Abstract
Objective
Although previous studies have reported the prognostic factors for functional remission, no reports have cited the predictive factors. Our aim was to study the predictive factors for functional remission, which is a treatment goal in rheumatoid arthritis (RA), after receiving biological disease-modifying antirheumatic drugs (bDMARDs) treatment for six months.
Methods
The study consisted of 333 RA patients treated with bDMARDs for six months. The following patient characteristics were investigated: age, gender, disease duration, type of bDMARDs, baseline steroid and methotrexate dosage, and levels of serum rheumatoid factor, matrix metalloprotease, anti-cyclic citrullinated peptides antibody, tumor necrosis factor-α, and interleukin-6. In our evaluation, we used the Simplified Disease Activity Index (SDAI) for RA disease activity, health assessment questionnaire disability index (HAQ-DI) for activity of daily living, Short Form (SF)-36 for quality of life, and Hamilton Depression Rating Scale (HAM-D) or Self-rating Depression Scale (SDS) to determine the patients' depression status. The subjects were divided into two groups: patients with HAQ-DI≤0.5 and HAQ-DI>0.5 at 6 months.
Results
A univariate analysis comparing a group of RA patients without functional remission (n=68) showed that the patients with functional remission (n=164) had the following in common compared with those without remission: younger age, shorter disease duration, lower baseline steroid dosage, lower SDAI, lower HAQ-DI, higher SF-36, and lower HAM-D. Only lower HAQ-DI scores and “mental health” score on the SF-36 were detected using a logistic regression analysis.
Conclusion
These findings suggested that RA patients with lower HAQ-DI and lower depression scores at baseline were more likely to achieve functional remission using bDMARDs treatment than those without these variables.
Keywords: rheumatoid arthritis, health assessment questionnaire disability index (HAQ-DI), quality of life (QOL), functional remission
Introduction
Recommendations for the treatment of rheumatoid arthritis (RA) have been well established (1), and the usage of methotrexate (MTX) as an anchor agent, in combination with biological disease-modifying antirheumatic drugs (bDMARDs), has contributed to an increased number of patients who have achieved clinical remission. As a result of this increase in the rate of clinical remission, the number of patients achieving radiographic remission and functional remission has also increased (2). Complete remission in a patient is defined as an individual who has achieved clinical, structural, and functional remissions (3). Although multiple studies regarding clinical and radiographic remissions have been reported to date, there are few reports regarding functional remission. It is important for patients to achieve functional remission as a final treatment outcome. Additionally, several reports have addressed the prognostic factors for clinical (4, 5) and radiographic (6) remissions; however, no prognostic factors for functional remission have been reported.
In this study, we analyzed the relationship between various baseline factors and functional outcomes after six-month biologic treatment to determine the prognostic factors for functional remission.
Materials and Methods
A retrospective study was performed in patients treated at a single hospital facility. RA patients who initiated bDMARDs treatment from 1 January 2007 to 31 December 2014 were examined. Among 333 patients treated with at least 1 of 6 biologic agents, 232 were deemed eligible to participate as subjects in this study. The bDMARDs used in the study included infliximab for 73 patients, etanercept for 37 patients, adalimumab for 39 patients, tocilizumab for 39 patients, abatacept for 34 patients, and golimumab for 10 patients, with no other bDMARDs used in any subjects. The selection of bDMARDs was deferred to each patient's primary physician.
The items described below were evaluated at baseline (before the treatment initiation) and six months after the treatment initiation. The patient background items included age, sex, experience of bDMARDs usage (either bio-naïve or bio-switch), disease duration, steroid dosage, and MTX dosage. The serological examination included assessment of anti-cyclic citrullinated peptides (CCP) antibody, rheumatoid factor (RF), matrix metalloproteinase 3 (MMP 3), tumor necrosis factor (TNF)-α, and interleukin (IL)-6. Disease activity was evaluated using the Simplified Disease Activity Index (SDAI) (7). The activity of daily living (ADL) was evaluated using the health assessment questionnaire disability index (HAQ-DI) (8), and nonspecific health-related quality of life (QOL) was evaluated using the Short Form 36 (SF-36) (7). The patient's level of depression was evaluated using the Hamilton Depression Rating Scale (HAM-D) (9) and Self-rating Depression Scale (SDS) (10). The usage of conventional synthetic DMARDs (csDMARDs), adrenocortical steroids (steroids), and non-steroidal anti-inflammatory drugs (NSAIDs) and their dosages before the initiation of biologic treatment as well as the patients' age or disease duration were not included in the study's inclusion or exclusion criteria.
As a primary outcome index, HAQ-DI ≤0.5 was defined as functional remission. In analyzing the relationship between baseline factors and functional outcomes, the baseline values of each item were analyzed based on the presence or absence of HAQ remission. The implementation, analysis, and evaluation of HAM-D and SDS were conducted under the supervision of SK. The study exclusion criteria included the following: use of antidepressant agents at the time of study initiation or during the treatment period; discontinuation of biologic treatment due to primary or secondary response failure or adverse effects; additional oral treatment using csDMARDs agents, steroids, or NSAIDs; complications, such as infection; unlikelihood of continuing the study because of situations such as hospital transfer, patients' withdrawal from the study, and other circumstances that the primary physician deemed inappropriate for the study.
All of the statistical analyses were performed using univariate and multivariate analyses in the JMP11 software program (SAS Institute, Cary, NC, USA). We obtained written informed consent from all of the patients who enrolled in the study. The study received approval from the Bio-Ethics Committee of the Department of Medicine, Showa University School of Medicine (No. 1435).
Results
Two hundred and thirty-two patients were included as study subjects as is shown in Figure. No patient used antidepressants at the time of study initiation or during the treatment period or received additional oral treatment with steroids or NSAIDs. As for the background parameters of the study subjects, there were 164 patients with functional remission (Group A) and 68 with no remission (Group B) (Table 1). Based on univariate analyses, Group A had a significantly lower age at the time of study initiation (p=0.001), higher ratio of naïve vs. switching (p=0.017), shorter disease duration (p=0.031), and lower steroid dosage (p=0.023) than Group B. Additionally, Group A had a significantly lower disease activity (p=0.001), lower HAQ-DI (p=0.001), and lower depression level (HAM-D: p=0.001) than Group B. With regard to QOL, Group A had significantly higher values in all 8 categories of the SF-36 than Group B (p<0.05).
Figure.
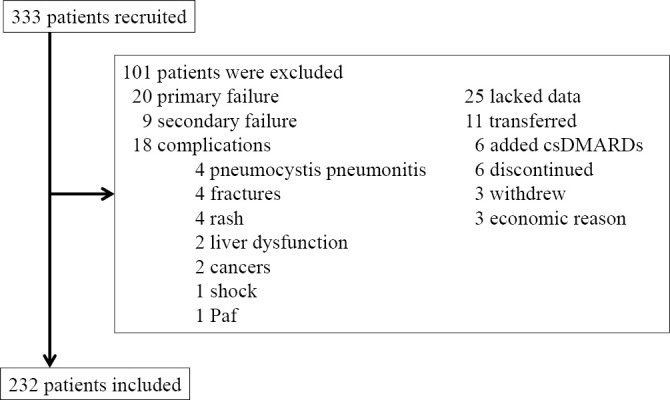
Flow chart of the study. Paf: paroxysmal atrial fibrillation, csDMARDs: conventional synthetic disease-modifying antirheumatic drugs
Table 1.
Univariable Analysis for the Summary of Demographics and Baseline Characteristics of 232 RA Patients.
| Remission (Group A) | No remission (Group B) | p | ||
|---|---|---|---|---|
| n | 164 | 68 | ||
| Age (years) | 54.8 ± 15.2 | 61.8 ± 13.3 | 0.001* | |
| Sex (female: male) | 138:26 | 55:13 | 0.680** | |
| Bio-naive or Switch | 121:43 | 38:29 | 0.017** | |
| Disease duration (year) | 7.1 ± 11.1 | 9.1 ± 8.5 | 0.031* | |
| Steroid dosage (mg/d) | 3.4 ± 3.7 | 4.5 ± 3.5 | 0.023* | |
| MTX dosage (mg/w) | 7.7 ± 4.3 | 6.7 ± 4.0 | 0.086* | |
| ACPA (U/mL) | 75.9 ± 141.9 | 332.1 ± 704.9 | 0.130* | |
| RF (IU/mL) | 126.4 ± 213.1 | 158.1 ± 261.4 | 0.718* | |
| MMP-3 (ng/mL) | 196.2 ± 234.4 | 321.2 ± 551.0 | 0.066* | |
| TNF-α (pg/mL) | 60.2 ± 176.1 | 73.3 ± 155.6 | 0.585* | |
| IL-6 (pg/mL) | 33.7 ± 81.6 | 7.7 ± 4.3 | 0.406* | |
| SDAI | 22.5 ± 13.4 | 32.1 ± 12.9 | 0.000* | |
| HAQ-DI | 0.39 ± 0.51 | 1.08 ± 0.54 | 0.000* | |
| SDS | 40.4 ± 9.9 | 43.5 ± 9.2 | 0.149* | |
| HAM-D | 5.0 ± 4.3 | 8.4 ± 5.0 | 0.004* | |
| SF-36 | Physical functioning | 35.1 ± 15.1 | 14.6± 15.6 | 0.000* |
| Role function | 35.3 ± 15.6 | 22.6 ± 13.9 | 0.000* | |
| Pain | 37.0 ± 9.4 | 30.9 ± 7.3 | 0.002* | |
| General health perception | 40.1 ± 8.5 | 34.2 ± 8.4 | 0.001* | |
| Fatigue | 45.2 ± 10.8 | 37.6 ± 10.7 | 0.003* | |
| Social functioning | 42.6 ± 14.1 | 32.6 ± 14.0 | 0.000* | |
| Role function(emotional) | 41.9 ± 14.9 | 28.0 ± 16.4 | 0.000* | |
| Mental health | 47.3 ± 10.8 | 41.8 ± 11.1 | 0.013* | |
Mean ± standard deviation value are used.
MTX: methotrexate, ACPA: anti-cyclic citrullinated peptides antibody, RF: rheumatoid factor, MMP-3: matrix metalloproteinase 3, TNF-α: tumor necrosis factor-α IL-6: interleukin-6, SDAI: Simplified Disease Activity Index, HAQ-DI: health assessment questionnaire disability index, SDS: Self-rating Depression Scale, HAM-D: Hamilton Depression Rating Scale, SF-36: short form-36
*analysis using Mann-Whitney U test.
**analysis using chi-squared test for independence test.
The multivariate analyses extracted HAQ-DI and mental health, 1 of 8 categories in the SF-36 [p=0.0001, odds ratio: 0.057, 95% confidence interval (CI): 0.013-0.244; and p=0.0189, odds ratio: 0.902, 95% CI: 0.828-0.983, respectively]. None of the other factors were extracted (Table 2).
Table 2.
Prognostic Factor Identified by Multivariate Analysis as Showing a Significant Association with Functional Remmission.
| Remission | No remission | Odd ratio (95% CI) | p | ||
|---|---|---|---|---|---|
| HAQ-DI | 0.39 ± 0.51 | 1.08 ± 0.54 | 0.057 (0.013-0.244) | 0.0001 | |
| SF-36 | Mental health | 47.3 ± 10.8 | 41.8 ± 11.1 | 0.902 (0.828-0.983) | 0.0189 |
Mean ± standard deviation values are used.
HAQ-DI: health assessment questionnaire disability index, SF-36: short form-36
Discussion
Multiple studies regarding RA prognostic factors have been reported (11). According to the literature, good prognostic factors for remission from disease activity include the following: male sex; young age; late-onset RA; short disease duration; nonsmoker; low baseline disease activity; mild functional impairment; low baseline radiographic damage; absence of RF and anti-CCP; low serum level of acute-phase reactant, IL 2, and receptor activator of nuclear factor kappa-B ligand (RANKL) at baseline; early treatment using csDMARDs combinations; use of anti-TNF; concurrent use of DMARDs in anti-TNF-treated patients; and moderate or good response to treatments during the first six months. A study on radiographic remission reported that MTX in combination with etanercept resulted in a better radiographic remission rate than MTX alone (12). After this report was published, similar outcomes were reported with other biologic agents (13). As a prognostic factor for complete remission, very early RA was reported (14). None of the studies, however, have focused on functional remission.
In the present study, we determined low HAQ-DI at baseline as a prognostic factor for functional remission after six months of bDMARDs treatment. Although depression scores determined by SDS or HAM-D were not extracted, mental health, one of the SF-36 categories, was determined as a prognostic factor for functional remission. Compared to the baseline HAQ-DI, however, mental health was a weaker prognostic factor based on multivariate analyses. Although disease activity, age, and sex were expected to influence functional outcomes, our analyses did not extract those variables as prognostic factors.
Our study had four limitations. First, we did not perform a radiographic evaluation of the joints, although we are aware that a radiographic evaluation is expected to influence the Damage-HAQ (15). The Total vdH-Sharp score is one of the factors influencing a functional remission and it is determined using a logistic regression analysis. Although HAQ-DI contributed to the functional remission according to a multiple logistic regression analysis, the Total vdH-Sharp score did not play a role.
In addition, due to the fact that radiographic evaluations were only performed in 50 patients, the subjects were therefore analyzed without the use of radiographic evaluations.
Second, we used actual clinical data accumulated over a long period, generating a bias in the biologics used in this study; therefore, the clinical outcomes may be different, depending on the type of biologic treatments. Third, the study design is not a prospective study but a retrospective one. Finally, no socioeconomic factors were included in our analysis.
Biological DMARDs for the treatment of RA demonstrate efficacy, leading to clinical remission as well as radiographic remission in many cases. High baseline HAQ-DI scores, however, prevent functional remission. This point is considered to be a limitation of bDMARDs treatment. To achieve functional remission, the establishment of a novel therapy algorithm is necessary.
In conclusion, our study demonstrated that low HAQ-DI and high mental health scores in SF-36 at baseline were significant prognostic factors for functional remission after a six-month bDMARDs treatment. To achieve functional remission, a novel therapy algorithm including useful prognostic factors must be established.
Author's disclosure of potential Conflicts of Interest (COI).
Yusuke Miwa: Research funding, Astellas Pharm Inc., Mitsubishi Tanabe Pharma Corporation, AbbVie CK, Pfizer Japan Inc., Chugai Pharmaceutical Co., Ltd., and Eizai Co., Ltd. Tsuyoshi Kasama: Research funding, Mitsubishi Tanabe Pharma Corporation and AbbVie CK.
References
- 1. Singh JA, Saag KG, Bridges SL Jr, et al. . 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol 68: 1-26, 2016. [DOI] [PubMed] [Google Scholar]
- 2. Haugeberg G, Boyesen P, Helgetveit K, Proven A. Clinical and radiographic outcomes in patients diagnosed with early rheumatoid arthritis in the first years of the biologic treatment era: a 10-year prospective observational study. J Rheumatol 42: 2279-2287, 2015. [DOI] [PubMed] [Google Scholar]
- 3. Izumi K, Kaneko Y, Yasuoka H, et al. . Tocilizumab is clinically, functionally, and radiographically effective and safe either with or without low-dose methotrexate in active rheumatoid arthritis patients with inadequate responses to DMARDs and/or TNF inhibitors: a single-center retrospective cohort study (KEIO-TCZ study) at week 52. Mod Rheumatol 25: 31-37, 2015. [DOI] [PubMed] [Google Scholar]
- 4. Kojima M, Kojima T, Suzuki S, et al. . Patient-reported outcomes as assessment tools and predictors of long-term prognosis: a 7-year follow-up study of patients with rheumatoid arthritis. Int J Rheum Dis (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 5. Odai T, Matsunawa M, Takahashi R, et al. . Correlation of CX3CL1 and CX3CR1 levels with response to infliximab therapy in patients with rheumatoid arthritis. J Rheumatol 36: 1158-1165, 2009. [DOI] [PubMed] [Google Scholar]
- 6. Bathon J, Robles M, Ximenes AC, et al. . Sustained disease remission and inhibition of radiographic progression in methotrexate-naive patients with rheumatoid arthritis and poor prognostic factors treated with abatacept: 2-year outcomes. Ann Rheum Dis 70: 1949-1956, 2011. [DOI] [PubMed] [Google Scholar]
- 7. Aletaha D, Ward MM, Machold KP, Nell VP, Stamm T, Smolen JS. Remission and active disease in rheumatoid arthritis: defining criteria for disease activity states. Arthritis Rheum 52: 2625-2636, 2005. [DOI] [PubMed] [Google Scholar]
- 8. Ziebland S, Fitzpatrick R, Jenkinson C, Mowat A. Comparison of two approaches to measuring change in health status in rheumatoid arthritis: the Health Assessment Questionnaire (HAQ) and modified HAQ. Ann Rheum Dis 51: 1202-1205, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56-62, 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zung WW. A self-rating depression scale. Arch Gen Psychiatry 12: 63-70, 1965. [DOI] [PubMed] [Google Scholar]
- 11. Katchamart W, Johnson S, Lin HJ, Phumethum V, Salliot C, Bombardier C. Predictors for remission in rheumatoid arthritis patients: a systematic review. Arthritis Care Res (Hoboken) 62: 1128-1143, 2010. [DOI] [PubMed] [Google Scholar]
- 12. van der Heijde D, Landewe R, van Vollenhoven R, Fatenejad S, Klareskog L. Level of radiographic damage and radiographic progression are determinants of physical function: a longitudinal analysis of the TEMPO trial. Ann Rheum Dis 67: 1267-1270, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ciubotariu E, Gabay C, Finckh A. Joint damage progression in patients with rheumatoid arthritis in clinical remission: do biologics perform better than synthetic antirheumatic drugs? J Rheumatol 41: 1576-1582, 2014. [DOI] [PubMed] [Google Scholar]
- 14. Bosello S, Fedele AL, Peluso G, Gremese E, Tolusso B, Ferraccioli G. Very early rheumatoid arthritis is the major predictor of major outcomes: clinical ACR remission and radiographic non-progression. Ann Rheum Dis 70: 1292-1295, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smolen JS, Aletaha D, Grisar JC, Stamm TA, Sharp JT. Estimation of a numerical value for joint damage-related physical disability in rheumatoid arthritis clinical trials. Ann Rheum Dis 69: 1058-1064, 2010. [DOI] [PubMed] [Google Scholar]


