Abstract
Scedosporium prolificans is a fungus that has demonstrated resistance against most currently available antifungal agents and which causes a rapidly disseminating and potentially fatal infection. A 68-year-old woman presented with a fever and consolidation in the lung field. Her symptoms and inflammatory reaction did not improve despite treatment with tazobactam/piperacillin, meropenem, and micafungin. Scedosporium prolificans was detected from the patient's bronchial lavage fluid, and we initiated treatment with voriconazole. Voriconazole was effective in shrinking the consolidation and suppressing the inflammatory reaction. The residual lesion was surgically resected because of the risk of systemic dissemination. The patient is currently alive without relapse or dissemination.
Keywords: Scedosporium prolificans, voriconazole, surgery, lung infection
Introduction
The Scedosporium species is a ubiquitous mold, which is distributed in environments such as soil, polluted water, and decaying food (1). Four species have been revealed as human pathogens: S. prolificans, S. aurantiacum, S. apiospermum (teleomorphic state, Pseudallescheria apiosperma), and S. boydii (teleomorphic state, P. boydii) (1, 2). Scedosporium is multidrug resistant and may cause various infections, such as epidural abscess, endocarditis, keratitis, pneumonia, and fungemia (3, 4). In particular, S. prolificans is resistant to most currently available antifungal agents, and is associated with a high mortality rate of up to 95% in immunocompromised patients (1, 2). We herein report a case of lung infection due to S. prolificans in an immunocompetent patient who was successfully treated with voriconazole and surgery.
Case Report
A 68-year-old woman underwent follow-up examinations for a pulmonary opacity at a different hospital several times in a year. The patient had no symptoms at two months prior to her admission to our hospital; however, follow-up chest computed tomography (CT) without contrast showed new consolidation of the right lower lung lobe (Fig. 1). A few days before admission, the patient developed a fever and pain in the right hypochondrium. She underwent a checkup at a different hospital. The laboratory data were as follows: white blood cell (WBC) count, 17,500 /μL with 83% neutrophils; aspartate aminotransferase (AST), 23 IU/L; alanine aminotransferase (ALT), 33 IU/L; alkaline phosphatase (ALP), 370 IU/L; γ-glutamyl transpeptidase (γ-GTP), 80 IU/L; and C-reactive protein (CRP), 15.6 mg/dL. Follow-up chest CT showed the enlargement of the patient's lung consolidation with a high-density area and bronchiectasis (Fig. 2). Treatment with antibiotics and antifungal agents (tazobactam/piperacillin 13.5 g/day, meropenem 1.5 g/day, and micafungin 100 mg/day) was initiated due to the possibility of a bacterial or fungal infection, such as Aspergillus pneumonia. However, there was no improvement in her symptoms or laboratory data after a few days of treatment, and she was referred to our hospital.
Figure 1.
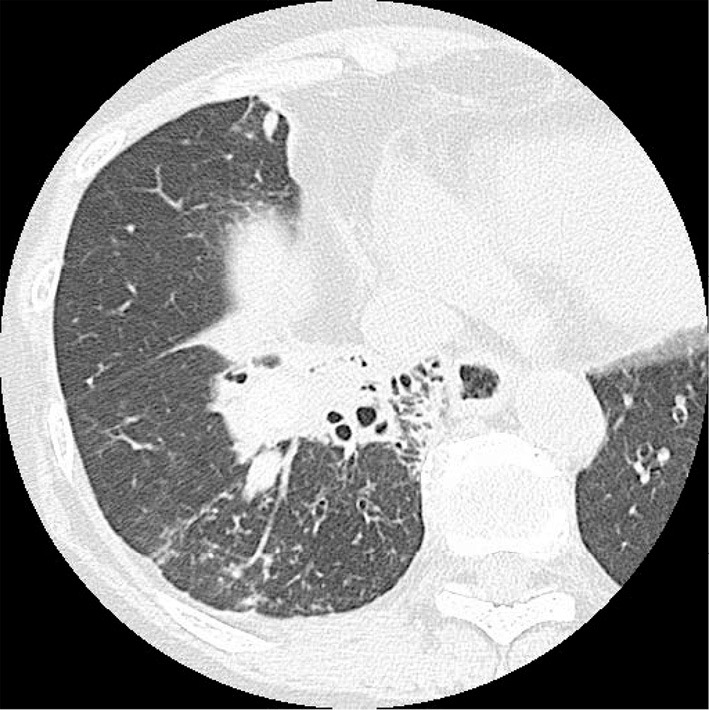
Chest computed tomography scan taken 2 months before admission, showing consolidation of the right lower lung lobe with a high-density area.
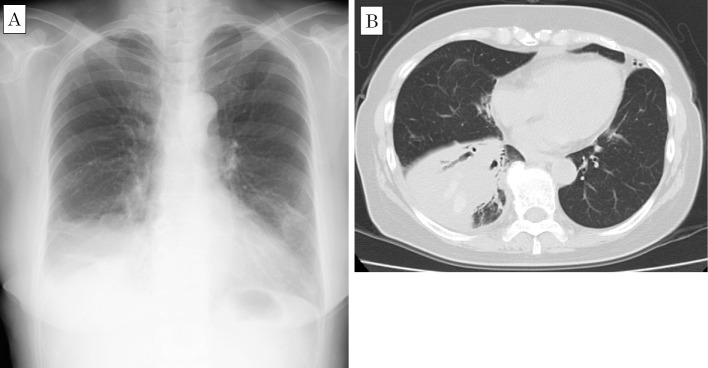
Figure 2.
Chest radiograph (A) and computed tomography scan (B) taken on hospitalization, showing enlargement of lung consolidation with a high-density area and bronchiectasis.
Her laboratory data revealed the further elevation of her WBC count, CRP level, and hepatobiliary enzyme levels (AST, ALT, ALP, and γ-GTP), suggesting inflammation of the gallbladder (Table 1). The patient was negative for 1, 3-β-D-glucan, Aspergillus galactomannan antigen, and Aspergillus antibodies. Other serological tests revealed that the patient's immunoglobulin (Ig) G, IgA, and IgM levels were within the normal ranges, and that the patient was negative for human immunodeficiency virus (HIV)-1, 2 antibody (by chemiluminescent enzyme immunoassay). These findings suggested that the patient was immunocompetent. No significant bacteria or fungi were detected in the patient's blood or sputum cultures.
Table 1.
Laboratory Findings on Admission.
| WBC | 16,400 | /μL | TP | 7.1 | g/dL | CRP | 12.4 | mg/dL |
| Neut | 71.9 | % | Alb | 2.8 | g/dL | PCT | 0.06 | ng/mL |
| Eosi | 4.3 | % | AST | 50 | IU/L | CEA | 12.9 | ng/mL |
| Baso | 0.7 | % | ALT | 61 | IU/L | SCC | 1.2 | ng/mL |
| Ly | 16.8 | % | LDH | 267 | IU/L | β-D glucan | <6.0 | pg/mL |
| Mono | 6.3 | % | ALP | 600 | IU/L | IgG | 1,659 | mg/dL |
| RBC | 357×104 | /μL | γ-GTP | 174 | IU/L | IgA | 207 | mg/dL |
| Hb | 10.7 | g/dL | BUN | 8 | mg/dL | IgM | 96 | mg/dL |
| Plt | 42.8×104 | /μL | Cre | 0.4 | mg/dL | IgE | 860 | IU/mL |
| PT-INR | 1.12 | T-Bil | 0.4 | mg/dL | HIV-1,2 antibody | negative | ||
| APTT | 31.6 | s | Na | 141 | mEq/L | Aspergillus galactomannan antigen | negative | |
| Fib | 848.4 | mg/dL | Cl | 101 | mEq/L | Aspergillus precipitating antibody | negative | |
| FDP | 10.2 | μg/mL | K | 4 | mEq/L | |||
| D-dimer | 3.9 | μg/mL | Ca | 9.1 | mg/dL | |||
Alb: albumin, ALP: alkaline phosphatase, ALT: alanine aminotransferase, APTT: activated partial thromboplastin time, AST: aspartate aminotransferase, Baso: basophils, BUN: blood urea nitrogen, Ca: calcium, CEA: carcinoembryonic antigen, Cl: chloride, Cre: creatinine, CRP: C-reactive protein, Eosi: eosinophils, FDP: fibrin degradation products, Fib: fibrinogen, γ-GTP: gamma-glutamyl transpeptidase, Hb: hemoglobin, HIV: human immunodeficiency virus, Ig: immunoglobulin, K: potassium, LDH: lactate dehydrogenase, Ly: lymphocytes, Mono: monocytes, Na: sodium, Neut: neutrophils, PCT: procalcitonin, Plt: platelets, PT-INR: prothrombin time-international normalized ratio, RBC: red blood cells, SCC: squamous cell carcinoma, T-Bil: total bilirubin, TP: total protein, WBC: white blood cells
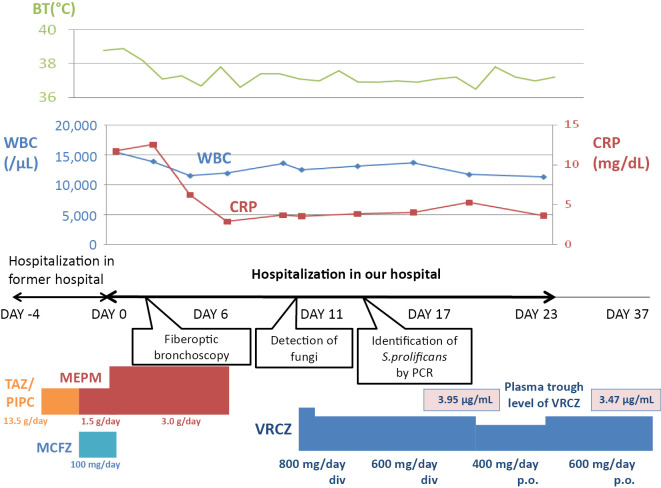
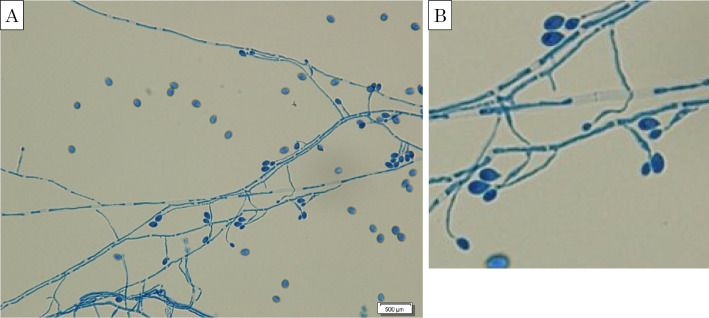
After increasing the patient's dose of meropenem to 3.0 g/day, her fever abated and the right hypochondralgia disappeared (Fig. 3). Her hepatobiliary enzyme (AST, ALT, ALP, and γ-GTP) levels declined, but her WBC count and CRP level remained high (12,000 /μL and 4.0 mg/dL, respectively) and the chest radiograph findings did not change. Because no significant bacteria or fungi were detected in the patient's blood and sputum cultures and because other infectious diseases for which meropenem is known to be ineffective were considered fiberoptic bronchoscopy was performed. Branching hyphae were detected in a bronchial lavage fluid culture, suggesting the possibility of a Scedosporium species (Fig. 4). A polymerase chain reaction was performed at the Department of Microbiology at Tokyo Medical University to identify the fungal species. The results of the polymerase chain reaction using primers for the 28S ribosomal RNA gene region showed 100% homology with S. prolificans when compared with the MycoBank Database (http://mycobank.org/).
Figure 3.
Clinical course. BT: body temperature, CRP: C-reactive protein, div: intravenous drip, MCFG: micafungin, MEPM: meropenem, PIPC: piperacillin, po: per os, TAZ: tazobactam, VRCZ: voriconazole, WBC: white blood cell
Figure 4.
(A) Light microscopic image of a bronchial lavage fluid culture, showing branching hyphae and conidia of Scedosporium prolificans (lactophenol cotton blue stain). (B) Magnified image of (A).
A colorimetric microdilution panel (ASTY; Kyokuto Pharmaceutical Industrial) was used to determine the minimal inhibitory concentration for the S. prolificans strain. Although the minimal inhibitory concentration against almost all antifungal agents was very high, the minimal inhibitory concentration against voriconazole was relatively low (Table 2). Thus, intravenous voriconazole [loading dose, 600 mg/day; maintenance dose, 400 mg/day (8 mg/kg)] was initiated with the monitoring of the plasma drug concentration (trough level, 3.95 μg/mL). After changing from intravenous to oral voriconazole (maintenance dose, 600 mg/day; trough level, 3.47 mg/day), the patient was discharged at approximately 1 month after hospitalization.
Table 2.
Minimum Inhibitory Concentration (MIC) of Scedosporium prolificans in Our Patient.
| Antifungal agent | MIC, mg/mL |
|---|---|
| Amphotericin B | 8 |
| Fluconazole | >64 |
| Miconazole | 4 |
| Itraconazole | >8 |
| Voriconazole | 2 |
| Micafangin | >16 |
| Flucytosine | >64 |
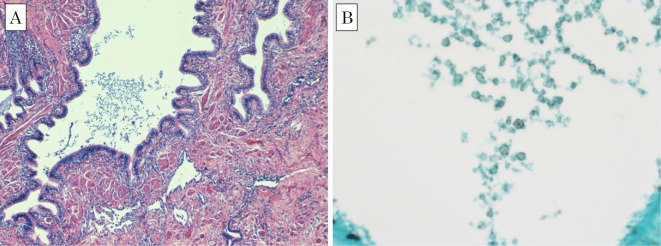
One month later, the patient's laboratory data revealed a WBC count of 6,600 /μL and a CRP level of 0.19 mg/dL, suggesting the improvement of inflammation. Chest CT showed the shrinkage of the consolidation in the right lower lung lobe; however, a residual lesion remained (Fig. 5). Right lower lobectomy was performed in the third month of outpatient visits, while the lesion was localized, based on the risk of resistance to voriconazole and systemic dissemination. The surgery was successfully completed, despite the presence of strong adhesion to the diaphragm and right lower lobe. The patient's postoperative course was uneventful. Inflammatory changes were revealed, and a fungus-like substance was detected in a Grocott methenamine silver-stained histopathological specimen from the bronchus (Fig. 6). The invasion of a fungus-like substance into the lung tissue was not definitive. Voriconazole was discontinued in the third month after surgery, after ensuring that there was no increase in the inflammatory response. At present, the patient is alive, after having completed approximately 7 months of treatment with voriconazole.
Figure 5.
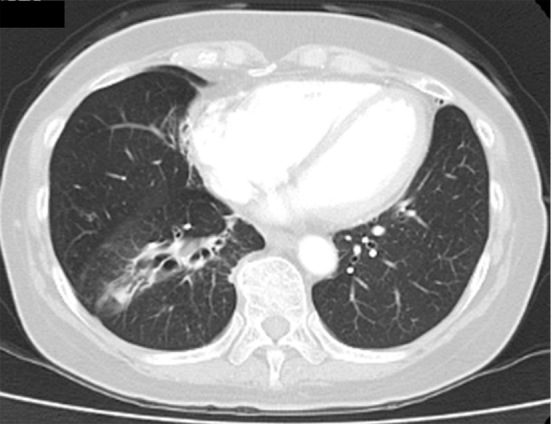
Chest computed tomography scan taken 3 months after initiation of voriconazole, showing decreased lung consolidation but a residual lesion.
Figure 6.
Light microscopic images of a histopathologic specimen, showing a fungus-like substance in the bronchus. Inflammatory cell infiltration and collagen fiber hyperplasia is noted, but invasion of fungus-like substance into the lung tissue is not definitive. (A) Hematoxylin and Eosin staining. (B) Grocott methenamine silver stain.
Discussion
S. prolificans infection is rare; however, the number of cases in Oceanian and European countries have gradually been increasing (2, 5). In Japan, only a few cases of lung infection due to S. prolificans have been reported (4, 6, 7). All of the patients in these cases died within a few days to a few months after the onset of disease; in contrast, our patient survived. We believe that the sensitivity to voriconazole and the patient's immune state had an impact on her survival.
S. prolificans is resistant to most clinically available antifungal agents (3, 6). As such, S. prolificans infection is often fatal after dissemination to multiple organs. In our case, minimum inhibitory concentration of voriconazole against S. prolificans was relatively low in comparison to other studies (Table 2), which implies that it had some influence on the effect of voriconazole. The plasma concentration of voriconazole should be monitored to minimize the adverse effects; however, the administration of a high dose of voriconazole should be considered when treating patients with S. prolificans.
In our case, it is possible that the patient had a mixed infection with bacteria at the time of hospitalization because of the slight decline that was observed in the inflammatory response after antibiotic treatment. However, no significant bacteria were detected in the patient's sputum culture. This is considered to be due to the effects of antibiotic treatment.
Surgical resection can enable a successful outcome if a patient with a Scedosporium infection has a localized lesion (2). In our case, surgical resection was performed because CT showed a residual lesion and a possible remnant fungal infection. The surgery was successful, without recurrence or dissemination. Because it had been proven that the fungi were still localized in the residual lesion, we considered that it would be difficult to achieve a complete cure with drug therapy alone. Thus, if the focus of infection can be removed, surgical resection should be actively recommended.
Immunocompromised or immunosuppressed patients, including patients with HIV, organ transplant recipients, hematopoietic stem cell transplant recipients, and patients with hematologic malignancies, are more likely to be infected with Scedosporium (3, 4, 6). However, one study reported that 21% of 270 patients with Scedosporium infection were immunocompetent (1). In our case, the patient was immunocompetent, but the decline in local immunization with bronchiectasis likely influenced the S. prolificans infection. All of the patients in the domestic cases of lung infection due to S. prolificans had a poor general condition, such as monoclonal gammopathy with undetermined significance (6), nephrotic syndrome (7), or Tsunami lung (4), which facilitated dissemination or respiratory failure. In contrast, our patient had a relatively good condition. Thus, although S. prolificans infection can be fatal, it is inferred that immunocompetent patients are less likely to have dissemination and that a local infection can be maintained.
The symptoms and imaging findings of lung scedosporiosis are similar to those of pulmonary aspergillosis, which makes a diagnosis difficult and often delays the provision of adequate treatment (8). In contrast with Aspergillus, S. prolificans displays multidrug resistance, and is resistant to voriconazole. When a fungal infection is suspected and principal antifungal agents are ineffective, as in our case, multidrug-resistant fungi such as Scedosporium should be considered.
In conclusion, S. prolificans infection is very rare, is associated with high rates of mortality, and is being increasingly encountered. Treatment with a high dose of voriconazole and surgical resection is recommended for immunocompetent patients without dissemination.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors would like to thank Prof. Kiyofumi Ohkusu, Department of Microbiology, Tokyo Medical University, for performing the polymerase chain reaction to identify S. prolificans.
References
- 1. Husain S, Muñoz P, Forrest G, et al. Infections due to Scedosporium apiospermum and Scedosporium prolificans in transplant recipients: clinical characteristics and impact of antifungal agent therapy on outcome. Clin Infect Dis 40: 89-99, 2004. [DOI] [PubMed] [Google Scholar]
- 2. Tortorano AM, Richardson M, Roilides E, et al. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin Microbiol Infect 3: 27-46, 2014. [DOI] [PubMed] [Google Scholar]
- 3. Nishimori M, Takahashi T, Suzuki E, et al. Fatal fungemia with Scedosporium prolificans in a patient with acute myeloid leukemia. Med Mycol J 55: E63-E70, 2014. [DOI] [PubMed] [Google Scholar]
- 4. Hatakeyama Y, Yamada Y, Furukawa K, et al. Three cases of tsunami lung in which genus Scedosporium was isolated. Nihon Rinsyou Biseibutsugaku Zasshi (Journal of Japanese Society for Clinical Microbiology) 22: 289-297, 2012(in Japanese). [Google Scholar]
- 5. Tintelnot K, Just-Nübling G, Horré R, et al. A review of German Scedosporium prolificans cases from 1993 to 2007. Med Mycol 47: 351-358, 2009. [DOI] [PubMed] [Google Scholar]
- 6. Ohashi R, Kato M, Katsura Y, et al. Breakthrough lung Scedosporium prolificans infection with multiple cavity lesions in a patient receiving voriconazole for probable invasive aspergillosis associated with monoclonal gammmopathy of undetermined significance (MGUS). Med Mycol J 52: 33-38, 2011. [DOI] [PubMed] [Google Scholar]
- 7. Kikuti K. Photo quiz: Deep-seated mycosis. Med Mycol J 53: 93-94, 2012. [DOI] [PubMed] [Google Scholar]
- 8. Ogata R, Hagiwara E, Shiihara J, Ogura T, Takahashi H, Kamei K. A case of lung scedosporiosis successfully treated with monitoring of plasma voriconazole concentration level. Nihon Kokyuki Gakkai Zasshi (Journal of Japanese Respiratory Society) 49: 388-392, 2011(in Japanese). [PubMed] [Google Scholar]