Abstract
The persistence of human immunodeficiency virus type 1 (HIV-1) infection in the presence of robust host immunity has been associated in part with variation in viral envelope proteins leading to antigenic variation and escape from neutralizing antibodies. Previous studies of natural neutralization escape mutants have predominantly focused on gp120 and gp41 ectodomain sequence variations that alter antibody binding via changes in conformation or glycosylation pattern of the Env, likely due to the immune pressure exerted on the exposed ectodomain component of the glycoprotein. Here, we show for the first time a novel mechanism by which point mutations in the intracytoplasmic tail of the transmembrane component (gp41) of envelope can render the virus resistant to neutralization by monoclonal antibodies and broadly neutralizing polyclonal serum antibodies. Point mutations in a highly conserved structural motif within the intracytoplasmic tail resulted in decreased binding of neutralizing antibodies to the Env ectodomain, evidently due to allosteric changes both in the gp41 ectodomain and in gp120. While receptor binding and infectivity of the mutant virus remained unaltered, the changes in Env antigenicity were associated with an increase in neutralization resistance of the mutant virus. These studies demonstrate the structurally integrated nature of gp120 and gp41 and underscore a previously unrecognized potentially critical role for even minor sequence variation of the intracytoplasmic tail in modulating the antigenicity of the ectodomain of HIV-1 envelope glycoprotein complex.
The characteristic mutability of human immunodeficiency virus type 1 (HIV-1) due to the error-prone nature of reverse transcriptase and high rates of viral replication in vivo poses a major challenge to host humoral and cellular immunity (17). In relation to viral escape from neutralizing antibody responses (4, 10, 24), currently defined mutations resulting in neutralization escape primarily map to the ectodomain of the envelope glycoprotein (Env) (19, 20, 22, 25, 27, 39), while the intracytoplasmic component of the Env remains largely unexamined. The 150-amino-acid-long intracytoplasmic tail (ICT) of the transmembrane protein (TM) is characteristic of the envelope glycoproteins of all lentiviruses, in distinct contrast to oncoretroviruses that have a relatively short ICT (20 to 30 amino acids) (15). In vitro the ICT of HIV-1 impacts viral replication in a cell type-dependent manner (23); primary peripheral blood mononuclear cells (PBMC) are absolutely dependent on the presence of an intact ICT to support viral replication (23). The ICT has been identified as a locus for attenuation of simian immunodeficiency virus (SIV) in experimentally infected macaques (32), thereby providing evidence for the in vivo functional significance of the ICT. The ICT of HIV-1 gp41 contains two structurally conserved amphipathic α-helical domains, lentivirus lytic peptides 1 and 2 (LLP-1 and LLP-2) (Fig. 1) (12). We have recently shown that site-directed mutations in the LLP-1 domain inhibit virion Env incorporation and viral replication in vitro, while similar mutations in the LLP-2 domain inhibit cell-cell fusogenicity of the envelope glycoprotein without any evident effects on viral replication (18). Previous studies involving large truncations of the ICT of SIV TM protein have implicated a function of the ICT in modulating the conformation of the ectodomain of the envelope glycoprotein (34). Owing to the highly ordered structural properties of the LLP-2 domain, we investigated in this study the contribution of the LLP-2 domain to HIV-1 neutralization and overall envelope structure.
FIG. 1.
Diagram of gp41 with location and amino acid sequence of WT and mutant LLP-2 domain. Mutations in the LLP-2 domain were engineered in the proviral clone of ME46. Helical wheel representations of LLP-2 domain are depicted here with arginine residues (shown by arrowheads) that were replaced with glutamate. Hydrophobic amino acids are shown by dark shaded circles; white circles represent hydrophilic amino acids. Arginine residues in the wild-type (WT) sequence that were replaced with glutamate are shown in boldface type. In the MX3 mutant, Δ147 refers to a deletion of 147 amino acids from the carboxy-terminal end of gp41 by replacing the glutamine residue at position 715 (Q715) with a stop codon (*).
MATERIALS AND METHODS
Cells and virus stocks.
293T cells were obtained from the American Type Culture Collection (Manassas, Va.) and maintained in Dulbecco's modified Eagle medium (GIBCO, Grand Island, N.Y.) containing 10% fetal bovine serum (FBS), l-glutamine (2 mM), penicillin G (100 U/ml), and streptomycin sulfate (0.1 mg/ml). MAGI-R5 cells (HeLa-CD4-LTR-β-galactosidase, CCR5 and CXCR4 coreceptors) (obtained from the National Institutes of Health [NIH] AIDS Research and Reference Reagent Program) were maintained in the same medium as 293T cells in the presence of G418 (0.2 μg/ml), hygromycin B (0.1 μg/ml), and puromycin (1 μg/ml). Human PBMC were isolated by Ficoll-Hypaque gradient centrifugation. Following isolation of PBMC, CD8+ T cells were depleted by magnetic separation with anti-CD8-conjugated magnetic beads (Miltenyi Biotec, Auburn, Calif.). Prior to HIV-1 infection, PBMC were activated by incubation in interleukin-2 (IL-2) cell culture medium containing 10 μg of phytohemagglutinin (PHA) (PHA-P; Difco Laboratories, Detroit, Mich.)/ml. IL-2 culture medium was RPMI 1640 medium containing (per milliliter) 100 U of penicillin, 100 μg of streptomycin, 2 mM l-glutamine, 10% heat-inactivated fetal calf serum (FCS), and 20 U of recombinant IL-2 (Roche Molecular Biochemicals, Indianapolis, Ind.). After 2 to 3 days of incubation with PHA, cells were washed and used for infection. All cell cultures were maintained in 5% CO2 incubators at 37°C.
HIV-1 strain ME46 was isolated at the University of Pittsburgh and has been characterized in detail previously (8). ME46 is a dual-tropic primary isolate that uses both CCR5 and CXCR4 as coreceptors for virus entry into target cells. The LLP-2 mutant virus (MX2 or LLP2 R775,793E) and ICT-deleted ME46 mutant (MX3 or ICTΔ147) were generated by PCR-based mutagenesis as described previously (18). Virus stocks of ME46, MX2, and MX3 were obtained by transfecting the wild-type and mutant full-length proviral genomes into 293T cells (50 to 80% confluence) by the calcium phosphate transfection method (CalPhos mammalian transfection kit; Clontech, Palo Alto, Calif.). To generate virus stocks from 293T cells, supernatants were collected about 2 to 3 days posttransfection, and virus stocks were made cell free by centrifugation at 1,000 × g and filtration though a 0.45-μm-pore-size filter. In both cases, viral stocks were concentrated by about 10-fold through a 100-kDa cutoff polyethersulfone filter (Centricon Plus Biomax filter; Millipore, Bedford, Mass.) according to the manufacturer's instructions. Virus aliquots were stored in the vapor phase of liquid nitrogen. Virus 50% tissue culture infectious doses were determined by the Reed and Muench method by endpoint titration assay in MAGI-R5 cells (18).
Determination of HIV-1 replication kinetics.
For measurement of viral replication kinetics, the transfected 293T cells were incubated at 37°C for 48 h, and supernatants were collected and filtered through a 0.45-μm-pore-size filter. Samples of filtered cell-free tissue culture supernatant containing similar amounts of p24 were then used to infect 5 × 106 PHA-stimulated PBMC. At an interval of 5 to 6 days, half of the culture medium was replaced with fresh medium containing 2.5 × 106 PHA-stimulated PBMC. Virus production was monitored by measuring the p24 antigen in the culture supernatant by an antigen-capture enzyme-linked immunosorbent assay (DuPont, Wilmington, Del.) as per the directions of the manufacturer.
Viral infection and flow cytometric analysis for intracellular expression of p24 antigen.
HIV-1 infection of PHA- and IL-2-stimulated PBMC was performed in 96-well round-bottom culture plates by combining 50 μl of virus stock with 20 μl of PBMC (1.0 × 105 cells). The multiplicity of infection (MOI) is indicated for individual experiments. After overnight incubation at 37°C, 150 μl of IL-2 culture medium was added, and the cells were continued in culture. PBMC were harvested for intracellular p24 antigen staining on specific days as described for individual experiments. PBMC were resuspended in IL-2 culture medium containing indinavir prior to being distributed into culture plates. The final concentration of indinavir was 1 μM, and this concentration was maintained throughout.
Cells were harvested for intracellular p24 antigen staining 2 days following infection. Staining was performed in the same U-bottom plates in which the cells were cultured. Cells were first washed once with phosphate-buffered saline (PBS) containing 5% FBS (wash buffer). Cells were then fixed in 1% paraformaldehyde for 1 h and permeabilized with PBS containing 5% FCS and 0.5% saponin (permeabilization buffer) for staining intracellular p24 with KC57-RD1 (Coulter Corporation, Miami, Fla.). Following 30 min of incubation with the selected antibody, cells were washed three times with permeabilization buffer. Finally, cells were washed twice with wash buffer, resuspended in 1% paraformaldehyde, and analyzed for intracellular p24 expression. A minimum of 100,000 gated live events were acquired on a FACSCalibur (Becton Dickinson, San Jose, Calif.) flow cytometer and analyzed with FlowJo batch analysis software (Treestar, San Carlos, Calif.).
Neutralization assays.
The single-cycle intracellular p24 antigen neutralization (21) assays were performed in 96-well culture plates by incubating 50 μl of virus stock with 10 μl of the appropriate antibody concentrations. All antibodies were obtained from the NIH AIDS Research and Reference Reagent Program. After incubation for 30 min at 37°C, 20 μl of PBMC (1.0 × 105 cells) was added to each well. PBMC were maintained in IL-2 culture medium containing 1 μM indinavir, and the cells were fed on day 1 with 150 μl of IL-2 culture medium containing indinavir. The concentration of virus stock was adjusted to provide a final MOI of about 0.2. PBMC were harvested for intracellular p24 antigen staining on day 2. To enumerate infected PBMC, cells were washed, fixed, permeabilized, and stained with the KC57 anti-p24 antibody as described above. After forward and side scatter gating, at least 100,000 events were counted and collected. The percent neutralization was defined as the reduction in the number of p24 antigen-positive cells compared with the number in control wells with no antibody. The inhibitory concentration that neutralized 50% (ND50) of virus was calculated from the antibody dose-response curves by nonlinear regression curve fit analyses (21). All neutralization experiments were performed with PBMC depleted of CD8+ cells.
Flow cytometric analysis of HIV-1 surface Env and intracellular p24 expression.
Parallel samples of 293T cells were transfected with wild-type virus or with MX2 virus as described above. At 2 to 3 days postinfection, the cells were stained for cell surface gp41 and gp120 with the indicated antibodies (obtained from the NIH AIDS Research and Reference Reagent Program) as described previously (18). Cy5- or allophycocyanin-conjugated mouse anti-human immunoglobulin G (IgG) was used as the secondary antibody (ICN, Costa Mesa, Calif.). Following incubation for 30 min, cells were washed three times with PBS containing 5% FBS (wash buffer) and fixed in 1% paraformaldehyde for 1 h. To assess coreceptor binding site exposure, transfected cells were first incubated with 200 μl of 1 μg of sCD4/ml in Dulbecco's modified Eagle medium for 120 min at 4°C (13), washed once with wash buffer, and stained with primary and secondary antibodies, as described above. Cells were then permeabilized with PBS containing 5% FCS and 0.5% saponin (permeabilization buffer) for staining intracellular p24 with KC57-RD1 (Coulter Corporation). Following 30 min of incubation, cells were washed three times with permeabilization buffer. Finally, cells were washed twice with wash buffer, resuspended in 1% paraformaldehyde, and analyzed for cell surface gp41 and intracellular p24 expression. A minimum of 50,000 gated live events were acquired on a FACSCalibur (Becton Dickinson) flow cytometer and analyzed with FlowJo batch analysis software (Treestar). Env expression was assessed in the p24-positive population of cells, and the mean fluorescence intensities of phycoerythrin and fluorescein isothiocyanate stainings were determined. Ratios of the mean fluorescence intensity of p24-phycoerythrin and Env-fluorescein isothiocyanate were calculated and normalized to that of wild-type Env.
Radioimmunoprecipitation.
Methods used for metabolic radiolabeling of transfected 293T cells, preparation of cell lysates, pelleting of labeled virions, and immunoprecipitation of cell- and virion-associated proteins with fractionated serum immunoglobulin from HIV-1-infected patients (HIV-1 neutralizing sera; obtained from NIH AIDS Research and Reference Reagent Program) were performed as described previously (18). Briefly, log-phase 293T cells (106) were seeded in T25 flasks (Falcon, Franklin Lakes, N.J.). Cells were transfected on the following day with the proviral constructs as described above and incubated for 48 h. The cells were then metabolically labeled overnight at 37°C with 0.5 mCi of [35S]methionine-cysteine protein labeling mix (NEN, Boston, Mass.). Thereafter, cells were washed with PBS and lysed, and the cell-associated viral proteins were immunoprecipitated by using HIV-1 patient sera. Labeled virus was pelleted from cell-free tissue culture supernatant by centrifugation through a 20% (vol/vol) glycerol cushion at 39,000 rpm in an SW55Ti rotor (Beckman, Fullerton, Calif.), and immunoprecipitation was performed with HIV-1 neutralizing sera. Following immunoprecipitation, proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis with a 4 to 15% gradient gel (GIBCO). Dried gels were exposed to film for 1 or more days prior to being developed and quantified by a GS710 calibrated imaging densitometer (Bio-Rad, Hercules, Calif.).
Protein analysis.
Clarified supernatants from transfected cells were centrifuged to pellet viral particles as described above. The viral pellets were then resuspended in PBS for protein analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting. SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, Ill.) was used to visualize the bands after blotting with appropriate monoclonal antibodies. The antibodies were obtained from the NIH AIDS Research and Reference Reagent Program.
Statistical analysis and calculations.
Nonlinear regression curve fit was used for calculation of ND50 values by using Prism, version 3.00, for Windows (GraphPad Software, San Diego, Calif.). GraphPad Prism was also employed for comparison of neutralization sensitivity of wild-type and LLP-2 mutant viruses by using Student's unpaired t test.
RESULTS
Analysis of gp41 and gp120 incorporation into virions by Western blot analysis and effects of mutations in LLP-2 on viral replication.
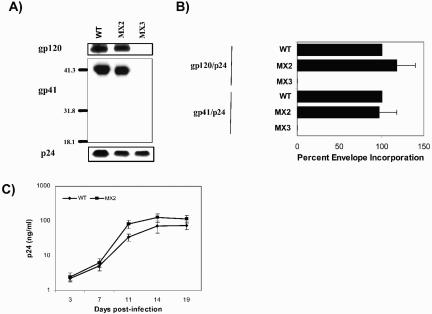
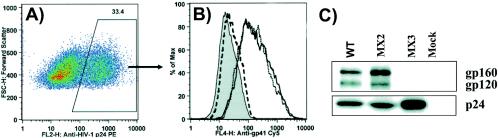
The previously described and characterized mutant HIV-1 MX2, bearing two point mutations in the LLP-2 region of a full-length proviral clone of a primary dual-tropic HIV-1 isolate (Fig. 1), was used. The mutations (775R/E and 793R/E) in MX2 were directed to cause structural perturbations in the ICT by dampening the net positive charge of the amphipathic α-helical motif LLP-2. As reported previously, these mutations did not alter incorporation of Env into the virions (Fig. 2A and B) as assessed by immunoblot analysis for virion-associated gp41. Correlating with wild-type levels of Env incorporation, these mutations in the LLP-2 domain also did not substantially affect viral replicative ability in primary human PBMC (Fig. 2C).
FIG. 2.
Analysis of gp41 and gp120 incorporation into virions by Western blot analysis and effects of mutations in LLP-2 on viral replication. Virion lysates were prepared on day 2 from culture supernatants of 293T cells transfected with wild-type (WT) and mutant proviral clones after ultracentrifugation. (A) Samples were transferred to polyvinylidene difluoride membranes and blotted with polyclonal goat anti-gp120, anti-gp41 monoclonal antibody (T32) and anti-p24 monoclonal antibody (AG3.0). gp120, gp41, and p24 protein bands were also quantitated by densitometry. (B) Relative Env incorporation was determined by calculating ratios of gp120 to p24 and gp41 to p24 and normalizing them to wild-type levels. The results are representative of duplicate experiments. Error bars indicate standard deviations. (C) Wild-type and LLP-2 mutant proviral clones were transfected into 293T cells to produce viral particles. Two days posttransfection, supernatants from the transfections were filtered through a 0.45-μm-pore-size filter, normalized to p24 levels, and used to infect human CD8− PBMC stimulated with PHA. Fresh CD8− PHA-PBMC samples were added every 5 days by replacing half of the culture supernatants. Viral replication kinetics was followed by measuring p24 levels in culture supernatants.
Neutralization properties of LLP-2 mutant virus.
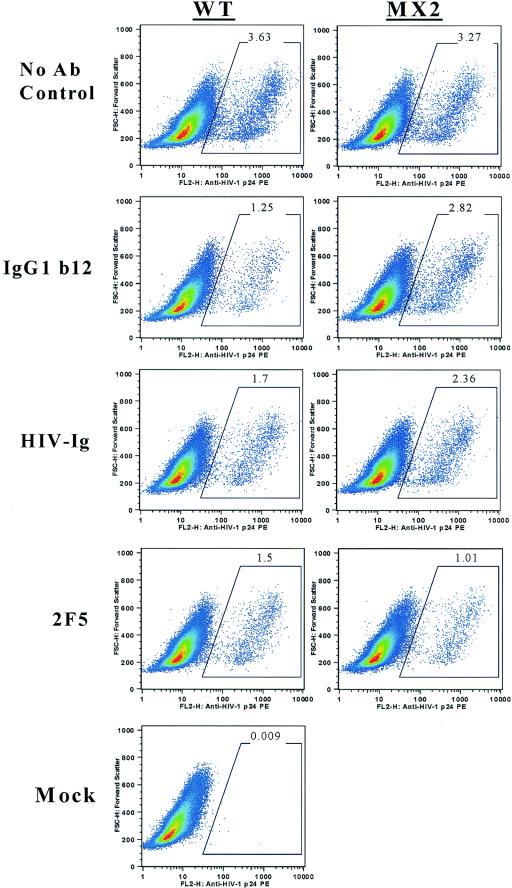
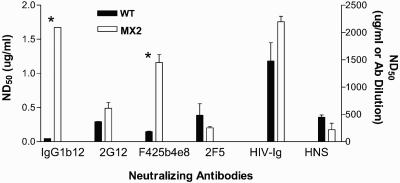
The effects of these mutations on the neutralization sensitivity of the LLP-2 mutant virus were assessed in primary human cells by highly sensitive flow cytometry-based detection of HIV-1 intracellular core antigen p24 2 days postinfection. In this assay, the replication of HIV-1 is restricted to a single round by inclusion of the viral protease inhibitor indinavir in PHA-stimulated PBMC cultures depleted of CD8+ cells (21). Broadly neutralizing monoclonal antibodies (IgG1b12 [5], 2G12 [37], F425b4e8 [6], and 2F5 [30]) with specificities to gp120 and gp41 and reference neutralizing polyclonal HIV-1 patient sera (patient immunoglobulin [HIV-Ig] and HIV-1 neutralizing sera) (38) were tested for their ability to neutralize the LLP-2 mutant virus. As shown in Fig. 3, both wild-type and LLP-2 mutant viruses exhibited similar levels of infection (3.63 and 3.27%, respectively) in the absence of neutralizing antibodies. Upon preincubation of the virus with a broadly neutralizing monoclonal antibody IgG1b12 (0.4 μg/ml) directed against the CD4 binding site, the infectivity of the wild-type virus was reduced to 1.25% of infected cells, reflecting a 65% decrease in infectivity by the neutralizing antibody. Strikingly, we found that the LLP-2 mutant virus was relatively neutralization resistant, as demonstrated by a significantly higher (P < 0.05) percentage of infected p24-positive cells in the presence of neutralizing antibody IgG1b12 (2.82%) compared with wild-type infected cells (1.25%). Relative to the 65% decrease in infectivity observed in the case of wild-type virus, the infectivity of the LLP-2 mutant virus was neutralized by only about 13% by the IgG1b12 monoclonal antibody. A reference polyclonal neutralizing patient immunoglobulin (HIV-Ig) reduced the infectivity of wild-type virus to about 1.7% compared with 3.63% infection in the absence of HIV-Ig. In contrast to the wild-type virus, the infectivity of the LLP-2 mutant virus was decreased to only about 2.36% of infected cells, reflecting about 25% neutralization by HIV-Ig (1,000 μg/ml) compared with about 50% neutralization observed in the case of wild-type virus. In contrast, the 2F5 monoclonal antibody effectively neutralized both wild-type and LLP-2 mutant viruses by about 60 to 70% (Fig. 3), as evident from 1.5 and 1.01% infection upon preincubation of the virus with 0.4 μg of antibody/ml.
FIG. 3.
Primary flow cytometry data for antibody neutralization of HIV-1 infection. CD8-depleted PBMC were isolated from seronegative donor blood by immunomagnetic depletion of CD8+ cells and stimulated with PHA for 3 days. PBMC were resuspended in IL-2 culture medium containing indinavir prior to infection. Wild-type (WT) and LLP-2 mutant viral stocks were obtained by concentrating supernatants from transfected 293T cells 2 days posttransfection by about 10-fold through a 100-kDa-cutoff polyethersulfone filter. Similar infectious doses (MOI ≥ 0.1) of wild-type and LLP-2 mutant viruses were preincubated with 0.4 μg of IgG1b12/ml, 1,000 μg of HIV-Ig/ml, 0.4 μg of 2F5/ml, or medium alone (no antibody control) for 30 min at 37°C prior to the addition of 100,000 PHA-stimulated CD8-depleted PBMC in medium containing indinavir. Infection was allowed to proceed for 48 h, following which cells were stained and analyzed for intracellular p24 expression. Representative primary data from the results of two independent experiments are presented here.
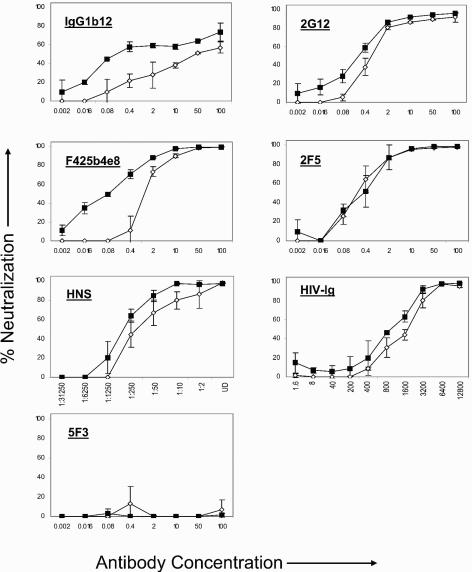
Neutralization resistance of the LLP-2 mutant virus to IgG1b12, 2G12, F425b4e8, HIV-Ig, and HIV-1 neutralizing sera was also evident from antibody titrations (Fig. 4). Neutralization curves demonstrated the neutralization resistance of the LLP-2 mutant virus relative to wild-type virus over a range of antibody concentrations. As expected, preincubation of the virus with previously established nonneutralizing monoclonal antibody 5F3 (3) did not neutralize infectivity of either the wild-type or the LLP-2 mutant viruses. Determination of antibody concentrations to mediate 50% neutralization (ND50) of the test viruses were calculated from the antibody titration curves (Fig. 5). Compared with about 0.04 μg of IgG1b12/ml that was required for decreasing the infectivity of wild-type virus by 50%, substantially higher (1.67 μg/ml) concentrations of the IgG1b12 antibody were required for 50% neutralization of the LLP-2 mutant virus. A 50% neutralization of the wild-type virus was achieved with about 0.3 μg of 2G12 monoclonal antibody/ml, 0.15 μg of F425b4e8/ml, and 1,491 μg of HIV-Ig/ml, while the LLP-2 mutant required comparatively higher concentrations of antibodies (0.5 μg of 2G12/ml, 1.17 μg of F425b4e8/ml, and 2,184 μg of HIV-Ig/ml). Similarly, the infectivity of the wild-type virus was neutralized by 50% in the presence of a 1:437 dilution of HIV-1 neutralizing sera, while a lower dilution of about 1:200 was required for mediating 50% neutralization of the LLP-2 mutant virus. In contrast, no evident differences between wild-type and LLP-2 mutant viruses were observed when comparing the of 2F5 monoclonal antibody. These results clearly demonstrated the relative neutralization resistance of LLP-2 mutant virus to a variety of monoclonal antibodies (IgG1b12, 2G12, and F425b4e8) and to the polyclonal HIV-Ig. The differences in neutralization of LLP-2 mutant and wild-type viruses by the test antibodies (IgG1b12 and F425b4e8) (Fig. 5) were found to be statistically significant (P < 0.05) in comparisons of the ND50 values for each monoclonal antibody for wild-type and LLP-2 mutant viruses.
FIG. 4.
Neutralization dose-response curves. CD8-depleted PBMC were isolated from seronegative donor blood by immunomagnetic depletion of CD8+ cells and stimulated with PHA for 3 days. PBMC were resuspended in IL-2 culture medium containing indinavir prior to infection. Wild-type and LLP-2 mutant viral stocks were obtained by concentrating supernatants from transfected 293T cells 2 days posttransfection by about 10-fold through a 100-kDa-cutoff polyethersulfone filter. Similar infectious doses (MOI ≥ 0.1) of wild-type and LLP-2 mutant viruses were preincubated with the indicated concentrations (in micrograms/milliliter) or dilutions of IgG1b12, 2G12, F425b4e8, 2F5, HIV-1 neutralizing sera, HIV-Ig, or medium alone (no antibody control) for 30 min at 37°C prior to the addition of 100,000 PHA-stimulated CD8− PBMC in medium containing indinavir. Infection was allowed to proceed for 48 h, following which cells were stained and analyzed for intracellular p24 expression. Comparison of antibody titration curves for wild-type (filled squares) and LLP-2 mutant (open diamonds) viruses is presented here with IgG1b12, 2G12, F425B4e8, HIV-Ig, 2F5, and 5F3 antibodies. Data represent averages from the results of two experiments ± standard deviations.
FIG. 5.
Antibody dose for 50% neutralization of HIV-1. Concentrations of antibodies required for 50% neutralization of wild-type (WT) and LLP-2 mutant viruses derived from neutralization curves shown in Fig. 4 are plotted here from two independent experiments. ND50 values for IgG1b12, 2G12, F425b4e8, and 2F5 monoclonal antibodies are plotted on the left y axis, and ND50 values for HIV-Ig and HIV-1 neutralizing sera (antibody [Ab] dilution) are plotted on the right y axis. An unpaired t test was used to compare the differences in neutralization sensitivity of LLP-2 mutant and wild-type viruses. *, P < 0.05.
Thus, these observations that two point mutations in the ICT of gp41 can markedly alter the neutralization phenotype of HIV-1, even to polyclonal serum antibodies, present a novel prospect that the gp41 ICT may hold potential significance in the process of in vivo neutralization resistance of HIV-1.
Expression and antigenicity of LLP-2 mutant envelope glycoprotein.
To define the mechanism by which point mutations in the ICT alter the neutralization sensitivity of HIV-1, we performed an extensive analysis of envelope structure. For these evaluations, we characterized the antigenicity of LLP-2 mutant Env relative to wild-type Env in the context of Env presented on the surface of virus-producing transfected 293T cells as well as in the context of Env associated with pelleted HIV-1 particles. The evaluation of antigenicity of Env expressed on the surface of transfected 293T cells was conducted at the level of a single transfected p24-positive cell by flow cytometry to provide a sensitive and quantitative measure of antibody binding. Critical to such an analysis is evaluation of the levels of total cellular and cell surface expression of LLP-2 mutant and wild-type Env proteins. We first characterized envelope expression of the LLP-2 mutant virus to assess the effects of the LLP-2 mutations on envelope expression and stability. 293T cells transfected with full-length proviral clones of wild-type and LLP-2 mutant viruses were analyzed for intracellular p24 expression to select transfected p24-positive cells (Fig. 6A) for further analysis of total Env expression with a mixture of gp41 antibodies directed to linear determinants (Fig. 6B). The MX3 (ICTΔ147) proviral clone lacking any detectable Env expression has been described previously (18) and was included as an appropriate negative control along with mock-transfected cells. Analysis of total Env expression in cells gated for intracellular viral core protein p24 expression indicated that the level of total Env expression on a per cell basis was similar for both LLP-2 mutant and wild-type Env (Fig. 6A and B). Analysis of total cellular Env expression by highly sensitive radioimmunoprecipitation procedures demonstrated similar expression levels in cells transfected by the wild-type or LLP-2 mutant virus (Fig. 6C). These data also confirmed that the processing of the polyprotein gp160 precursor into gp120 and gp41 was unaffected in the LLP-2 mutant.
FIG. 6.
Total expression and processing of LLP-2 mutant Env. 293T cells transfected with wild-type (WT) and LLP-2 mutant proviral clones were harvested 2 days posttransfection and stained for Env with specific antibodies as well as intracellular p24. For analysis of total envelope expression, cells were permeabilized prior to staining with Env-specific antibodies. (A) An example of gating for p24 positive cells is shown with cells transfected with wild-type proviral clone. (B) Histogram plots of total Env expression in p24-gated populations are shown for wild-type (solid line) and LLP-2 mutant viruses (dotted line). The ICTΔ147 (dashed line) mutant MX3 virus lacking any detectable Env was used as a negative control along with mock-transfected cells (shaded histogram). Monoclonal antibodies 246D, 240D, and 2F5 directed to linear gp41 determinants were used to detect total Env expression as primary antibodies, followed by anti-human Cy5 antibody as the secondary antibody. (C) Radioimmunoprecipitation analyses of total Env expression in transfected 293T cells were performed with HIV patient sera. Cellular expression of gp160 and processed gp120 along with p24 was assessed by autoradiography.
Since sequence motifs in the ICT can function to regulate the cell surface expression of Env (1, 2), the trafficking of LLP-2 mutant Env to the cell surface was next determined by flow cytometry with polyclonal Ig fraction from HIV-1-infected patient sera, HIV-Ig, and a monoclonal antibody 2F5 directed to a linear gp41 epitope (30). Flow cytometric single cell analysis of cell surface Env in p24-gated cells revealed similar mean fluorescence intensity (MFI) of staining by polyclonal HIV-Ig (Fig. 7) and 2F5, indicating similar levels of cell surface Env expression for wild-type and LLP-2 mutant viruses.
FIG. 7.
Comparison of antibody binding to cell surface-expressed wild-type (WT) and LLP-2 mutant Env. 293T cells transfected with wild-type and LLP-2 mutant proviral clones were harvested 2 days posttransfection and stained for intracellular p24 and Env with the indicated antibodies. For cell surface Env expression studies, intact cells were stained for cell surface Env and then fixed and permeabilized for intracellular p24 staining. Primary flow cytometry data for binding of the indicated antibodies to cell surface wild-type (solid line) and LLP-2 mutant (dotted line) envelope glycoproteins is shown in p24-gated 293T cells along with mock-transfected controls (shaded histogram). Data are representative of the results from at least two independent experiments.
Having established similar levels of cell surface expression of LLP-2 mutant and wild-type Env, we next performed a comparative analysis of the structural properties of the two glycoproteins. Thus, the structural integrity and antigenicity of the LLP-2 mutant Env were tested by a detailed immunophenotypic analysis with a panel of Env-specific monoclonal antibodies with defined specificities to defined linear and conformational determinants of gp120 and gp41 (Table 1). Envelope antibody reactivity determinations were made by using quantitative antibody binding studies to envelope glycoprotein expressed on the cell surface of transfected cells at the level of a single p24-positive cell to maximize sensitivity. First, the oligomeric structure of the LLP-2 mutant Env was determined by the reactivity of Md-1 monoclonal antibody to Env on the surface of virus-producing p24-gated cells. Md-1 recognizes a conformational epitope expressed exclusively on oligomeric forms of Env (29). As shown in Fig. 7, both wild-type and LLP-2 mutant envelope glycoproteins possessed similar reactivity to Md-1 as demonstrated by similar MFI of staining for Md-1, indicating that the oligomeric potential of the LLP-2 mutant Env was not altered. Reactivities of individual monoclonal antibodies to LLP-2 mutant Env are summarized in Table 1, and primary data for representative monoclonal antibodies are presented in Fig. 7. In general, the antigenicity analyses indicated that the conformation of the gp120 and gp41 ectodomain of the LLP-2 mutant Env was specifically modulated to alter the binding of a variety of Env-specific monoclonal antibodies (Table 1). Certain gp41 and gp120 monoclonal antibodies (246D [14], 2F5, and F105 [28]) (Table 1 and Fig. 7) exhibited near wild-type levels of reactivity to LLP-2 mutant Env. In contrast, other reference antibodies (F240 [7], 697-30D [14], IgG1b12, 2G12, and F425b4e8) (Table 1 and Fig. 7) showed a decrease in reactivity to LLP-2 mutant Env relative to wild-type Env. Differential reactivities of monoclonal antibodies directed to linear determinants on gp41 (2F5, F240, 5F3, 50-69 [14], and 246D) (Table 1) showed that point mutations in the ICT of gp41 caused structural alterations in the ectodomain of gp41 leading to differences in epitope exposure compared to wild-type Env. These structural perturbations in the gp41 ectodomain were also transmitted to the noncovalently associated gp120 component as evidenced by decreased antibody recognition of linear (697-30D), conformational (F425B4e8, IgG1b12, and F105), and carbohydrate-dependent epitopes (2G12 and 654-30D) (42). Notably, the neutralizing monoclonal antibodies IgG1b12 (Fig. 7), 2G12 (Fig. 7), and F425b4e8 (Fig. 7), which were less efficient at neutralizing the LLP-2 mutant virus, also exhibited decreased reactivities to LLP-2 mutant Env compared to wild-type Env (about 52, 40, and 48%, respectively) (Table 1 and Fig. 7). In contrast, the reactivity of 2F5 monoclonal antibody, which exhibited similar neutralizing potential towards wild-type and LLP-2 mutant viruses, was unaltered toward LLP-2 mutant Env.
TABLE 1.
Quantitation of reference envelope-specific antibody binding to LLP-2 mutant envelope glycoproteina
| Antibody | Target protein(s) | Epitope specificity and neutralization properties | Antibody binding |
|---|---|---|---|
| HIV-Ig | gp120 and gp41 | Polyclonal Ig fraction from patient sera, neutralizing | 102.6 ± 20.3 |
| Linear gp41 epitopes | |||
| 2F5 | gp41 | aa 662-667, adjacent to cluster II, neutralizing (30) | 121 ± 27 |
| F240 | gp41 | aa 592-606, nonneutralizing antibody (7) | 54.8 ± 4.1 |
| 5F3 | gp41 | aa 526-543, nonneutralizing antibody (3) | 79.8 ± 1.6 |
| 246D | gp41 | aa 579-604, nonneutralizing antibody (14) | 97.1 ± 15.6 |
| 50-69 | gp41 | aa 579-603, cluster, nonneutralizing (14) | 114.8 ± 5.8 |
| Conformational gp41 epitope, Md-1 | gp41 | Conformational epitope on oligomeric form of envelope (29) | 122.2 ± 26.3 |
| Linear gp120 epitopes | |||
| F425B4e8 | gp120 | Base of V3 loop, neutralizing (6) | 52.1 ± 0.2 |
| 697-30D | gp120 | Weakly binds linear sequence aa 161-180, neutralizing (14) | 60.4 ± 4.1 |
| Conformational gp120 epitopes | |||
| 2G12 | gp120 | Carbohydrate-dependent epitope, neutralizing (37) | 60.1 ± 3.8 |
| 697-30D | gp120 | aa 161-180, neutralizing, primarily conformation dependent (14) | 60.4 ± 4.1 |
| Receptor binding site epitopes | |||
| b12 | gp120 | CD4 binding site, neutralizing (5) | 47.4 ± 15.0 |
| F105 | gp120 | Discontinuous or conformational, nonneutralizing (28) | 108.4 ± 20.8 |
| CD4-Ig | gp120 | CD4 binding site (4) | 90.3 ± 6.8 |
| 654-30D | gp120 | Discontinuous carbohydrate-dependent epitope, blocks sCD4 binding (42) | 82.7 ± 1.9 |
| Coreceptor Binding site epitopes | |||
| 17b | gp120 | CD4-induced epitope, neutralizing (36) | 99.8 ± 1.2 |
| 48d | gp120 | CD4-induced epitope, neutralizing (36) | 85.9 ± 14.3 |
Transfected 293T cells were stained for cell surface envelope glycoprotein by using indicated reference envelope-specific antibodies and for intracellular p24. The MFI of envelope staining was determined and normalized for transfection efficiency by calculating the ratio of MFI of envelope staining to MFI of p24 staining. The level of antibody binding to LLP-2 mutant envelope is presented, with antibody binding to wild-type envelope being set at 100%. Averages ± standard deviation are presented for the results from two to five independent experiments. Numbers in parentheses represent select references for the antibodies. aa, amino acids.
In summary, the differential antibody reactivity to wild-type and LLP-2 mutant Env by flow cytometric measurements of cell surface Env indicated perturbations in the overall conformation of LLP-2 mutant Env. Furthermore, changes in antigenicity of the LLP-2 mutant Env correlated with the neutralization resistance of the LLP-2 mutant virus.
CD4 binding and coreceptor binding site exposure of LLP-2 mutant Env.
The observed decrease in reactivities of antibodies recognizing the CD4 binding site (654-30D and IgG1b12) (Table 1) prompted us to examine the CD4 binding ability of the LLP-2 mutant Env. Thus, we next assessed the ability of the LLP-2 mutant Env to bind the natural receptor CD4 and to induce subsequent conformational changes to expose the coreceptor binding site for HIV-1 entry. Flow cytometric analysis of CD4 binding to oligomeric Env expressed on the cell surface (Fig. 8A and Table 1) revealed that the ability of the LLP-2 mutant Env to bind CD4 receptor remained largely unaltered by the structural perturbation of the ICT (Table 1). The differential reactivities of CD4 binding site monoclonal antibodies F105, 654-30D, and IgG1b12 to LLP-2 mutant Env compared with the reactivity of the CD4 molecule are consistent with previous observations that demonstrated that CD4 binding site antibody reactivity profiles may not directly correlate with actual CD4 binding (40).
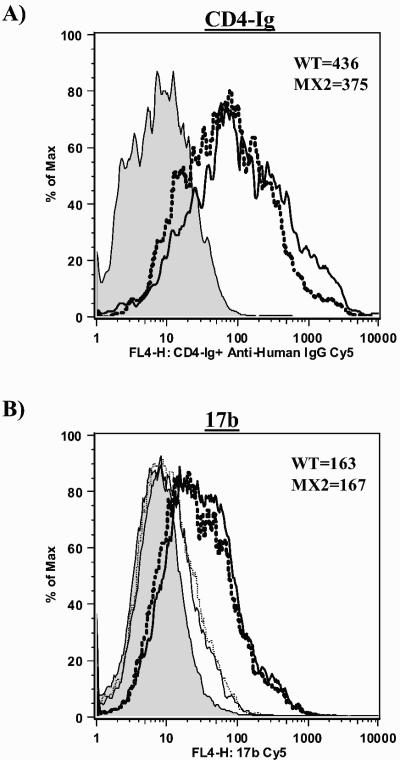
FIG. 8.
Comparison of CD4 binding and coreceptor binding site exposure in wild-type (WT) and LLP-2 mutant Env. 293T cells transfected with wild-type and LLP-2 mutant proviral clones were harvested 2 days posttransfection and stained for intracellular p24 and Env with CD4-Ig (A) or 17b (B) monoclonal antibody as described for Fig. 7. Primary flow cytometry data for binding of the indicated antibodies to cell surface wild-type (thick solid line) and LLP-2 mutant (thick dotted line) envelope glycoproteins following sCD4 triggering is shown in p24-gated 293T cells along with mock-transfected controls (shaded histogram). The corresponding thin solid and thin dotted lines represent antibody binding to wild-type and LLP-2 mutant envelope glycoproteins in the absence of sCD4 preincubation.
During the process of HIV-1 entry into target cells, the gp120 component of Env binds the CD4 receptor, which induces a conformational change in the gp120 protein, thereby releasing the coreceptor binding site on the envelope (33). Thus, we next assessed the ability of soluble CD4 (sCD4) to trigger the exposure of coreceptor binding. Single-cell flow cytometric analysis of coreceptor binding site exposure following sCD4 binding to cell surface-expressed Env was performed by using defined monoclonal antibodies directed to the coreceptor binding site 17b and 48d (36). The data demonstrated wild-type levels of reactivities of coreceptor binding site monoclonal antibodies 17b and 48d to LLP-2 mutant Env (Fig. 8B and Table 1), indicating that sCD4 binding to the LLP-2 mutant Env successfully triggered a conformational change in gp120 to mediate the exposure of the coreceptor binding site.
In summary, the observations that mutations in the LLP-2 domain do not affect the ability of the mutant Env to bind the CD4 receptor and undergo subsequent conformational changes are in conformity with wild-type levels of Env incorporation into virions and wild-type levels of replication potential of the LLP-2 mutant virus in primary human peripheral blood mononuclear cells (Fig. 2).
DISCUSSION
Previous studies of HIV-1 evasion of host neutralizing antibody responses identified variations in the ectodomain of gp120 and gp41 that resulted in altered antibody binding due to changes in conformation or glycosylation patterns (19, 20, 22, 25, 27, 39). Besides the ectodomain component, the HIV-1 Env possesses a 150-amino-acid-long ICT. However, due to the presumed sequestration of the ICT of gp41 within the cytoplasm of the cell, most studies of antibody neutralization escape have discounted the ICT as a determinant of neutralization escape. Studies presented here define the HIV-1 gp41 ICT as a novel locus that can potentially mediate virus neutralization escape in vivo.
Point mutations in the highly conserved structural motif LLP-2 within the ICT caused alterations in the conformation of gp41 and gp120, resulting in decreased binding of defined neutralizing antibodies to the Env ectodomain without altering the CD4 binding or coreceptor binding site exposure and infectivity of the virus. Prior to monoclonal antibody binding studies, levels of total Env expression, Env processing, and cell surface Env expression were determined by radioimmunoprecipitation and flow cytometry with polyclonal patient sera to ensure that any measured differences in Env antigenicity were independent of differences in levels of cell surface Env expression. Furthermore, the fact that the mutant Env protein was transported to the cell surface and efficiently mediated infection also illustrated the formation of a biologically functional oligomer. Correlating with decreased monoclonal antibody binding to LLP-2 mutant Env, the LLP-2 mutant virus was significantly neutralization resistant to several neutralizing antibodies (IgG1b12 and F425b4e8). The binding of 2F5 monoclonal antibody to LLP-2 mutant gp41 was unaltered compared to wild-type gp41, which correlated with wild-type levels of neutralization of LLP-2 mutant virus by 2F5. Similar to previous reports, these studies indicate a direct correlation between the level of antibody binding to envelope glycoprotein and the level of virus neutralization. Direct quantitative measurements of antibody binding to oligomeric envelope glycoprotein expressed on the surface of cells were performed to closely reflect the expected Env structure on the surface of virions. Such measurements of antibody binding to oligomeric Env have been shown to better predict the neutralization properties of the virus compared with antibody binding studies involving monomeric Env (26). The structural changes in the ICT did not result in a global neutralization resistance of the virus, further strengthening the observation that virion Env incorporation and presumable Env oligomerization were unaffected, which would result in more global effects on antibody binding. However, the resistance of LLP-2 mutant virus to polyclonal patient serum antibodies and other neutralizing monoclonal antibodies was observed to varying degrees. Such escape of HIV-1 from neutralization by a few specific antibodies may provide the virus with increased chances to mutate and gain resistance to other antibodies and to persist in vivo.
The significance of full-length ICT in viral replication in primary human PBMC and in rhesus macaques is well established (23, 32). We have previously demonstrated the contribution of the LLP-1 domain to viral replication and infectivity (18). Current studies demonstrating the contribution of ICT to overall Env conformation and neutralization sensitivity of HIV-1 provide yet another reason for the length of the ICT to be preserved in vivo. Studies of neutralization escape by HIV-1 have thus far focused on the ectodomain of the Env protein alone. Our results indicate that although the HIV-1 envelope glycoprotein consists of two noncovalently associated gp120 and gp41 subunits, crucial structural information can be potentially transmitted from the intracytoplasmic segment of gp41 to the ectodomain of gp41 and between gp41 and gp120 subunits. Previous studies involving large truncations in the ICT also present similar structural implications of the ICT (11). Thus, evaluation of the ICT sequences of gp41 in addition to ectodomain sequences of neutralization escape variants should be considered an integral part of neutralization escape studies. The presence of amphipathic α-helical LLP domains is well conserved across various HIV-1 sequence variants (35), although amino acid sequence changes within the motifs are observed. Thus, a fine tuning of the ICT structure via amino acid changes in the LLP domains is quite likely.
The modulation of Env conformation by ICT sequences may also influence Env immunogenicity in vivo in addition to antigenicity in vitro. In light of the present observations, it is relevant to consider the potential impact of ICT deletions on the conformation and immune specificity of recombinant soluble Env proteins currently employed in various AIDS vaccine trials. The use in experimental AIDS vaccines of soluble recombinant Env immunogens lacking the ICT as protein subunits or in DNA or live vector expression vectors is based on the assumption that the truncated soluble protein will have similar conformation and immunogenic properties to the complete membrane-associated Env complex presented on the virion or infected cell surface. Thus, a careful comparison of host humoral and cellular immune responses elicited by soluble Env vaccines with host immunity elicited to native Env in infected patients is warranted to validate the use of ICT-truncated Env proteins as optimal vaccine immunogens.
While previous studies involving large truncations of the SIV and HIV-1 gp41 ICT have indicated a contribution of the ICT to the overall envelope structure (11, 41), our studies define the LLP-2 domain as a critical determinant of Env structure and antigenicity. Amphipathic peptide sequences of LLP-2 domains form α-helical structures in hydrophobic environments such as lipid membranes and interact with the negative membrane phospholipid head groups via their positively charged residues. Preliminary structural circular dichroism spectroscopic analyses of peptides corresponding to wild-type and mutant LLP-2 sequences demonstrated that the helical content of mutant LLP-2 was evidently reduced by about 60% compared to wild-type LLP-2 peptides (V. Kalia and R. Montelaro, unpublished data). While these structural perturbations were observed in vitro at the level of LLP-2 peptides, it is intriguing that similar mutations in the context of full-length envelope glycoprotein caused dramatic changes in the overall conformation of the Env. Thus, it is interesting to speculate that reduction of the positively charged amphipathic helical content of the LLP-2 peptide within the ICT of gp41 may cause decreased membrane interaction and that these structural perturbations may be transmitted via the TM domain to the ectodomain of gp41. While the precise topology of the ICT is not defined, observations that monoclonal antibodies directed to ICT determinants recognize cell surface Env further support the prediction that the LLP domains may potentially interact with and insert into the plasma membrane to expose a segment of the ICT on the extracellular aspect of an infected cell (9, 31).
The present studies implicate allosteric effects of highly ordered structural LLP motifs within the ICT of gp41 in modulating the overall conformation of the HIV-1 Env. Such transmission of structural information from the cytoplasmic domain to the ectodomain of envelope may be considered analogous to the transmission of receptor-ligand interactions to the cytoplasmic cellular milieu. While the precise mechanism of ligand-binding information transfer to the cytoplasm is not known, an induction of conformational change is quite plausible and may likely occur in a bidirectional manner. Our observations that the structural properties of the gp120 and gp41 ectodomains can be influenced by a helical motif within the cytoplasmic tail of gp41 suggests that this structural motif may play a role in modulating Env antigenicity to mediate the escape of HIV-1 from neutralization. These observations suggest that similar structural motifs may impact bidirectional transmembrane signals during a variety of information-exchange processes including cellular activation, adhesion, migration, proliferation, differentiation, and specific gene transcription. Indeed, integrins have been shown to undergo conformational alterations in response to extracellular or intracellular stimuli such that structural elements within their ecto- and cytoplasmic domains cooperate during TM signaling (16).
Acknowledgments
This research was supported in part by NIH grants RO1 AI47758 and RO1 AI39415.
We thank Deena Dampf for technical assistance with isolation of CD8-depleted human PBMC samples and Mary White for p24 antigen assays. We also thank Opendra Sharma at the National Institutes of Health AIDS Research and Reference Reagent Program for providing antibody samples and indinavir.
REFERENCES
- 1.Berlioz-Torrent, C., B. L. Shacklett, L. Erdtmann, L. Delamarre, I. Bouchaert, P. Sonigo, M. C. Dokhelar, and R. Benarous. 1999. Interactions of the cytoplasmic domains of human and simian retroviral transmembrane proteins with components of the clathrin adaptor complexes modulate intracellular and cell surface expression of envelope glycoproteins. J. Virol. 73:1350-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boge, M., S. Wyss, J. S. Bonifacino, and M. Thali. 1998. A membrane-proximal tyrosine-based signal mediates internalization of the HIV-1 envelope glycoprotein via interaction with the AP-2 clathrin adaptor. J. Biol. Chem. 273:15773-15778. [DOI] [PubMed] [Google Scholar]
- 3.Buchacher, A., R. Predl, K. Strutzenberger, W. Steinfellner, A. Trkola, M. Purtscher, G. Gruber, C. Tauer, F. Steindl, and A. Jungbauer. 1994. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res. Hum. Retrovir. 10:359-369. [DOI] [PubMed] [Google Scholar]
- 4.Burton, D. R. 2002. Antibodies, viruses and vaccines. Nat. Rev. Immunol. 2:706-713. [DOI] [PubMed] [Google Scholar]
- 5.Burton, D. R., J. Pyati, R. Koduri, S. J. Sharp, G. B. Thornton, P. W. Parren, L. S. Sawyer, R. M. Hendry, N. Dunlop, and P. L. Nara. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024-1027. [DOI] [PubMed] [Google Scholar]
- 6.Cavacini, L., M. Duval, L. Song, R. Sangster, S. H. Xiang, J. Sodroski, and M. Posner. 2003. Conformational changes in env oligomer induced by an antibody dependent on the V3 loop base. AIDS 17:685-689. [DOI] [PubMed] [Google Scholar]
- 7.Cavacini, L. A., C. L. Emes, A. V. Wisnewski, J. Power, G. Lewis, D. Montefiori, and M. R. Posner. 1998. Functional and molecular characterization of human monoclonal antibody reactive with the immunodominant region of HIV type 1 glycoprotein 41. AIDS Res. Hum. Retrovir. 14:1271-1280. [DOI] [PubMed] [Google Scholar]
- 8.Chen, M., M. K. Singh, R. Balachandran, and P. Gupta. 1997. Isolation and characterization of two divergent infectious molecular clones of HIV type 1 longitudinally obtained from a seropositive patient by a progressive amplification procedure. AIDS Res. Hum. Retrovir. 13:743-750. [DOI] [PubMed] [Google Scholar]
- 9.Cleveland, S. M., L. McLain, L. Cheung, T. D. Jones, M. Hollier, and N. J. Dimmock. 2003. A region of the C-terminal tail of the gp41 envelope glycoprotein of human immunodeficiency virus type 1 contains a neutralizing epitope: evidence for its exposure on the surface of the virion. J. Gen. Virol. 84:591-602. [DOI] [PubMed] [Google Scholar]
- 10.Cohen, J. 2003. HIV. Escape artist par excellence. Science 299:1505-1508. [DOI] [PubMed] [Google Scholar]
- 11.Edwards, T. G., S. Wyss, J. D. Reeves, S. Zolla-Pazner, J. A. Hoxie, R. W. Doms, and F. Baribaud. 2002. Truncation of the cytoplasmic domain induces exposure of conserved regions in the ectodomain of human immunodeficiency virus type 1 envelope protein. J. Virol. 76:2683-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenberg, D., and M. Wesson. 1990. The most highly amphiphilic alpha-helices include two amino acid segments in human immunodeficiency virus glycoprotein 41. Biopolymers 29:171-177. [DOI] [PubMed] [Google Scholar]
- 13.Finnegan, C. M., W. Berg, G. K. Lewis, and A. L. DeVico. 2001. Antigenic properties of the human immunodeficiency virus envelope during cell-cell fusion. J. Virol. 75:11096-11105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorny, M. K., V. Gianakakos, S. Sharpe, and S. Zolla-Pazner. 1989. Generation of human monoclonal antibodies to human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 86:1624-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunter, E., and R. Swanstrom. 1990. Retrovirus envelope glycoproteins. Curr. Top. Microbiol. Immunol. 157:187-253. [DOI] [PubMed] [Google Scholar]
- 16.Hynes, R. O. 2002. Integrins: bidirectional, allosteric signaling machines. Cell 110:673-687. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, W. E., and R. C. Desrosiers. 2002. Viral persistance: HIV's strategies of immune system evasion. Annu. Rev. Med. 53:499-518. [DOI] [PubMed] [Google Scholar]
- 18.Kalia, V., S. Sarkar, P. Gupta, and R. C. Montelaro. 2003. Rational site-directed mutations of the LLP-1 and LLP-2 lentivirus lytic peptide domains in the intracytoplasmic tail of human immunodeficiency virus type 1 gp41 indicate common functions in cell-cell fusion but distinct roles in virion envelope incorporation. J. Virol. 77:3634-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwong, P. D., M. L. Doyle, D. J. Casper, C. Cicala, S. A. Leavitt, S. Majeed, T. D. Steenbeke, M. Venturi, I. Chaiken, M. Fung, H. Katinger, P. W. Parren, J. Robinson, D. Van Ryk, L. Wang, D. R. Burton, E. Freire, R. Wyatt, J. Sodroski, W. A. Hendrickson, and J. Arthos. 2002. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature 420:678-682. [DOI] [PubMed] [Google Scholar]
- 20.Lue, J., M. Hsu, D. Yang, P. Marx, Z. Chen, and C. Cheng-Mayer. 2002. Addition of a single gp120 glycan confers increased binding to dendritic cell-specific ICAM-3-grabbing nonintegrin and neutralization escape to human immunodeficiency virus type 1. J. Virol. 76:10299-10306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mascola, J. R., M. K. Louder, C. Winter, R. Prabhakara, S. C. De Rosa, D. C. Douek, B. J. Hill, D. Gabuzda, and M. Roederer. 2002. Human immunodeficiency virus type 1 neutralization measured by flow cytometric quantitation of single-round infection of primary human T cells. J. Virol. 76:4810-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mo, H., L. Stamatatos, J. E. Ip, C. F. Barbas, P. W. Parren, D. R. Burton, J. P. Moore, and D. D. Ho. 1997. Human immunodeficiency virus type 1 mutants that escape neutralization by human monoclonal antibody IgG1b12. J. Virol. 71:6869-6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murakami, T., and E. O. Freed. 2000. The long cytoplasmic tail of gp41 is required in a cell type-dependent manner for HIV-1 envelope glycoprotein incorporation into virions. Proc. Natl. Acad. Sci. USA 97:343-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nabel, G. J. 2001. Challenges and opportunities for development of an AIDS vaccine. Nature 410:1002-1007. [DOI] [PubMed] [Google Scholar]
- 25.Park, E. J., L. K. Vujcic, R. Anand, T. S. Theodore, and G. V. Quinnan, Jr. 1998. Mutations in both gp120 and gp41 are responsible for the broad neutralization resistance of variant human immunodeficiency virus type 1 MN to antibodies directed at V3 and non-V3 epitopes. J. Virol. 72:7099-7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parren, P. W., I. Mondor, D. Naniche, H. J. Ditzel, P. J. Klasse, D. R. Burton, and Q. J. Sattentau. 1998. Neutralization of human immunodeficiency virus type 1 by antibody to gp120 is determined primarily by occupancy of sites on the virion irrespective of epitope specificity. J. Virol. 72:3512-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parren, P. W., M. Wang, A. Trkola, J. M. Binley, M. Purtscher, H. Katinger, J. P. Moore, and D. R. Burton. 1998. Antibody neutralization-resistant primary isolates of human immunodeficiency virus type 1. J. Virol. 72:10270-10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Posner, M. R., L. A. Cavacini, C. L. Emes, J. Power, and R. Byrn. 1993. Neutralization of HIV-1 by F105, a human monoclonal antibody to the CD4 binding site of gp120. J. Acquir. Immune Defic. Syndr. 6:7-14. [PubMed] [Google Scholar]
- 29.Poumbourios, P., K. A. Wilson, R. J. Center, W. El Ahmar, and B. E. Kemp. 1997. Human immunodeficiency virus type 1 envelope glycoprotein oligomerization requires the gp41 amphipathic alpha-helical/leucine zipper-like sequence. J. Virol. 71:2041-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Purtscher, M., A. Trkola, G. Gruber, A. Buchacher, R. Predl, F. Steindl, C. Tauer, R. Berger, N. Barrett, and A. Jungbauer. 1994. A broadly neutralizing human monoclonal antibody against gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 10:1651-1658. [DOI] [PubMed] [Google Scholar]
- 31.Reading, S. A., C. J. Heap, and N. J. Dimmock. 2003. A novel monoclonal antibody specific to the C-terminal tail of the gp41 envelope transmembrane protein of human immunodeficiency virus type 1 that preferentially neutralizes virus after it has attached to the target cell and inhibits the production of infectious progeny. Virology 315:362-372. [DOI] [PubMed] [Google Scholar]
- 32.Shacklett, B. L., C. J. Weber, K. E. Shaw, E. M. Keddie, M. B. Gardner, P. Sonigo, and P. A. Luciw. 2000. The intracytoplasmic domain of the Env transmembrane protein is a locus for attenuation of simian immunodeficiency virus SIVmac in rhesus macaques. J. Virol. 74:5836-5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sodroski, J. G. 1999. HIV-1 entry inhibitors in the side pocket. Cell 99:243-246. [DOI] [PubMed] [Google Scholar]
- 34.Spies, C. P., G. D. Ritter, Jr., M. J. Mulligan, and R. W. Compans. 1994. Truncation of the cytoplasmic domain of the simian immunodeficiency virus envelope glycoprotein alters the conformation of the external domain. J. Virol. 68:585-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tencza, S. B., T. A. Mietzner, and R. C. Montelaro. 1997. Calmodulin-binding function of LLP segments from the HIV type 1 transmembrane protein is conserved among natural sequence variants. AIDS Res. Hum. Retrovir. 13:263-269. [DOI] [PubMed] [Google Scholar]
- 36.Thali, M., J. P. Moore, C. Furman, M. Charles, D. D. Ho, J. Robinson, and J. Sodroski. 1993. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J. Virol. 67:3978-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trkola, A., M. Purtscher, T. Muster, C. Ballaun, A. Buchacher, N. Sullivan, K. Srinivasan, J. Sodroski, J. P. Moore, and H. Katinger. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 70:1100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vujcic, L. K., and G. V. Quinnan, Jr. 1995. Preparation and characterization of human HIV type 1 neutralizing reference sera. AIDS Res. Hum. Retrovir. 11:783-787. [DOI] [PubMed] [Google Scholar]
- 39.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307-312. [DOI] [PubMed] [Google Scholar]
- 40.Xiang, S. H., P. D. Kwong, R. Gupta, C. D. Rizzuto, D. J. Casper, R. Wyatt, L. Wang, W. A. Hendrickson, M. L. Doyle, and J. Sodroski. 2002. Mutagenic stabilization and/or disruption of a CD4-bound state reveals distinct conformations of the human immunodeficiency virus type 1 gp120 envelope glycoprotein. J. Virol. 76:9888-9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zingler, K., and D. R. Littman. 1993. Truncation of the cytoplasmic domain of the simian immunodeficiency virus envelope glycoprotein increases env incorporation into particles and fusogenicity and infectivity. J. Virol. 67:2824-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zolla-Pazner, S., J. O'Leary, S. Burda, M. K. Gorny, M. Kim, J. Mascola, and F. McCutchan. 1995. Serotyping of primary human immunodeficiency virus type 1 isolates from diverse geographic locations by flow cytometry. J. Virol. 69:3807-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]