Abstract
Patients with B cell lymphomas bearing MYC translocation combined with translocation involving other genes, such as BCL2, BCL3, or BCL6, defined as double-hit lymphoma (DHL), have a poor prognosis. Recent studies expanded the concept to include double-expressing lymphoma (DEL) that co-overexpresses MYC protein with either of those proteins. Accordingly, we defined cytogenetic DHL and DEL as primary DHL. An adoptive T cell immunotherapy with a chimeric antigen receptor (CAR) has been clinically shown to exhibit cytotoxicity in refractory neoplasias. We revealed the marked cytotoxicity of anti-CD19- and/or anti-CD38-CAR T cells against primary DHL cells from patients. CD19- and/or CD38-specific T cells were co-cultured with cytogenetic DHL (n = 3) or DEL (n = 2) cells from five patients for 3 days. We examined whether T cells retrovirally transduced with each vector showed cytotoxicity against DHL cells. Anti-CD19- and/or anti-CD38-CAR T cells were co-cultured with primary DHL cells at an E:T ratio of 1:2 for 3 days. Anti-CD19- and anti-CD38-CAR T cells completely abrogated these DHL cells, respectively. Anti-CD19-CAR T cells synergistically exerted collaborative cytotoxicity against these primary DHL cells with anti-CD38-CAR T cells. Therefore, refractory DHL cells can be efficiently abrogated by the clinical use of T cells with anti-CD19- and/or anti-CD38-CAR.
Electronic supplementary material
The online version of this article (doi:10.1186/s13045-017-0488-x) contains supplementary material, which is available to authorized users.
Keywords: T cell immunotherapy, Double-hit lymphoma, Double-expressing lymphoma, Chimeric antigen receptor (CAR), Anti-CD19-CAR, Anti-CD38-CAR
Letter to the Editor
Patients with B cell lymphoma bearing MYC translocation combined with an additional translocation involving other genes, such as BCL2, BCL3, BCL6, or CCND1, whose category is defined as double-hit lymphoma (DHL), have a dismal prognosis [1]. Li et al. reported that the prognosis of B cell lymphoma patients expressing concurrent MYC and BCL2 proteins without translocations was also dismal as well as that of DHL-bearing translocations of MYC/BCL2 genes in terms of the prognosis [1–4]. Thus, recent studies have expanded the concept to include double-expressing lymphoma (DEL) that co-overexpresses MYC protein with those proteins. Accordingly, we defined cytogenetic DHL and DEL as primary DHL. An adoptive T cell immunotherapy with anti-CD19 chimeric antigen receptors (CAR) has been clinically shown to exhibit marked cytotoxicity in patients with relapsed and refractory B cell lymphoid neoplasias [5–7]. We also developed anti-CD38-CAR and demonstrated its marked cytotoxicity against various hematological malignancies [8, 9]. However, it has not been elucidated whether CAR therapy could be effective for patients with cytogenetic DHL and DEL. Here, we revealed the marked cytotoxicity of anti-CD19- and/or anti-CD38-CAR T cells as well as the synergy of both CARs against primary DHL cells.
Cytogenetic DHL (n = 3) or DEL (n = 2) cells of the lymph nodes were collected from five patients (Table 1) after obtaining appropriate informed consent. CD19+ CD20+ lymphoma cells accounted for over 90% (90–97%). DHL (DEL) cell line cells, bearing the translocation of the IgH/MYC gene as well as overexpression of BCL2 protein (KPUM-UH1) or these primary cells were cultured in RPMI-1640 complete medium.
Table 1.
Patients’ profiles and cytotoxicity of T cells expressing anti-CD19- or anti-CD38-CAR against primary DHL cells
| Cells | Karyotype (major abnormalities) | IHC-positive | FISH-positive | aExpression of CD38 in CD19+ cells (%) | aSpecific cytotoxicity of anti-CD19-CAR T cells (%) | aSpecific cytotoxicity of anti-CD38-CAR T cells (%) |
|---|---|---|---|---|---|---|
| Patient 1 | t(8;22)(q24;q11.2),t(14;18)(q32;q21) | BCL2 MYC: ND |
BCL2 MYC | 98.69 ± 0.37 | 92.67 ± 0.55 | 97.49 ± 0.19 |
| Patient 2 | add(8)(q24),t(8;14)(q24;q32), t(3;22)(q27;q11.2) | BCL2 BCL6 MYC |
BCL6 MYC |
98.74 ± 0.59 | 95.53 ± 2.88 | 99.88 ± 0.73 |
| Patient 3 | ND | BCL2 BCL6 MYC |
BCL6 | 97.37 ± 0.02 | 98.94 ± 0.03 | 99.60 ± 0.27 |
| Patient 4 | ND | BCL2 MYC: ND |
BCL2 MYC |
98.47 ± 0.26 | 98.26 ± 0.78 | 99.47 ± 0.04 |
| Patient 5 | +8,add(3)(q27) | BCL2 BCL6 MYC |
BCL6 | 97.14 ± 0.83 | 97.41 ± 0.16 | 97.70 ± 0.23 |
Specific cytotoxicity was evaluated by flow cytometry following the co-incubation of T cells bearing anti-CD19- or anti-CD38-CAR (E) with DHL cells (T) at an E:T ratio of 1:2 for 3 days. The cutoffs for positivity for BCL2, BCL6, or MYC were 50, 30, and 40% of the cells, respectively
ND not determined
aResults are the mean ± SD of three experiments
The cutoffs for immunohistochemical positivity for BCL2, BCL6, and MYC (Abcam, Cambridge, MA, USA) were 50, 30, and 40% of microscopically observed lymphoma cells, respectively. FISH analyses were performed by SRL (Tokyo, Japan).
The retroviral vector of anti-CD19- and anti-CD38-CAR was previously developed [8–10]. To produce a RD114-pseudotyped retrovirus, MSCV-IRES-EGFP-anti-CD19-CAR or MSCV-IRES-EGFP-anti-CD38-CAR, pEQ-PAM3(-E), and pRDF were used to co-transfect 293T cells with Lipofectamine plus (Invitrogen, Carlsbad, CA, USA). Peripheral blood mononuclear cells of donors were cultured for 48 h with 7 μg/ml PHA-M (Sigma, St Louis, MO, USA), 200 IU/ml interleukin-2 (PeproTech, London, UK) in the complete medium as described previously [8–10]. These T cells were retrovirally transduced in the presence of 4 μg/ml polybrene (Sigma) in a retronectin-coated tube (Takara-Bio, Otsu, Japan). For the transduction of anti-CD38-CAR, an anti-CD38 antibody (CPK-H; MBL, Nagoya, Japan) was added to the culture medium to protect transduced T cells from autolysis through cross-linkage of the anti-CD38-CAR with intrinsic CD38 [8, 9]. For the subsequent co-culture experiments, transduced T cells expressing green fluorescent protein (GFP) were sorted by FACSAria (BD). The specimens from patients and donors were used after approval by the institutional review board of Hiroshima University.
Primary DHL cells co-cultured with anti-CD19- and/or anti-CD38-CAR T cells were harvested and stained with an anti-CD19 antibody-PE and anti-CD38 antibody-APC (BD). These cells were then analyzed by a flow cytometer. Specific cytotoxicity of anti-CD19- and/or anti-CD38-CAR T cells against CD19+ primary DHL cells was evaluated using the formula (B-A)/B, where A is the number of CD19+ GFP− cells or CD38+ GFP− cells after incubation with anti-CD19- or anti-CD38-CAR-expressing T cells, respectively, and B is the number of CD19+ GFP− or CD38+ GFP− cells after incubation with vector-transduced T cells [8–10].
We initially detected cytogenetic DHL and DEL (Additional file 1: Figure S1 and Table 1). Next, we confirmed that goat anti-mouse-IgG-PerCP, which cross-reacts with CAR and GFP of the vector, were co-expressed as an internal control in T cells retrovirally transduced (transduction efficiency: 67.42 ± 14.43% (n = 5) for anti-CD19-CAR, 63.21 ± 10.89% (n = 5) for anti-CD38-CAR).
Prior to co-culture experiments, we examined whether CD19+ primary DHL cells expressed CD38. We showed that >97% of DHL cells obtained from five patients expressed CD38 (Table 1). DHL (DEL) cell line cells (KPUM-UH1) expressing CD19 and CD38 were co-cultured with anti-CD19- or anti-CD38-CAR T cells at an effector (E) target (T) ratio of 1:2 for 3 days. Co-culture experiments showed that either anti-CD19- or anti-CD38-CAR T cells almost completely eradicated KPUM-UH1 cells (Fig. 1a). As further experiments, CD19- or CD38-specific T cells were co-cultured with cytogenetic DHL (n = 3) or DEL (n = 2) cells from five patients at an E:T ratio of 1:2 for 3 days. Similarly, anti-CD19- or anti-CD38-CAR T cells completely abolished the primary DHL cells, respectively (Fig. 1a, c and Table 1). Using DHL cells from patient 2, we confirmed that each of the CAR T cells eliminated DHL cells in a cell-number-dependent manner (Fig. 1b, c). Additionally, anti-CD19-CAR T cells synergistically exerted a collaborative cytotoxicity against DHL cells from patient 2 with anti-CD38-CAR T cells, as shown in Fig. 1c. The simultaneous combination index was less than 1.0, leading to the synergy according to Calcusyn software (Biosoft, Cambridge, UK).
Fig. 1.
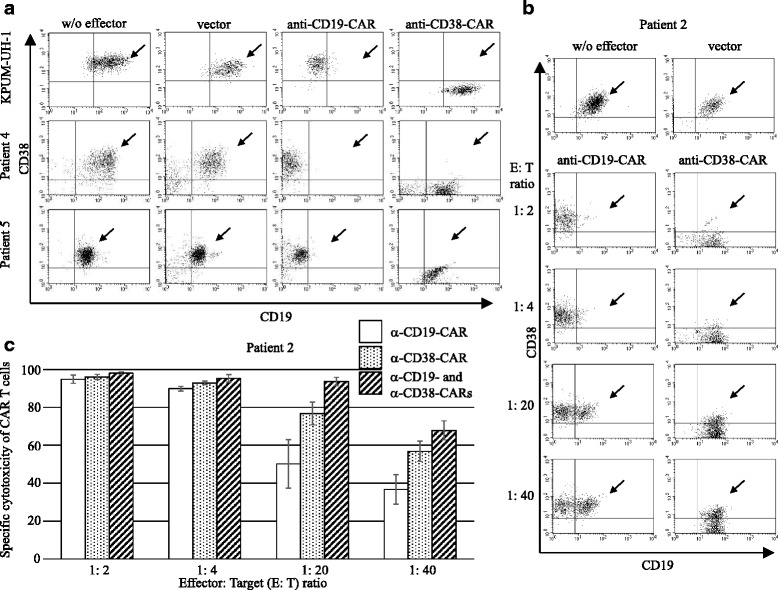
Cytotoxic effect of T cells with anti-CD19- and/or anti-CD38-CAR against DHL cells. a KPUM-UH1(DHL cell line) cells were co-cultured with mock, anti-CD19-, or anti-CD38-CAR T cells at an E:T ratio of 1:2 for 3 days. The cells were harvested and stained with an anti-CD38 antibody-APC and anti-CD19 antibody-PE. These cells were then analyzed by a flow cytometer. Anti-CD19- or anti-CD38-CAR T cells killed KPUM-UH1 cells, respectively (upper panels). Primary DHL cells from patients (patients 4 (cytogenetic DHL) and 5 (DEL)) were co-cultured with either of mock, anti-CD19-, or anti-CD38-CAR T cells at an E:T ratio of 1:2 for 3 days. Anti-CD19- or anti-CD38-CAR T cells eliminated primary DHL cells, respectively (middle and lower panels). The viable primary DHL cell population is indicated by the arrowhead. b Cytogenetic DHL cells from patient 2 (1 × 105 cells) were co-cultured with anti-CD19- or anti-CD38-CAR T cells for 3 days at various ratios to effector cells (0.5 × 105, 0.25 × 105, 0.05 × 105, and 0.025 × 105 cells). Each type of CAR T cells abrogated cytogenetic DHL cells in a cell-number-dependent manner. The viable cytogenetic DHL cell population is indicated by the arrowhead. c The specific cytotoxic effect of anti-CD19- and/or anti-CD38-CAR transduced T cells against DHL cells was cell-number-dependent
These results showed that primary DHL cells, which are refractory or resistant to existing chemotherapeutic agents, can be efficiently abrogated by the clinical use of T cells with anti-CD19- and/or anti-CD38-CAR. Taken together, these results may warrant adoptive immunotherapy with T cells transduced with anti-CD19- and/or anti-CD38-CAR for patients with refractory cytogenetic DHL and DEL.
Additional files
Morphology of cells in the specimens on hematoxylin-eosin staining is shown. MYC expression is shown in lymph node specimens from patient 3. LPF, MPF, and HPF denote low-power, middle-power, and high-power fields, respectively. (PPTX 1063 kb)
Acknowledgements
We thank Sachiko Fukumoto and Ryoko Matsumoto (Department of Hematology and Oncology, Hiroshima University) for providing us with experimental assistance.
Funding
This study was supported in part by grants from the Ministry of Health, Labour, and Welfare of Japan.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
KM designed and performed the experiments, analyzed the data, and wrote the paper. JK provided us with KPUM-UH1 cells and comments on writing the paper. TY and YT analyzed the data. YT, NS, and TI helped write the manuscript. All authors contributed to the interpretation of the results. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The specimens from the patients and donors were used after obtaining appropriate informed consent and approval by the institutional review board of Hiroshima University.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- CAR
Chimeric antigen receptor
- DEL
Double-expressing lymphoma
- DHL
Double-hit lymphoma
- GFP
Green fluorescent protein
- PerCP
Peridinin chlorophyll protein complex
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s13045-017-0488-x) contains supplementary material, which is available to authorized users.
Contributor Information
Keichiro Mihara, Phone: +81-82-257-5861, Email: kmmihara@hiroshima-u.ac.jp.
Tetsumi Yoshida, Email: c35medalist2@gmail.com.
Yoshifumi Takei, Email: takei@dpc.agu.ac.jp.
Naomi Sasaki, Email: n-sasaki@kure-kyosai.jp.
Yoshihiro Takihara, Email: takihara@hiroshima-u.ac.jp.
Junya Kuroda, Email: junkuro@koto.kpu-m.ac.jp.
Tatsuo Ichinohe, Email: nohe@hiroshima-u.ac.jp.
References
- 1.Cheah CY, Oki Y, Westin JR, Turturro F. A clinician’s guide to double hit lymphomas. Br J Haematol. 2015;168(6):784–95. doi: 10.1111/bjh.13276. [DOI] [PubMed] [Google Scholar]
- 2.Friedberg JW. Double-hit diffuse large B-cell lymphoma. J Clin Oncol. 2012;30(28):3439–43. doi: 10.1200/JCO.2012.43.5800. [DOI] [PubMed] [Google Scholar]
- 3.Li S, Lin P, Fayad LE, Lennon PA, Miranda RN, Yin CC, Lin E, Medeiros LJ. B-cell lymphomas with MYC/8q24 rearrangements and IGH@BCL2/t(14;18)(q32;q21): an aggressive disease with heterogeneous histology, germinal center B-cell immunophenotype and poor outcome. Mod Pathol. 2012;25(1):145–56. doi: 10.1038/modpathol.2011.147. [DOI] [PubMed] [Google Scholar]
- 4.Perry AM, Alvarado-Bernal Y, Laurini JA, Smith LM, Slack GW, Tan KL, Sehn LH, Fu K, Aoun P, Greiner TC, Chan WC, Bierman PJ, Bociek RG, Armitage JO, Vose JM, Gascoyne RD, Weisenburger DD. MYC and BCL2 protein expression predicts survival in patients with diffuse large B-cell lymphoma treated with rituximab. Br J Haematol. 2014;165(3):382–91. doi: 10.1111/bjh.12763. [DOI] [PubMed] [Google Scholar]
- 5.Park JH, Geyer MB, Brentjens RJ. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood. 2016;127(26):3312–20. doi: 10.1182/blood-2016-02-629063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davila ML, Sadelain M. Biology and clinical application of CAR T cells for B cell malignancies. Int J Hematol. 2016;104(1):6–17. doi: 10.1007/s12185-016-2039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maude S, Barrett DM. Current status of chimeric antigen receptor therapy for haematological malignancies. Br J Haematol. 2016;172(1):11–22. doi: 10.1111/bjh.13792. [DOI] [PubMed] [Google Scholar]
- 8.Mihara K, Yanagihara K, Takigahira M, Imai C, Kitanaka A, Takihara Y, Kimura A. Activated T-cell-mediated immunotherapy with a chimeric receptor against CD38 in B-cell non-Hodgkin lymphoma. J Immunother. 2009;32(7):737–43. doi: 10.1097/CJI.0b013e3181adaff1. [DOI] [PubMed] [Google Scholar]
- 9.Mihara K, Bhattacharyya J, Kitanaka A, Yanagihara K, Kubo T, Takei Y, Asaoku H, Takihara Y, Kimura A. T-cell immunotherapy with a chimeric receptor against CD38 is effective in eliminating myeloma cells. Leukemia. 2012;26(2):365–7. doi: 10.1038/leu.2011.205. [DOI] [PubMed] [Google Scholar]
- 10.Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, Campana D. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18(4):676–84. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Morphology of cells in the specimens on hematoxylin-eosin staining is shown. MYC expression is shown in lymph node specimens from patient 3. LPF, MPF, and HPF denote low-power, middle-power, and high-power fields, respectively. (PPTX 1063 kb)
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


