Abstract
IMPORTANCE
Recent studies have yielded conflicting results as to whether testosterone treatment increases cardiovascular risk.
OBJECTIVE
To test the hypothesis that testosterone treatment of older men with low testosterone slows progression of noncalcified coronary artery plaque volume.
DESIGN, SETTING, AND PARTICIPANTS
Double-blinded, placebo-controlled trial at 9 academic medical centers in the United States. The participants were 170 of 788 men aged 65 years or older with an average of 2 serum testosterone levels lower than 275 ng/dL (82 men assigned to placebo, 88 to testosterone) and symptoms suggestive of hypogonadism who were enrolled in the Testosterone Trials between June 24, 2010, and June 9, 2014.
INTERVENTION
Testosterone gel, with the dose adjusted to maintain the testosterone level in the normal range for young men, or placebo gel for 12 months.
MAIN OUTCOMES AND MEASURES
The primary outcome was non calcified coronary artery plaque volume, as determined by coronary computed tomographic angiography. Secondary outcomes included total coronary artery plaque volume and coronary artery calcium score (range of 0 to >400 Agatston units, with higher values indicating more severe atherosclerosis).
RESULTS
Of 170 men who were enrolled, 138 (73 receiving testosterone treatment and 65 receiving placebo) completed the study and were available for the primary analysis. Among the 138 men, the mean (SD) age was 71.2 (5.7) years, and 81%were white. At baseline, 70 men (50.7%) had a coronary artery calcification score higher than 300 Agatston units, reflecting severe atherosclerosis. For the primary outcome, testosterone treatment compared with placebo was associated with a significantly greater increase in noncalcified plaque volume from baseline to 12 months (from median values of 204 mm3 to 232 mm3 vs 317 mm3 to 325 mm3, respectively; estimated difference, 41 mm3; 95%CI, 14 to 67 mm3; P = .003). For the secondary outcomes, the median total plaque volume increased from baseline to 12 months from 272 mm3 to 318 mm3 in the testosterone group vs from 499 mm3 to 541 mm3 in the placebo group (estimated difference, 47 mm3; 95%CI, 13 to 80 mm3; P = .006), and the median coronary artery calcification score changed from 255 to 244 Agatston units in the testosterone group vs 494 to 503 Agatston units in the placebo group (estimated difference, −27 Agatston units; 95%CI, −80 to 26 Agatston units). No major adverse cardiovascular events occurred in either group.
CONCLUSIONS AND RELEVANCE
Among older men with symptomatic hypogonadism, treatment with testosterone gel for 1 year compared with placebo was associated with a significantly greater increase in coronary artery noncalcified plaque volume, as measured by coronary computed tomographic angiography. Larger studies are needed to understand the clinical implications of this finding.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT00799617
Although testosterone replacement is increasingly being used clinically,1 the cardiovascular benefits and risks of testosterone administration to older men with age-related decline in testosterone levels remain uncertain. Several observational studies show an inverse association between serum testosterone concentration and adverse cardiovascular outcomes, the metabolic syndrome, diabetes, and mortality,2–5 independent of traditional cardiovascular risk factors.
Studies of the effects of testosterone on clinical cardiovascular outcomes are conflicting.6 Meta-analyses of clinical trials have shown no association between testosterone treatment and cardiovascular adverse events, but none of the individual trials included in the meta-analyses were designed to assess these events prospectively. A clinical trial in older men with mobility limitation showed an excess of cardiovascular adverse events in men treated with testosterone compared with placebo,7 but another trial in a similar population did not.8 These trials were also not designed to assess cardiovascular adverse events. Retrospective analyses of electronic medical records to evaluate the possible association of testosterone treatment with cardiovascular adverse events have also yielded conflicting results.9–13
The Testosterone Trials (TTrials), a group of 7 placebo-controlled, coordinated trials, were designed to determine the efficacy of testosterone treatment of men aged 65 years or older with low testosterone concentrations for no apparent reason other than age. The Cardiovascular Trial was designed to test the hypothesis that testosterone treatment of older men with low testosterone slows the progression of noncalcified coronary artery plaque volume, assessed by coronary computed tomographic angiography (CCTA), as an indicator of coronary atherosclerosis.
Methods
Study Design
The overall study design of the TTrials14 as well as the design of the Cardiovascular Trial15 have been published. To qualify for the TTrials overall, a participant had to qualify for at least 1 of the 3 main trials (Sexual Function Trial, Physical Function Trial, and Vitality Trial). Those who qualified overall could participate in any of the others for which they qualified. Participants were allocated to receive testosterone or placebo gel for 1 year. This article describes the results of the Cardiovascular Trial.
The TTrials and the Cardiovascular Trial protocols were approved by the institutional review boards of the participating sites. The trial protocols for the TTrials and the Cardiovascular Trial are available in Supplement 1. All participants provided written informed consent. Participant safety and trial conduct were overseen by an independent data and safety monitoring board.
Participants
Participants were recruited primarily by mass mailings.16 Respondents were screened by telephone and then during 2 clinic visits. The main overall inclusion criteria were men aged 65 years or older, serum testosterone levels that averaged lower than 275 ng/dL (to convert to nanomoles per liter, multiply by 0.0347) on 2 morning samples, and subjective complaints and objective evidence of sexual dysfunction, physical dysfunction, and/or reduced vitality. The main exclusion criterion was high risk of prostate cancer.14 Men who had a history of myocardial infarction or stroke within the previous 3 months and who had a systolic blood pressure higher than 160 mm Hg or diastolic blood pressure higher than 100 mmHg were excluded.
Key Points.
Question
Is testosterone treatment of older men with low testosterone associated with a decrease in noncalcified coronary artery plaque volume?
Findings
In a controlled clinical trial, 1 year of testosterone treatment of men aged 65 years or older with a low serum testosterone level was associated with a significant increase in noncalcified coronary artery plaque volume of 41 mm3 more than placebo.
Meaning
Testosterone treatment of older men was associated with an increase in coronary artery plaque volume, but additional studies are needed to determine the clinical significance.
Additional exclusion criteria for the Cardiovascular Trial were related to CCTA: conditions that either increased the risk of performing the procedure (estimated glomerular filtration rate <60 mL/min/1.73 m2 or known allergy to iodinated contrast medium) or made the procedure technically impractical (weight >136 kg, inability to hold the breath for 10 seconds, a prior diagnosis of tachycardia or irregular heart rhythm [eg, atrial fibrillation], or history of coronary artery bypass graft surgery). Self-report of race and ethnicity, by fixed categories, was collected as required by the National Institutes of Health.
Testosterone Treatment
Participants were allocated to treatment by minimization, a computerized allocation technique that calculates the assignment that provides the best balance across groups on specified baseline characteristics. A random component was included by assigning the optimally balancing treatment with 80% probability.14 The balancing variables included participation in the main trials, trial site, age younger than or older than 75 years, screening testosterone concentration lower than or higher than 200 ng/dL, and use of antidepressants and phosphodiesterase type 5 (PDE5) inhibitors.14 There was only 1 treatment assignment that applied to all trials in which a man participated.
Testosterone was administered as a 1% gel in a pump bottle (AndroGel). Placebo gel was similar. The dose was initially 5 g/d and was adjusted, if necessary, on the basis of testosterone levels measured at a central laboratory (Quest Clinical Trials) at months 1, 2, 3, 6, and 9, to try to keep the serum concentration within the normal range for young men (280–873 ng/dL). To maintain blinding when the dose was adjusted in a man receiving testosterone treatment, the dose was changed simultaneously in a man receiving placebo.
Assessments
The concentrations of testosterone, free testosterone, and estradiol were measured on serum samples drawn at baseline and months 3, 6, 9, and 12 and stored at −80°C. These assays were performed at the Brigham Research Assay Core as previously described.17
Coronary artery plaque volume was assessed by CCTA at 9 of the 12 TTrials clinical sites. Each of these sites had at least a 64-slice CCTA scanner and staff experienced in CCTA, as determined by a questionnaire.15 Precontrast scans for evaluation of coronary artery calcium density and post contrast scans for evaluation of coronary artery plaque volume were performed at baseline and 12 months. If a participant who had a baseline scan developed an allergy to contrast medium or experienced a decrease in estimated glomerular filtration rate to 60 mL/min/1.73 m2 or less before the month 12 scan, only the precontrast scan was performed at month 12.
All scans were evaluated at a central reading center (Harbor-UCLA Medical Center) by an investigator blinded to treatment assignment. Coronary images were transferred to the workstation with the use of semiautomated plaque analysis software (QAngio CT Research Edition Version 2.0.5; Medis Medical Imaging Systems) and evaluated using a protocol for quantitative plaque assessment.18,19 Vessel diameters greater than 1.5 mm were evaluated and assessed based on a Society of Cardiovascular Computed Tomography 17-segment coronary artery model.20
The readers (R.N., N.N., and S.M.), blinded to both treatment group and date of scan, evaluated the baseline and month 12 scans side by side to ensure that the same segments were compared and measured. The area of each coronary plaque visualized in at least 2 adjacent slices (reconstructed slice thickness of 0.6 mm) was determined on all affected slices. The total plaque volume per segment was summed over all segments with plaque. In addition, each coronary territory (right coronary artery, left main artery, left anterior descending artery, and left circumflex artery) was scored according to presence of the most significant lesion.
The volumes of 4 types of coronary artery plaque—low attenuation, fibrous-fatty, fibrous, and dense calcium—were calculated by the Hounsfield unit threshold. The Hounsfield unit threshold was changed dynamically by the software, as plaque attenuation values are affected by luminal contrast densities.21 The primary outcome was noncalcified plaque volume, defined as the sum of the low attenuation, fibrous-fatty, and fibrous plaque volumes. (The primary outcome was originally [October 2010] total plaque volume but was changed to noncalcified plaque volume in the final protocol of October 2012 [Supplement 1]. Determination of plaque volume was not made until all participants had completed the trial in June 2014.) The secondary outcomes were (1) total plaque volume, defined as the sum of the volume of all 4 types of coronary artery plaque volume (low attenuation, fibrous-fatty, fibrous, and dense calcium); and (2) coronary artery calcium score. The exploratory outcomes were the 4 individual components of plaque volume.
Reproducibility of the coronary artery plaque volume readings in the reading center was assessed by dividing the analyzed scans into quartiles based on noncalcified plaque volume and selecting 10 scans from each quartile to ensure representation of the full range of plaque volumes. One original reader read each of the 40 scans once, and a new reader read each twice, a week apart and in a different order. Intraobserver and interobserver reliability for the primary outcome of noncalcified plaque volume was assessed using linear regression and Bland-Altman plots. The intraclass correlations (ICCs) and coefficients of variation (CVs) were also calculated. For noncalcified plaque volume, the intraobserver CV was 7.8%, the intraobserver ICC was 0.99, the interobserver CV was 19.9%, and the interobserver ICC was 0.95. The intraobserver and interobserver variability was greater for some of the individual plaque components. Details are provided in the eAppendix, eFigure 1, eFigure 2, and eTable 1 in Supplement 2.
Coronary artery calcium score, a secondary outcome, was determined on precontrast scans as previously described.22
Statistical Analyses
The association of testosterone with outcomes related to coronary artery plaque and calcium scores was assessed using a multivariable linear model that adjusted for baseline plaque volume and all balancing variables used in the minimization procedure: study site, indicator variables of participation in each primary efficacy trial, baseline testosterone concentration (≤200 ng/dL or >200 ng/dL), age (≤75 years or >75 years), use of antidepressants, and use of PDE5 inhibitors as covariables. The adjusted mean difference was calculated for the change in plaque volume from baseline to month 12 for men allocated to testosterone compared with placebo. All participants with measurements of both baseline and 12-month plaque volumes were included in the analysis. Significance of the association between testosterone treatment and plaque volume was assessed using the 2-sided Wald test and 95% confidence interval. Assessment of the association of the use of statins and assessment of change in testosterone, free testosterone, and estradiol levels with change in plaque volume for men assigned to testosterone treatment were done by regression analysis, adjusting for balancing variables and baseline plaque volume. A sensitivity analysis to assess the potential effect of missing data was performed by multiple imputation with all known and measured risk factors for cardiovascular disease (age; body mass index [calculated as weight in kilograms divided by height in meters squared]; smoking status; diabetes; hypertension; prior myocardial infarction; prior stroke; revascularization; sleep apnea; use of medications for diabetes, hypertension, or lipid lowering; baseline plaque volumes; and baseline testosterone level) in addition to the balancing factors used to develop the model. The Markov chain Monte Carlo method was used to impute the missing values under the assumption of an arbitrary missing pattern. No adjustments were made for multiple testing. All hypothesis testing used a 2-sided P = .05 significance threshold. All analyses were performed using SAS version 9.4 statistical software (SAS Institute Inc).
The sample size for this trial was initially estimated to be 400 men in the protocol of October 2010 but was later reduced to 140 men when the primary outcome was changed from total to noncalcified plaque volume, because the latter has a smaller standard deviation.23 Using a standard deviation of 26 mm3 for the change in volume from scans obtained at 2 time points approximately 1 year apart in that study, 140 men (70 per group) with both baseline and month 12 scans would provide 80% power to detect a difference of 12 mm3 in the change in noncalcified plaque volume from baseline to 12 months (protocol of October 2012, Supplement 1). This difference was chosen to be smaller than the 14- to 15-mm3 difference seen between statin users and nonusers.23
Results
Participants
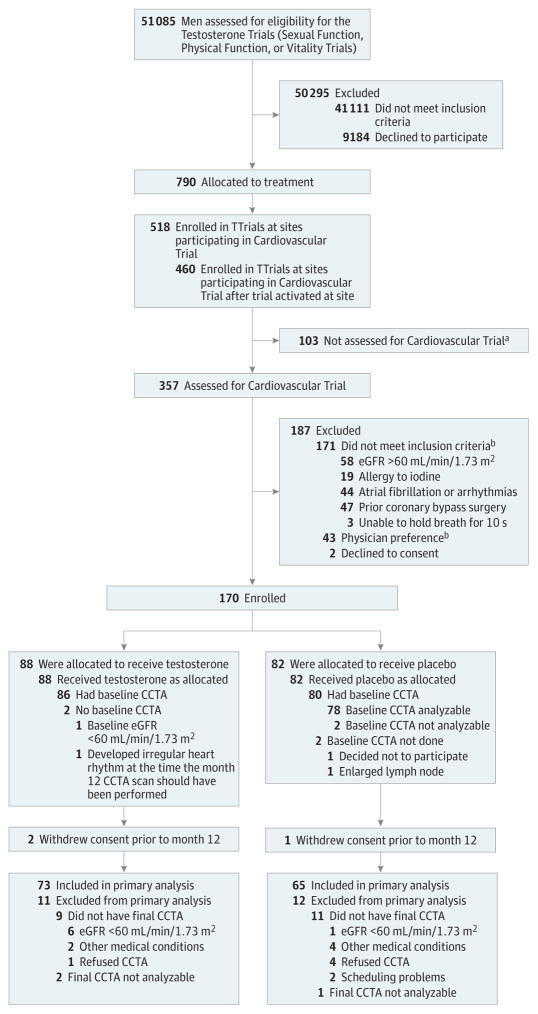
Recruitment began on April 28, 2011. Targeted enrollment was completed on June 11, 2013, and treatment was completed on June 16, 2014. Of the 460 men enrolled in the TTrials at the 9 sites participating in the Cardiovascular Trial, 170 consented and enrolled, and 166 had a baseline scan, 86 in the testosterone group and 80 in the placebo group (Figure). A total of 138 men had a month 12 scan, 73 in the testosterone group and 65 in the placebo group, that could be analyzed. Nine men in the testosterone group and 11 men in the placebo group did not have month 12 scans even though they were still enrolled, because they either developed a reason for exclusion or refused to have the second scan. eTable 2 in Supplement 2 shows the numbers of men in this trial who participated in the 3 main trials of the TTrials.
Figure. Screening and Retention of Participants in the Cardiovascular Trial.
CCTA indicates coronary computed tomographic angiography; eGFR, estimated glomerular filtration rate; and TTrials, Testosterone Trials.
aReasons unknown; no assessment form submitted.
bSome men had more than 1 reason.
At baseline, the mean (SD) age was 71.2 (5.7) years, and 81% were white. The participants had relatively high rates of obesity and concomitant illnesses, such as hypertension, hyperlipidemia, and diabetes, as well as relatively high 10-year risk of a cardiovascular event by the American College of Cardiology/American Heart Association risk calculator24 (a mean risk of 24% [95% CI, 2.6%–45.4%] in the testosterone group and 27% [95% CI, 6.4%–47.6%] in the placebo group) (Table 1). The prevalence of atherosclerosis, assessed radiographically by a coronary artery calcification score higher than 300 Agatston units, was also high (70 men [50.7%] overall; 60.3% in the placebo group and 43.8% in the testosterone group).
Table 1.
Baseline Characteristics of Men in the Cardiovascular Trial
| Characteristic | Treatment Group | |
|---|---|---|
| Testosterone (n = 73) | Placebo (n = 65) | |
| Demographics | ||
| Agea | ||
| Mean (SD), y | 70.5 (5.7) | 72.0 (5.7) |
| No. (%) | ||
| ≤75 y | 63 (86.3) | 51 (78.5) |
| >75 y | 10 (13.7) | 14 (21.5) |
| Race, No. (%) | ||
| White | 60 (82.2) | 52 (80.0) |
| Black | 7 (9.6) | 8 (12.3) |
| Otherb | 6 (8.2) | 5 (7.7) |
| Ethnicity, No. (%) | ||
| Hispanic | 5 (6.8) | 2 (3.1) |
| Non-Hispanic | 68 (93.2) | 63 (96.9) |
| College graduate, No. (%) | 41 (56.2) | 36 (55.4) |
| Married or living with partner, No. (%) | 48 (65.8) | 48 (73.8) |
| Study site, No. (%)a | ||
| Albert Einstein College of Medicine | 5 (6.8) | 8 (12.3) |
| Baylor College of Medicine | 9 (12.3) | 9 (13.8) |
| Boston University Medical Center | 1 (1.4) | 0 |
| Northwestern University | 6 (8.2) | 10 (15.4) |
| Harbor-UCLA Medical Center | 21 (28.8) | 16 (24.6) |
| University of Florida | 4 (5.5) | 5 (7.7) |
| University of Pittsburgh | 8 (11.0) | 6 (9.2) |
| VA Puget Sound Health Care System | 7 (9.6) | 2 (3.1) |
| Yale School of Medicine | 12 (16.4) | 9 (13.8) |
| Main trial participation, No. (%)a | ||
| Sexual Function Trial | 49 (67.1) | 39 (60.0) |
| Physical Function Trial | 34 (46.6) | 35 (53.8) |
| Vitality Trial | 51 (69.9) | 40 (61.5) |
| Concomitant conditions | ||
| BMI | ||
| Mean (SD) | 30.6 (3.8) | 30.4 (3.5) |
| >30, No. (%) | 45 (61.6) | 37 (56.9) |
| Alcohol use, No. of drinks/wk, median (IQR) | 0 (0–2) | 1.0 (0–2) |
| Smoking, No. (%) | ||
| Current smoker | 4 (5.5) | 10 (15.4) |
| Ever smoker | 48 (65.8) | 43 (66.2) |
| Diabetes, No. (%) | 23 (31.5) | 19 (29.2) |
| Hypertension, No. (%) | 49 (67.1) | 42 (64.6) |
| High cholesterol, No. (%) | 46 (63.0) | 42 (64.6) |
| History of myocardial infarction, No. (%) | 6 (8.2) | 6 (9.2) |
| History of stroke, No. (%) | 0 | 2 (3.1) |
| Sleep apnea, No. (%) | 14 (19.2) | 12 (18.5) |
| Coronary artery revascularization, No. (%) | 4 (5.5) | 6 (9.2) |
| ACC/AHA risk score, mean (SD), %c | 24 (10.9) | 27 (10.5) |
| Coronary artery calcium score in Agatston units, No. (%)d | ||
| 0 | 7 (9.6) | 3 (4.8) |
| >0 to <300 | 34 (46.6) | 22 (34.9) |
| ≥300 | 32 (43.8) | 38 (60.3) |
| Medication use, No. (%) | ||
| Antidiabetics | 20 (27.4) | 18 (27.7) |
| Statins | 45 (61.6) | 40 (61.5) |
| Antihypertensives | 46 (63.0) | 39 (60.0) |
| Antidepressantsa | 8 (12.3) | 12 (16.4) |
| PDE5 inhibitorsa | 3 (4.1) | 4 (6.2) |
| Sex hormones | ||
| Testosteronea | ||
| Mean (SD), ng/dL | 225.0 (56.8) | 252.3 (56.4) |
| No. (%) | ||
| ≤200 ng/dL | 23 (31.5) | 12 (18.5) |
| >200 ng/dL | 50 (68.5) | 53 (81.5) |
| Free testosterone, mean (SD), pg/mL | 59.1 (18.2) | 67.6 (22.9) |
| Dihydrotestosterone, mean (SD), ng/dL | 22.7 (16.0) | 21.6 (10.0) |
| Estradiol, mean (SD), pg/mL | 19.7 (5.2) | 21.2 (6.2) |
| Sex hormone–binding globulin, mean (SD), μg/mL | 3.4 (1.7) | 3.3 (1.5) |
Abbreviations: ACC/AHA, American College of Cardiology/American Heart Association; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IQR, interquartile range; PDE5, phosphodiesterase type 5.
SI conversion factors: To convert testosterone to nanomoles per liter, multiply by 0.0347; estradiol to picomoles per liter, multiply by 3.671; and sex hormone–binding globulin to nanomoles per liter, multiply by 8.896.
Factor used to balance the treatment assignment using the minimization procedure.
Includes Asian, North American Indian/Alaska Native, multiracial, and other.
The 10-year risk of heart disease or stroke by the ACC/AHA risk calculator (http://www.cvriskcalculator.com).24
Higher scores indicate more coronary artery calcium.
Testosterone treatment increased the serum testosterone concentrations from unequivocally low to midnormal for young men by month 3 and maintained that level through month 12 (eFigure 3 in Supplement 2). Testosterone treatment also increased the levels of free testosterone and estradiol to midnormal for young men.
CCTA and Coronary Artery Calcium Results
At baseline, noncalcified plaque volume showed considerable variability, and the median in the testosterone group (204 mm3 [interquartile range, 60 to 420 mm3]) was somewhat lower than that in the placebo group (317 mm3 [interquartile range, 168 to 589 mm3]) (Table 2). The components of noncalcified plaque volume (low-attenuation plaque, fibrous-fatty plaque, and fibrous plaque) and total plaque volume (noncalcified plaque plus dense calcium plaque) were also somewhat lower in the testosterone group (Table 2).
Table 2.
Change From Baseline and Estimated Differences for Primary, Secondary, and Exploratory Outcomes in the Cardiovascular Trial
| Outcome | Treatment Group | Estimated Difference (95% CI)a | P Valueb | |
|---|---|---|---|---|
| Testosterone (n = 73) | Placebo (n = 65) | |||
| Primary | ||||
| Noncalcified plaque volume, mm3 | ||||
| Baseline, median (IQR) | 204 (60 to 420) | 317 (168 to 589) | ||
| Month 12, median (IQR) | 232 (103 to 473) | 325 (172 to 560) | ||
| Change from baseline value, unadjusted mean (95% CI) | 40 (23 to 56) | 4 (−14 to 22) | ||
| LS mean (95% CI)c | 54 (12 to 97) | 14 (−29 to 56) | 41 (14 to 67) | .003 |
| Secondary | ||||
| Total plaque volume, mm3 | ||||
| Baseline, median (IQR) | 272 (84 to 600) | 499 (246 to 925) | ||
| Month 12, median (IQR) | 318 (133 to 693) | 541 (248 to 950) | ||
| Change from baseline value, unadjusted mean (95% CI) | 57 (35 to 78) | 21 (0 to 42) | ||
| LS mean (95% CI)c | 75 (22 to 128) | 28 (−24 to 81) | 47 (13 to 80) | .006 |
| Coronary artery calcium score, Agatston unitsd | ||||
| Baseline, median (IQR) | 255 (43 to 963) | 494 (146 to 1892) | ||
| Month 12, median (IQR) | 244 (52 to 1013) | 503 (146 to 2108) | ||
| Change from baseline value, unadjusted mean (95% CI) | 53 (25 to 82) | 118 (73 to 164) | ||
| LS mean (95% CI)c | 64 (−19 to 146) | 91 (7 to 174) | −27 (−80 to 26) | .31 |
| Exploratory | ||||
| Low-attenuation plaque volume, mm3 | ||||
| Baseline, median (IQR) | 7.1 (1.5 to 32.4) | 15.3 (2.6 to 31.1) | ||
| Month 12, median (IQR) | 9.5 (2.1 to 24.2) | 11.0 (3.2 to 30.8) | ||
| Change from baseline value, unadjusted mean (95% CI) | 6 (0 to 12) | 2 (−2 to 6) | ||
| LS mean (95% CI)c | 8 (−4 to 20) | 3 (−9 to 14) | 5 (−2 to 13) | .14 |
| Fibrous-fatty plaque volume, mm3 | ||||
| Baseline, median (IQR) | 40.0 (11.5 to 72.6) | 43.7 (18.9 to 107) | ||
| Month 12, median (IQR) | 46.3 (14.0 to 100) | 54.5 (14.7 to 107) | ||
| Change from baseline value, unadjusted mean (95% CI) | 9 (1 to 17) | 1 (−6 to 9) | ||
| LS mean (95% CI)c | 12 (−7 to 30) | 2 (−17 to 21) | 10 (−2 to 21) | .11 |
| Fibrous plaque volume, mm3 | ||||
| Baseline, median (IQR) | 160 (51.5 to 305) | 254 (122 to 426) | ||
| Month 12, median (IQR) | 177 (64.1 to 320) | 253 (138 to 471) | ||
| Change from baseline value, unadjusted mean (95% CI) | 25 (14 to 35) | 1 (−13 to 15) | ||
| LS mean (95% CI)c | 31 (0 to 62) | 7 (−24 to 37) | 24 (5 to 43) | .01 |
| Dense calcium plaque volume, mm3 | ||||
| Baseline, median (IQR) | 69.5 (13.6 to 211) | 173 (35.2 to 351) | ||
| Month 12, median (IQR) | 74.8 (13.9 to 245) | 177 (47.2 to 323) | ||
| Change from baseline value, unadjusted mean (95% CI) | 17 (7 to 27) | 17 (6 to 28) | ||
| LS mean (95% CI)c | 17 (−8 to 42) | 11 (−14 to 36) | 5 (−11 to 21) | .51 |
Abbreviations: IQR, interquartile range; LS, least squares.
Mean difference in change from baseline for participants assigned to testosterone vs those assigned to placebo, with adjustment for balancing factors: baseline total testosterone level (≤200 or >200 ng/dL [to convert to nanomoles per liter, multiply by 0.0347]), age (≤75 or >75 years), trial site, participation in the main trials, use or nonuse of antidepressants, use or nonuse of phosphodiesterase type 5 inhibitors, and baseline value of the outcome variable.
Determined by a linear mixed model with all balancing factors and baseline outcome value as covariates and a random effect for participant.
Adjusted treatment group mean of the change from baseline to month 12.
Higher values indicate more coronary artery calcium.
For the primary outcome, noncalcified coronary artery plaque volume, testosterone treatment was associated with a significantly greater increase from baseline to month 12 (from median of 204 mm3 to 232 mm3; change: mean, 40 mm3; 95% CI, 23 to 56 mm3) than placebo (from median of 317 mm3 to 325 mm3; change: mean, 4 mm3; 95% CI, −14 to 22 mm3) (estimated difference, 41 mm3; 95% CI, 14 to 67 mm3; P = .003) (Table 2). Sensitivity analysis using multiple imputation to account for missing data resulted in similar point estimates, and confidence intervals were almost identical (estimated difference, 39 mm3; 95% CI, 13 to 65 mm3). The P value for the difference in plaque volume in this analysis was .003.
For the secondary outcome of total plaque volume, testosterone was significantly associated with a greater increase from baseline to month 12 (from a median of 272 mm3 to 318 mm3; change: mean, 57 mm3; 95% CI, 35 to 78 mm3) than placebo (from a median of 499 mm3 to 541 mm3; change: mean, 21 mm3; 95%CI, 0 to 42 mm3) (estimated difference, 47 mm3; 95% CI, 13 to 80 mm3; P = .006). For the secondary outcome of coronary artery calcium score, testosterone was not statistically significantly associated with a change from baseline to 12 months (change in testosterone group: mean, 53 Agatston units; 95% CI, 25 to 82 Agatston units; change in placebo group: mean, 118 Agatston units; 95% CI, 73 to 164 Agatston units). The median scores changed from 255 to 244 Agatston units in the testosterone group vs 494 to 503 Agatston units in the placebo group (estimated difference, −27 Agatston units; 95% CI, −80 to 26 Agatston units; P = .31).
Exploratory analyses of the individual components of noncalcified plaque showed that testosterone treatment was associated with a significantly greater increase in fibrous plaque volume (change, 25 mm3; 95% CI, 14 to 35 mm3) than placebo (change, 1 mm3; 95%CI, −13 to 15 mm3) (estimated difference, 24 mm3; 95%CI, 5 to 43 mm3; P = .01) (Table 2). Testosterone was also associated with greater increases in low-attenuation plaque volume and fibrous-fatty plaque volume, but neither difference reached statistical significance. Testosterone and placebo were associated with almost identical (and not statistically significant) changes in dense calcium plaque volume.
The change in noncalcified coronary artery plaque volume in men in the testosterone group was not associated with changes in levels of total testosterone (r = −0.04; P = .74), free testosterone (r = −0.006; P = .96), or estradiol (r = −0.08; P = .50). The changes were also not associated with statin use (P = .35). Differences in plaque volume were similar for men with baseline coronary artery calcium scores higher and lower than the median value.
Adverse Events
Among the 170 menenrolled in the Cardiovascular Trial, none in either the testosterone treatment group or the placebo group were reported to have a major adverse cardiovascular event.
Discussion
One year of testosterone treatment of men aged 65 years or older with low testosterone was associated with an increase in noncalcified coronary artery plaque volume, as determined by CCTA. Testosterone treatment was also associated with increased total plaque volume, but not with changes in coronary artery calcium score.
Most of the men participating in the Cardiovascular Trial had a severe amount of coronary atherosclerosis at baseline: 70 of 138 men (50.7%) had a coronary artery calcification score higher than 300 Agatston units. Although men in the placebo group at baseline had a somewhat greater mean coronary artery calcium score, as well as a somewhat greater mean noncalcified plaque volume by CCTA, than men in the testosterone group, these differences did not affect the comparison of changes from baseline to month 12; analyses of associations between testosterone and coronary artery calcium score were adjusted for baseline values, and results of men with baseline values higher than the median did not differ appreciably from those with baseline values below the median.
Few prior studies have examined the effect of testosterone on atherosclerosis in men. In a placebo-controlled trial in middle-aged and older men, testosterone treatment for 3 years did not affect the change from baseline in coronary artery calcium score or common carotid artery intima-media thickness.25 In the Cardiovascular Trial, testosterone treatment, although for 1 year only, was not associated with a change in the coronary artery calcium score.
Coronary computed tomographic angiography, as used in the present trial, has the advantage over the coronary artery calcium score of being able to detect noncalcified coronary artery plaque and its components as well as calcified plaque. Although a relatively new technique, it appears to give similar quantitative results to intravascular ultrasonography,26,27 and its excellent intraobserver and interobserver reproducibility for noncalcified plaque volume28,29 makes it feasible for longitudinal studies. Noncalcified plaque volume, as determined by CCTA, has been associated with myocardial ischemia30 and subsequent cardiovascular adverse events.31
The increase in coronary artery noncalcified and total plaque volumes in men treated with testosterone is concerning because any limitation of the vascular lumen could be considered deleterious.32 The clinical significance of these increases could depend on the differential effects of testosterone on the individual components of noncalcified plaque. Testosterone treatment was associated with a significant increase in the volume of fibrous plaque, which may be more stable than other types of plaque.33 A recent review, however, concluded that total plaque burden may be more important than the radiologic characteristics of individual plaques.32
This trial had several strengths, including a placebo-controlled design, selection of men with unequivocally low testosterone, and a relatively high retention rate. This study also has limitations. One limitation is that the results apply only to men aged 65 years or older who have low testosterone. Another limitation is that the assumptions about the composition of plaque components as detected by CCTA have not been confirmed by direct radiologic-pathologic studies. Furthermore, the volume and radiologic characteristics of coronary artery plaques are only surrogate outcomes and do not account for other factors that can influence the frequency and extent of plaque rupture and thrombosis. The major limitation is that the trial was not large enough or long enough to draw conclusions about the risk of testosterone treatment on major adverse cardiovascular events.
Conclusions
Among older men with symptomatic hypogonadism, treatment with testosterone gel for 1 year compared with placebo was associated with a significantly greater increase in coronary artery noncalcified plaque volume, as measured by CCTA. Larger studies are needed to understand the clinical implications of this finding.
Supplementary Material
Acknowledgments
Funding/Support: The Testosterone Trials were supported by grant U01 AG030644 from the National Institute on Aging, supplemented by funds from the National Heart, Lung, and Blood Institute. Additional funding was provided by the National Institute of Neurological Diseases and Stroke and the National Institute of Child Health and Human Development. AbbVie provided funding, AndroGel, and placebo gel. Dr Lewis was supported by grant DK079626 from the National Institute of Diabetes and Digestive and Kidney Diseases to the University of Alabama at Birmingham Diabetes Research and Training Center. Dr Gill is the recipient of Academic Leadership Award K07AG043587 from the National Institute on Aging, and the Yale Field Center is supported in part by the Claude D. Pepper Older Americans Independence Centers grant P30-AG021342 from the National Institute on Aging.
Footnotes
Role of the Funder/Sponsor: The National Institute on Aging and the National Heart, Lung, and Blood Institute gave advice on the design and conduct of the trial. None of the sponsoring organizations participated in collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Author Contributions: Drs Budoff and Ellenberg had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Budoff, Ellenberg, Lewis, Mohler, Wenger, Bhasin, Cunningham, Gill, A. M. Matsumoto, Molitch, Snyder.
Acquisition, analysis, or interpretation of data: Budoff, Ellenberg, Lewis, Mohler, Bhasin, Barrett- Connor, Swerdloff, Stephens-Shields, Cauley, Crandall, Cunningham, Ensrud, Gill, A. M. Matsumoto, Molitch, Nakanishi, Nezarat, S. Matsumoto, Hou, Basaria, Diem, Wang, Cifelli, Snyder.
Drafting of the manuscript: Budoff, Ellenberg, Mohler, Wenger, Bhasin, A. M. Matsumoto, Hou, Snyder.
Critical revision of the manuscript for important intellectual content: Budoff, Ellenberg, Lewis, Mohler, Wenger, Bhasin, Barrett-Connor, Swerdloff, Stephens-Shields, Cauley, Crandall, Cunningham, Ensrud, Gill, A. M. Matsumoto, Molitch, Nakanishi, Nezarat, S. Matsumoto, Basaria, Diem, Wang, Cifelli, Snyder.
Statistical analysis: Ellenberg, Swerdloff, Stephens-Shields, Hou.
Obtained funding: Budoff, Ellenberg, Lewis, Bhasin, A. M. Matsumoto, S. Matsumoto, Snyder.
Administrative, technical, or material support: Lewis, Swerdloff, Cauley, Nakanishi, Nezarat, Snyder.
Study supervision: Budoff, Ellenberg, Bhasin, Barrett-Connor, Stephens-Shields, Crandall, Cunningham, Ensrud, Gill, A. M. Matsumoto, Molitch, Basaria, Diem, Wang, Cifelli, Snyder.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Budoff reported receiving a grant from General Electric. Dr Ellenberg reported receiving a grant from AbbVie. Dr Mohler reported serving as a consultant to Clarus Therapeutics and AbbVie. Dr Wenger reported receiving grants from Alnylam Pharmaceuticals, National Heart, Lung, and Blood Institute, Pfizer, and Society for Women’s Health Research; grants and personal fees from Gilead Sciences; and personal fees from Amgen, AstraZeneca, and Merck. Dr Bhasin reported receiving grants administered by Brigham and Women’s Hospital from AbbVie, Lilly, Transition Therapeutics, and Regeneron; receiving consulting fees from AbbVie, Lilly, and Regeneron; having a patent pending on a free testosterone calculator; and having equity interest in FPT, LLC. Dr Swerdloff reported receiving grants from Clarus Therapeutics, Lipocine, and Antares; and serving as a consultant to Clarus Therapeutics and Antares. Dr Cunningham reported serving as a consultant to AbbVie, Apricus, Besins, Clarus Therapeutics, Endo Pharma, Ferring, and Repros Therapeutics; and serving on advisory boards for AbbVie, Apricus, Clarus Therapeutics, Endo Pharma, Ferring, Lilly, Pfizer, and Repros Therapeutics. Dr A. M. Matsumoto reported receiving research support from AbbVie; receiving consulting fees from AbbVie, Endo, Lilly, the US Anti-Doping Agency, and Lipocine; receiving personal fees from Clarus Therapeutics; receiving royalties from UpTo Date; and serving on the scientific advisory board for the Partnership for Clean Competition. Dr Molitch reported serving as a consultant to AbbVie (Abbott Laboratories), Eli Lilly & Co, and Pfizer. Dr Basaria reported serving as a consultant to Eli Lilly and Takeda Pharmaceuticals. Dr Wang reported receiving research support from Clarus Therapeutics, Lipocine, Antares, and Besins; and serving on advisory committees for Lipocine, Antares, Teso RX, and Besins. Dr Snyder reported serving as a consultant to Watson Laboratories. No other disclosures were reported.
Additional Contributions: We thank Evan Hadley, MD, and Sergei Romashkan, MD, PhD (both from the National Institute on Aging), for their support throughout the trials. Sina Rahmani, MD (Division of Cardiology, Los Angeles Biomedical Research Institute, Harbor-UCLA Medical Center), read computed tomographic angiography scans for the reproducibility study. They received no compensation for their contributions.
References
- 1.Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173(15):1465–1466. doi: 10.1001/jamainternmed.2013.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett-Connor E, Khaw KT. Endogenous sex hormones and cardiovascular disease in men: a prospective population-based study. Circulation. 1988;78(3):539–545. doi: 10.1161/01.cir.78.3.539. [DOI] [PubMed] [Google Scholar]
- 3.Khaw KT, Dowsett M, Folkerd E, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European Prospective Investigation Into Cancer in Norfolk (EPIC-Norfolk) prospective population study. Circulation. 2007;116(23):2694–2701. doi: 10.1161/CIRCULATIONAHA.107.719005. [DOI] [PubMed] [Google Scholar]
- 4.Oh JY, Barrett-Connor E, Wedick NM, Wingard DL Rancho Bernardo Study. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo Study. Diabetes Care. 2002;25(1):55–60. doi: 10.2337/diacare.25.1.55. [DOI] [PubMed] [Google Scholar]
- 5.Smith GD, Ben-Shlomo Y, Beswick A, Yarnell J, Lightman S, Elwood P. Cortisol, testosterone, and coronary heart disease: prospective evidence from the Caerphilly study. Circulation. 2005;112(3):332–340. doi: 10.1161/CIRCULATIONAHA.104.489088. [DOI] [PubMed] [Google Scholar]
- 6.Kloner RA, Carson C, III, Dobs A, Kopecky S, Mohler ER., III Testosterone and cardiovascular disease. J Am Coll Cardiol. 2016;67(5):545–557. doi: 10.1016/j.jacc.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95(2):639–650. doi: 10.1210/jc.2009-1251. [DOI] [PubMed] [Google Scholar]
- 9.Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9(1):e85805. doi: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829–1836. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 11.Baillargeon J, Urban RJ, Kuo YF, et al. Risk of myocardial infarction in older men receiving testosterone therapy. Ann Pharmacother. 2014;48(9):1138–1144. doi: 10.1177/1060028014539918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muraleedharan V, Marsh H, Kapoor D, Channer KS, Jones TH. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol. 2013;169(6):725–733. doi: 10.1530/EJE-13-0321. [DOI] [PubMed] [Google Scholar]
- 13.Shores MM, Smith NL, Forsberg CW, Anawalt BD, Matsumoto AM. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab. 2012;97(6):2050–2058. doi: 10.1210/jc.2011-2591. [DOI] [PubMed] [Google Scholar]
- 14.Snyder PJ, Ellenberg SS, Cunningham GR, et al. The Testosterone Trials: seven coordinated trials of testosterone treatment in elderly men. Clin Trials. 2014;11(3):362–375. doi: 10.1177/1740774514524032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abd Alamir M, Ellenberg SS, Swerdloff RS, et al. The Cardiovascular Trial of the Testosterone Trials: rationale, design, and baseline data of a clinical trial using computed tomographic imaging to assess the progression of coronary atherosclerosis. Coron Artery Dis. 2016;27(2):95–103. doi: 10.1097/MCA.0000000000000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cauley JA, Fluharty L, Ellenberg SS, et al. Recruitment and screening for the Testosterone Trials. J Gerontol A Biol Sci Med Sci. 2015;70(9):1105–1111. doi: 10.1093/gerona/glv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snyder PJ, Bhasin S, Cunningham GR, et al. Testosterone Trials Investigators. Effects of testosterone treatment in older men. N Engl J Med. 2016;374(7):611–624. doi: 10.1056/NEJMoa1506119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto S, Nakanishi R, Li D, et al. Aged garlic extract reduces low attenuation plaque in coronary arteries of patients with metabolic syndrome in a prospective randomized double-blind study. J Nutr. 2016;146(2):427S–432S. doi: 10.3945/jn.114.202424. [DOI] [PubMed] [Google Scholar]
- 19.Papadopoulou SL, Neefjes LA, Garcia-Garcia HM, et al. Natural history of coronary atherosclerosis by multislice computed tomography. JACC Cardiovasc Imaging. 2012;5(3 suppl):S28–S37. doi: 10.1016/j.jcmg.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Raff GL, Abidov A, Achenbach S, et al. Society of Cardiovascular Computed Tomography. SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr. 2009;3(2):122–136. doi: 10.1016/j.jcct.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Dalager MG, Bøttcher M, Andersen G, et al. Impact of luminal density on plaque classification by CT coronary angiography. Int J Cardiovasc Imaging. 2011;27(4):593–600. doi: 10.1007/s10554-010-9695-z. [DOI] [PubMed] [Google Scholar]
- 22.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 23.Zeb I, Li D, Nasir K, et al. Effect of statin treatment on coronary plaque progression—a serial coronary CT angiography study. Atherosclerosis. 2013;231(2):198–204. doi: 10.1016/j.atherosclerosis.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 24.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basaria S, Harman SM, Travison TG, et al. Effects of testosterone administration for 3 years on subclinical atherosclerosis progression in older men with low or low-normal testosterone levels: a randomized clinical trial. JAMA. 2015;314(6):570–581. doi: 10.1001/jama.2015.8881. [DOI] [PubMed] [Google Scholar]
- 26.Boogers MJ, Broersen A, van Velzen JE, et al. Automated quantification of coronary plaque with computed tomography: comparison with intravascular ultrasound using a dedicated registration algorithm for fusion-based quantification. Eur Heart J. 2012;33(8):1007–1016. doi: 10.1093/eurheartj/ehr465. [DOI] [PubMed] [Google Scholar]
- 27.Fischer C, Hulten E, Belur P, Smith R, Voros S, Villines TC. Coronary CT angiography versus intravascular ultrasound for estimation of coronary stenosis and atherosclerotic plaque burden: a meta-analysis. J Cardiovasc Comput Tomogr. 2013;7(4):256–266. doi: 10.1016/j.jcct.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Hamirani YS, Kadakia J, Pagali SR, et al. Assessment of progression of coronary atherosclerosis using multidetector computed tomography angiography (MDCT) Int J Cardiol. 2011;149(2):270–274. doi: 10.1016/j.ijcard.2011.02.056. [DOI] [PubMed] [Google Scholar]
- 29.Schuhbaeck A, Dey D, Otaki Y, et al. Interscan reproducibility of quantitative coronary plaque volume and composition from CT coronary angiography using an automated method. Eur Radiol. 2014;24(9):2300–2308. doi: 10.1007/s00330-014-3253-3. [DOI] [PubMed] [Google Scholar]
- 30.Gaur S, Øvrehus KA, Dey D, et al. Coronary plaque quantification and fractional flow reserve by coronary computed tomography angiography identify ischaemia-causing lesions. Eur Heart J. 2016;37(15):1220–1227. doi: 10.1093/eurheartj/ehv690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miszalski-Jamka T, Klimeczek P, Banyś R, et al. The composition and extent of coronary artery plaque detected by multislice computed tomographic angiography provides incremental prognostic value in patients with suspected coronary artery disease. Int J Cardiovasc Imaging. 2012;28(3):621–631. doi: 10.1007/s10554-011-9799-0. [DOI] [PubMed] [Google Scholar]
- 32.Arbab-Zadeh A, Fuster V. Themyth of the “vulnerable plaque”: transitioning from a focus on individual lesions to atherosclerotic disease burden for coronary artery disease risk assessment. J Am Coll Cardiol. 2015;65(8):846–855. doi: 10.1016/j.jacc.2014.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samady H, Eshtehardi P, McDaniel MC, et al. Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation. 2011;124(7):779–788. doi: 10.1161/CIRCULATIONAHA.111.021824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.