Abstract
Objectives:
Irritable bowel syndrome (IBS) is a functional bowel disorder characterized by symptoms including abdominal pain and altered bowel function. Up to 75% of individuals with IBS may be undiagnosed. The aim of this study was to characterize symptoms, healthcare utilization, and treatments in populations with both diagnosed and undiagnosed IBS.
Methods:
An online survey was conducted to compare gastrointestinal (GI) symptoms, healthcare visits, well-being, symptom management, and treatment satisfaction in individuals with and without medically diagnosed IBS (Rome III criteria). Symptom severity, disruptiveness, and treatment satisfaction were rated using a 7-point scale. Adjustments to daily life were determined by predefined survey responses.
Results:
A total of 1,924 individuals with a history of GI symptoms were eligible and completed the survey. Of these, 1,094 individuals (56.9%) met the criteria for IBS; 830 individuals (43.1%) had no medical diagnosis of IBS despite meeting diagnostic criteria. Most participants received a diagnosis from either gastroenterologists (45%) or primary care physicians (42%). A greater percentage of diagnosed patients had severe GI symptoms (score ≥6) vs. undiagnosed individuals (16% vs. 8%, respectively; P<0.05); diagnosed patients were more likely to report that GI symptoms adversely affected their quality of life. Approximately 40% of participants received IBS-related treatment from primary care physicians; 26% and 43% of diagnosed and undiagnosed individuals, respectively, were not receiving treatment for GI symptoms.
Conclusions:
Many individuals with IBS-related symptoms have not been medically diagnosed with IBS. IBS-related symptoms impact quality of life, yet more than one-third of individuals are not receiving treatment for IBS.
Introduction
Irritable bowel syndrome (IBS) is a functional bowel disorder characterized by chronic or recurrent symptoms of abdominal pain that is associated with altered bowel function (i.e., pain related to defecation, changes in stool frequency, or appearance) (1, 2, 3). Additional symptoms of IBS may include straining, fecal urgency, and bloating (1). IBS can further be subdivided by stool consistency, namely, constipation-predominant IBS (IBS-C), diarrhea-predominant IBS (IBS-D), or mixed bowel habit pattern (IBS-M). Importantly, patients with IBS report reduced health-related quality of life (HRQOL) compared with individuals without IBS (4).
As many as 48 million individuals in the United States are thought to be affected by IBS annually (5), yet up to 75% of patients with IBS may lack a formal medical diagnosis of IBS (6). Although diagnostic symptom criteria exist for IBS (1, 3), they are mainly used in clinical research studies, rather than used routinely in clinical practice. In a US study using a large health insurance database, patients with IBS were most likely to receive a diagnosis from an internist (68%) rather than a gastroenterologist (13%) (7). Hungin et al. (8) conducted a European study that surveyed 3,880 participants with IBS symptoms and suggested that primary care physicians may have an even greater role in IBS diagnosis and management; the authors found that most patients with medically diagnosed IBS sought medical care from primary care physicians (90%) rather than gastroenterologists (28%). Of symptomatic individuals lacking a medical diagnosis of IBS, Hungin et al. (8) found, for a European population, that 37% did not receive care, whereas 55 and 12% sought medical care from primary care physicians and gastroenterologists, respectively. A comparable, but smaller, US community survey study conducted by the same group found similar results (6). The reasons for the lack of a formal diagnosis of IBS for many individuals are not entirely clear, but likely are multifactorial. Individuals with IBS symptoms often describe a range of gastrointestinal (GI) symptoms, including reflux-like symptoms, dyspepsia, and IBS-related symptoms (e.g., fecal urgency, bloating) (9). IBS-D may pose a relatively greater diagnostic challenge than the other bowel habit subtypes, as celiac disease and inflammatory bowel disease, among other conditions, need to be considered in patients with chronic or recurrent diarrhea (10). In fact, current evidence suggests that celiac serologies and inflammatory markers such as C-reactive protein and fecal calprotectin should be obtained in these patients (11).
Previous studies have compared GI and psychologic symptoms between individuals with IBS symptoms who seek healthcare and those who do not; however, differences between individuals with diagnosed and undiagnosed IBS (including those who had visited a physician for their IBS-related symptoms) have not been well studied. The European and US surveys performed by Hungin et al. (6, 8) were conducted >10 years ago, used older diagnostic criteria for IBS and bowel habit subtypes, and did not focus on patients with IBS-D.
Thus, the objective of this investigation was to compare symptom characteristics, healthcare utilization, HRQOL, treatments, and perceived explanations for GI symptoms in patients diagnosed with IBS-D using contemporary criteria compared with individuals who remain undiagnosed despite having sought medical attention for IBS-related symptoms.
Methods
Study participants
Individuals ≥18 years of age from a general US population sample who had previously responded to invitations to participate in various surveys for two different firms and who indicated in a profile questionnaire that they had experienced GI issues were eligible for inclusion in the survey. An invitation to complete an online survey was sent by e-mail to eligible individuals in the two databases, with up to three additional e-mail reminders sent to nonresponders. The survey was conducted between 1 September 2014 and 15 September 2014, and assessed the frequency and severity of GI symptoms, number and type of healthcare visits, general well-being, management of symptoms, and treatment satisfaction. A point system redeemable for various rewards (e.g., gift cards) was used as an incentive for participation in the survey.
Participants were classified as patients with diagnosed IBS if they indicated that a healthcare provider had diagnosed them as having IBS (i.e., “diagnosed patients”). Participants were classified as individuals with undiagnosed IBS-D (i.e., “undiagnosed individuals”) if their symptoms were compatible with IBS according to Rome III criteria (1) based on their survey responses, but they had not received a medical diagnosis of IBS. Hence, undiagnosed individuals with IBS-D symptoms reported abdominal pain and discomfort at least 2 to 3 days per month for at least the previous 3 months. Furthermore, these symptoms were accompanied by more frequent bowel movements and looser stools, and individuals experienced improvement in abdominal pain or discomfort with a bowel movement. Individuals were not included in the study if they reported constipation as “always” occurring or if their associated stomach issues were predominantly accompanied by constipation. Individuals were also not included in the analysis if they reported having irregular hematochezia or anorectal bleeding in the previous month or if they had previous GI surgery.
Assessments
Symptom severity was scored on a 7-point scale (range: 1=very mild to 7=very severe). Disruptiveness of symptoms was also scored on a 7-point scale (range: 1=not at all disruptive to 7=extremely disruptive), as was satisfaction with current treatments (range: 1=extremely unsatisfied to 7=extremely satisfied). Adjustments to daily living invoked to control symptoms were determined based on 11 predefined responses, or a choice of “other” or “none of the above” to the question: “Which, if any, of the following things do you do in your day-to-day life in order to manage your (IBS/stomach problems)?” Detailed information regarding questions and response options are included in Table 1 in the Supplementary Information online.
Table 1. Population demographics and symptom history.
|
Individuals,
N
(%) |
||
|---|---|---|
| Parameter | Diagnosed with IBS-D (n=1,094) | Undiagnosed (n=830) |
| Male/female (%) | 241:853a (22:78a) | 299b:531 (36b:64) |
| Age range, years (n, %) | ||
| 18–39 | 416 (38) | 332 (40) |
| 40–59 | 437 (40) | 398 (48)a |
| ≥60 | 241 (22)a | 100 (12) |
| Race/ethnicity (n, %) | ||
| White | 1,017 (93)a | 714 (86) |
| Black | 33 (3) | 50 (6)b |
| Hispanic/Latino | 33 (3) | 42 (5) |
| Asian-American/Asian | 22 (2) | 33 (4)b |
| Other ethnicity | 11 (1) | 25 (3) |
| Symptom duration, years (n, %) | ||
| <5 | 272 (25) | 546 (66)a |
| 5–10 | 218 (20)b | 134 (16) |
| ≥10 | 604 (55)a | 150 (18) |
| Symptom intensity (n, %) | ||
| Mild (score, 1–2) | 33 (3) | 50 (6)a |
| Moderate (score, 3–5) | 886 (81) | 714 (86) |
| Severe (score, 6–7) | 175 (16)a | 66 (8) |
| Consulted ≥3 physicians (n, %) | ||
| Symptoms <5 years | 60 (22)a,c | 33 (6)d |
| Symptoms 5–10 years | 83 (38)a,e | 23 (17)f |
| Symptoms ≥10 years | 302 (50)a,g | 44 (29)h |
| Consultation with gastroenterologist (n, %) | ||
| Symptoms <5 years | 139 (51)a,c | 137 (25)d |
| Symptoms 5–10 years | 122 (56)a,e | 40 (30)f |
| Symptoms ≥10 years | 405 (67)a,g | 68 (45)h |
IBS-D, diarrhea-predominant irritable bowel syndrome.
P<0.05 vs. undiagnosed.
P<0.05 vs. diagnosed.
n=272.
n=546.
n=218.
n=134.
n=604.
n=150.
Statistically significant differences between diagnosed and undiagnosed populations were calculated using a z-test for proportions and only calculated as P<0.05, which was considered statistically significant, or P≥0.05, which was considered not statistically significant.
Role of the sponsor
The study was sponsored by Salix Pharmaceuticals, which had a role in study design and data analysis.
Results
Study participants
A total of 126,057 individuals pooled from 2 separate groups of potential respondents were sent an invitation by e-mail to participate in the survey. Of the 23,707 individuals (18.8%) who accessed the link to the survey, 1,924 (1.5%) completed the survey, thus meeting the eligibility criteria for inclusion in the study (Supplementary Figure S1 online). Of these individuals, 1,094 (56.9%) met the criteria for an IBS-D diagnosis; despite meeting the Rome III criteria for IBS-D, 830 individuals (43.1%) had not received a medical diagnosis of IBS. In the undiagnosed group, 53% of individuals reported that they had never spoken with a physician regarding their stomach problems.
Of the diagnosed patients, 45% and 42% of patients received a diagnosis from either a gastroenterologist or a primary care physician, respectively. Internists diagnosed 11% of patients with IBS-D. Compared with undiagnosed individuals, diagnosed patients were more commonly white and female (P<0.05; Table 1). Older individuals (≥60 years of age) were also more likely to be diagnosed with IBS. Furthermore, diagnosed patients had consulted a greater number of physicians and had more GI consultations as compared with undiagnosed individuals (P<0.05 for all comparisons), with the percentage of diagnosed and undiagnosed individuals consulting healthcare providers increasing with a longer duration of symptoms.
GI symptom history and impact on HRQOL
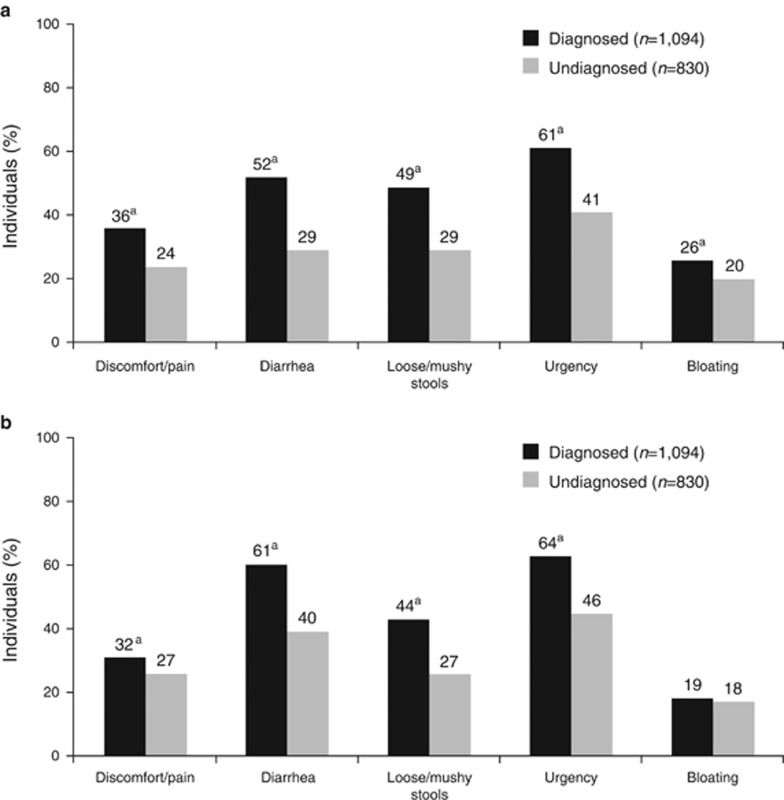
A significantly greater percentage of diagnosed patients had GI symptoms for ≥10 years compared with undiagnosed individuals (55% vs. 18%, respectively; P<0.05). Conversely, a significantly greater percentage of undiagnosed individuals had symptoms for <3 years compared with diagnosed patients (51% vs. 14%, respectively; P<0.05). Diagnosed patients were twice as likely to have severe GI symptoms (i.e., severity score, 6 or 7) than undiagnosed individuals (16% vs. 8%, respectively; P<0.05), whereas undiagnosed individuals were significantly more likely to have mild symptoms (i.e., severity score, 1 or 2) compared with diagnosed patients (6% vs. 3%, respectively; P<0.05). A greater percentage of diagnosed patients had more severe symptoms compared with individuals in the undiagnosed group for discomfort/pain (diagnosed, 36% vs. undiagnosed, 24%), diarrhea (52% vs. 29%), loose/mushy stools (49% vs. 29%), urgency (61% vs. 41%), and bloating (26% vs. 20%); P<0.05 for all comparisons (Figure 1a).
Figure 1.
Severity and disruptiveness of bowel movement characteristics. Severity (a) was determined by a score of 6 or 7 on a 7-point scale (range: 1=very mild to 7=very severe). Disruptiveness (b) was determined by a score of 6 or 7 on a 7-point scale (range: 1=not at all disruptive to 7=extremely disruptive). aP<0.05 vs. undiagnosed group.
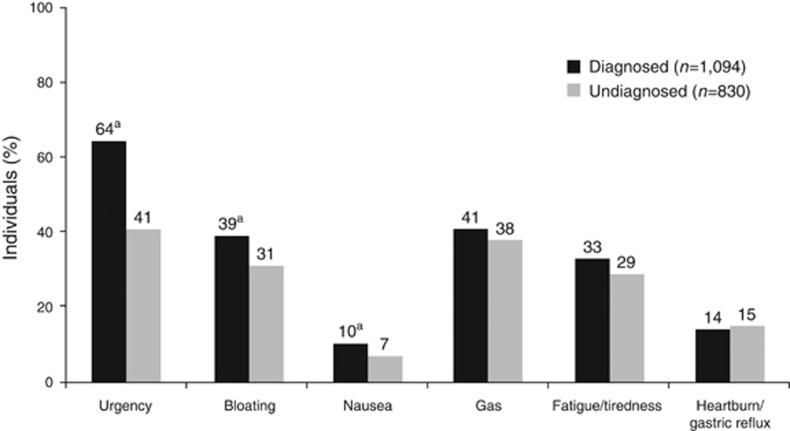
Diagnosed patients had more disruptive IBS-associated symptoms than individuals in the undiagnosed group, including discomfort/pain (32% vs. 27%), diarrhea (61% vs. 40%), loose/mushy stools (44% vs. 27%), and urgency (64% vs. 46%); P<0.05 for all comparisons (Figure 1b). Bloating occurred in a comparable percentage of individuals (19% vs. 18%). Yet, undiagnosed individuals had more “confounder” symptoms (i.e., heartburn, gastric reflux, constipation) than diagnosed patients. When experiencing GI symptoms of pain or discomfort and diarrhea, diagnosed patients reported IBS-associated symptoms (e.g., bowel movement urgency, bloating, nausea) “always or most of the time” with greater frequency than undiagnosed individuals (Figure 2).
Figure 2.
Additional symptoms experienced “always or most of the time.” aP<0.05 vs. undiagnosed group.
In addition, both diagnosed and undiagnosed patients reported that HRQOL was adversely impacted by GI symptoms (Supplementary Table S2 online). However, a significantly greater percentage of diagnosed patients than undiagnosed individuals reported cancelling or changing plans at the last minute (52% vs. 39%, respectively), premedicating with antidiarrheal agents (50% vs. 33%), and avoiding food consumption before important events (52% vs. 39%), events with poor bathroom access (e.g., outdoor activity; 42% vs. 27%), work activities (38% vs. 28%), or dinner or social events with friends (34% vs. 23%), because of symptoms of IBS (P<0.05 for all comparisons).
Management of GI symptoms
Diagnosed patients reported using a greater mean number of treatments in the past compared with undiagnosed individuals (4.9 vs. 3.4, respectively), including in the past 3 months (2.6 vs. 2.1, respectively; Table 2). However, few individuals with either diagnosed or undiagnosed IBS reported satisfaction with treatment (20% vs. 18%, respectively). A significantly greater percentage of diagnosed patients reported satisfaction with specific treatments than undiagnosed individuals, notably antidiarrheals (30% vs. 24%, respectively; P<0.05) and dietary adjustments (26% vs. 20% P<0.05). Furthermore, diagnosed patients were significantly more likely to have received antidepressants or psychologic therapies compared with undiagnosed individuals (antidepressants, 29% vs. 16%, respectively; psychological therapies, 15% vs. 9% P<0.05 for both comparisons). In addition, diagnosed patients were more likely to report adjustments to daily activities as a result of GI symptoms compared with undiagnosed individuals (Table 3).
Table 2. Treatments administered to help manage symptoms of irritable bowel syndrome.
|
Individuals,
N
(%) |
||||
|---|---|---|---|---|
|
Current treatmentsb |
Past treatments |
|||
| Treatment with recommendation score and quality of evidence (in brackets)a | Diagnosed with IBS-D (n=1,094) | Undiagnosed (n=830) | Diagnosed with IBS-D (n=1,094) | Undiagnosed (n=830) |
| Level 2 recommendations | ||||
| Antidepressants (2A) | 175 (16)b | 66 (8) | 317 (29)b | 133 (16) |
| Bulking agents or fiber supplements (2B) | 219 (20)c | 75 (9) | 558 (51)c | 216 (26) |
| Antibiotics (2B) | 33 (3) | 25 (3) | 153 (14) | 100 (12) |
| Alosetron (2B) | 11 (1) | 0 (0) | 44 (4) | 8 (1) |
| Antispasmodics (2C) | 131 (12)c | 17 (2) | 328 (30)c | 33 (4) |
| Probiotics (2C) | 372 (34)b | 199 (24) | 689 (63)b | 349 (42) |
| Dietary manipulation (2D) | 459 (42)b | 307 (37) | 755 (69)b | 481 (58) |
| Psychological therapies to reduce stress (2D) | 66 (6)b | 42 (5) | 164 (15)b | 75 (9) |
| Level 1 recommendation of insufficient evidence to recommend use | ||||
| Antidiarrheals (1D) | 394 (36)b | 208 (25) | 711 (65)b | 382 (46) |
| No recommendation provided; not evaluated | ||||
| OTC agents for upset stomach (NA) | 306 (28) | 324 (39)c | 667 (61) | 498 (60) |
| Lifestyle adjustments to reduce stress (NA) | 252 (23)b | 141 (17) | 449 (41)b | 232 (28) |
| Incorporation of more exercise into routine (NA) | 186 (17)b | 100 (12) | 372 (34)b | 183 (22) |
| Treatments, mean | 2.6 | 2.1 | 4.9 | 3.4 |
IBS-D, diarrhea-predominant irritable bowel syndrome; NA, not applicable; OTC, over the counter.
Scoring of recommendations and quality of evidence based on GRADE approach: 1=strong recommendation for or against use; 2=weak recommendation for or against use; A=high quality of evidence; B=moderate quality of evidence; C=low quality of evidence; D=very low quality of evidence. Recommendation and quality of evidence data from Ford et al. (2).
Used within the past 3 months.
P<0.05 vs. undiagnosed.
P<0.05 vs. diagnosed.
Table 3. Lifestyle modifications used to manage symptoms of irritable bowel syndrome.
| Positive response to “Which, if any, |
Individuals,
N
(%) |
|
|---|---|---|
| of the following things do you do in your day-to-day life in order to manage your (IBS/stomach problems)?” | Diagnosed with IBS-D (n=1,094) | Undiagnosed (n=830) |
| Avoid foods that I think will upset my stomach | 799 (73)a | 506 (61) |
| Always know where bathrooms are located | 733 (67)a | 407 (49) |
| Keep OTC medications or supplements handy at all times | 569 (52)a | 374 (45) |
| Avoid stressful situations | 350 (32)a | 199 (24) |
| Always stay near a bathroom | 350 (32)a | 183 (22) |
| Carry wipes when you're on the go | 252 (23)a | 133 (16) |
| Avoid drinking alcohol | 219 (20)a | 133 (16) |
| Put off or avoid intimacy with a partner | 197 (18)a | 116 (14) |
| Wear different clothes such as oversized, looser, or dark-colored items | 186 (17) | 116 (14) |
| Carry an extra set of clothing | 142 (13)a | 66 (8) |
| Avoid exercise | 44 (4) | 25 (3) |
| Other | 55 (5)a | 17 (2) |
| None of the above | 44 (4) | 83 (10)b |
| Current lifestyle adjustments, mean | 3.7 | 2.9 |
IBS, irritable bowel syndrome; IBS-D, diarrhea-predominant irritable bowel syndrome; OTC, over the counter.
P<0.05 vs. undiagnosed.
P<0.05 vs. diagnosed.
The largest share of participants received treatment for their IBS symptoms from primary care physicians (41% of diagnosed patients and 38% of undiagnosed individuals). Of these, internists treated 8% and 6% of diagnosed and undiagnosed individuals for IBS symptoms, respectively (P<0.05). A significantly greater percentage of diagnosed patients received treatment from a gastroenterologist compared with undiagnosed individuals (23% vs. 9%, respectively; P<0.05). In all, 26% of diagnosed patients and 43% of undiagnosed individuals were currently not receiving treatment for their IBS symptoms.
Perceptions about etiology of IBS symptoms
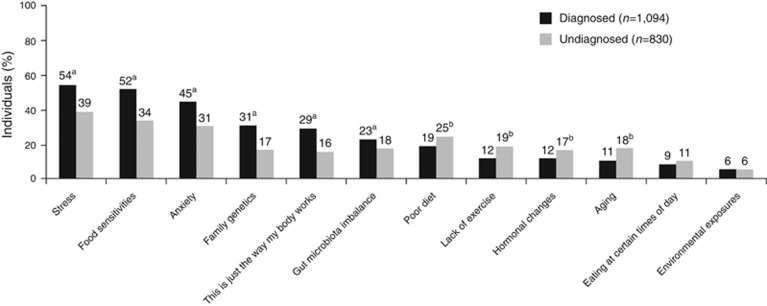
More than half of diagnosed patients believed that stress (54%) and sensitivities to specific foods (52%) caused IBS as compared with 39% and 34% of undiagnosed individuals, respectively (P<0.05 for both comparisons; Figure 3). Furthermore, diagnosed patients were significantly more likely than undiagnosed individuals to believe anxiety (45% vs. 31%, respectively), genetics (31% vs. 17%, respectively), and/or imbalances in the gut microbiota (“imbalance of bacteria within my stomach or gut” 23% vs. 18%, respectively) caused IBS (P<0.05 for all comparisons). Finally, 29% and 16% of diagnosed and undiagnosed participants, respectively, believed “this is just the way my body works” was a cause for IBS (P<0.05).
Figure 3.
Participant perceptions of irritable bowel syndrome (IBS) etiology. This figure shows the percentage of individuals responding “definitely a cause” to the question “To the best of your knowledge, which of the following do you believe are causing your IBS/stomach problems?”. aP<0.05 vs. undiagnosed group. bP<0.05 vs. diagnosed group.
Almost half (47%) of undiagnosed individuals reported speaking with their physician about stomach problems, with the majority of these individuals speaking with 1 or 2 physicians (54% and 33%, respectively). Most undiagnosed individuals had considered that they might have IBS (67% Supplementary Figure S2 online). However, 61% of undiagnosed individuals never considered they might have the IBS-D form of IBS. Another 20% of undiagnosed individuals reported that they had considered that they might have IBS-D, but had never asked their physician about it, and 19% of undiagnosed individuals reported speaking with their physician, who stated that they did not have IBS-D.
Diagnosed and undiagnosed participants reported that their physicians had described a mean of 3.1 and 2.3 factors, respectively, that may be contributing to their IBS/stomach problems. Only 15% of participants reported that they were provided only one cause of their IBS/stomach problems by physicians. Factors provided by physicians varied significantly between diagnosed and undiagnosed participants and included stress or anxiety (67% vs. 37%, respectively; P<0.05), specific food sensitivities (58% vs. 32%, P<0.05), gut microbiota imbalances (34% vs. 16% P<0.05), genetics (32% vs. 14% P<0.05), and “this is just the way my body works” (21% vs. 7% P<0.05).
Discussion
In this study, we report on a cohort of internet survey responders who met Rome III criteria for IBS-D, comparing the clinical features of participants formally “diagnosed” with IBS-D with those of “undiagnosed” individuals similarly affected by IBS-D symptoms. The survey revealed that more than two in every five individuals experiencing IBS-D symptoms had not been formally diagnosed with the condition.
Although this undiagnosed group reflects a substantial portion of individuals reporting IBS-like symptoms, a smaller US study by Hungin et al. (6) previously reported that most individuals (>75%) meeting IBS diagnostic criteria had not been formally diagnosed by a physician with IBS (any subtype). However, only 53% of those not medically diagnosed with IBS had visited a medical professional at some point for their condition; this rate is in line with that observed in the current study. Although this study employed different study methodologies and criteria to define IBS, and limited its focus to diarrhea-predominant symptoms, we interpret this improvement in rates of IBS diagnosis to, in part, reflect an enhanced awareness of IBS on behalf of patients and physicians alike. Earlier research has suggested that gastroenterologists have reasonable familiarity with Rome diagnostic criteria, whereas general practitioners may be less familiar with these criteria (12, 13), and perhaps less comfortable in making an IBS diagnosis based on symptom-based criteria alone (14). This latter observation might continue to pose a challenge in establishing definitive IBS diagnoses, particularly in light of these data suggesting that less than half of potential IBS diagnoses are made by gastroenterologists. Gastroenterologist consultation ultimately may be an important step in expediting IBS diagnoses; in those experiencing IBS-D symptoms for <5 years in this survey, gastroenterologist consultations were reported by IBS-D-diagnosed individuals at rates double those of undiagnosed participants (51% vs. 25%, respectively). It should be acknowledged that the IBS-D-diagnosed patients generally utilized more healthcare services, with higher rates of physician consultation (even when stratified by duration of symptoms), and significantly greater rates of GI/abdominal surgery. These health-seeking behaviors of IBS-D-diagnosed patients alone may have facilitated physician diagnoses.
Individuals diagnosed with IBS-D in this study reported more severe and disruptive symptoms, particularly abdominal pain and alterations in bowel frequency/consistency, and endorsed greater impact of their symptoms on HRQOL. Chronic symptoms without revelation of an organic process should further facilitate an IBS diagnosis, and, indeed, more than half of IBS-diagnosed participants in this survey reported having symptoms for >10 years; conversely, 51% of undiagnosed individuals reported symptoms of <3 years in duration. Undiagnosed individuals in this study reported proportionately greater numbers of GI symptoms not typically associated with IBS-D, such as heartburn/GI reflux disease, that may have further confounded physicians' abilities to confidently diagnose IBS-D. Taken together, these data highlight the importance of maintaining a high index of suspicion in establishing a diagnosis of IBS, particularly in cases with milder symptoms of shorter durations, or in the context of less typical symptom presentations.
The data suggest that formal IBS-D diagnoses may be important for two reasons. First, an IBS diagnosis may facilitate the dialogue between the patient and physician about treatment options. Patients diagnosed with IBS-D were offered more treatments and were found to have greater access to evidence-based, proven therapies. Indeed, a greater percentage of patients with diagnosed IBS received prescription treatment for symptoms compared with undiagnosed individuals with IBS symptoms (50% vs. 30%, respectively) (8). Although dietary modification and antidiarrheals were tried at some point by approximately half to two-thirds of individuals with undiagnosed or diagnosed IBS-D, respectively, antibiotics, antispasmodics, and serotonin (5-hydroxytryptamine type 3) antagonists that have beneficial effects in IBS-D (2, 15) were tried in only a small subset of patients. Patients diagnosed with IBS were also significantly more likely (P<0.05) in this study to manage symptoms by engaging in lifestyle modifications for which there are data supporting their use, such as abstaining from problematic foods and avoidance of stressful situations (16, 17). Minimizing the use of prescription medications, when possible, has been suggested for patients with IBS, as patient education and reassurance are considered key aspects of disease management (18). Symptom severity clearly is an important factor influencing therapeutic interventions; a previous study demonstrated that patients with severe IBS had a greater mean number of physician visits compared with patients with mild or moderate IBS (19). Results of this same study indicated that patients with severe IBS were receiving a greater mean number of medications compared with patients with mild or moderate IBS (2.5 vs. 1.6 and 1.9, respectively) (19).
The second reason why a formal IBS-D diagnosis is valuable is that patients with this distinction appeared to be better informed about IBS pathophysiology, reporting explanations for their symptoms that are more scientifically derived (e.g., diet, genetics, and intestinal microbiota) compared with undiagnosed individuals. Conversely, one-third of those without an IBS-D diagnosis reported that they had been provided no explanation by their healthcare provider for their GI symptoms. Once established, a diagnosis enables the symptomatic individual to seek out additional informational and support resources; such knowledge is central to patient empowerment strategies that have recently proven useful in the management of a variety of chronic pain conditions (20, 21) and may similarly enhance IBS-D treatment outcomes.
Limitations of this study include those inherent to any survey-based design (e.g., potential for sampling and recall bias). Recall bias may play a role in reporting of symptom severity (22), and comorbid conditions could potentially influence recall of symptom severity (22). The cross-sectional nature of the study clearly does not allow for the investigation of causal relationships, and the analyses do not allow one to entirely separate the influences of symptom severity and IBS diagnosis. This study also did not comprehensively assess for comorbid functional disorders or structural diagnoses; without access to the patient medical records, physician diagnoses of IBS could not be confirmed. Nevertheless, this study employed measures that were both reliable and validated; the study also successfully highlights several important clinical distinctions between “real-world” samples of symptomatic individuals with or without an IBS diagnosis (23). Although we only studied patients with IBS-D symptoms using Rome III criteria, we anticipate that similar findings likely would be present in IBS patients with constipation and mixed bowel patterns, and largely would extrapolate to the new Rome IV criteria (3), although this needs to be studied.
In conclusion, a substantial percentage of individuals meeting Rome III criteria for IBS-D have not been formally diagnosed with IBS. Individuals with undiagnosed IBS generally experience milder and less disruptive GI symptoms, experience fewer supportive symptoms of IBS (e.g., urgency, bloating), and report symptoms for fewer years than their counterparts diagnosed with IBS-D; all these factors may challenge the clinician's ability to make a definitive IBS-D diagnosis, particularly in the primary care setting. Nevertheless, it is important that providers maintain an appropriate index of suspicion in diagnosing IBS, as clinical recognition of this condition may enhance patient insight into potential etiopathologic factors that trigger symptoms (i.e., diet, stress) and may facilitate physician implementation of more proven, evidence-based treatment approaches.
Study Highlights
Acknowledgments
Technical editorial assistance was provided, under the direction of the authors, by Mary Beth Moncrief, PhD, and Sophie Bolick, PhD, Synchrony Medical Communications, West Chester, PA. Funding for this support was provided by Salix Pharmaceuticals (Raleigh, NC).
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
Guarantor of the article: Lin Chang, MD.
Specific author contributions: Lin Chang and Gregory S. Sayuk analyzed data and wrote and edited the manuscript. Ray Wolf critically reviewed and edited the manuscript. All authors approved the final version of the manuscript.
Financial support: This study was funded by Salix Pharmaceuticals.
Potential competing interests: Lin Chang has served on scientific advisory boards for QOL Medical, AstraZeneca, Ardelyx, Commonwealth Laboratories, a Division of Valeant Pharmaceuticals North America, Synergy Pharmaceuticals, Actavis, and Bioamerica. Gregory Sayuk is a speaker for Ironwood/Allergan. Ray Wolf is a former employee of Salix Pharmaceuticals.
Supplementary Material
References
- Longstreth GF, Thompson WG, Chey WD et al. Functional bowel disorders. Gastroenterology 2006;130:1480–1491. [DOI] [PubMed] [Google Scholar]
- Ford AC, Moayyedi P, Lacy BE et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol 2014;109:S2–S26. [DOI] [PubMed] [Google Scholar]
- Lacy BE, Mearin F, Chang L et al. Bowel disorders. Gastroenterology 2016;150:1393–1407. [DOI] [PubMed] [Google Scholar]
- Nellesen D, Yee K, Chawla A et al. A systematic review of the economic and humanistic burden of illness in irritable bowel syndrome and chronic constipation. J Manag Care Pharm 2013;19:755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito YA, Schoenfeld P, Locke GR III. The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol 2002;97:1910–1915. [DOI] [PubMed] [Google Scholar]
- Hungin APS, Chang L, Locke GR et al. Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Aliment Pharmacol Ther 2005;21:1365–1375. [DOI] [PubMed] [Google Scholar]
- Ladabaum U, Boyd E, Zhao WK et al. Diagnosis, comorbidities, and management of irritable bowel syndrome in patients in a large health maintenance organization. Clin Gastroenterol Hepatol 2012;10:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungin AP, Whorwell PJ, Tack J et al. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther 2003;17:643–650. [DOI] [PubMed] [Google Scholar]
- Molinder H, Agreus L, Kjellstrom L et al. How individuals with the irritable bowel syndrome describe their own symptoms before formal diagnosis. Ups J Med Sci 2015;120:276–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt LJ, Chey WD, Foxx-Orenstein AE et al. An evidence-based systematic review on the management of irritable bowel syndrome. Am J Gastroenterol 2009;104:S8–S28. [DOI] [PubMed] [Google Scholar]
- Menees SB, Powell C, Kurlander J et al. A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am J Gastroenterol 2015;110:444–454. [DOI] [PubMed] [Google Scholar]
- Charapata C, Mertz H. Physician knowledge of Rome symptom criteria for irritable bowel syndrome is poor among non-gastroenterologists. Neurogastroenterol Motil 2006;18:211–216. [DOI] [PubMed] [Google Scholar]
- Longstreth GF, Burchette RJ. Family practitioners' attitudes and knowledge about irritable bowel syndrome: effect of a trial of physician education. Fam Pract 2003;20:670–674. [DOI] [PubMed] [Google Scholar]
- Shivaji UN, Ford AC. Beliefs about management of irritable bowel syndrome in primary care: cross-sectional survey in one locality. Prim Health Care Res Dev 2015;16:263–269. [DOI] [PubMed] [Google Scholar]
- Chang L, Lembo A, Sultan S. American Gastroenterological Association technical review on the pharmacological management of irritable bowel syndrome. Gastroenterology 2014;147:1149–1172. [DOI] [PubMed] [Google Scholar]
- Rao SS, Yu S, Fedewa A. Systematic review: dietary fibre and FODMAP-restricted diet in the management of constipation and irritable bowel syndrome. Aliment Pharmacol Ther 2015;41:1256–1270. [DOI] [PubMed] [Google Scholar]
- Khan S, Chang L. Diagnosis and management of IBS. Nat Rev Gastroenterol Hepatol 2010;7:565–581. [DOI] [PubMed] [Google Scholar]
- Sayuk GS, Gyawali CP. Irritable bowel syndrome: modern concepts and management options. Am J Med 2015;128:817–827. [DOI] [PubMed] [Google Scholar]
- Drossman DA, Morris CB, Schneck S et al. International survey of patients with IBS: symptom features and their severity, health status, treatments, and risk taking to achieve clinical benefit. J Clin Gastroenterol 2009;43:541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister M, Dunn G, Payne K et al. Patient empowerment: the need to consider it as a measurable patient-reported outcome for chronic conditions. BMC Health Serv Res 2012;12:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Boveldt N, Vernooij-Dassen M, Leppink I et al. Patient empowerment in cancer pain management: an integrative literature review. Psychooncology 2014;23:1203–1211. [DOI] [PubMed] [Google Scholar]
- Mujagic Z, Leue C, Vork L et al. The Experience Sampling Method—a new digital tool for momentary symptom assessment in IBS: an exploratory study. Neurogastroenterol Motil 2015;27:1295–1302. [DOI] [PubMed] [Google Scholar]
- Whitehead WE, Validation Working Team in Association with the Rome Questionnaire Committee. Development and validation of the Rome III diagnostic questionnaire. In:Drossman DA, Corazziari E, Delvaux M, et al(eds). Rome III: The Functional Gastrointestinal Disorders 3rd edn Degnon Associates: McLean, VA. 2006. pp 835–853. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.