Abstract
Ebola virus, a member of the family Filoviridae, causes one of the most severe forms of viral hemorrhagic fever. In the terminal stages of disease, symptoms progress to hypotension, coagulation disorders, and hemorrhages, and there is prominent involvement of the mononuclear phagocytic and reticuloendothelial systems. Cells of the mononuclear phagocytic system are primary target cells and producers of inflammatory mediators. Ebola virus efficiently produces four soluble glycoproteins during infection: sGP, delta peptide (Δ-peptide), GP1, and GP1,2Δ. While the presence of these glycoproteins has been confirmed in blood (sGP) and in vitro systems, it is hypothesized that they are of biological relevance in pathogenesis, particularly target cell activation. To gain insight into their function, we expressed the four soluble glycoproteins in mammalian cells and purified and characterized them. The role of the transmembrane glycoprotein in the context of virus-like particles was also investigated. Primary human macrophages were treated with glycoproteins and virus-like particles and subsequently tested for activation by detection of several critical proinflammatory cytokines (tumor necrosis factor alpha, interleukin-6 [IL-6], and IL-1 beta) and the chemokine IL-8. The presentation of the glycoprotein was determined to be critical since virus-like particles, but not soluble glycoproteins, induced high levels of activation. We propose that the presentation of GP1,2 in the rigid form such as that observed on the surface of particles is critical for initiating a sufficient signal for the activation of primary target cells. The secreted glycoproteins do not appear to play any role in exogenous activation of these cells during Ebola virus infection.
Ebola virus (EBOV), a member of the family Filoviridae, is a negative-sense, single-stranded, enveloped RNA virus. The prototype virus, Zaire ebolavirus (ZEBOV), strain Mayinga, was responsible for severe hemorrhagic disease in 1976 with high morbidity and mortality (39). Since the initial outbreak in 1976, there have been over 1,500 cases of human infection, with the most recent outbreaks occurring in Gabon and the Republic of the Congo in 2003 (http://www.who.int/mediacentre/factsheets/fs103/en/). The hemorrhagic disease caused by EBOV is characterized by generalized fluid distribution problems, hypotension, coagulation disorders, and bleeding tendency, and it finally results in fulminant shock (25). Primary target cells include mononuclear phagocytic cells, in which the viruses lytically replicate (8, 9, 12, 21). Vascular instability and dysregulation are thought to be disease-decisive symptoms during severe infection and are likely caused by virus-induced activation of mononuclear phagocytic cells and the subsequent production of active mediator molecules, such as proinflammatory cytokines and chemokines (2, 8, 14, 27, 28). Our data indicate that these target cells are activated early upon infection and that activation is independent of virus replication (27). Although all viral components may contribute to disease development, the filoviral glycoproteins are thought to be the major pathogenic determinants (11).
The glycoprotein (GP) gene is the fourth of seven genes in the linear gene order and gives rise to several distinct proteins. The predominant products of the GP gene, sGP and delta peptide (Δ-peptide), are generated through furin cleavage from a precursor (pre-sGP) that is produced from nonedited mRNA species and are efficiently released from infected cells (33, 34). Full-length GP1,2, found on the surface of mature particles, is produced through transcriptional RNA editing (23, 30) and facilitates receptor binding and fusion with target cells (11). GP1,2 is proteolytically processed into GP1 and GP2, which are disulfide linked and form the mature spike protein. During processing, GP1,2 becomes unstable and GP1 is released from GP2, the transmembrane portion of the protein (24, 32). Recently, a fourth secreted glycoprotein, the product of metalloprotease cleavage of GP1,2, was identified and designated GP1,2Δ (6). sGP is identical to GP1 in its 295 N-terminal amino acids (aa) but differs in the 69 C-terminal aa and has been shown to form homodimers in antiparallel orientation (33). GP1,2 is known to form trimeric heterodimers on the surface of particles, and it is speculated that trimerization is mediated through the GP2 component of the protein (24). This idea was supported by a structural analysis of the crystallized GP2 portion in comparison to human immunodeficiency virus type 1 gp41, influenza hemagglutinin (HA), and the fusion protein of paramyxoviruses (16, 18, 37, 38). Formation of virus-like particles (VLPs), following expression of GP1,2 with virion protein 40 (VP 40), has been previously described (4, 20). VLPs are strikingly similar in morphology to wild-type EBOV and have proven to be useful tools to study early events of EBOV pathogenesis as well as viral egress (35, 36, 41).
The various glycoproteins of ZEBOV have been the subject of intense investigation in the past 10 years and recently have been implicated as major pathogenic determinants for infection (5, 10, 11, 40). While the function of GP1,2 is known to mediate receptor binding and fusion, the function of the secreted glycoproteins remains unknown. In contrast to other studies (14), ours have shown that the activation of macrophages is independent of virus replication (27). This finding led to the hypothesis that initial interactions of mononuclear phagocytic cells with either GP1,2 or the secreted glycoproteins may be the critical factor for activation. We have expressed, purified, and characterized recombinant secreted glycoproteins of ZEBOV to test their ability to activate macrophages. While these proteins resemble authentic ZEBOV secreted glycoproteins, we found that they were not capable of activating primary human macrophages. Interestingly, purified VLPs were able to cause a robust activation of macrophages, thereby suggesting that the presentation of GP1,2 into the structure of EBOV particles is an efficient immune stimulator. These results strongly indicate that secreted glycoproteins play no role in the exogenous activation of primary target cells during ZEBOV infection.
(This work was performed in partial fulfillment of the requirements for the Ph.D. thesis of V.W.-J.)
MATERIALS AND METHODS
Cell lines.
The human embryonic kidney cell line 293T was maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and l-glutamine (2 mM). The 293T cell line contains the gene for the temperature-sensitive simian virus 40 large T antigen and is a derivative of the 293 cell line (7). Cells were maintained at 37°C in 5% CO2.
Isolation of PBMC.
Peripheral blood mononuclear cells (PBMC) consisting of monocytes and lymphocytes were separated from whole blood from healthy donors by using Ficoll-Paque Plus density gradient centrifugation (Amersham Biosciences, Baie d'Urfé, Canada). Donor blood was nonpooled. PBMC were seeded into Primaria 24-well culture plates (Becton Dickinson, Mississauga, Canada) and allowed to adhere for 1 h. Following 1 h of incubation, monolayers were washed extensively to remove nonadherent cells. Cells were then incubated at 37°C in a humidified (95%) 5% CO2 environment for 7 days prior to treatment. The macrophages were cultured in RPMI 1640 (Invitrogen, Burlington, Canada) containing 20% heat-inactivated human AB serum (Sigma, Oakville, Canada), penicillin (100 U/ml), streptomycin (100 μg/ml), and l-glutamine (2 mM).
Construction of recombinant plasmids for transient expression.
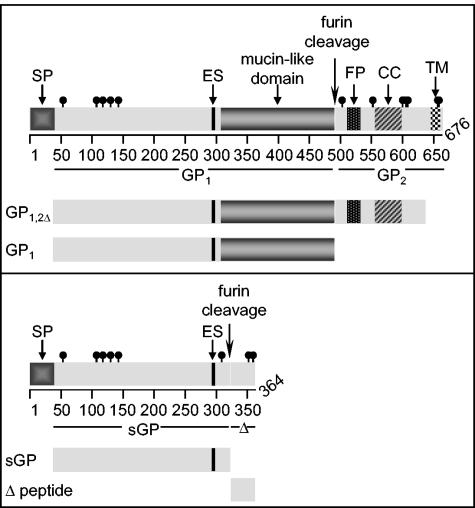
The template cDNA for GP1,2, GP1,2Δ, and GP1 originated from the prototype ZEBOV strain Mayinga (GenBank accession no. AF272001) GP gene containing eight adenosine (A) residues at the RNA editing site, which was kindly provided by Viktor Volchkov (Lyon, France) (32). Template cDNA for sGP and Δ peptide contained seven As. The regions amplified for each protein are shown diagrammatically in Fig. 1. The regions amplified include nucleotide positions 238 to 1629 for GP1 (aa 33 to 496), 238 to 2052 for GP1,2Δ (aa 33 to 637), 238 to 1101 for sGP (aa 33 to 320), and 1114 to 1236 for Δ-peptide (aa 325 to 364) (GenBank accession no. U23187). All PCR products were cloned into the expression vector pDisplay (Invitrogen) such that recombinant proteins contain the amino-terminal influenza HA epitope tag but lack the carboxy-terminal myc tag and transmembrane domain. Plasmids for VLPs were generated by cloning full-length GP1,2 (nucleotide positions 142 to 2172; aa 1 to 676) and the open reading frame of ZEBOV VP40 individually into the eukaryotic expression vector pCAGGS (19), which was kindly provided by Yoshihiro Kawaoka (University of Tokyo, Tokyo, Japan).
FIG. 1.
Schematic representation of cloning strategy for recombinant proteins. GP1,2Δ and GP1 were cloned by using template DNA with eight adenosines in the editing site (ES). sGP and Δ-peptide were cloned by using template DNA with seven adenosines in the ES. Known significant domains are shown with respect to their amino acid positions, including the signal peptide (SP), ES, mucin-like domain, furin cleavage site, fusion peptide (FP), coiled coil (CC), and transmembrane domain (TD). Cysteine residues are represented by black lines with closed circles.
Expression and purification of recombinant proteins and VLPs.
293T cells were seeded into poly-d-lysine (Sigma)-coated triple-layer (500 cm2) flasks (VWR, Mississauga, Canada) 24 h prior to transfection in Dulbecco's modified Eagle medium containing 10% fetal bovine serum without antibiotics. Plasmids (sGP, GP1, GP1,2Δ, Δ-peptide, and control pDisplay vector) were individually transfected by using FuGENE6 (Roche, Laval, Canada) according to the manufacturer's instructions. Flasks were incubated at 37°C for 72 h, after which point the supernatants were harvested and clarified by centrifugation at 250 × g for 10 min at 4°C. The clarified supernatants were concentrated by using Centricon Plus-80 filters (Millipore, Nepean, Canada) with molecular weight cutoffs of 10,000 (sGP, GP1,2Δ, GP1, and control pDisplay) or 5,000 (Δ-peptide). Following concentration, supernatants were immunoaffinity purified by using an anti-HA matrix (Roche) per the manufacturer's instructions. HA peptide (used for elution) was removed from the final eluates by washing four times with TNE buffer (20 mM Tris [pH 7.5], 0.1 M NaCl, 0.1 mM EDTA), and proteins were further concentrated by using Centricon YM filters with molecular weight cutoffs of 3,000 (Δ-peptide), 10,000 (sGP), or 100,000 (GP1 and GP1,2Δ). The final concentrations of proteins were determined by using a detergent-compatible protein assay (Bio-Rad, Mississauga, Canada). All proteins were aliquoted and stored at −20°C.
VLPs were generated through transient transfection of both GP1,2 and VP40 plasmids in 293T by using FuGENE6 as described above, while VP40 was transfected alone to generate VLPs without GP1,2. pCAGGS transfected alone served as a mock control. Supernatants were harvested at 72 h posttransfection and clarified by centrifugation at 3,000 × g for 10 min at 4°C. Supernatants were then layered on TNE buffer containing 20% sucrose and spun at 28,000 rpm at 4°C for 2 h by using an SW28 rotor with a Beckman Optima L-70K ultracentrifuge. Supernatants were discarded, and pellets were washed once with TNE buffer and spun at 28,000 rpm at 4°C for 30 min. Finally, the pellets were resuspended in TNE buffer and stored at 4°C for no more than 2 days prior to the experiments. The total protein concentration of purified VLPs was determined by using a detergent-compatible protein assay (Bio-Rad). The VLP concentration was determined by direct particle counting as previously described (13).
The Limulus amebocyte lysate test (Mandel Scientific, Guelph, Canada) was used to determine endotoxin levels for all proteins, controls, and VLPs used for these studies, and values were less than or equal to those with tissue culture media (<0.5 endotoxin units per ml).
Characterization of recombinant proteins and VLPs.
All secreted glycoproteins were tested for proper carbohydrate processing (glycosylation) by digestion with the enzymes N-glycosidase F, sialidase A, and O-glycosidase (PROzyme, San Leandro, Calif.). Reactions were performed according to the manufacturer's instructions, and results were analyzed by sodium dodecyl-sulfate polyacrylamide electrophoresis (SDS-PAGE). Proteins were transferred to polyvinylidene difluoride membranes and detected by using anti-HA antibodies (1:2,000). The expected oligomerization was confirmed by SDS-PAGE analysis under reducing and nonreducing conditions. Proteins were detected by using Coomassie brilliant blue. The expression of GP1,2 and VP40 was confirmed by Western blot analysis using GP-specific (ZGP12/1.1) and VP40-specific (anti-VP40) monoclonal antibodies (1:4,000) kindly provided by Ayato Takada and Yoshihiro Kawaoka, respectively (University of Tokyo, Tokyo, Japan). Purified VLPs were incubated with macrophages for 1 h and binding at 37°C confirmed by immunofluorescence microscopy. Cells were washed extensively, fixed with 2% paraformaldehyde, and stained with ZGP12/1.1 (1:200) and anti-mouse Alexa 488 secondary antibody (1:400).
Negative stain and immunoelectron microscopy of VLPs.
Purified VLPs were prepared for negative stain and immunoelectron microscopy examination essentially as previously described (13). Briefly, purified VLPs were diluted in 50 μl of 0.1% glutaraldehyde in phosphate-buffered saline [pH 7.2], allowed to fix for 10 min at 4°C, and centrifuged directly onto Formvar-coated, carbon-stabilized 400-mesh copper or nickel electron microscopy grids (Airfuge EM-90 rotor; Beckman, Palo Alto, Calif.) at 26 lb/in2 for 30 min. For indirect immunolabeling, VLPs pelleted to nickel grids were incubated with ZGP12/1.1 for 60 min at 20°C. The primary antibody was detected with polyclonal anti-mouse immunoglobulin G gold conjugates (18 nm) (Jackson ImmunoResearch, West Grove, Pa.) and fixed with 0.1% glutaraldehyde to stabilize the reactions. Samples were negatively stained with 1.2 mM phosphotungstic acid, pH 7.0, and examined with a Phillips model 201 electron microscope. Images were recorded at machine magnifications of ×30,000 and ×70,000 on Direct Positive film 5302 (Kodak, Rochester, N.Y.) and printed on Kodak Polycontrast III paper.
Treatment of macrophages with secreted glycoproteins.
At 6 days postseeding, medium was removed from macrophages and replaced with fresh RPMI 1640 containing 2% human AB serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and l-glutamine (2 mM). Cells were incubated for 24 h in a 37°C humidified (95%) 5% CO2 environment. After 24 h, cells were treated with either 10 or 50 μg of each secreted glycoprotein/ml, HA peptide, pDisplay control, lipopolysaccharide (LPS; 10 ng/ml), VLPs (107), or mock VLPs (pCAGGS control) by adding samples directly to wells. Cells were incubated with samples for 1, 6, 12, or 24 h. After incubation, the supernatants were removed from the macrophage cell cultures and clarified from cell debris by centrifugation (8,000 × g at 4°C for 10 min), aliquoted, and stored at −80°C.
Relative quantification of transcript levels.
Cells were lysed by using a guanidinium isothiocyanate-based buffer system (RNeasy mini kit; QIAGEN, Mississauga, Canada). The generation of cDNA was performed with TaqMan Reverse Transcriptase reagents (Applied Biosystems, Foster City, Calif.), followed by real-time PCRs using Sybr Green Master Mix (Applied Biosystems) as recommended by the manufacturers. The real-time PCRs also contained 40 nM concentrations of each high-pressure liquid chromatography-purified primer, 25 ng of yeast tRNA (Sigma), and 40 ng of cDNA and were performed in triplicate using an ABI Prism 5700 detection system. The amplification specificity was monitored by dissociation curves. Relative quantification was performed by the comparative computed tomography method (Applied Biosystems user bulletin 2, 11 December 1997), by using the mRNA level of the GAPDH housekeeping gene as an endogenous reference for normalization.
ELISA.
Supernatants from cell cultures were thawed only once, at the time of testing. Precoated enzyme-linked immunosorbent assay (ELISA) plates for tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) and enzyme immunoassay plates for IL-8 were purchased from PromoCell (Heidelberg, Germany). All samples were tested in duplicate and according to the procedures provided by the manufacturer.
RESULTS
Production and characterization of recombinant secreted glycoproteins.
Plasmids coding for sGP, GP1, GP1,2Δ, and Δ-peptide were transfected into 293T cells in 500-cm2 flasks for large-scale protein production. After several purification steps, including immunoaffinity chromatography, targeting of the HA epitope tag present on proteins, and size exclusion centrifugation, protein concentrations were determined. On average, concentrations in the range of 2 μg/μl were obtained with sGP, GP1, and GP1,2Δ, while Δ-peptide proved more challenging to produce, with concentrations in the range of 0.5 to 1 μg/μl (data not shown). In order to prove that these recombinant proteins were homologous to those produced during live virus infection, all proteins were subjected to characterization methods similar to those previously described (24, 33, 34). Treatment with N-glycosidase F to remove all N-linked carbohydrates resulted in a shift of 15 kDa for sGP, while a shift of 40 kDa was seen for GP1 and GP1,2Δ (Fig. 2A). These results are consistent with current data in the literature (29). All three proteins also experienced decreases in their molecular mass following digestion with sialidase A and O-glycosidase, indicating the presence of O-linked carbohydrates. Δ-peptide does not contain N-linked carbohydrates; however, a shift of approximately 10 kDa was observed following treatment with sialidase A and O-glycosidase (Fig. 2A). All proteins were resistant to treatment with endoglycosidase H, indicating the absence of high-mannose type carbohydrates and proper transport from the endoplasmic reticulum into the Golgi compartments, where N-linked glycosylation is completed and O glycosylation occurs (data not shown).
FIG. 2.
Characterization of recombinant glycoproteins. Purified soluble glycoproteins were treated with N-glycosidase F, sialidase A, and O-glycosidase as indicated (A). Samples were analyzed under reducing SDS-PAGE and immunoblotted with anti-HA antibodies (1:2,000). All proteins demonstrate shifts in molecular mass following digestion with sialidase A and O-glycosidase. Additionally, N-linked sugars were removed from GP1, GP1,2Δ, and sGP. Purified proteins were analyzed for conformation by reducing (left panels) or nonreducing (right panels) SDS-PAGE and detected by Coomassie brilliant blue (B).
The conformation of the recombinant proteins was assessed since structure may dictate proper function. It is known that sGP occurs as a homodimer that is linked through two disulfide bonds (33), while Δ-peptide is not known to form oligomers (34). The structure of GP1,2 on the surface of particles is a trimer (24), and the secreted forms of GP1,2Δ and GP1 were expected to be trimeric and monomeric, respectively (6, 32). Proteins were analyzed by SDS-PAGE under reducing and nonreducing conditions. We found that under nonreducing conditions, recombinant sGP forms the predicted dimers (Fig. 2B), while Δ-peptide occurred, as expected, in monomeric form (33, 34). When analyzed under nonreducing conditions, both GP1 and GP1,2Δ were found to form trimers (Fig. 2B). The ability of GP1 to form trimers in the absence of GP2 was unexpected and is currently the subject of further investigation.
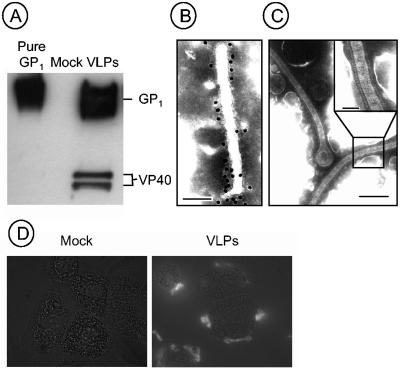
VLPs were produced through transient transfection, and purified particles were positive by immunoblotting for the presence of both GP1,2 and VP40 as determined by using monoclonal antibodies (Fig. 3A). Electron microscopy studies confirmed the conformation of VLPs as filamentous, and virion glycoprotein spikes were identified on the surface (Fig. 3B and C).
FIG. 3.
Analysis of VLPs. VLPs purified from transfected 293T cells were subjected to reducing SDS-PAGE and probed with monoclonal antibodies (ZGP12/1.1 and anti-VP40). Purified GP1 and the supernatant from mock-transfected cells were used as controls (A). Morphology of purified VLPs was determined by immunogold labeling (ZGP12/1.1) (B) and negative staining transmission electron microscopy (C). Size bars are 100 (B), 200 (C), and 50 (C, inset) nm. Binding of VLPs to macrophages was determined by incubating cells with VLPs or supernatants derived from mock-transfected cells for 1 h at 37°C (D). Cells were washed extensively, fixed, and stained with ZGP12/1.1 antibody (1:200). Specific staining for GP1,2 is seen with VLPs but not mock-treated cells. Images were acquired at ×1,000 magnification.
VLPs but not the soluble glycoproteins induce activation of human macrophages.
Human macrophages were treated with secreted glycoproteins at concentrations of 10 or 50 μg/ml, and VLPs (10 particles/cell) and the expression of selected cytokines/chemokines were measured at 1, 6, 12, and 24 h posttreatment. Prior to all experiments, media and samples were tested for the presence of endotoxin, and levels were below 0.5 endotoxin units/ml. Real-time PCR was used to determine the levels of IL-1 beta, TNF-α, IL-6, and IL-8, mediators previously shown to be significantly up-regulated during ZEBOV infection in vivo and in vitro (2, 14, 27, 28). Interestingly, all of the secreted glycoproteins failed to induce significant changes in the transcription of these cytokines/chemokines (Fig. 4; results with 50 μg/ml are shown). This finding is in agreement with the lack of binding of these glycoproteins to macrophages, as investigated by fluorescence-activated cell sorter analysis with a HA-specific antibody (data not shown). In contrast, VLPs were able to bind macrophages, as demonstrated by immunostaining (Fig. 3D). VLP binding induced expression of all four mediators at levels equal to or above that of the positive control, LPS, and, more importantly, equal to infection with ZEBOV as previously shown (27). Transcriptional upregulation of TNF-α, IL-6, and IL-8 occurred as early as 1 h posttreatment, whereas IL-1 beta was not detected until 6 h posttreatment. Transcriptional upregulation of TNF-α and IL-6 was short-lived, being already undetectable 6 or 12 h posttreatment, respectively. Transcriptional upregulation of the chemokine IL-8 was maintained over 24 h and, interestingly, to a higher degree over time than the LPS control (Fig. 4). Interestingly, VLPs lacking GP1,2 are able to induce low levels of macrophage activation, but the addition of GP1,2 in VLPs greatly enhances the effect (Fig. 4).
FIG. 4.
Transcriptional activation of macrophages. Macrophages were treated with 50 μg of soluble glycoproteins (sGP, Δ-peptide, GP1, and GP1,2Δ)/ml, VLPs with or without GP1,2 (10 particles/cell), and controls. Cellular RNA was analyzed by real-time PCR for transcriptional upregulation of TNF-α, IL-6, IL-8, and IL-1 beta. VLPs with GP1,2 and LPS induced early and robust increases in the amounts of transcripts. Levels of cytokine/chemokine transcripts were similar for soluble glycoproteins and negative controls.
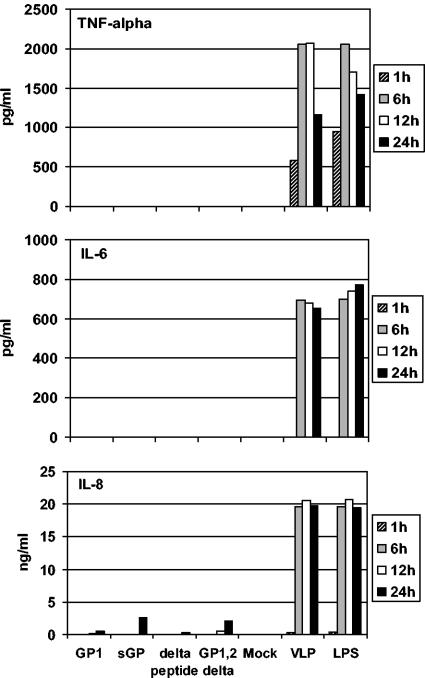
ELISA was used to determine the secretion levels of proinflammatory cytokines and IL-8 into the supernatants of treated macrophages (Fig. 5). As expected from the results on the transcriptional level (Fig. 4), only VLPs, not the secreted glycoproteins, were able to trigger secretion of such mediators. Secreted TNF-α was already detectable at 1 h posttreatment, slightly earlier than IL-6 and IL-8, which appeared as early as 6 h posttreatment. The levels of secreted TNF-α peaked at 6 to 12 h posttreatment, whereas IL-6 and IL-8 remained at higher levels over a 24-h period. Thus, the pattern of secretion is delayed but consistent with transcriptional activation as measured by real-time PCR (Fig. 5). Of note, VLPs induced expression of all three products with kinetics similar to that of LPS and similar to ZEBOV-infected cells, as shown earlier (27).
FIG. 5.
Release of mediators (cytokines/chemokines) in macrophage supernatants. Supernatants from soluble glycoprotein (50 μg/ml), VLP, and control-treated macrophages were analyzed for cytokine expression by ELISA (TNF-α and IL-6) and chemokine expression by enzyme immunoassay (IL-8). Supernatants from LPS- and VLP-treated cells were positive for all mediators tested.
DISCUSSION
The role of EBOV secreted glycoproteins in the pathogenesis of EBOV hemorrhagic fever has been a point of speculation and controversy since their discovery. Virus-infected cells produce these proteins in tissue culture, and sGP has also been detected in the blood of infected patients (22-24, 32). Once released, these proteins are free to interact with host cells, including cells of the mononuclear phagocytic system and the endothelium. The interaction of these proteins with host cells and their role in pathogenesis has largely been hampered due to an inability to biosynthesize large quantities of protein equivalent to those seen during a natural virus infection. Heavy glycosylation and posttranslational modifications of the native proteins limited the use of less expensive and more conventional methods of protein expression, such as bacterial or yeast systems. While viral vectors, such as the vesicular stomatitis virus, are efficient, the inherent cytotoxicity makes them suboptimal for activation studies. An additional obstacle for recombinant secreted glycoprotein detection and purification is the lack of antibodies specific for these proteins, particularly for Δ-peptide. For these reasons, we sought to biosynthesize the secreted glycoproteins by using a mammalian system that incorporated N-terminal influenza hemagglutinin epitope tags on all proteins. These tags were used for both detection and immunoaffinity purification. Once the proteins were produced and purified, it was then imperative to ascertain the nature of the proteins with respect to posttranslational modifications (Fig. 2). To this end, we first analyzed the N-linked and O-linked sugars of the expressed and purified soluble glycoproteins, and the results supported the authenticity of the recombinant proteins compared to previously published data (Fig. 2A) (24, 29, 33, 34). Glycosylation of full-length GP1,2, particularly with respect to the mucin-like domain, is thought to play a significant role in the function of this protein. Indeed, other groups have reported that when O-linked sugars were removed there was a reduction in cytopathic effects or that a loss of adherence resulting from GP1,2 expression was also observed (26, 31, 40). O-linked glycosylation is believed to be a rate-limiting step in glycoprotein transport (15). We believe that the heavy O glycosylation of Δ-peptide may be one reason for lower total protein yields, together with a loss of this polypeptide during the purification procedures.
Following characterization of glycosylation, proper conformation of the recombinant proteins was assessed. It is well established that sGP is expressed as a dimer and its smaller cleavage fragment, Δ-peptide, is released as a monomer (33, 34). As it seems reasonable to assume that any function of sGP may depend on proper conformation, we confirmed the structure of the recombinant sGP to be dimeric following purification (Fig. 2B). Similarly, Δ-peptide was detected in monomeric conformation under both reducing and nonreducing conditions (data not shown). The conformation of released GP1,2Δ was reported to occur as a trimer, much the same as the attachment protein found in virion form (6, 32). Mutational analysis of GP1,2 has implicated cysteine 53 in disulfide bond formation to GP2 (15) and based on similarity to retroviral GPs, others have speculated that trimerization is initiated through the GP2 component of EBOV GP1,2 (24). Indeed, recombinantly expressed GP1,2Δ was secreted in trimeric form (Fig. 2B). Interestingly, a trimeric structure was also found with recombinantly expressed and purified GP1 in the absence of GP2 (Fig. 2B). It seems likely that cysteine 53 is also involved in the oligomerization of GP1. While this result was somewhat unexpected, it was fortuitous since our recombinant proteins are present in a conformation which might mimic that of the virion surface GP1,2.
Several studies on infected humans and nonhuman primates have implicated the interference of EBOV with innate immunity (2, 3, 14, 17, 27, 28). Despite differences in the details of the responses among the studies, the presence of IL-1 beta and elevated concentrations of IL-6 in plasma have been postulated as markers of nonfatal human infections, whereas the release of IL-10 and high levels of neopterin and IL-1 receptor antagonist in the plasma early after the onset of symptoms seems to be indicative of a fatal outcome (9). Cultured primary human monocytes/macrophages were also activated upon EBOV infection, resulting in an increase of several mediators (14, 27). In contrast to results of a study by Hensley and colleagues, who did not find macrophage activation with gamma-irradiated, inactivated ZEBOV (14), previous studies by our group have reported that UV-inactivated viral stocks could induce expression of several cytokines/chemokines when applied to human monocytes and macrophages (27). This observation led to the hypothesis that viral replication was not a prerequisite for activation and that secreted glycoproteins, also present in viral stocks, may contribute to the activation. In contrast to our hypothesis, none of the secreted glycoproteins, even at high concentrations, were able to activate macrophages as demonstrated in the present study (Fig. 4 and 5). However, this is the first report of purified VLPs, in the absence of other soluble factors (including viral secreted glycoproteins) being sufficient for binding and activation (Fig. 3D, 4, and 5). It is interesting, however, that although our recombinant GP1 and GP1,2Δ are essentially secreted forms of the spike glycoprotein, they are not sufficient to induce macrophage activation (Fig. 4 and 5). Therefore, we propose that the EBOV glycoprotein activation may occur in a manner similar to that of vesicular stomatitis virus induction of B cells (1). Specifically, we hypothesize that the rigid form of the glycoprotein, spaced 5 nm apart on VLPs and virions, presents a repetitive antigenic stimulus to macrophages and may function by cross-linking receptors, resulting in strong activation. Presentation of the antigen in a specific organization may be more important than simply supplying the protein in solution. In the absence of this rigid presentation of antigen, as in the case with soluble GP1 and GP1,2Δ, a potent signal may not be induced. Previous studies, which have shown that VLPs were immunogenic and sufficient to activate dendritic cells as demonstrated by their ability to confer complete protection from a lethal challenge of mouse-adapted ZEBOV (35), support this concept.
In conclusion, we demonstrated here that primary target cell activation is mediated by the viral glycoprotein GP1,2 and not the different soluble glycoproteins released during virus infection. GP1,2 needs to be presented in the rigid form, such as that observed on the surface of VLPs or virions. Only this form seems to present a repetitive antigenic stimulus to macrophages and may function by cross-linking receptors, resulting in strong activation. While the secreted glycoproteins do not seem to play a role in macrophage activation, they may induce changes in secondary target cells, such as endothelial cells, or function in binding neutralizing antibodies (6, 11). Thus, the roles of the different soluble glycoproteins remain speculative and require further investigation.
Acknowledgments
We thank Allison Groseth, Hideki Ebihara, and Alex Silaghi for discussion and editorials on the manuscript. We thank Yoshihiro Kawaoka and Ayato Takada for kindly providing the vector pCAGGS and monoclonal antibodies (ZGP12/1.1 and anti-VP40) and Viktor Volchkov for providing the template cDNA for the generation of the ZEBOV glycoprotein clones.
This study was supported by grants from the Canadian Institutes of Health Research (MOP-43921) awarded to H.F. and the National Institutes of Health (AI 48053) awarded to D.R.B. V.W.-J. was supported by fellowships from the Manitoba Health Research Council and the Department of Medical Microbiology, University of Manitoba.
REFERENCES
- 1.Bachmann, M. F., H. Hengartner, and R. M. Zinkernagel. 1995. T helper cell-independent neutralizing B cell response against vesicular stomatitis virus: role of antigen patterns in B cell induction? Eur. J. Immunol. 25:3445-3451. [DOI] [PubMed] [Google Scholar]
- 2.Baize, S., E. M. Leroy, A. J. Georges, M. C. Georges-Courbot, M. Capron, I. Bedjabaga, J. Lansoud-Soukate, and E. Mavoungou. 2002. Inflammatory responses in Ebola virus-infected patients. Clin. Exp. Immunol. 128:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baize, S., E. M. Leroy, M. C. Georges-Courbot, M. Capron, J. Lansoud-Soukate, P. Debre, S. P. Fisher-Hoch, J. B. McCormick, and A. J. Georges. 1999. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat. Med. 5:423-426. [DOI] [PubMed] [Google Scholar]
- 4.Bavari, S., C. M. Bosio, E. Wiegand, G. Ruthel, A. B. Will, T. W. Geisbert, M. Hevey, C. Schmaljohn, A. Schmaljohn, and M. J. Aman. 2002. Lipid raft microdomains: a gateway for compartmentalized trafficking of Ebola and Marburg viruses. J. Exp. Med. 195:593-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan, S. Y., M. C. Ma, and M. A. Goldsmith. 2000. Differential induction of cellular detachment by envelope glycoproteins of Marburg and Ebola (Zaire) viruses. J. Gen. Virol. 81:2155-2159. [DOI] [PubMed] [Google Scholar]
- 6.Dolnik, O., V. Volchkova, W. Garten, C. Carbonnelle, S. Becker, J. Kahnt, U. Stroher, H. D. Klenk, and V. Volchkov. 2004. Ectodomain shedding of the glycoprotein GP of Ebola virus. EMBO J. [DOI] [PMC free article] [PubMed]
- 7.DuBridge, R. B., P. Tang, H. C. Hsia, P. M. Leong, J. H. Miller, and M. P. Calos. 1987. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol. Cell. Biol. 7:379-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldmann, H., H. Bugany, F. Mahner, H. D. Klenk, D. Drenckhahn, and H. J. Schnittler. 1996. Filovirus-induced endothelial leakage triggered by infected monocytes/macrophages. J. Virol. 70:2208-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldmann, H., S. Jones, H. D. Klenk, and H. J. Schnittler. 2003. Ebola virus: from discovery to vaccine. Nat. Rev. Immunol. 3:677-685. [DOI] [PubMed] [Google Scholar]
- 10.Feldmann, H., V. E. Volchkov, V. A. Volchkova, and H. D. Klenk. 1999. The glycoproteins of Marburg and Ebola virus and their potential roles in pathogenesis. Arch. Virol. Suppl. 15:159-169. [DOI] [PubMed] [Google Scholar]
- 11.Feldmann, H., V. E. Volchkov, V. A. Volchkova, U. Stroher, and H. D. Klenk. 2001. Biosynthesis and role of filoviral glycoproteins. J. Gen. Virol. 82:2839-2848. [DOI] [PubMed] [Google Scholar]
- 12.Geisbert, T. W., L. E. Hensley, T. Larsen, H. A. Young, D. S. Reed, J. B. Geisbert, D. P. Scott, E. Kagan, P. B. Jahrling, and K. J. Davis. 2003. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection. Am. J. Pathol. 163:2347-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hazelton, P. R., and K. M. Coombs. 1999. The reovirus mutant tsA279 L2 gene is associated with generation of a spikeless core particle: implications for capsid assembly. J. Virol. 73:2298-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hensley, L. E., H. A. Young, P. B. Jahrling, and T. W. Geisbert. 2002. Proinflammatory response during Ebola virus infection of primate models: possible involvement of the tumor necrosis factor receptor superfamily. Immunol. Lett. 80:169-179. [DOI] [PubMed] [Google Scholar]
- 15.Jeffers, S. A., D. A. Sanders, and A. Sanchez. 2002. Covalent modifications of the Ebola virus glycoprotein. J. Virol. 76:12463-12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klenk, H., H. Feldmann, V. Volchkov, V. Volchkova, and W. Weissenhorn. 2001. Structure and function of the proteins of Marburg and Ebola viruses, p. 233-245. In G. L. Smith, W. L. Irving, J. W. McCauley, and D. J. Rowlands (ed.), New challenges to health: the threat of virus infection. SGM symposium, vol. 60. Cambridge University Press, Cambridge, United Kingdom.
- 17.Leroy, E. M., S. Baize, V. E. Volchkov, S. P. Fisher-Hoch, M. C. Georges-Courbot, J. Lansoud-Soukate, M. Capron, P. Debre, J. B. McCormick, and A. J. Georges. 2000. Human asymptomatic Ebola infection and strong inflammatory response. Lancet 355:2210-2215. [DOI] [PubMed] [Google Scholar]
- 18.Malashkevich, V. N., B. J. Schneider, M. L. McNally, M. A. Milhollen, J. X. Pang, and P. S. Kim. 1999. Core structure of the envelope glycoprotein GP2 from Ebola virus at 1.9-A resolution. Proc. Natl. Acad. Sci. USA 96:2662-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193-199. [DOI] [PubMed] [Google Scholar]
- 20.Noda, T., H. Sagara, E. Suzuki, A. Takada, H. Kida, and Y. Kawaoka. 2002. Ebola virus VP40 drives the formation of virus-like filamentous particles along with GP. J. Virol. 76:4855-4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez, A., A. Khan, S. R. Zaki, G. J. Nabel, T. G. Ksiazek, and C. J. Peters. 2001. Filoviridae: Marburg and Ebola viruses, p. 1279-1304. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields Virology, 4th ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 22.Sanchez, A., T. G. Ksiazek, P. E. Rollin, M. E. Miranda, S. G. Trappier, A. S. Khan, C. J. Peters, and S. T. Nichol. 1999. Detection and molecular characterization of Ebola viruses causing disease in human and nonhuman primates. J. Infect. Dis. 179(Suppl. 1):S164-S169. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez, A., S. G. Trappier, B. W. Mahy, C. J. Peters, and S. T. Nichol. 1996. The virion glycoproteins of Ebola viruses are encoded in two reading frames and are expressed through transcriptional editing. Proc. Natl. Acad. Sci. USA 93:3602-3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez, A., Z. Y. Yang, L. Xu, G. J. Nabel, T. Crews, and C. J. Peters. 1998. Biochemical analysis of the secreted and virion glycoproteins of Ebola virus. J. Virol. 72:6442-6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schnittler, H. J., and H. Feldmann. 2003. Viral hemorrhagic fever—a vascular disease? Thromb. Haemost. 89:967-972. [PubMed] [Google Scholar]
- 26.Simmons, G., R. J. Wool-Lewis, F. Baribaud, R. C. Netter, and P. Bates. 2002. Ebola virus glycoproteins induce global surface protein down-modulation and loss of cell adherence. J. Virol. 76:2518-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stroher, U., E. West, H. Bugany, H. D. Klenk, H. J. Schnittler, and H. Feldmann. 2001. Infection and activation of monocytes by Marburg and Ebola viruses. J. Virol. 75:11025-11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villinger, F., P. E. Rollin, S. S. Brar, N. F. Chikkala, J. Winter, J. B. Sundstrom, S. R. Zaki, R. Swanepoel, A. A. Ansari, and C. J. Peters. 1999. Markedly elevated levels of interferon (IFN)-gamma, IFN-alpha, interleukin (IL)-2, IL-10, and tumor necrosis factor-alpha associated with fatal Ebola virus infection. J. Infect. Dis. 179(Suppl. 1):S188-S191. [DOI] [PubMed] [Google Scholar]
- 29.Volchkov, V. E. 1999. Processing of the Ebola virus glycoprotein. Curr. Top. Microbiol. Immunol. 235:35-47. [DOI] [PubMed] [Google Scholar]
- 30.Volchkov, V. E., S. Becker, V. A. Volchkova, V. A. Ternovoj, A. N. Kotov, S. V. Netesov, and H. D. Klenk. 1995. GP mRNA of Ebola virus is edited by the Ebola virus polymerase and by T7 and vaccinia virus polymerases. Virology 214:421-430. [DOI] [PubMed] [Google Scholar]
- 31.Volchkov, V. E., V. A. Volchkova, E. Muhlberger, L. V. Kolesnikova, M. Weik, O. Dolnik, and H. D. Klenk. 2001. Recovery of infectious Ebola virus from complementary DNA: RNA editing of the GP gene and viral cytotoxicity. Science 291:1965-1969. [DOI] [PubMed] [Google Scholar]
- 32.Volchkov, V. E., V. A. Volchkova, W. Slenczka, H. D. Klenk, and H. Feldmann. 1998. Release of viral glycoproteins during Ebola virus infection. Virology 245:110-119. [DOI] [PubMed] [Google Scholar]
- 33.Volchkova, V. A., H. Feldmann, H. D. Klenk, and V. E. Volchkov. 1998. The nonstructural small glycoprotein sGP of Ebola virus is secreted as an antiparallel-orientated homodimer. Virology 250:408-414. [DOI] [PubMed] [Google Scholar]
- 34.Volchkova, V. A., H. D. Klenk, and V. E. Volchkov. 1999. Delta-peptide is the carboxy-terminal cleavage fragment of the nonstructural small glycoprotein sGP of Ebola virus. Virology 265:164-171. [DOI] [PubMed] [Google Scholar]
- 35.Warfield, K. L., C. M. Bosio, B. C. Welcher, E. M. Deal, M. Mohamadzadeh, A. Schmaljohn, M. J. Aman, and S. Bavari. 2003. Ebola virus-like particles protect from lethal Ebola virus infection. Proc. Natl. Acad. Sci. USA 100:15889-15894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanabe, S., T. Watanabe, T. Noda, A. Takada, H. Feldmann, L. D. Jasenosky, and Y. Kawaoka. 2004. Production of novel Ebola virus-like particles from cDNAs: an alternative to Ebola virus generation by reverse genetics. J. Virol. 78:999-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weissenhorn, W., L. J. Calder, S. A. Wharton, J. J. Skehel, and D. C. Wiley. 1998. The central structural feature of the membrane fusion protein subunit from the Ebola virus glycoprotein is a long triple-stranded coiled coil. Proc. Natl. Acad. Sci. USA 95:6032-6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weissenhorn, W., A. Carfi, K. H. Lee, J. J. Skehel, and D. C. Wiley. 1998. Crystal structure of the Ebola virus membrane fusion subunit, GP2, from the envelope glycoprotein ectodomain. Mol. Cell 2:605-616. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization.1978. Ebola haemorrhagic fever in Zaire, 1976. Bull. W. H. O. 56:271-293. [PMC free article] [PubMed] [Google Scholar]
- 40.Yang, Z. Y., H. J. Duckers, N. J. Sullivan, A. Sanchez, E. G. Nabel, and G. J. Nabel. 2000. Identification of the Ebola virus glycoprotein as the main viral determinant of vascular cell cytotoxicity and injury. Nat. Med. 6:886-889. [DOI] [PubMed] [Google Scholar]
- 41.Yasuda, J., M. Nakao, Y. Kawaoka, and H. Shida. 2003. Nedd4 regulates egress of Ebola virus-like particles from host cells. J. Virol. 77:9987-9992. [DOI] [PMC free article] [PubMed] [Google Scholar]