Abstract
Strategies to prime CD8+ T cells against Murine gammaherpesvirus 68 (γHV68; MHV68) latency have, to date, resulted in only limited effects. While early forms of latency (<21 days) were significantly reduced, effects were not seen at later times, indicating loss of control by the primed CD8+ T cells. In the present study, we evaluated CD8+ T cells in an optimized system, consisting of OTI T-cell-receptor (TCR) transgenic mice, which generate clonal CD8+ T cells specific for Kb-SIINFEKL of OVA, and a recombinant γHV68 that expresses OVA (γHV68.OVA). Our aim was to test whether this optimized system would result in more effective control not only of acute infection but also of later forms of latent infection than was seen with previous strategies. First, we show that OTI CD8+ T cells effectively controlled acute replication of γHV68.OVA in liver, lung, and spleen at 8 and 16 days after infection of OTI/RAG mice, which lack expression of B and CD4+ T cells. However, we found that, despite eliminating detectable acute replication, the OTI CD8+ T cells did not prevent the establishment of latency in the OTI/RAG mice. We next evaluated the effectiveness of OTI T cells in OTI/B6 animals, which express B cells—a major site of latency in wild-type mice—and CD4+ T cells. In OTI/B6 mice OTI CD8+ T cells not only reduced the frequency of cells that reactivate from latency and the frequency of cells bearing the viral genome at 16 days after infection (similar to what has been reported before) but also were effective at reducing latency at 42 days after infection. Together, these data show that CD8+ T cells are sufficient, in the absence of B cells and CD4+ T cells, for effective control of acute replication. The data also demonstrate for the first time that a strong CD8+ T-cell response can limit long-term latent infection.
Infection of immunocompetent mice with gammaherpesvirus 68 (γHV68) occurs as an acute phase of replication in several organs, which is cleared after 16 days, followed by a latent phase that is limited largely to B cells, macrophages, and dendritic cells and lasts for the life of the animal (13, 15, 35, 39, 40, 42, 43). Previous studies have indicated that all T- and B-cell subsets are important for control of both acute and latent γHV68 infection. This has been demonstrated for T cells by evaluating mice that lack both CD8+ and CD4+ T cells; such mice do not control acute replication and die by around 25 days after infection. On the other hand, single T-cell deficiency is less detrimental. For example, major histocompatibility complex (MHC) class II−/− or CD8−/− mice, or mice immunodepleted of either CD4+ or CD8+ T cells, have only slight delays in clearance of acute replication (14). The situation during chronic infection is similar in that both virus-specific, CD4+-T-cell-dependent antibody (Ab) (17, 21) and CD8+ T cells (20, 35) have been shown to regulate latency, and MHC class II−/− animals succumb to a wasting disease (6) and vasculitis (41), diseases that are both associated with persistent virus replication. Thus, both T-cell subsets have important roles in preventing disease associated with chronic infection.
Attempts have been made to augment the immune response to latent γHV68 infection by priming CD8+ T-cell responses against defined lytic (4, 32) or latent (36, 37) antigens. The principal aim of these studies was to assess the potential of CD8+ T cells primed against single antigens to reduce latent infection. Two outcomes were reported. After induction of immunity by heterologous priming with a vaccinia virus expressing the epitope of the latency-associated M2 protein, no reduction in acute replication was observed while early latency (16 days after infection) was significantly reduced; this effect was only transient, as long-term latency (>21 days) was the same as in nonprimed mice. A different strategy, using vaccination with dendritic cells pulsed with individual lytic cycle antigens, resulted in control of acute replication in the lung (from 10- to 100-fold reduction) and a decrease in splenic latency at 14 days after infection, but again, long-term effects on latency were not seen.
For the present study we had two principal aims, both of which rely on the use of a defined model system. First, we wanted to formally test whether CD8+ T cells are sufficient for control of γHV68 infection. Second, we wanted to test the published finding that virus-specific CD8+ T cells are unable to effect a long-term (>21-day-postinfection) reduction in γHV68 latency (32, 36).
To address the first aim, we evaluated infection of OTI T-cell receptor (TCR) transgenic T cells on a RAG background with γHV68.OVA. The OTI TCR has been used extensively on both wild-type B6 and RAG backgrounds to detect MHC class I presentation of the Kb-specific epitope of OVA SIINFEKL (9, 23). The central question that we sought to answer was whether virus-specific CD8+ T cells, without other adaptive immune cells, could control acute replication and prevent, or delay, lethal γHV68.OVA infection. We also wanted to know whether, if acute γHV68.OVA replication were effectively controlled by the OTI T cells, latent infection would be established in the OTI/RAG mice. Our initial hypothesis was that the very high number of virus-specific OTI CD8+ T cells in the OTI/RAG mice would be sufficient, even in the absence of B and CD4+ T cells, to prevent the establishment of latent infection.
To address the second aim, we evaluated latency in OTI/B6 mice, which express both B and CD4+ T cells in addition to the OTI CD8+ T cells (9). These mice were used instead of OTI/RAG mice because published reports studying long-term latency have largely evaluated mice that have all of the lymphocyte subsets (4, 8, 11, 15, 16, 18, 21, 24, 32, 33, 35, 36, 37, 38, 39, 40, 41, 42, 43). Especially important in this regard, OTI/B6 mice contain B cells, a major reservoir of γHV68 latency (15, 42, 43) though they are not required for the establishment of latency (40). As in the studies with OTI/RAG mice, our hypothesis was that the high number of virus-specific OTI CD8+ T cells in the OTI/B6 mice would be sufficient to effect a reduction latency, but in this case it would be long-term latency.
MATERIALS AND METHODS
Mice.
Mice were housed and bred in a pathogen-free environment at Washington University School of Medicine in accordance with all federal and university policies. C57BL/6J (B6) (000664) and OTI transgenic (003831) mice were obtained from the Jackson Laboratory. OTI/B6 mice were bred to B6/RAG1−/− mice, and the offspring were genotyped (OTI+/−/B6RAG1−/−) by flow cytometry of peripheral blood leukocytes (PBL) for CD8α and for the α chain of the OTI TCR, Vα2. 2C+/−/B6RAG1−/− mice (kindly provided by Jianzhu Chen) express CD8+ T cells that recognize the peptide SIYRYYGL as presented on Kb in B6 mice and were also genotyped by flow cytometry but with the clonotypic Ab 1B2 (7). 2C/B6 mice, bred by crossing 2C/B6RAG1 and C57BL/6J mice, were also genotyped by flow cytometry with the 1B2 Ab. All mice were between 8 and 12 weeks of age when used.
Cells and virus cultures.
NIH 3T12 cells and mouse embryonic fibroblasts (40) were maintained at 37°C in a 5% CO2 environment in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (HyClone Laboratories, Inc., Logan, Utah), 100 U of penicillin, 100 mg of streptomycin per ml, 10 mM HEPES (pH 7.0), 1 mM sodium pyruvate, and 2 mM l-glutamine (cDMEM). Virus passage and maintenance were performed as described previously (40). γHV68 strain WUMS (ATCC VR-1465) was used as wild-type virus for all experiments or as the parent of recombinant viruses, as described below.
Recombinant viruses.
Details of the construction of the γHV68.v-cyclin.OVA virus (which will be referred to in this paper as γHV68.OVA) are available upon request. In brief, a 3.5-kb fragment from the plasmid pL3700-CMV-Tfn-OVA/IRES/GFP, consisting of the human cytomegalovirus (CMV) promoter, a fusion of the transferrin transmembrane domain and OVA (Tfn.OVA) (22), an internal ribosome entry site (IRES), and enhanced green fluorescent protein (eGFP), was inserted by homologous recombination into the v-cyclin locus of γHV68 after transfection of the plasmid and γHV68.v-cyclin.LacZ (38) viral genomic DNA into 3T12 cells. Tfn.OVA is known to be processed in cells for presentation of the Kb-restricted SIINFEKL peptide (22). The use of γHV68.v-cyclin.LacZ allowed for selection of the desired recombinant by loss of β-galactosidase activity. pL3700-CMV-Tfn-OVA/IRES/GFP also contains flanking sequences homologous to viral genomic sequences bp 101654 to 102692, for one flanking arm, and bp 103179 to 105383, for the other arm, which allows for homologous recombination between the plasmid and the viral DNA. This method of producing recombinant γHV68 viruses has been described elsewhere (8, 18, 38). The CMV promoter, IRES, and eGFP sequences were derived from plasmid pIRES2-EGFP (Clontech; catalog no. 6029-1). Recombinants identified by expression of eGFP were plaque purified three times and then screened by Southern blotting (28) for insertion of the ∼3.5-kb CMV-Tfn.OVA/IRES/GFP fragment in place of nucleotides 102692 to 103179 of γHV68. A DNA fragment specific for the v-cyclin region (38) was radiolabeled with 32P by random hexamer primer synthesis (Megaprime labeling kit; Amersham) and used as probe.
To confirm OVA expression in infected cells, Western blotting was performed (28) on total cell lysates of 3T12 fibroblasts infected for 36 h. Briefly, proteins separated on duplicate sodium dodecyl sulfate-10% polyacrylamide gels were transferred to a polyvinylidene fluoride membrane (PolyScreen; NEN Life Science Products) and then incubated with either a polyclonal γHV68 primary Ab (41) (1:5,000) and horseradish peroxidase (HRP)-goat anti-rabbit secondary Ab (Pharmingen; catalog no. 554021) or a biotin-conjugated polyclonal OVA primary Ab (1:1,000) (RDI Research Diagnostics, Inc.; catalog no. RDI-OVALBabr-BT) followed by HRP-conjugated streptavidin secondary Ab (Perkin-Elmer Life Sciences, Inc.). HRP+ proteins were visualized by chemiluminescence (ECL Plus; Amersham) on Biomax ML film (Kodak).
After Southern and Western screening, a single clone of γHV68.OVA was amplified by infection of 3T12 cells at a multiplicity of infection of 0.05 and prepared as a stock for subsequent experiments. Stocks of γHV68.v-cyclin.LacZ, γHV68.v-cyclin.STOP (38), and wild-type γHV68 were diluted in cDMEM and injected (4 × 103 PFU in 0.5 ml) into the peritoneum (i.p.) or given (400 PFU in 25 μl) intranasally. The in vitro multistep growth kinetics assay (18) and the plaque assay (40) were performed as described previously. The limit of detection of the plaque assay is 50 PFU per organ.
Quantitation of latency and persistent replication.
The ex vivo reactivation assay has been reported extensively (40). The assay discriminates between persistent and latent infection because identical numbers of cell dilutions of infected cells are plated onto indicator mouse embryonic fibroblasts, except that for one set the cells are previously mechanically disrupted; this process reveals the amount of preformed infectious virus in cells. The readout of the assay is cytopathic effect of the fibroblasts, which indicates the presence of replicating virus. Briefly, serial twofold dilutions of peritoneal cells (24 wells/dilution starting at 4 × 104 cells/well) were plated onto permissive mouse embryonic fibroblasts for 21 days and then scored for cytopathic effect. Replicate cell aliquots were mechanically disrupted in 1/3× DMEM in the presence of 0.5-mm-diameter silica beads to determine the amount of preformed virus in the cells. To determine the frequency of cells carrying the viral genome, single-copy sensitive nested PCR assays for the γHV68 gene50 were performed on serial dilutions of cells as previously described (40). Briefly, cells were serially diluted threefold starting at 104 cells/reaction in a background of uninfected 3T12 cells such that a total of 104 cells were present in each sample. Single copies of a plasmid containing gene50 in a background of 3T12 cells were in included as positive controls. 3T12 samples with no plasmid were included as a negative control. After overnight lysis of cells in proteinase K, nested PCR was performed and products were visualized on a 1.5% agarose gel. For the PCR experiments reported here there were no false positives and the frequencies for control plasmids were as follows: 10 copies, 38 of 42 positive (90%); 1 copy, 17 of 42 (40%); 0.1 copy, 1 of 42 (2%).
Statistical analyses.
Frequencies of reactivation from latency were calculated as previously described (35). Lethality experiments were analyzed for significance by the chi-square test. For limiting dilution reactivation experiments, cells from three mice were pooled. The frequencies of cells reactivating from latency and of genome-positive cells were determined by Poisson analysis, and significance was calculated by paired t test. All analyses were performed with GraphPad Prism software (San Diego, Calif.).
Cell proliferation assay.
Carboxyfluorescein diacetate succinimidyl ester (CFSE) was used according to the manufacturer's instructions (Molecular Probes, Eugene, Oreg.). Briefly, splenocytes were incubated for 15 min at 37°C in prewarmed phosphate-buffered saline (PBS) plus 1 μM CFSE and then washed and resuspended in cDMEM and incubated for an additional 30 min at 37°C. After two more washes the cells were resuspended in cDMEM at the appropriate concentration for use.
Depletion of lymphocyte subsets, in vivo.
Monoclonal Abs (MAbs) specific for CD4 (YTS191.1) (10) and CD8 (H35) (30) were used to deplete mice of lymphocyte subsets. Hybridomas producing the MAbs were grown in HyClone ADCF-MAb protein-free medium with Integra CELLine CL1000 flasks (Integra Biosciences, Inc., Ijamsville, Md.). The Ab-containing supernatants were sterile filtered and measured for immunoglobulin G by enzyme-linked immunosorbent assay. For the in vivo depletions, beginning 1 day prior to challenge, 1 mg of each Ab or an isotype-matched control Ab (SFR3-DR5, ATCC HB-151) was injected i.p. This was repeated on the fourth day after infection. The efficacy of depletion was >98%, as determined by flow cytometry of peripheral blood.
Immunohistochemistry.
Liver sections were stained as described previously (12) with a polyclonal γHV68 Ab (1:1,000) followed by an HRP-conjugated goat anti-rabbit secondary Ab (1:250). The secondary Ab signal was amplified with tyramide (1:50) (Perkin-Elmer Life Sciences, Inc.; catalog no. NEL746B) followed by incubation with HRP-conjugated streptavidin (1:250). Detection of Ab conjugates was visualized with 3,3′-diaminobenzidine substrate (Vector Laboratories; catalog no. SK-4100); the sections were then counterstained with hematoxylin and eosin.
Cells, Abs, and flow cytometry.
Splenocytes were harvested by passing spleens through 100-μm-pore-size nylon cell strainers. Enriched CD8+ T cells (>95% purity by flow cytometry) were prepared from total splenocytes with CD8+ T-cell enrichment columns according to the manufacturer's instructions (R&D Systems; catalog no. MCD8C-1000). For flow cytometry, splenocytes were washed with PBS and then the red blood cells were lysed by treatment with ammonium chloride (Red Blood Cell Lysing Buffer; Sigma). The lymphocytes were washed twice with PBS, counted, and then resuspended in fluorescence-activated cell sorting (FACS) buffer (PBS, 1.0% bovine serum albumin, 0.1% sodium azide) at a concentration of 106 cells/ml. PBL were obtained from blood collected after severing the renal artery. Erythrocytes were lysed, and the PBL were washed and resuspended in FACS buffer at a concentration of 106/ml.
Cell populations were stained after incubation in FACS blocking buffer (FACS buffer plus rat anti-mouse CD16/CD32 [clone 2.4G2, ATCC no. HB-197] and 5% rat serum) for 30 min on ice. All staining for cell surface markers was performed in 96-well plates (106 cells/ml) in 50 μl of FACS buffer plus Abs on ice for 30 min; this was followed by three washes with (200 μl each) ice-cold FACS buffer. The cells were immediately processed by flow cytometry or else fixed in 1% paraformaldehyde (in PBS) and then stored in FACS buffer until analyzed. Phycoerythrin (PE)-CD43 (1B11), PE-CD69 (H1.2F3), PE-gamma interferon (IFN-γ) (XMG1.2), and fluorescein isothiocyanate-Vα2 (B20.6) were obtained from Pharmingen. Tricolor-CD8+ (CT-CD8a) and PE-CD62L (H1.2F3) were obtained from Caltag. A hybridoma producing the Kb-SIINFEKL-specific Ab 25-D1.16 (27) was kindly provided by Jon Yewdell. Stained cell populations were processed with a FACSCalibur flow cytometer (Becton Dickinson), and the data were analyzed with either CellQuest (Becton Dickinson) or FCS Express Version 2 (De Novo Software, Thornhill, Ontario, Canada) software.
RESULTS
Construction of γHV68.OVA.
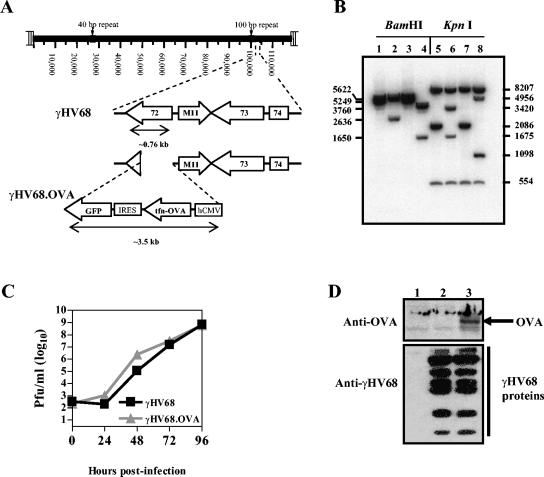
γHV68.OVA expresses OVA in place of the viral cyclin gene of wild-type γHV68. Other recombinant γHV68 viruses containing gene substitutions for the viral cyclin (v-cyclin) gene lack defects in the capacity to replicate during acute infection or to establish latency (38). We performed homologous recombination in 3T12 cells between a targeting plasmid and γHV68 viral DNA, which resulted in the specific replacement of sequences of the viral DNA with an expression cassette consisting of OVA and eGFP (Fig. 1A). eGFP+ virus plaques that grew from the NIH 3T12 fibroblasts were first plaque purified three times, and viral genomic DNA was then analyzed by Southern blot hybridization with a DNA probe spanning the v-cyclin locus (Fig. 1B). The results demonstrated that the OVA-GFP cassette was inserted into the viral genome in place of v-cyclin. We ruled out alterations elsewhere in the genome with additional Southern blot hybridizations with viral genomic DNA as probe (data not shown). After purifying a single clone of γHV68.OVA, we next characterized both its replication in vitro and expression of OVA in γHV68.OVA-infected cells. Multistep growth kinetics in NIH 3T12 fibroblasts demonstrated that γHV68.OVA replicates similarly to wild-type γHV68 (Fig. 1C). Western blotting of total cell extracts 36 h postinfection demonstrated that OVA is expressed during the lytic cycle of γHV68 infection (Fig. 1D).
FIG. 1.
Generation of γHV68.OVA. (A) Schematic illustration of the γHV68 genome (coordinates in kilobases) including an expanded region demonstrating where the OVA-expressing cassette replaced most of the viral cyclin (ORF72) gene during homologous recombination to create γHV68.OVA. (B) Southern blot hybridization comparing viral genomic DNAs of wild-type γHV68 (lanes 1 and 5), γHV68. OVA (lanes 2 and 6), γHV68.v-cyclin.STOP (lanes 3 and 7), and γHV68.v-cyclin.LacZ (lanes 4 and 8) viruses. DNAs were digested with either BamHI (lanes 1 to 4) or KpnI (lanes 5 to 8). Both γHV68.v-cyclin.STOP and γHV68.v-cyclin.LacZ were included as a controls; they are recombinant viruses produced previously (38) by the same method used to produce γHV68.OVA. The bands seen in lanes 2 and 6 are expected in γHV68.OVA because ∼3,480 bp of the OVA cassette are substituted for 489 bp of the viral cyclin gene. (C) Multistep growth of γHV68.OVA and wild-type γHV68 in NIH 3T12 fibroblasts. Infections were initiated at a multiplicity of infection of 0.05 and monitored for 96 h. The results are representative of two independent experiments. (D) Western blot assay on total cell lysates from 3T12 cells either mock treated (lane 1) or infected for 18 h with wild-type γHV68 (lane 2) or γHV68.OVA (lane 3). The arrow indicates OVA protein only in cells that were infected with γHV68.OVA.
γHV68.OVA-infected cells express OVA and Kb-SIINFEKL in vivo.
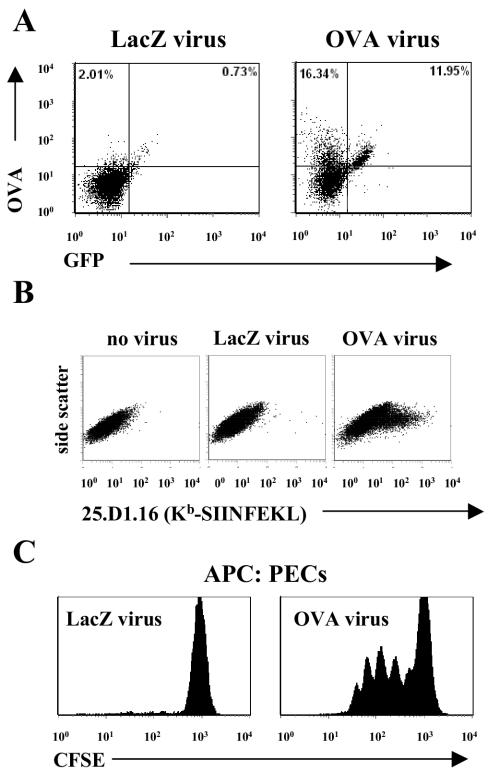
To confirm that expression of OVA occurred in cells during infection in vivo, we isolated peritoneal cells from B6 mice infected with γHV68.OVA 4 days previously and analyzed them by flow cytometry for expression of both OVA and eGFP; we also prepared peritoneal cells from mice infected with the control virus γHV68.v-cyclin.LacZ. The results demonstrated that peritoneal cells infected with γHV68.OVA expressed OVA and eGFP (Fig. 2A), though some of the γHV68.OVA-infected cells expressed OVA but not eGFP, which we interpreted as variable IRES-driven GFP expression. To determine if expression of OVA led to presentation of Kb-SIINFEKL on infected cells, we stained NIH 3T12 fibroblasts infected for 36 h with either γHV68.OVA or γHV68.v-cyclin.LacZ. As expected only cells infected with γHV68.OVA expressed Kb-SIINFEKL (Fig. 2B).
FIG. 2.
γHV68.OVA-infected cells express both OVA and Kb-SIINFEKL and activate OTI CD8+ T cells. (A) Peritoneal cells from B6 mice infected (107 PFU i.p.) for 4 days with either γHV68.v-cyclin.LacZ (left) or γHV68.OVA were stained with a polyclonal OVA Ab and then analyzed by flow cytometry. OVA+/GFP+ cells are clearly present in the γHV68.OVA-infected peritoneal cells. (B) Kb-SIINFEKL expression occurs on 3T12 cells infected with γHV68.OVA (right) but not with γHV68.v-cyclin.LacZ (middle). (C) OTI CD8+ T cells proliferate in response to γHV68.OVA-infected peritoneal cells harvested from B6 mice infected for 72 h. The peritoneal cells (PECs) were fixed in 1% paraformaldehyde for 30 min on ice before being used as antigen-presenting cells (APC) to CFSE-labeled OTI CD8+ T cells obtained from OTI/RAG spleens. After incubation together for 36 h, the cells were analyzed by flow cytometry. The histograms are gated on the CD8+ T cells. These data are representative of two independent experiments.
γHV68 encodes at least one gene product that can interfere with class I presentation (25, 33, 45). To use the OTI:γHV68.OVA system for further immunological experiments, we needed to demonstrate that expression of Kb-SIINFEKL on γHV68.OVA-infected cells was sufficient to stimulate OTI T cells. To do this, we assessed proliferation of OTI cells after incubating CFSE-labeled OTI T cells with peritoneal cells from B6 mice infected with either γHV68.OVA or γHV68.v-cyclin.LacZ. Flow cytometry on the cells demonstrated that proliferation of OTI T cells occurred only after exposure to cells infected with γHV68.OVA (Fig. 2C). These data demonstrated that Kb-SIINFEKL expressed on γHV68.OVA-infected cells is sufficient for activation of OTI T cells.
OTI CD8+ T cells decrease the virulence of γHV68.OVA.
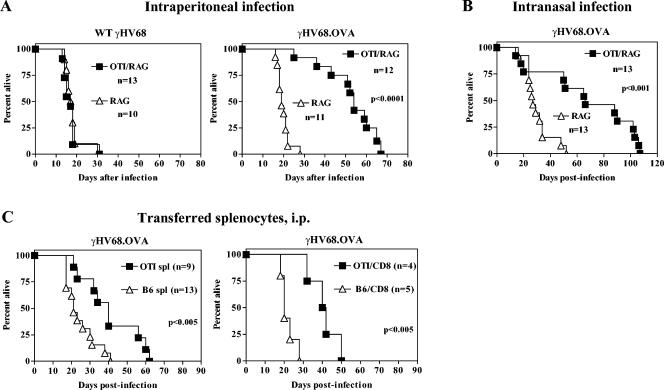
We next determined whether the presence of OTI T cells, in the absence of B and CD4+ T cells, provided protection against lethal infection by γHV68. OTI/RAG and RAG mice were infected with either wild-type γHV68 or γHV68.OVA and monitored over time. Normally, γHV68 infection of immunodeficient strains such as RAG causes 100% lethality within 35 days (14). Consistent with this, all RAG mice infected with either wild-type γHV68 (Fig. 3A) or γHV68.OVA (Fig. 3B) died by 35 days after infection. The same kinetics of lethality were observed in OTI/RAG mice infected with wild-type γHV68 (Fig. 3A), an expected result since these mice have no adaptive immunity other than the OVA-specific T cells. In contrast, OTI/RAG mice survived significantly longer after either i.p. (P < 0.0001) or intranasal (P < 0.001) inoculation with γHV68.OVA (Fig. 3B). To address whether OTI T cells that develop in the presence of other lymphocytes also prolong survival of RAG mice, total splenocytes or CD8+ T cells enriched from OTI/B6 mice were transferred to RAG mice, which were then challenged with γHV68.OVA. Adoptive transfer of either 5 × 105 total OTI splenocytes or 5 × 105 CD8+ OTI T cells resulted in a significant delay of lethality (P < 0.005 and P < 0.005, respectively) compared to cells from B6 control mice (Fig. 3B). This result demonstrated that OTI T cells from a mouse with other lymphocytes have a protective capacity in common with OTI T cells that develop in a RAG background. Together, the above data demonstrated that OTI CD8+ T cells, in the absence of B and CD4+ T cells, can significantly prolong survival of γHV68.OVA-infected mice.
FIG. 3.
OTI CD8+ T cells control lethal infection by γHV68.OVA. (A) Lethality from wild-type γHV68 infection is identical in both RAG and OTI/RAG mice, whereas lethality from γHV68.OVA is significantly delayed in OTI/RAG mice after i.p. (4 × 103 PFU) infection (P < 0.0001). (B) Similar results to those in panels A were obtained after intranasal infection with 400 PFU. (C) Significant reductions in lethality were seen after OTI T-cell transfer to RAG mice, both with total splenocytes from OTI/B6 (OTI spl) versus B6 splenocytes (B6 spl) (P < 0.005) and with CD8+ T cells enriched from OTI/B6 splenocytes (OTI/CD8) versus CD8+ T cells enriched from B6 splenocytes (B6/CD8) (P < 0.005). These data are representative of between two and four experiments for each condition. n, number of mice.
Antigen-specific activation of OTI T cells is maintained throughout infection.
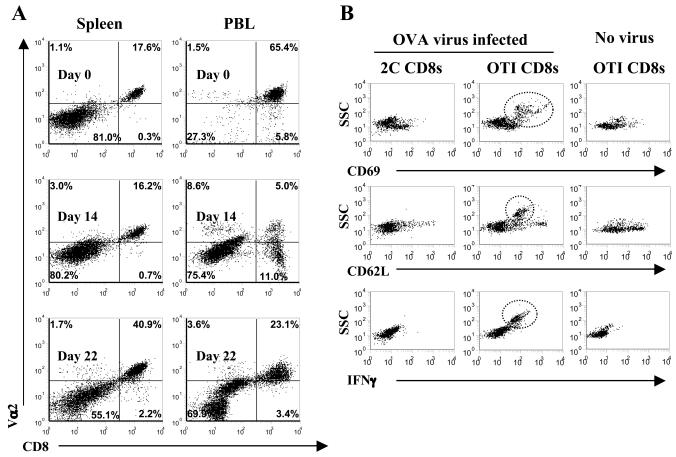
Because of the correlation between the presence of the OTI CD8+ T cells and an antiviral phenotype during acute infection, we looked directly at the OTI T cells from infected mice for signs of antigen-specific activation. Both PBL and total splenocytes from γHV68.OVA-infected OTI/RAG mice were evaluated by flow cytometry for expression of TCR (Vα2) and CD8α. Both molecules were decreased on PBL 14 days after infection, which is consistent with exposure to antigen presented on MHC class I (1). By 22 days a population of Vα2(hi)/CD8(hi) cells had returned (similar to that seen in naïve mice), and in addition a large population of cells that displayed low levels of both cell surface markers was present, a phenotype consistent with resolution of the acute CD8+ T-cell response by contraction of the activated OTI cells and repopulation of the periphery by cells with normal levels of TCR and CD8 (Fig. 4A).
FIG. 4.
Antigen-specific activation of OTI CD8+ T cells in γHV68.OVA-infected mice. (A) PBL and splenocytes were isolated from naïve OTI/RAG mice or from OTI/RAG mice infected with γHV68.OVA (104 PFU i.p.) for 14 or 22 days. The cells were stained for cell surface expression of CD8α and Vα2. (B) Equal numbers of OTI and 2C CD8+ T cells were coinjected into RAG recipients, which were then infected with γHV68.OVA. CD8+ T cells were evaluated 16 days later for cell surface expression of activation markers and for increased granularity. The plots are side scatter by either cell surface molecule or intracellular IFN-γ and are gated on CD8+ (for 2C T cells) or CD8+/Vα2+ (for OTI T cells). The circles highlight OTI T cells that have both increased granularity and levels of surface marker or IFN-γ consistent with activation. These data are representative of two independent experiments.
Activation of CD8+ T cells through a β2-microglobulin-independent mechanism during γHV68 infection has been reported elsewhere (5, 11, 16, 19), which suggests that factors other than MHC class I-peptide complexes may contribute to CD8+ T-cell activation. To demonstrate that the activation phenotype of the OTI T cells was due to exposure to antigen directly, we performed adoptive transfer experiments in which OTI/RAG splenocytes were mixed with an equal number of splenocytes from a second CD8+ TCR transgenic mouse, 2C/RAG, and then transferred to RAG mice 1 day prior to infection with γHV68.OVA. As a control, OTI/RAG splenocytes alone were transferred to RAG mice that were left uninfected. Fourteen days after infection we harvested and stained splenocytes from all the mice for CD8α, for Vα2, for the activation-associated cell surface markers CD69 and CD62L, or for intracellular IFN-γ, and for increased granularity (i.e., increased side scatter). Only the OTI CD8+ T cells contained a subset of cells that had a phenotype consistent with activation, i.e., increased granularity and expression of activation cell surface molecules, e.g., CD69hi and CD62Llow, and expression of intracellular IFN-γ (Fig. 4B). These results demonstrated that OTI CD8+ T cells become activated in an antigen-specific manner during γHV68.OVA infection. They also ruled out bystander activation, β2-microglobulin-independent T-cell activation, and contributions of virus-expressed or -induced superantigens as causes of activation of CD8+ T cells in the OTI:γHV68.OVA experimental system.
In the absence of B and CD4+ T cells, OTI T cells control acute replication of γHV68.OVA but cannot prevent establishment of latency.
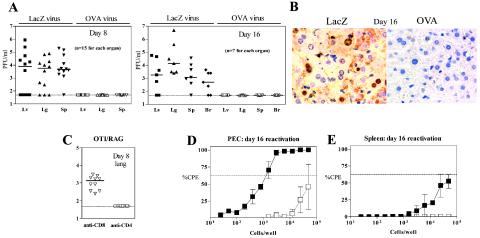
To determine if the significantly prolonged survival of the OTI/RAG mice correlated with a reduction in acute virus replication, we compared viral titers (40) in organs from OTI/RAG mice infected with either γHV68.OVA or γHV68.v-cyclin.LacZ for 8 and 16 days. At both time points the majority of γHV68.v-cyclin.LacZ-infected mice exhibited high titers of virus in all organs (Fig. 5A); in contrast, no virus was detected in any of the γHV68.OVA-infected mice. We also evaluated liver sections by immunohistochemistry with a polyclonal γHV68 Ab (41). Viral antigen was present in γHV68.v-cyclin.LacZ-infected mice while none was detected in the γHV68.OVA-infected mice (Fig. 5B). Finally, since in addition to >99% OTI CD8+ T cells OTI/RAG mice generate <1.0% CD4+ T cells (9) that express the OTI TCR, we immunodepleted T-cell subsets to prove that the OTI CD8+ T cells are required for the antiviral phenotype. Injection of anti-CD4 Ab had no effect on the antiviral phenotype whereas injection of anti-CD8 Ab resulted in an increase in γHV68.OVA virus titer (Fig. 5C). Together, these data formally demonstrated that OTI T cells are sufficient for effective control of acute replication.
FIG. 5.
OTI CD8+ T cells are sufficient for control of acute replication of γHV68.OVA. (A) Plaque assay results showing titers of organs from OTI/RAG animals at 8 (left) and 16 (right) days after infection (106 PFU, i.p.). Each symbol represents a single animal. The results are pooled from three independent experiments. (B) Immunohistochemistry of liver sections from OTI/RAG animals infected for 16 days with γHV68.OVA or γHV68.v-cyclin.LacZ. Paraffin sections were stained with the polyclonal γHV68 Ab; no viral antigen was seen in the OTI/RAG animals. These results are representative of at least six independent experiments. (C) Immunodepletion of lymphocyte subsets, beginning at day −1 and a second injection of Ab on day 4, after γHV68.OVA infection demonstrates a rise in titer only after treatment with the Ab specific for CD8+ T cells. Each symbol represents plaque assay results of titers of an organ from a single animal (n = 10 for each group). The results are representative of two independent experiments. (D and E) Results of the ex vivo limiting dilution reactivation assay 16 days after infection with 103 PFU i.p., demonstrating that both splenocytes (E) and peritoneal cells (PEC) (D) reactivate γHV68.OVA virus at frequencies of ∼1:100,000 and ∼1:2,000 cells, respectively. Dilutions of the cell population in question are plotted against the number of wells that scored positive for cytopathic effect (CPE). Open symbols are samples that were mechanically disrupted and thus represent persistent virus in the samples. The data were subjected to Poisson analysis, which gave the cell dilution at which single reactivation events occurred (i.e., where 63% of wells were positive for cytopathic effect). The data are pooled from two independent experiments.
Having demonstrated that OTI T cells control acute infection in multiple organs, we next evaluated if they would be sufficient to prevent the establishment of latency. In immunocompetent B6 mice latency is readily measured after resolution of acute replication, as measured by plaque assay at 16 days after infection. We thus examined OTI/RAG mice infected for 16 days with γHV68.OVA for the presence of latently infected cells in the spleen and peritoneum. RAG mice infected with γHV68.OVA and OTI/RAG mice infected with γHV68.v-cyclin.LacZ could not be evaluated similarly since these mice contain high levels of virus. Splenocytes and peritoneal exudate cells from the γHV68.OVA-infected OTI/RAG mice were purified and subjected to an ex vivo limiting dilution reactivation assay, which can detect both reactivation from latency and the presence of preformed virus known as “persistent virus,” a genetically distinct form of virus that may be related to the process of maintenance of latency (18). The results demonstrated that ∼1:2,000 peritoneal exudate cells (Fig. 5D) and ∼1:100,000 splenocytes (Fig. 5E) harbor latently infected cells that reactivate virus. In addition, cells that were disrupted before plating on the fibroblast indicator monolayer indicated that cells in the peritoneum harbor persistent virus (Fig. 5D, open squares). Together, these data show that latency of γHV68.OVA is established in OTI/RAG mice, even in the absence of detectable acute replication in multiple organs, including the spleen, and that the peritoneum harbors cells that contain preformed virus.
OTI T cells reduce the frequency of both reactivation and genome-bearing cells during late latency in mice that express B and CD4+ T cells.
To determine if OTI T cells could alter latency at late times (42 days) after infection, we evaluated the frequency of cells that reactivate from latency in OTI/B6 mice, which contain B and CD4+ T cells in addition to OTI CD8+ T cells. We chose to use OTI/B6 mice to evaluate late latency because the OTI/RAG mice lack B cells, an important latency reservoir. As has been extensively reported (26, 35, 38), mice that contain all the lymphocyte subsets clear acute and persistent infection by 16 days after infection and, importantly, can be evaluated at late times for both reactivation and the frequency of cells that bear the viral genome.
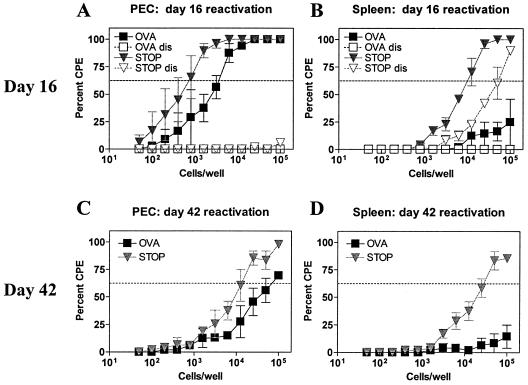
We first evaluated reactivation frequencies in OTI/B6 mice that were infected with either γHV68.OVA or the control virus γHV68.v-cyclin.STOP, a virus containing a stop mutation in the v-cyclin gene (38). At 16 days after infection, the frequency of peritoneal cells that reactivated from latency ex vivo was ∼1:5,000 for γHV68.OVA (filled squares) and ∼1:800 for γHV68.v-cyclin.STOP (filled inverted triangles) (Fig. 6A); for splenocytes, the frequency of cells that reactivated from latency was >1:100,000 for γHV68.OVA (filled squares) and ∼1:10,000 for γHV68.v-cyclin.STOP (filled inverted triangles) (Fig. 6B). In addition, in contrast to what we found in the peritoneal cells, we observed a significant amount of persistent virus in spleen cells from γHV68.v-cyclin.STOP-infected mice (open inverted triangles, Fig. 6B), while none was observed in the γHV68.OVA-infected samples (open squares, Fig. 6B). Thus, at 16 days after infection OTI T cells completely controlled persistent replication of γHV68.OVA but not γHV68.v-cyclin.STOP, and there was a greater-than-fivefold reduction in the frequency of splenocytes that reactivate γHV68.OVA from latency compared to the frequency of splenocytes that reactivate γHV68.v-cyclin.STOP from latency.
FIG. 6.
OTI T cells control reactivation from latency of γHV68.OVA. Peritoneal cells (PEC) (A) and splenocytes (B) from OTI/B6 mice, infected with 106 PFU, i.p., of either γHV68.OVA (filled squares) or γHV68.v-cyclin.STOP (filled inverted triangles) for 16 days, were evaluated by the ex vivo reactivation assay. Open symbols are samples that were mechanically disrupted and thus represent persistent virus in the samples. Peritoneal cells (C) and splenocytes (D) from OTI/B6 mice were also evaluated at 42 days after infection. No persistent virus was seen in these samples (data not shown). The data are pooled from two independent experiments. CPE, cytopathic effect.
Mice were next evaluated at 42 days after infection. We were particularly interested in looking at the effect of the OTI T cells at a late time after infection of OTI/B6 mice, since previous reports have shown that γHV68-specific CD8+ T cells that reduce acute replication and latency at 21 days after infection have no effect on latency measured at later times (32, 36). In contrast to those reports, we found that both peritoneal cells and splenocytes from OTI/B6 mice infected for 42 days with γHV68.OVA had lower frequencies of reactivating cells compared to mice infected with γHV68.v-cyclin.STOP (for peritoneal cells, ∼1:90,000 for γHV68.OVA versus ∼1:11,000 for γHV68.v-cyclin.STOP [Fig. 6C]; for splenocytes, >1:100,000 for γHV68.OVA versus ∼1:40,000 for γHV68.v-cyclin.STOP [Fig. 6D]). Thus, even at 42 days after infection OTI T cells reduced the frequency of splenocytes that reactivate γHV68.OVA from latency compared to the frequency of splenocytes that reactivate γHV68.v-cyclin.STOP from latency.
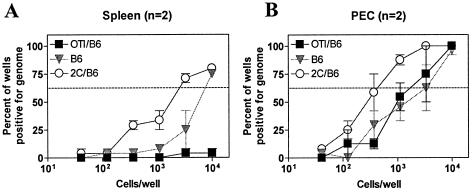
We next wanted to determine if the frequency of genome-bearing cells in OTI/B6 mice was also reduced at 42 days after infection with γHV68.OVA. To measure this, we performed a limiting dilution PCR assay, which gives the frequency of viral genomes in a cell population, on peritoneal and spleen cells from OTI/B6 mice; results from OTI/B6 cells were compared to the frequencies of genome-positive cells in B6 and 2C/B6 infected mice (2C/B6 is a CD8+ TCR transgenic strain with irrelevant CD8+ T cells). Surprisingly, we could not detect genome-positive cells in the spleen of OTI/B6 mice (Fig. 7A), while in 2C/B6 mice ∼1:3,000 splenocytes contained viral genome. The genome frequency in cells from B6 mice was intermediate between those seen with OTI/B6 mice and those seen with 2C/B6 mice. Because both OTI/B6 and 2C/B6 are CD8+ TCR transgenic animals, their frequencies of reactivation can be directly compared. For peritoneal cells, while no difference was found in the frequency of genome-bearing cells between OTI/B6 and B6 animals (∼1:2,000), there was a significant difference (P < 0.032) between OTI/B6 and 2C/B6 animals (∼1:2,000 versus ∼1:500, respectively) (Fig. 7B). Together, the above results demonstrated that the OTI T cells effect a significant long-term reduction in both the frequency of cells that reactivate from latency and the frequency of genome-positive cells.
FIG. 7.
OTI T cells control the frequency of genome-positive cells during latency at 42 days after infection. Shown are the results of a limiting dilution PCR assay, specific for gene50, on splenocytes (A) and peritoneal cells (PEC) (B) from either OTI/B6 (filled squares), 2C/B6 (open circles), or B6 (filled inverted triangles) animals infected with γHV68.OVA (106 PFU, i.p.) for 42 days. In panel B the difference between OTI/B6 (filled squares) and 2C/B6 (open circles) is statistically significant (P < 0.032). n, number of experiments.
DISCUSSION
The establishment of and reactivation from latency and the occurrence of persistent virus are three defined stages of chronic γHV68 infection that have been found to be differentially, and often independently, regulated by components of the adaptive immune system (18, 26, 35, 38). In previous studies of these stages, the extent to which any single arm of the adaptive immune system could alter them was studied genetically or by depletion with MAb, leaving, in both cases, other arms of the adaptive immune system intact and present during the analysis.
In this study we used a different approach. We evaluated mice that contain OTI TCR transgenic CD8+ T cells infected with γHV68 that expresses the defined model antigen OVA from a promoter active during lytic infection. In one set of experiments, the OTI T cells were expressed in RAG mice, which enabled us to test whether large numbers of epitope-specific TCR transgenic CD8+ T cells could eliminate acute virus replication independently of B and CD4+ T cells and, if so, whether this was sufficient for preventing the establishment of and reactivation from latency, and for control of persistent infection. In a second set of experiments, we evaluated the potential of the TCR transgenic CD8+ T cells to effect a reduction in frequencies of reactivation and genome-positive cells during latency at relatively late times after infection, e.g., 42 days, in OTI/B6 mice, which express both B and CD4+ T cells.
Control of γHV68 infection by OTI CD8+ T cells in the absence of CD4+ T-cell help.
The central question that we wanted to address first was whether γHV68.OVA acute replication would be controlled in an animal that expresses OTI T cells but no other lymphocytes. We clearly demonstrated that OTI/RAG mice infected with γHV68.OVA contain no detectable virus in the spleen, liver, lung, or brain at 8 or 16 days after infection and that this control is lost when mice are treated with a CD8, but not CD4, T-cell-depleting Ab. We chose to limit our evaluation of the infected OTI/RAG mice to 16 days postinfection and earlier because this allowed us to compare results directly to the level of γHV68.OVA replication in RAG control mice, which begin to die by 16 to 20 days after infection and which do not resolve acute replication. In contrast, γHV68.OVA-infected OTI/RAG mice do not begin to die until around 30 days after infection. However, eventually all the OTI/RAG mice die, demonstrating that the OTI CD8+ T cells are not sufficient for long-term prevention of lethal disease.
Several studies have implicated the lack of CD4+ T-cell help in the decline of effective CD8+ T-cell responses, which is one possible explanation for the insufficiency of the OTI T cells to prevent lethal disease. The requirement for CD4+ T-cell help for development of functional secondary CD8+ T-cell responses to pathogens has been recently demonstrated (29, 34). The general conclusions were that memory CD8+ T cells from mice without CD4 T cells have defective recall responses compared to CD8+ T cells from wild-type mice. Consistent with this, at least two studies have concluded that there are functional deficiencies in cytotoxic T lymphocytes (CTLs) from CD4-deficient mice (I-Ab−/−) persistently infected with γHV68 (6, 31) or from γHV68-infected CD4-deficient mice that were boosted with lytic antigen CTL epitopes (4). However, a more recent analysis failed to demonstrate a functional deficit in CD8+ T cells from CD4-deficient mice challenged with γHV68 (3). As far as these issues relate to the case of γHV68.OVA infection of OTI/RAG mice, it is clear that OTI T cells can function to control acute replication without CD4+ T-cell help. In future studies it will be important to determine, however, if the OTI T-cell response can be improved by the presence of CD4+ T cells and if the survival of OTI/RAG mice infected with γHV68.OVA can be prolonged by adding CD4+ T cells.
Effective control of acute replication does not prevent the establishment of latency.
Since OTI T cells effectively eliminated acute replication in the OTI/RAG mice, we next wanted to determine if the OTI T cells would prevent the establishment of latency. To do this, we measured both the frequency at which cells reactivate from latency and the presence of persistent virus infection. At 16 days after γHV68.OVA infection, both peritoneal and spleen cells from OTI/RAG mice reactivated latent virus, and persistent virus was observed in peritoneal cells. These data demonstrated that the OTI T cells could neither prevent the establishment of latency nor eliminate persistent virus; these results were surprising because of the effectiveness of control of acute replication. These data indicate that establishment of latency is a robust phenomenon, being quite refractory to even large numbers of virus-specific CD8+ T cells present at the time of challenge and which are effective at controlling acute replication. Possible explanations for the apparent inability of the large number of preexisting OTI T cells to prevent or eliminate latent infection include the following.
The limit of detection for the plaque assay used to assess acute replication is ∼50 PFU/organ; thus, it is formally possible that the OTI T-cell response, while very effective, does not eliminate a small amount of replication that remains undetectable by this assay.
A more interesting explanation for the establishment of latency derives from a model postulated for infection by Epstein-Barr virus (2), according to which establishment of latency requires infection of cells only during the initial stage of virus attack of the mucosal epithelium of the host. These infected cells then seed the latent pool, which expands independently of acute replication by mechanisms that drive proliferation and differentiation of the cells to an immunologically privileged state.
Another possible explanation for the establishment of latency despite effective control of acute infection by the OTI T cells is that OVA may not be expressed in the relevant cell type or at the proper stage in the viral life cycle. In the recombinant virus γHV68.OVA, OVA is expressed from the highly active CMV promoter and thus should be expressed in all productively infected cells. However, constitutive expression of OVA may not in fact occur in all cells due to regulation of the viral genome during different states of the virus life cycle.
Lastly, establishment of latency in the OTI/RAG mice could result because latently infected cells fail to be recognized by CD8+ T cells, compared to recognition of lytically infected cells. The mechanism of such immune evasion might include either expression of a specific gene program only in latent cells that result in decreased MHC class I expression (25, 33, 45) or direct interference with known pathways of antigen processing into peptides for presentation on MHC (44).
OTI CD8+ T cells control γHV68 reactivation and frequency of genome-positive cells at late times after infection.
Possible explanations for the failure of previous studies to effect long-term control of latency by virus-specific CD8+ T cells (32, 36) include failure of the priming regimens to sufficiently stimulate adequate numbers of antigen-specific CD8+ T cells and the specificity of the CD8+ T cells for recognized epitopes that were not ideal for the desired outcome (i.e., are not expressed at the right place or time to eliminate latently infected cells).
In contrast to those results we observed a reduction in long-term latency in OTI/B6 mice. At 42 days after infection we found that both the frequency of reactivation and the frequency of genome-positive cells were lower in peritoneal and spleen cells in OTI/B6 mice infected with γHV68.OVA, compared to infection with γHV68.v-cyclin.STOP. These data provide the first indication that strategies aimed at limiting the extent of latent gammaherpesvirus infection by stimulating CTL immunity will be effective, although, for the aim of preventing the establishment of latency in the first place, CTLs do not appear to be sufficient.
OTI/B6 mice express a large clonal population of cells (at a minimum, 5 × 107 OTI T cells in the spleen and lymph nodes [9]), and present at the time of infection, this high number of preexisting immune cells likely accounts for the reductions in latency that were seen in the γHV68.OVA-infected mice. It is also important to keep in mind that OVA is expressed from the viral genome by a highly active nonviral promoter, which, compared to endogenous viral proteins, likely dramatically affects the time and place at which OVA is expressed during the various stages of gammaherpesvirus infection. This brings up an important point. While we have demonstrated that OVA is expressed during lytic infection, we do not know about its expression during other stages of infection. Unfortunately, at present it is not technically possible to determine if OVA is also expressed in latently infected cells. The most significant reason for this is the fact that a molecular definition of γHV68 latency is not known, and so it is impossible to correlate expression of OVA with cells that are definitely “latently infected. ” It is thus not possible to say whether OTI T cells have their effects via recognition of both lytically and latently infected cells or via that of cells in the process of reactivating from latency or whether it is the result of some combination of recognition during all three stages. By whichever means, it is nevertheless clear in the OTI/B6:γHV68.OVA system that virus-specific OTI CD8+ T cells can effect a long-term reduction of latency.
Several important question left unaddressed in the present work include the mechanism of control of the antiviral OTI T cells, the number of OTI T cells required to effect the reductions in latency, and whether the reduction in latency occurs throughout the life of infected animals. The reductive model system that we describe here will be useful for clearly determining answers to these and further questions.
Acknowledgments
H.W.V. and S.H.S. were supported by grants RO1CA74730, RO160090, and CA96511. D.C.B. was supported by a fellowship from the Leukemia and Lymphoma Society and by training grants T32 AI07172-22 and −21. R.L.S.-T. was supported by a fellowship from the American Heart Association and training grant 5-T32-CA09547.
We thank Darren Kreamalmeyer for expert assistance in the generation of transgenic mice. We thank the members of the laboratory of H.W.V. for helpful discussions.
REFERENCES
- 1.Alcover, A., and B. Alarcon. 2000. Internalization and intracellular fate of TCR-CD3 complexes. Crit. Rev. Immunol. 20:325-346. [PubMed] [Google Scholar]
- 2.Babcock, G. J., L. L. Decker, M. Volk, and D. A. Thorley-Lawson. 1998. EBV persistence in memory B cells in vivo. Immunity 9:395-404. [DOI] [PubMed] [Google Scholar]
- 3.Belz, G. T., H. Liu, S. Andreansky, P. C. Doherty, and P. G. Stevenson. 2003. Absence of a functional defect in CD8+ T cells during primary murine gammaherpesvirus-68 infection of I-A(b−/−) mice. J. Gen. Virol. 84:337-341. [DOI] [PubMed] [Google Scholar]
- 4.Belz, G. T., P. G. Stevenson, M. R. Castrucci, J. D. Altman, and P. C. Doherty. 2000. Postexposure vaccination massively increases the prevalence of gamma-herpesvirus-specific CD8+ T cells but confers minimal survival advantage on CD4-deficient mice. Proc. Natl. Acad. Sci. USA 97:2725-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackman, M. A., E. Flano, E. Usherwood, and D. L. Woodland. 2000. Murine gamma-herpesvirus-68: a mouse model for infectious mononucleosis? Mol. Med. Today 6:488-490. [DOI] [PubMed] [Google Scholar]
- 6.Cardin, R. D., J. W. Brooks, S. R. Sarawar, and P. C. Doherty. 1996. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J. Exp. Med. 184:863-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, J., H. N. Eisen, and D. M. Kranz. 2003. A model T-cell receptor system for studying memory T-cell development. Microbes Infect. 5:233-240. [DOI] [PubMed] [Google Scholar]
- 8.Clambey, E. T., H. W. Virgin, and S. H. Speck. 2000. Disruption of the murine gammaherpesvirus 68 M1 open reading frame leads to enhanced reactivation from latency. J. Virol. 74:1973-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke, S. R., M. Barnden, C. Kurts, F. R. Carbone, J. F. Miller, and W. R. Heath. 2000. Characterization of the ovalbumin-specific TCR transgenic line OT-I: MHC elements for positive and negative selection. Immunol. Cell Biol. 78:110-117. [DOI] [PubMed] [Google Scholar]
- 10.Cobbold, S. P., A. Jayasuriya, A. Nash, T. D. Prospero, and H. Waldmann. 1984. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature 312:548-551. [DOI] [PubMed] [Google Scholar]
- 11.Coppola, M. A., E. Flano, P. Nguyen, C. L. Hardy, R. D. Cardin, N. Shastri, D. L. Woodland, and M. A. Blackman. 1999. Apparent MHC-independent stimulation of CD8+ T cells in vivo during latent murine gammaherpesvirus infection. J. Immunol. 163:1481-1489. [PubMed] [Google Scholar]
- 12.Dal Canto, A. J., H. W. Virgin, and S. H. Speck. 2000. Ongoing viral replication is required for gammaherpesvirus 68-induced vascular damage. J. Virol. 74:11304-11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doherty, P. C., J. P. Christensen, G. T. Belz, P. G. Stevenson, and M. Y. Sangster. 2001. Dissecting the host response to a gamma-herpesvirus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356:581-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehtisham, S., N. P. Sunil-Chandra, and A. A. Nash. 1993. Pathogenesis of murine gammaherpesvirus infection in mice deficient in CD4 and CD8 T cells. J. Virol. 67:5247-5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flano, E., S. M. Husain, J. T. Sample, D. L. Woodland, and M. A. Blackman. 2000. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J. Immunol. 165:1074-1081. [DOI] [PubMed] [Google Scholar]
- 16.Flano, E., D. L. Woodland, and M. A. Blackman. 1999. Requirement for CD4+ T cells in V beta 4+CD8+ T cell activation associated with latent murine gammaherpesvirus infection. J. Immunol. 163:3403-3408. [PubMed] [Google Scholar]
- 17.Gangappa, S., S. B. Kapadia, S. H. Speck, and H. W. Virgin. 2002. Antibody to a lytic cycle viral protein decreases gammaherpesvirus latency in B-cell-deficient mice. J. Virol. 76:11460-11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gangappa, S., L. F. Van Dyk, T. J. Jewett, S. H. Speck, and H. W. Virgin. 2002. Identification of the in vivo role of a viral bcl-2. J. Exp. Med. 195:931-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy, C. L., L. Lu, P. Nguyen, D. L. Woodland, R. W. Williams, and M. A. Blackman. 2001. Identification of quantitative trait loci controlling activation of TRBV4 CD8+ T cells during murine gamma-herpesvirus-induced infectious mononucleosis. Immunogenetics 53:395-400. [DOI] [PubMed] [Google Scholar]
- 20.Husain, S. M., E. J. Usherwood, H. Dyson, C. Coleclough, M. A. Coppola, D. L. Woodland, M. A. Blackman, J. P. Stewart, and J. T. Sample. 1999. Murine gammaherpesvirus M2 gene is latency-associated and its protein a target for CD8+ T lymphocytes. Proc. Natl. Acad. Sci. USA 96:7508-7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim, I. J., E. Flano, D. L. Woodland, and M. A. Blackman. 2002. Antibody-mediated control of persistent γ-herpesvirus infection. J. Immunol. 168:3958-3964. [DOI] [PubMed] [Google Scholar]
- 22.Kurts, C., W. R. Heath, F. R. Carbone, J. Allison, J. F. Miller, and H. Kosaka. 1996. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J. Exp. Med. 184:923-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, M., G. M. Davey, R. M. Sutherland, C. Kurts, A. M. Lew, C. Hirst, F. R. Carbone, and W. R. Heath. 2001. Cell-associated ovalbumin is cross-presented much more efficiently than soluble ovalbumin in vivo. J. Immunol. 166:6099-6103. [DOI] [PubMed] [Google Scholar]
- 24.Liu, L., E. J. Usherwood, M. A. Blackman, and D. L. Woodland. 1999. T-cell vaccination alters the course of murine herpesvirus 68 infection and the establishment of viral latency in mice. J. Virol. 73:9849-9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lybarger, L., X. Wang, M. R. Harris, H. W. Virgin, and T. H. Hansen. 2003. Virus subversion of the MHC class I peptide-loading complex. Immunity 18:121-130. [DOI] [PubMed] [Google Scholar]
- 26.McClellan, J. S., S. A. Tibbetts, S. Gangappa, K. A. Brett, and H. W. Virgin IV. 2004. Critical role of CD4 T cells in an antibody-independent mechanism of vaccination against gammaherpesvirus latency. J. Virol. 78:6836-6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porgador, A., J. W. Yewdell, Y. Deng, J. R. Bennink, and R. N. Germain. 1997. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity 6:715-726. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 29.Shedlock, D. J., and H. Shen. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300:337-339. [DOI] [PubMed] [Google Scholar]
- 30.Smith, S. C., and P. M. Allen. 1991. Myosin-induced acute myocarditis is a T cell-mediated disease. J. Immunol. 147:2141-2147. [PubMed] [Google Scholar]
- 31.Stevenson, P. G., G. T. Belz, J. D. Altman, and P. C. Doherty. 1998. Virus-specific CD8+ T cell numbers are maintained during gamma-herpesvirus reactivation in CD4-deficient mice. Proc. Natl. Acad. Sci. USA 95:15565-15570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevenson, P. G., G. T. Belz, M. R. Castrucci, J. D. Altman, and P. C. Doherty. 1999. A gamma-herpesvirus sneaks through a CD8+ T cell response primed to a lytic-phase epitope. Proc. Natl. Acad. Sci. USA 96:9281-9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevenson, P. G., S. Efstathiou, P. C. Doherty, and P. J. Lehner. 2000. Inhibition of MHC class I-restricted antigen presentation by gamma 2-herpesviruses. Proc. Natl. Acad. Sci. USA 97:8455-8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun, J. C., and M. J. Bevan. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300:339-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tibbetts, S. A., L. Van Dyk, S. H. Speck, and H. W. Virgin IV. 2002. Immune control of the number and reactivation phenotype of cells latently infected with a gammaherpesvirus. J. Virol. 76:7125-7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Usherwood, E. J., D. J. Roy, K. Ward, S. L. Surman, B. M. Dutia, M. A. Blackman, J. P. Stewart, and D. L. Woodland. 2000. Control of gammaherpesvirus latency by latent antigen-specific CD8+ T cells. J. Exp. Med. 192:943-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Usherwood, E. J., K. A. Ward, M. A. Blackman, J. P. Stewart, and D. L. Woodland. 2001. Latent antigen vaccination in a model gammaherpesvirus infection. J. Virol. 75:8283-8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Dyk, L. F., H. W. Virgin, and S. H. Speck. 2000. The murine gammaherpesvirus 68 v-cyclin is a critical regulator of reactivation from latency. J. Virol. 74:7451-7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Virgin, H. W., and S. H. Speck. 1999. Unraveling immunity to gamma-herpesviruses: a new model for understanding the role of immunity in chronic virus infection. Curr. Opin. Immunol. 11:371-379. [DOI] [PubMed] [Google Scholar]
- 40.Weck, K. E., M. L. Barkon, L. I. Yoo, S. H. Speck, and H. W. Virgin. 1996. Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus 68. J. Virol. 70:6775-6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weck, K. E., A. J. Dal Canto, J. D. Gould, A. K. O'Guin, K. A. Roth, J. E. Saffitz, S. H. Speck, and H. W. Virgin. 1997. Murine gammaherpesvirus 68 causes severe large vessel arteritis in mice lacking interferon-gamma responsiveness: a new model for virus induced vascular disease. Nat. Med. 3:1346-1353. [DOI] [PubMed] [Google Scholar]
- 42.Weck, K. E., S. S. Kim, H. W. Virgin, and S. H. Speck. 1999. B cells regulate murine gammaherpesvirus 68 latency. J. Virol. 73:4651-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weck, K. E., S. S. Kim, H. W. Virgin, and S. H. Speck. 1999. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J. Virol. 73:3273-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yewdell, J. W., and A. B. Hill. 2002. Viral interference with antigen presentation. Nat. Immunol. 3:1019-1025. [DOI] [PubMed] [Google Scholar]
- 45.Yu, Y. Y. L., M. R. Harris, L. Lybarger, L. A. Kimpler, N. B. Myers, H. W. Virgin IV, and T. H. Hansen. 2002. Physical association of the K3 protein of gamma-2 herpesvirus 68 with major histocompatibility complex class I molecules with impaired peptide and β2-microglobulin assembly. J. Virol. 76:2796-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]