Abstract
Expression of Kaposi's sarcoma-associated herpesvirus (KSHV) lytic genes is thought to be essential for the establishment and progression of KSHV-induced diseases. The inefficiency of lytic reactivation in various in vitro systems hampers the study of lytic genes in the context of whole virus. We report here increased expression of KSHV lytic genes and increased release of progeny virus when synchronized cultures of body cavity-based lymphoma-1 cells are treated with a phorbol ester during S phase of the cell cycle.
Kaposi's sarcoma-associated herpesvirus (KSHV; also called human herpesvirus 8) is a gamma-2 herpesvirus and is widely accepted to be the causative agent of Kaposi's sarcoma as well as the lymphoproliferative disorders primary effusion lymphoma (3, 4) and multicentric Castleman's disease (16). KSHV DNA is consistently found to be associated with almost all clinical forms of Kaposi's sarcoma (6), localizing specifically to endothelial and spindle cells (2) as well as infiltrating monocytes (1). In vivo, the majority of infected cells maintain the virus as a latent infection, with only a small percentage of cells spontaneously entering the lytic replication cycle (17, 20). The biological signals that promote lytic induction are not fully understood, though hypoxia (7), coinfection with other viruses (10, 18), and expression of KSHV open reading frame (ORF) 50 (9), a homolog of Epstein-Barr virus Rta, have been implicated. In vitro, KSHV-infected cells can be induced to enter the lytic cycle by treatment with phorbol esters or sodium butyrate or by overexpression of ORF 50 (19). Chemical induction significantly increases the percentage of cells that express lytic gene products above the typical background of 0.5 to 5%, but in most cases the maximum level of lytic induction does not exceed 20% (5, 21). Efforts to study lytic cycle genes in vitro within the context of whole virus are thus complicated by the inefficiency of chemical induction.
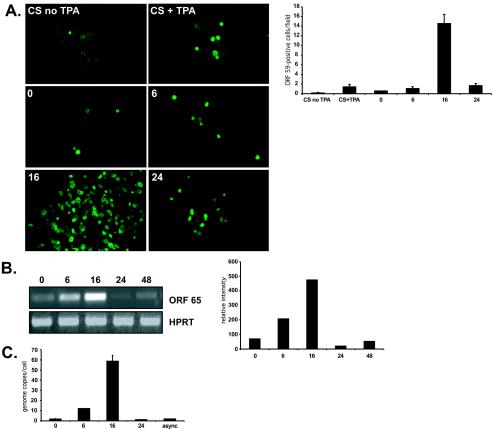
DNA viruses, including herpesviruses, are known to influence cell cycle progression in host cells to optimize the cellular environment for viral replication (13-15). We hypothesized, therefore, that inducing lytic reactivation in synchronized cultures of KSHV-infected cells at different stages of the cell cycle might reveal a point of maximum inducibility. To test this hypothesis, asynchronous cultures of BCBL-1 cells, a primary effusion lymphoma cell line persistently infected with KSHV but not Epstein-Barr virus (obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Disease, National Institutes of Health; contributed by Michael McGrath and Don Ganem), were synchronized at G0 by 24 h of incubation in serum-free RPMI 1640 medium (SFM) supplemented with 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 2 mM glutamine. Asynchronously dividing cells (1.2 × 107) were pelleted, resuspended in 20 ml of SFM, and then distributed evenly between four T25 flasks (3 × 106 cells in 5 ml of SFM/culture). After 24 h, 500 μl of fetal calf serum (10% final concentration) was added to induce reentry into the cell cycle. At 0, 6, 16, and 24 h following the addition of serum, lytic replication was induced by the addition of tetradecanoyl phorbol acetate (TPA; 20 ng/ml), and cultures were incubated for an additional 48 h (Fig. 1). Parallel unsynchronized cultures, TPA treated and untreated, were prepared as controls. Trypan blue exclusion verified that cell viability was not appreciably affected by any of the experimental conditions employed (data not shown). Following TPA induction, cultures were spotted onto glass slides and air dried, fixed with 2% paraformaldehyde, permeabilized with 0.05% Triton, stained for a KSHV early lytic marker (ORF 59) with a monoclonal antibody generously provided by Bala Chandran (The University of Kansas Medical Center, Kansas City) followed by goat anti-mouse immunoglobulin fluorescein isothiocyanate-labeled secondary antibody (Biosource International, Camarillo, Calif.), and examined on a Zeiss fluorescent microscope. Figure 2A shows representative images for each treatment. Calculation of the average number of ORF 59-positive cells from nine random fields shows a 10-fold increase in ORF 59 reactivity in the synchronized BCBL-1 culture that was induced with TPA 16 h after release from G0 block compared with the unsynchronized TPA-treated culture (Fig. 2A, bar graph, 16 h versus constant serum). This observation suggests that the cellular environment 16 h into the cell cycle is better able to support lytic replication following chemical induction.
FIG. 1.
Schematic of cell synchronization protocol. Asynchronously dividing BCBL-1 cells were arrested in G0 by 24 h of incubation in SFM. Following the reintroduction of serum, TPA was added to induce lytic replication at 0, 6, 16, and 24 h after release from G0 block (asterisks). Parallel unsynchronized BCBL-1 cells, TPA treated or untreated, were prepared as controls.
FIG. 2.
Staining BCBL-1 cells for an early lytic KSHV protein (ORF 59, green) revealed that cells were most susceptible to lytic induction by TPA treatment when synchronized cells were induced 16 h following release from G0 block (A, panel 16). The average number of ORF 59-positive cells counted over nine random fields was 10-fold higher in synchronized cells 16 h following the reintroduction of serum than in cells incubated in constant serum (CS) treated with TPA (A, bar graph, CS + TPA versus 16 [error bars show standard errors of the means from quadruplicate determinations]). Expression of a late lytic gene (ORF 65, determined by RT-PCR analysis) was also greatest at this time point (B, lane 16 of agarose-ethidium bromide gel). Densitometry analysis of PCR band intensity showed an approximately sevenfold elevation of ORF 65 expression at 16 h compared with the 0-h sample (B, bar graph,16 versus 0). Increased expression of lytic genes at 16 h was accompanied by a 30-fold increase in release of viral progeny as determined by quantitative SYBR-green PCR (C).
We next compared expression levels of a late lytic gene, ORF 65, by reverse transcriptase-PCR (RT-PCR) in synchronized cultures prepared as described for Fig. 1. Total cellular RNA was harvested with an RNeasy RNA isolation kit (Qiagen Inc., Valencia, Calif.). DNase-treated RNA (5 ng) was used as the template for RT-PCR for ORF 65 with a Titan one-tube RT-PCR kit (Roche, Indianapolis, Ind.) per the manufacturer's instructions (ORF 65 forward primer, 5′-GGCGTTAATTAAGCTAGCATGTCCAACTTTAAGGTGAGA-3′; ORF 65 reverse primer, 5′-AAACCTATTTCTTTTTGCCAGAGG-3′; cycle conditions: 1 cycle of 50°C for 30 min and 94°C for 5 min, 40 cycles of 94°C for 30 s, 56°C for 1 min, and 68°C for 1 min, and 1 cycle of 72°C for 10 min). The cellular hypoxanthine-guanine phosphoribosyltransferase gene was amplified from each sample as a control for cDNA synthesis and yielded consistent amplification products from sample to sample. Parallel reactions using Vent polymerase (New England Biolabs, Beverly, Mass.) showed that there was no DNA contamination in template RNAs (data not shown). Cultures without TPA induction had ORF 65 levels below the level of detection, as did TPA-induced cultures treated with ganciclovir, a drug that blocks viral replication (11) (data not shown). Figure 2B shows the levels of ORF 65 expression when RT-PCR products were visualized by electrophoresis through agarose-ethidium bromide gels. Densitometry analysis of band intensity revealed that ORF 65 expression was highest in the synchronized BCBL-1 culture that was induced with TPA 16 h after release from G0 block (Fig. 2B, bar graph), again indicating that the intracellular environment of BCBL-1 cells is most conducive to KSHV lytic reactivation by chemical induction 16 h following release from G0 block.
To determine if the increase in lytic gene expression seen in the previous experiments represents abortive reactivation or if there is a concomitant increase in release of viral progeny, numbers of KSHV genome copies in culture supernatants were determined by quantitative SYBR-green PCR. Culture supernatants from synchronized and unsynchronized cultures prepared as for Fig. 1 were centrifuged for 1 h at 22,000 rpm in a Beckman L8M ultracentrifuge with rotor SW28 over 5 ml of 20% sorbitol. DNA from the resultant pellets was harvested by using a Purgene genomic DNA purification kit (Gentra Systems, Minneapolis, Minn.) according to the manufacturer's instructions and resuspended in 10 μl of distilled H2O; 1 μl was used as the template per reaction using TaqMan universal PCR Master Mix (Applied Biosystems, Foster City, Calif.). Quantitative PCR was performed on an ABI-PRISM 7700 sequence detection system (Applied Biosystems) under standard reaction conditions. Full-length ORF 65 was cloned into pGEM-TEasy (pGEM-TEasy vector system; Promega, Madison, Wis.; ORF 65 forward primer, 5′-CAGGAGCGACTGGATCATGA-3′, ORF 65 reverse primer, 5′-TTTCCCTGATCCAGGGTATTCA-3′) and serially diluted for construction of a standard curve. Culture supernatant from synchronized BCBL-1 cells chemically induced 16 h following release from G0 block yielded an average of approximately 60 KSHV genomes/cell, a 30-fold increase in virus production compared with the unsynchronized TPA-induced culture (Fig. 2C), indicating that the increased expression of lytic genes under these culture conditions is reflective of increased productive rather than abortive lytic replication.
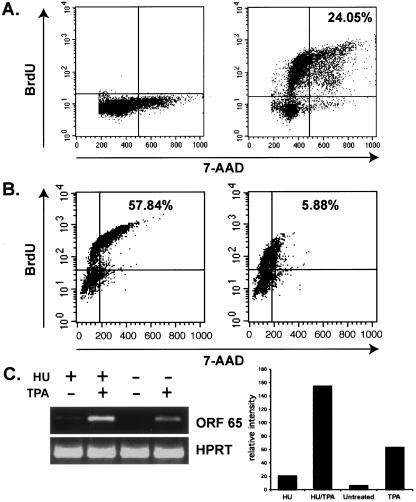
The S phase of the cell cycle is reached between 12 and 16 h after the addition of serum to cultures synchronized by serum starvation (12). To determine whether synchronized BCBL-1 cells were in S phase 16 h following release from G0 block, we determined the DNA content of these cells with a bromodeoxyuridine (BrdU) flow kit (BD Pharmingen, San Diego, Calif.) per the manufacturer's instructions. As expected, BCBL-1 cells synchronized by 24 h of serum starvation were predominantly stalled in G0, indicated by a 2N DNA content (7-amino actinomycin D [7-AAD] staining of total DNA content) (Fig. 3A, lower left quadrant of left panel) and the absence of DNA replication (BrdU staining of actively replicating DNA) (Fig. 3A, upper left and right quadrants of left panel). In contrast, 16 h following the reintroduction of serum, 70% of cells began DNA synthesis (Fig. 3A, upper left and right quadrants of right panel) with 24% of the total achieving a 4N DNA content (upper right quadrant only). TPA treatment did not alter the progression through the cell cycle past S phase (data not shown). Therefore, G0-synchronized BCBL-1 cells are predominantly in S phase 16 h after reentering the cell cycle.
FIG. 3.
Quantification of DNA content showed that after 24 h incubation in serum free medium synchronized BCBL-1 cells were arrested in G0, as shown by the majority of cells having a 2N DNA content and no BrdU incorporation (A, left panel). However, 16 h after release from G0 block, cells reach S phase of the cell cycle, as shown by an increase in the percentage of cells that have incorporated BrdU into newly synthesized DNA (A, right panel). Blockade at early S phase following HU treatment was verified by the same method (B). HU-treated cultures had significantly reduced numbers of cells progressing through S phase (6%; B, upper right quadrant of right panel) compared with untreated cultures (58%; upper right quadrant of left panel). Expression of the late lytic gene ORF 65 (determined by RT-PCR analysis) was greater in TPA-induced cultures of BCBL-1 cells that had been previously synchronized at early S phase of the cell cycle by incubation with HU (C).
We next employed hydroxyurea (HU; 1.5 mM; Fisher, Pittsburgh, Pa.) to halt cell cycle progression in S phase; HU inhibits ribonucleotide reductase, thereby limiting the ribonucleotide pool and blocking DNA synthesis (8). We verified that HU treatment blocked BCBL-1 cells in S phase by measuring the DNA content of HU-treated and untreated cells by BrdU and 7-AAD staining as described above. BCBL-1 cultures were first synchronized by serum starvation for 24 h; 10% serum was then added, and cultures were allowed to progress through the cell cycle for 20 h in the presence or absence of 1.5 mM HU plus 10 μM BrdU. Figure 3B demonstrates that approximately 58% of gated cells from untreated cultures were able to achieve a 4N DNA content (upper right quadrant of left panel), whereas 10-fold-fewer HU-treated cells (upper right quadrant of right panel) were able to proceed past early S phase, thus verifying that HU-treatment synchronized BCBL-1 cells at S phase. To corroborate our evidence that BCBL-1 cells are most responsive to lytic induction during S phase, BCBL-1 cultures with and without HU were divided into TPA-treated and untreated pools. ORF 65 expression, determined by RT-PCR as described for Fig. 2B, was approximately 2.5-fold greater in TPA-induced BCBL-1 cultures that had been previously synchronized at early S phase with HU than in asynchronous cells treated with TPA (Fig. 3C, bar graph). This finding is consistent with other data presented here and indicates that the intracellular environment of BCBL-1 cells is most able to support KSHV lytic replication following chemical induction during S phase of the cell cycle.
This simple method may prove useful in the study of KSHV lytic cycle genes in the context of whole virus as well as increasing the efficiency of high-titer KSHV stock preparation. More broadly, determination of specific S-phase cellular products which account for the increased inducibility of infected cells as demonstrated here may shed further light on the molecular mechanisms governing induction of KSHV lytic replication.
Acknowledgments
This work was supported by RO1-HL61928-S1 and P51-RR00163 (A.V.M.) and NIA AG20719 (J.N.-Z.).
We acknowledge Andrew Townsend, Imaging Core Manager at Oregon Health & Science University and Vaccine & Gene Therapy Institute, for assistance with manuscript preparation and Arlene Bitmansour for assistance with quantitative PCR.
REFERENCES
- 1.Blasig, C., C. Zietz, B. Haar, F. Neipel, S. Esser, N. H. Brockmeyer, E. Tschachler, S. Colombini, B. Ensoli, and M. Sturzl. 1997. Monocytes in Kaposi's sarcoma lesions are productively infected by human herpesvirus 8. J. Virol. 71:7963-7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boshoff, C., T. F. Schulz, M. M. Kennedy, A. K. Graham, C. Fisher, A. Thomas, J. O. McGee, R. A. Weiss, and J. J. O'Leary. 1995. Kaposi's sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat. Med. 1:1274-1278. [DOI] [PubMed] [Google Scholar]
- 3.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomase. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 4.Cesarman, E., R. G. Nador, K. Aozasa, G. Delsol, J. W. Said, and D. M. Knowles. 1996. Kaposi's sarcoma-associated herpesvirus in non-AIDS related lymphomas occurring in body cavities. Am. J. Pathol. 149:53-57. [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, J., R. Renne, D. Dittmer, and D. Ganem. 2000. Inflammatory cytokines and the reactivation of Kaposi's sarcoma-associated herpesvirus lytic replication. Virology 266:17-25. [DOI] [PubMed] [Google Scholar]
- 6.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 7.Davis, D. A., A. S. Rinderknecht, J. P. Zoeteweij, Y. Aoki, E. L. Read-Connole, G. Tosato, A. Blauvelt, and R. Yarchoan. 2001. Hypoxia induces lytic replication of Kaposi sarcoma-associated herpesvirus. Blood 97:3244-3250. [DOI] [PubMed] [Google Scholar]
- 8.Donehower, R. 1996. Hydroxyurea, p. 253-261. In D. Longo (ed.), Cancer chemotherapy and biotherapy, 2nd ed. Lippincott-Raven, Philadelphia, Pa.
- 9.Gradoville, L., J. Gerlach, E. Grogan, D. Shedd, S. Nikiforow, C. Metroka, and G. Miller. 2000. Kaposi's sarcoma-associated herpesvirus open reading frame 50/Rta protein activates the entire viral lytic cycle in the HH-B2 primary effusion lymphoma cell line. J. Virol. 74:6207-6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrington, W., Jr., L. Sieczkowski, C. Sosa, S. Chan-a-Sue, J. P. Cai, L. Cabral, and C. Wood. 1997. Activation of HHV-8 by HIV-1 tat. Lancet 349:774-775. [DOI] [PubMed] [Google Scholar]
- 11.Kedes, D. H., and D. Ganem. 1997. Sensitivity of Kaposi's sarcoma-associated herpesvirus replication to antiviral drugs. Implications for potential therapy. J. Clin. Investig. 99:2082-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krek, W., and J. A. DeCaprio. 1995. Cell synchronization. Methods Enzymol. 254:114-124 [DOI] [PubMed] [Google Scholar]
- 13.Kudoh, A., T. Daikoku, Y. Sugaya, H. Isomura, M. Fujita, T. Kiyono, Y. Nishiyama, and T. Tsurumi. 2004. Inhibition of S-phase cyclin-dependent kinase activity blocks expression of Epstein-Barr virus immediate-early and early genes, preventing viral lytic replication. J. Virol. 78:104-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kudoh, A., M. Fujita, T. Kiyono, K. Kuzushima, Y. Sugaya, S. Izuta, Y. Nishiyama, and T. Tsurumi. 2003. Reactivation of lytic replication from B cells latently infected with Epstein-Barr virus occurs with high S-phase cyclin-dependent kinase activity while inhibiting cellular DNA replication. J. Virol. 77:851-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogris, E., I. Mudrak, and E. Wintersberger. 1992. Polyomavirus large and small T antigens cooperate in induction of the S phase in serum-starved 3T3 mouse fibroblasts. J. Virol. 66:53-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soulier, J., L. Grollet, E. Oksenhendler, P. Cacoub, D. Cazals-Hatem, P. Babinet, M. F. d'Agay, J. P. Clauvel, M. Raphael, L. Degos, et al. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276-1280. [PubMed] [Google Scholar]
- 17.Staskus, K. A., W. Zhong, K. Gebhard, B. Herndier, H. Wang, R. Renne, J. Beneke, J. Pudney, D. J. Anderson, D. Ganem, and A. T. Haase. 1997. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J. Virol. 71:715-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vieira, J., P. O'Hearn, L. Kimball, B. Chandran, and L. Corey. 2001. Activation of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) lytic replication by human cytomegalovirus. J. Virol. 75:1378-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vieira, J., and P. M. O'Hearn. 2004. Use of the red fluorescent protein as a marker of Kaposi's sarcoma-associated herpesvirus lytic gene expression. Virology 325:225-240. [DOI] [PubMed] [Google Scholar]
- 20.Zhong, W., H. Wang, B. Herndier, and D. Ganem. 1996. Restricted expression of Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi sarcoma. Proc. Natl. Acad. Sci. USA 93:6641-6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zoeteweij, J. P., A. S. Rinderknecht, D. A. Davis, R. Yarchoan, and A. Blauvelt. 2002. Minimal reactivation of Kaposi's sarcoma-associated herpesvirus by corticosteroids in latently infected B cell lines. J. Med. Virol. 66:378-383. [DOI] [PubMed] [Google Scholar]