on PBMC target cells, but the degree of enhancement was less than that observed with any of the R5 strains of virus (Fig. 1).
Taken together, our data indicate that β-chemokines are responsible for some, but not most, of the FcγR-mediated enhancement of virus neutralization.
DISCUSSION
We determined the impact of virus inoculum and target cells on polyclonal antibody neutralization of HIV-1. Our findings indicate that virus inhibition is enhanced when monocyte-depleted PBMCs, rather than CD4+ lymphocytes, are used as target cells. Although enhanced inhibition is greater with larger quantities of virus, enhancement is nonetheless apparent at quantities as low as 100 TCID50. Furthermore, virus inhibition is more effective on PBMC target cells due to the presence in PBMCs of FcγR-bearing NK cells. The critical nature of the interaction between the Fc segment of antibody and FcγRs on the NK cells is strongly suggested by the inability of F(ab′)2 to enhance virus neutralization on PBMCs. Finally, part (but not most) of the enhanced virus inhibition is due to β-chemokine production triggered by antibody or antibody complexes in the presence of PBMCs.
Antibody is thought to neutralize HIV-1 by directly blocking virus from binding to cellular receptors or by interfering with postbinding events (3, 7, 40, 42). In this study, we found that antibodies can also neutralize HIV-1 indirectly through FcγR triggering of β-chemokines. However, the rather small effect that anti-β-chemokine antibodies had in reducing the enhanced virus inhibition on PBMC target cells and the enhanced neutralization observed with an X4 strain make it likely that additional antiviral effector mechanisms result from FcγR triggering. In this regard, we have not yet explored the possibility that other soluble antiviral substances might be involved, including stromal-derived factor 1 on the X4 strain. Furthermore, we were unable to consistently demonstrate target cell cytotoxicity, due to FcγR triggering (data not shown) (6). Although high concentrations of goat anti-β-chemokine antibodies were used in these experiments and similar effects on enhanced neutralization were seen with monoclonal anti-β-chemokine antibodies at concentrations of 50 μg/ml each (data not shown), it is possible that β-chemokine neutralization was incomplete. We also unexpectedly found that adding recombinant β-chemokines to neutralizing assays enhanced virus inhibition on PBMCs (although only slightly) but not on CD4+ lymphocytes. We interpret this finding as indicating that the enhancing effect of β-chemokines in the presence of antibody and NK cells may not be due to blocking or down-modulating CCR5 on CD4+ lymphocytes but may be due to chemotaxis or activating effects on NK or other cells in the PBMCs (19, 24, 25, 41, 46). Further experiments will be required to confirm this.
Antibodies may trigger FcγRs on NK cells by forming antibody-antigen complexes or by binding to target cells expressing foreign antigens (37, 38). In most experiments, antibody was left on infected cells for 4 days prior to washing, which could allow antibody to bind to HIV glycoproteins on cell surfaces produced by virus that initially escaped neutralization. However, similar enhancement of virus neutralization on PBMCs was observed when antibody was washed off cells only 1 h after the addition of antibody and virus, making it unlikely that infected target cells were required for FcγR triggering. Furthermore, enhanced neutralization on PBMCs was observed in a single-round infection assay. Thus, mechanisms of enhancement acting prior to integration and viral gene expression must be involved. One such mechanism was directly tested by adsorbing antibody-coated virus to CD4+ lymphocytes at 4°C, thereby blocking fusion and infection. Our results indicate that antigen-antibody complexes bound to uninfected cells trigger an antiviral response from NK cells. Antibody bound to adsorbed virus by its Fab could interact through its Fc segment with NK cells, with the resultant release of β-chemokines or other soluble antiviral substances (13, 32). In this scenario, the attached virus might still be capable of infecting the cell, if the antibody doesn't interfere with events that occur after coreceptor binding or if cytotoxicity does not occur; however, adjacent cells would be protected due to the presence of soluble antiviral factors. In any event, these results provide one explanation for how antibodies inhibit postbinding steps in virus replication when FcγR-bearing cells are present (40).
Enhanced inhibition on PBMCs was more prominent with larger quantities of virus, whereas with smaller amounts, virus was well inhibited on both PBMCs and CD4+ lymphocytes. Thus, mechanisms of neutralization that are independent of Fc-FcγR interactions, such as direct interference with attachment, are sufficient at inhibiting small amounts of HIV-1. The requirement of Fc-FcγR interactions for enhanced inhibition of smaller quantities of virus was confirmed by the lack of enhancement on PBMCs when F(ab′)2 rather than IgG was used. The F(ab′)2 did partially inhibit virus on both PBMCs and CD4+ lymphocytes, and the degree of inhibition by F(ab′)2 was approximately equivalent to that of whole IgG on CD4+ lymphocytes. Thus, inhibition of virus by IgG on CD4+ lymphocytes reflects Fc-independent mechanisms of virus neutralization.
It is not known exactly how antibody functions in vivo to inhibit HIV-1 or other lentiviruses. Assays measuring neutralization of cell-free lentivirus have generally correlated with preventing infection (27, 34). However, antibodies that do not neutralize virus in vitro are also capable of preventing lentivirus infection in monkeys (43). Our study indicates that in vitro neutralizing assays that limit the possibility of Fc-FcγR interactions could underestimate the degree to which an antibody might be effective in vivo, where FcγR-bearing cells would be available to interact with antibody. It is thus possible that Fc-FcγR interactions on cell-free virus might contribute, along with AOCC, to the ability of nonneutralizing antibodies to prevent lentivirus infection in animal models.
Several studies have correlated functional antibody activities with markers of HIV disease progression (4, 8, 9, 13). Our present findings show that large amounts of virus (up to 20,000 TCID50) can be inhibited in vitro by antibody, but predominantly through an FcγR-mediated mechanism. Since antibodies capable of modulating established infections must do so in the presence of large quantities of virus, it is reasonable to postulate that the FcγR-mediated antibody function we describe plays a role in the ability of antibody to control infection.
To our knowledge, this study is the first to show that nontarget cells present in PBMCs, namely NK cells, have a marked effect on the ability of polyclonal antibody to inhibit HIV-1. Furthermore, since NK cells alone accounted for all of the enhanced neutralization of PBMCs, it is unlikely that CD8+ T-lymphocytes played any role; this is to be expected, given that CD8+ T-lymphocytes (with the exception of those bearing the γδ T-cell receptor) generally do not express FcγRs and would be unlikely to interact with antibody (1, 22).
If the ultimate ability of antibody to inhibit HIV-1 in vivo depends in part on Fc-FcγR interactions, the functional status of the FcγR-bearing cell will be critically important to consider. Thus, although antibodies with appropriate binding and functional characteristics must be present to prevent or modulate infection, NK cells or macrophages must also be capable of interacting with the antibody and of producing an antiviral effect. In this regard, binding of Fc to FcγRs is influenced by polymorphisms in the FcγRIIa and FcγRIIIa genes (11). Furthermore, based on AOCC assays, there is considerable variability in NK cell function among immunocompetent individuals, and effector cell function is reduced as HIV infection progresses (14, 23, 31). Thus, both immunization and antibody therapy may benefit from approaches that maximize the effector functions of FcγR-bearing cells.
Acknowledgments
This work was funded by Public Health Service grant R01 AI52039 from the National Institutes of Health.
This research complies with University of California, Irvine, human subjects' research policies.
REFERENCES
- 1.Alamo, A. L., and S. J. Melnick. 2000. Clinical application of four and five-color flow cytometry lymphocyte subset immunophenotyping. Cytometry 42:363-370. [DOI] [PubMed] [Google Scholar]
- 2.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. [DOI] [PubMed] [Google Scholar]
- 3.Barbato, G., E. Bianchi, P. Ingallinella, W. H. Hurni, M. D. Miller, G. Ciliberto, R. Cortese, R. Bazzo, J. W. Shiver, and A. Pessi. 2003. Structural analysis of the epitope of the anti-HIV antibody 2F5 sheds light into its mechanism of neutralization and HIV fusion. J. Mol. Biol. 330:1101-1115. [DOI] [PubMed] [Google Scholar]
- 4.Baum, L. L., K. J. Cassutt, K. Knigge, R. Khattri, J. Margolick, C. Rinaldo, C. A. Kleeberger, P. Nishanian, D. R. Henrard, and J. Phair. 1996. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J. Immunol. 157:2168-2173. [PubMed] [Google Scholar]
- 5.Binley, J. M., B. Clas, A. Gettie, M. Vesanen, D. C. Montefiori, L. Sawyer, J. Booth, M. Lewis, P. A. Marx, S. Bonhoeffer, and J. P. Moore. 2000. Passive infusion of immune serum into simian immunodeficiency virus-infected rhesus macaques undergoing a rapid disease course has minimal effect on plasma viremia. Virology 270:237-249. [DOI] [PubMed] [Google Scholar]
- 6.Bleul, C. C., M. Farzan, H. Choe, C. Parolin, I. Clark-Lewis, J. Sodroski, and T. A. Springer. 1996. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 382:829-833. [DOI] [PubMed] [Google Scholar]
- 7.Burrer, R., S. Haessig-Einius, A. M. Aubertin, and C. Moog. 2003. Polyclonal immunoglobulin G from patients neutralizes human immunodeficiency virus type 1 primary isolates by binding free virions, but without interfering with an initial CD4-independent attachment of the virus to primary blood mononuclear cells. J. Virol. 77:11385-11397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carotenuto, P., D. Looij, L. Keldermans, F. de Wolf, and J. Goudsmit. 1998. Neutralizing antibodies are positively associated with CD4+ T-cell counts and T-cell function in long-term AIDS-free infection. AIDS 12:1591-1600. [DOI] [PubMed] [Google Scholar]
- 9.Cecilia, D., C. Kleeberger, A. Munoz, J. V. Giorgi, and S. Zolla-Pazner. 1999. A longitudinal study of neutralizing antibodies and disease progression in HIV-1-infected subjects. J. Infect. Dis. 179:1365-1374. [DOI] [PubMed] [Google Scholar]
- 10.Chakraborty, H., P. K. Sen, R. W. Helms, P. L. Vernazza, S. A. Fiscus, J. J. Eron, B. K. Patterson, R. W. Coombs, J. N. Krieger, and M. S. Cohen. 2001. Viral burden in genital secretions determines male-to-female sexual transmission of HIV-1: a probabilistic empiric model. AIDS 15:621-627. [DOI] [PubMed] [Google Scholar]
- 11.Dijstelbloem, H. M., J. G. van de Winkel, and C. G. Kallenberg. 2001. Inflammation in autoimmunity: receptors for IgG revisited. Trends Immunol. 22:510-516. [DOI] [PubMed] [Google Scholar]
- 12.Fiore, J. R., B. Suligoi, A. Saracino, M. Di Stefano, R. Bugarini, A. Lepera, A. Favia, L. Monno, G. Angarano, and G. Pastore. 2003. Correlates of HIV-1 shedding in cervicovaginal secretions and effects of antiretroviral therapies. AIDS 17:2169-2176. [DOI] [PubMed] [Google Scholar]
- 13.Forthal, D. N., G. Landucci, and E. S. Daar. 2001. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J. Virol. 75:6953-6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forthal, D. N., G. Landucci, and B. Keenan. 2001. Relationship between antibody-dependent cellular cytotoxicity, plasma HIV type 1 RNA, and CD4+ lymphocyte count. AIDS Res. Hum. Retrovir. 17:553-561. [DOI] [PubMed] [Google Scholar]
- 15.Gauduin, M. C., G. P. Allaway, W. C. Olson, R. Weir, P. J. Maddon, and R. A. Koup. 1998. CD4-immunoglobulin G2 protects Hu-PBL-SCID mice against challenge by primary human immunodeficiency virus type 1 isolates. J. Virol. 72:3475-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gauduin, M. C., P. W. Parren, R. Weir, C. F. Barbas, D. R. Burton, and R. A. Koup. 1997. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat. Med. 3:1389-1393. [DOI] [PubMed] [Google Scholar]
- 17.Gauduin, M. C., J. T. Safrit, R. Weir, M. S. Fung, and R. A. Koup. 1995. Pre- and postexposure protection against human immunodeficiency virus type 1 infection mediated by a monoclonal antibody. J. Infect. Dis. 171:1203-1209. [DOI] [PubMed] [Google Scholar]
- 18.Hofmann-Lehmann, R., J. Vlasak, R. A. Rasmussen, B. A. Smith, T. W. Baba, V. Liska, F. Ferrantelli, D. C. Montefiori, H. M. McClure, D. C. Anderson, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, H. Katinger, G. Stiegler, L. A. Cavacini, M. R. Posner, T. C. Chou, J. Andersen, and R. M. Ruprecht. 2001. Postnatal passive immunization of neonatal macaques with a triple combination of human monoclonal antibodies against oral simian-human immunodeficiency virus challenge. J. Virol. 75:7470-7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inngjerdingen, M., B. Damaj, and A. A. Maghazachi. 2001. Expression and regulation of chemokine receptors in human natural killer cells. Blood 97:367-375. [DOI] [PubMed] [Google Scholar]
- 20.Kalichman, S. C., M. Cage, T. Barnett, P. Tharnish, D. Rompa, J. Austin, W. Luke, J. O'Mowrey, and R. F. Schinazi. 2001. Human immunodeficiency virus in semen and plasma: investigation of sexual transmission risk behavioral correlates. AIDS Res. Hum. Retrovir. 17:1695-1703. [DOI] [PubMed] [Google Scholar]
- 21.Kärber, G. 1931. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Arch. Exp. Pathol. Pharmakol. 162:480-483. [Google Scholar]
- 22.Lafont, V., J. Liautard, J. P. Liautard, and J. Favero. 2001. Production of TNF-α by human Vγ9Vδ2 T cells via engagement of FcγRIIIA, the low affinity type 3 receptor for the Fc portion of IgG, expressed upon TCR activation by nonpeptidic antigen. J. Immunol. 166:7190-7199. [DOI] [PubMed] [Google Scholar]
- 23.Liljefors, M., B. Nilsson, A. L. Hjelm Skog, P. Ragnhammar, H. Mellstedt, and J. E. Frodin. 2003. Natural killer (NK) cell function is a strong prognostic factor in colorectal carcinoma patients treated with the monoclonal antibody 17-1A. Int. J. Cancer 105:717-723. [DOI] [PubMed] [Google Scholar]
- 24.Loetscher, P., M. Seitz, I. Clark-Lewis, M. Baggiolini, and B. Moser. 1996. Activation of NK cells by CC chemokines. Chemotaxis, Ca2+ mobilization, and enzyme release. J. Immunol. 156:322-327. [PubMed] [Google Scholar]
- 25.Maghazachi, A. A., A. al-Aoukaty, and T. J. Schall. 1994. C-C chemokines induce the chemotaxis of NK and IL-2-activated NK cells. Role for G proteins. J. Immunol. 153:4969-4977. [PubMed] [Google Scholar]
- 26.Mascola, J. R., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mascola, J. R., M. G. Lewis, T. C. VanCott, G. Stiegler, H. Katinger, M. Seaman, K. Beaudry, D. H. Barouch, B. Korioth-Schmitz, G. Krivulka, A. Sambor, B. Welcher, D. C. Douek, D. C. Montefiori, J. W. Shiver, P. Poignard, D. R. Burton, and N. L. Letvin. 2003. Cellular immunity elicited by human immunodeficiency virus type 1/simian immunodeficiency virus DNA vaccination does not augment the sterile protection afforded by passive infusion of neutralizing antibodies. J. Virol. 77:10348-10356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mascola, J. R., M. K. Louder, C. Winter, R. Prabhakara, S. C. De Rosa, D. C. Douek, B. J. Hill, D. Gabuzda, and M. Roederer. 2002. Hum. immunodeficiency virus type 1 neutralization measured by flow cytometric quantitation of single-round infection of primary human T cells. J. Virol. 76:4810-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207-210. [DOI] [PubMed] [Google Scholar]
- 30.Montefiori, D. C., R. G. Collman, T. R. Fouts, J. Y. Zhou, M. Bilska, J. A. Hoxie, J. P. Moore, and D. P. Bolognesi. 1998. Evidence that antibody-mediated neutralization of human immunodeficiency virus type 1 by sera from infected individuals is independent of coreceptor usage. J. Virol. 72:1886-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murayama, T., Q. Cai, and C. R. Rinaldo, Jr. 1990. Antibody-dependent cellular cytotoxicity mediated by CD16+ lymphocytes from HIV-seropositive homosexual men. Clin. Immunol. Immunopathol. 55:297-304. [DOI] [PubMed] [Google Scholar]
- 32.Oliva, A., A. L. Kinter, M. Vaccarezza, A. Rubbert, A. Catanzaro, S. Moir, J. Monaco, L. Ehler, S. Mizell, R. Jackson, Y. Li, J. W. Romano, and A. S. Fauci. 1998. Natural killer cells from human immunodeficiency virus (HIV)-infected individuals are an important source of CC-chemokines and suppress HIV-1 entry and replication in vitro. J. Clin. Investig. 102:223-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parren, P. W., H. J. Ditzel, R. J. Gulizia, J. M. Binley, C. F. Barbas III, D. R. Burton, and D. E. Mosier. 1995. Protection against HIV-1 infection in hu-PBL-SCID mice by passive immunization with a neutralizing human monoclonal antibody against the gp120 CD4-binding site. AIDS 9:F1-F6. [DOI] [PubMed] [Google Scholar]
- 34.Parren, P. W., P. A. Marx, A. J. Hessell, A. Luckay, J. Harouse, C. Cheng-Mayer, J. P. Moore, and D. R. Burton. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 75:8340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piatak, M., Jr., M. S. Saag, L. C. Yang, S. J. Clark, J. C. Kappes, K. C. Luk, B. H. Hahn, G. M. Shaw, and J. D. Lifson. 1993. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science 259:1749-1754. [DOI] [PubMed] [Google Scholar]
- 36.Quinn, T. C., M. J. Wawer, N. Sewankambo, D. Serwadda, C. Li, F. Wabwire-Mangen, M. O. Meehan, T. Lutalo, R. H. Gray, et al. 2000. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N. Engl. J. Med. 342:921-929. [DOI] [PubMed] [Google Scholar]
- 37.Ravetch, J. V., and S. Bolland. 2001. IgG Fc receptors. Annu. Rev. Immunol. 19:275-290. [DOI] [PubMed] [Google Scholar]
- 38.Rönnelid, J., A. Tejde, L. Mathsson, K. Nilsson-Ekdahl, and B. Nilsson. 2003. Immune complexes from SLE sera induce IL10 production from normal peripheral blood mononuclear cells by an FcγRII dependent mechanism: implications for a possible vicious cycle maintaining B cell hyperactivity in SLE. Ann. Rheum. Dis. 62:37-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shibata, R., T. Igarashi, N. Haigwood, A. Buckler-White, R. Ogert, W. Ross, R. Willey, M. W. Cho, and M. A. Martin. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat. Med. 5:204-210. [DOI] [PubMed] [Google Scholar]
- 40.Spenlehauer, C., A. Kirn, A. M. Aubertin, and C. Moog. 2001. Antibody-mediated neutralization of primary human immunodeficiency virus type 1 isolates: investigation of the mechanism of inhibition. J. Virol. 75:2235-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taub, D. D., T. J. Sayers, C. R. Carter, and J. R. Ortaldo. 1995. Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J. Immunol. 155:3877-3888. [PubMed] [Google Scholar]
- 42.Ugolini, S., I. Mondor, P. W. Parren, D. R. Burton, S. A. Tilley, P. J. Klasse, and Q. J. Sattentau. 1997. Inhibition of virus attachment to CD4+ target cells is a major mechanism of T cell line-adapted HIV-1 neutralization. J. Exp. Med. 186:1287-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Rompay, K. K., C. J. Berardi, S. Dillard-Telm, R. P. Tarara, D. R. Canfield, C. R. Valverde, D. C. Montefiori, K. S. Cole, R. C. Montelaro, C. J. Miller, and M. L. Marthas. 1998. Passive immunization of newborn rhesus macaques prevents oral simian immunodeficiency virus infection. J. Infect. Dis. 177:1247-1259. [DOI] [PubMed] [Google Scholar]
- 44.Wang, C. Y., L. S. Sawyer, K. K. Murthy, X. Fang, A. M. Walfield, J. Ye, J. J. Wang, P. D. Chen, M. L. Li, M. T. Salas, M. Shen, M. C. Gauduin, R. W. Boyle, R. A. Koup, D. C. Montefiori, J. R. Mascola, W. C. Koff, and C. V. Hanson. 1999. Postexposure immunoprophylaxis of primary isolates by an antibody to HIV receptor complex. Proc. Natl. Acad. Sci. USA 96:10367-10372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307-312. [DOI] [PubMed] [Google Scholar]
- 46.Xiang, J., S. L. George, S. Wunschmann, Q. Chang, D. Klinzman, and J. T. Stapleton. 2004. Inhibition of HIV-1 replication by GB virus C infection through increases in RANTES, MIP-1α, MIP-1β, and SDF-1. Lancet 363:2040-2046. [DOI] [PubMed] [Google Scholar]
- 47.Zhou, J. Y., and D. C. Montefiori. 1997. Antibody-mediated neutralization of primary isolates of human immunodeficiency virus type 1 in peripheral blood mononuclear cells is not affected by the initial activation state of the cells. J. Virol. 71:2512-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]